Abstract
Jones, Dorothy (American Meat Institute Foundation, Chicago, Ill.), R. H. Deibel, and C. F. Niven, Jr. Catalase activity of two Streptococcus faecalis strains and its enhancement by aerobiosis and added cations. J. Bacteriol. 88:602–610. 1964.—The nature of catalase activity noted in two unusual Streptococcus faecalis strains was determined. Enzyme activity was lost slowly when cultures were maintained by daily transfer in test tubes of broth media. Loss of activity could be prevented by aerobic culture. Supplementation of the growth medium with ferric, manganese, and zinc ions, as well as aerobiosis, enhanced catalase activity. However, addition of these cations to cell suspensions or to cell-free extracts did not increase catalase activity. Although oxygen was observed to be one of the reaction end products, the catalase activity was not inhibited by cyanide or azide, and the iron-porphyrin coenzyme of classical catalase was not detected. The enzyme was purified 185-fold by precipitation with ammonium sulfate, followed by chromotography on a diethylaminoethyl cellulose column.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCHANAN B. B., LOVENBERG W., RABINOWITZ J. C. A comparison of clostridial ferredoxins. Proc Natl Acad Sci U S A. 1963 Mar 15;49:345–353. doi: 10.1073/pnas.49.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAIX P., FLAMENS P. Pigments hématiniques de Bacillus coagulans cultivé en aérobiose ou en anaérobiose. Biochim Biophys Acta. 1953 Aug;11(4):530–534. doi: 10.1016/0006-3002(53)90091-2. [DOI] [PubMed] [Google Scholar]
- CLAYTON R. K. The induced synthesis of catalase in Rhodopseudomonas spheroides. Biochim Biophys Acta. 1960 Jan 29;37:503–512. doi: 10.1016/0006-3002(60)90507-2. [DOI] [PubMed] [Google Scholar]
- DACRE J. C., SHARPE M. E. Catalase production by Lactobacilli. Nature. 1956 Sep 29;178(4535):700–700. doi: 10.1038/178700a0. [DOI] [PubMed] [Google Scholar]
- DEIBEL R. H., EVANS J. B. Modified benzidine test for the detection of cytochrome-containing respiratory systems in microorganisms. J Bacteriol. 1960 Mar;79:356–360. doi: 10.1128/jb.79.3.356-360.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEIBEL R. H., NIVEN C. F., Jr Comparative study of Gaffkya homari, Aerococcus viridans, tetrad-forming cocci from meat curing brines, and the genus Pediococcus. J Bacteriol. 1960 Feb;79:175–180. doi: 10.1128/jb.79.2.175-180.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
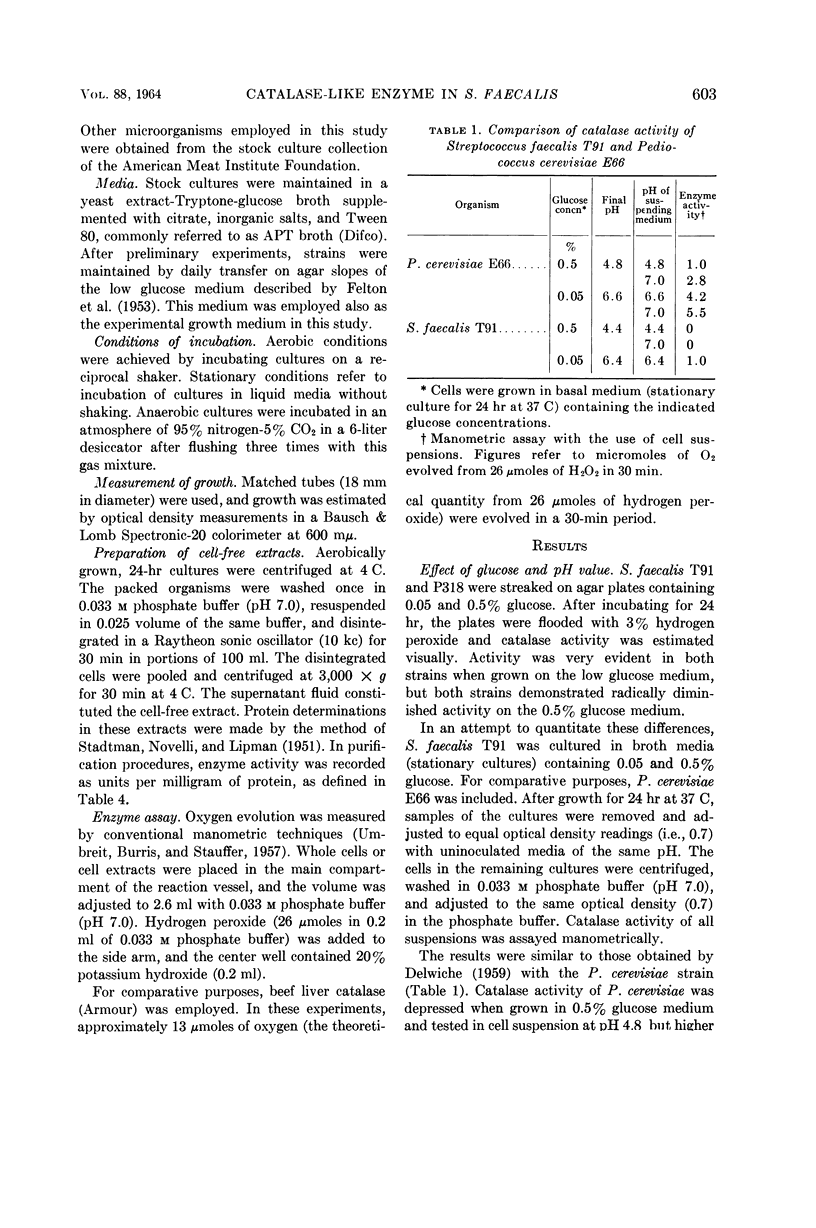
- DELWICHE E. A. Catalase of Pedicoccus cerevisiae. J Bacteriol. 1961 Mar;81:416–418. doi: 10.1128/jb.81.3.416-418.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBROGOSZ W. J., STONE R. W. Oxidative metabolism in Pediococcus pentosaceus. I. Role of oxygen and catalase. J Bacteriol. 1962 Oct;84:716–723. doi: 10.1128/jb.84.4.716-723.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBROGOSZ W. J., STONE R. W. Oxidative metabolism in Pediococcus pentosaceus. II. Factors controlling the formation of oxidative activities. J Bacteriol. 1962 Oct;84:724–729. doi: 10.1128/jb.84.4.724-729.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., LEVY J. B., GIBOR A. Some enzymatic changes accompanying the shift from anaerobiosis to aerobiosis in Pasteurella pestis. J Bacteriol. 1954 Aug;68(2):178–185. doi: 10.1128/jb.68.2.178-185.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELTON E. A., EVANS J. B., NIVEN C. F., Jr Production of catalase by the pediococci. J Bacteriol. 1953 Apr;65(4):481–482. doi: 10.1128/jb.65.4.481-482.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTEKUNST R. R., DELWICHE E. A., SEELEY H. W. Catalase activity in Pediococcus cerevisiae as related to hydrogen ion activity. J Bacteriol. 1957 Nov;74(5):693–695. doi: 10.1128/jb.74.5.693-695.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George P. A comparison of the decomposition of hydrogen peroxide by catalase, ferrous and ferric ions, haemin and ferrous phthalocyanine. Biochem J. 1948;43(2):287–295. doi: 10.1042/bj0430287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES D., DEIBEL R. H., NIVEN C. F., Jr APPARENT PIGMENT PRODUCTION BY STREPTOCOCCUS FAECALIS IN THE PRESENCE OF METAL IONS. J Bacteriol. 1963 Jul;86:171–172. doi: 10.1128/jb.86.1.171-172.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGSTON C. W., BOUMA C. A study of the microorganisms from grass silage. I. The cocci. Appl Microbiol. 1960 Jul;8:212–222. doi: 10.1128/am.8.4.212-222.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANGSTON C. W., GUTIERREZ J., BOUMA C. Catalase-producing strains of streptococci. J Bacteriol. 1960 Nov;80:693–695. doi: 10.1128/jb.80.5.693-695.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
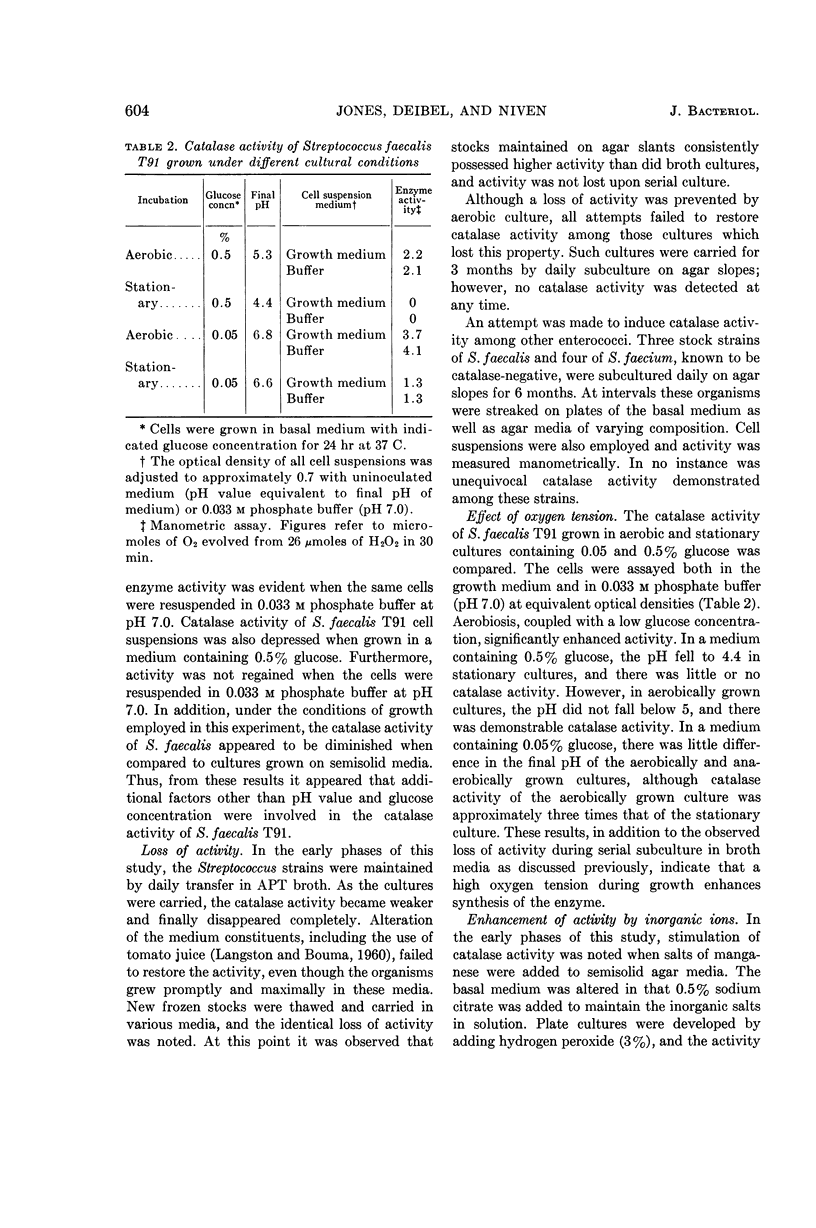
- MELNYKOVYCH G., SNELL E. E. Nutritional requirements for the formation of arginine decarboxylase in Escherichia coli. J Bacteriol. 1958 Nov;76(5):518–523. doi: 10.1128/jb.76.5.518-523.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTENSON L. E., VALENTINE R. C., CARNAHAN J. E. An electron transport factor from Clostridium pasteurianum. Biochem Biophys Res Commun. 1962 Jun 4;7:448–452. doi: 10.1016/0006-291x(62)90333-9. [DOI] [PubMed] [Google Scholar]
- MOSS F. The separation of a cytochrome-containing fraction from Aerobacter aerogenes. Aust J Exp Biol Med Sci. 1954 Aug;32(4):571–575. doi: 10.1038/icb.1954.58. [DOI] [PubMed] [Google Scholar]
- SEELEY H. W., VANDEMARK P. J. An adaptive peroxidation by Streptococcus faecalis. J Bacteriol. 1951 Jan;61(1):27–35. doi: 10.1128/jb.61.1.27-35.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R., NOVELLI G. D., LIPMANN F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J Biol Chem. 1951 Jul;191(1):365–376. [PubMed] [Google Scholar]
- VANKOVA J. Motile catalase-producing strains of Lactobacillus delbrückii. Nature. 1957 Jan 26;179(4552):204–204. doi: 10.1038/179204a0. [DOI] [PubMed] [Google Scholar]
- WHEATER D. M. The characteristics of Lactobacillus plantarum, L. helveticus and L. casei. J Gen Microbiol. 1955 Feb;12(1):133–139. doi: 10.1099/00221287-12-1-133. [DOI] [PubMed] [Google Scholar]
- WHITTENBURY R. Two types of catalase-like activity in lactic acid bacteria. Nature. 1960 Jul 30;187:433–434. doi: 10.1038/187433a0. [DOI] [PubMed] [Google Scholar]



