Abstract
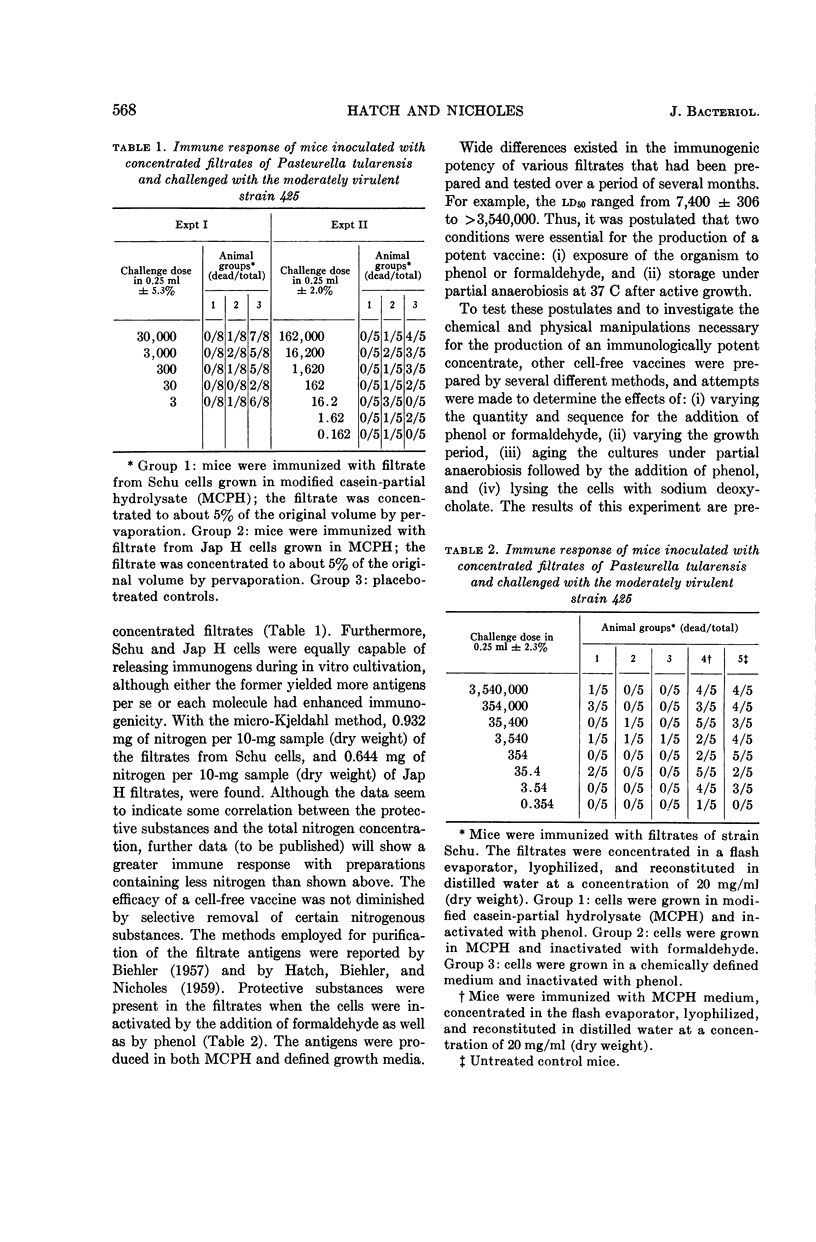
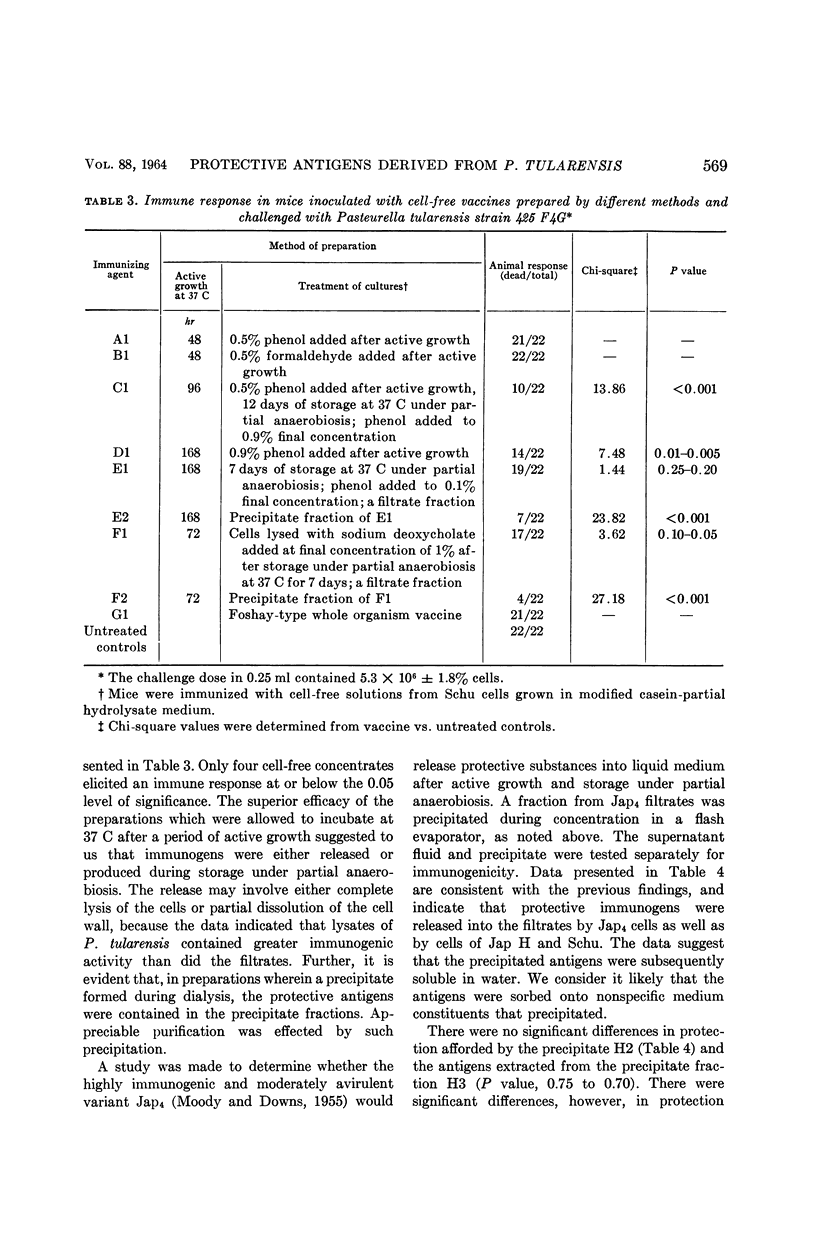
Hatch, Melvin T. (University of Utah, Salt Lake City), and Paul S. Nicholes. Immunogenic substances in culture filtrates and lysates of Pasteurella tularensis. J. Bacteriol. 88:566–573. 1964.—Culture filtrates and lysates of Pasteurella tularensis were tested for immunogenicity in mice subsequently infected with either strain 425 or 425 F4G. The efficacy of the vaccines varied with dosage and was significantly dependent upon methods of preparation. The optimal procedures for the production of an immunologically potent vaccine included: (i) aging the cultures after growth under partial anaerobiosis at 37 C, and (ii) inactivating the cells with phenol or formaldehyde. An unusual “survival phenomenon” was suggested when mice were administered large doses of cell-free vaccines and subsequently large doses of moderately virulent P. tularensis. The data indicated that the filtrates and lysates elicited an immune response sufficient to protect against an active infection with strains 425 or 425 F4G, and that the challenge dose per se stimulated an enhanced immunity. Furthermore, this survival phenomenon was demonstrable when immunized mice were subsequently given massive doses of strain 425 and challenged with approximately 1,000 ld50 of the fully virulent strain Schu. On the basis of our data, we have hypothesized that the protective antigens were released into the suspending medium as a result of alterations in the permeability of cells undergoing either complete or partial enzymatic degradation. We believe that the envelope antigens were released from the cell by mechanisms analogous to those causing leakage of intracellular constituents in cells maintained at an incubation temperature in an unfavorable growth environment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- EIGELSBACH H. T., DOWNS C. M. Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and guinea pig. J Immunol. 1961 Oct;87:415–425. [PubMed] [Google Scholar]
- HERBST E. J., DOCTOR B. P. Inhibition of ribonucleic acid degradation in bacteria by spermine. J Biol Chem. 1959 Jun;234(6):1497–1500. [PubMed] [Google Scholar]
- MANDELSTAM J. Turnover of protein in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):110–119. doi: 10.1042/bj0690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOODY M. D., DOWNS C. M. Studies on tularemia. I. The relation between certain pathogenic and immunogenic properties of variants of Pasteurella tularensis. J Bacteriol. 1955 Sep;70(3):297–304. doi: 10.1128/jb.70.3.297-304.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEELY W. B. ACTION OF FORMALDEHYDE ON MICROORGANISMS. I. CORRELATION OF ACTIVITY WITH FORMALDEHYDE METABOLISM. J Bacteriol. 1963 May;85:1028–1031. doi: 10.1128/jb.85.5.1028-1031.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEELY W. B. ACTION OF FORMALDEHYDE ON MICROORGANISMS. III. BACTERICIDAL ACTION OF SUBLETHAL CONCENTRATIONS OF FORMALDEHYDE ON AEROBACTER AEROGENES. J Bacteriol. 1963 Sep;86:445–448. doi: 10.1128/jb.86.3.445-448.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMSBEE R. A., BELL J. F., LARSON C. L. Studies on Bacterium tularense antigens. I. The isolation, purification, and biologic activity of antigen preparations from Bacterium tularense. J Immunol. 1955 May;74(5):351–358. [PubMed] [Google Scholar]
- PANNELL L., CORDLE M. S. Further studies on protective filtrates of Pasteurella tularensis broth cultures. J Infect Dis. 1962 Jul-Aug;111:49–54. doi: 10.1093/infdis/111.1.49. [DOI] [PubMed] [Google Scholar]
- PIZZI M. Sampling variation of the fifty percent end-point, determined by the Reed-Muench (Behrens) method. Hum Biol. 1950 Sep;22(3):151–190. [PubMed] [Google Scholar]
- POSTGATE J. R., HUNTER J. R. The survival of starved bacteria. J Gen Microbiol. 1962 Oct;29:233–263. doi: 10.1099/00221287-29-2-233. [DOI] [PubMed] [Google Scholar]
- Roberts M. H., Rahn O. The Amount of Enzyme Inactivation at Bacteriostatic and Bactericidal Concentrations of Disinfectants. J Bacteriol. 1946 Dec;52(6):639–644. doi: 10.1128/jb.52.6.639-644.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEKEVITZ P. Uptake of radioactive alanine in vitro into the proteins of rat liver fractions. J Biol Chem. 1952 Apr;195(2):549–565. [PubMed] [Google Scholar]
- STRANGE R. E. Induced enzyme synthesis in aqueous suspensions of starved stationary phase Aerobacter aerogenes. Nature. 1961 Sep 23;191:1272–1273. doi: 10.1038/1911272a0. [DOI] [PubMed] [Google Scholar]
- Schweet R., Lamfrom H., Allen E. THE SYNTHESIS OF HEMOGLOBIN IN A CELL-FREE SYSTEM. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1029–1035. doi: 10.1073/pnas.44.10.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAMECNIK P. C., KELLER E. B. Relation between phosphate energy donors and incorporation of labeled amino acids into proteins. J Biol Chem. 1954 Jul;209(1):337–354. [PubMed] [Google Scholar]


