Abstract
Background
Selenium is an antioxidant micronutrient with potential interest for cardiovascular disease prevention. Few studies have evaluated the association between selenium and hypertension, with inconsistent findings. We explored the relationship of serum selenium concentrations with blood pressure and hypertension in a representative sample of the US population.
Methods and Results
Cross-sectional analysis of 2,638 adults ≥40 year old who participated in the National Health and Nutrition Examination Survey (NHANES) 2003–2004. Serum selenium was measured by inductively coupled plasma-dynamic reaction cell-mass spectrometry. Hypertension was defined as blood pressure ≥140/90 mmHg or current use of antihypertensive medication. Mean serum selenium was 137.1 µg/L. The multivariable adjusted differences (95% confidence interval) in blood pressure levels comparing the highest (≥150 µg/L) to the lowest (<122 µg/L) quintile of serum selenium were 4.3 (1.3, 7.4), 1.6 (−0.5, 3.7) and 2.8 (0.8, 4.7) mmHg for systolic, diastolic, and pulse pressure, respectively. The corresponding odds ratio (95% CI) for hypertension was 1.73 (1.18, 2.53). In spline regression models, blood pressure levels and the prevalence of hypertension increased with increasing selenium concentrations up to 160 µg/L.
Conclusions
High serum selenium concentrations were associated with higher prevalence of hypertension. These findings call for a thorough evaluation of the risks and benefits associated with high selenium status in the US.
Keywords: Selenium, blood pressure, hypertension, National Health and Nutrition Examination Survey, NHANES
INTRODUCTION
Selenium is an essential element with antioxidant properties mediated through glutathione peroxidases and other selenoenzymes. Because oxidative stress is involved in hypertension development,1 it has been suggested that selenium may be involved in blood pressure control and hypertension prevention.2–4 The few studies that have evaluated the association between selenium and blood pressure have been inconsistent, reporting inverse3,5, null,6,7 or positive8 associations. These studies were relatively small and were conducted in European countries with moderately low selenium intake. As a consequence, the association of selenium with blood pressure levels is still uncertain, particularly in selenium replete populations such as the US.
In the US, selenium intake ranges from 60 to 220 µg/d,4,9,10 well above the recommended dietary allowance of 55 µg/d.11,12 High selenium intake in the US is a consequence of the high selenium content of US soil, particularly in the Northern Plains. Organ meats, seafoods, muscle meats, and cereals and grains are the main sources of selenium in the diet, although the widespread use of vitamin / mineral supplements also contributes to US selenium intake.13 At these intake levels, additional selenium intake does not increase glutathione peroxidase synthesis or activity, but rather increases plasma selenium concentration by nonspecific incorporation of selenomethionine into plasma proteins.9 Furthermore, selenium has a narrow safety range,13 and high selenium concentrations in US studies have been associated with increased lipid levels14 and diabetes.15,16 The objective of this study was thus to assess the association of serum selenium concentrations with blood pressure levels and with the prevalence of hypertension in the US National Health and Nutrition Examination Survey (NHANES) 2003–2004.
METHODS
NHANES is conducted by the National Center for Health Statistics using a complex multistage sampling design to obtain a probability sample of the civilian non-institutionalized US population. We used data from NHANES 2003–2004,17 as this was the most recent release with selenium data available in adults. Serum selenium measurements were restricted to participants aged ≥40 years (N = 3,299). Among these, 2,903 participants had serum selenium measurements, and 2,699 participants had also blood pressure measurements available. We excluded 2 pregnant women and 59 participants with missing data on relevant covariates (i.e., body mass index, education, cotinine concentration, and tobacco consumption). The final sample size was 2,638.
Serum selenium
Collection materials were screened for potential selenium contamination. After blood collection, serum aliquots were frozen at −20°C and shipped to the laboratory. Serum selenium was measured at the Trace Elements Laboratory at the Wadsworth Center of the New York State Department of Health using inductively coupled plasma-dynamic reaction cell-mass spectrometry (ICP-DRC-MS). The laboratory procedures and quality control methods for serum selenium measurement have been described in detail elsewhere.18 The between-assay coefficients of variation for quality control pooled samples analyzed throughout the duration of the survey ranged from 2.5 to 2.9%.
Hypertension
Blood pressure readings were obtained by trained and certified physicians following procedures developed by the American Heart Association.19 Study participants rested sitting quietly for 5 minutes before three consecutive blood pressure readings were obtained using a mercury sphygmomanometer with an appropriate size cuff (five sizes available) placed on the bare right arm. If one reading failed, a fourth attempt could be made. Systolic and diastolic blood pressure were registered at the appearance (phase I) and disappearance (phase V) of Korotkoff sounds, respectively. Repeated measurements of systolic and diastolic blood pressure were averaged discarding the first one.19 Pulse pressure was calculated as the difference between systolic and diastolic blood pressure. Quality control and assurance procedures included extensive initial training, quarterly recertification, procedural checklists, and continuous review of data for systematic errors.20 Hypertension was defined as having an average systolic blood pressure ≥ 140 mmHg, an average diastolic blood pressure ≥ 90 mmHg, or current use of antihypertensive medications.
Other variables
Information on sex, age, race-ethnicity, education, menopausal status, smoking and use of vitamin / mineral supplements was based on self-report. Body mass index was calculated by dividing measured weight in kilograms by measured height in meters squared. Nutrient intake data were obtained from two 24-hour dietary recall interviews,21 one conducted in-person and a second phone follow-up interview 3 to 10 days later. Average intakes were used when information was available from both interviews. Serum cotinine was measured by isotope-dilution high-performance liquid chromatography / atmospheric pressure chemical ionization tandem mass spectrometry.22
Statistical methods
Participants were divided in quintiles of serum selenium concentration based on the weighted population distribution. Adjusted means for blood pressure differences and odds ratios for hypertension status comparing each quintile of serum selenium to the lowest quintile were calculated using multivariable linear and logistic regression, respectively. We used 3 models with progressive degrees of adjustment. Model 1 was adjusted for sex, age (continuous), race / ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American or other) and education (high school or higher vs. less than high school). Model 2 was further adjusted for body mass index (continuous), smoking (never, former, current), cotinine (continuous), and menopausal status (yes, no). Model 3 was further adjusted for use of vitamin / mineral supplements (yes, no) and, in linear regression models, for use of antihypertensive medications (yes, no). Continuous covariables were included as linear terms in the models. Since adjusting for hypertension treatment may result in biased estimates of the association between selenium and continuous blood pressure outcomes, we conducted an additional analysis using censored linear regression (Model 4) to correct for antihypertensive treatment using NHANES III survey weights.23 Tests for linear trend were calculated by including serum selenium as a continuous variable in the models. To further explore the shape of the relationship between serum selenium and blood pressure measurements and hypertension, we used restricted quadratic splines24 with knots at the 5th, 50th and 95th percentiles of the serum selenium distribution in models adjusted as previously described for Model 3. We also evaluated the interactions between selenium (modeled as quadratic restricted splines) and sex, age, race / ethnicity, education, body mass index, smoking status or use of vitamin / mineral supplements by fitting separate models for every interaction, including all the adjustment variables described for Model 3 but each with interaction terms for the selenium splines and the variable of interest. Statistical analyses were performed using the survey package25 (version 3.6.13) in R26 (version 2.6.1) to account for the complex sampling design and weights in NHANES 2003–2004. Censored regression models were estimated using the cnreg command in Stata27 (version 9.2) weighted for NHANES survey weights.
RESULTS
The mean (standard deviation) serum selenium concentration in the study population was 137.1 (19.3) µg/L. The overall prevalence of hypertension was 45.2%. Participants with hypertension were more likely to be older, Non-Hispanic Black and to have a higher body mass index, and less likely to have a high school education and to be current smokers compared to participants without hypertension (Table 1). Serum selenium concentrations were positively associated with age and with the use of vitamin / mineral supplements, and inversely associated with current smoking (Table 2). Men had higher mean serum selenium than women (139.7 vs. 134.7 µg/L). Non-Hispanic Blacks had lower mean serum selenium compared to Non-Hipanic Whites and to Mexican Americans (130.7, 137.7 and 140.4 µg/L, respectively).
Table 1.
Characteristics of the study population by hypertension status.
| Overall | Normal | Hypertension | P | |
|---|---|---|---|---|
| N | 2638 | 1227 (54.8 %) | 1411 (45.2 %) | |
| Age (years) | 56.7 (12.4) | 52.5 (10.6) | 61.6 (12.5) | <0.001 |
| Gender (% female) | 51.8 | 51.4 | 52.4 | 0.74 |
| Race | 0.007 | |||
| Non-Hispanic White (%) | 78.8 | 79.7 | 77.9 | |
| Non-Hispanic Black (%) | 9.5 | 7.6 | 11.9 | |
| Mexican American (%) | 4.9 | 5.7 | 3.9 | |
| Other (%) | 6.7 | 7.0 | 6.3 | |
| Education (% High school) | 81.4 | 84.4 | 77.7 | 0.004 |
| Body Mass Index (kg/m2) | 28.5 (5.7) | 27.5 (5.3) | 29.7 (5.8) | <0.001 |
| Current smoker (%) | 21.1 | 25.1 | 16.3 | <0.001 |
| Dietary Supplements (%) | 62.4 | 60.5 | 64.7 | 0.18 |
| Selenium (µg/L) | 137.1 (19.3) | 136.1 (18.8) | 138.3 (19.8) | 0.02 |
| Systolic BP (mmHg) | 127.9 (19.5) | 118.1 (11.5) | 139.8 (20.5) | - |
| Diastolic BP (mmHg) | 72.4 (13.2) | 71.4 (9.4) | 73.6 (16.6) | - |
| Pulse Pressure (mmHg) | 55.6 (20.9) | 46.7 (12.2) | 66.3 (24.1) | - |
Values are survey weighted means (standard deviation) or percentages for continuous or categorical variables, respectively.
Table 2.
Characteristics of the study population by serum selenium quintile.
| Quintile of serum selenium (interval in µg/L) | ||||||
|---|---|---|---|---|---|---|
| 1st < 122 |
2nd 122 – 131 |
3rd 132 – 139 |
4th 140 – 149 |
5th ≥ 150 |
p trend | |
| N | 504 | 570 | 524 | 481 | 559 | |
| Age (years) | 56.6 | 56.3 | 57.2 | 55.4 | 57.7 | 0.02 |
| Gender (% female) | 64.3 | 57.9 | 49.8 | 43.3 | 44.4 | <0.001 |
| Race | ||||||
| Non-Hispanic White (%) | 76.5 | 75.6 | 76.6 | 82.3 | 83.1 | 0.03 |
| Non-Hispanic Black (%) | 15.3 | 10.9 | 10.3 | 6.3 | 5.3 | 0.004 |
| Mexican American (%) | 2.3 | 5.4 | 5.8 | 4.6 | 6.1 | 0.04 |
| Other (%) | 5.9 | 8.1 | 7.2 | 6.9 | 5.4 | 0.73 |
| Education (% High school) | 77.1 | 79.6 | 82.1 | 84.2 | 83.6 | 0.12 |
| Body Mass Index (kg/m2) | 28.6 | 28.7 | 28.7 | 28.6 | 28.0 | 0.05 |
| Current smoker (%) | 33.4 | 20.6 | 18.5 | 20.7 | 13.7 | <0.001 |
| Dietary Supplements (%) | 54.6 | 57.5 | 68.9 | 61.4 | 69.2 | 0.001 |
| Selenium (µg/L) | 113.1 | 126.7 | 135.2 | 144.4 | 163.7 | - |
Values are survey weighted means or percentages for continuous or categorical variables, respectively.
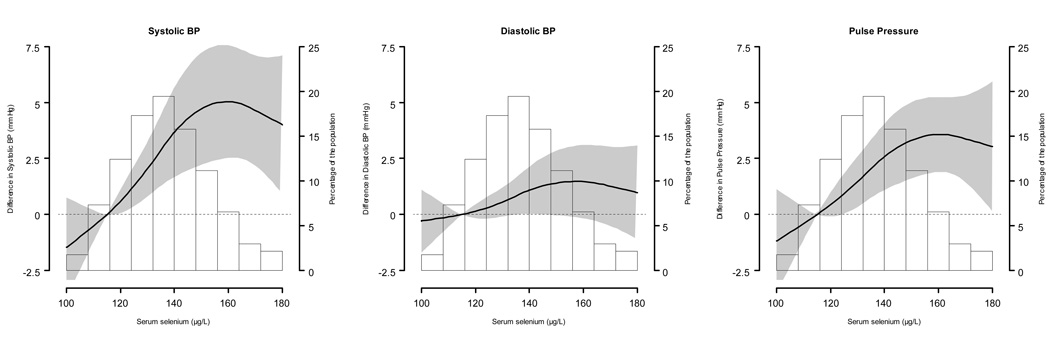
Mean serum selenium concentrations were higher in participants with hypertension compared to those without it (138.3 vs 136.1 µg/L, p = 0.02). In multivariable adjusted models (Table 3), the average differences (95% CI) comparing the highest (≥150 µg/L) to the lowest (<122 µg/L) selenium quintiles were 4.3 (1.3, 7.4), 1.6 (−0.5, 3.7), and 2.8 (0.8, 4.7) mmHg for systolic, diastolic, and pulse pressure, respectively. After correcting for use of medication for hypertension in censored regression models, these differences were 6.3 (3.4, 9.2), 2.8 (1.1, 4.6), and 4.6 (1.8, 7.4) mmHg for systolic, diastolic, and pulse pressure, respectively. In spline regression models, blood pressure levels increased with increasing selenium concentrations up to 160 µg/L (Figure 1).
Table 3.
Adjusted differences (95% CI) in blood pressure levels comparing the four highest quintiles to the first quintile of serum selenium.
| Quintile of serum selenium (interval in µg/L) | ||||||
|---|---|---|---|---|---|---|
| 1st < 122 |
2nd 122 – 131 |
3rd 132 – 139 |
4th 140 – 149 |
5th ≥ 150 |
p trend | |
|
Systolic BP (mmHg) |
125.4 | 127.4 | 127.5 | 129.4 | 129.7 | |
| Model 1 * | 0.0 (Reference) |
2.3 (−0.6, 5.2) |
2.0 (0.0, 4.1) |
5.4 (3.0, 7.8) |
4.4 (1.5, 7.4) |
0.008 |
| Model 2 † | 0.0 (Reference) |
2.4 (−0.5, 5.4) |
2.1 (−0.1, 4.2) |
5.3 (2.7, 7.9) |
4.6 (1.5, 7.7) |
0.007 |
| Model 3 ‡ | 0.0 (Reference) |
2.4 (−0.7, 5.5) |
2.2 (−0.1, 4.4) |
5.1 (2.3, 7.8) |
4.3 (1.3, 7.4) |
0.009 |
| Model 4 ** | 0.0 (Reference) |
3.3 (0.5, 6.1) |
2.3 (−0.6, 5.2) |
6.9 (4.0, 9.8) |
6.3 (3.4, 9.2) |
<0.001 |
|
Diastolic BP (mmHg) |
70.7 | 72.9 | 71.6 | 74.0 | 72.4 | |
| Model 1 * | 0.0 (Reference) |
2.0 (0.2, 3.8) |
0.8 (−0.9, 2.6) |
2.5 (0.8, 4.2) |
1.8 (−0.3, 3.9) |
0.15 |
| Model 2 † | 0.0 (Reference) |
1.9 (0.0, 3.7) |
0.6 (−1.1, 2.4) |
2.2 (0.5, 4.0) |
1.5 (−0.6, 3.6) |
0.20 |
| Model 3 ‡ | 0.0 (Reference) |
1.9 (0.0, 3.7) |
0.6 (−1.1, 2.4) |
2.3 (0.5, 4.0) |
1.6 (−0.5, 3.7) |
0.19 |
| Model 4 ** | 0.0 (Reference) |
2.1 (0.4, 3.8) |
0.5 (−1.2, 2.3) |
3.2 (1.4, 5.0) |
2.8 (1.1, 4.6) |
0.004 |
|
Pulse Pressure (mmHg) |
54.7 | 54.4 | 55.9 | 55.4 | 57.3 | |
| Model 1 * | 0.0 (Reference) |
0.3 (−1.3, 2.0) |
1.2 (−1.0, 3.4) |
2.8 (1.1, 4.5) |
2.6 (0.7, 4.6) |
0.03 |
| Model 2 † | 0.0 (Reference) |
0.6 (−1.1, 2.3) |
1.5 (−0.9, 3.8) |
3.1 (1.4, 4.8) |
3.1 (1.1, 5.1) |
0.02 |
| Model 3 ‡ | 0.0 (Reference) |
0.5 (−1.2, 2.3) |
1.5 (−0.9, 3.9) |
2.8 (0.8, 4.8) |
2.8 (0.8, 4.7) |
0.03 |
| Model 4 ** | 0.0 (Reference) |
0.9 (−1.8, 3.7) |
1.5 (−1.3, 4.3) |
4.3 (1.5, 7.2) |
4.6 (1.8, 7.4) |
<0.001 |
In bold, unadjusted (survey-weighted) averages. Models 1 – 3 used multiple linear regression models with survey weights, strata, and clusters to account for complex survey design. Model 4 used censored regression with survey weights only. Continuous covariables were included as linear terms in the models.
Model 1. Adjusted for sex, age (continuous), race / ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American or other) and education (high school or higher vs. less than high school).
Model 2. Further adjusted for body mass index (continuous), smoking (never, former, current), cotinine (continuous), and menopausal status (yes, no).
Model 3. Further adjusted for use of vitamin / mineral supplements (yes, no), and use of antihypertensive medications (yes, no).
Model 4. Censored linear regression to correct for the effect of medication for hypertension, adjusted for the same variables as model 3.
Figure 1. Adjusted differences (95% confidence intervals) for blood pressure levels by serum selenium concentrations.
Serum selenium was modeled as restricted quadratic splines with nodes at the 5th, 50th, and 95th percentiles. Multivariable linear regression models were adjusted for sex, age (continuous), race / ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American or other), education (high school or higher vs. less than high school), body mass index (continuous), smoking (never, former, current), cotinine (continuous), menopausal status (yes, no), use of vitamin / mineral supplements (yes, no), and use of antihypertensive medications (yes, no). Blood pressure levels at the 10th percentile (115 µg/L) of the serum selenium distribution were used as reference. The histogram shows the distribution of selenium concentrations in the study population.
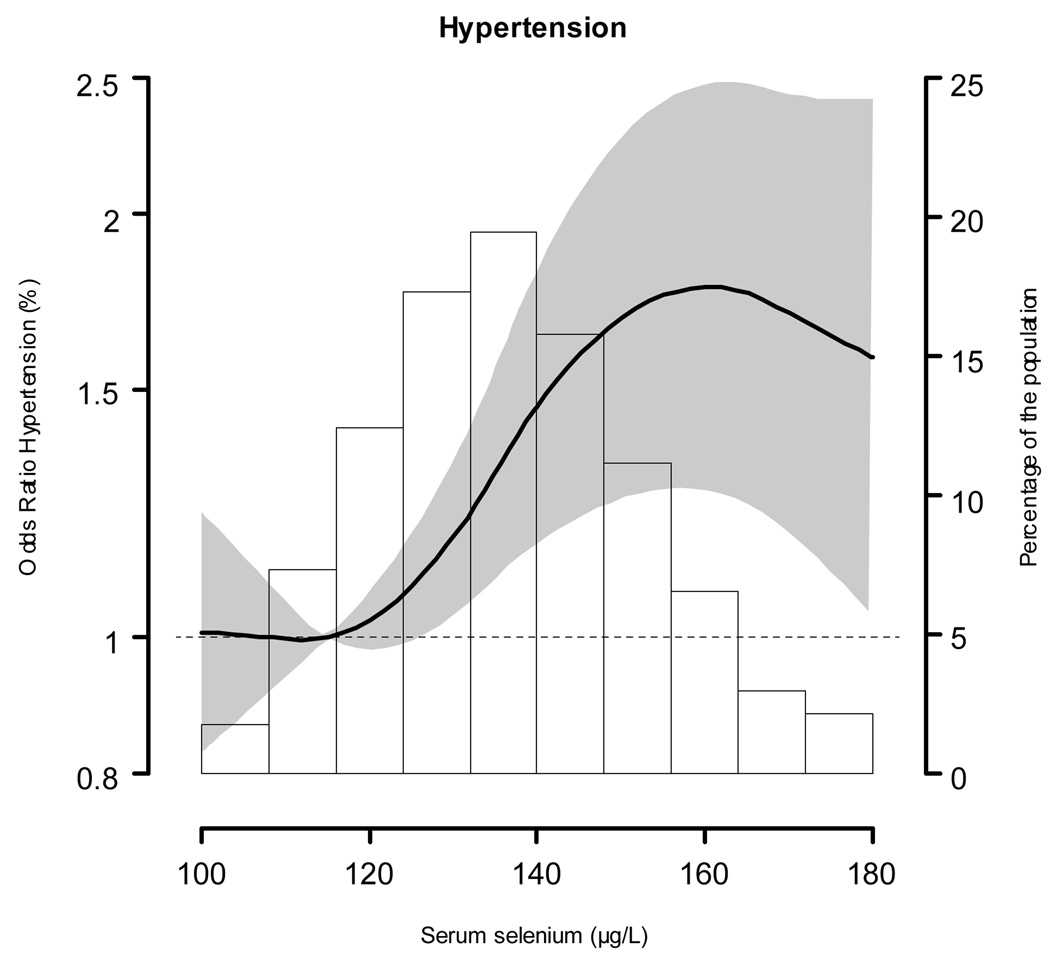
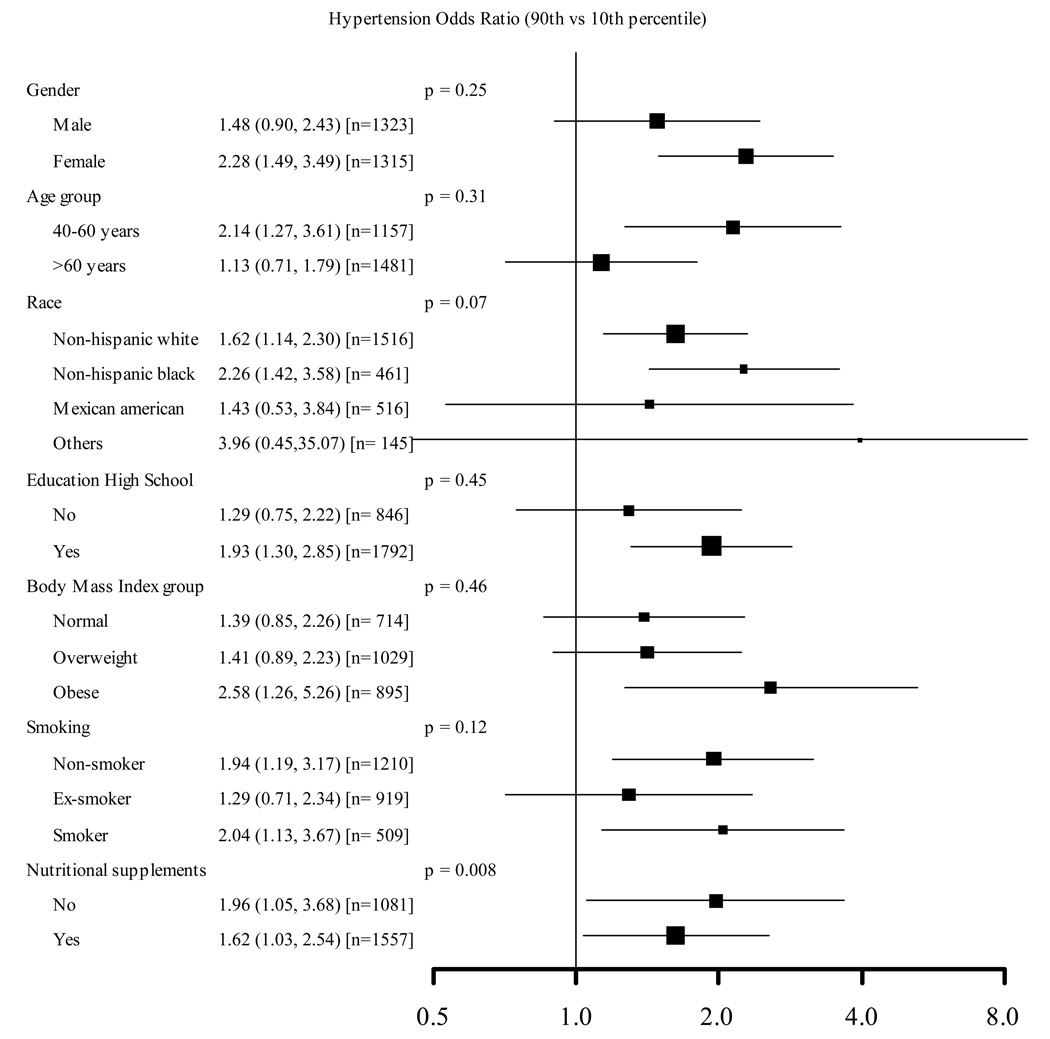
The multivariable adjusted odds ratio (95% CI) for hypertension comparing the highest to the lowest selenium quintiles was 1.73 (1.18, 2.53) (Table 4). In spline regression models, the prevalence of hypertension increased with increasing selenium concentrations up to 160 µg/L (Figure 2). The adjusted odds ratio for hypertension comparing the 90th (160 µg/L) to the 10th (115 µg/L) percentiles of the selenium distribution was 1.77 (1.27, 2.47), with consistent findings across clinically relevant subgroups (Figure 3).
Table 4.
Adjusted odds ratios (95% CI) for the presence of hypertension comparing the four highest quintiles to the first quintile of serum selenium.
| Quintile of serum selenium (interval in µg/L) | ||||||
|---|---|---|---|---|---|---|
| 1st < 122 |
2nd 122 – 131 |
3rd 132 – 139 |
4th 140 – 149 |
5th ≥ 150 |
p trend | |
| Hypertension (%) | 39.2 | 44.3 | 43.6 | 49.7 | 49.0 | |
| Model 1 * | 1.00 (Reference) |
1.37 (0.99, 1.90) |
1.25 (1.01, 1.55) |
1.95 (1.50, 2.55) |
1.63 (1.13, 2.33) |
0.05 |
| Model 2 † | 1.00 (Reference) |
1.40 (1.01, 1.94) |
1.25 (1.00, 1.56) |
1.99 (1.43, 2.78) |
1.72 (1.19, 2.50) |
0.02 |
| Model 3 ‡ | 1.00 (Reference) |
1.40 (1.01, 1.94) |
1.26 (0.99, 1.59) |
2.00 (1.42, 2.81) |
1.73 (1.18, 2.53) |
0.02 |
Model 1. Adjusted for sex, age (continuous), race / ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American or other) and education (high school or higher vs. less than high school).
Model 2. Further adjusted for body mass index (continuous), smoking (never, former, current), cotinine (continuous), and menopausal status (yes, no).
Model 3. Further adjusted for use of vitamin / mineral supplements (yes, no).
Figure 2. Adjusted odds ratios (95% confidence intervals) for hypertension by serum selenium concentrations.
Serum selenium was modeled as restricted quadratic splines with nodes at the 5th, 50th, and 95th percentiles. The multivariable logistic regression model was adjusted for sex, age (continuous), race / ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American or other), education (high school or higher vs. less than high school), body mass index (continuous), smoking (never, former, current), cotinine (continuous), menopausal status (yes, no), and use of vitamin / mineral supplements (yes, no). The odds for hypertension at the 10th percentile (115 µg/L) of the serum selenium distribution were used as reference. The histogram shows the distribution of selenium concentrations in the study population.
Figure 3. Adjusted odds ratios (95% confidence intervals) for hypertension comparing the 90th (160 µg/L) vs. the 10th (115 µg/L) percentiles of the serum selenium distribution.
Serum selenium was modeled as restricted quadratic splines with nodes at the 5th, 50th, and 95th percentiles. Multivariable logistic regression models were adjusted for sex, age (continuous), race / ethnicity (non-Hispanic White, non-Hispanic Black, Mexican American or other), education (high school or higher vs. less than high school), body mass index (continuous), smoking (never, former, current), cotinine (continuous), menopausal status (yes, no), and use of vitamin / mineral supplements (yes, no). Separate models for every interaction were fitted, including all these covariables but each with interaction terms for the selenium splines and the variable of interest. The size of the square indicates the number of participants in each stratum.
Sensitivity analyses showed similar results after excluding participants with self-reported cancer or cardiovascular disease, and after excluding participants taking any anti-hypertensive medications. Similarly, results from Model 3 did not substantially change after additional adjustment for dietary variables from the 24-h dietary recalls (total caloric intake, polyunsaturated to saturated fat intake ratio, sodium, calcium, magnesium, fiber, β-carotene, vitamin E, vitamin C, and alcohol intake), after separate additional adjustment for serum levels of β-carotene, vitamin E, and vitamin C, or after separate additional adjustment for the presence of diabetes and the presence of hypercholesterolemia. Adjustment for the NHANES pseudo-primary sampling units had negligible effects on the results (not shown).
DISCUSSION
In this representative cross-sectional study of the US population, high serum selenium concentrations were associated with higher blood pressure levels and with higher prevalence of hypertension. Compared to the lowest quintile, participants in the highest selenium quintile had increased systolic, diastolic, and pulse pressure by 4.3, 1.6, and 2.8 mmHg, respectively (6.3, 2.8, and 4.6 mmHg after correcting for use of medication for hypertension in censored regression models, respectively). These differences are epidemiologically and clinically important,28,29 and they add to the concerns raised by the observation of elevated lipid levels14 and increased risk of diabetes15,16 with high selenium concentrations in studies conducted in the US, a country with high selenium intake.
Few observational studies have evaluated the association of selenium with blood pressure levels. A study in elderly Finnish men6 from areas with very low mean serum selenium found no relationship between selenium and blood pressure. Conversely, the Kuopio Ischaemic Heart Disease Risk Factor Study,5 a Finnish cohort of middle-aged men, found an inverse association between serum selenium and systolic blood pressure. The Flemish Study on Environment Genes and Health Outcomes (FLEMENGHO)3 also found an inverse cross-sectional association between blood selenium concentrations and systolic and diastolic blood pressure in men, but this association was largely due to markedly higher blood pressure levels in the lowest selenium quintile (<78 µg/L) with little variation across the remaining quintiles. Baseline blood selenium concentrations were also inversely associated with the risk of hypertension after 5.2 years of follow-up in men. No association was observed among women, either cross-sectionally or prospectively.3 A cross-sectional analysis of the French Etude du Viellissement Artérial (EVA),8 reported that hypertensive men, but not women, had higher selenium concentrations compared to those without major chronic diseases or risk factors. Plasma selenium concentrations were not correlated with systolic blood pressure in either men or women.8 Finally, serum selenium concentrations were not associated with blood pressure levels in the Olivetti Heart Study.7 All these studies were conducted in European countries and had mean selenium concentrations below 100 µg/L.
Compared to previous observational studies, our study was conducted in the US, a country with high mean serum selenium concentrations (137 µg/L). The increased blood pressure levels and prevalence of hypertension at high selenium levels in NHANES 2003–2004 is consistent with previous studies reporting a positive association of selenium concentration with adverse lipid profile and diabetes risk.14–16 Indeed, it is possible that high risk factor levels at high selenium concentrations may explain the U-shaped relationship between selenium concentration and cardiovascular endpoints observed in several US studies.12,30,31 Given the high selenium intake in the US population and the popularity of selenium supplements, it is important to elucidate the mechanisms underlying the association of high selenium exposure and cardiovascular risk factors in selenium-replete populations.
Unfortunately, no data are available on the effect of selenium supplementation on blood pressure endpoints in randomized controlled trials using single selenium supplements. In the HDL-Atherosclerosis Treatment Study (HATS) trial, selenium (100 µg/d) was administered along with vitamin E (800 IU/d), vitamin C (1000 mg/d), and β-carotene (25 mg/d), with no effect on blood pressure levels.32 In China (Linxian), antioxidant supplementation (selenium 50 µg/d, β-carotene 15 mg/d, and vitamin E 60 mg/d) in a nutritionally deficient population was linked only to increased isolated diastolic hypertension, but other blood pressure endpoints were not significantly different.33
The concerns with potential metabolic side effects of elevated selenium levels add to the lack of efficacy of selenium supplements recently shown in the Selenium and Vitamin E Cancer Prevention Trial (SELECT), a mega-trial aimed to evaluate the efficacy of selenium in preventing prostate cancer. SELECT was prematurely stopped by the Data and Safety Monitoring Committee for lack of benefit on the prostate cancer prevention and for safety concerns.34
The strengths of our study come from the rigorous sampling design, the strict adherence to study protocols in measurements and laboratory assays, and the representativeness of the NHANES sample. Several limitations, however, need to be considered in the interpretation of these findings. The cross-sectional design limits our ability to determine the direction and the causality of the observed association. It is possible that pathophysiological changes of hypertension modify serum selenium concentration or that participants with hypertension change their health behaviors, including selenium intake through diet and dietary supplements. While the observed association persisted among participants who did not use multivitamin / mineral supplements and after adjusting for several intake variables, our findings must be confirmed in prospective studies with incident cases of hypertension. Also, dietary information in NHANES was based on 24-h recalls, that may not be sufficiently reliable to estimate the nutrient intakes of individuals resulting in potential residual confounding. Another limitation is the use of a single measurement of serum selenium, which may be subject to relatively high within person variability and may bias our findings towards the null.35 In addition, because only total serum Se was measured in NHANES, we do not have information of selenoprotein levels or activity or about nonspecific incorporation of selenium as selenomethionine in other plasma proteins. More detailed analysis of different selenoproteins and related activities will be needed to better understand the association of selenium with hypertension.
Our findings apply to high-selenium intake countries such as the US, and may not be generalized to other populations with marginal selenium intake. Furthermore, there is not yet a clear mechanism that could explain the effects of high selenium concentrations on cardiovascular risk factors. While substantial attention has been paid to explain the mechanisms for a potential benefit of increasing serum selenium in low-selenium intake populations, the mechanistic explanation for the effects of selenium above the levels required to maximize glutathione peroxidase activity are unknown.
In summary, high serum selenium concentrations were associated with higher prevalence of hypertension in a representative sample of US adults. The differences between the extreme quintiles, over 4 mmHg for systolic blood pressure, may be associated with a substantial number of cardiovascular events and with complications in blood pressure control. Recently, high selenium concentrations have been linked to increased prevalence of hyperlipidemia and diabetes,14 and to a U-shaped relationship with cardiovascular events.30 From a clinical perspective, selenium supplements cannot currently be recommended for cardiovascular protection. Furthermore, for individuals living in regions with high selenium intake, selenium supplementation could potentially increase risk of hypertension, diabetes, and/or hypercholesterolemia. Our findings call for a thorough evaluation of the risks and benefits associated high selenium status in the US.
Acknowledgments
Funding sources
Supported by grants 1 R01 ES012673 from the National Institute of Environmental Health Sciences and 0230232N from the American Heart Association.
Footnotes
Disclosures
There are no conflicts of interest regarding the contents of this article.
References
- 1.Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care. 2008;31 Suppl 2:S181–S184. doi: 10.2337/dc08-s245. [DOI] [PubMed] [Google Scholar]
- 2.Das UN. Nutritional factors in the pathobiology of human essential hypertension. Nutrition. 2001;17:337–346. doi: 10.1016/s0899-9007(00)00586-4. [DOI] [PubMed] [Google Scholar]
- 3.Nawrot TS, Staessen JA, Roels HA, Den Hond E, Thijs L, Fagard RH, Dominiczak AF, Struijker-Boudier HA. Blood pressure and blood selenium: a cross-sectional and longitudinal population study. Eur Heart J. 2007;28:628–633. doi: 10.1093/eurheartj/ehl479. [DOI] [PubMed] [Google Scholar]
- 4.Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 5.Salonen JT, Salonen R, Ihanainen M, Parviainen M, Seppanen R, Kantola M, Seppanen K, Rauramaa R. Blood pressure, dietary fats, and antioxidants. Am J Clin Nutr. 1988;48:1226–1232. doi: 10.1093/ajcn/48.5.1226. [DOI] [PubMed] [Google Scholar]
- 6.Virtamo J, Valkeila E, Alfthan G, Punsar S, Huttunen JK, Karvonen MJ. Serum selenium and the risk of coronary heart disease and stroke. Am J Epidemiol. 1985;122:276–282. doi: 10.1093/oxfordjournals.aje.a114099. [DOI] [PubMed] [Google Scholar]
- 7.Jossa F, Trevisan M, Krogh V, Farinaro E, Giumetti D, Fusco G, Galasso R, Panico S, Frascatore S, Mellone C, et al. Serum selenium and coronary heart disease risk factors in southern Italian men. Atherosclerosis. 1991;87:129–134. doi: 10.1016/0021-9150(91)90015-u. [DOI] [PubMed] [Google Scholar]
- 8.Coudray C, Roussel AM, Mainard F, Arnaud J, Favier A. Lipid peroxidation level and antioxidant micronutrient status in a pre-aging population; correlation with chronic disease prevalence in a French epidemiological study (Nantes, France) J Am Coll Nutr. 1997;16:584–591. [PubMed] [Google Scholar]
- 9.Institute of Medicine (U.S.) Panel on Dietary Antioxidants and Related Compounds: Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids : a report of the Panel on Dietary Antioxidants and Related Compounds, Subcommittees on Upper Reference Levels of Nutrients and Interpretation and Uses of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Washington, D.C.: National Academy Press; 2000. [Google Scholar]
- 10.Combs GF., Jr Selenium in global food systems. Br J Nutr. 2001;85:517–547. doi: 10.1079/bjn2000280. [DOI] [PubMed] [Google Scholar]
- 11.Navas-Acien A, Bleys J, Guallar E. Selenium intake and cardiovascular risk: what is new? Curr Opin Lipidol. 2008;19:43–49. doi: 10.1097/MOL.0b013e3282f2b261. [DOI] [PubMed] [Google Scholar]
- 12.Bleys J, Navas-Acien A, Guallar E. Serum selenium levels and all-cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–410. doi: 10.1001/archinternmed.2007.74. [DOI] [PubMed] [Google Scholar]
- 13.Rayman MP. Food-chain selenium and human health: emphasis on intake. Br J Nutr. 2008;100:254–268. doi: 10.1017/S0007114508939830. [DOI] [PubMed] [Google Scholar]
- 14.Bleys J, Navas-Acien A, Stranges S, Menke A, Miller ER, 3rd, Guallar E. Serum selenium and serum lipids in US adults. Am J Clin Nutr. 2008;88:416–423. doi: 10.1093/ajcn/88.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bleys J, Navas-Acien A, Guallar E. Serum selenium and diabetes in U.S. adults. Diabetes Care. 2007;30:829–834. doi: 10.2337/dc06-1726. [DOI] [PubMed] [Google Scholar]
- 16.Stranges S, Marshall JR, Natarajan R, Donahue RP, Trevisan M, Combs GF, Cappuccio FP, Ceriello A, Reid ME. Effects of long-term selenium supplementation on the incidence of type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:217–223. doi: 10.7326/0003-4819-147-4-200708210-00175. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS): Hyattsville, MD: U.S., Department of Health and Human Services, Centers for Disease Control and Prevention, 2003–2004. Reprint: http://www.cdc.gov/nchs/nhanes.htm.
- 18.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS): National Health and Nutrition Examination Survey Laboratory Protocol: Selenium. Hyattsville, MD: U.S., Department of Health and Human Services, Centers for Disease Control and Prevention, 2003–2004. Reprint: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l39_c_met_selenium.pdf.
- 19.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS): National Health and Nutrition Examination Survey Physician Examination Procederes Manual. Hyattsville, MD: U.S., Department of Health and Human Services, Centers for Disease Control and Prevention, 2003–2004. Reprint: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/PE.pdf.
- 20.Ostchega Y, Prineas RJ, Paulose-Ram R, Grim CM, Willard G, Collins D. National Health and Nutrition Examination Survey 1999–2000: effect of observer training and protocol standardization on reducing blood pressure measurement error. J Clin Epidemiol. 2003;56:768–774. doi: 10.1016/s0895-4356(03)00085-4. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS): National Health and Nutrition Examination Survey MEC In-Person Dietary Interviewers Procedures Manual. Hyattsville, MD: U.S., Department of Health and Human Services, Centers for Disease Control and Prevention, 2003–2004. Reprint: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/DIETARY_MEC.pdf.
- 22.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS): National Health and Nutrition Examination Survey Laboratory Protocol: Cotinine. Hyattsville, MD: U.S., Department of Health and Human Services, Centers for Disease Control and Prevention, 2003–2004. Reprint: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l06_c_met_cotinine.pdf.
- 23.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 24.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Lumley T. Survey: analysis of complex survey samples. R package version 3.6-13. Reprint: http://faculty.washington.edu/tlumley/survey/
- 26.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; Reprint: http://www.R-project.org. [Google Scholar]
- 27.StataCorp. Stata Statistical Software: Release 9.2. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- 28.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 29.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 30.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. 2006;84:762–773. doi: 10.1093/ajcn/84.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvini S, Hennekens CH, Morris JS, Willett WC, Stampfer MJ. Plasma levels of the antioxidant selenium and risk of myocardial infarction among U.S. physicians. Am J Cardiol. 1995;76:1218–1221. doi: 10.1016/s0002-9149(99)80344-0. [DOI] [PubMed] [Google Scholar]
- 32.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 33.Mark SD, Wang W, Fraumeni JF, Jr, Li JY, Taylor PR, Wang GQ, Dawsey SM, Li B, Blot WJ. Do nutritional supplements lower the risk of stroke or hypertension? Epidemiology. 1998;9:9–15. [PubMed] [Google Scholar]
- 34.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, Parnes HL, Minasian LM, Gaziano JM, Hartline JA, Parsons JK, Bearden JD, 3rd, Crawford ED, Goodman GE, Claudio J, Winquist E, Cook ED, Karp DD, Walther P, Lieber MM, Kristal AR, Darke AK, Arnold KB, Ganz PA, Santella RM, Albanes D, Taylor PR, Probstfield JL, Jagpal TJ, Crowley JJ, Meyskens FL, Jr, Baker LH, Coltman CA., Jr Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longnecker MP, Stampfer MJ, Morris JS, Spate V, Baskett C, Mason M, Willett WC. A 1-y trial of the of high-selenium bread on selenium concentrations in blood and toenails. Am J Clin Nutr. 1993;57:408–413. doi: 10.1093/ajcn/57.3.408. [DOI] [PubMed] [Google Scholar]