Abstract
Aquaporin-1 (AQP-1), the universal water channel, is responsible for rapid response of cell volume to changes in plasma tonicity. In the membrane of the red cell the concentration of the protein is tightly controlled. Here, we show that AQP-1 is partially lost during in vitro maturation of mouse reticulocytes and that it is associated with exosomes, released throughout this process. AQP-1 in young reticulocytes localizes to the plasma membrane and also in endosomal compartments and exosomes, formed both in vitro and in vivo. During maturation a part of the total pool of AQP-1 is differentially sorted and released via the exosomal pathway. A proteasome inhibitor, MG132, suppresses secretion of AQP-1, implying that ubiquitination is a sorting signal for its release. We further show that modulation of medium tonicity in vitro regulates the secretion of AQP-1, thus showing that extracellular osmotic conditions can drive sorting of selected proteins by the exosomal pathway. These results lead us to suggest that AQP-1 sorting into exosomes may be the mechanism by which the reticulocyte adapts to environmental changes during its maturation.
Introduction
The final stage of erythropoiesis consists of maturation of reticulocytes into erythrocytes. Young immature reticulocytes are generated through the process of enucleation, which occurs within bone marrow erythroid niches called erythroblastic islands. During steady state, reticulocyte maturation occurs over a period of approximately 72 hours, two-thirds of this time in the marrow, one-third in the circulation.1,2 This maturation process involves complete loss of intracellular organelles, including mitochondria, endoplasmic reticulum, Golgi apparatus, and endocytic vesicles.3 Equally important is the extensive remodelling of the plasma membrane, with progressive loss of various membrane proteins.4 These include the transferrin receptor (TfR), because iron for heme synthesis is no longer required, and adhesion receptors such as β1-integrin,5 the presence of which could otherwise cause mature circulating red cells to adhere to vascular endothelial cells. To date the best studied of these is the TfR, which is completely shed from the reticulocyte in exosomes, small vesicles released into the circulation.6 During maturation, the TfR is rerouted from a recycling pathway into a secretion pathway.7 The plasma membrane–bound receptors are sorted into endocytic vesicles, which are internalized and assembled into multivesicular bodies. These fuse with the plasma membrane and release their contents into the plasma in the form of exosomes. Although the molecular mechanisms of TfR sorting into exosomes have been explored,8 they are far from completely understood. Nevertheless, the identification of the components involved in the formation of multivesicular bodies affords some insight into the biogenesis of exosomes.9 Four protein complexes, ESCRT-0, -1, -2, and -3 (referred to as endosomal sorting complexes, required for transport), were shown to play important roles in the formation of multivesicular bodies (MVBs).10,11 Mono-ubiquitination has been identified as a key signal for the sorting of proteins into the intraluminal vesicles of MVBs.12 Other cells besides reticulocytes also secrete exosomes into various biologic fluids (eg, plasma,13 pleural effusion,14 and urine15).
A recent study showed that aquaporin-2 (AQP-2) is secreted into urine in association with vesicles that share some characteristics with exosomes.16 Aquaporins represent a family of integral proteins expressed in many tissues.17 To date, 10 mammalian aquaporins and 4 aquaglyceroporins have been identified. Their main function is to regulate water permeability of membranes. The first aquaporin, AQP-1, was discovered in the erythrocyte plasma membrane18 and was shown to be the water channel. In the absence of AQP-1, the red cell's response to changes in plasma osmolality is decreased by more than an order of magnitude.19
Here, we show that a subpopulation of AQP-1 is associated with endosomes and colocalizes with the TfR both at the plasma membrane and in endosomal compartments of reticulocytes. Moreover, exosomal release of AQP-1 is inhibited by the proteasome inhibitor MG132, implying that mono-ubiquitination of the protein may be involved in AQP-1 exosomal sorting. Finally, the addition of sorbitol to the in vitro culture medium to induce osmotic stress inhibits AQP-1 release. This leads us to conclude that exosomal secretion allows immature red cells to deplete their AQP-1 pool in response to the osmotic disturbances. Importantly, AQP-1 is also present in exosomes directly isolated from the plasma of anemic mice, implying that this process is physiologically relevant.
Methods
Cells
Reticulocytosis (> 40%) was induced in 6- to 9-month-old C57BL/6 mice by phlebotomy. Blood (0.5 mL) was removed from the retroorbital vein on days 0 and 2. On day 4, animals were killed, and blood was collected by cardiac puncture. Mature red cells were obtained from the blood of normal mice with reticulocyte count less than 2%. Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the New York Blood Center.
Antibodies
Mouse monoclonal anti–human TfR, raised against the cytoplasmic tail of the receptor, was from Zymed Laboratories. Rabbit polyclonal antibodies against AQP-1 and mouse monoclonal antibody against NHE-1 were from Millipore Corporation. Rabbit polyclonal antibody raised against mouse Hsc70 (SPA-816) was from Nventa Biopharmaceuticals. Rabbit polyclonal antibody against Glut-4 was from Abcam Inc. Mouse monoclonal antibody against flotillin-2 was from BD Transduction Laboratories. Mouse monoclonal anti–β-actin clone AC-15 was from Sigma-Aldrich. Purified rat anti–mouse TER-119 was from BD PharMingen. Rabbit polyclonal antibodies against β-spectrin, CD47, ICAM-4, Band3, glycophorin C, 4.1R, 4.2, 4.9, and adducin were generated in our laboratory. Peroxidase-conjugated donkey anti–rabbit IgG and donkey anti–mouse IgG were from Jackson ImmunoResearch Laboratories Inc. Alexa Fluor 488– and Alexa Fluor 568–conjugated donkey anti–rabbit IgG and donkey anti–mouse IgG were from Invitrogen.
Reticulocyte maturation and exosome isolation
Plasma was separated from red blood cells by centrifugation (3000g for 5 minutes). After 3 washes in phosphate-buffered saline (PBS), reticulocytes from anemic mice were purified by layering red cells on top of a Percoll/NaCl density gradient (1.096-1.058 g/mL) and centrifuged at 250g for 30 minutes. Purified reticulocytes were then cultured for either 24 or 48 hours at 37°C in RPMI 1640, supplemented with 5 mM glutamine, 5 mM adenosine, 10 mM inosine, 3% calf serum, 50 U/mL penicillin, and 50 μg/mL streptomycin at a concentration of 30 μL packed cells/mL as previously described.20 To avoid any possible contamination by exogenous exosomes in the culture medium, the calf serum was precentrifuged to remove bovine exosomal vesicles. For inhibition of the ubiquitin-proteasome degradation pathway, during reticulocyte maturation, cells were cultured in the presence of 20 μM MG132 (Calbiochem). To induce hypertonic stress, sorbitol (Sigma-Aldrich) at indicated concentrations was added to the culture medium. For dynamin inhibition, reticulocytes were treated with 80 μM Dynasore (Sigma-Aldrich) or with the equivalent amount of dimethyl sulfoxide (DMSO). After 24 or 48 hours, cells were pelleted, and the culture supernatant was centrifuged (20 000g for 20 minutes) to remove cellular debris. Exosomes were isolated from the supernatant by ultracentrifugation (100 000g for 2 hours in a Beckman TLA110 rotor; Beckman Coulter) and resuspended in PBS. Exosomes contained in the plasma of anemic mice were isolated by the same differential centrifugation method.
Western blot analysis
Solubilized protein preparations were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) in 10% polyacrylamide gels or 4% to 18% gradient polyacrylamide gel and were electrophoretically transferred to nitrocellulose membrane (Bio-Rad). Membranes were blocked for 1 hour in PBS-T (137 mM NaCl, 10 mM phosphate, pH 7.4; 2.7 mM KCl; 0.1% Tween 20), containing 4% (wt/vol) nonfat milk and 1% (wt/vol) bovine serum albumin (BSA), followed by 1-hour incubation at room temperature with the indicated primary antibody. Blots were then washed 3 times in PBS-T and incubated for 1 hour at room temperature with the second antibody (horseradish peroxidase [HRP] conjugated). Immunoreactive bands were detected by the enhanced chemiluminescence method (Pierce Chemical).
Reticulocyte subcellular fractionation
Freshly prepared reticulocytes were washed 3 times in PBS, resuspended in lysis buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 1 mM EGTA [ethylene glycol-bis(β-aminoethyl ester)-N,N,N′,N′-tetraacetic acid], 1 mM MgSO4; 7H2O, 3 mM NaN3, 50 mM NaCl, 50 mM KCl, 0.2 mM PMSF [phenylmethylsulfonyl fluoride], supplemented with a protease inhibitor cocktail) and subjected to 2 cycles of freezing/thawing. The lysed cells were pelleted at 1500g for 5 minutes, and the supernatant was centrifuged at 10 000g for 10 minutes to remove cellular debris. Endosomes were separated from the remaining cytosolic fraction by ultracentrifugation (60 000g for 30 minutes in a Beckman TLA110 rotor). After a wash in PBS, the endosomal fraction was resuspended in PBS. Reticulocyte plasma membranes (ghosts) were prepared by the method of Steck and Kant21 with the use of blood from anemic mice.
Immunofluorescence and confocal microscopy
Reticulocytes or erythrocytes were washed 3 times in PBG buffer (PBS, 0.1% BSA, 10 mM glucose). Four microliters packed cell volume (4 × 107 cells) were fixed in 100 μL 0.5% acrolein-PBG for 5 minutes at room temperature and resuspended in PBG buffer to a final concentration of 107 cells/mL. Cells were then plated on to poly-l-lysine–coated coverslips and permeabilized with 0.05% Triton X-100–PBS for 1 minute at room temperature and washed 3 times with 0.1 M glycine-PBS. After saturation of nonspecific binding sites with 0.1 M glycine-PBS; 1% BSA for 1 hour at room temperature, labeling with primary antibodies was performed for 1 hour at room temperature, and the coverslips were washed 3 times in 0.1 M glycine-PBS. Staining with the Alexa 488– or Alexa 568–conjugated antibodies was carried out at room temperature for 1 hour, and, after 3 washes in 0.1 M glycine-PBS, slides were mounted with Vecta Shield (Vector Laboratories). Images were obtained with a Zeiss LSM META 510 confocal microscope.
Immunogold electron microscopy
Reticulocytes were fixed with 4% paraformaldehyde, 0.1% glutaraldehyde, and 1% sucrose in 0.1 M cacodylate buffer for 1 hour at 4°C; washed in 0.1 M buffer, pH 7.4; and treated with 50 mM ammonium chloride to quench remaining aldehydes. The fixed cells were then dehydrated and embedded in LR-White Resin (Electron Microscopy Sciences). Thin sections of embedded cells were mounted on parlodion-coated nickel grids, blocked with 0.5% gelatin, 0.01 M glycine for 30 minutes at 37°C, and then incubated with antibodies to either AQP-1 or TfR overnight at 4°C, and washed in buffer containing bovine serum albumin and Tween 20. The sections were incubated with goat anti–mouse IgG or goat anti–rabbit IgG, coupled to 5-nm and 10-nm gold particles, respectively (Amersham Biosciences). After staining with uranyl acetate, sections were examined in a Philips-410 electron microscope.
Results
Reticulocyte proteins are selectively sorted into exosomes
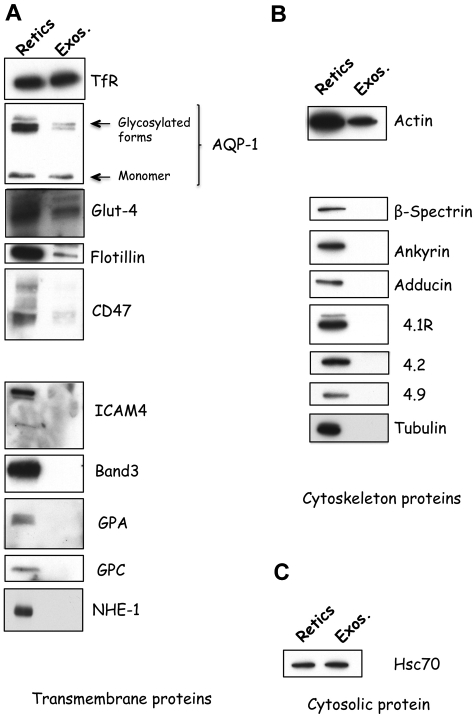
We first undertook a comprehensive analysis of the membrane proteins associated with the exosomes released during reticulocyte maturation. We collected exosomes from conditioned medium after in vitro maturation of enriched reticulocyte populations by differential ultracentrifugation as previously described.20 Western blot analysis was used as a qualitative approach to assess the protein composition of exosomes compared with reticulocytes. In additionto TfR and flotillin, 2 proteins known to be associated with exosomes, we found that they also contained several other proteins, AQP-1, Glut-4, and CD47 (Figure 1A) that have important functions. Note that in the case of AQP-1, both the monomeric (28 kDa) and the glycosylated (30-40 kDa) forms were present in exosomes. Neither the anion transporter, band 3, or the sodium proton exchanger NHE-1 nor the sialoglycoproteins glycophorin A (GPA) and glycophorin C or the adhesion protein ICAM-4 were detected in exosomes. Actin was the only cytoskeletal component detected in significant amount (Figure 1B), whereas the cytosolic protein, Hsc70, was enriched (Figure 1C). These findings imply that sorting of proteins into exosomes during reticulocyte maturation is highly selective.
Figure 1.
Specificity of protein sorting into exosomes during reticulocyte maturation. Reticulocytes (Retics; 106 cells; left lanes) and exosomes (Exos.; 50 μg protein; right lanes) were assessed for the presence of transmembrane (A), cytoskeletal (B), or cytosolic (C) proteins by SDS-PAGE and immunoblotting for the proteins, as indicated on the right.
AQP-1 is released into exosomes generated during reticulocyte maturation both in vitro and in vivo
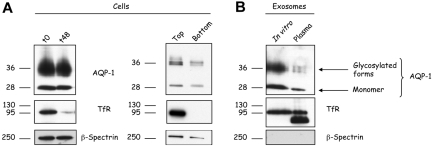
In the present study we elected to focus on selective loss of a subpopulation of AQP-1 during reticulocyte maturation. Because AQP-1 is associated with exosomes, it is to be expected that the content of AQP-1 in reticulocyte membranes should decrease in the course of maturation. During in vitro maturation of reticulocytes for 48 hours, the membrane content of both the monomeric and the glycosylated forms of AQP-1 were reduced by approximately 15% to 20%. Over the same period, there was, as expected, a marked decrease in TfR but no change in β-spectrin content (Figure 2A). A similar pattern was observed on analyzing the top and bottom fractions of a Percoll density separation of reticulocytes or erythrocytes collected from anemic mice. These in vivo data confirm our findings on reticulocytes cultured in vitro.
Figure 2.
AQP-1 is released in association with exosomes during in vitro maturation of reticulocytes and in vivo. (A) Reticulocytes (106 cells) before (t0) and after 48 hours (t48) in vitro maturation (left); top and bottom fractions were collected after cell separation on a Percoll gradient (right) were subjected to SDS-PAGE, followed by immunoblotting for the proteins indicated. (B) Exosomes (50 μg protein) released during in vitro maturation (left lane) or isolated directly from the plasma of anemic mice (right lane) were subjected to SDS-PAGE, followed by immunoblotting for the proteins indicated.
To confirm that AQP-1 exosomal release is not an artifact of in vitro maturation, we isolated exosomes from the plasma of anemic animals and compared them with exosomes generated in vitro (Figure 2B). Both contained the monomeric as well as the glycosylated forms of AQP-1. We also compared the TfR and β-spectrin contents of the same samples. TfR staining showed an additional lower-molecular-weight band in the in vivo–generated exosomes, corresponding probably to a proteolytically truncated form of the protein.22 Consistent with our earlier observations, β-spectrin was not found in the in vivo–generated exosomes. Collectively, these data imply that partitioning of AQP-1 into exosomes is a physiologic event.
AQP-1 is located in endosomes within reticulocytes
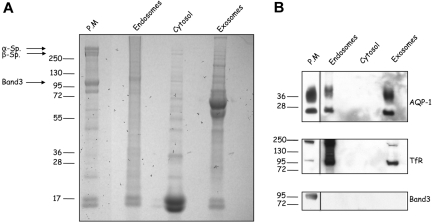
We searched by 4 methods for the presence of AQP-1 in the endosomal compartment, where the exosomes originate. We first prepared reticulocyte subfractions (plasma membrane, cytosol, and endosomal vesicles) as previously described.20 As shown in Figure 3A, the protein compositions of these subfractions clearly differed. The absence of α- and β-spectrin and band 3 from the endosomal fraction excluded cross-contamination with components of the plasma membrane fraction and showed the purity of the endosomal preparation. Western blot analysis of the endosomal fraction showed that AQP-1 and TfR, but not band 3, are associated with this compartment (Figure 3B). Moreover, our finding that the same proteins are associated with both endosomes and exosomes confirms that the AQP-1 of exosomes originates, like TfR, from the endosomal compartment.
Figure 3.
Subcellular localization of reticulocyte AQP-1. Constituents of mouse reticulocytes were fractionated after lysis as described in “Methods.” The various fractions (50 μg protein), plasma membrane, endosomes, cytosol, and exosomes were separated by SDS-PAGE and (A) stained with Coomassie Blue or (B) immunoblotted for the proteins indicated. Arrows indicate cytoskeleton proteins present in the mature red cell plasma membrane. Blots of plasma membrane fraction were exposed for a shorter period of time than those of the other 3 samples as indicated by the solid lines.
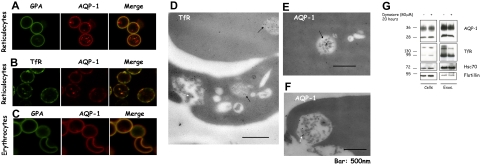
In parallel with this biochemical approach, we performed confocal microscopy to establish the cellular localization of AQP-1. Figure 4A shows that AQP-1 is present both on the plasma membrane and in punctate structures in the cytoplasm. In contrast, GPA, a specific marker for the red cell plasma membrane, is absent from the punctated structures. Importantly, there is partial colocalization of AQP-1 with the TfR, both on the plasma membrane and in intracellular structures (Figure 4B), indicating that these 2 proteins occur in the same endosomal compartments. In the mature red cell there is no discernable intracellular staining for AQP-1, consistent with its loss by exocytosis (Figure 4C).
Figure 4.
A part of the pool of AQP-1 colocalizes with the TfR and is found in multivesicular endosomes in reticulocytes. After purification by Percoll gradient, reticulocytes (A-B) and red blood cells (C) were fixed with acrolein and deposited on cover slips pretreated with poly-l-lysine. After permeabilization (Triton X-100), the cells were immunostained for GPA (green) and AQP-1 (red) (A,C), or TfR (green) and AQP-1 (red) (B). The slides were examined in a confocal microscope (Zeiss LSM 510 META camera, Zeiss Plan Apochromat 100×/1.4 NA oil objective, scan zoom 2.0) and pictures acquired and processed with Zeiss Laser Scanning Microscope LSM 510 software, Version 3.2 SP2. For visualization of TfR and AQP-1 in multivesicular endosomes and exosomes, reticulocytes were fixed with a mixture of paraformaldehyde, glutaraldehyde, and sucrose. After dehydration and embedding, thin sections of cells were mounted on nickel grids, blocked, and then immunostained for the TfR (D) or AQP-1 (E-F). Sections were examined in a Philips-410 electron microscope equipped with AMT XR41 Biological 2k side-mounted CCD camera, and images were acquired with AMT600 software and analyzed with Photoshop CS (Adobe Systems). After treatment with dynasore 80 μM or the solvent, DMSO, alone for 20 hours (G), 106 cells (left) and exosomes, resuspended in the same volume of Laemmli buffer,23 (right) were examined by SDS-PAGE and immunoblotting for the proteins indicated.
Because exosomal release is a consequence of the fusion of the intracellular structures, termed multivesicular endosomes (MVEs), with the plasma membrane, we next sought to establish that AQP-1 was indeed associated with MVEs, with the aid of immunoelectron microscopy. Figure 4D shows that the TfR is predominantly in intraluminal vesicles of MVEs (black arrows) and in exosomes released after fusion of these structures with the plasma membrane (white arrow). Importantly, we detected AQP-1 both in MVEs (Figure 4E black arrow) and in exosomes (Figure 4F white arrow). Note that the gold particles labeling TfR are more abundant than those labeling AQP-1, consistent with the larger proportion of TfR sorted into exosomes during reticulocyte maturation.
Because exosomes derive from the endocytic compartment, we hypothesized that dynasore, a specific inhibitor of dynamin,24 a key participant in the generation of endocytic vesicles, should inhibit the secretion of exosomal membrane proteins by depleting the available endosomal pool. As shown in Figure 4G, in vitro reticulocyte maturation in the presence of dynasore is accompanied by reduced secretion of both TfR and AQP-1 in exosomes, whereas neither flotillin, a protein internalized independently of clathrin,25 nor the cytosolic protein hsc70 was affected.
We conclude from the outcome of all 4 independent approaches that reticulocytes endocytose AQP-1 and package it into intraluminal vesicles of MVEs to induce its secretion by the exosomal pathway.
MG132 can inhibit AQP-1 release into exosomes
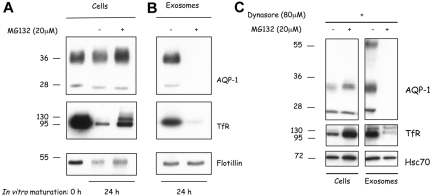
Because previous studies have suggested that the molecular basis of exosomal sorting may be related to the mechanism of MVB targeting, by way of mono-ubiquitination of proteins,26 we explored the possibility that ubiquitination might be involved in AQP-1 sorting into reticulocyte exosomes. For these studies, we took advantage of the fact that MG132, a selective proteasome inhibitor, induces depletion of the intracellular pool of free ubiquitin,27,28 thereby further inhibiting protein ubiquitination. Reticulocytes were cultured in vitro for 24 hours in the presence or absence of MG132 (20 μM), and exosomes were collected and analyzed. As shown in Figure 5A, MG132 inhibited the loss of both cell-associated AQP-1 (top) and TfR (bottom). AQP-1 release into exosomes was suppressed by the addition of MG132 (Figure 5B). TfR sorting into exosomes is also affected by MG132 during reticulocyte maturation, as previously described.8
Figure 5.
Inhibition of AQP-1 trafficking and sorting by MG132. (A) Freshly obtained mouse reticulocytes (0 hour; left lane) were cultured for 24 hours with the proteasome inhibitor MG132 (20 μM; 24 hours; right lane). Freshly obtained mouse reticulocytes were also cultured with an equivalent volume of the solvent, DMSO (24 hours; middle lane). (B) Exosomes were isolated from the culture medium after 24 hours and resuspended in the same volume of Laemmli buffer.23 Cellular and exosomal proteins were separated by SDS-PAGE and immunoblotted for the proteins indicated on the right. In each lane, 106 cells were loaded. (C) After inhibition of endocytosis, the cells were allowed to mature in the presence or absence of MG132 for 24 hours. Exosomes were then collected, and the compositions of both cells and the vesicles released from them were analyzed by SDS-PAGE and immunoblotted for the same proteins.
Because ubiquitination of membrane proteins, as well as targeting to MVBs, has also been found to induce its endocytosis through clathrin-coated pits, we attempted to determine the contribution of a ubiquitination-induced increase in endocytosis to exosomal release of AQP-1. Reticulocytes were cultured for 20 hours in the presence of dynasore to empty the intracellular endosomal compartments, and, after the removal of dynasore, the cells were cultured for a further 24 hours in the presence of MG132. As shown in Figure 5C, both TfR and AQP-1 of dynasore pretreated cells, matured in the presence of MG132, were mainly found in the cell rather than in exosomes. Note that in exosomes collected after maturation in the absence of MG132, an additional form of AQP-1 appeared, with a molecular weight corresponding to that of a dimer. Dimerization of the AQP-1 may thus accompany exosome biogenesis, as has been previously reported in the case of TfR.29 Of note, hsc70, the exosome marker, was released with unchanged efficiency. When DMSO-treated cells were then chased in the presence or absence of MG132 (supplemental Figure 1, available on the Blood website; see the Supplemental Materials link at the top of the online article), no clear change in cell-associated TfR or AQP-1 could be seen, and concomitantly very low amounts of exosomes were released by both sets of cells.
Modulation of AQP-1 levels by osmolality
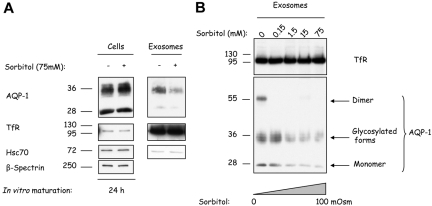
To determine whether a change in tonicity of the culture medium could affect AQP-1 sorting and release during reticulocyte maturation, we cultured reticulocytes for 24 hours in the presence of various concentrations of sorbitol as osmolyte. As shown in Figure 6A, the addition of 75 mM sorbitol resulted in an increased cell association of AQP-1, whereas TfR and hsc70 levels were unaffected. Exosomes collected after in vitro maturation contained less AQP-1 when the cells were cultured under hypertonic conditions (Figure 6A top right). This inhibition of AQP-1 sorting into exosomes is specific, because exosomal TfR and hsc70 are unaffected regardless of the treatment (Figure 6A center and bottom right, respectively). We then explored AQP-1 sorting as a function of medium osmolality. Cells were cultured for 24 hours in the presence of increasing concentrations of sorbitol, from isotonic 300 mOsm to hypertonic 400 mOsm. As shown in Figure 6B, whereas the exosomal TfR levels remained constant (top), the AQP-1 levels were severely reduced. Furthermore, the water channel was almost absent from exosomes obtained after reticulocyte maturation in the presence of 75 mM sorbitol (bottom). These results establish that osmotic stress has an inhibitory effect on AQP-1 sorting into exosomes.
Figure 6.
Hypertonic stress progressively inhibits AQP-1 sorting into exosomes. (A) Mouse reticulocytes were cultured for 24 hours with or without sorbitol 75 mM. Exosomes were then collected, and both cells (106) and exosomes (resuspended in the same volume of Laemmli buffer) were examined by SDS-PAGE and immunoblotted for the proteins indicated on the right. (B) Mouse reticulocytes were cultured for 24 hours, with increasing concentrations of sorbitol, and exosomes were collected, resuspended in the same volume of Laemmli buffer,23 and examined by SDS-PAGE and immunoblotted for TfR and AQP-1.
Discussion
During the last step of erythropoiesis, the reticulocyte plasma membrane is extensively remodelled. This process can be partly explained by the loss of proteins released into the bloodstream in association with exosomes. It has been well documented that the TfR is the major protein component of reticulocyte-secreted exosomes.30 Here, we have shown that, during reticulocyte maturation, proteins are selectively targeted to these vesicles and that a subpopulation of the water channel, AQP-1, is selectively directed into MVBs and exosomes. A striking feature of our results is that the extent of this sorting process changes in response to the extracellular tonicity. This previously unreported means of regulating protein concentrations in exosomes released by reticulocytes has implications for the regulation of cell volume.
There is as yet no comprehensive list of the proteins resident in exosomes secreted during reticulocyte maturation. On the basis of our comparative analysis between reticulocytes and exosomes, we could highlight the presence of several proteins, including Glut-4. A recent study showed that Glut-1 is not expressed by adult murine red cells, where it is replaced by Glut-4.31 We infer that in adult mice the total pool of Glut-4 is down-regulated by its loss in exosomes, whereas in newborn animals, glucose transport regulation is linked to Glut-1 turnover and transcriptional switching to Glut-4. The fact that CD47 is also associated with exosomes is intriguing. Indeed, CD47 has been described as a “do not eat me” signal, preventing, eg, phagocytosis of the red cell throughout its lifespan in the circulation,32 until eventually aged erythrocytes present other signals (eg, phosphatidylserine exposure33). The effect of the do not eat me signaling molecule CD47 on exosomes is unknown. If exosomes are indeed phagocytosed, it may be that there are not enough copies of CD47 present or that it is structurally different in the exosomal membrane compared with the mature erythrocyte membrane, resulting in CD47 molecules that do not function as do not eat me signals.
The mechanisms that trigger the protein-sorting process are not fully understood. We hypothesized that ubiquitination might be a signal for directing AQP-1 into exosomes. In fact, ubiquitination of AQP-1 has been shown in fibroblasts.34 Furthermore. earlier studies showed mono-ubiquitination to be a key signal for the recruitment and assembly of the ESCRT machinery, which initiates sorting of integral proteins into intraluminal vesicles of MVBs.8,35,36 To test our hypothesis, MG132 was used to inhibit putative ubiquitination of the water channel, and AQP-1 sorting into exosomes was indeed inhibited by this drug.
Regulation of cell volume is an important attribute of the red blood cell, and the presence of AQP-1 within exosomes is striking because it fulfils the essential function in the mature red cell of enabling passage of water through the membrane. Although sorting of AQP-1 has never been investigated in the reticulocyte system, the water channel was found in urine exosomes, based on a proteomic approach.16 In the present study, we demonstrated that sorting of AQP-1 into exosomes accompanies maturation. Moreover, the fact that AQP-1 is also found in exosomes isolated from the plasma of anemic animals supports the view that sorting of the water channel during reticulocyte maturation is a physiologic process, occurring in vivo. Importantly, our finding that the extent of AQP-1 sorting into exosomes is modulated by suspending medium osmolality is probably physiologically relevant in the context of terminal erythroid differentiation. We speculate that the extracellular osmotic conditions that erythroblasts and young reticulocytes are exposed to in the bone marrow are different from those encountered in the peripheral circulation, necessitating a higher level of expression of AQP-1.
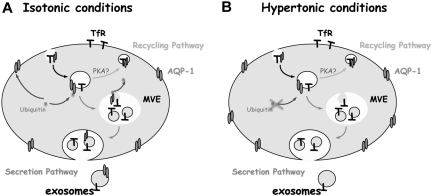
In this context, we present a model for AQP-1 sorting during reticulocyte maturation, as governed by the osmotic conditions. We infer that in the isotonic state (Figure 7A) approximately 15% to 20% of the total pool of AQP-1 in reticulocytes is lost through endocytosis and sorting into MVEs, leading to its final release within exosomes. Under hypertonic conditions (Figure 7B), conversely, this exosomal sorting pathway is blocked, such that more AQP-1 is mobilized on the plasma membrane to counter the osmotic disturbance and to prevent damage to the cell. By analogy with vasopressin-mediated AQP-2 translocation in renal cells,37 and osmotic stress-mediated AQP-1 mobilization from an intracellular pool in peritoneal mesothelial cells,38 we may conjecture that PKA-mediated phosphorylation is involved in AQP-1 recycling from the endosomal compartment to the plasma membrane in reticulocytes.
Figure 7.
Proposed model of AQP-1 sorting into exosomes during reticulocyte maturation. Under physiologic conditions (A), AQP-1 levels are regulated through the exosomal pathway. During osmotic stress, by contrast (B), AQP-1 secretion is inhibited, probably by regulation of its ubiquitination or by phosphorylation. It is consequently rerouted from the secretion pathway into the recycling pathway, thereby augmenting the AQP-1 concentration on the plasma membrane, and thus assisting the cell to nullify the osmotic shock. TfR trafficking, by contrast, is not affected by the osmotic disturbance and continues to be sorted and secreted through the exosomal pathway.
Supplementary Material
Acknowledgments
We thank Yelena Oksov for help with immunogold labeling and electron microscopy, and Lyudmil Angelov for help with confocal microscopy. We thank Dr Walter Gratzer for helpful discussions and critical reading of the manuscript.
This work was supported by the National Institutes of Health (grants DK26263, DK32094, and HL31579).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.B. designed and performed research, analyzed data, and wrote the manuscript; J.L. performed research and analyzed data; and M.V., J.A.C., X.A., and N.M. designed research, analyzed data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lionel Blanc or Xiulu An, Red Cell Physiology Laboratory, New York Blood Center, 310 East 67th St, New York, NY 10065; e-mail: lblanc@nybloodcenter.org or xan@nybloodcenter.org.
References
- 1.Mel HC, Prenant M, Mohandas N. Reticulocyte motility and form: studies on maturation and classification. Blood. 1977;49(6):1001–1009. [PubMed] [Google Scholar]
- 2.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112(3):470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gronowicz G, Swift H, Steck TL. Maturation of the reticulocyte in vitro. J Cell Sci. 1984;71(1):177–197. doi: 10.1242/jcs.71.1.177. [DOI] [PubMed] [Google Scholar]
- 4.Johnstone RM. The Jeanne Manery-Fisher Memorial Lecture 1991. Maturation of reticulocytes: formation of exosomes as a mechanism for shedding membrane proteins. Biochem Cell Biol. 1992;70(3):179–190. doi: 10.1139/o92-028. [DOI] [PubMed] [Google Scholar]
- 5.Rieu S, Geminard C, Rabesandratana H, Sainte-Marie J, Vidal M. Exosomes released during reticulocyte maturation bind to fibronectin via integrin alpha4beta1. Eur J Biochem. 2000;267(2):583–590. doi: 10.1046/j.1432-1327.2000.01036.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420. [PubMed] [Google Scholar]
- 7.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 8.Geminard C, De Gassart A, Blanc L, Vidal M. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic. 2004;5(3):181–193. doi: 10.1111/j.1600-0854.2004.0167.x. [DOI] [PubMed] [Google Scholar]
- 9.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140(1):13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 10.Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell. 2002;3(2):283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 11.Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev Cell. 2002;3(2):271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- 12.Saksena S, Sun J, Chu T, Emr SD. ESCRTing proteins in the endocytic pathway. Trends Biochem Sci. 2007;32(12):561–573. doi: 10.1016/j.tibs.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 14.Andre F, Schartz NE, Movassagh M, et al. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360(9329):295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 15.Knepper MA, Pisitkun T. Exosomes in urine: who would have thought? Kidney Int. 2007;72(9):1043–1045. doi: 10.1038/sj.ki.5002510. [DOI] [PubMed] [Google Scholar]
- 16.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101(36):13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agre P, King LS, Yasui M, et al. Aquaporin water channels–from atomic structure to clinical medicine. J Physiol. 2002;542(1):3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 19.Mathai JC, Mori S, Smith BL, et al. Functional analysis of aquaporin-1 deficient red cells. The Colton-null phenotype. J Biol Chem. 1996;271(3):1309–1313. doi: 10.1074/jbc.271.3.1309. [DOI] [PubMed] [Google Scholar]
- 20.Blanc L, Barres C, Bette-Bobillo P, Vidal M. Reticulocyte-secreted exosomes bind natural IgM antibodies: involvement of a ROS-activatable endosomal phospholipase iPLA2. Blood. 2007;110(9):3407–3416. doi: 10.1182/blood-2007-04-085845. [DOI] [PubMed] [Google Scholar]
- 21.Steck TL, Kant JA. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31(1):172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- 22.Ahn J, Johnstone RM. Origin of a soluble truncated transferrin receptor. Blood. 1993;81(9):2442–2451. [PubMed] [Google Scholar]
- 23.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10(6):839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Langhorst MF, Reuter A, Jaeger FA, et al. Trafficking of the microdomain scaffolding protein reggie-1/flotillin-2. Eur J Cell Biol. 2008;87(4):211–226. doi: 10.1016/j.ejcb.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 27.Melikova MS, Kondratov KA, Kornilova ES. Two different stages of epidermal growth factor (EGF) receptor endocytosis are sensitive to free ubiquitin depletion produced by proteasome inhibitor MG132. Cell Biol Int. 2006;30(1):31–43. doi: 10.1016/j.cellbi.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 28.van Kerkhof P, Alves dos Santos CM, Sachse M, Klumperman J, Bu G, Strous GJ. Proteasome inhibitors block a late step in lysosomal transport of selected membrane but not soluble proteins. Mol Biol Cell. 2001;12(8):2556–2566. doi: 10.1091/mbc.12.8.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vidal M, Mangeat P, Hoekstra D. Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J Cell Sci. 1997;110(16):1867–1877. doi: 10.1242/jcs.110.16.1867. [DOI] [PubMed] [Google Scholar]
- 30.Johnstone RM. Revisiting the road to the discovery of exosomes. Blood Cells Mol Dis. 2005;34(3):214–219. doi: 10.1016/j.bcmd.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Montel-Hagen A, Blanc L, Boyer-Clavel M, et al. The Glut1 and Glut4 glucose transporters are differentially expressed during perinatal and postnatal erythropoiesis. Blood. 2008;112(12):4729–4738. doi: 10.1182/blood-2008-05-159269. [DOI] [PubMed] [Google Scholar]
- 32.Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 33.Connor J, Pak CC, Schroit AJ. Exposure of phosphatidylserine in the outer leaflet of human red blood cells. Relationship to cell density, cell age, and clearance by mononuclear cells. J Biol Chem. 1994;269(4):2399–2404. [PubMed] [Google Scholar]
- 34.Leitch V, Agre P, King LS. Altered ubiquitination and stability of aquaporin-1 in hypertonic stress. Proc Natl Acad Sci U S A. 2001;98(5):2894–2898. doi: 10.1073/pnas.041616498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buschow SI, Liefhebber JM, Wubbolts R, Stoorvogel W. Exosomes contain ubiquitinated proteins. Blood Cells Mol Dis. 2005;35(3):398–403. doi: 10.1016/j.bcmd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Olver C, Vidal M. Proteomic analysis of secreted exosomes. Subcell Biochem. 2007;43(2):99–131. doi: 10.1007/978-1-4020-5943-8_7. [DOI] [PubMed] [Google Scholar]
- 37.Hoffert JD, Fenton RA, Moeller HB, et al. Vasopressin-stimulated increase in phosphorylation at Ser269 potentiates plasma membrane retention of aquaporin-2. J Biol Chem. 2008;283(36):24617–24627. doi: 10.1074/jbc.M803074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakurada T, Kuboshima S, Ogimoto G, et al. Aquaporin-1 is recruited to the plasma membrane by hyperosmotic stimuli via a protein kinase A-dependent pathway in rat peritoneal mesothelial cells. Adv Perit Dial. 2004;20(1):37–42. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.