Abstract
Purpose
Although chemoradiotherapy plus resection is considered standard treatment for operable rectal carcinoma, the optimal time to administer this therapy is not clear. The NSABP R-03 (National Surgical Adjuvant Breast and Bowel Project R-03) trial compared neoadjuvant versus adjuvant chemoradiotherapy in the treatment of locally advanced rectal carcinoma.
Patients and Methods
Patients with clinical T3 or T4 or node-positive rectal cancer were randomly assigned to preoperative or postoperative chemoradiotherapy. Chemotherapy consisted of fluorouracil and leucovorin with 45 Gy in 25 fractions with a 5.40-Gy boost within the original margins of treatment. In the preoperative group, surgery was performed within 8 weeks after completion of radiotherapy. In the postoperative group, chemotherapy began after recovery from surgery but no later than 4 weeks after surgery. The primary end points were disease-free survival (DFS) and overall survival (OS).
Results
From August 1993 to June 1999, 267 patients were randomly assigned to NSABP R-03. The intended sample size was 900 patients. Excluding 11 ineligible and two eligible patients without follow-up data, the analysis used data on 123 patients randomly assigned to preoperative and 131 to postoperative chemoradiotherapy. Surviving patients were observed for a median of 8.4 years. The 5-year DFS for preoperative patients was 64.7% v 53.4% for postoperative patients (P = .011). The 5-year OS for preoperative patients was 74.5% v 65.6% for postoperative patients (P = .065). A complete pathologic response was achieved in 15% of preoperative patients. No preoperative patient with a complete pathologic response has had a recurrence.
Conclusion
Preoperative chemoradiotherapy, compared with postoperative chemoradiotherapy, significantly improved DFS and showed a trend toward improved OS.
INTRODUCTION
Radiotherapy and surgical resection are standard components of therapy for patients with stage II/III carcinoma of the rectum.1,2 Numerous randomized trials have investigated the impact of dose modifications and preoperative/postoperative administration in an effort to improve safety without compromising effectiveness, reduce the incidence of local recurrence, and significantly prolong survival.3,4
The Dutch Colorectal Cancer Group administered 25 Gy during 5 days followed by immediate total mesorectal resection and significantly reduced locoregional tumor recurrence at 2 years from 8.2% in the surgery-only arm to 2.4%.5 The addition of radiotherapy did not prolong survival compared with surgery alone. The European Organisation for Research and Treatment of Cancer (EORTC) evaluated the value of preoperative chemoradiotherapy or preoperative radiotherapy alone and postoperative chemotherapy versus preoperative radiotherapy and surgery alone. The addition of fluorouracil and leucovorin to the preoperative administration of 45 Gy during 5 weeks reduced locoregional recurrence from 17.1% to 8.5%; the 5-year overall survival (OS) did not improve with the addition of chemotherapy.6,7 A similar study conducted by the Fédération Francophone de Cancérologie Digestive in France (FFCD) compared the combination of preoperative chemotherapy (fluorouracil and leucovorin) and radiotherapy versus preoperative radiotherapy alone, with all patients receiving postoperative chemotherapy.8 The administration of postoperative chemotherapy reduced local recurrence compared with radiotherapy alone; 5-year survival did not differ between the two groups. The German Rectal Cancer Study Group trial compared preoperative to postoperative chemoradiotherapy in patients with clinical stage T3 or T4 or node-positive disease.9 The 5-year locoregional recurrence rate decreased from 13% in the postoperative group to 6%. Survival was not different between the two groups.
The NSABP R-03 (National Surgical Adjuvant Breast and Bowel Project R-03) trial was designed to determine the best time to administer multimodality therapy to patients with stage II/III carcinoma of the rectum. The primary aim of this study was to determine whether there is a difference in disease-free survival (DFS) and OS when the radiation therapy and chemotherapy are administered preoperatively compared with all therapy being administered postoperatively. Additional aims were to determine if preoperative therapy results in improvement in local recurrence rates compared with postoperative therapy, compare the proportion of patients receiving sphincter-saving surgery (SSS) in the two treatment arms, and correlate the response to preoperative therapy with DFS and OS.
PATIENTS AND METHODS
Patients
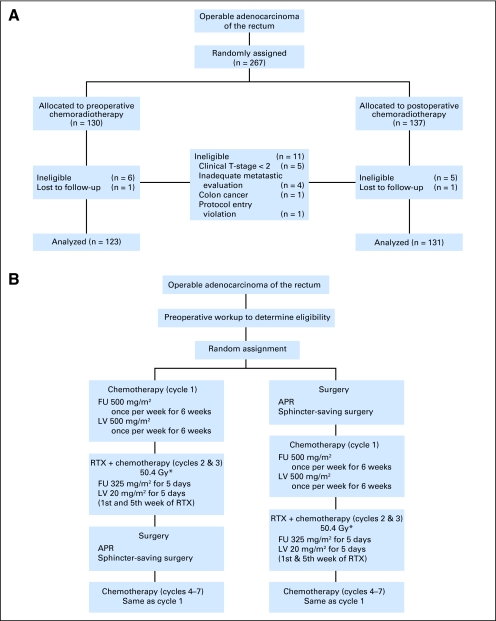
These studies were approved by institutional review committees, with assurances approved by the Department of Health and Human Services, and are in accordance with the Helsinki Declaration. Informed consent was required for participation. Patients were randomly assigned to preoperative (group 1) or postoperative (group 2) administration of adjuvant chemotherapy with radiotherapy, as indicated in the CONSORT diagram (Fig 1A). Patients were eligible if they had a histologic diagnosis of rectal adenocarcinoma, as defined by the distal border of the tumor being no more than 15 cm from the anal verge as measured using a rigid proctoscope or sigmoidoscope. Patients must have been able to begin treatment (surgery or chemotherapy) within 49 days from the histologic diagnosis. Other eligibility criteria included no radiologic evidence of metastatic disease on abdominal and pelvic computed tomography (CT) scans, an Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, adequate blood counts, and adequate hepatic and renal function. Endoscopic ultrasound was optional as a staging technique. Table 1 lists criteria for patient eligibility or ineligibility.
Fig 1.
(A) CONSORT diagram showing the flow of participants through each stage of National Surgical Adjuvant Breast and Bowel Project R-03 trial. (B) Diagram of chemotherapy and radiotherapy treatment regimens. (*) Forty-five Gy in 25 fractions with a 5.4 Gy boost within the original margins of treatment. FU, fluorouracil; LV, leucovorin; APR, anterior-posterior resection; RTX, radiation therapy.
Table 1.
NSABP R-03 Trial Patient Eligibility and Ineligibility Criteria
Eligibility criteria*
|
Ineligibility criteria
|
Abbreviations: NSABP R-03, National Surgical Adjuvant Breast and Bowel Project R-03; CT, computed tomography.
Eligible patients having histologic diagnosis by proctoscopic incisional biopsy of invasive rectal adenocarcinoma will be considered for entry in this study.
Performance status key: 0, normal activity; 1, symptoms but ambulatory; 2, in bed ≤ 50% of the time; 3, in bed > 50% of the time; 4, 100% bedridden.
Treatment
Patients were stratified according to sex and age (≤ 60 or > 60 years). Table 2 lists patient characteristics. The prescribed amount of chemotherapy according to the protocol consisted of seven cycles; the duration of cycle 1 and cycles 4 to 7 was 8 weeks including rest periods. Chemotherapy administered during radiotherapy was considered cycles 2 and 3. A schematic of chemotherapy and radiation therapy regimens is provided in Figure 1B. The pelvis was treated with 45 Gy in 25 fractions to the isocenter using a four-field box technique with a 5.4-Gy boost in three fractions to a restricted volume. All simulation portal films and dosimetry data were centrally reviewed.
Table 2.
Characteristics of Eligible Patients in NSABP R-03 Trial
| Characteristic | Preoperative (n = 123) |
Postoperative (n = 131) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| ≤ 60 | 53 | 43.1 | 59 | 45.0 |
| > 60 | 70 | 56.9 | 72 | 55.0 |
| Sex | ||||
| Male | 85 | 69.1 | 89 | 67.9 |
| Female | 38 | 30.9 | 42 | 32.1 |
| Intended procedure | ||||
| SSS | 43 | 35.0 | 44 | 32.8 |
| Non-SSS | 80 | 65.0 | 88 | 67.2 |
| Multiple tumors | ||||
| Yes | 4 | 3.3 | 1 | 0.8 |
| No | 119 | 96.8 | 130 | 99.2 |
| Palpable tumor* | ||||
| Yes | 94 | 79.0 | 111 | 85.4 |
| No | 25 | 21.0 | 19 | 14.6 |
Abbreviations: NSABP R-03, National Surgical Adjuvant Breast and Bowel Project R-03; SSS, sphincter-saving surgery.
Excludes patients with multiple tumors.
The treating physician determined the type of surgical procedure for each patient. Acceptable procedures included an abdominoperineal resection, low anterior resection (including coloanal), and local excision. Group 1 patients who were found after surgery to have a stage I tumor were to continue protocol therapy because it was not evident in which patients this was a true stage I tumor and in which this was a result of the downstaging effect of chemotherapy/radiotherapy. Group 2 patients who were found after surgery to have a stage I tumor were treated at the discretion of the investigator.
Group 1 patients who developed progressive inoperable disease (advanced locoregional or distant) while receiving chemotherapy or radiotherapy before surgery were considered to have experienced treatment failures and were treated at the discretion of the investigator. Patients who developed progressive operable disease while receiving radiotherapy and chemotherapy (cycles 2 and 3) had interruption of therapy and were offered immediate surgery. Postoperatively, the patients received the remaining four cycles of chemotherapy. Group 2 patients who were found at surgery to have inoperable or metastatic disease were classified as having experienced treatment failures (representing protocol diagnostic failures) and were treated at the discretion of the investigator. Adverse effects were graded according to National Cancer Institute Common Toxicity Criteria, version 1.
Follow-Up
Patients were assessed before being randomly assigned, every week before chemotherapy during radiotherapy, during chemotherapy every 8 weeks before the next cycle, and post-therapy every 3 months during the first and second year; during years 3 to 5, they were assessed every 6 months, and after 5 years, every 12 months. The baseline assessment included a history and physical examination, tumor measurements, performance status, measurement of carcinoembyronic antigen level, hematologic studies, serum chemistries, chest radiography, CT of abdomen and pelvis, and barium enema and/or full colonoscopic examination. Clinical response to preoperative therapy in group 1 was assessed with proctosigmoidoscopy after patients completed the first cycle of chemotherapy (within 2 weeks of the beginning of radiotherapy) and on completion of radiotherapy (no sooner than 2 weeks after completion of radiotherapy, but before tumor removal).
The diagnosis of recurrence was made on the basis of imaging and, if possible, cytologic analysis or biopsy. An elevated carcinoembyronic antigen as a solitary finding was not acceptable evidence of treatment failure.
Statistical Methods
Patients were randomly assigned to either pre- or postoperative chemotherapy and radiation and were stratified by age (≤ 60 years or > 60 years), sex, and randomizing institution using a biased coin minimization algorithm.10
The protocol-specified primary end points were DFS and OS. Data on all patients who were eligible and had follow-up were used in the primary analyses, and patients were analyzed according to their randomly assigned therapy regardless of therapy actually received. DFS was defined as the time from random assignment to recurrence, second primary cancer (excluding basal cell carcinomas of the skin and carcinoma in situ of the cervix), or death without evidence of recurrence or second primary cancer. OS was defined as the time from random assignment to death as a result of all causes. These analyses were supplemented by analyses of time to locoregional recurrence (after the completion of therapy, including surgery, evidence of tumor in the pelvis, including the presacrum, pelvic sidewalls, base of the bladder and the perineum, or at the anastomotic site) and time to recurrence (time to locoregional or distant recurrence of rectal cancer) as a first event. Patients who had inoperable disease, gross residual disease, or involved surgical margins were also considered to have locoregional recurrence. Patients diagnosed concurrently with both locoregional and distant recurrence were considered to have locoregional recurrence.
The Kaplan-Meier method was used to construct OS and DFS curves. Plots showing the incidence of recurrence and locoregional recurrence by time were generated by using a cause-specific incidence approach as defined by Gaynor et al.11 Although plots show data through 7 years, all follow-up data were used in the statistical calculations. The log-rank test was used to compare distributions, stratifying by age and sex. Cox proportional hazards models were used to compute relative risks and 95% CIs. Analyses were stratified by age and sex. Because of the small number of events, local recurrence models and outcomes by pathologic response status were not stratified. Hazard ratios (HRs) compared preoperative relative to postoperative arms.
Analyses are based on the cohort of patients eligible with follow-up, except where otherwise specified. All P values are two-sided. The χ2 test was used to compare proportions. The study was designed to have a power greater than 0.81 to detect a 33% reduction in death rate in the preoperative group. The required sample size was 900 patients.
For the analyses of pathologic response and SSS, results are presented as proportions. Patients with missing data who had a treatment-related death or recurrence/progression of disease before evaluation of a response (or within 7 months of being randomly assigned for preoperative patients who refused surgery) were included as treatment failures. Patients with missing data who were event free or had a non–treatment-related death or a second primary cancer were not included. Ten patients who were randomly assigned to postoperative therapy refused their assigned treatment (crossovers) and received preoperative therapy instead. These 10 patients were removed from the pathologic response and SSS analyses. One postoperative patient with squamous cell cancer was removed from the pathologic nodal status analysis.
RESULTS
Accrual
The first patient was entered on August 12, 1993, and accrual continued until June 30, 1999. Two hundred sixty-seven patients were accrued (130 to the preoperative therapy arm and 137 to the postoperative therapy arm; Fig 1B).
After 6 years of slower-than-anticipated accrual, the trial was terminated short of the planned goal of 900 patients. The combination of fewer patients but longer follow-up than originally anticipated resulted in a reduction in the planned power of 0.81 to 0.54 for the primary end point of OS and 0.61 for DFS.
Pathologic Response in Preoperative Patients
Ten patients were considered to be not evaluable for pathologic response. Two patients died before surgery as a result of a cardiac event that was considered to be non–treatment related, one developed cancer of the lung and did not have surgery, five refused surgery, and two patients were free of tumor at surgery but their nodal status was unknown. The two latter patients had clinical responses (one complete and one partial) before surgery. Seven patients who did not have surgery because of treatment-related causes were included in the analysis as failures. Of the 113 evaluable patients, 17 (15.0%) were determined to be free of disease on the basis of pathology. When we examined tumor response only and ignored nodal status, 19 (16.5%) of 115 patients had complete pathologic responses (cPRs). Of those patients who had a resection and did not have an event at surgery, there were 17 complete pathologic responders and 86 who were not complete responders. There were no recurrences among the pathologic responders, but there were three deaths as a first event. The 5-year OS from surgery was 87.8% for responders and 79.9% for those who were not complete responders (P = .42). Corresponding values were 87.8% and 70.6% (P = .22) for DFS and 0% and 24.7% (P = .04) for the cumulative incidence of recurrence, respectively.
Nodal Status
Nodal status was available for 105 preoperative and 118 postoperative patients. There was a significant benefit relative to nodal status because 66.7% of the preoperative patients had no positive lymph nodes versus 52.5% of the postoperative patients (P = .04). The percentages of patients with three or fewer positive nodes were 86.7% for preoperative and 69.5% for postoperative (P = .004).
SSS
In this study, 47.8% (55 of 115) of the preoperative patients and 39.2% (47 of 120) of the postoperative patients had SSS (P = .227). At 5 years after random assignment, 33.9% (39 of 115) of the preoperative patients maintained their sphincter and were free of disease versus 24.2% (29 of 120) of the postoperative patients (P = .13).
Postoperative Complications
Postoperative complication rates were similar in both arms. Of the preoperative patients, 25.0% had a complication compared with 22.6% of postoperative patients.
Toxicities
Toxicity data were collected for all patients who began protocol chemotherapy for each cycle of therapy and for the 3-month period after completion of protocol therapy. All available toxicity data were analyzed (126 preoperative, 99 postoperative) and are listed in Table 3. The difference in available data is largely due to those postoperative patients who were not given protocol therapy after being diagnosed as stage I or IV. Virtually all the patients on the study experienced some toxicity. Toxicities were reasonably balanced between arms with the exception of diarrhea; 24% of the patients on the preoperative therapy arm experienced grade 4 diarrhea versus 13% on the postoperative therapy arm. Further analysis indicated that this difference occurred primarily in the first three cycles of chemotherapy. During the first chemotherapy cycle of the preoperative therapy arm, 11% of the patients experienced grade 4 diarrhea versus 7% of those on the postoperative arm. The corresponding percentages during the chemotherapy plus radiation cycles were 12% v 3%, respectively. In the remaining chemotherapy cycles, the percentages of patients with grade 4 diarrhea were 7% and 5%, respectively. Overall, grade 5 toxicity occurred in 5% of preoperative patients and 3% of postoperative patients.
Table 3.
Worst Grade of Toxicity per Patient by Arm in NSABP R-03 Trial According to National Cancer Institute Common Toxicity Criteria, Version 1.0
| Toxicity | Any Toxicity (%) |
Grade 3, 4, or 5 (%) |
Grade 4 or 5 (%)* |
|||
|---|---|---|---|---|---|---|
| Preoperative Arm(n = 126) | Postoperative Arm(n = 99) | Preoperative Arm(n = 126) | Postoperative Arm(n = 99) | Preoperative Arm(n = 126) | Postoperative Arm(n = 99) | |
| Overall toxicity | 99 | 97 | 52 | 49 | 33 | 23 |
| Diarrhea | 82 | 88 | 36 | 29 | 24 | 14 |
| Nausea | 66 | 59 | 12 | 7 | NA | NA |
| Vomiting | 31 | 28 | 7 | 8 | 3 | 3 |
| Stomatitis | 30 | 27 | 3 | 3 | 1 | 2 |
| Leukopenia | 71 | 68 | 10 | 8 | 2 | 1 |
| Granulocytopenia | 52 | 52 | 13 | 15 | 3 | 4 |
NOTE. In National Cancer Institute Common Toxicity Criteria, Version 1.0, grade 3 diarrhea was defined as an increase of seven to nine stools over baseline every 24 hours (severe). Grade 4 diarrhea was defined as having 10 or more stools per day, grossly bloody diarrhea, or need for parenteral support.
Abbreviations: NSABP R-03, National Surgical Adjuvant Breast and Bowel Project R-03; NA, not applicable.
Deaths within 30 days of last chemotherapy dose or random assignment occurred in 5% of preoperative patients and 3% of postoperative patients. However, several of these deaths appeared to be unrelated to treatment. Probable or possible treatment-related mortality was 3% for preoperative and 1% for postoperative patients. A total of nine deaths occurred within 30 days of random assignment or last dose of chemotherapy administration; four deaths did not appear to be related to protocol therapy. Other deaths comprised two patients with a history of severe coronary artery disease who experienced cardiac arrest with no evidence of chemotherapy adverse effects, one patient with a dissecting thoracic aortic aneurysm, and one patient who died postoperatively of aspiration pneumonia who had not received any chemotherapy. The number of deaths considered possibly or probably related to protocol therapy were four (3.2%) of 126 in the preoperative group; two were due to complications from chemotherapy-induced dehydration related to diarrhea, nausea, and/or vomiting; one was due to perforated sigmoid colon in a patient with fixation of the bowel to a large rectal tumor; one was due to postoperative acute respiratory distress syndrome and peritonitis. In the postoperative group, the only death (one [1%] of 99) was due to dehydration related to chemotherapy-induced diarrhea, nausea, and vomiting. We believe these rates of treatment-related fatality are within the acceptable range for combined modality adjuvant therapy of high-risk rectal cancer in clinical practice. Clinicians should be aware of the potential for severe diarrhea, nausea, and vomiting, which can lead to dehydration; should monitor patients carefully, particularly during radiation therapy; and should treat dehydration aggressively with intravenous fluids.
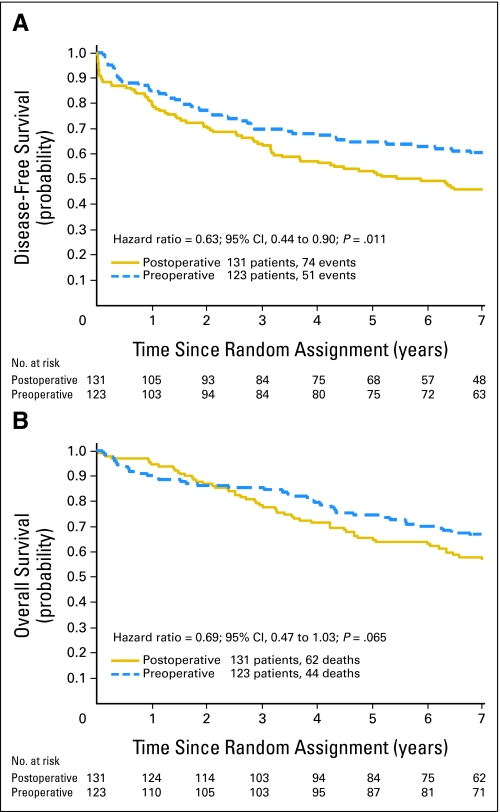
DFS
One hundred twenty-five patients (51 preoperative, 74 postoperative) had a recurrence, had a second primary cancer, or have died. The 5-year DFS for preoperative patients was 64.7% v 53.4% for postoperative patients (Fig 2A). The HR was 0.629 (95% CI, 0.439 to 0.902; P = .011), indicating a benefit for preoperative therapy.
Fig 2.
(A) Disease-free survival of 254 patients randomly assigned to preoperative or postoperative chemoradiotherapy, National Surgical Adjuvant Breast and Bowel Project R-03 (NSABP R-03) trial. (B) Overall survival of 254 patients randomly assigned to preoperative or postoperative chemoradiotherapy, NSABP R-03.
OS
Of 254 patients, 106 (44 preoperative, 62 postoperative) have died. Surviving patients were observed for a median of 8.4 years (range, 10.9 months to 12.9 years). The 5-year OS for preoperative patients was 74.5% v 65.6% for postoperative patients (Fig 2B). The HR comparing preoperative with postoperative patients was 0.693 (95% CI, 0.468 to 1.026; P = .065), suggesting a possible survival benefit for preoperative therapy.
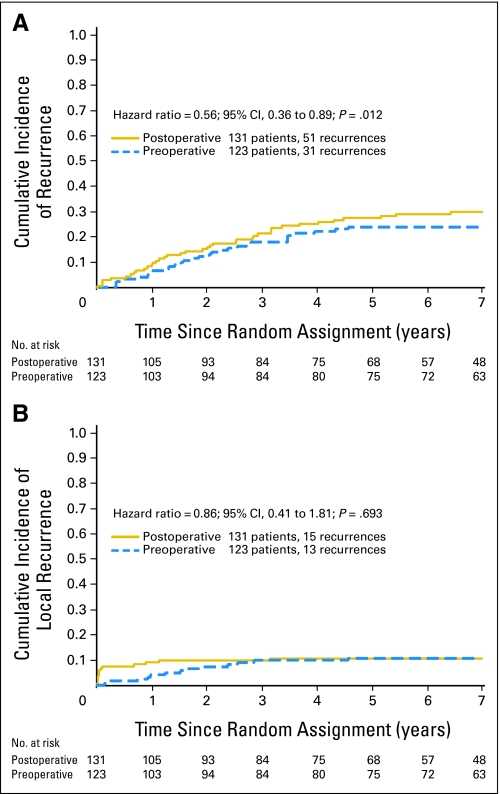
Recurrence-Free Interval
Thirty-one preoperative patients and 51 postoperative patients had a recurrence as a first event. The 5-year cumulative incidence of recurrence was 23.9% for preoperative and 27.5% for postoperative patients (HR, 0.564; 95% CI, 0.360 to 0.885; P = .0115), indicating a benefit for preoperative therapy (Fig 3A).
Fig 3.
(A) Cumulative incidence of recurrence in 254 patients who underwent complete resection of rectal cancer and chemoradiotherapy, according to treatment group, National Surgical Adjuvant Breast and Bowel Project R-03 (NSABP R-03) trial. (B) Cumulative incidence of local recurrence in 254 patients who underwent complete resection of rectal cancer and chemoradiotherapy, according to treatment group, NSABP R-03.
Locoregional Recurrence
Thirteen preoperative patients and 15 postoperative patients had a locoregional recurrence as a first event. The 5-year cumulative incidence of locoregional recurrence was 10.7% for each treatment arm (HR, 0.86; 95% CI, 0.41 to 1.81; P = .693; Fig 3B).
DISCUSSION
The NSABP R-03 trial has shown that the preoperative administration of radiation therapy and chemotherapy significantly prolonged DFS compared with postoperative administration and demonstrated a trend toward improved OS. We were not able to demonstrate any decrease in the local recurrence rates nor any significant increase in the proportion of patients undergoing sphincter-saving procedures in the preoperative group, although the statistical power to detect such differences was low because of the small number of events in our trial.
Local recurrence in this trial for each treatment arm of 10.7% at 5 years was greater than that reported in the Dutch Colorectal Cancer Group.5 The rate of local recurrence at 2 years was reduced from 8.2% to 2.4% with the addition of preoperative radiotherapy. The difference in recurrence between the Dutch study and NSABP R-03 could be due to the length of follow-up (2 v 5 years), dose (25 v 50.4 Gy), timing of radiotherapy (5 days v 5 weeks), type of surgical procedure (mandatory total mesorectal excision in the Dutch trial), and patient eligibility (inclusion of stage I patients in the Dutch study). Equivalent rates of local recurrence between preoperative and postoperative therapy in the NSABP R-03 trial are difficult to interpret because there were only 28 locoregional events observed in this trial, and the statistical power to detect a 33% reduction in local recurrence was only 18%.
Differences in locoregional recurrence rates between preoperative and postoperative groups were observed in the German Rectal Cancer Study Group trial. The 5-year cumulative incidence of local recurrence was significantly reduced from 13% to 6% (P < .006) with the preoperative administration of chemoradiotherapy.9 The difference could be influenced by the type of surgical procedure. Not every patient in NSABP R-03 underwent a total mesorectal excision, compared with 100% of the patients in the German trial.
To our knowledge, the NSABP R-03 trial is the first to demonstrate a significant improvement in recurrence-free survival and DFS with preoperative multimodality therapy compared with postoperative treatment. This observation is contrary to the results from other phase III trials with different treatment regimens.5–9 In the German Rectal Cancer Study Group, the 5-year DFS was 68% for the preoperative and 65% for the postoperative group.9 The radiotherapy dose in the NSABP R-03 study was similar to that in the German study, but the chemotherapy regimens were different. The NSABP R-03 5-year cumulative incidence of recurrence was superior to that in the German trial in the preoperative (23.9% v 36%) and postoperative (27.5% v 38%) groups. However, preoperative therapy in the NSABP R-03 trial increased the incidence of grade 4 or 5 toxicities to 33%, compared with 23% in the postoperative group. This may be a biased comparison, given that only the subset of postoperative patients who had stage II or III disease were to have protocol therapy. Some of the postoperative patients (stages I and IV) may not have received any chemotherapy, because the treatment was at the discretion of the treating physician.
Fifteen percent of patients achieved a cPR, and no recurrence had occurred at 5 years. A cPR did not significantly correlate with improved OS and DFS in our study, perhaps because of the small number of events and low statistical power to detect significant differences between complete responders and and those who were not complete responders. Other trials have demonstrated a significant correlation between tumor regression and improved DFS.12–14 An important difference between NSABP R-03 and these studies is the classification of a pathologic response. In the other studies, treatment response was assessed using a standardized 5-point grading system for tumor regression, as initially described by Dworak.15 It is not known whether using a more rigorous tumor regression grading system in the current trial might have increased the accuracy of a complete response and demonstrated an improved DFS.
The combined modality regimen used in NSABP R-03 was generally well tolerated but does have the capability of causing severe toxicity (particularly diarrhea) in a minority of patients. Clinicians should be aware of the potential for several diarrhea, nausea, and vomiting, which can lead to dehydration; should monitor patients carefully particularly during radiation therapy; and should treat dehydration aggressively with intravenous fluids.
In conclusion, a significant DFS benefit was achieved with preoperative compared with postoperative chemoradiotherapy and is the recommended treatment for patients with locally advanced rectal cancer.
Acknowledgment
We thank Barbara C. Good, PhD, and Wendy L. Rea for editorial assistance, and Christine Rudock for assistance in preparing figures.
Footnotes
See accompanying editorial on page 5115
Supported by Public Health Service Grants No. U10-CA-37377, U10-CA-69974, U10-CA-12027, U10-CA-69651, and U24-CA-114732 from the National Cancer Institute, Department of Health and Human Services.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: PDQ: NSABP-R-03.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Melvin Deutsch
Administrative support: Greg Yothers, Luis Baez-Diaz, Norman Wolmark
Provision of study materials or patients: Melvin Deutsch, Luis Baez-Diaz
Collection and assembly of data: Mark S. Roh, Greg Yothers, Melvin Deutsch, Luis Baez-Diaz, Carol S. Ursiny
Data analysis and interpretation: Mark S. Roh, Linda H. Colangelo, Michael J. O'Connell, Greg Yothers, Melvin Deutsch, Carmen J. Allegra, Norman Wolmark
Manuscript writing: Mark S. Roh, Linda H. Colangelo, Michael J. O'Connell, Greg Yothers, Carmen J. Allegra, Nicholas J. Petrelli
Final approval of manuscript: Mark S. Roh, Linda H. Colangelo, Michael J. O'Connell, Greg Yothers, Melvin Deutsch, Carmen J. Allegra, Morton S. Kahlenberg, Luis Baez-Diaz, Carol S. Ursiny, Nicholas J. Petrelli, Norman Wolmark
REFERENCES
- 1.Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: A systematic overview of 8,507 patients from 22 randomized trials. Lancet. 2001;358:1291–1304. doi: 10.1016/S0140-6736(01)06409-1. [DOI] [PubMed] [Google Scholar]
- 2.Cammà C, Giunta M, Fiorica F, et al. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA. 2000;284:1008–1015. doi: 10.1001/jama.284.8.1008. [DOI] [PubMed] [Google Scholar]
- 3.Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324:709–715. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 4.Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92:388–396. doi: 10.1093/jnci/92.5.388. [DOI] [PubMed] [Google Scholar]
- 5.Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 6.Bosset JF, Calais G, Mineur L, et al. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: Preliminary results–EORTC 22921. J Clin Oncol. 2005;23:5620–5627. doi: 10.1200/JCO.2005.02.113. [DOI] [PubMed] [Google Scholar]
- 7.Bosset JF, Calais G, Mineur L, et al. Preoperative radiation in rectal cancer: Effect and timing of additional chemotherapy, 5-year results of the EORTC 22921 trial. J Clin Oncol. 2005;23(suppl):247s. doi: 10.1200/JCO.2005.02.113. abstr 3505. [DOI] [PubMed] [Google Scholar]
- 8.Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: Results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 9.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 10.White SJ, Freedman LS. Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer. 1978;37:849–857. doi: 10.1038/bjc.1978.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: Examples from clinical oncology data. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 12.Kaminsky-Forrett MC, Conroy T, Luporsi E, et al. Prognostic implications of downstaging following preoperative radiation therapy for operable T3–T4 rectal cancer. Int J Radiat Oncol Biol Phys. 1998;42:935–941. doi: 10.1016/s0360-3016(98)00345-9. [DOI] [PubMed] [Google Scholar]
- 13.Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol. 2005;23:8688–8696. doi: 10.1200/JCO.2005.02.1329. [DOI] [PubMed] [Google Scholar]
- 14.Vecchio FM, Valentini V, Minsky BD, et al. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:752–760. doi: 10.1016/j.ijrobp.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]