Abstract
In 2006, 6 million beneficiaries who were dually eligible for Medicare and Medicaid switched from Medicaid to Medicare Part D coverage of their prescription drugs. This change led to a significant expansion of Medicare’s role in financing psychotropic medications for this group. A reduction in the number of plans serving dual-eligibles and an increase in utilization restrictions for some psychotropics since 2006 raises concerns about medication access for dual-eligibles with mental disorders and point to potential problems with adverse selection. To improve access to medication for this population, Medicare might consider changes to the enrollment and risk-sharing systems.
In 2006, 6 million Medicare beneficiaries dually eligible for Medicaid transitioned from Medicaid coverage of prescription drugs to Medicare Part D drug plans. Dual-eligibles are randomly assigned to one of multiple Part D plans serving their region each varying in the generosity of coverage of medications. Approximately 60% of disabled and 20% of elderly dual-eligibles have mental disorders.1 Because of their low educational attainment, very low incomes, poor health status, and greater likelihood of cognitive and physical impairments, dual-eligibles with mental disorders may be particularly vulnerable to major changes in health care coverage and have difficulty navigating a complex market.2
In this paper, we assess how dual-eligibles with mental disorders are faring under Part D. First, we describe how payment for psychotropic medications for dual-eligibles has changed as a result of Part D by providing data on psychotropic drug financing for Medicare beneficiaries overall and dual-eligibles in particular. We then provide background on the dual-eligible Part D plan market, which presents a more complicated set of choices for dual-eligibles than what they faced under Medicaid. We go on to describe the policies put into place by Medicare to ensure medication access for vulnerable populations. In order to assess how dual-eligibles with mental disorders have fared since the transition to Part D, we (1) examine formulary coverage of psychotropic drugs in Part D, (2) provide data on changes in the out-of-pocket costs for psychotropic medications for dual-eligibles since Part D, (3) review evidence on medication discontinuities under Part D for dual-eligibles with mental disorders, and (4) discuss the implications of changes in plan participation in the dual-eligible market for medication access. Finally, we evaluate the impact of the dual-eligibles’ transition to Part D on tax-payers by reviewing evidence on price changes for psychotropics.
Background
Medicare’s changing role in financing psychotropic medications
We used data from the Medical Expenditure Panel Survey (MEPS) to examine the distribution of expenditures by payor in 2005 and 2006 for three classes of medications accounting for a large share of pharmacy expenditures, especially among dual-eligibles: antidepressants, antipsychotics, and anticonvulsants.
Part D brought about a dramatic change in the distribution of payments for psychotropics and other drugs for the U.S. civilian, non-institutionalized population (Exhibit 1). In 2005, Medicaid covered 14% of all antidepressant, 55% of antipsychotic, and 33% of anticonvulsant expenditures compared to 14% of all drug expenditures. In 2006, Medicaid’s share of spending for these three classes was cut approximately in half and Medicare financed 16% of antidepressant, 21% of antipsychotic, 16% of anticonvulsant expenditures, and 20% of medications overall.
Exhibit 1.
Medication use, spending, and distribution of spending by source for psychotropic medications and all medications in 2005 and 2006, U.S. Civilian Non-institutionalized Population
| % With | Total | Percent Distribution of Spending by Source |
||||||
|---|---|---|---|---|---|---|---|---|
| Use | Spending $ | OOP | Medicare | Medicaid | Private | Other | ||
| 2005 | ||||||||
| Antidepressants (all) | 8.5 | 13.3b | 36 | 2 | 14 | 43 | 6 | |
| SSRI, SSNRI other newer | 7.8 | 12.9b | 35 | 2 | 14 | 43 | 6 | |
| Antipsychotics (all) | 1.3 | 5.5b | 23 | 1 | 55 | 14 | 6 | |
| Atypicals | 1.2 | 5.1b | 23 | 1 | 57 | 13 | 6 | |
| Anticonvulsants | 2.7 | 5.5b | 29 | 2 | 33 | 30 | 7 | |
| ALL PRESCRIPTION DRUGS | 63.1 | 213b | 39 | 3 | 14 | 37 | 7 | |
| 2006 | ||||||||
| Antidepressants (all) | 8.4 | 13.2b | 35 | 16* | 8* | 36* | 5 | |
| SSRI, SSNRI, other newer | 7.6 | 12.7b | 36 | 16* | 7* | 37* | 5 | |
| Antipsychotics (all) | 1.3 | 5.7b | 26 | 21* | 26* | 20 | 7 | |
| Atypicals | 1.1 | 5.3b | 25 | 21* | 26* | 21 | 7 | |
| Anticonvulsants | 2.8 | 5.7b | 34 | 16* | 19* | 26 | 5 | |
| ALL PRESCRIPTION DRUGS | 62.6 | 224b | 35* | 20* | 7* | 33* | 6* | |
SOURCE: 2005–2006 MEPS
Difference between 2005 and 2006 significant at p=0.05 level. Exhibit does not include in “clawback” payments made by the states to the federal government.
Part D led to even greater changes in the financing of psychotropics for dual-eligibles (Exhibit 2). In 2005, Medicaid covered 70% of antidepressant, 84% of antipsychotic, and 82% of anticonvulsant spending for non-institutionalized dual-eligibles. In 2006, Medicaid’s share of spending in these classes fell to 5%, 11% and 7%, respectively, as Medicare’s share increased to 84%, 84% and 78%.
Exhibit 2.
Medication use, spending, and distribution of spending by source for psychotropic medications and all medications in 2005 and 2006, Dually Eligible Civilian Non-institutionalized Population
| % With | Total | Percent Distribution of Spending by Source |
||||||
|---|---|---|---|---|---|---|---|---|
| Use | Spending $ | OOP | Medicare | Medicaid | Private | Other | ||
| 2005 | ||||||||
| Antidepressants (all) | 18.8 | 0.9b | 20 | 5 | 70 | 3 | 2 | |
| SSRI, SSNRI other newer | 16.2 | 0.9b | 18 | 5 | 73 | 1 | 3 | |
| Antipsychotics (all) | 8.5 | 1.5b | 12 | 2 | 84 | 0 | 1 | |
| Atypicals | 7.4 | 1.5b | 12 | 2 | 85 | 0 | 1 | |
| Anticonvulsants | 13.0 | 0.9b | 15 | 3 | 82 | 1 | 0 | |
| ALL PRESCRIPTION DRUGS | 88.0 | 18.7b | 19 | 5 | 73 | 1 | 2 | |
| 2006 | ||||||||
| Antidepressants (all) | 20.8 | 1.0b | 9* | 84* | 5* | 0 | 3 | |
| SSRI, SSNRI, other newer | 19.2 | 0.9b | 9* | 83* | 5* | 0 | 3 | |
| Antipsychotics (all) | 8.4 | 1.0b | 6 | 83* | 11* | 0 | 0 | |
| Atypicals | 7.7 | 0.9b | 6 | 83* | 11* | 0 | 0 | |
| Anticonvulsants | 11.5 | 0.6b* | 12 | 78* | 7* | 1 | 2 | |
| ALL PRESCRIPTION DRUGS | 87.0 | 17.7b | 17 | 77* | 5* | 0* | 1 | |
SOURCE: 2005–2006 MEPS
Difference between 2005 and 2006 significant at P=0.05 level. Exhibit does not include in “clawback” payments made by the states to the federal government.
For the total Medicare population, Medicare’s share increased from 8% to 52% for antidepressants, from 4% to 61% for antipsychotics, and from 7% to 45% for all drugs between 2005 and 2006 (Exhibit 3). Psychotropic medication classes were the second most costly drug category used by Medicare beneficiaries overall, and the most costly among dual-eligibles.3
Exhibit 3.
Medication use, spending, and distribution of spending by source for psychotropic medications and all medications in 2005 and 2006, Civilian Non-Institutionalized Medicare Population
| % With | Total | Percent Distribution of Spending by Source |
||||||
|---|---|---|---|---|---|---|---|---|
| Use | Spending | OOP | Medicare | Medicaid | Private | Other | ||
| 2005 | ||||||||
| Antidepressants (all) | 16.0 | 3.8b | 41 | 8 | 18 | 23 | 10 | |
| SSRI, SSNRI other newer | 13.7 | 3.6b | 41 | 7 | 18 | 23 | 10 | |
| Antipsychotics (all) | 3.3 | 2.1b | 25 | 4 | 64 | 4 | 3 | |
| atypicals | 2.7 | 2.0b | 24 | 4 | 66 | 3 | 3 | |
| Anticonvulsants | 7.0 | 2.1b | 29 | 4 | 35 | 22 | 9 | |
| ALL DRUGS | 91.0 | 88.5b | 43 | 7 | 16 | 23 | 11 | |
| 2006 | ||||||||
| Antidepressants (all) | 18.1* | 4.1b | 32* | 52* | 1* | 10* | 5* | |
| SSRI, SSNRI, other newer | 16.1* | 3.8b | 32* | 51* | 1* | 11* | 5* | |
| Antipsychotics (all) | 3.6 | 1.9b | 22 | 61* | 6* | 4 | 7 | |
| atypicals | 2.9 | 1.8b | 23 | 61* | 5* | 4 | 6 | |
| Anticonvulsants | 7.2 | 1.9b | 31 | 48* | 2* | 12 | 7 | |
| ALL DRUGS | 91.2 | 96.3b | 31* | 45* | 1* | 14 | 8* | |
SOURCE: 2005–2006 MEPS
Difference between 2005 and 2006 significant at P=0.05 level. Exhibit does not include in “clawback” payments made by the states to the federal government.
Part D Market for Dual-Eligibles
Plan enrollment
Part D is a voluntary benefit that relies on a market-based, consumer choice model of health care delivery. Non-dual-eligible beneficiaries must choose to participate and select a plan to meet their needs. To maintain continuity of coverage from 2005 to 2006, dual-eligibles were auto-assigned in November 2005 to a plan with a premium at or below a regional benchmark set by the Centers for Medicare and Medicaid Services (CMS) (hereafter, “benchmark plan”) using a methodology discussed below.
Medicare beneficiaries, including dual-eligibles, may be enrolled in two types of Part D coverage. They may remain in Medicare’s fee-for-service program and enroll in a stand-alone Prescription Drug Plan (PDP), or enroll in a Medicare-Advantage Prescription Drug (MA-PD) plan for all covered benefits including drugs. In many areas, dual-eligibles may enroll in a Special Needs Plan (SNP), a MA-PD designed specifically for dual-eligibles, those in nursing homes, or beneficiaries with severe, disabilities. Because only 11% of dual eligibles were enrolled in SNPs in 2008,4 this paper focuses on the PDP market.
Although dual-eligibles are auto-assigned to a benchmark plan initially, it is their responsibility to switch plans if their assigned plan is a poor match. Switching plans requires beneficiaries to understand their medication needs as well as multiple plans’ formularies and utilization management requirements. Beneficiaries with schizophrenia, bipolar or major depressive disorder, whose decision-making ability is impaired,5 may have difficulty evaluating plan options and switching plans. CMS reassigns to another benchmark plan beneficiaries whose current plan’s premium bid for the following year is above benchmark and therefore no longer eligible for random assignment of dual-eligibles. In fact, 2.1 million dual-eligibles were reassigned for this reason in 2008 and 1.6 million in 2009.6 However, CMS does not reassign the minority (approximately 6%)7 of beneficiaries who choose their own plan if the plan’s premium for the next year is above benchmark. These beneficiaries must either pay the difference between the benchmark premium, which ranged from $16 to $36 ($28 on average) in 2008,8 and the plan's premium or choose another plan. Part D plan premiums (benchmark and non-benchmark) varied from $10 to $108 in 2008.9
Adverse Selection
The Part D market may be more prone to adverse selection, the tendency for plans with relatively generous coverage to draw a disproportionate share of enrollees with high expected costs, than other health insurance markets because of the persistence and predictability of drug expenditures.10 The high expected drug spending of dual-eligibles with mental disorders creates incentives for PDPs to avoid enrolling them. In general, plans can influence beneficiary enrollment primarily through the structure of their formulary, cost sharing/benefit design, and utilization management.
However, CMS has put into place a number of policies to reduce plans’ incentives and abilities to influence enrollee selection and ensure medication access. These policies include risk adjustment, risk-sharing between Medicare and plans, and formulary protections. If properly structured these policies can minimize adverse selection among plans, and mitigate the incentives for plans to reduce coverage of drugs used by enrollees with high expected costs. Thus, these policies could help to ensure access to medications for vulnerable populations like dual-eligibles with mental disorders.
To account for differences in the level of risk faced by plans based on the composition of their enrollees, Medicare risk adjusts payments to PDPs using an age, sex, low-income status, and diagnosis-based model similar to that used for the Medicare Advantage program.11 Notably, schizophrenia was among the conditions associated with the largest drug costs.12 Hsu and colleagues evaluated the Part D risk adjustment model and reported a significant difference between the amount of variation it explained in drug expenditures (12%), and the amount explained by a model that added prior-year drug expenditures (40%).13 In addition, the model over-predicts costs for beneficiaries with low expenditures and under-predicts costs for beneficiaries with high expenditures. These findings suggest that, even after plan premiums are risk-adjusted, PDPs have incentives to manage the composition of their enrollees by potentially limiting coverage of drugs used by beneficiaries with high expected costs such as those with mental disorders.
Improving the performance of the risk adjustment system is particularly important as the financial risk faced by plans increased in 2008. Medicare established risk corridors to limit PDPs’ overall losses and gains. For example, in 2006–2007, if a PDP’s actual costs were more than 5% above their expected costs, Medicare assumed 80% of losses (and likewise kept 80% of gains if the PDP’s costs were less than 5% below expected costs). Beginning in 2008 and through 2011, Medicare assumes only 50% of losses from 5% to 10% above expected costs and 80% of losses above 10% of expected costs. CMS has authority to further increase the level of plan risk in 2012.
Medicare put into place policies to limit PDPs’ ability to restrict coverage of medications. For example, institutionalized dual-eligibles pay no cost sharing and non-institutionalized dual-eligibles pay one fixed copayment for generics and a slightly higher fixed copayment for brand drugs.14 As a result, PDPs cannot use cost-sharing tiers to influence use. In addition, for major classes of psychiatric drugs – antidepressants, antipsychotics, and anticonvulsants – PDPs must cover at least one formulation of every drug, a further limitation on their ability to influence use and/or affect enrollee selection. However, recent legislation codifying the requirement that PDPs list “all or substantially all” drugs in these classes allows CMS to establish exceptions that permit PDPs to either exclude a drug in the protected classes from its formulary or to impose utilization restrictions.15
PDP Participation in the Dual-Eligible Market
The percentage of PDPs offering benchmark plans eligible for auto-assignment of dual-eligibles and other low-income subsidy (LIS) beneficiaries fell from 29% (409) of all plans in 2006 to 18% (308) in 2009, compared to an overall increase in the number of non-benchmark plans from 1,020 in 2006 to 1,381 in 2009.16 More than a third of plans qualified to serve low-income beneficiaries in 2008 did not offer benchmark plans for 2009.17 Most of the large PDPs accounting for the bulk of Part D enrollment have reduced the number of plan offerings for dual-eligibles.18
There is substantial state-to-state variation in the number of plans available to dual-eligibles. In 2009, six states each have 5 or fewer benchmark plans; Nevada has only one.19
In some cases the withdrawal of PDPs from the LIS market is part of a deliberate business strategy while in other cases it is a function of the methodology CMS uses to calculate the annual benchmark. The benchmark is the average monthly premium for PDP and MA-PD plans in the region, weighted by beneficiary enrollment.20 Regions with high MA-PD penetration tend to have lower benchmark premiums. Plan exit from the LIS market could lead to disruptions in medication use for dual-eligibles who have to switch plans.
Impact on Medication Access
Formulary coverage and utilization management
Because of the low fixed copayments for dual-eligibles and formulary rules for psychotropic drugs mentioned above, plans must rely primarily on utilization management tools like prior authorization (requiring preapproval before coverage) and step therapy (requiring enrollees to initiate treatment with a specified drug(s) before receiving coverage for others) to influence psychotropic medication use and/or discourage enrollment of those with mental disorders. Some advocates for dual-eligibles were concerned that utilization management requirements under Part D would increase barriers to psychotropic drug access despite the special protections governing these classes. However, appropriate application of utilization management may lead to a more efficient allocation of resources and possibly higher quality of care. We examined trends at the plan- and drug-level in formulary coverage and utilization management for antidepressants, atypical antipsychotics, and anticonvulsants approved to treat bipolar disorder among benchmark plans using CMS data. We present data for the classes as a whole as well as for specific, commonly-used medications.
In the atypical antipsychotic class the trend between 2006 and 2008 was toward greater formulary coverage (Exhibit 4). For example, 16% of plans excluded Zyprexa Zydis in 2006 but none excluded it in 2008. In the antidepressant category, where a number of reformulations (e.g., controlled-release forms) have been introduced, plans cover at least one but typically not all formulations. For example, 100% of benchmark PDPs listed generic paroxetine on their formularies in 2008 but only 52% covered Paxil CR (controlled-release paroxetine) (Exhibit 4).
Exhibit 4.
Formulary Coverage of Selected Atypical Antipsychotics, Newer Antidepressants and Anticonvulsants for PDPs that Serve Dual Eligibles,2006–2008
| Drug Product | % Covered (Yes/No) | ||
|---|---|---|---|
| ′06 | ′07 | ′08 | |
| Antipsychotics | |||
| Abilify | 100% | 100% | 100% |
| Abilify Discmelt | N/A | 83% | 100% |
| Risperdal | 100% | 100% | 100% |
| Risperdal Consta (IM) | 93% | 100% | 100% |
| Risperdal M-TAB ODT | 93% | 100% | 100% |
| Zyprexa | 100% | 100% | 100% |
| Zyprexa IM | 74% | 90% | 100% |
| Zyprexa Zydis | 84% | 100% | 100% |
| Antidepressants | |||
| Celexa | 17% | 29% | 28% |
| Citalopram | 100% | 100% | 100% |
| Lexapro | 71% | 83% | 88% |
| Cymbalta | 100% | 100% | 100% |
| Paroxetine | 100% | 100% | 100% |
| Paxil | 17% | 29% | 28% |
| Paxil CR | 64% | 59% | 52% |
| Anticonvulsants | |||
| Carbamazepine | 98% | 100% | 94% |
| Carbamazepine chewable | 99% | 100% | 100% |
| Tegretol | 97% | 98% | 100% |
| Tegretol chewable | 64% | 58% | 63% |
| Lamictal | 100% | 100% | 100% |
| Lamictal chewable | 79% | 41% | 40% |
| Lamotragine chewable | 67% | 95% | 100% |
| Depakote | 92% | 100% | 100% |
| Depakote ER | 98% | 99% | 100% |
| Depakote sprinkles | 90% | 100% | 100% |
| Valproic acid | 100% | 100% | 100% |
| Valproate IV | 100% | 95% | 99% |
Note: The source of these data is the January 2006, 2007, and 2008 CMS Prescription Drug Formulary and Pharmacy Network Files. “N/A” refers to drug products not yet available as of January of the year in question. There were 409 PDPs serving dual eligibles in 2006,642 in 2007, and 495 in 2008.
Utilization management requirements are common in these classes, and the number of plans using them has generally increased since 2006 (Exhibit 5). The proportion of plans requiring prior authorization or step therapy for anticonvulsants and antidepressants doubled while the proportion for antipsychotics increased 12%. At the drug-level, on average in 2008, plans required prior authorization for 10% of atypical antipsychotics and 2% of antidepressants (data not shown), compared to an average of 5% of 169 drugs (both psychotropic and non-psychotropic) commonly used by Medicare beneficiaries.21
Exhibit 5.
Percentage of Benchmark PDPs Requiring Either Step Therapy or Prior Authorization for Any Drug in Selected Psychotropic Classes, 2006–2008
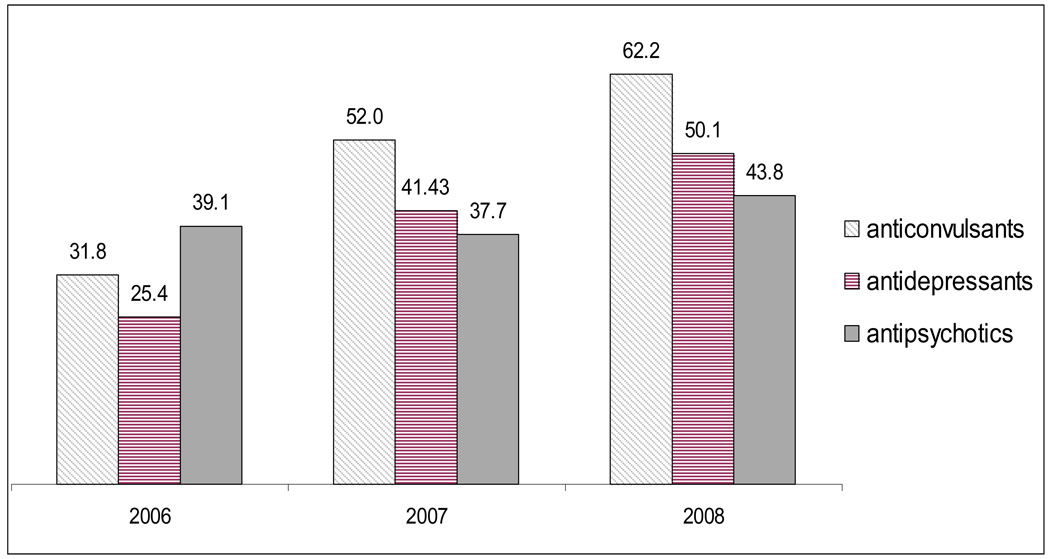 |
SOURCE: CMS Prescription Drug Plan Formulary and Pharmacy Network Files, January 2006, 2007, and 2008.
Plans have become somewhat less likely to use prior authorization and more likely to use step therapy for atypical antipsychotics (Exhibit 6). In the antidepressant category, in which several generics are available, plans have increased step therapy use. For example, in 2006, no benchmark PDPs required step therapy for Cymbalta, a drug with no generic. However, 24% of benchmark PDPs imposed this limit in 2007 and 33% in 2008. Few plans imposed either restriction on anticonvulsants used as mood stabilizers (Exhibit 6).
Exhibit 6.
Use of Utilization Management Tools for Selected Atypical Antipsychotics, Newer Antidepressants and Anticonvulsants for PDPs that Serve Dual Eligibles, 2006–2008
| Of Plans that Cover Drug, % that Require Each Utilization Management Tool |
|||||||
|---|---|---|---|---|---|---|---|
| Drug Product | Prior Authorization |
Step Therapy | |||||
| ′06 | ′07 | ′08 | ′06 | ′07 | ′08 | ||
| Antipsychotics | |||||||
| Abilify | 14% | 14% | 12% | 0% | 5% | 7% | |
| Abilify Discmelt | N/A | 16% | 18% | N/A | 5% | 7% | |
| Risperdal | 11% | 0% | 0% | 0.5% | 0% | 0% | |
| Risperdal Consta (IM) | 26% | 17% | 15% | 0.5% | 0% | 0% | |
| Risperdal M-TAB ODT | 11% | 6% | 6% | 0.5% | 0% | 0% | |
| Zyprexa | 11% | 10% | 7% | 0.5% | 3% | 7% | |
| Zyprexa IM | 23% | 16% | 7% | 0.5% | 4% | 7% | |
| Zyprexa Zydis | 7% | 19% | 18% | 0.5% | 0.2% | 2% | |
| Antidepressants | |||||||
| Celexa | 0% | 2% | 2% | 0% | 3% | 51% | |
| Citalopram | 0% | 0% | 0% | 1% | 0% | 0% | |
| Lexapro | 0% | 0% | 0% | 0% | 14% | 26% | |
| Cymbalta | 15% | 1% | 2% | 0% | 24% | 33% | |
| Paroxetine | 0% | 0% | 0% | 4% | 0% | 0% | |
| Paxil | 0% | 2% | 2% | 0% | 3% | 51% | |
| Paxil CR | 5% | 0% | 1% | 5% | 7% | 39% | |
| Anticonvulsants | |||||||
| Carbamazepine | 0% | 0% | 0% | 0% | 0% | 0% | |
| Carbamazepine chewable | 0% | 0% | 0% | 0% | 0% | 0% | |
| Tegretol | 0% | 1% | 0% | 0% | 0% | 0% | |
| Tegretol chewable | 0% | 1% | 0% | 0% | 0% | 0% | |
| Lamictal | 11% | 23% | 0% | 0% | 0% | 5% | |
| Lamictal chewable | 0% | 2% | 0% | 0% | 0% | 0% | |
| Lamotragine chewable | 5% | 14% | 0% | 0% | 0% | 5% | |
| Depakote | 0% | 0% | 0% | 0% | 0% | 0% | |
| Depakote ER | 0% | 1% | 0% | 0% | 0% | 0% | |
| Depakote sprinkles | 0% | 1% | 0% | 0% | 0% | 0% | |
| Valproic acid | 3% | 0% | 0% | 0% | 0% | 0% | |
| Valproate IV | 6% | 2% | 1% | 0% | 0% | 0% | |
Note: The source of these data is the January 2006, 2007, and 2008 CMS Prescription Drug Formulary and Pharmacy Network Files. “N/A” refers to drug products not yet available as of January of the year in question. There were 409 PDPs serving dual eligibles in 2006, 642 in 2007, and 495 in 2008.
These data tell us that a substantial number of dual-eligibles with mental disorders will face utilization restrictions that may lead to medication discontinuities. However, duals may be no more exposed to utilization management in Part D than if they had remained in Medicaid. In 2006, 25 states required prior authorization for one or more atypical antipsychotics.22 Studies suggest that Medicaid atypical antipsychotics prior authorization programs have led to modest reductions in pharmacy spending yet have increased risk of treatment discontinuities.23 How prior authorization programs in Medicaid and Part D differ with respect to the administrative burden they pose for providers and approval rates is not known.
Given that (a) newer drugs in the antidepressant and antipsychotic categories have similar rates of treatment response and side effects, on average,24 (b) we currently lack biological and/or environmental markers that clearly inform providers’ treatment decisions,25 and (c) the significant cost differences within these categories, it may be appropriate to require a Medicare beneficiary to start on a lower-cost agent (i.e., step therapy). However, given the vulnerability of beneficiaries with mental disorders, safeguards such as the CMS requirement that utilization management tools not be applied to patients on stable medication regimens,26 are important for maintaining continuity of care.
Out-of-Pocket Costs
Many Medicaid programs charge modest copays similar to those charged dual-eligibles under Part D. However, copayments for brand drugs are higher under Part D than under some Medicaid programs.27 In addition, unlike some Medicaid programs, PDPs do not exempt any medication classes from copayments. Finally, while most Medicaid programs require pharmacies to waive copayments for beneficiaries unable to pay,28 there is no similar requirement under Part D.
Despite these changes, dual-eligibles, who had relatively low out-of-pocket drug costs under Medicaid, saw little to no change in their out-of-pocket share of psychotropic drug costs in 2006 (Exhibit 2). Similarly, a study using data on 5 drug classes from a single pharmacy chain reported that average copayments for elderly dual-eligibles decreased between 2005 and 2006 in 4 out of the 5 classes.29
Medication discontinuities
A few published studies offer indirect evidence of problems with medication access under Part D for dual-eligibles with mental disorders. One study based on a survey of non-elderly dual-eligibles with disabilities, including those with mental disorders, in one state found that 20% reported medication access problems in the first few months of 2006.30 Another study, based on a survey of psychiatrists, found that nearly a third of dual-eligibles (30.6%) could not access a clinically-indicated refill during the first four months of Part D and 19.8% could not fill a new prescription because the drug was not covered or approved.31 A survey conducted at the end of 2006 suggests that rates of access problems remained stable throughout the first year of the program.32 Finally, a study using pre-part D data, projected that 9–10% of antipsychotic users, 7% of antidepressant users, and 4% of mood stabilizer users would switch medications due to PDP restrictions, with as many as 26% of beneficiaries switching psychotropic medications in some plans.33 Medication discontinuities among individuals with mental disorders like schizophrenia have been shown to be associated with high rates of symptom relapse and hospitalization.34
Beneficiary plan assignment and switching
Little is known about what represents a “good plan choice” for beneficiaries in Part D, and the optimal choice likely varies with beneficiary preferences and needs. For instance, some beneficiaries may prefer to first try a generic medication that would carry a lower copayment than a brand drug as required by a step therapy program than seek prior authorization to secure coverage. In addition, dual-eligibles with mental disorders who, on average, take multiple drugs may view some drugs as more important for their health.
Although permitted to change plans monthly, only 11% of non-institutionalized dual-eligibles changed plans in 200635 despite evidence of wide variability in plan features that can lead to differences in medication access. Dual-eligibles enrolled in PDPs whose 2008 premium bids exceeded the benchmark had to be reassigned to new PDPs with potentially different coverage and utilization management rules and may have experienced medication discontinuities as a result.36 To mitigate these problems CMS typically reassigns beneficiaries to benchmark plan offerings from the same PDP sponsor wherever possible.
CMS has used random assignment to ensure roughly equal numbers of dual-eligibles across plans and reduce the likelihood of adverse selection in order to boost plan participation.37 However, some state Medicaid programs have implemented “intelligent assignment” systems (i.e., taking into account the beneficiary’s medications when making plan assignment) for dual-eligibles. For example, Maine reassigned beneficiaries initially assigned to plans that covered less than 85% of their drugs. A recent MedPAC report concluded that it would be feasible to adopt such a system nation-wide.38
In designing an assignment algorithm, a tradeoff exists between exacerbating selection incentives and achieving good beneficiary/plan matches. Given that dual-eligibles have very high drug spending for which the current risk adjustment system is unlikely to completely account, a “best-fit” algorithm might create incentives for plans to reduce coverage of psychotropics, as they are used by beneficiaries with high expected costs. However, experience with intelligent assignment of dual-eligibles suggests these programs have led to increased concentration of beneficiaries in a smaller number of plans but have apparently not increased plan exit.39
Impact on taxpayers
Psychotropics Prices
The expanded role for Medicare as a purchaser of psychotropic medications has important implications for taxpayers. Medicare’s approach to drug pricing must balance the need to minimize costs to the federal government against the desire to maintain manufacturers’ incentives to invest in drug development. Through the Medicaid rebate program, states are entitled to pay the lowest prices available in the private market. The Medicare Prescription Drug Improvement and Modernization Act (MMA) specifically prohibited the federal government from directly negotiating drug prices, relying instead on the purchasing power of PDPs. While PDPs are not entitled to the best private price like Medicaid, they can negotiate prices below Medicaid’s “best price” without those prices being counted as a best price (i.e., the manufacturer need not rebate the lower amount to Medicaid).40 This confers a potential advantage on PDPs over other private purchasers in negotiating rebates with manufacturers.
Frank and Newhouse (2008) reviewed pharmaceutical manufacturers’ Form 10-Q filings with the Securities and Exchange Commission and found that manufacturers of the four most widely-used atypical antipsychotics all noted favorable price changes that resulted from the shift of dual-eligibles to Part D in 2006, suggesting that Medicare is paying higher prices for these drugs than Medicaid did.41
Conclusion
Medicare now finances a large share of psychotropic drug expenditures overall and for dual-eligibles in particular. While we know relatively little about the impact of Part D on health outcomes among dual-eligibles, we do know something about how PDPs have responded to the economic incentives in Part D and can point to some new directions for policymakers to consider.
Some PDPs that served dual-eligibles when the program was implemented have exited the LIS market.42 Re-assignment of dual-eligibles to other PDPs might cause medication discontinuities and possibly decrements in health. Also, if plan exit continues at current levels, plan choice (and the competitive effects that might result) will become limited in more areas.
The doubling of the PDP risk corridors (the proportion of financial gains/losses that a PDP assumes responsibility for) in 2008 exposes plans to greater risk, and may exacerbate incentives to limit coverage for medications used by high-cost beneficiaries. Increased plan risk may also result in additional benchmark plan exits if the risk adjustment system does not accurately adjust for expected costs of dual-eligibles. The risk adjustment system could be updated to take drug utilization into account. In addition, CMS could consider exposing benchmark PDPs to less financial risk for dual-eligibles than other PDPs. For vulnerable populations like dual-eligibles, Medicare may opt to face higher program costs in order to improve medication access and plan participation.
Formulary coverage of psychotropic medications has been relatively generous overall since Part D’s implementation due to the special protections for antidepressants, antipsychotics, and anticonvulsants. There are, however, gaps in coverage for certain formulations. In addition, utilization management requirements for psychotropic drugs vary across PDPs and have increased in the first three years of the program. Many state Medicaid programs use similar tools, so the switch from Medicaid to PDPs for dual-eligibles may not represent more restrictive coverage. However, the devil is in the details with respect to the impact of tools like prior authorization on access to psychotropics. For example, some PDPs may grant few approvals, while others approve most requests. CMS might consider monitoring prior authorization approval rates and possibly including them in plan performance rankings so that beneficiaries can make better plan choices.
Because of the variation across PDPs in formulary coverage and utilization management for psychotropics, duals with mental disorders may be better served by an intelligent random assignment process, although such a process may exacerbate concerns about adverse selection. However, the degree to which plans vary in the restrictions they place on psychotropics, the cognitive and other limitations preventing dual-eligibles from making fully-informed choices, and the vulnerability of this population to medication discontinuities suggest that CMS might consider experimenting with alternatives to random assignment.
Contributor Information
Julie M. Donohue, University of Pittsburgh, Graduate School of Public Health.
Haiden A. Huskamp, Harvard Medical School, Department of Health Care Policy.
Samuel H. Zuvekas, Center for Financing, Access, and Cost Trends, Agency for Healthcare Research and Quality.
Notes
- 1.Donohue JM. Mental Health in the Medicare Part D Drug Benefit: a New Regulatory Model? Health Affairs. 2006;25(no 3):707–719. doi: 10.1377/hlthaff.25.3.707. [DOI] [PubMed] [Google Scholar]
- 2.Morden NE, Garrison LP. Implications of Part D for Mentally Ill Dual Eligibles: A Challenge for Medicare. Health Affairs. 2006;25(no 2):491–500. doi: 10.1377/hlthaff.25.2.491. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Medicare and Medicaid Services (CMS) Medicare releases Part D data for 2006 and 2007 at Medicare Prescription Drug Benefit Symposium. Available on-line at http://www.cms.hhs.gov/PrescriptionDrugCovGenIn/08_PartDData.asp#TopOfPage.
- 4.Grabowski DC. Special Needs Plans and the Coordination of Benefits and Services for Dual Eligibles. Health Affairs. 28(1):136–146. doi: 10.1377/hlthaff.28.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For example Murphy FC, Rubinsztein JS, Rogers AMRD, et al. Decision-Making Cognition in Mania and Depression. Psychological Medicine. 2001;31:679–693. doi: 10.1017/s0033291701003804.
- 6.Hoadley J, Hargrave E, Cubanski J. Medicare Part D 2008 Data Spotlight: Low-Income Subsidy Plan Availability. Washington DC: Kaiser Family Foundation; 2008. Apr, [Google Scholar]
- 7.Authors’ calculation based on data from Hoadley J. Medicare Part D 2008 Data Spotlight
- 8.Hoadley, et al. Medicare Part D 2008 Data Spotlight [Google Scholar]
- 9.Kaiser Family Foundation. Medicare Prescription Drug Benefit Fact Sheet. Menlo Park, CA: 2008. Feb, [Google Scholar]
- 10.Pauly MV, Zeng Y. Adverse Selection and the Challenges to Stand-alone Prescription Drug Insurance. Frontiers in Health Policy Research. 2004;7(no 1):55–74. doi: 10.2202/1558-9544.1051. [DOI] [PubMed] [Google Scholar]
- 11.Robst J, Levy JM, Ingber MJ. Diagnosis-Based Risk Adjustment for Medicare Prescription Drug Plans. Health Care Financing Review. 2007;28(no 4):15–30. [PMC free article] [PubMed] [Google Scholar]
- 12.Robst, et al. Diagnosis-based risk adjustment [Google Scholar]
- 13.Hsu J, et al. Distributing $800 billion: an Early Assessment of the Medicare Part D Risk Adjustment Approach. Health Affairs. 2009 doi: 10.1377/hlthaff.28.1.215. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Full-benefit dual-eligibles pay $1.10–$2.40 for generic and $3.20–$6.00 for brand drugs in 2009.
- 15.Department of Health and Human Services. Medicare Program: Medicare Advantage and Prescription Drug Program MIPPA Drug Formulary and Protected Classes Policies. Federal Register. 2009 January Friday,16;74(no 11):2881–2888. [PubMed] [Google Scholar]
- 16.Hoadley, et al. Medicare Part D 2008 Data Spotlight [Google Scholar]
- 17.Avalere Health. Low-income Medicare beneficiaries Will Have Fewer Part D Options in 2009. Available on-line: http://www.avalerehealth.net/news.
- 18.Avalere Health. Low-income Medicare beneficiaries [Google Scholar]
- 19.Hoadley, et al. Medicare Part D 2008 Data Spotlight [Google Scholar]
- 20.Beginning in 2009, CMS weights premiums not by total Medicare but by LIS beneficiary enrollment to minimize the number of beneficiaries subject to plan reassignment.
- 21.Hoadley J, Hargrave E, Cubanski J, Neuman T. Medicare Prescription Drug Plans in 2008 and Key Changes Since 2006. Kaiser Family Foundation. 2008 April [Google Scholar]
- 22.Polinski JM, Wang PS, Fischer MA. Medicaid’s Prior Authorization Program and Access to Atypical Antipsychotic Medications. Health Affairs. 2007;26(no 3):750–760. doi: 10.1377/hlthaff.26.3.750. [DOI] [PubMed] [Google Scholar]
- 23.Soumerai SB, et al. Use of Atypical Antipsychotic Drugs For Schizophrenia in Maine Medicaid Following a Policy Change. Health Affairs. 2008;27(no 3):w185–w195. doi: 10.1377/hlthaff.27.3.w185. [DOI] [PubMed] [Google Scholar]
- 24.Haiden A, Huskamp Managing Psychotropic Drug Costs: Will Formularies Work? Health Affairs. 2003 September/October;22(5):84–96. doi: 10.1377/hlthaff.22.5.84. [DOI] [PubMed] [Google Scholar]
- 25.Arranz MJ, Kapur S. Pharmacogenetics in Psychiatry: Are We Ready for Widespread Clinical Use? Schizophr Bull. 2008 November 1;34(6):1130–1144. doi: 10.1093/schbul/sbn114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CMS. Chapter 6. Medicare Prescription Drug Benefit Manual. 2008 July; available on-line at http://www.cms.hhs.gov/PrescriptionDrugCovContra/Downloads/R2PDB.pdf.
- 27.Crowley JS, Ashner D, Elam L. State Medicaid outpatient prescription drug policies: findings from a national survey, 2005 update. Washington DC: Kaiser Family Foundation; 2005. [Google Scholar]
- 28.Crowley State Medicaid outpatient prescription drug policies [Google Scholar]
- 29.Shrank WH, Patrick AR, Pedan A, et al. The Effect of transitioning to Medicare Part D Drug Coverage in Seniors Dually Eligible for Medicare and Medicaid. Journal of the American Geriatric Society. 2008 doi: 10.1111/j.1532-5415.2008.02025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall JP, Kurth NK, Moore JM. Transition to Medicare Part D: an Early Snapshot of Barriers Experienced by Younger Dual Eligibles with Disabilities. American Journal of Managed Care. 2007;13:14–18. [PubMed] [Google Scholar]
- 31.West JC, et al. Medication access and continuity: the experiences of dual-eligible psychiatric patients during the first 4 months of the Medicare prescription drug benefit. Am J Psychiatry. 2007;164(no 5):789–796. doi: 10.1176/ajp.2007.164.5.789. [DOI] [PubMed] [Google Scholar]
- 32.West JC, Wilk JE, Rae DS, et al. Medicare Prescription Drug Benefits: Medication Access and Continuity Among Dual Eligible Psychiatric Patients. Journal of Clinical Psychiatry. 2009 doi: 10.4088/JCP.08m04608whi. in press. [DOI] [PubMed] [Google Scholar]
- 33.Donohue JM, Frank RG. Estimating Medicare Part D’s Impact on Medication Access Among Dually Eligible Beneficiaries with Mental Disorders. Psychiatric Services. 2007;58:1285–1291. doi: 10.1176/ps.2007.58.10.1285. [DOI] [PubMed] [Google Scholar]
- 34.Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Archives of General Psychiatry. 1999;56:241–247. doi: 10.1001/archpsyc.56.3.241. [DOI] [PubMed] [Google Scholar]; Soumerai S, et al. Effects of Medicaid drug payment limits on admission to hospitals and nursing homes. NEJM. 1991;325:1072–1077. doi: 10.1056/NEJM199110103251505. [DOI] [PubMed] [Google Scholar]
- 35.Neuman T, et al. Medicare Prescription Drug Benefit Progress Report: Findings From a 2006 National Survey of Seniors. Health Affairs. 2007;26(no5):w630–w643. doi: 10.1377/hlthaff.26.5.w630. [DOI] [PubMed] [Google Scholar]
- 36.Hoadley, et al. Medicare Part D 2008 Data Spotlight [Google Scholar]
- 37.Hoadley, et al. The Role of Beneficiary-Centered Assignment [Google Scholar]
- 38.ibid
- 39.ibid
- 40.Frank RG, Newhouse JP. Should Drug Prices Be Negotiated Under Part D of Medicare? And if so, How? Health Affairs. 2008;27(no 1):33–34. doi: 10.1377/hlthaff.27.1.33. [DOI] [PubMed] [Google Scholar]
- 41.Ibid
- 42.Hoadley, et al. Medicare Part D 2008 Data Spotlight [Google Scholar]


