Abstract
Purpose
To evaluate the maximum-tolerated dose (MTD), safety profile, and immunogenicity of two chimeric, B-cell epitopes derived from the human epidermal growth factor receptor (HER2) extracellular domain in a combination vaccine with a promiscuous T-cell epitope (ie, MVF) and nor-muramyl-dipeptide as adjuvant emulsified in SEPPIC ISA 720.
Patients and Methods
Eligible patients with metastatic and/or recurrent solid tumors received three inoculations on days 1, 22, and 43 at doses of total peptide that ranged from 0.5 to 3.0 mg. Immunogenicity was evaluated by enzyme-linked immunosorbent assay, flow cytometry, and HER2 signaling assays.
Results
Twenty-four patients received three inoculations at the intended dose levels, which elicited antibodies able to recognize native HER2 receptor and inhibited both the proliferation of HER2-expressing cell lines and phosphorylation of the HER2 protein. The MTD was determined to be the highest dose level of 3.0 mg of the combination vaccine. There was a significant increase from dose level 1 (0.5 mg) to dose level 4 (3.0 mg) in HER2-specific antibodies. Four patients (one each with adrenal, colon, ovarian, and squamous cell carcinoma of unknown primary) were judged to have stable disease; two patients (one each with endometrial and ovarian cancer) had partial responses; and 11 patients had progressive disease. Patients with stable disease received 6-month boosts, and one patient received a 20-month boost.
Conclusion
The combination vaccines were safe and effective in eliciting antibody responses in a subset of patients (62.5%) and were associated with no serious adverse events, autoimmune disease, or cardiotoxicity. There was preliminary evidence of clinical activity in several patients.
INTRODUCTION
ErbB2 (human epidermal growth factor-2; HER2) is overexpressed in many epithelial-derived cancers,1 including breast cancer, and its expression is associated with a poor prognosis and a high risk of cancer relapse.2 Trastuzumab (Herceptin; Genentech, San Francisco, CA), a humanized, immunoglobulin G1 (IgG1), HER2, monoclonal antibody strongly inhibits the growth of HER2-overexpressing cell lines and xenografts in preclinical models.3,4 In patients with metastatic, HER2-overexpressing breast cancer, administration of trastuzumab alone or in combination with paclitaxel produced response rates between 20% and 50%5,6 and prolonged disease-free survival.7,8 Targeting HER2 with another recombinant humanized monoclonal antibody (ie, 2C4), pertuzumab (Omnitarg; Genentech) prevents ligand-induced heterodimerization with other HER2 receptors. Combination of these agents could disrupt both signaling pathways, resulting in additive/synergistic tumor growth inhibition.9
To date, most peptide, cancer vaccine strategies seek to induce a cellular, antigen-specific, T-cell response.10–12 T-cell immune responses also have been identified in patients with cancer whose tumors overexpress HER2.13–15 Disis et al13 demonstrated that patients with HER2-positive breast cancer have pre-existent T- and B-cell immunity against HER216 and found that CD4+ T-cell peptides elicited immunity to HER217 in three clinical trials.18–20
Peptide vaccines against weakly immunogenic self proteins, such as HER2, are attractive because of the safety profiles. B-cell epitopes do not have specific human leukocyte antigen (HLA) restrictions, as cytotoxic T lymphocytes or T helper cell epitopes do, and therefore are potential vaccine candidates. Vaccines that target B-cell responses could avoid the limitations of trastuzumab/pertuzumab by eliciting a long-lasting, endogenous immune response. Despite the clinical success of passive immunotherapy with trastuzumab, humanized monoclonal antibody therapy is associated with limitations that stem from the short half-life of IgG, the need for repeated treatments, and the high cost of treatment. Active immunotherapy with peptide vaccines by using HER2, B-cell epitopes as chimeric peptides with a promiscuous T-cell epitope (ie, MVF) derived from the measles virus fusion protein could trigger the patient's immune system to respond by producing antibodies to the epitope.
We identified sequences 316 to 339 and 628 to 647 of HER2 as potential B-cell epitopes by computer-aided analysis, and we have demonstrated preclinical in vitro and in vivo antitumor properties.21,22 The study described here is a phase I, active-immunotherapy, clinical trial in patients with metastatic cancer that used a combination of MVF-316 to MVF-339 and MVF-628 to MVF-647 with nor-muramyl-dipeptide (n-MDP) adjuvant emulsified in Montanide ISA 720 (SEPPIC, Inc., Paris, France). The goals of this study were to determine the safety, assess the optimal dosing, and measure the immunogenicity of the vaccine.
PATIENTS AND METHODS
Eligibility Criteria
The clinical trial was conducted under an investigational new drug application (BB-IND-9803) approved by the Food and Drug Administration and the Ohio State University human institutional review board. Patients were required to meet the following eligibility criteria: stable disease for at least 3 months with a prior history of treated brain metastases; ambulatory, with an European Cooperative Oncology Group Zubrod performance status of 0, 1, or 2; and at least 4 weeks past any prior surgery, cytotoxic chemotherapy, other immunotherapy, hormonal therapy, or radiation therapy. Patients were excluded if they had a significant concurrent illness; uncontrolled or severe cardiac disease; active viral hepatitis, HIV, or other infectious agents; autoimmune disease; anaphylactic response to the vaccine; or corticosteroid requirement. Eligible patients were assessed by physical examination, medical history, chest x-ray, and computed tomography (CT) scan of the brain. Patients were skin-tested for immediate hypersensitivity; those with a positive skin test (> 20 mm) were not eligible for the trial. Patients gave signed informed consent before initiation of therapy.
Chimeric Peptide Vaccines, Adjuvant, and Vehicle
The combination vaccine was a mixture of two synthetic peptides that consisted of B-cell epitopes of HER2 that corresponded to amino acids 628 to 647 (INGTHSCVDLDDKGCPAEQR) and 316 to 339 (LHNQEVTAEDGTQRAEKCSKPCA) and that were fused via a four-residue linker sequence (GPSL) to the Th (MVF) epitope that corresponded to amino acids 288 to 302 (KLLSLIKGVIVHRLEGVE).22 The GMP peptide and n-MDP (N-acetyl-glucosamine-3yl-acetyl-L-alanyl-D-isoglutamine) met the all Food and Drug Administration and US Pharmacopeia requirements for sterility (ie, bacterial/fungal), endotoxins, and potency. The saline-oil phase vehicle (Montanide ISA 720; SEPPIC Inc), was a kind gift from SEPPIC (Paris, France) and was used as an emulsifying agent with approved certificate of analyses for toxicity, emulsifying property, and sterility.
Vaccination Protocol
The study was performed as a dose-escalating safety trial. The vaccine emulsified with n-MDP and Montanide ISA 720 vehicle was administered intramuscularly into the gluteus maximus muscle, and subsequent injections were in alternating muscles. Six patients at each dose level received three inoculations of the combination vaccines at 3-week intervals. The dose levels were 0.25, 0.5, 1.0, and 1.5 mg each. Three patients were treated at each dose level and were observed for a minimum of 9 weeks. If no patients or one of these patients experienced dose-limiting toxicity (DLT), an additional three patients were entered at that dose level and were observed for a minimum of 9 weeks. If two or three of the initial three patients at the dose level experienced DLT, additional enrollment at the dose level was terminated and the previous dose level was identified as the maximum-tolerable dose (MTD). In the absence of dose-limiting toxicity, successive cohorts of patients were entered onto the protocol at increasing doses of peptide vaccine. Thus, the MTD was defined as dose level 4, because no patients or only one patient experienced DLT for each dose level.
Toxicity and Response Assessment
Toxicity was assessed by using the National Cancer Institute Common Terminology Criteria of Adverse Events, version 3.0. Patients who experienced any grade 3 nonhematologic or hematologic toxicity, including grade 3 flu-like symptoms or any grade 3 injection-site reaction, were considered to have experienced a DLT. In the absence of DLT, successive cohorts of patients were entered onto the protocol at increasing dose levels. Radiologic assessment was done by CT or magnetic resonance imagine (MRI; as long as the same consistent measure was used serially) every 8 weeks, and responses were measured according to RECIST (Response Evaluation Criteria in Solid Tumors.
Procurement of Patient Serum
Peripheral blood (40 ml) samples were drawn from patients into heparinized tubes, were centrifuged for the isolation of plasma, and were frozen at −70°C after purification. Protein concentration of each sample was measured by Coomassie. Blood samples (8 mL) were collected monthly after the final vaccination until no antibody response was detected.
Cell Lines, Antibodies, and Protein
Cell culture medium, fetal calf serum and supplements were purchased from Invitrogen Life Technologies (Carlsbad, CA). The human breast tumor cell line BT-474 (American Type Culture Collection, Manassas, VA) was maintained according to the supplier's guidelines. AG825, a selective HER2/neu kinase inhibitor (Calbiochem, San Diego, CA), human recombinant HER2 protein (rhHER2, MBS1432470, HER2 amino acid sequence 23-419; BioSource, San Diego, CA) and Herceptin (Genentech) were provided by William Carson, MD.
Enzyme-Linked Immunosorbent Assay
The presence of antibodies specific for the HER2 peptide vaccine in patient serum was directly assessed by using enzyme-linked immunosorbent assay (ELISA), as described previously.21 Briefly, 96-well plates were coated with individual immunogen (ie, MVF-316 to MVF-339, MVF-628 to MVF-647) or rhHER2 antigen overnight, patient serum (in a 1:4 dilution) in phosphate-buffered saline (PBS) containing 0.1% BSA and 0.01% Tween-20 (PBT buffer) was added in duplicate, and the serum was serially diluted at a 1:2 ratio in PBT. Goat-antihuman IgG conjugated to horseradish peroxidase and substrate were added to the plate for detection. Matched-patient preserum was subtracted from all samples. For the detection of antibody isotype, secondary antibodies against human IgG1, 2, 3, and 4 conjugated to alkaline phosphatase were used. p-nitrophenyl phosphate disodium salt substrate was added, and the reaction was stopped with 2N NaOH.
Flow Cytometry
BT-474 (1 × 106) cells were incubated with patient serum in 100 μL of 2% FCS in phosphate-buffered saline (PBS) for 2 hours at 4°C. Presera was used as a negative control, and trastuzumab was used as a positive control. Unbound antibodies were removed with PBS, and the cells were incubated with fluorescein isothiocyanate–conjugated antihuman antibody for 30 minutes at 4°C in 100 μL of 2% FCS in PBS. Cells were washed in PBS and were fixed in 1% formaldehyde before they were analyzed by Coulter ELITE flow cytometer (Coulter, Hialeah, FL). A total of 10,000 cells were gated by light-scatter assessment before single-parameter histograms were drawn and smoothed.
The proliferation assay was performed as previously described23,24 with BT-474 (2 × 104 per well) in 96-well, flat-bottom plates overnight. Purified patient sera were added, were incubated for 1 hour at 37°C followed by the addition of 10% heregulin (R&D Systems, Minneapolis, MN), and were additionally incubation for 72 hours before addition of MTT. After extraction with buffer, plates were incubated overnight at 37°C and were read on an ELISA reader at 570 nm with a 655-nm background.
The HER2 phosphorylation assay was performed by using BT-474 (1 × 106 per well), as described previously23,24 in six-well plates, which were incubated at 37°C overnight. Then, 100 μg of patient sera were added and were incubated at room temperature for 1 hour before addition of HRG (5 nmol/L per well). Then, 1 mL of RIPA lyses buffer was added after incubation and removal of buffer; plates were rocked at 4°C for 30 minutes; lysates were removed; sera were spun at 13,000 × g; and supernatants collected. Phosphorylation was determined by DuoSet IC (R&D Systems) for total human phospho-HER2, according to the manufacturer directions.
Antibody-Dependent Cellular Cytotoxicity
Antibody-dependent cellular cytotoxicity (ADCC) was measured by using the nonradioactive aCella-TOX kit (Cell Technology, Mountain View, CA) according to the manufacturer's recommendations. A total of 10,000 BT-474 target cells, 50 μg of each of the patient antibodies, trastuzumab (50 μg as positive control) and human peripheral-blood mononuclear cells (PBMCs; effector cells, with a target ratio of 100:1, 20:1, and 4:1) were used. Normal human and mouse IgGs served as negative controls.
RESULTS
Patients
Twenty-four patients were enrolled (Table 1) on the study; the mean age was 62 years; and patients had a variety of malignancies, which included breast (n = 5), ovarian (n = 5), colorectal (n = 4), endometrial (n = 2), cervical (n = 1), pancreatic (n = 1), adrenal (n = 1), gastrointestinal stromal tumor (n = 1), leiomyosarcoma (n = 1), and unspecified squamous cell cancer (n = 1). Among the patients, there were 19 women and five men. Twenty-one patients were white, and three were African American.
Table 1.
Patient Demographic and Clinical Characteristics
| Patient by Cohort | Characteristic |
|||||||
|---|---|---|---|---|---|---|---|---|
| Age (years) | Sex | Malignancy | ECOG PS | Metastatic Site | Prior Chemotherapy | Clinical Benefit | HER2 Positivity Status | |
| Cohort 1:1A | 73 | F | Colon | 0 | Lung | 2 | +SD | — |
| 1B | 75 | F | SCC | 0 | Thigh | 2 | +SD | FISH |
| 1C | 65 | F | Ovarian | 1 | Liver cyst | 5 | — | FISH |
| 1D | 78 | F | Endometrial | 2 | Peritoneum | 2 | +PR*** | — |
| 1E | 74 | F | Breast | 1 | Breast, bone | 8 | — | IHC3 |
| 1F | 37 | F | Breast | 0 | Lung | 3 | — | IHC3 |
| Cohort 2:2A | 57 | M | Adrenal | 1 | Lung, liver | 2 | +SD | — |
| 2B | 55 | M | Pancreas | 0 | Pancreas | 3 | — | — |
| 2C | 67 | F | Ovarian | 1 | Liver | 9 | — | — |
| 2D | 59 | M | Rectal | 1 | Lung, liver | 3 | — | — |
| 2E | 53 | F | Leiomyosarcoma | 0 | Lung | 4 | — | — |
| 2F | 75 | F | Endometrial | 1 | Lung | 2 | — | FISH |
| Cohort 3:3A | 66 | F | Breast | 1 | Liver | 6 | — | IHC3 |
| 3B | 41 | F | Breast | 0-1 | Liver | 4 | — | IHC3 |
| 3C | 46 | F | Rectal | 2 | Periaortic | 2 | — | — |
| 3D | 58 | M | Colon | 0 | Liver, lung | 3 | — | — |
| 3E | 61 | M | Colorectal | 0 | Liver, bone | 6 | — | — |
| 3F | 66 | F | Breast | 0 | Lung, chest | 3 | — | IHC2 |
| Cohort 4:4A | 65 | F | Ovarian | 0 | Pelvis | 1 | — | IHC2 |
| 4B | 35 | F | GIST | — | Lung | 1 | — | — |
| 4C | 70 | F | Cervical | 1 | Small intestine | 3 | — | — |
| 4D | 74 | F | Ovarian | 0 | Abdomen | 7 | +SD | — |
| 4E | 71 | F | Ovarian | 0 | Abdomen | 3 | +SD | — |
| 4F | 49 | F | NSCLC | 0 | Abdomen | 3 | — | — |
Patients with histologically confirmed metastatic and/or recurrent solid tumors were eligible for enrollment after having received standard therapy and were no longer responding. Patients were not required to have HER-2 over-expression for enrollment.
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; HER2, human epidermal growth factor receptor 2; +, clinical response; —, no clinical response; SD, stable disease; SCC, squamous cell carcinoma; FISH, fluorescent in situ hybridization; PR, partial response; IHC, imunohistochemistry; GIST, gastrointestinal stromal tumor; NSCLC, non–small-cell lung cancer.
Toxicity
Patients were evaluated for toxicities at each visit. Five patients (21%) experienced serious adverse events (SAEs); two were hospitalized for reasons unrelated to vaccine therapy; and one patient developed grade 3 diarrhea. One patient had grade 3 pain that was grade 2 pain at baseline. One patient died on day 118 but had been removed from the study on day 63 because of progressive disease. This same patient had grade 3 pain and hyperglycemia that was grade 1 pain and hyperglycemia at baseline. None of the patients who received the vaccine exhibited cardiac toxicity.
Clinical Response/Benefit
Of 24 patients who received three immunizations of the HER2 peptide vaccines (Table 1), six patients (25%) demonstrated clinical benefit after radiologic reassessment. Only one patient (1B) was HER2 positive, whereas the others were all negative. Two patients (1A with colon cancer and 1B with squamous cell carcinoma) had high antibody response and demonstrated stable disease. One patient (1D with endometrial cancer) showed high antibody response and had a partial response after a fourth 6-month injection. This patient received additional boosts three times yearly, and the last vaccination in May 2008 showed extended clinical benefit at 4 years after the initial vaccination. One patient at dose level 2 (2A with adrenal cancer) produced high antibody levels and had stable disease. Two patients with ovarian cancer (4D, who had partial response, and 4E, who had stable disease) produced high IgG antibody responses and had clinical benefit. Three of the six patients received a fourth inoculation. Our results indicate that, at dose level 4, combination vaccines (1.5 mg each) produced a sustained, high antibody (IgG3 and IgG4) response (Figs 1 and 2) against both MVF-316 to MVF-339 and MVF-628 to MVF-647.
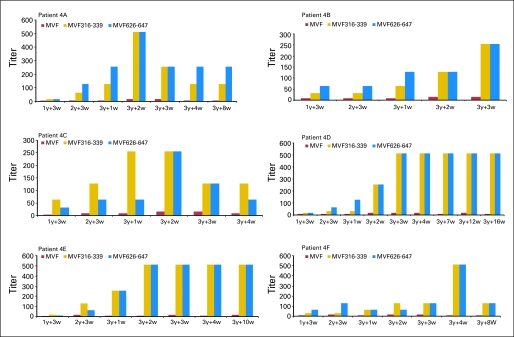
Fig 1.
Dose level 4 immunoglobulin G responses to human epidermal growth factor receptor 2 peptide vaccines. Enzyme-linked immunosorbent assay was carried out for patients A through F, as discussed in Patients and Methods. Antibody titers were defined as the reciprocal of the highest serum dilution with an absorbance of 0.2. 1y + 3w, blood drawn 3 weeks after the first immunization; 3y + 1w, blood drawn 1 week after third immunization. The y-axis represents the titer after subtracting the patient presera value and the blank.
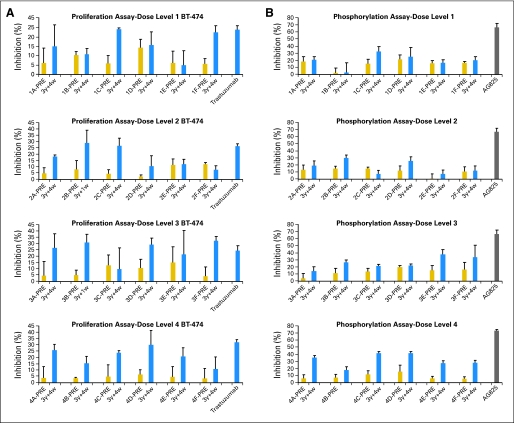
Fig 2.
Effects of purified antipeptide antibodies on (A) proliferation and on (B) phosphorylation with BT-474 cells. Cells were incubated with medium alone or were treated with preantibodies, peptide antibodies, and trastuzumab (100 μg). The inhibition rate was calculated with the following formula: (optical density 570 nm [OD]medium alone − ODtreated) ÷ ODmedium alone × 100. Error bars represent standard error of the mean.
Antibody Response to Peptide Vaccine and Recombinant HER2 Protein
ELISA assays were performed to determine patient IgG antibody responses (Table 2; Fig 1). Patients were classified as having HER2 peptide–specific antibody responses according to optical density values of 415 nm with a 1:16 dilution of serum antibodies; high response was greater than 0.6, and intermediate response was 0.2 to 0.6. From dose level 1 to dose level 3, three patients (50%) had high antibody responses, and three patients (50%) had intermediate responses. At dose level 4 (Fig 1), four patients (67%; 4A, 4D, 4E, 4F) showed high antibody responses, whereas two patients (4B and 4C) had intermediate responses. Dose level 4 was determined to be the MTD. Importantly, the six patients in dose level 4 also elicited HER2-specific recombinant protein antibodies, as presented in Table 3. These results confirm that one of the combination vaccines (MVF-316 to MVF-339) elicited pertuzumab-like antibodies.
Table 2.
IgG Responses to Individual Peptide Immunogens, MVF 316-339 and MVF 628-647, by ELISA
| Peptide | Individual Patient OD Values to Immunogenic Vaccine Constructs |
||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | Dose Level | |
| 316-339 | 0.934 | 0.778 | 0.544 | 0.611 | 0.537 | 1.242 | 1 |
| 628-647 | 1.098 | 0.701 | 0.409 | 0.396 | 0.311 | 1.985 | 1 |
| 316-339 | 1.899 | 0.915 | 0.602 | 0.739 | 0.400 | 0.505 | 2 |
| 628-647 | 2.443 | 0.829 | 1.719 | 1.265 | 0.201 | 0.568 | 2 |
| 316-339 | 0.351 | 0.268 | 1.246 | 0.739 | 1.382 | 0.505 | 3 |
| 628-647 | 0.421 | 0.842 | 0.837 | 1.265 | 1.394 | 0.568 | 3 |
| 316-339 | 1.207 | 0.753 | 0.675 | 1.323 | 2.369 | 0.934 | 4 |
| 628-647 | 1.265 | 0.698 | 0.469 | 1.740 | 2.590 | 1.098 | 4 |
NOTE. Individual patients A through F are in cohorts 1 through 4. The maximum absorbance value for each patient's serum at a 1:16 dilution after subtracting preserum value was determined against MVF 316-339 and MVF 628-647. The numbers correspond to the maximum absorbance for each patient at the indicated dose level.
Abbreviations: IgG, immunoglobulin G; ELISA, enzyme-linked immunosorbent assay; OD, optical density 415 nm.
Table 3.
IgG Responses to Human Recombinant ErbB-2 Protein MBS1432470, HER-2 Sequence 1-398, by ELISA
| Serum Antibody | Trastuzumab-Negative Control | Individual Patient OD (415 nm) Values to Human Recombinant HER2 by Patient |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1A | 1B | 1D | 2A | 4A | 4B | 4C | 4D | 4E | 4F | ||
| OD value, 415 nm | −0.2 | 0.589 | 0.786 | 0.225 | 0.419 | 0.23 | 0.86 | 0.427 | 0.695 | 0.361 | 0.468 |
NOTE. Individual patients A through F are in cohort 4. Results of each patient in dose level 4 (ie patients 4A through 4F) and those with a clinical response (ie, patients 1A, 1B, 1D, and 2A) are shown, using a 1:16 dilution of patient sera. The y-axis represents the absorbance after subtracting patient presera (ie, day 1) value.
Abbreviations: IgG, immunoglobulin G; HER-2, human epidermal growth factor receptor 2; ELISA, enzyme-linked immunosorbent assay; OD, optical density.
Reactivity of peptide antibodies with the native HER2 receptor.
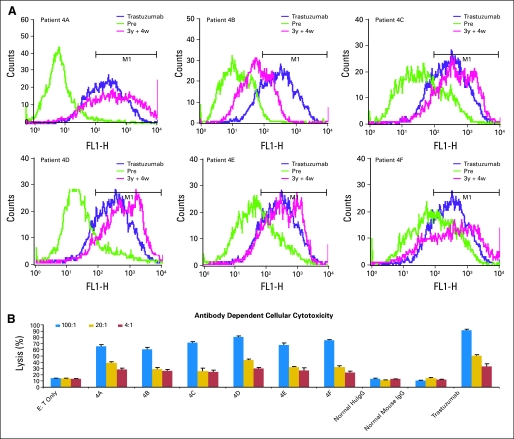
Binding of the peptide antibodies to the intact HER2 receptor was evaluated by immunofluorescence staining of single-cell suspension of BT-474. Differential binding was obtained at each dose level. In cohort 4, antibodies elicited by each patient bound similarly or better to the HER2 receptor compared with the positive control (trastuzumab), and presera served as negative control (Fig 3). These results demonstrate that the peptide vaccine recognized the native receptor.
Fig 3.
(A) Antipeptide antibodies recognize human epidermal growth factor receptor 2 (HER2). Flow cytometry was used to assess the binding capabilities of the antipeptide antibodies of patients (4A through 4F) to HER2. Purified antibodies (50 μg) from presera and immunized sera were tested against BT-474 breast cancer cells. Histograms contain overlays of antipresera postsera (3y + 4w) and trastuzumab. (B) Purified antipeptide antibodies cause antibody-dependent cellular cytotoxicity. BT474 target cells were incubated with different amounts of effector cells (ie, peripheral-blood mononuclear cells) after adding 100 μg of antipeptide antibodies for patients 4A through 4F. Trastuzumab was used as the positive control, and normal human and mouse immunoglobulin G (IgG) were used as negative controls. The percentage cytotoxicity was calculated according to instructions provided by the reagent kit.
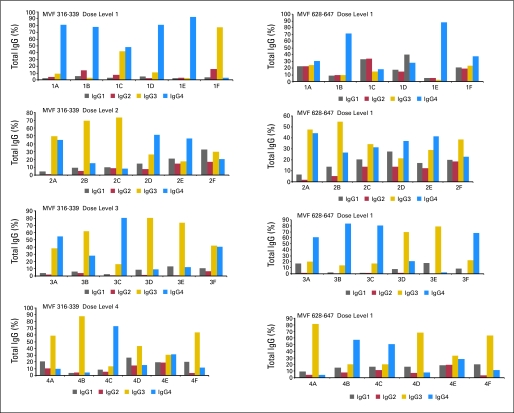
Vaccine Elicits IgG3 and IgG4 Antipeptide Antibodies
All four cohorts elicited a mixture of IgG antibodies (IgG1, IgG2, IgG3, and IgG4), with a preponderance of IgG3 and four isotopes; with increasing dose of vaccine, the level of IgG3 increased (data not shown, Appendix Fig A1, online only).
Antiproliferative Effects of Peptide Antibodies
The effects of the peptide antibodies on proliferation were tested by using BT-474 cells in the presence of HRG. The patient sera were able to inhibit proliferation of the HER2-overexpressing cell BT-474 (Fig 2A). In most of the occurrences, the difference in inhibition between the antibodies from the presera and postsera (3 weeks after third vaccination) was significant at dose level 4. Presera antibodies showed some antiproliferative effects, which indicated that the patients had mounted weak immune responses to the tumors and that vaccination has boosted that response.
HER2 Phosphorylation Inhibition
MVF-316 to MVF-339 represent a sequence overlapping the pertuzumab-binding site and could similarly disrupt ligand-dependent receptor complexes independent of HER2 expression. To evaluate if the patients sera containing HER2-specific antibodies were able to function as dimerization inhibitors, we used a total phospho-HER2 ELISA. BT-474 cells were treated with serum antibodies, and HRG was used to activate HER3 before cell lysates were captured with an anti-HER2 monoclonal antibody and were probed with a phospho-HER2 antibody. Eighty-four percent of patients at dose level 4 lowered the concentration of the phosphotyrosine on BT-474 cells (Fig 2B) by 40% to 50% compared with the HER2 phosphorylation inhibitor AG825, which was used as the positive control.
DISCUSSION
To our knowledge, this clinical trial described here is the first male/female trial to consist of a vaccine strategy that used two B-cell epitope peptides, MVF-316 to MVF-339 and MVF-628 to MVF-647, in combination with n-MDP adjuvant in an oil-in-water vehicle. The majority of peptide vaccines that target the HER2 oncogene have targeted T-cell immunity by utilizing either CTL or Th-cell peptide epitopes. The HER2 CTL epitope E75 (HER2 sequence 369 to 377) was originally identified by Fisk et al25 as an immunodominant, HLA-A2–binding epitope recognized by tumor-associated lymphocytes of ovarian cancer. One HER2, T-cell trial reported with the E75 peptide in combination with granulocyte-macrophage colony-stimulating factor showed an increase in disease-free survival.19 The Disis group26 has vaccinated patients with HER2-overexpressing breast cancer, non–small-cell lung cancer, and ovarian cancers with peptide derived from potential Th epitopes of the HER2 protein in combination with granulocyte-macrophage colony-stimulating factor.20,26 Ninety-two percent of patients developed T-cell immunity to the HER2 peptides.
Several preclinical studies have demonstrated that the induction of anti-HER2/neu antibodies are both necessary and sufficient for protection of BALB-neuT mice,27,28 whereas DNA- and adenovirus-based HER2 vaccines have shown efficacy but not protection.27,29,30 Our preclinical studies underscored the importance of eliciting a humoral immune response against HER2.23,24 A clinical trial that used HER2 peptide 328 to 345–induced antibodies in patients suppressed the phosphorylation of HER2 on tyrosine 1248.31
We demonstrate here that the peptide vaccines elicited IgG antibodies at all dose levels (data not shown; Appendix Fig A1), which indicates that T-cell activation was provided by the promiscuous MVF T-cell epitope.32,33 Dose level 4 was determined to be the MTD dose because of the lack of DLT and because of a significant, dose-dependent increase in the IgG antibody response in patients compared with that of dose levels 1 to 3 (Table 2).
It is important to underscore that our vaccine epitopes correspond to overlapping sequences of trastuzumab and pertuzumab binding sites that target different extracellular regions of the HER2 tyrosine kinase receptor. The crystal structure34,35 of HER2 with trastuzumab and/or pertuzumab show that trastuzumab binds domain IV, causes ADCC, and inhibits proliferation; conversely, pertuzumab binds to extracellular domain II of the HER2 receptor and blocks its ability to dimerize with other HER receptors. In this paper, we have demonstrated that the patient sera containing HER2-specific antipeptide antibodies were able to recognize the native HER2 receptor, as they bound to BT-474–expressing cells in similar fashion to trastuzumab (Fig 3). The sera in cohort-4 patients who experienced clinical benefit bound to the truncated rhHER2 antigen by ELISA (Table 3; specific for pertuzumab binding site) and were able to inhibit the proliferation of HER2-overexpressing cells35 (Fig 2A) as well as block phosphorylation (Fig 2B) and, indirectly, receptor dimerization.
Although the intent of this study was not to determine the efficacy, we did find evidence of preliminary activity in six patients who had either partial response or stable disease. Interestingly, only one of these six patients was HER2 positive as determined by fluorescent in situ hybridization, and the remaining five patients did not overexpress HER2. Of the six patients who demonstrated a clinical benefit, five patients (1A, 1B, 2A, 4D, and 4E) had a strong (ie, high) antibody response against both vaccines, whereas one patient (1C) had high and intermediate IgG. Of significance, the two-combination vaccine that binds a distinct site of HER2 can target both HER2-positive and -negative patients with cancer; thus, it can synergistically inhibit the tumorigenic growth of cancer cells by different mechanisms (ie, ADCC v dephosphorylation). However, it is difficult to infer clinical benefit to any immune mechanisms in this study, given the small number of patients.
In conclusion, this phase I study demonstrates that the peptide vaccine is safe and that it elicited IgG antibodies in a population of patients who have metastatic disease that has been heavily pretreated. These antibodies recognize HER2 and specifically suppress its phosphorylation and inhibit cell proliferation. This study, to our knowledge, is the first to show that a combination, B-cell epitope, peptide vaccine can elicit antibodies that disrupt two different HER2 signaling methods. The design of HER2-based peptides should offer safe alternatives, given that clinicians still face many uncertainties concerning the optimal use of tyrosine kinase inhibitors and humanized monoclonal antibody therapy. The clinical benefit of this vaccine remains to be investigated in future phase II, clinical trials.
Appendix
Fig A1.
Isotype of human epidermal growth factor receptor 2 (HER2) peptide antibodies elicited for dose levels 1 to 4. Isotypes of serum antibodies specific for MVF-316 to MVF-339 and MVF-628 to MVF-647 from patients were determined by using a 1:16 dilution of patient serum. Secondary antibodies used were specific for the indicated isotypes conjugated to alkaline phosphatase. Values shown are percentages of total immunoglobulin G (IgG) after subtracting individual patient preserum.
Footnotes
Supported in part by National Institutes of Health National Cancer Institute Grant No. CA094555 (P.T.P.K.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Gregory A. Otterson, Genentech, Abraxix BioScience, Pfizer Expert Testimony: None Other Remuneration: Gregory A. Otterson, Celga, WIRB
AUTHOR CONTRIBUTIONS
Conception and design: Pravin T.P. Kaumaya
Financial support: Pravin T.P. Kaumaya
Administrative support: Pravin T.P. Kaumaya, Catherine R. Balint, Michael R. Grever
Provision of study materials or patients: Pravin T.P. Kaumaya, Donald Chalupa, Gregory A. Otterson, Charles L. Shapiro, Jeffrey M. Fowler, Tanios S. Bekaii-Saab, William E. Carson III
Collection and assembly of data: Pravin T.P. Kaumaya, Kevin Chu Foy, Joan Garrett, Sharad V. Rawale, Daniele Vicari, Jennifer M. Thurmond, Tammy Lamb, Aruna Mani, Yahaira Kane, Tanios S. Bekaii-Saab
Data analysis and interpretation: Pravin T.P. Kaumaya, Kevin Chu Foy, Joan Garrett, Sharad V. Rawale, Aruna Mani, Gregory A. Otterson, Charles L. Shapiro, William E. Carson III
Manuscript writing: Pravin T.P. Kaumaya, Kevin Chu Foy, Joan Garrett, Aruna Mani, Tanios S. Bekaii-Saab, William E. Carson III
Final approval of manuscript: Pravin T.P. Kaumaya, Kevin Chu Foy, Joan Garrett, Daniele Vicari, Jennifer M. Thurmond, Tammy Lamb, Aruna Mani, Catherine R. Balint, Donald Chalupa, Gregory A. Otterson, Charles L. Shapiro, Jeffrey M. Fowler, Michael R. Grever, Tanios S. Bekaii-Saab, William E. Carson III
REFERENCES
- 1.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Hudziak RM, Lewis GD, Winget M, et al. P185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989;9:1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter P, Presta L, Gorman CM, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 6.Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 7.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 8.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 9.Nahta R, Hung MC, Esteva FJ. The HER2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 10.Weber J. Peptide vaccines for cancer. Cancer Invest. 2002;20:208–221. doi: 10.1081/cnv-120001149. [DOI] [PubMed] [Google Scholar]
- 11.Buteau C, Markovic SN, Celis E. Challenges in the development of effective peptide vaccines for cancer. Mayo Clin Proc. 2002;77:339–349. doi: 10.4065/77.4.339. [DOI] [PubMed] [Google Scholar]
- 12.Lazoura E, Apostolopoulos V. Rational Peptide-based vaccine design for cancer immunotherapeutic applications. Curr Med Chem. 2005;12:629–639. doi: 10.2174/0929867053202188. [DOI] [PubMed] [Google Scholar]
- 13.Disis ML, Pupa SM, Gralow JR, et al. High-titer HER2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol. 1997;15:3363–3367. doi: 10.1200/JCO.1997.15.11.3363. [DOI] [PubMed] [Google Scholar]
- 14.Ioannides CG, Fisk B, Fan D, et al. Cytotoxic T cells isolated from ovarian malignant ascites recognize a peptide derived from the HER2/neu proto-oncogene. Cell Immunol. 1993;151:225–234. doi: 10.1006/cimm.1993.1233. [DOI] [PubMed] [Google Scholar]
- 15.Yoshino I, Goedegebuure PS, Peoples GE, et al. HER2/neu-derived peptides are shared antigens among human non–small-cell lung cancer and ovarian cancer. Cancer Res. 1994;54:3387–3390. [PubMed] [Google Scholar]
- 16.Disis ML, Calenoff E, McLaughlin G, et al. Existent T-cell and antibody immunity to HER2/neu protein in patients with breast cancer. Cancer Res. 1994;54:16–20. [PubMed] [Google Scholar]
- 17.Disis ML, Gralow JR, Bernhard H, et al. Peptide-based, but not whole protein, vaccines elicit immunity to HER2/neu, oncogenic self-protein. J Immunol. 1996;156:3151–3158. [PubMed] [Google Scholar]
- 18.Disis ML, Grabstein KH, Sleath PR, et al. Generation of immunity to the HER2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res. 1999;5:1289–1297. [PubMed] [Google Scholar]
- 19.Peoples GE, Gurney JM, Hueman MT, et al. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23:7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 20.Knutson KL, Schiffman K, Disis ML. Immunization with a HER2/neu helper peptide vaccine generates HER2/neu CD8 T-cell immunity in cancer patients. J Clin Invest. 2001;107:477–484. doi: 10.1172/JCI11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dakappagari NK, Douglas DB, Triozzi PL, et al. Prevention of mammary tumors with a chimeric HER2 B-cell epitope peptide vaccine. Cancer Res. 2000;60:3782–3789. [PubMed] [Google Scholar]
- 22.Dakappagari NK, Pyles J, Parihar R, et al. A chimeric multi-human epidermal growth factor receptor-2 B cell epitope peptide vaccine mediates superior antitumor responses. J Immunol. 2003;170:4242–4253. doi: 10.4049/jimmunol.170.8.4242. [DOI] [PubMed] [Google Scholar]
- 23.Allen SD, Garrett JT, Rawale SV, et al. Peptide vaccines of the HER2/neu dimerization loop are effective in inhibiting mammary tumor growth in vivo. J Immunol. 2007;179:472–482. doi: 10.4049/jimmunol.179.1.472. [DOI] [PubMed] [Google Scholar]
- 24.Garrett JT, Rawale S, Allen SD, et al. Novel engineered trastuzumab conformational epitopes demonstrate in vitro and in vivo antitumor properties against HER2/neu. J Immunol. 2007;178:7120–7131. doi: 10.4049/jimmunol.178.11.7120. [DOI] [PubMed] [Google Scholar]
- 25.Fisk B, Blevins TL, Wharton JT, et al. Identification of an immunodominant peptide of HER2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med. 1995;181:2109–2117. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Disis ML, Gooley TA, Rinn K, et al. Generation of T-cell immunity to the HER2/neu protein after active immunization with HER2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 27.Nanni P, Landuzzi L, Nicoletti G, et al. Immunoprevention of mammary carcinoma in HER2/neu transgenic mice is IFN-gamma and B cell dependent. J Immunol. 2004;173:2288–2296. doi: 10.4049/jimmunol.173.4.2288. [DOI] [PubMed] [Google Scholar]
- 28.Park JM, Terabe M, Sakai Y, et al. Early role of CD4+ Th1 cells and antibodies in HER2 adenovirus vaccine protection against autochthonous mammary carcinomas. J Immunol. 2005;174:4228–4236. doi: 10.4049/jimmunol.174.7.4228. [DOI] [PubMed] [Google Scholar]
- 29.Quaglino E, Rolla S, Iezzi M, et al. Concordant morphologic and gene expression data show that a vaccine halts HER2/neu preneoplastic lesions. J Clin Invest. 2004;113:709–717. doi: 10.1172/JCI19850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quaglino E, Iezzi M, Mastini C, et al. Electroporated DNA vaccine clears away multifocal mammary carcinomas in HER2/neu transgenic mice. Cancer Res. 2004;64:2858–2864. doi: 10.1158/0008-5472.can-03-2962. [DOI] [PubMed] [Google Scholar]
- 31.Montgomery RB, Makary E, Schiffman K, et al. Endogenous anti-HER2 antibodies block HER2 phosphorylation and signaling through extracellular signal-regulated kinase. Cancer Res. 2005;65:650–656. [PubMed] [Google Scholar]
- 32.Partidos CD, Steward MW. Prediction and identification of a T cell epitope in the fusion protein of measles virus immunodominant in mice and humans. J Gen Virol. 1990;71:2099–2105. doi: 10.1099/0022-1317-71-9-2099. [DOI] [PubMed] [Google Scholar]
- 33.Baxevanis CN, Sotiriadou NN, Gritzapis AD, et al. Immunogenic HER2/neu peptides as tumor vaccines. Cancer Immunol Immunother. 2006;55:85–95. doi: 10.1007/s00262-005-0692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho HS, Mason K, Ramyar KX, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–760. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 35.Franklin MC, Carey KD, Vajdos FF, et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–328. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]