Abstract
Rapid down-regulation of the anti-apoptotic Bcl-2 family protein Mcl-1 is required for UV-induced apoptosis, underlining an important role for Mcl-1 in epidermal pathology. To determine if Mcl-1 has a specific role in normal keratinocyte (KC) biology, Mcl-1 was down-regulated in human KCs by RNAi and these KCs were induced to differentiate in organotypic raft cultures. Mcl-1 shRNA organotypic cultures showed increased levels of spontaneous premature apoptosis, implicating Mcl-1 as an essential KC survival protein. Mcl-1 down-regulated cultures also had reduced granular and cornified layers, and produced lower levels of cross-linked protein and cornified envelopes. Cornification could only partially be rescued with the general caspase inhibitor z-VAD, suggesting that reduced cornification was not entirely due to premature apoptosis. Differentiation markers (K1, K10, filaggrin, loricrin, cleaved caspase-14) were normally expressed in control organotypic cultures, but were expressed at reduced levels in organotypic cultures with down-regulated Mcl-1. The defect in differentiation marker expression was independent of apoptosis as it could not be rescued by z-VAD. Thus, Mcl-1 serves two important, independent functions in epidermal KCs: acting as a major survival protein by inhibiting premature apoptosis in the spinous and granular layers to promote conification, and promoting the robust induction of KC differentiation markers.
INTRODUCTION
Homeostasis in the skin is maintained by a balance between two processes: cell proliferation of basal KCs and terminal differentiation of suprabasal KCs. Differentiation involves irreversible exit of KCs from the cell cycle and transit through the suprabasal spinous, granular and ultimately cornified layers of the epidermis. It is essential for KCs to survive during the differentiation processes in order to carry out their barrier and systemic functions before ultimately dying via cornification. It is also essential for KCs to be able to rapidly respond to genotoxic stresses, most notably UV radiation from the sun, in order to eliminate damaged KCs which may be at risk of malignant transformation. The important tumor suppressor function of UV-induced apoptosis is illustrated by the increased cancer incidence in transgenic mice over-expressing cell survival proteins (Nischt et al., 1988; Pena et al., 1998). Thus, epidermal KCs have a need for both constitutive (i.e. terminal differentiation) and inducible (i.e. apoptosis) cell death pathways. While much is known about the control of KC differentiation marker expression in viable cells, the regulatory determinants of the terminal cornification process are complex and poorly understood (Nickoloff et al., 2002; Lippens et al., 2005; Candi et al., 2005). The KC apoptotic machinery and response to UV radiation has been extensively studied, and consists of both intrinsic/mitochondrial and receptor-mediated cell-death pathways (Ziegler et al., 1994; Sitailo et al., 2002; Sheikh et al., 1998; Rehemtulla et al., 1997; Schwarz et al., 1995; Aragane et al., 1998). Critical mediators of UV apoptosis include activation of p53, down-regulation of the anti-apoptotic Mcl-1 protein, release of cytochrome c, and subsequent activation of a caspase cascade effecting the apoptotic elimination of damaged KCs (Nijhawan et al., 2003; Sitailo et al., 2002; Chaturvedi et al., 2005).
The transition of KCs from the granular to cornified layer during differentiation resembles a special form of apoptosis in some aspects (Lippens et al., 2005; Polakowska et al., 1994). The process is clearly genetically pre-programmed, and KCs become enucleated, lose all organelles, shrink and die without inducing an inflammatory response. Several studies have examined the relationship between KC terminal differentiation and classical apoptosis, however few common features have been identified (Gandarillas et al., 1999; Mitra et al., 1997; Lippens et al., 2000). While classical apoptotic caspases (caspase-3, -8, -9) are not activated during KC terminal differentiation, the squamous differentiation-associated caspase-14 is selectively activated in differentiating KCs, and plays a role in the terminal differentiation process (Lippens et al., 2000; Chien et al., 2002; Raymond et al., 2007). The mechanism of caspase-14 activation during differentiation is unclear as this caspase is not activated by either genotoxic or death receptor apoptotic agents (Lippens et al., 2000).
The myeloid cell leukemia 1 or Mcl-1 protein was initially discovered in ML-1 human myeloblastic leukemia cells after initiating differentiation of these cells by the phorbol ester TPA (Kozopas et al., 1993). Mcl-1 is an anti-apoptotic member of the Bcl-2 family of proteins involved in preserving outer mitochondrial membrane integrity by sequestering the pro-apoptotic Bcl-2 proteins Bak and Bax, although the binding to these pro-apoptotic proteins can be disrupted by BH3-only family members (i.e. tBid, Puma, Noxa, Bmf, Bim) (Willis et al., 2005; Zhou et al., 1997; Clohessy et al., 2006; Kuwana et al., 2005; Gomez-Bougie et al., 2005). Unique features of Mcl-1 include its dynamic regulation at multiple levels, and its rapid response to a wide variety of pro-apoptotic stimuli and growth factors (Michels et al., 2005; Iglesias-Serret et al., 2003). For example, Mcl-1 is rapidly down-regulated following UV irradiation, and this is required for cytochrome c release and apoptosis (Nijhawan et al., 2003; Sitailo et al., 2006). Both Mcl-1 null and Bcl-x null mice die in utero (Rinkenberger et al., 2000; Motoyama et al., 1995), although Mcl-1 null mice die much earlier (E4.0 vs. E13, respectively) indicating that these anti-apoptotic proteins have functions not compensated by other proteins. Of interest, the very early lethality observed in Mcl-1 null mice was associated with delayed development and not increased apoptosis, while the Bcl-x null embryos exhibited widespread spontaneous apoptosis. These studies, together with its initial identification as a differentiation-associated gene, suggest that Mcl-1 has additional functions in development and/or differentiation which are not shared by other Bcl-2 family members (Rinkenberger et al., 2000; Kozopas et al., 1993). We addressed two basic questions in this paper: what is the role of Mcl-1 protein in the survival of human KCs in the context of epidermal differentiation, and does the loss of Mcl-1 influence the KC differentiation process.
RESULTS
Mcl-1 protein is localized throughout the normal human epidermis
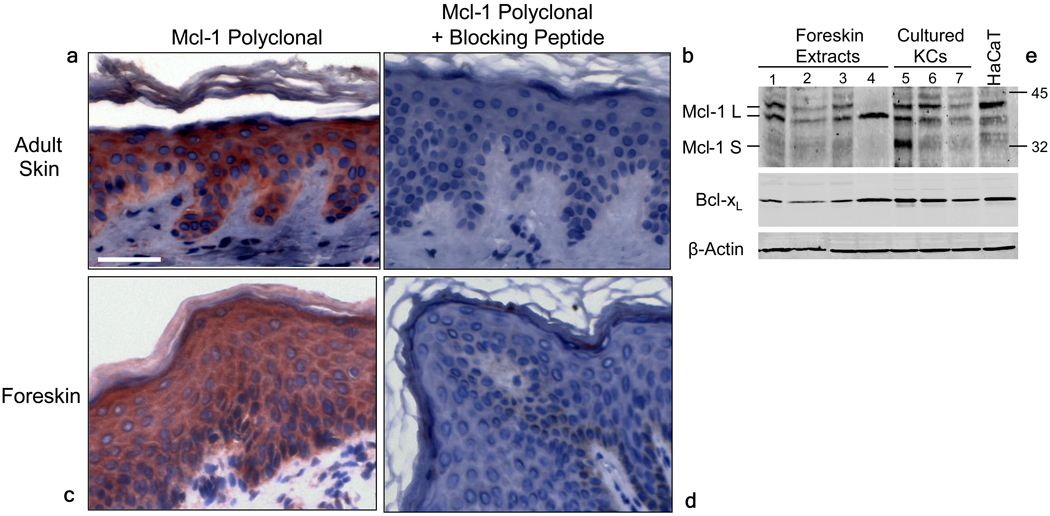
To evaluate the role of Mcl-1 in skin biology we performed immunohistochemical staining for Mcl-1 in formalin-fixed skin tissue (Figure 1 A–D). We found that Mcl-1 was highly expressed throughout the adult human epidermis, with stronger staining in the suprabasal compartment. The antibody staining was specific as it could be prevented by preincubation with Mcl-1 blocking peptide. Western blot analysis of protein extracted from foreskin or KCs growing in culture identified Mcl-1 as 42/40 kDa protein doublet, corresponding to the anti-apoptotic Mcl-1L, although one KC culture sample also contained a prominent 32 kDa Mcl-1 band. Bcl-xL was also readily detected at 33 kDa by western blot in all samples (Figure 1E).
Figure 1.
Altered epidermal morphology in organotypic cultures with down-regulated Mcl-1
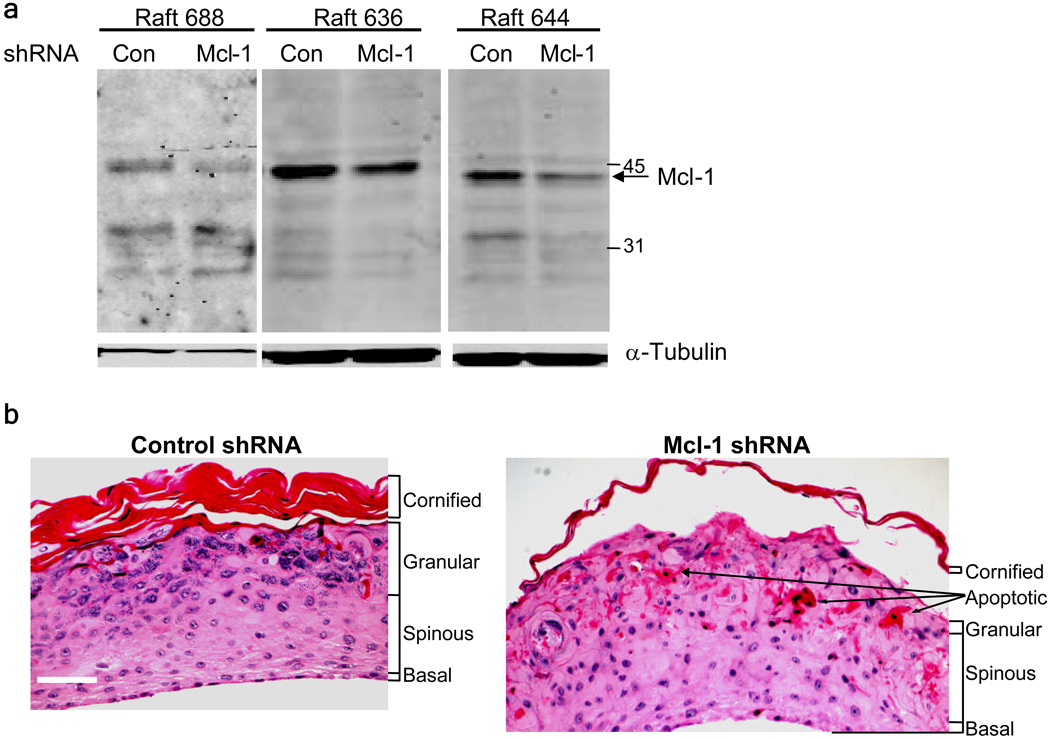
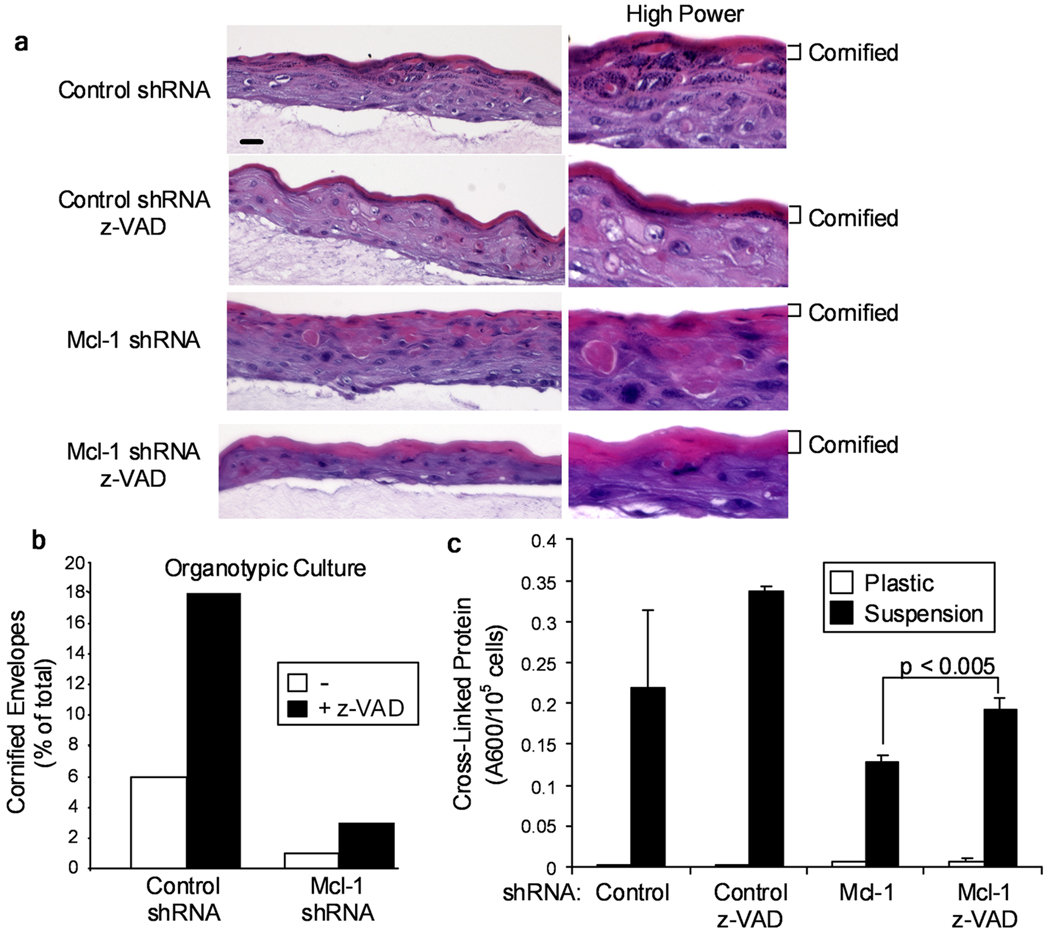
To determine the role of Mcl-1 in KC survival, we infected normal human KCs with a retrovirus encoding a specific shRNA targeting Mcl-1 (Sitailo et al., 2006), plated the cells on a collagen-fibroblast matrix, and lifted them to the air-liquid interface to generate organotypic raft cultures. As shown in Figure 2A, the Mcl-1 shRNA retrovirus reduced Mcl-1 protein levels to ~45% of control in organotypic raft cultures. Hematoxylin and eosin staining of sections revealed striking differences in histology of Mcl-1 shRNA cultures compared to control (Figure 2B). Control shRNA organotypic cultures had a well-developed histological appearance with clearly visible spinous, granular and cornified layers. In contrast, the granular layer in Mcl-1 shRNA raft was largely absent and poorly developed, and the cornified layer was much thinner than in control cultures. The Mcl-1 shRNA organotypic raft cultures also had numerous cells with eosinic cytoplasm in the upper spinous layer, suggestive of apoptotic cells. Organotypic cultures prepared from KCs transfected with a pool of Mcl-1 siRNA oligonucleotides had a similar morphological appearance (Supplementary Figure 1).
Figure 2.
Elevated spontaneous apoptosis in KCs with down-regulated Mcl-1
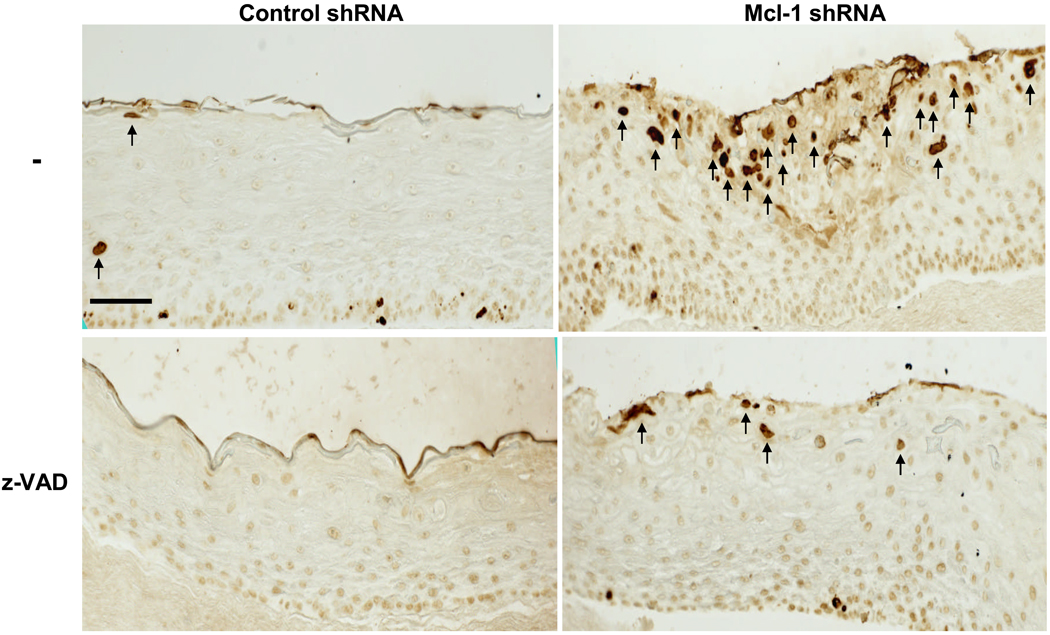
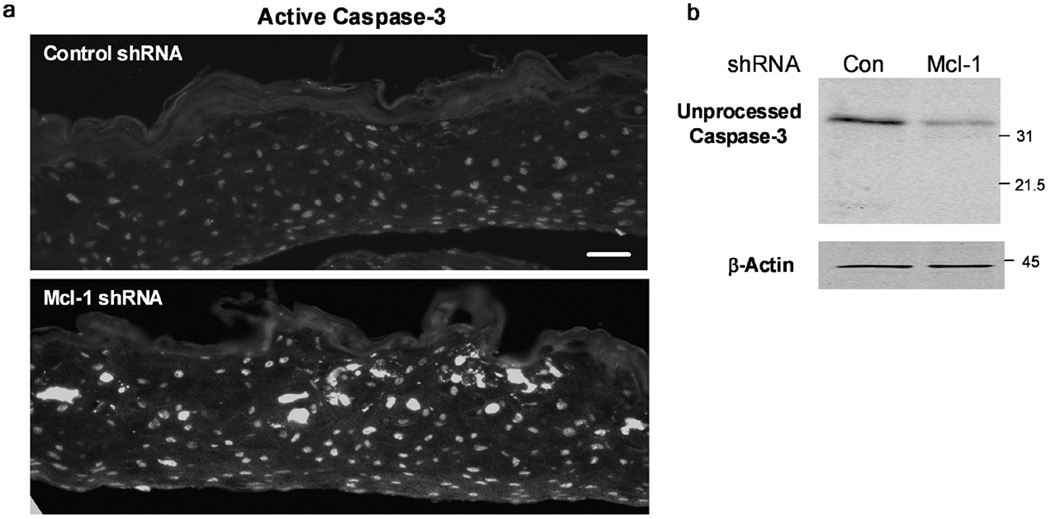
Figure 3 shows TUNEL staining performed on paraffin sections of Mcl-1 shRNA organotypic cultures, and demonstrates increased spontaneous apoptosis in the upper spinous layers (5 ± 1 cells/field control shRNA versus 20.3 ± 4.2 cells/field Mcl-1 shRNA; p<0.05). z-VAD, a general caspase inhibitor, was added to both control and Mcl-1 shRNA cultures after air-lifting to prevent apoptosis, and this effectively reduced the number of TUNEL positive cells from 20.3 ± 4.2 cells/field in Mcl-1 shRNA to 3 ± 0.3 cells/field in Mcl-1 shRNA with z-VAD (p<0.01). Increased spontaneous apoptosis in organotypic cultures with Mcl-1 down-regulated was confirmed by immunofluorescence staining for active caspase-3 (Figure 4A). Activation of caspase-3 was detected throughout the Mcl-1 shRNA epidermis with highest level detected in the upper spinous layers. Western blot analysis for full-length caspase-3 also confirmed increased proteolytic activation of caspase-3 in Mcl-1 down-regulated organotypic cultures compared to control (Figure 4B).
Figure 3.
Figure 4.
Inhibition of caspase-dependent apoptosis promotes cornification
To determine if the increased spontaneous apoptosis in the spinous layer of Mcl-1 shRNA organotypic cultures was responsible for decreased histological differentiation and/or cornification, we established organotypic raft cultures in the presence of z-VAD. Histological analysis of these organotypic cultures revealed increased cornified layers in z-VAD-treated control and especially Mcl-1 shRNA cultures (Figure 5A). To quantify cornification, we trypsinized the epidermis of organotypic cultures and determined the percent of cornified envelopes produced. Figure 5B shows that Mcl-1 shRNA cultures had fewer cornified envelopes than control shRNA cultures, and that z-VAD treatment increased cornified envelope formation in both control and Mcl-1 down-regulated cultures, consistent with the morphological assessment in Figure 5A. As an alternative system for differentiation induction, we used suspension culture in methylcellulose and assayed cross-linked protein formation (Tibudan et al., 2002). Figure 5C demonstrates a large induction of cross-linked protein induced by suspension culture and a reduction in protein cross-linking in the Mcl-1 shRNA cultures, although this was not a statistically significant decrease. Addition of z-VAD to the Mcl-1 shRNA suspension cultures significantly (p<0.005) increased the production of cross-linked protein (Figure 5C). Based on the ability of z-VAD to inhibit caspase-dependent apoptosis and increase cornification, we conclude that the reduced cornification in Mcl-1 shRNA KCs is in part due to increased apoptosis.
Figure 5.
Abnormal expression of differentiation markers in KCs with down-regulated Mcl-1
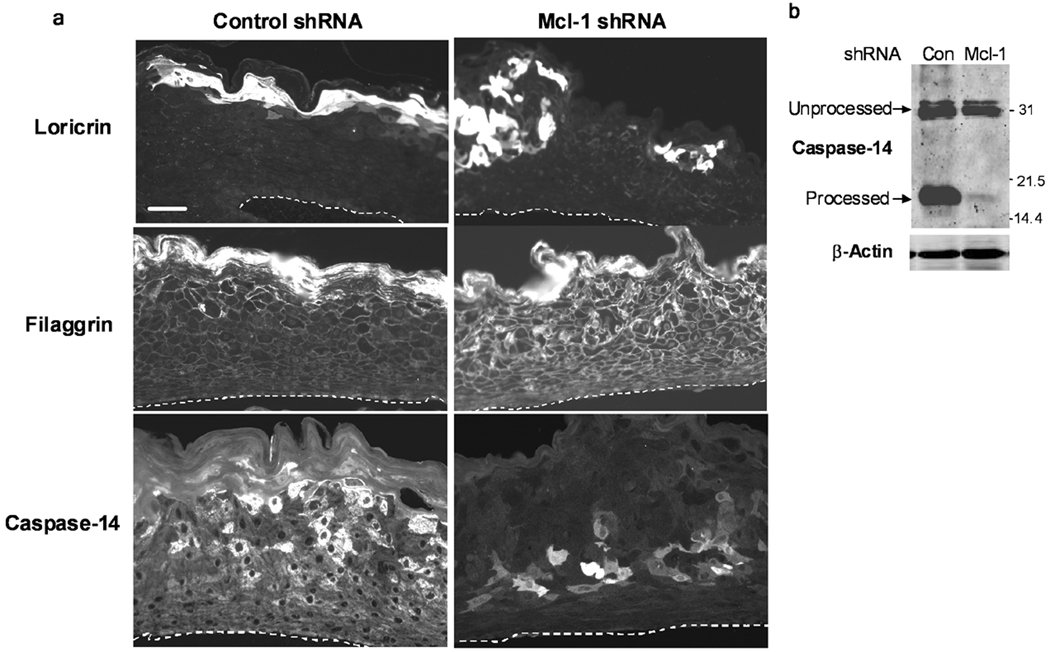
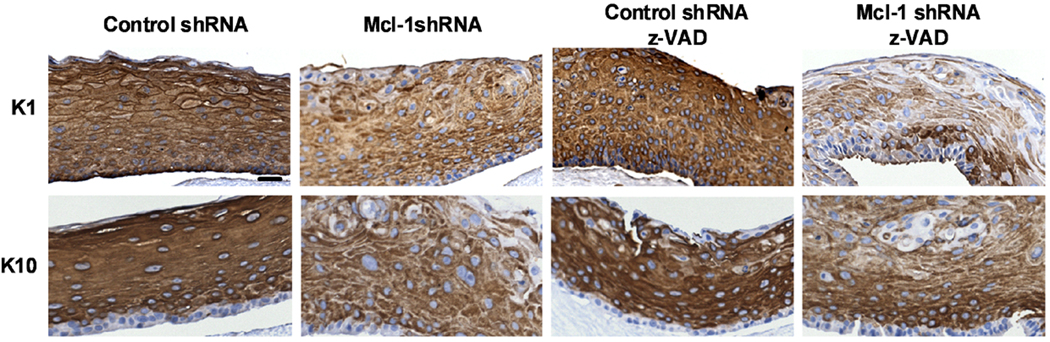
We performed immunofluorescence staining for KC differentiation markers in control and Mcl-1 shRNA organotypic cultures to determine if down-regulation of Mcl-1 affected differentiation marker expression. Figure 6 shows that loricrin and filaggrin were detected in a continuous band in the granular layer of control shRNA cultures, consistent with their expression in normal epidermis. In contrast, Mcl-1 shRNA cultures had discontinuous, patchy expression of loricrin and premature expression of filaggrin throughout the organotypic culture. Caspase-14 was strongly expressed in a patchy pattern in upper spinous and granular layers of control cultures, but was prematurely expressed in lower spinous layer of Mcl-1 shRNA organotypic cultures. Caspase-14 proteolytic processing/activation was also dramatically inhibited in Mcl-1 shRNA cultures, as determined by western blot analysis (Figure 6B). Immunohistochemical staining of early differentiation markers K1 and K10 also revealed reduced expression in Mcl-1 shRNA organotypic cultures, indicating an overall inhibition of KC differentiation by Mcl-1 down-regulation (Figure 7). A similar alteration in caspase-14 expression and reduction in K10 was observed in organotypic cultures from KCs transfected with Mcl-1 siRNA oligonucleotides (Supplementary Figure 1).
Figure 6.
Figure 7.
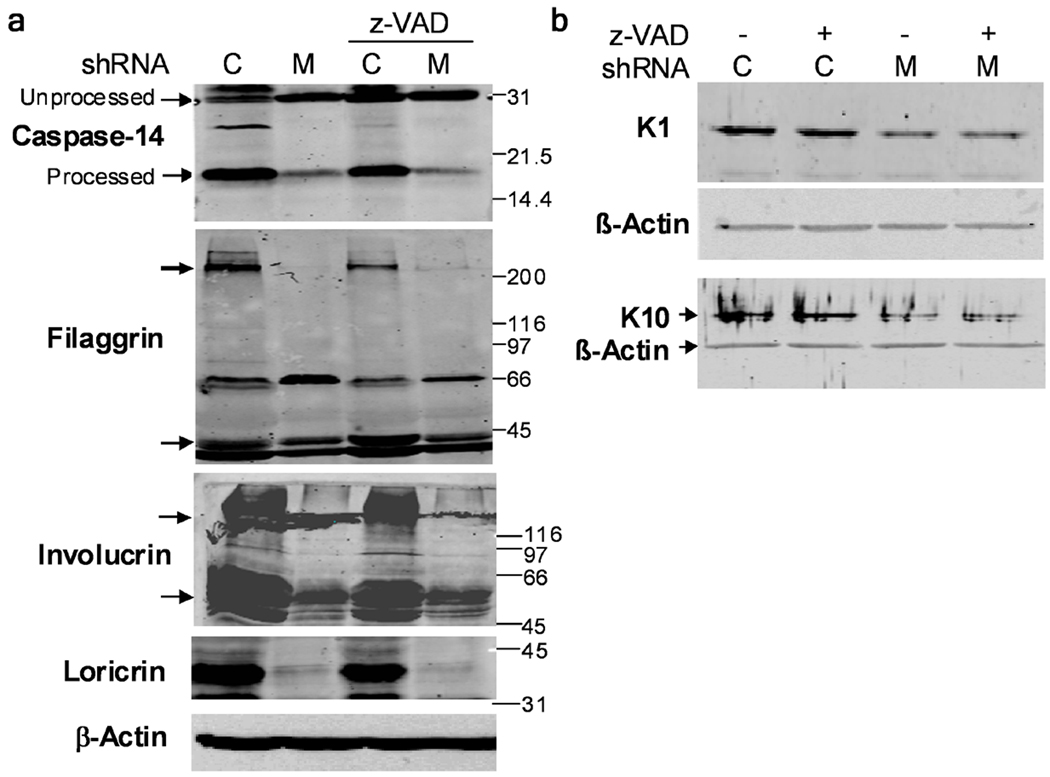
To determine if the decreased KC differentiation marker expression in Mcl-1 down-regulated organotypic cultures was due to elevated apoptosis, differentiation markers were examined in cultures air-lifted in the presence of z-VAD, which inhibits spontaneous apoptosis in Mcl-1 shRNA cultures (Fig. 3). Western blot analysis in Figure 8 confirmed reduced expression of loricrin, filaggrin, involucrin, K1 and K10, and lack of proteolytic processing (activation) of caspase-14 in Mcl-1 shRNA cultures. The addition of z-VAD did not significantly alter the expression of any differentiation markers in either control or Mcl-1 shRNA cultures, indicating that the increase in caspase-dependent apoptosis was not responsible for the decreased differentiation marker expression in Mcl-1 shRNA cultures.
Figure 8.
DISCUSSION
The coordinated regulation of epidermal KC proliferation, differentiation and apoptosis are vital to the function of skin under physiological conditions, as well as in response to physical and genotoxic stresses (Raj et al., 2006). In this study, we have identified Mcl-1 as a critical anti-apoptotic protein for KCs that also is involved in the regulation of the KC differentiation program. We were specifically interested in the epidermal function of Mcl-1 due to its involvement in the UV radiation apoptotic pathway, and its critical role in early embryonic development (Nijhawan et al., 2003; Sitailo et al., 2006; Rinkenberger et al., 2000). Our studies (Figure 1) localized Mcl-1 within the human epidermis with higher expression in the spinous and especially granular layers, consistent with previous reports (Krajewski et al., 1995; Thomkova et al., 1999). While the increased expression of Mcl-1 in the more differentiated layers is suggestive of a role for Mcl-1 in differentiation, it may also simply reflect increased protection from apoptosis as KCs proceed through their terminal differentiation program. We tested this possibility by down-regulating Mcl-1 in human keratinocytes induced to terminally differentiate in organotypic raft cultures.
Knock-down of Mcl-1 in organotypic cultures of human KC induced spontaneous apoptosis in the upper spinous and granular layers (Figure 3 and Figure 4), indicating an important survival role for Mcl-1 which was not compensated by other anti-apoptotic proteins. A variety of other anti-apoptotic proteins have been evaluated for a role in keratinocyte survival, including Bcl-xL, NF-κB, p53, and phosphoinositide 3-kinase/Akt (Raj et al., 2006; Umeda et al., 2003; Calautti et al., 2005; Chaturvedi et al., 2005; Chaturvedi et al., 1999). Bcl-x deficient mice are embryonic lethal due to extensive hematopoietic and neuronal cell death, although these mice survive much longer than Mcl-1 deficient mice (E13 vs. E3.5, respectively) (Motoyama et al., 1995; Rinkenberger et al., 2000). Mice with targeted disruption of Bcl-x in their epidermis were viable and did not show alteration in skin development, but spontaneous apoptotic epidermal KCs were detected (Umeda et al., 2003). Thus, Bcl-x is also a non-redundant survival protein for epidermal keratinocytes. Bax and Bcl-2 null mice do not exhibit any increased spontaneous epidermal apoptosis, although they expressed elevated levels of loricrin and K1 (Cho et al., 2001). Pharmacological inhibition of PI3K induced spontaneous apoptosis and reduced differentiation marker expression in cultured KCs induced to differentiate with extracellular calcium, implicating that the PI3K/AKT pathway in control between differentiation and apoptosis (Calautti et al., 2005). We previously reported that knock-down of Mcl-1 in monolayer culture induced a much smaller increase in spontaneous KC apoptosis than what we observed here in organotypic culture, possibly due to increased apoptotic signals present during terminal differentiation (Sitailo et al., 2006).
Inhibition of apoptosis with the caspase inhibitor z-VAD partially rescued the reduced cornification observed in the Mcl-1 shRNA cultures, indicating that apoptosis was an alternative cell fate to cornification (Figure 3 and Figure 5). In contrast, we clearly demonstrated that expression of both early (K1, K10) and late (involucrin, loricrin, filaggrin) differentiation markers were not altered by inhibition of caspases with z-VAD (Figure 7 and Figure 8). The incomplete rescue of cornification by z-VAD is likely due to the requirement for proper differentiation marker expression to produce mature, detergent-resistant cornified envelopes. Thus, the effects of Mcl-1 down-regulation on differentiation marker expression are independent of apoptosis and constitute a novel function for Mcl-1. Other studies found that general caspase inhibition either reduced the loss of nuclei during cornification (Weil et al., 1999) or inhibited expression of differentiation markers involucrin and loricrin (Chaturvedi et al., 2006), leading to the conclusion that differentiation and cornification in part require apoptotic caspase activation. Several important differences exist between the experimental system used in these studies and our current study. Organotypic culture systems lacking fibroblasts in the dermal layer appear to be primed for apoptosis as significant apoptotic caspase activation are detected after lifting to the air-liquid interface (Weil et al., 1999; Chaturvedi et al., 2006). Other in vitro differentiation model systems support our conclusion that differentiation and apoptosis are alternative cell fates (Gandarillas et al., 1999; Mitra et al., 1997; Lippens et al., 2000).
Caspase-14 is a unique member of the caspase family of cysteine proteases due to its selective expression and proteolytic processing in terminally differentiating KCs (Eckhart et al., 2000; Lippens et al., 2000; Chien et al., 2002). The proteolytic activation of caspase-14 is mediated by an uncharacterized protease which generates p20 large and p10 small subunits. Processed and activated caspase-14 has a substrate specificity similar to other caspases, W/Y-X-X-D, although it is not activated by conventional apoptotic stimuli (Chien et al., 2002; Park et al., 2006; Hu et al., 1998). Caspase-14 null mice have defective epidermal barrier function and altered profilaggrin processing, indicating a role for caspase-14 in epidermal terminal differentiation (Denecker et al., 2007). Immunofluorescence staining for caspase-14 in control organotypic cultures showed granular and upper spinous layer expression (Figure 6), consistent with other reports examining caspase-14 in vivo (Eckhart et al., 2000; Kuechle et al., 2001). A significant proportion (~50%) of the caspase-14 we detected from our organotypic cultures was processed and presumably activated (Figure 6 and Figure 8). We did not detect processing of caspase-14 in monolayer culture of human KCs, even after two days at confluence in high calcium media or in KC induced to differentiate in suspension culture (data not shown). Thus, caspase-14 expression and proteolytic processing are tightly linked to the KC differentiation program and can be considered a bona fide differentiation marker. Caspase-14 expression in Mcl-1 shRNA organotypic cultures was reduced, expressed prematurely in the lower spinous layers, and was not proteolytically processed/activated (Figure 6 and Figure 8). Caspase inhibition with z-VAD had no effect on the expression or processing of caspase-14, indicating that the differentiation signal(s) inducing caspase-14 activation are independent from the canonical caspase-dependent apoptotic pathway. The inability of z-VAD to prevent processing of caspase-14 is consistent with the non-caspase cleavage sites identified for caspase-14, although the enzymatic activity of processes caspase-14 would be expected to be inhibited by z-VAD (Chien et al., 2002).
Overall, our studies demonstrate that Mcl-1 is required for proper KC differentiation, although the mechanism(s) of regulation of KC differentiation by Mcl-1 is currently unclear. The vast majority of functional studies on Mcl-1 have focused on its anti-apoptotic function via sequestering pro-apoptotic Bcl-2 family members to prevent permeability of the mitochondrial outer membrane (Willis et al., 2005; Gomez-Bougie et al., 2005; Clohessy et al., 2006; Chen et al., 2005; Qin et al., 2006). However other Mcl-1-binding proteins have been identified that may be involved in KC differentiation control. Fujise and colleagues identified the calcium-binding protein fortilin/TCTP as being bound and stabilized by Mcl-1 (Zhang et al., 2002; Graidist et al., 2007; Haghighat and Ruben, 1992). Given the important role of calcium in KC differentiation, it is conceivable that the stabilization of fortilin by Mcl-1 may help regulate the calcium scavenging function of fortilin (Hennings et al., 1980; Li et al., 1995). Mcl-1 has also been shown to bind proliferating cell nuclear antigen, PCNA, and this binding was partially required for Mcl-1 to inhibit S phase progression (Fujise et al., 2000). A proteolytic fragment of Mcl-1 also binds to Cdk1 and inhibits its kinase activity, providing another potential mechanism for Mcl-1 to inhibit proliferation (Jamil et al., 2005). Thus, down-regulation of Mcl-1 may impede KC differentiation by altering cell cycle progression to favor cell proliferation over differentiation. Consistent with this, we found an increase in KCs in the S phase of the cell cycle when Mcl-1 was down-regulated (data not shown).
MATERIALS AND METHODS
Antibodies and chemicals
Mcl-1 was detected using an antibody against an internal part of Mcl-1 protein (sc-819, Santa Cruz Biotechnology Inc., Santa Cruz, CA) which can detect both the Mcl-1L 42-40 kDa and Mcl-1S 32 kDa isoforms. This antibody was used for western blotting at 1:1000 and for tissue immunostaining at 1:100. Total caspase-3 was detected by western blot (sc-2727, Santa Cruz Biotech), and active caspase-3 detected in tissue sections by immunofluorescence (AF835, R&D Systems, Minneapolis, MN). The following differentiation marker antibodies were used: loricrin (PRB-145P, Covance Research Products, Inc., Berkeley, CA ) at 1:1000 for western blotting and 1:250 for immunofluorescence; filaggrin (PRB-417P, Covance) at 1:1000 for western blotting and 1:250 for immunofluorescence; caspase-14 (sc-5628, Santa Cruz Biotech., Inc.) at 1:500 for western blotting and 1:100 for immunofluorescence; involucrin (sc-21748, Santa Cruz Biotechnology, Inc.) at 1:500 for western blotting. The general caspase inhibitor z-VAD-FMK (51-69361U, BD Biosciences Pharmingen, San Jose, CA) and prepared as 10 mM stock solution in DMSO, aliquoted and stored at −20° C.
Cell culture
Primary human KCs were isolated from neonatal foreskin tissue as described previously, and approved by the Loyola University Medical Center Institutional Review Board (Denning et al., 1998). KCs were maintained in Media 154CF with Human KC Growth Supplement and 0.07 mM Ca+2 (Cascade Biologics, Portland, OR). For suspension-induced differentiation, KCs were trypsinized, suspended in a small volume (50–100 µl) of Dulbecco Modified Eagles Medium and 10% fetal bovine serum (DMEM/10% FBS), and mixed into the same medium containing 1.75% methylcellulose with or without 10 µM z-VAD. After 24 hours, the cells were washed twice in PBS and analyzed for western blotting or cross-linked protein formation.
Organotypic cultures
Organotypic raft cultures were generated based on the protocol described by Meyers et al. (Meyers et al., 1994). Control shRNA or Mcl-1 shRNA-infected KCs were initially selected in the presence of 1 µg/ml puromycin in subconfluent monolayer cultures. In some experiments, KCs were transfected with SMARTpool siRNAs targeting Mcl-1 (MV-004501-04, Dharmacon, Inc, Lafayette, CO). Collagen (type I rat tail, 3.79 mg/ml, BD Biosciences, Bedford, MA) plugs with 105 J2-3T3 fibroblasts per ml were made in 12 well cell culture inserts (1.0 µm pore size, PET track-etched membrane, Becton Dickinson, Franklin Lakes, NJ), and incubated for 1 day. 2 × 106 Control shRNA or Mcl-1 shRNA KCs were plated on top of each plug in E-media supplemented with 5 ng/ml of EGF. After two days, KC differentiation was initiated by lifting organotypic cultures to the air-liquid interface. 1 ml of E media (+/− 10 µM z-VAD) was added to the bottom of each wells and changed daily for the next 5–6 days. Organotypic cultures were then fixed in neutral buffered formalin and processed for histology, or the epidermis removed for either protein or cornified envelope analysis.
shRNA cloning and retrovirus production
Retroviruses encoding small interfering RNA hairpins were constructed using pSUPER.retro.puro vector system (OligoEngine, Seattle, WA) as described previously (Sitailo et al., 2006). Briefly, double-stranded oligonucleotides targeting Mcl-1 or no human gene (control) were cloned into pSUPER.retro.puro, and retroviruses generated by transfecting Phoenix-Ampho cells (ATCC, Manassas, VA). Packing cells were selected with puromycin (1 µg/ml), and the viruses were used to infect normal human KC, which were also selected with puromycin.
Western blots
KCs were washed with PBS- buffer, scrapped in lysis buffer: 20 mM Tris-HCl, pH 7.5, 5 mM EDTA, 1% Triton X-100, 1X Complete Protease Inhibitor Cocktail, 1 mM sodium fluoride, 1 mM sodium orthovanadate, 10 mM β-glycerophosphate. For epidermal extracts, foreskins were incubated in 60° C water for 45 seconds, cooled in ice water, and the epidermis removed with a razor blade and placed into lysis buffer. Protein extracts were briefly sonicated, centrifuged at 10,000 × g for 5 min, and protein concentration was estimated by Bradford assay. Usually 30 µg/well was loaded on 8.5% or 12.5% SDS-PAGE gel. For western blot analysis of filaggrin, involucrin and loricrin, 0.5% SDS was included in the lysis buffer and the extract was heated for 5 min at 95° C to increase protein extraction. Quantitation of band intensities was done using an Odyssey Infrared Imaging System.
Staining of tissues
For immunofluorescence staining paraffin sections of rafts on glass slides were deparaffinized by soaking in xylene 5 min, then another xylene 5 min, dipping up and down 10X in 100% ethanol, 10X in 95% ethanol and 10X in ddH2O and rinsed once in FA buffer. For antigen retrieval of formalin-fixed, paraffin-embedded samples, slides were placed in a rack for heating in the pressure cooker in 10 mM citrate buffer (pH 6.0) for 10 minutes, and then cooled for 20 min with lid off. Slides were removed from cooker and equilibrated in FA buffer for 10 minutes. 50 µl primary antibody was used per slide staining in moisture chamber for 1 hour at room temp. Then slides washed for 5 min in FA buffer and stained with AlexaFluor 488 (anti-mouse) or AlexaFluor 568 (anti-rabbit) secondary antibodies at 1:400 (A11001 and A11036, Molecular Probes, Eugene, OR) for 1 hour at room temp. After washing 5 min in FA buffer slides were mounted with coverslips on gelvatol (polyvinyl alcohol, 30,000–70,000 MW, Sigma, St. Louis, MO) plus DABCO (1,4-diazabicyclo-[2.2.2]octane, Sigma). Immunoperoxidase staining was performed using peroxidase-labeled secondary antibody, followed by color detection with the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) and diaminobenzidine as a substrate. Sections were counterstained with hematoxylin for 3 min and mounted in Aqua-Mount (cat # 13800, Lerner Lab, Pittsburgh, PA). For TUNEL staining, 8 µm sections were cut from Control shRNA and Mcl-1 shRNA organotypic culture paraffin blocks. Peroxidase staining of sections was performed with ApopTag Peroxidase In Situ Detection Kit as described by the manufacturer (S7100, Chemicon International Inc., Temecula, CA). For quantitation, treatment groups were concealed and TUNEL positive cells counted in three randomly selected fields (20× objective). Photographs were taken with Olympus AX80 fluorescence microscope equipped with a Retiga 4000R 4.2 Mpixel monochrome camera.
Quantitation of cross-linked protein and cornified envelopes
Cornified envelopes resistant to heating in SDS/dithiothreitol, DTT, were quantified by a membrane binding assay or by counting cornified envelopes in a hematocytometer. Total cells were collected from suspension culture or trypsinized from raft epidermis and counted. Cornified envelopes were prepared by heating cells at 90° C for 10 min in 2% SDS, 20 mM DTT, and resistant cornified cells counted directly under the hemacytometer. For cross-linked protein measurements, the envelopes was washed 2X in SDS/DTT buffer by spinning at 11,000 × g to remove soluble protein before applying to a RC60 membrane (Schleicher & Schuell, pore size 1.0 µm, Keene, NH) on slot blot vacuum manifold (Hough-Monroe and Milstone, 1991). 105 cells in 50 µl were applied per slot. Wells were washed 3X with SDS/DTT buffer and the membrane was air dried, submerged in 7.5% TCA at 80° C for 30 min. The membrane was stained with constant rocking in 1% Coomasie Blue G250 in 7% acetic acid for 15 min at 50° C. After washing with 7% acetic acid at 50° C for 5 min, the stained spots were cut out and placed in tube, and the dye eluted in 400 µl of 66% methanol, 1% ammonium hydroxide. Absorbance was read at 600 nm to quantify the cross-linked protein. All statistics were performed using a two-tailed Student’s t-Test.
Supplementary Material
Acknowledgments
We would like to acknowledge all members of the Skin Cancer Research Program within the Oncology Institute of Loyola University Chicago, especially Dr. Brian J. Nickoloff, Dr. Jian-Zhong Qin and Mrs. Vijaya Chaturvedi. We also thank Mr. Vipin Yadav for help with shRNA design. This work was supported by NIH grant CA083784 (MFD).
REFERENCES
- Aragane Y, Kulms D, Metze D, Wilkes G, Poppelmann B, Luger TA, et al. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J Cell Biol. 1998;140:171–182. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti E, Li J, Saoncella S, Brissette JL, Goetinck PF. Phosphoinositide 3-kinase signaling to AKT promotes keratinocyte differentiation versus death. J Biol Chem. 2005 doi: 10.1074/jbc.M506119200. [DOI] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, Qin JZ, Denning MF, Choubey D, Diaz MO, Nickoloff BJ. Apoptosis in proliferating, senescent, and immortalized keratinocytes. J Biol Chem. 1999;274:23358–23367. doi: 10.1074/jbc.274.33.23358. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, Sitailo LA, Bodner B, Denning MF, Nickoloff BJ. Defining the caspase-containing apoptotic machinery contributing to cornification in human epidermal equivalents. Exp Dermatol. 2006;15:14–22. doi: 10.1111/j.0906-6705.2005.00383.x. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, Sitailo LA, Qin JZ, Bodner B, Denning MF, Curry J, et al. Knockdown of p53 levels in human keratinocytes accelerates Mcl-1 and Bcl-xL reduction thereby enhancing UV-light induced apoptosis. Oncogene. 2005;24:5299–5312. doi: 10.1038/sj.onc.1208650. [DOI] [PubMed] [Google Scholar]
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Chien AJ, Presland RB, Kuechle MK. Processing of native caspase-14 occurs at an atypical cleavage site in normal epidermal differentiation. Biochem Biophys Res Commun. 2002;296:911–917. doi: 10.1016/s0006-291x(02)02015-6. [DOI] [PubMed] [Google Scholar]
- Cho SH, Delehedde M, Rodriguez-Villanueva J, Brisbay S, McDonnell TJ. Bax gene disruption alters the epidermal response to ultraviolet irradiation and in vivo induced skin carcinogenesis. Int J Mol Med. 2001;7:235–241. doi: 10.3892/ijmm.7.3.235. [DOI] [PubMed] [Google Scholar]
- Clohessy JG, Zhuang J, de Boer J, Gil-Gomez G, Brady HJ. Mcl-1 interacts with truncated Bid and inhibits its induction of cytochrome c release and its role in receptor-mediated apoptosis. J Biol Chem. 2006;281:5750–5759. doi: 10.1074/jbc.M505688200. [DOI] [PubMed] [Google Scholar]
- Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, et al. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–674. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- Denning MF, Wang Y, Nickoloff BJ, Wrone-Smith T. Protein kinase Cδ is activated by caspase-dependent proteolysis during ultraviolet radiation-induced apoptosis of human keratinocytes. J Biol Chem. 1998;273:29995–30002. doi: 10.1074/jbc.273.45.29995. [DOI] [PubMed] [Google Scholar]
- Eckhart L, Declercq W, Ban J, Rendl M, Lengauer B, Mayer C, et al. Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J Invest Dermatol. 2000;115:1148–1151. doi: 10.1046/j.1523-1747.2000.00205.x. [DOI] [PubMed] [Google Scholar]
- Fujise K, Zhang D, Liu J, Yeh ET. Regulation of apoptosis and cell cycle progression by MCL1. Differential role of proliferating cell nuclear antigen. J Biol Chem. 2000;275:39458–39465. doi: 10.1074/jbc.M006626200. [DOI] [PubMed] [Google Scholar]
- Gandarillas A, Goldsmith LA, Gschmeissner S, Leigh IM, Watt FM. Evidence that apoptosis and terminal differentiation of epidermal keratinocytes are distinct processes. Exp Dermatol. 1999;8:71–79. doi: 10.1111/j.1600-0625.1999.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Bougie P, Bataille R, Amiot M. Endogenous association of Bim BH3-only protein with Mcl-1, Bcl-xL and Bcl-2 on mitochondria in human B cells. Eur J Immunol. 2005;35:971–976. doi: 10.1002/eji.200425878. [DOI] [PubMed] [Google Scholar]
- Graidist P, Yazawa M, Tonganunt M, Nakatomi A, Lin CC, Chang JY, et al. Fortilin binds Ca2+ and blocks Ca2+-dependent apoptosis in vivo. Biochem J. 2007;408:181–191. doi: 10.1042/BJ20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighat NG, Ruben L. Purification of novel calcium binding proteins from Trypanosoma brucei: properties of 22-, 24- and 38-kilodalton proteins. Mol Biochem Parasitol. 1992;51:99–110. doi: 10.1016/0166-6851(92)90205-x. [DOI] [PubMed] [Google Scholar]
- Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Hough-Monroe L, Milstone LM. Quantitation of cross-linked protein: an alternative to counting cornified envelopes as an index of keratinocyte differentiation. Anal Biochem. 1991;199:25–28. doi: 10.1016/0003-2697(91)90264-t. [DOI] [PubMed] [Google Scholar]
- Hu S, Snipas SJ, Vincenz C, Salvesen G, Dixit VM. Caspase-14 is a novel developmentally regulated protease. J Biol Chem. 1998;273:29648–29653. doi: 10.1074/jbc.273.45.29648. [DOI] [PubMed] [Google Scholar]
- Iglesias-Serret D, Pique M, Gil J, Pons G, Lopez JM. Transcriptional and translational control of Mcl-1 during apoptosis. Arch Biochem Biophys. 2003;417:141–152. doi: 10.1016/s0003-9861(03)00345-x. [DOI] [PubMed] [Google Scholar]
- Jamil S, Sobouti R, Hojabrpour P, Raj M, Kast J, Duronio V. A proteolytic fragment of Mcl-1 exhibits nuclear localization and regulates cell growth by interaction with Cdk1. Biochem J. 2005;387:659–667. doi: 10.1042/BJ20041596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski S, Bodrug S, Krajewska M, Shabaik A, Gascoyne R, Berean K, et al. Immunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1 in control of programmed cell death in vivo. Am J Pathol. 1995;146:1309–1319. [PMC free article] [PubMed] [Google Scholar]
- Kuechle MK, Predd HM, Fleckman P, Dale BA, Presland RB. Caspase-14, a keratinocyte specific caspase: mRNA splice variants and expression pattern in embryonic and adult mouse. Cell Death Differ. 2001;8:868–870. doi: 10.1038/sj.cdd.4400897. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Li L, Tucker RW, Hennings H, Yuspa SH. Chelation of intracellular Ca2+ inhibits murine keratinocyte differentiation in vitro. J Cell Physiol. 1995;163:105–114. doi: 10.1002/jcp.1041630112. [DOI] [PubMed] [Google Scholar]
- Lippens S, Denecker G, Ovaere P, Vandenabeele P, Declercq W. Death penalty for keratinocytes: apoptosis versus cornification. Cell Death Differ. 2005;12 Suppl 2:1497–1508. doi: 10.1038/sj.cdd.4401722. [DOI] [PubMed] [Google Scholar]
- Lippens S, Kockx M, Knaapen M, Mortier L, Polakowska R, Verheyen A, et al. Epidermal differentiation does not involve the pro-apoptotic executioner caspases, but is associated with caspase-14 induction and processing. Cell Death Differ. 2000;7:1218–1224. doi: 10.1038/sj.cdd.4400785. [DOI] [PubMed] [Google Scholar]
- Meyers C, Frattini MG, Laiminis LA. Cell Biology: A Laboratory Handbook. Academic Press, Inc.; 1994. Tissue culture techniques for the study of human papillomaviruses in stratified epithelia; pp. 491–499. [Google Scholar]
- Michels J, Johnson PW, Packham G. Mcl-1. Int J Biochem Cell Biol. 2005;37:267–271. doi: 10.1016/j.biocel.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Mitra RS, Wrone-Smith T, Simonian P, Foreman KE, Nunez G, Nickoloff BJ. Apoptosis in keratinocytes is not dependent on induction of differentiation. Lab Invest. 1997;76:99–107. [PubMed] [Google Scholar]
- Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Qin JZ, Chaturvedi V, Bacon P, Panella J, Denning MF. Life and death signaling pathways contributing to skin cancer. J Investig Dermatol Symp Proc. 2002;7:27–35. doi: 10.1046/j.1523-1747.2002.19633.x. [DOI] [PubMed] [Google Scholar]
- Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, et al. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nischt R, Roop DR, Mehrel T, Yuspa SH, Rentrop M, Winter H, et al. Aberrant expression during two-stage mouse skin carcinogenesis of a type I 47-kDa keratin, K13,normally associated with terminal differentiation of internal stratified epithelia. Molecular Carcinogenesis. 1988;1:96–108. doi: 10.1002/mc.2940010205. [DOI] [PubMed] [Google Scholar]
- Park K, Kuechle MK, Choe Y, Craik CS, Lawrence OT, Presland RB. Expression and characterization of constitutively active human caspase-14. Biochem Biophys Res Commun. 2006;347:941–948. doi: 10.1016/j.bbrc.2006.06.156. [DOI] [PubMed] [Google Scholar]
- Pena JC, Rudin CM, Thompson CB. A Bcl-xL transgene promotes malignant conversion of chemically initiated skin papillomas. Cancer Res. 1998;58:2111–2116. [PubMed] [Google Scholar]
- Polakowska RR, Piacentini M, Bartlett R, Goldsmith LA, Haake AR. Apoptosis in human skin development: morphogenesis, periderm, and stem cells. Dev Dyn. 1994;199:176–188. doi: 10.1002/aja.1001990303. [DOI] [PubMed] [Google Scholar]
- Qin JZ, Xin H, Sitailo LA, Denning MF, Nickoloff BJ. Enhanced Killing of Melanoma Cells by Simultaneously Targeting Mcl-1 and NOXA. Cancer Res. 2006;66:9636–9645. doi: 10.1158/0008-5472.CAN-06-0747. [DOI] [PubMed] [Google Scholar]
- Raj D, Brash DE, Grossman D. Keratinocyte apoptosis in epidermal development and disease. J Invest Dermatol. 2006;126:243–257. doi: 10.1038/sj.jid.5700008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AA, Mechin MC, Nachat R, Toulza E, Tazi-Ahnini R, Serre G, et al. Nine procaspases are expressed in normal human epidermis, but only caspase-14 is fully processed. Br J Dermatol. 2007;156:420–427. doi: 10.1111/j.1365-2133.2006.07656.x. [DOI] [PubMed] [Google Scholar]
- Rehemtulla A, Hamilton CA, Chinnaiyan AM, Dixit VM. Ultraviolet radiation-induced apoptosis is mediated by activation of CD-95 (Fas/APO-1) J Biol Chem. 1997;272:25783–25786. doi: 10.1074/jbc.272.41.25783. [DOI] [PubMed] [Google Scholar]
- Rinkenberger JL, Horning S, Klocke B, Roth K, Korsmeyer SJ. Mcl-1 deficiency results in peri-implantation embryonic lethality. Genes Dev. 2000;14:23–27. [PMC free article] [PubMed] [Google Scholar]
- Schwarz A, Bhardwaj R, Aragane Y, Mahnke K, Riemann H, Metze D, et al. Ultraviolet-B-induced apoptosis of keratinocytes: evidence for partial involvement of tumor necrosis factor-alpha in the formation of sunburn cells. J Invest Dermatol. 1995;104:922–927. doi: 10.1111/1523-1747.ep12606202. [DOI] [PubMed] [Google Scholar]
- Sheikh MS, Antinore MJ, Huang Y, Fornace AJ., Jr. Ultraviolet-irradiation-induced apoptosis is mediated via ligand independent activation of tumor necrosis factor receptor 1. Oncogene. 1998;17:2555–2563. doi: 10.1038/sj.onc.1202292. [DOI] [PubMed] [Google Scholar]
- Sitailo LA, Tibudan SS, Denning MF. Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J Biol Chem. 2002;277:19346–19352. doi: 10.1074/jbc.M200401200. [DOI] [PubMed] [Google Scholar]
- Sitailo LA, Tibudan SS, Denning MF. The protein kinase Cδ atalytic fragment targets Mcl-1 for degradation to trigger apoptosis. J Biol Chem. 2006 doi: 10.1074/jbc.M607351200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomkova H, Fujimoto W, Arata J. Expression of the bcl-2 family of genes in the course of keratinocyte differentiation. Eur J Dermatol. 1999;9:191–196. [PubMed] [Google Scholar]
- Tibudan SS, Wang Y, Denning MF. Activation of protein kinase C triggers irreversible cell cycle withdrawal in human keratinocytes. J Invest Dermatol. 2002;119:1282–1289. doi: 10.1046/j.1523-1747.2002.19625.x. [DOI] [PubMed] [Google Scholar]
- Umeda J, Sano S, Kogawa K, Motoyama N, Yoshikawa K, Itami S, et al. In vivo cooperation between Bcl-xL and the phosphoinositide 3-kinase-Akt signaling pathway for the protection of epidermal keratinocytes from apoptosis. FASEB J. 2003;17:610–620. doi: 10.1096/fj.02-0597com. [DOI] [PubMed] [Google Scholar]
- Weil M, Raff MC, Braga VM. Caspase activation in the terminal differentiation of human epidermal keratinocytes. Curr Biol. 1999;9:361–364. doi: 10.1016/s0960-9822(99)80162-6. [DOI] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005 doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Li F, Weidner D, Mnjoyan ZH, Fujise K. Physical and functional interaction between myeloid cell leukemia 1 protein (MCL1) and Fortilin. The potential role of MCL1 as a fortilin chaperone. J Biol Chem. 2002;277:37430–37438. doi: 10.1074/jbc.M207413200. [DOI] [PubMed] [Google Scholar]
- Zhou P, Qian L, Kozopas KM, Craig RW. Mcl-1, a Bcl-2 family member, delays the death of hematopoietic cells under a variety of apoptosis-inducing conditions. Blood. 1997;89:630–643. [PubMed] [Google Scholar]
- Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.