Abstract
For the nervous system to translate experience into memory and behavior, lasting structural change at synapses must occur. This requirement is clearly evident during critical periods of activity-dependent neural development, and accumulating evidence has established a surprising role for the major histocompatibility complex class I (MHCI) proteins in this process.
During critical periods of activity-dependent neural development, early experience sculpts connections to establish adult circuits via the selection and strengthening of subsets of synapses, combined with weakening and elimination of others. This selection process can even begin well before sensory experience. For example, retinal ganglion cell (RGC) axons from the two eyes are initially intermixed with each other within the lateral geniculate nucleus (LGN) of the thalamus and later sort from each other to achieve the eye-specific and topographically ordered layers of the LGN. Key experiments indicate that appropriate correlated patterns of neural activity are required for segregation (Stellwagen and Shatz, 2002; Torborg and Feller, 2005; Huberman et al, 2008). But just how these patterns of early activity contribute to synapse remodeling and ultimately to lasting structural change remain unclear.
An unbiased screen leads to a surprising discovery
Several years ago, we initiated studies of LGN synapse remodeling motivated by the hypothesis that changes in gene expression are required to translate initial physiological alterations in synaptic strength into stable long term structural changes in axonal branching and connectivity. Quite unexpectedly, reductions in neuronal MHCI (Class I Major Histocompatibility Complex, also known as HLA in humans) mRNA was discovered in an unbiased PCR-based differential screen for gene expression changes in response to blockade of spontaneous neural activity in the developing fetal cat visual system (Corriveau et al, 1998). In these experiments, weblocked pre- and post-synaptic activity by minipump infusions of the sodium channel blocker TTX during the time of synapse remodeling and formation of eye-specific layers in the LGN. Such blockades prevent the formation of the eye-specific layers but allow growth and branching of LGN RGC axons- though in a now unrestricted manner in which eye-specific layers fail to form (Sretavan et al, 1988; Katz and Shatz, 1996). This selective deficit results in mutant mice that have “grossly normal” brain histology and organization, and underscores the importance of studying the detailed patterning of synaptic connections. Indeed, changes in the details of activity-driven synaptic patterning may underlie many cognitive and behavioral disorders ranging from Autism to Schizpohrenia.
Class I MHCs are transmembrane molecules of the Ig Superfamily that comprise a large and highly polymorphic gene family (Maenaka and Jones, 1999; over 50 gene sequences are annotated presently in GenBank), subdivided into “classical” (Class Ia) or “non-classical” (Class Ib; Amadou et al., 1999). The classical MHCI genes are best known for their roles in cellular-mediated immunity, where one of their primary functions is to present antigenic peptides to cytotoxic T-lymphocytes. Loading of foreign peptides, such as those derived from viral infection, into the MHCI cleft triggers cell killing consequent to ligation and signaling by the T Cell receptor (TCR) and a required subunit, CD3 zeta (CD3z; Love et al, 1993; Kane et al, 2000). For cell surface expression, the vast majority of MHCI molecules also require the beta 2 microglobulin (B2m) light chain (Zijlstra et al, 1990). mRNA for B2m is also present in neurons (Corriveau et al, 1998). Cell surface expression of most Class Ia MHCs also requires peptide loading, which occurs in the ER via the TAP1 transporter (e.g. Shastri et al, 2002). Much less is known about the non-classical MHC I genes. Expression is more restricted to specific tissues, they are less polymorphic, and they can be involved in diverse functions ranging from immune function to transferrin receptor trafficking (Shawar et al, 1994).
Our identification of MHCI in our unbiased screen implied an unexpected and novel role for MHC Class I in nervous system development and function. Yet, there had been much controversy over whether or not neurons express MHCI (mRNA or protein). Until relatively recently, it had been thought that, with the exception of damage or viral infection in vivo and/or cytokine stimulation in vitro, neurons did not express MHCI (Lampson, 1995; Joly et al, 1991; Neumann et al, 1997; Rall et al, 1995; Oliveria et al, 2004). These findings have contributed in part to the idea that the brain is “immune-privileged”. Others had argued that neurons express MHCI only when they are electrically silenced (Neumann et al, 1997; Rall et al, 1995)- that is, under pathological conditions. However, it is important to note that in those experiments, fetal hippocampal neurons were dissociated, cultured in vitro and then stimulated to express MHCI with cytokines followed by TTX. In our experiments, we found that MHCI genes are dynamically regulated in neurons in the healthy, unmanipulated brain during development; expression also remains high in specific regions of adult brain (Corriveau et al, 1998; Huh et al, 2000). Following blockade of action potentials, there was a clear decrease in mRNAs encoding MHCI in the LGN (Corriveau et al, 1998, Goddard et al, 2007), which is why we had initially picked up this gene in our unbiased screen. Further, MHCI mRNA can be down-regulated in LGN neurons not only by blocking spontaneous retinal waves early in development, but also simply by occluding normal vision in one eye during early postnatal life. Conversely, following kainate-induced seizures, MHCI mRNA is increased in adult hippocampal neurons (Corriveau et al, 1998). Together, these findings clearly demonstrated that neurons in the healthy brain not only normally express MHCI mRNA, but that expression can be regulated by neural activity and is correlated with times and places of known synaptic plasticity. Thus, MHCIs are excellent candidates for linking neural activity to structural changes at synapses, and imply an unexpected and novel role for MHC Class I in nervous system development and function.
The discovery that neurons normally express MHCI mRNA in vivo without exogenous cytokine stimulation, and that expression is downregulated by activity blockade and upregulated by seizure, is opposite the prior in vitro observations. Many recent publications using more sensitive amplification methods to detect both mRNA and protein have demonstrated directly that neurons in rat, mouse and cat under normal circumstances and in pathogen-free animals express MHCI genes- both classical and nonclassical, as well as B2m (Corriveau et al, 1998; Huh et al, 2000; McConnell et al, 2009; reviewed in Boulanger et al, 2004; see Boulanger Figs. 1, 3 in this issue). Other notable examples include olfactory receptor neurons in the mouse vomeronasal organ where the M10 family of nonclassical MHCI genes are expressed (Loconto et al, 2003; Ishi et al, 2008), motoneurons and substantia nigral neurons (Linda et al, 1999; Thams et al, 2008), and cortical neurons (Miralves et al, 2007). Thus, these in vivo observations extend, and should not be confused with prior in vitro experimental results.
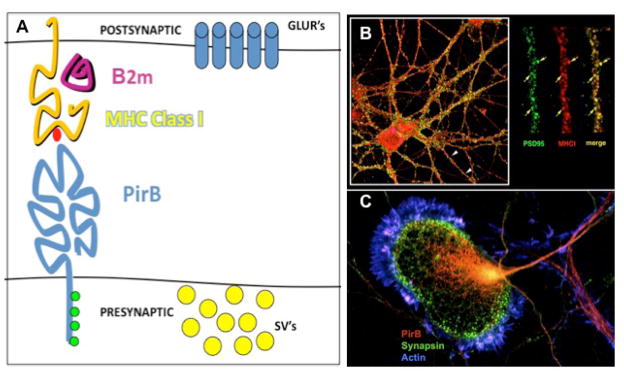
FIGURE 1.
Working model (A) for how MHCI might interact with PirB at or near synapses to regulate plasticity. It is proposed that MHCI is located postsynaptically near glutamate receptors (GLUR’s) based on immunostaining of hippocampal neuron dendrites and colocalization with PSD-95 shown in B. The expression of PirB in axonal growth cones of cortical neurons in vitro (C) suggests a presynaptic location. The iintracellular domain of PirB carries 4 motifs whose phosphorylation (green circles) activates signaling that opposes MAP Kinase and integrin cascades. In this model, PirB would signal when bound to MHCI located across the synapse. Since neural activity regulates MHCI expression levels, PirB could also regulate downstream signaling cascades in an activity-dependent way. (B: modified from Goddard et al, 2007. C: modified from Syken et al, 2006.)
Indirect Evidence for MHCI Function in Synaptic Remodeling and Plasticity
Since there are more than 50 MHCI genes in mice, how can one even begin to examine a requirement for MHCI in the CNS without knowing exactly which ones might be involved? Immunologists have generated mice with greatly diminished levels of most MHCI proteins by deleting beta 2 microglobulin (B2m), a protein required for stable cell surface expression (Dorfman et al, 1997), or by deleting TAP1, a protein required for loading of peptide and proper folding of MHC Class I in the endoplasmic reticulum (Tourne et al, 1996). The initial studies assessing general roles for MHC class I proteins in synapse remodeling and plasticity used mice lacking both of these genes. (Hereafter, referred to as B2m/TAP−/− mice.) We explicitly searched for phenotypes associated with abnormalities of activity-dependent synapse remodeling and plasticity because the original differential display screen demonstrated that activity blockade downregulates MHCI mRNA in the LGN (Corriveau et al, 1998). Thus, we reasoned that mice lacking B2m/TAP−/− might be similar to mice in which neural activity has been blocked if MHCI proteins play a critical role in this process.
Indeed, these mutant mice have defects in developmental remodeling of RGC axons in the LGN that resemble deficits known to result from blocking neural activity. In addition, rules of hippocampal synaptic plasticity in adult mutant mice are shifted: In CA1, long term potentiation (LTP) is enhanced and long term depression (LTD) is absent at typical Schaeffer collateral stimulus frequencies (Huh et al, 2000). The phenotypes of these mice are consistent with the hypothesis that neuronal MHCI proteins regulate synaptic plasticity in the hippocampus, as well as structural regression of synapses in the LGN during development. However, it should be stressed that these conclusions were based on indirect evidence from mice lacking molecules required for the folding and stable cell surface expression of many MHCI proteins, rather than loss or gain-of-function studies of specific MHCI molecules. Clearly, it is now essential to study mice lacking specific MHCI genes, and mice with conditional alleles to examine cell type specificity directly. The field desperately needs these new lines of mice, as well as other reagents including antibodies that can recognize individual MHCI proteins in aldehyde-fixed tissue sections, permitting ultrastructural localization.
Discovery of Neuronal MHCI Receptors
In the immune system, MHCI family members have very short intracellular domains not thought to function in intracellular signaling cascades, but instead by interacting with a variety of receptors during cell-mediated immunity. The most famous immune cell receptor is the T-cell receptor. While our results suggested a role for MHC1 in neuronal plasticity, it was unclear whether neurons actually expressed MHC receptors.. We first examined if neurons express TCR. Although a TCR transcript is selectively expressed and developmentally regulated in cortical layer 6 neurons, no transcribed protein could be detected (Syken and Shatz, 2003), making the TCR an unlikely candidate MHCI receptor. However CD3z, a component of the TCR needed for signaling (Kane et al, 2000), is expressed in the LGN transiently during development and is also expressed in the adult hippocampus (Huh et al, 2000; Baudouin et al, 2008). In mice lacking CD3z, RGC axons fail to remodel in the LGN and LTP is enhanced in hippocampus (Huh et al, 2000), implying potential roles for CD3z-containing MHCI receptor(s) in the CNS.
Another immune receptor thought to bind MHCI is PirB (Paired immunoglobulin-like receptor B), an Ig-like transmembrane receptor expressed on various types of immune cells. PirB was discovered in a search for receptors already known to bind MHCI proteins in the immune system. Studies of B cells in vitro have shown that MHCI proteins can bind and signal via PirB (Takai, 2005). Signaling through PirB is dependent on four tyrosines located within distinct immunoreceptor tyrosine-based inhibitory motifs (ITIM) in the intracellular domain, and in immune cells activation of PirB is thought to antagonize integrin and MAP Kinase signaling cascades (Takai, 2005). Our in situ hybridization screen revealed that PirB mRNA is highly expressed in certain regions of mouse CNS, particularly in neurons of cerebral cortex, olfactory bulb and granule cells in cerebellum. PirB protein is located in growth cones and axons of cerebral cortical neurons in vitro (Syken et al, 2006). Notably, expression of PirB was not detected in the LGN during the period of activity-dependent synapse remodeling, implying that other (possibly CD3 zeta-containing) MHCI receptors might be present. Indeed, several labs have recently added other innate immune receptors to the list of potential neuronal MHCI receptors. For example, Ly49, a member of the NK (natural killer) family of innate immune receptors (Zohar et al, 2008), as well as KIR (killer cell immunoglobulin-like receptor; Bryceson et al, 2005), have been observed in a variety of CNS neurons.
In the absence of functional PirB, the brains of mutant mice are grossly normal (Syken et al, 2006). Since PirB mRNA is highly expressed in cortex (but not LGN), we examined if the ocular dominance of neurons in primary visual cortex is normal by using a convenient immediate early gene induction method for Arc mRNA to map functionally inputs from each eye to cortical neurons (Tagawa et al, 2005). While the initial development of eye input to cortex occurs normally in PirB−/− mutant mice, ocular dominance (OD) plasticity is far from normal. Following monocular enucleation or monocular visual deprivation in PirB−/− mice during the critical period, OD plasticity is significantly enhanced over WT (Syken et al, 2006). Enhanced OD plasticity is even apparent in adulthood. Once again, these results stress the need to assess detailed aspects of synaptic patterning and activity when examining mutant phenotypes. Importantly, there are very few examples where loss of gene function results in enhanced plasticity; Nogo Receptor (NgR) mutant mice have a OD plasticity phenotype in adult visual cortex similar to that of PirB mutant mice (McGee et al., 2005). Recently, in a most unexpected intersection of research on axonal regeneration with that on neural plasticity, PirB was also found to bind Nogo peptide and to regulate growth cone inhibition on myelin substrates (Atwal et al, 2008), implying that in some instances PirB and NgR may function in a complex that also includes Nogo peptide. While these results are intriguing, there are a number of key unanswered questions including whether growth cone inhibition also requires the participation of MHCI proteins. It is also essential to determine if MHCI mutant mice have phenotypes similar to those of PirB−/− and/or NgR−/− mice. Similar neuronal phenotypes would be consistent with PirB acting as a receptor for neuronal MHCI.
A Working Model for MHCI Function in Healthy Neurons
Similarities in synaptic plasticity phenotypes would lend support to a working model of neuronal MHCI interacting and signaling via PirB or other immune receptors expressed on neurons. Based on observations of MHCI immunostaining localized to the postsynaptic densities and dendrites of hippocampal neurons in culture (Goddard et al, 2007) and Purkinje cell proximal dendrites in fixed tissue sections (McConnell et al, 2009), a first iteration model would suggest that MHCI proteins are located postsynaptically at or near synapses (Fig. 1). MHCI might then interact across the synapse with immune receptors such as PirB, located presynaptically based on the observation that PirB immunostaining is localized near synapsin- positive vesicles in the growth cones of cortical neurons in culture (Syken et al, 2006). PirB signaling activates SHP-1,2 phosphatases in neurons (Syken et al, 2006) as well as in immune cells, where PirB signaling is also known to oppose MAP Kinase and Integrin signaling (Takai, 2005) pathways also involved in long term synaptic plasticity and OD plasticity (Barco et al, 2005; Hensch, 2004; Taha and Stryker, 2005). MHCI levels are decreased by activity-blockade (Corriveau et al, 1998; Goddard et al, 2007) and have recently been shown to increase in transgenic mice expressing a constitutively active form of CREB (Barco et al, 2005). Thus it is possible that MHCI acts downstream of synaptic activity, changes in intracellular Ca++ and CREB, to regulate the degree and possibly the sign of synaptic plasticity. Note that this is not to imply that MHCI molecules are acting as cell-type specific markers (such as eye-specific markers in the LGN). Indeed, the normal expression patterns, along with the phenotypes seen in mutant mice, do not seem consistent with this idea. However another, not mutually exclusive, possibility is that MHCI molecules could alter trafficking of glutamate receptors by acting in cis as “chaperones”, based on analogy with certain nonclassical MHCIs and their role in trafficking of transferrin receptors (Bennett et al, 2000). Clearly the unresolved question of how MHCI signals in neurons is a key to understanding the function of this large gene family. Given examples in the immune system, there could be multiple modes of signaling in the CNS as well.
MHCI Function and Dysfunction in the CNS
These are early days for newly proposed roles of MHCI in neurons. Consequently few labs have considered whether or how dysregulation of MHCI in the CNS might contribute to pathology. Since the initial report of MHCI expression and activity-regulation in healthy neurons (Corriveau et al, 1998), fascinating hints about MHCI function in the context of synapse plasticity, learning and memory have come to light. For example, work from the Kandel lab (Barco et al; 2005) demonstrated that the same set of MHCI genes known to be expressed in normal hippocampal neurons (Huh et al 2000) are also regulated by CREB. Transgenic mice that express CREB in the hippocampus under a constitutive (VP16) promoter have highly elevated mRNAs for H2-K, H2-D, among other MHCIs, implying that these may be part of a process whereby LTP is read out. Screens for dendritic mRNAs in hippocampal neurons have identified MHCIs (Zhong et al, 2006), and MHCI mRNAs are reported to be enriched over 4-fold in the FMRP-mRNP complex in dendrites (Brown et al, 2001). These observations suggest that MHCI could be synthesized locally in dendrites and regulated by Fragile-X protein. Mouse models of Fragile X have alterations in hippocampal synaptic plasticity, and OD plasticity in visual cortex (Huber et al, 2002; Dolen et al, 2007) related to those seen in B2m/TAP−/− and PirB−/− mutant mice. MHCI protein may be upregulated in cortex of mice expressing the mutant form of MeCP2 that is known to be involved in the pathogenesis of Rett Syndrome (Miralves et al, 2007). It is even conceivable that altered MHCI expression contributes to synaptic changes and learning defects in Fragile-X and Rett (Moretti et al, 2006; Chahrour et al, 2008). In this regard, it will be important to determine whether or not mice lacking MHCI function also have changes in behaviors related to learning and memory. It is already known that mice lacking expression of just two of the more that 60 MHCI molecules (H2-K and H-2D) have enhanced motor learning on the rotarod, as well as lower threshold LTD in the cerebellum (McConnell et al, 2009). Given these considerations, it is possible to imagine that altered MHCI function in the human brain could also result in changes in learning and memory, some of which could even result in enhancements to behavior and cognition.
The presence of MHCI in neurons also suggests new ways to understand and ultimately to treat neurological and psychiatric disorders including those with known autoimmune components such as Multiple Sclerosis (Bhat and Steinman, this issue). It is also known that exogenous cytokines such as TNF alpha can alter MHCI cell surface expression on neurons (Neumann et al, 1997), and recent elegant studies have now demonstrated a normal role for cytokines such as TNF alpha in LTD (Beattie et al, 2002) and OD plasticity in vivo (Kaneko et al, 2008). Thus, damage and inflammation might lead to changes in synaptic plasticity and memory function via dysregulation of MHCI expression. In a series of 3 genome-wide studies of large populations published recently (Shi et al, 2009; Stefansson et al, 2009; International Schizophrenia Consortium et al, 2009), polygenic variations on human chromosome 6p22.1 at the MHCI locus have now been implicated in Schizophrenia and Bipolar Disorder. While many common gene variants in the MHC region (both Class I and Class II) are strongly associated with Schizophrenia and Bipolar disorder, there was no association with several non-psychiatric disorders. The authors of the studies related their observations regarding the MHCI region to the popular idea that there is a link between Schizophrenia and infection or autoimmunity (Patterson, 2009). While this proposal is reasonable, a burning question remains: how can early infection or autoimmune disorders change brain circuits and behavior? If neuronal MHCI indeed functions at synapses, then the immune system would have a variety of rather direct ways of communicating with and altering activity-dependent synaptic plasticity and circuit tuning. Major challenges for the future will be not only to identify MHCI molecules and receptors in the human brain, but also to understand how the extraordinary polymorphism at the MHCI locus contributes to brain function and dysfunction.
Acknowledgments
I wish to thank the many members of my lab both past and present for their contributions to work cited here, which was supported by NIH R01 EY02858, NIH R01 MH071666, the G. Harold and Leila Y. Mathers Charitable Foundation, and the Dana Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amadou C, Kumanovics A, Jones EP, Lambracht-Washington D, Yoshino M, Lindahl KF. The mouse major histocompatibility complex: some assembly required. Immunological Rev. 1999;167:211–221. doi: 10.1111/j.1600-065x.1999.tb01394.x. [DOI] [PubMed] [Google Scholar]
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz CJ, Tessier-Lavigne MT. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005 Oct 6;48(1):123–37. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Baudouin SJ, Angibaud J, Loussouarn G, Bonnamain V, Matsuura A, Kinebuchi M, Naveilhan P, Boudin H. The signaling adaptor protein CD3zeta is a negative regulator of dendrite development in young neurons. Mol Biol Cell. 2008;19:2444–2456. doi: 10.1091/mbc.E07-09-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNF-alpha. Science. 2002 Mar 22;295(5563):2282–5. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Lebron JA, Bjorkman PJ. Crystal structure of the hereditary hemochromatosisprotein HFE complexed with the transferrin receptor. Nature. 2000;403:46–53. doi: 10.1038/47417. [DOI] [PubMed] [Google Scholar]
- Bhat and Steinman, this issue.
- Brown V, Jin P, Ceman S, Darnell JC, O’Donnell WT, Tenenbaum SA, Jin X, Feng Y, Wilkinson KD, Keene JD, Darnell RB, Warren ST. Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome. Cell. 2001 Nov 16;107(4):477–87. doi: 10.1016/s0092-8674(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Boulanger, this issue.
- Boulanger LM, Shatz CJ. Immune Signaling in Neural Development, Synaptic Plasticity, and Disease. Nature Reviews Neurosci. 2004;5:521–531. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- Bryceson YT, Foster JA, Kuppusamy SP, Herkenham M, Long EO. Expression of a killer cell receptor-like gene in plastic regions of the central nervous system. J Neuroimmunol. 2005 Apr;161(1–2):177–82. doi: 10.1016/j.jneuroim.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Butts DA, Kanold PO, Shatz CJ. A burst-based ‘Hebbian’ learning rule at retinogeniculate synapses links retinal waves to activity dependent refinement. PLoS Biology. 2007;5:651–661. doi: 10.1371/journal.pbio.0050061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008 May 30;320(5880):1224–9. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corriveau RA, Huh GS, Shatz CJ. Regulation of Class I MHC gene expression in the developing and mature CNS by neural activity. Neuron. 1998;21:505–520. doi: 10.1016/s0896-6273(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Dölen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007 Dec 20;56(6):955–62. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman JR, Zerrahn J, Coles MC, Raulet DH. The basis for self-tolerance of natural killer cells in beta2-microglobulin- and TAP-1- mice. J Immunol. 1997;159:5219–5225. [PubMed] [Google Scholar]
- Dustin ML, Colman DR. Neural and immunological synaptic relations. Science. 2002 Oct 25;298(5594):785–9. doi: 10.1126/science.1076386. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Butts D, Shatz CJ. Regulation of CNS synapses by neuronal MHC Class I. PNAS. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–79. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc Natl Acad Sci U S A. 2002 May 28;99(11):7746–50. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh GS, Boulanger, Du HLM, Riquelme P, Brotz TM, Shatz CJ. Functional requirement for Class I MHC in CNS development and plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Schizophrenia Consortium. Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Mombaerts P. Expression of nonclassical class I major histocompatibility genes defines a tripartite organization of the mouse vomeronasal system. J Neurosci. 2008 Mar 5;28(10):2332–41. doi: 10.1523/JNEUROSCI.4807-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly E, Mucke L, Oldstone MBA. Viral persistence in neurons explained by lack of major histocompatibility class I expression. Science. 1991;253:1283–1285. doi: 10.1126/science.1891717. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kane LP, Lin J, Weiss A. Signal transduction by the TCR for antigen. Curr Opin Immunol. 2000;12:242–249. doi: 10.1016/s0952-7915(00)00083-2. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008 Jun 12;58(5):673–80. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson LA. Interpreting MHC class I expression and class I/class II reciprocity in the CNS: reconciling divergent findings. Microsc Res Tech. 1995;32:267–285. doi: 10.1002/jemt.1070320402. [DOI] [PubMed] [Google Scholar]
- Linda H, Hammarberg H, Piehl F, Khandemi M, Olsson T. Expression of MHC Class I heavy chain and B2 microglobulin in rat brainstem motoneurons and nigral dopaminergic neurons. J Neuroimmunol. 1999;101:76–86. doi: 10.1016/s0165-5728(99)00135-6. [DOI] [PubMed] [Google Scholar]
- Loconto J, et al. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112:607–18. doi: 10.1016/s0092-8674(03)00153-3. [DOI] [PubMed] [Google Scholar]
- Maenaka K, Jones EY. MHC superfamily structure and the immune system. Curr Opin Struct Biol. 1999;9:745–753. doi: 10.1016/s0959-440x(99)00039-1. [DOI] [PubMed] [Google Scholar]
- McConnell MJ, Huang YH, Datwani A, Shatz CJ. H2-Kb and H2-Db regulate cerebellar long term depression and limit motor learning. PNAS. 2009;106:6784–6789. doi: 10.1073/pnas.0902018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309(5744):2222–6. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralvès J, Magdeleine E, Kaddoum L, Brun H, Peries S, Joly E. High levels of MeCP2 depress MHC class I expression in neuronal cells. PLoS ONE. 2007 Dec 26;2(12):e1354. doi: 10.1371/journal.pone.0001354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006 Jan 4;26(1):319–27. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann H, Schmidt H, Cavalie A, Jenne D, Wekerle H. Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-gamma and tumor necrosis factor (TNF)-alpha. J Exp Med. 1997;185:305–316. doi: 10.1084/jem.185.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AL, Thams S, Lidman O, Piehl F, Hökfelt T, Kärre K, Lindå H, Cullheim S. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc Natl Acad Sci U S A. 2004 Dec 21;101(51):17843–8. doi: 10.1073/pnas.0408154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parachikova A, Agadjanyan MG, Cribbs DH, Blurton-Jones M, Perreau V, Rogers J, Beach TG, Cotman CW. Inflammatory changes parallel the early stages of Alzheimer disease. Neurobiol Aging. 2007 Dec;28(12):1821–33. doi: 10.1016/j.neurobiolaging.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005 Dec;17(6):485–95. doi: 10.1080/02646830500381930. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Rall GF, Mucke L, Oldstone MB. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J Exp Med. 1995;182:1201–1212. doi: 10.1084/jem.182.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Levinson DF, Duan J, Sanders AR, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawar SM, Vyas JM, Rodgers JR, Rich RR. Antigen presentation by major histocompatibility complex class I-b molecules. Ann Rev Immunol. 1994;12:839–880. doi: 10.1146/annurev.iy.12.040194.004203. [DOI] [PubMed] [Google Scholar]
- Shastri N, Schwab S, Serwold T. Producing nature’s gene chips: the generation of peptides for display by MHC Class I molecules. Ann Rev Immunol. 2002;20:463–493. doi: 10.1146/annurev.immunol.20.100301.064819. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Shatz CJ. An instructive role for retinal waves in the development of retinogeniculate connectivity. Neuron. 2002;33:357–367. doi: 10.1016/s0896-6273(02)00577-9. [DOI] [PubMed] [Google Scholar]
- Syken J, Shatz CJ. Expression of T Cell Receptor Beta Locus in CNS neurons. PNAS. 2003;100:13048–13053. doi: 10.1073/pnas.1735415100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, Shatz CJ. PIRB restricts ocular dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- Sretavan DW, Shatz CJ, Stryker MP. Modification of retinal ganglion cell axon morphology by prenatal infusion of tetrodotoxin. Nature. 1988;336:468–471. doi: 10.1038/336468a0. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Ophoff RA, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagawa Y, Kanold PO, Majdan M, Shatz CJ. Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nature Neuroscience. 2005;3:380–388. doi: 10.1038/nn1410. [DOI] [PubMed] [Google Scholar]
- Taha SA, Stryker MP. Molecular substrates of plasticity in the developing visual cortex. Prog Brain Res. 2005;147:103–114. doi: 10.1016/S0079-6123(04)47008-3. [DOI] [PubMed] [Google Scholar]
- Takai T. Paired immunoglobulin-like receptors and their MHC class I recognition. Immunology. 2005;115:433–440. doi: 10.1111/j.1365-2567.2005.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thams S, Oliveira A, Cullheim S. MHC class I expression and synaptic plasticity after nerve lesion. Brain Res Rev. 2008;57:265–269. doi: 10.1016/j.brainresrev.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Torborg CL, Feller MB. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog Neurobiol. 2005 Jul;76(4):213–35. doi: 10.1016/j.pneurobio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Tourne S, van Santen HM, van Roon M, Berns A, Benoist C, Mathis D, Ploegh H. Biosynthesis of major histocompatibility complex molecules and generation of T cells in TAP1 double-mutant mice. Proc Natl Acad Sci USA. 1996;93:1464–1469. doi: 10.1073/pnas.93.4.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohar O, Reiter Y, Bennink JR, Lev A, Cavallaro S, Paratore S, Pick CG, Brooker G, Yewdell JW. Cutting edge: MHC class I-Ly49 interaction regulates neuronal function. J Immunol. 2008 May 15;180(10):6447–51. doi: 10.4049/jimmunol.180.10.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Zhang T, Bloch LM. Dendritic mRNAs encode diversified functionalities in hippocampal pyramidal neurons. BMC Neurosci. 2006;7:17–31. doi: 10.1186/1471-2202-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M, Bix M, Simister NE, Loring JM, Raulet DH, Jaenish R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990;344:742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]