Abstract
Background
Adipose tissue is a readily available source of multipotent adult stem cells for use in tissue engineering/regenerative medicine. Various growth factors have been used to stimulate acquisition of endothelial characteristics by adipose-derived stem cells (ASC). Herein we study the effects of endothelial cell growth supplement (ECGS) and physiologic shear force on the differentiation of ASC into endothelial cells.
Methods
Human ASC (CD13+29+90+31−45−) were isolated from peri-umbilical fat, cultured in ECGS media (for up to three weeks) and exposed to physiological shear force (12 dynes for up to eight days) in vitro. Endothelial phenotype was defined by cord formation on Matrigel, acetylated-LDL (acLDL) uptake, and expression of nitric oxide synthase (eNOS), von Willebrand factor (vWF), and CD31 (PECAM). Additionally, cell thrombogenicity was evaluated by seeding canine autologous ASC onto vascular grafts implanted within the canine arterial circulation for two weeks.
Results
We found that undifferentiated ASC did not display any of the noted endothelial characteristics. After culture in ECGS, ASC formed cords in Matrigel, but failed to take up acLDL or express the molecular markers. Subsequent exposure to shear resulted in stem cell realignment, acLDL uptake and expression of CD31; eNOS and vWF expression was still not observed. Grafts seeded with cells grown in ECGS (±shear) remained patent (six of seven) at two weeks but had a thin coat of fibrin along the luminal surfaces.
Conclusions
This study suggests that: 1) ECGS and shear promote the expression of several endothelial characteristics in human adipose-derived stem cells, but not eNOS or vWF, 2) their combined effects appear synergistic, and 3) stem cells differentiated in ECGS appear mildly thrombogenic in vivo, possibly related, in part, to insufficient eNOS expression. Thus, while the acquisition of several endothelial characteristics by adult stem cells derived from adipose tissue suggests these cells are a viable source of autologous cells for cardiovascular regeneration, further stimulation/modifications are necessary prior to using them as a true endothelial cell replacement.
Introduction
Adult stem cells represent a source of autologous cells for cardiovascular therapy. These cells have been evaluated for the treatment of myocardial infarction1,2 and peripheral vascular disease,3,4 and used to create tissue-engineered vascular grafts.5,6 Adult stem cells are typically isolated from bone marrow or circulating blood; however, availability of cells from these sources is limited by advanced patient age and co-morbidity,7 highlighting the need for alternate tissue sources. Adipose tissue is an easily obtainable tissue from which stem cells are isolated in abundance. Our recent evaluation of adipose-derived stem cells (ASC) in patients with vascular disease demonstrated that advanced age, gender, obesity, renal failure and vascular disease does not alter isolation efficacy,8 suggesting autologous ASC would be obtainable in the patient population most like to benefit from cardiovascular stem cell therapies.
This study examines the ability of human ASC to differentiate into endothelial cells, i.e. cells whose phenotypes would be useful for regenerative medicine purposes. Given the possible future translational value to this technology, particular attention is paid to the isolation process of these adult stem cells. First, cells isolated from the adipose-tissue of patients undergoing vascular surgical procedures are examined. This differs from much of the current literature on ASC, which are typically isolated from young, healthy patients undergoing plastic surgical procedures. Second, isolated ASC that have undergone an additional purification step (negative selection for CD31+ and CD45+ cells to remove co-cultured microvascular endothelial and monocytes cells) are studied. This step allows the changes noted in the stem cells to be ascribed to differentiation rather than a selection bias.
We specifically evaluate the effects of endothelial cell growth supplement (ECGS) and shear force. ECGS has a long-recognized and important role in maintaining the viability and proliferation of differentiated endothelial cells in culture.9,10 Similarly, as the natural environment of differentiated endothelial cells, shear has well-known effects on endothelial cell function11–13 and has been used to stimulate differentiation in other stem cells towards endothelium.14–16
This study tests the hypothesis that ECGS and shear stimulate the acquisition of endothelial characteristics in stem cells derived from adipose tissue. Human ASC isolated from patients undergoing vascular surgical procedures were grown in culture medium containing ECGS and exposed to physiological shear. Phenotypic characteristics examined included both functional and molecular markers. Furthermore, we evaluated the thrombogenicity of stem cells isolated from adipose-tissue in vivo using a canine model. The results of this study aid in defining a potential role for adipose-derived stem cells in regenerative cardiovascular medicine.
Methods and Materials
Thomas Jefferson University’s IRB and IACU approved each of these human and animal studies. Each human subject gave informed consent, and all animal care complied with the Guide for the Care and Use of Laboratory Animals.17
1. ASC isolation and culture
Stem cells were isolated from human and canine adipose tissue. Peri-umbilical fat was donated by six patients undergoing elective vascular surgery (four bypass procedures, two amputations; three males; age 53±7 years; body mass index = 27±6; four diabetics); after instillation of tumescence solution, 14±7 gm of fat was removed by liposuction. Canine tissue was collected from the falciform ligament of one-year-old female mongrel dogs (Marshall Farms USA, Inc., North Rose, NY) weighing 20–25 kg (n=8); following general anesthesia induced by Propofol (10 mg/kg IV) and maintained with isoflurane (2–4% via endotracheal intubation), 10 cm of ligament was removed though abdominal incision and minced. Following collection, human and canine specimens were similarly digested in crude collagenase (Worthington Biochemical, Lakewood, NJ; 4mg/gm of fat) × 1 h at 37°C and centrifuged at 300g × 5 min. After discarding the supernatant, the stromal-vascular pellet (168,000±131,000 cells/gm of fat) was suspended in non-differentiating media consisting of Media-199 (Mediatech, Herndon, VA) supplemented with fetal bovine serum (13%; Gemini Bio-Products, West Sacramento, CA), antibiotics (12 mL/L; Mediatech) and heparin (7.5 U/mL; American Pharmaceutical Partners, Schaumberg, IL) and plated on gelatin-coated culture flasks (Corning, Corning, NY). After 8±3 days in culture at 37°C, 5% CO2, the adherent cells (passage 0) underwent negative selection using magnetic beads (MACS, Miltenyi Biotec, Auburn, CA) to remove contaminating endothelial (CD31+) and mononuclear (CD45+) cells. Briefly, cells were released by trypsin and centrifuged at 300g. The pellet was suspended in 2mM EDTA and 0.5% BSA (MACS buffer), mixed with CD45-tagged microbeads and incubated at 4°C × 15 min. The cells were centrifuged, suspended in MACS Buffer and passed through an LS Separation Column mounted in the QuadroMACS separator magnetic unit. The effluent underwent repeat procedure using CD31-tagged microbeads. The resultant CD31−45− cell population defined the adipose-derived stem cells.
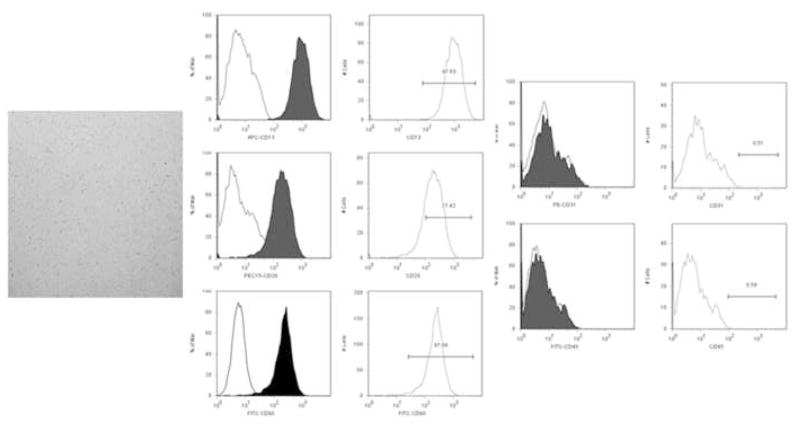
To assess stem cell markers and the effectiveness of the negative selection, isolates underwent FACS analysis for CD13, 29, 31, 45, and 90. CD 13, 29 and 90 are known markers associated with adipose-derived stem cells.18 Cultured cells were released with 0.25% trypsin/EDTA (Mediatech), washed and suspended in 0.1% BSA. Individually, primary antibody (APC-anti-human CD13, PE-Cy5-anti-human CD29, PE-anti-human CD31, FITC-anti-human CD45, FITC-anti-human CD90 (BD Pharmingen, San Jose, CA)) was added at 1:100 dilution and incubated × 20min at 37°C, 5% CO2. Samples were analyzed using a Dako MoFlo Cell Sorter equipped with a 488nm Argon laser, using Summit v.4 software (Dako, Fort Collins, CO). Analysis revealed the ASC cells were positive for CD13, 29 and 90; importantly, the cultures were devoid of CD31 and CD45 cells (≤0.6%)(Figure 1).
Figure 1. FACS analysis of human adipose-derived stem cells.
Photomicrograph of stem cells immediately after isolation, culture and negative selection for CD31 and 45 (phase-contrast, 40x) demonstrating homogeneous, spindle-shaped morphology. Representative FACS analysis of an isolate used in this study illustrates expression of the mesenchymal stem cells markers CD13, 29 and 90. Negative selection successfully removed co-isolated CD31 (endothelial) and CD45 (mononuclear) cells, allowing for the conclusion that subsequent expression of endothelial characteristics were due to stem cell differentiation and not culture selection.
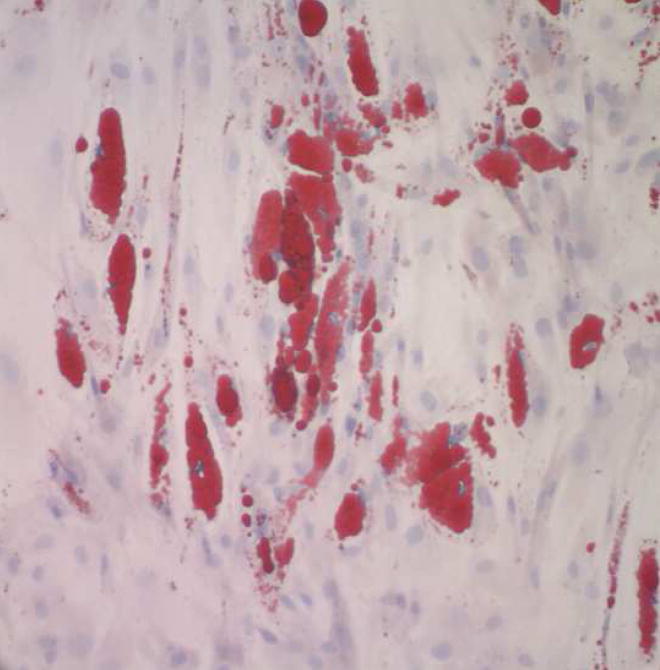
To demonstrate that the cells isolated in this study are indeed multi-potent, we elected to demonstrate differentiation into a second cell type, namely mature adipocytes. Human ASC (n=3 lines, passage 3) were grown in Adipogenic Differentiation Medium (Cambrex Bioscience, Walkersville, MD) per the manufacturer’s instructions, unmodified by the authors. After 3.5 weeks, cultures were stained with Oil Red O, counterstained with Modified Mayer’s hematoxylin, and observed with light microscopy (Figure 2). At this time point, approximately one half of the ASC accumulated lipid droplets, indicating differentiation into adipocytes.
Figure 2. Differentiation of human adipose-derived stem cells into adipocytes.
Photomicrograph of stem cells grown for 3.5 weeks (passage 3) in media promoting adipogenic differentiation (Oil Red O with Modified Mayer’s hematoxylin counter stain; 200x) reveals uptake of lipid within the cells. This finding demonstrates that cells used in this study are capable of differentiating into a cell line different than endothelial cells.
2.a. Stimulation of differentiation with ECGS
Following ASC isolation (including negative selection), Endothelial Cell Growth Supplement (Upstate Biotechnology, Lake Placid, NY; 50 μg/mL) derived from bovine hypothalamus was added to the culture medium. Cultures were fed fresh media twice weekly and split 1:3 when 80% confluent. Passages one (one week in medium) through five (three weeks in medium) were used for experimentation and analysis.
2.b. Stimulation of differentiation with shear force
Shear force was applied to cell cultures using an orbital shaker as described by Dardik.19 Cells were plated onto gelatin-coated 6-well plates at 5×104 cells/cm2. After one day in static culture, plates seated on an orbital shaker (Bellco Biotechnology, Vineland, NJ) maintained at 37°C, 5% CO2 were rotated at 210 cycles/min producing 12 dynes at the periphery of the wells, representing the average shear noted within human common femoral artery.20,21
3. Evaluation of endothelial characteristics
Differentiation was determined by response to extracellular matrix, acLDL uptake, expression of endothelial cell molecules (eNOS, vWF, CD31), and inspection of vascular grafts seeded with ASC. Human dermal microvessel endothelial cells (Cell Applications, San Diego, CA) and aortic smooth muscle cells were used as positive and negative controls, respectively.
3.a. Response to extracellular matrix
Twenty four-well plates chilled at 4°C were loaded with 100 μL Matrigel (BD Biosciences, San Jose, CA) in each well. The plate was incubated at 37°C × 30 min to set the Matrigel. Cells (undifferentiated ASC or differentiated ASC or endothelial cell controls; 100,000 cells/well; n=3 each group) were seeded onto the surface of the Matrigel in each well. After 24h of culture, three randomly selected fields (100x magnification) were photographed with a phase-contrast microscopy; subsequently, three blinded reviewers counted all of the branch points and branches within each photograph. The scores of each reviewer for each photograph were averaged for each specimen (n=3 for each cell line).
3.b. acLDL uptake
Cultured cells were plated on gelatin-coated 6-well plates at 5×105cells/cm2. After one day in culture, cells were washed with PBS and incubated with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled acetylated LDL (Biomedical Technologies, Stoughton, MA, 2 μg/mL) × 4 h at 37°C, 5% CO2. Cells were washed with phenol red-free M199 and uptake assessed qualitatively with an inverted fluorescent microscope.
3.c. Expression of endothelial molecules
The presence of molecular markers was detected by RT-PCR, immunoblot and immunohistochemistry.
3.c.i. RT-PCR
Total RNA was isolated from cultured cells using the RNeasy Mini Kit (Qiagen, Valencia, CA) and quantified using spectrophotometry. RT reaction was performed using the Promega Reverse Transcription System (Madison, WI) using 1 mg total RNA. RT-PCR was performed with primer pairs for CD31 (forward 5′-ACTGGACAAGAAAGA GGCCAT CCA-3′, reverse 5′-TCCTTCTGGATG GTGAAGTTGGCT-3′), vWF (forward 5′-ACTCAG TGCATTGGTGAGGATGGA-3′, reverse 5′-TCGGACACACTC ATTGAT GAGGCA-3′), and eNOS (forward 5′-AGATGTTCCAGGCTACAATCC GCT-3′, reverse 5′-TGTATGCCAGCACAG CTACAGTGA-3′). Electrophoresis was performed on 1% agarose gel treated with ethidium bromide and visualized using UV light.
3.c.ii. Immunoblot
Total protein was extracted from cultured cells using an SDS-derived buffer and quantified using the BCA Protein Assay (Pierce Biotechnology Inc., Rockford, IL). Proteins were separated on an 8% tris-glycine gel (Invitrogen, Carlsbad, CA) and transferred to a nitrocellulose membrane. Membranes were blocked with 5% nonfat milk/0.1% Tween20 and incubated with primary antibody × 1 h in 5% nonfat milk/0.1% Tween 20. Bound primary antibody was labeled with bovine anti-goat HRP (Santa Cruz) × 45 min in 5% nonfat milk/0.1% Tween 20. Signals were detected with enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
3.c.iii. Immunohistochemistry
Cultured cells were incubated with 1% BSA × 30 min at 37°C to prevent non-specific binding of antibody. Cells were washed with 0.1% BSA and incubated with 5 μL PE anti-human CD31 (BD Pharmingen) per 1 mL 0.1% BSA × 20 min at 37°C. Cells were washed of unbound antibody with 0.1% BSA and assessed qualitatively with an inverted fluorescent microscope.
3.d. In vivo evaluation of stem cell thrombogenicity
Vascular grafts seeded with differentiated autologous canine ASC were implanted into the carotid circulation of canine recipient animals for a period of two weeks. Subsequent evaluation included duplex ultrasound and histological examination.
A total of eight one-year-old female mongrel dogs (Marshall Farms USA, Inc., North Rose, NY) weighing 20–25kg were used to create and implant tissue-engineered grafts composed of ASC. Autologous canine ASC were isolated as above and cultured in media containing ECGS for two weeks. Differentiated canine ASC formed cords on Matrigel and re-aligned with flow similar to human cells (data not shown). Within a vascular graft bioreactor chamber (Tissue Growth Technologies, Minnetonka, MN), ASC (2×105 cells/cm2) were seeded onto the luminal surface of canine venous allografts (5 mm average diameter, 5 cm length) that had been decellularized with 0.075% sodium dodecyl sulfate as previous described22 and pre-coated with fibronectin (BD Biosciences; Bedford, MA). The grafts were rotated 90° every 15min × 1h after which luminal flow of media was begun at 3 ml/h × 24 h (<0.1 dyne). These statically seeded grafts (n=4) were then implanted as below. Three additional grafts, created in the same fashion, underwent flow conditioning wherein the luminal flow of media was increased incrementally by a roller pump from 0 to 12 dyne over 4 days. The measured pressures within the graft throughout this period did not exceed 30mmHg. Unseeded control grafts (n=7) for both groups underwent “sham” seeding under static conditions and remained in the identical culture media for the same period of time as the experiment grafts; they did not, however, undergo flow conditioning. Two grafts (one control, one experimental) were lost to infection in the flow conditioned group.
Tissue-engineered grafts were implanted into the carotid circulation of recipient animals such that each animal received an unseeded control graft and one seeded with autologous, differentiated ASC. Under general anesthesia the carotid arteries were dissected bilaterally through a mid-line cervical incision. The animals were systemically heparinized (100 U/kg IV) and the carotid arteries clamped proximally and distally. Four cm of intervening carotid artery was excised; the grafts were spatulated and sutured as interposition grafts using running 6-0 polypropylene suture. The heparin was reversed with Protamine (1 mg/kg IV), the incision closed, and the animal recovered. Aspirin (81 mg po daily) was begun one day pre-operatively and maintained throughout the study. After two weeks, under general anesthesia, duplex ultrasound was used to determine graft patency. The cervical incision was opened and the carotid arteries proximal and distal to the grafts were isolated and cannulated. The grafts were perfusion fixed in 4% formaldehyde (30 min at 150 mmHg), excised and further fixed overnight at 4°C. The grafts were opened longitudinally for gross examination; mid-graft specimens were cut transversely and submitted for histological analysis.
Results
ECGS stimulates cord formation, but not molecular markers
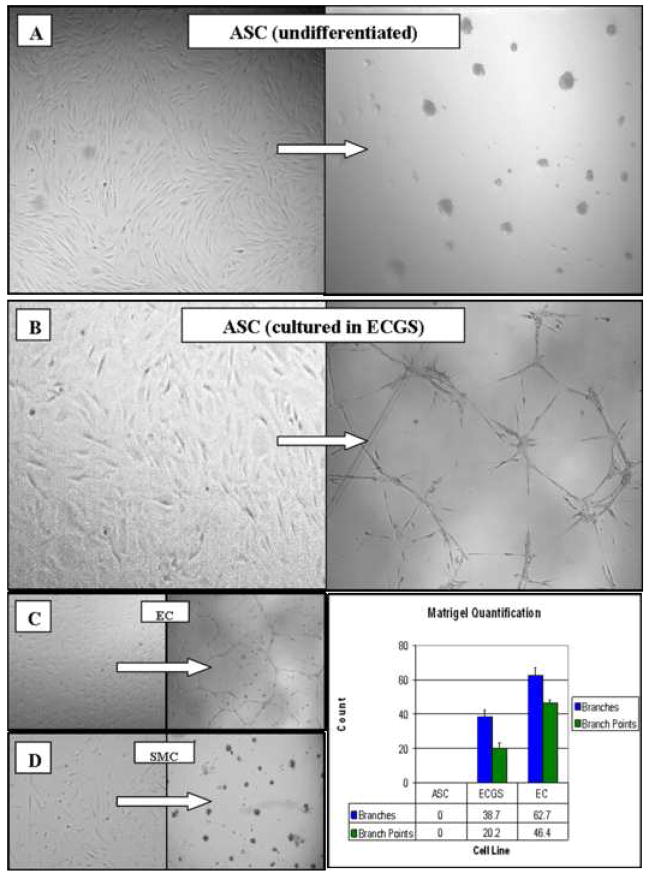
Prior to culture in differentiating medium, the stem cells did not form cords on Matrigel, uptake acLDL, or express molecular markers (eNOS, vWF, CD31). After seven days in medium containing ECGS, ASC formed cord networks when plated onto Matrigel for 24h, but not as robustly when compared to mature endothelial cell controls (Figure 3). ASC cultured for up to three weeks in ECGS did not uptake acLDL significantly nor express the molecular markers by RT-PCR (data not shown).
Figure 3. Effect of ECGS on the differentiation of human adipose-derived stem cells (ASC).
Photomicrographs of cells plated onto Matrigel (left panels) and subsequently cultured for 24 h (right panels; phase-contrast, 100x). Undifferentiated stem cells (A), naïve to ECGS, recoil and ball up in response to Matrigel, similar to smooth muscle cell (SMC) controls (D). In contrast, stem cells grown in ECGS for a minimum of 7 days (B) form cord-like structures similar to the endothelial cell (EC) controls (C).
Shear force enhances CD31 expression and acLDL uptake in cells differentiated in ECGS
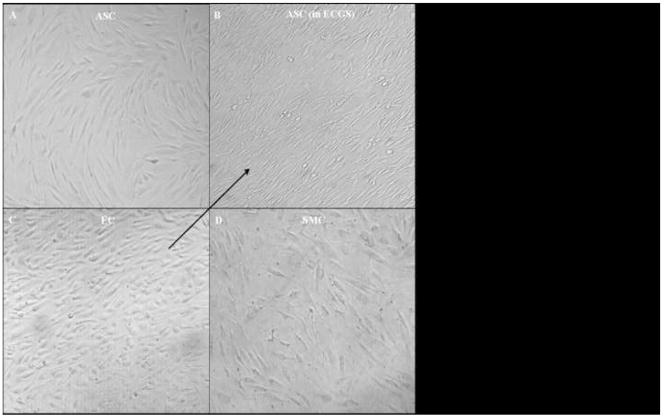
Prior to culture in ECGS, ASC exposed to 12 dyne of shear up to 8 days did not re-align in the direction of shear, significantly take up acLDL, or express molecular markers by RT-PCR (data not shown). However, after culture in ECGS for two weeks, ASC exposed to shear realigned as early as two days, a pattern that was complete by four days (Figure 4). Additionally, shear up-regulated CD31 expression and acLDL uptake (Figure 5). After two days, RT-PCR detected the presence of CD31 message; after four days, both immunoblot and immunohistochemistry demonstrated CD31 protein. Finally, after eight days of shear, a significant number of the ASC took up acLDL. Significant expression of eNOS or vWF was not observed under these conditions (data not shown).
Figure 4. Morphological effects of shear force on the differentiation of human adipose-derived stem cells (ASC).
Photomicrographs of cells exposed to 12 dynes of shear in vitro for 4 days (phase-contrast, 100x). The arrow indicates the direction of shear in all four panels. Undifferentiated stem cells (A), naïve to ECGS, remain randomly oriented, similar to smooth muscle cell (SMC) controls (D). In contrast, stem cells grown in ECGS for 2 weeks and subsequently exposed to shear (B) orient in the direction of flow similar to the endothelial cell (EC) controls (C).
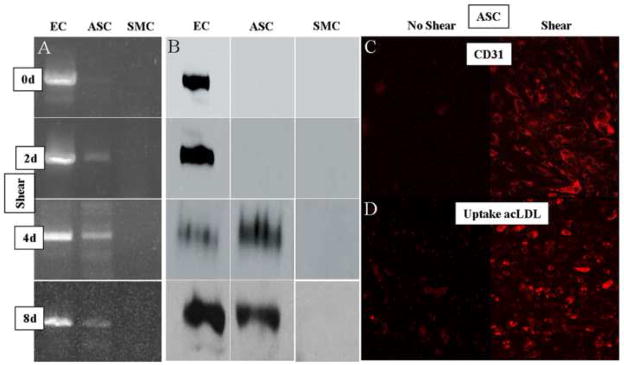
Figure 5. Molecular effects of shear force on the differentiation of human adipose-derived stem cells.
Panel A. RT-PCR results (CD31). In these experiments, endothelial (EC), differentiated stem (ASC), and smooth muscle (SMC) cells were exposed to shear for up to 8 days. Examination of the ASC results reveals the expression of CD31 message beginning 2 days after shear was introduced, and continuing throughout 8 days. Panel B. Immunoblot results (CD31). In experiments parallel to those in panel A, each of the cell lines were exposed to shear for up to 8 days. Examination of the ASC results reveals the expression of CD31 protein beginning 4 days after shear was introduced, and continuing throughout 8 days. Panel C. Immunohistochemical results (CD31). Fluorescent photomicrograph of differentiated ASC stained for CD31 before (left) and after 4 days (right) of shear demonstrating significant expression of this protein in response to shear (100x). Panel D. acLDL uptake. Fluorescent photomicrograph of differentiated ASC stained for acLDL before (left) and after 8 days (right) of shear demonstrating significant uptake in response to shear (100x).
Differentiated ASC appear mildly thrombogenic after implantation in the arterial circulation
All animals survived the procedures through to harvest without neurological deficit to suggest embolization of thrombus. Duplex ultrasound examination of the implanted grafts at two weeks demonstrated similar graft patency between seeded and unseeded grafts (Figure 6). Gross examination of the seeded grafts (both sheared and statically seeded) revealed a homogeneous lining of the luminal surface; histological analysis demonstrated that this layer was composed of fibrin.
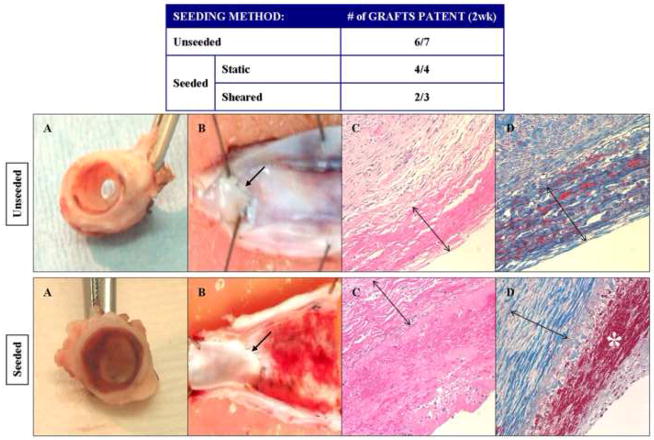
Figure 6. In vivo evaluation of the thrombogenicity of human adipose-derived stem cells.
In these experiments, autologous canine adipose-derived stem cells were cultured in ECGS and seeded onto canine decellularized vein grafts with (static) and without (shear) flow conditioning. Unseeded grafts were used as controls. The grafts were then implanted into the carotid circulation of the animal from which the stem cells were harvested. The Table reveals that patency of the grafts were similar amongst the seeded (6 of 7) and unseeded (6 of 7) groups. After explant, gross examination (Panels A and B) of the seeded grafts revealed a thin layer of thrombus throughout the graft, not seen on the unseeded grafts. The proximal anastomotic suture lines are marked with an arrow. Microscopic evaluation (Panels C and D, H&E and trichrome stain, respectively; 100x) confirms that this layer contains fibrin (red staining marked by * in Panel D), and is absent on the unseeded controls. The decellularized vein allografts (marked by double arrows in Panels C and D) are noted to be largely composed of collagen (blue staining in Panels D).
Discussion
This study demonstrates acquisition of endothelial cell characteristics by human adipose-derived stem cells (ASC). The main stimulus—endothelial cell growth supplement (ECGS)—enabled the stem cells to respond to extracellular matrix and shear in fashions similar to endothelial cells; ECGS alone, however, did not promote expression of molecular markers. A second additional stimulus—shear force—elicited CD31 (PECAM) expression and acLDL uptake. ASC did not express significant amounts of eNOS in response to either stimulus. Finally, in vivo study suggested that the stem cells are mildly thrombogenic, although grafts seeded with ECGS/shear differentiated ASC remained patent at two weeks. These studies illustrate the capability of ASC to differentiate into cells potentially useful for cardiovascular regenerative medicine under simple and inexpensive culture conditions.
Adipose-derived stem cells have been shown to have adipogenic, osteogenic, myogenic and neurogenic potentials.23–25 Recently, endothelial cell differentiation by ASC has been evaluated. In separate studies, both Miranville and Planat-Bernard demonstrated expression of CD31 and vWF, along with participation in postnatal neovascularization, by ASC stimulated with vascular endothelial growth factor (VEGF) and insulin-like growth factor (IGF).26,27 Cao confirmed these findings and noted differentiation was blocked by a PI3 kinase inhibitor.28 Wosnitza found that CD31+ cells in the stromal-vascular fraction maintain their ability to commit to adipocytes, suggesting that endothelial cells and adipocytes may have a common progenitor.29
The present study confirms the ability of human ASC to acquire endothelial cell traits, and is the first to describe effects of ECGS. The rationale for this choice is based on ECGS (50 μg/mL) use as a growth factor for endothelial cell culture as described by Maciag and colleagues.10,30 ECGS is derived from bovine hypothalamus, mainly contains acidic fibroblast growth factor, and is mitogenic for endothelial and smooth muscle cells, keratinocytes, melanocytes and hybridomas. Our findings suggest that ECGS directly stimulated the differentiation of ASC. Although it is possible these results arose secondary to selection of microvessel endothelial cells still present within the stromal-vascular pellet, two lines of evidence point to true differentiation. First, the primary cultures underwent negative selection for CD31 and CD45 cells to remove lineages related to endothelium and monocytes. Subsequent to this process, both flow cytometry and RT-PCR demonstrated a population of cells that were devoid of each of the endothelial molecular markers examined. Second, the morphological studies (cord formation, re-alignment) demonstrated homogeneity within the observed cultures, without evidence of the presence of two different cell lines.
Ultimately, the effect of ECGS alone on stem cell differentiation appeared limited. After culture, the cells did uniformly form cords in response to seeding onto extracellular matrix components, but this response was not as robust as the differentiated endothelial cells. Additionally, ECGS alone did not induce expression of the molecular markers; rather, it appeared to “prime” the cells for subsequent changes induced by shear. Endothelial growth supplement, as a medium component, traditionally has been used for culture and expansion of differentiated endothelial cells. Given the positive effect of Vascular Endothelial Growth Factor (VEGF) on stem cell differentiation,26 - 28 it is likely that VEGF-containing media such as Endothelial Growth Media-2 will have more impact than media containing ECGS as its main stimulant. These studies are now ongoing in our laboratory.
This study is the first to describe the differentiating effect of shear on ASC. The rationale for using shear as a stimulus is based its well-known effects on endothelial morphology and function. In response to shear, endothelial cells elongate and decrease their profile in proportion to the magnitude of shear.31,32 Additionally, shear modulates cell proliferation, cell cycle arrest, and nitric oxide (NO) production, thereby affecting vascular tone.12,13,33,34 This stimulus has also been used to promote endothelial differentiation of embryonic stem15,16 and endothelial progenitor cells.35 In this study, we observed that shear up-regulated the expression of CD31 (PECAM) and promoted acLDL uptake in cells cultured in ECGS. CD31 is a transmembrane glycoprotein localized at the cell junction with neighboring endothelial cells.36 Its location at the cell-cell junction may explain its activation in response to shear force on the cell, as it has been implicated as a mechanotransductive protein.37 Uptake of acLDL by endothelial cells largely occurs by endocytosis; this process has been shown to be modulated by shear force.38
In examining the acquisition of endothelial phenotypic characteristics by ASC, we tested for thrombogenicity. Endothelial cells play a significantly role in promoting the non-thrombogenic luminal surface of a blood vessel. We chose to examine this property in vivo by placing the differentiated ASC directly in the milieu of an endothelial cell. We observed that ASC cultured in ECGS for two weeks, with and without subsequent exposure to gradually increased shear force, formed thin fibrin layer along the luminal surface suggesting that the cells are thrombogenic at this stage. The thrombogenic properties of undifferentiated stem cells has not been studied extensively. Hashi and colleagues have demonstrated that platelet adhesion to bone marrow mesenchymal stem cells in vitro is similar to that on endothelial cells.39 They note that “as endothelial cells” the seeded stem cells improve the patency of nanofibrous grafts.
Although the cells appeared thrombogenic in this model, it should be noted that we can not present direct evidence as to stem cell fate after two weeks of implantation. A stem cell-specific marker for the canine cells is not currently available, and our attempts to examine the canine stem cells in vitro or in vivo using anti-human CD31 or vWF monoclonal antibodies proved unsuccessful. We are presently developing an adenovirus to transfect the stem cells with green fluorescent protein that will be useful in tracking the stem cells.
The finding of fibrin on ASC cultured in ECGS is possibly due, in part, to lack of eNOS expression within the differentiated ASC. In addition to its vasoactive and atheroprotective effects, nitric oxide (NO) produced by eNOS is an important mechanism by which endothelium inhibit thrombus formation within a blood vessel. NO limits platelet activation, adhesion, and aggregation by activating guanylyl cyclase, inhibiting phosphoinositide 3 kinase, impairing capacitative calcium influx, and inhibiting cyclooxygenase-I.40 Suppression of intracellular calcium flux further suppresses activation of glycoprotein IIb/IIIa required for binding fibrinogen. Inhibition of cyclooxygenase-I decreases the conversion of arachidonic acid to prostaglandins G2 and H2, thus reducing thromboxane A2 synthesis.41 The acquisition of eNOS by a stem cell naturally or by genetic manipulation would be advantageous for its use in the creation of a vascular graft. Kanki-Horimoto and Zhang have both demonstrated the effectiveness of improving graft patency with stem cells that overexpress NO.42,43 As noted, we did not observe eNOS message within ASC cultured in ECGS and/or exposed to shear. In each of the other investigations evaluating endothelial differentiation from adipose-derived stem cells, only Cao demonstrated eNOS by RT-PCR.28 This later study did not report protein expression or NO production. It is therefore possible that these stems cells are limited in their ability to express eNOS.
Fibrin formation within the stem cell-seeded grafts may be the result of other stem cell characteristics suggestive of a partially differentiated state, but unrelated to lack of eNOS expression. We have recently begun to evaluate thrombogenicity in vitro, and have observed that human stem cells up-regulate tissue plasminogen activator and down-regulate Plasminogen Activator Inhbitor-1 in response to shear force, but not to the levels seen within mature endothelial cell controls (unpublished results). An additional possibility includes de-differentiation of the cells in vivo. Upon implantation, these cells are theoretically no longer stimulated by growth factor supplied by our culture conditions; however, so long as they remain in contact with luminal blood flow, theoretically physiological shear force continues to stimulate them in vivo.
Although the primary purpose of the canine model was to test the thrombogenic properties of the stem cells in vivo, this study suggests proof of concept for using autologous ASC to create a vascular graft. A potential advantage of this strategy, largely based on the availability of this particular stem cell, is the limited amount of time necessary for graft creation (here, less than two weeks). Many tissue-engineered vascular grafts, including that of L’Hereaux which has reached human implantation,44 require months of ex vivo cell and graft culture to create, resulting in significant expense and narrowed clinical application.
In summary, human adult stem cells derived from adipose tissue acquire several endothelial-like characteristics when cultured in endothelial cell growth supplement and exposed to physiological shear force. These include cord formation in response to extracellular matrix, uptake of acLDL, expression of CD31 (PECAM) and re-alignment in the direction of flow. Despite the acquisition of these traits, in vivo evaluation of these cells suggests that the stem cells differentiated in medium containing endothelial growth supplement are mildly thrombogenic, possibly related to the lack of eNOS expression. Further work, perhaps evaluating other soluble growth factors and shear force, is necessary to determine if adipose-derived stem cells are capable of more fully expressing endothelial cell characteristics.
Supplementary Material
Acknowledgments
This work was supported by the following grants: NIH K08 HL076300-01 (PJD, TNT), American Heart Association Beginning Grant-in-Aid (PJD), American Vascular Association (PJD), and the Pacific Vascular Research Foundation Wylie Scholar Award (PJD).
Appendix
The following information is provided to further understand the canine model used in these experiments and how the results may be translatable to humans.
-
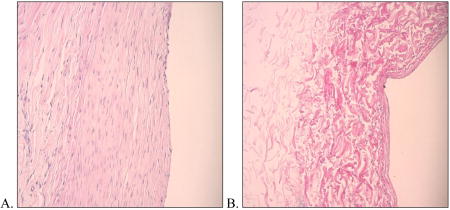
Canine decellularized vein. Using methods previously described by us,22,45 we removed all of the vascular wall cells from canine external jugular vein. Shown here are photomicrographs of canine vein before (A) and after (B) decellularization, representative of the vascular conduit that was seeded with canine adipose-derived stem cells prior to carotid interposition grafting (H&E; 100x).
-
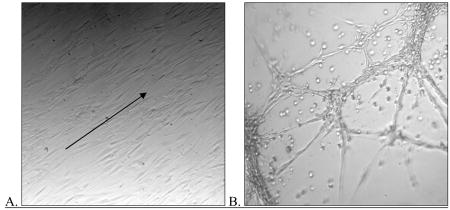
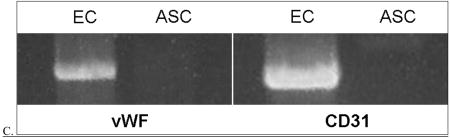
Differentiation of canine adipose-derived stem cells (ASC). Canine ASC were isolated from adipose tissue residing within the animal’s falciform ligament. After isolation identical to that used for isolation of human ASC, we differentiated the cells in vitro using media containing endothelial cell growth factor (ECGS; identical to that used for differentiation of human cells). Shown here are photomicrographs demonstrating that the differentiated canine cells re-align with in the direction of shear force (arrow)(A) and form cords when seeded onto Matrigel (B)(phase-contrast microscopy; 100x). Additionally, these cells not express message for vWF or CD31(C)(RT-PCR). Each of these results is similar to those found in their human counterparts.
-
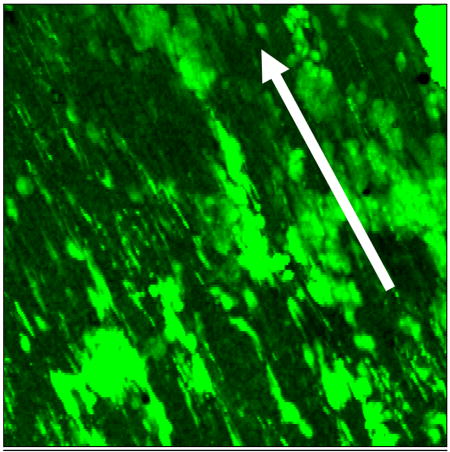
Creation of tissue-engineered stem cell graft in canine model. Shown here is a laser confocal micrograph of canine adipose-derived stem cells differentiated in medium containing ECGS, seeded onto canine decellularized vein allograft, and flow conditioned for three days (up to 12dynes)(Cell tracker green; 100x). This demonstrates that the cells are retained on the graft surface at physiologic levels of shear, and indeed re-align in the direction of shear force (arrow), prior to implantation into the canine arterial circulation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lauren J. Fischer, Department of Surgery, Thomas Jefferson University, Philadelphia, PA.
Stephen McIlhenny, Department of Surgery, Thomas Jefferson University, Philadelphia, PA.
Thomas Tulenko, Department of Surgery, Thomas Jefferson University, Philadelphia, PA.
Negar Golesorkhi, Department of Surgery, Thomas Jefferson University, Philadelphia, PA.
Ping Zhang, Department of Surgery, Thomas Jefferson University, Philadelphia, PA.
Robert Larson, Department of Surgery, Thomas Jefferson University, Philadelphia, PA.
Joseph Lombardi, Department of Surgery, Thomas Jefferson University, Philadelphia, PA.
Irving Shapiro, Department of Orthopedic Research, Thomas Jefferson University, Philadelphia, PA.
Paul J. DiMuzio, Department of Surgery, Thomas Jefferson University, Philadelphia, PA.
References
- 1.Fukuda K, Yuasa S. Stem Cells as a Source of Regenerative Cardiomyocytes. Circ Res. 2006;98:1002–1013. doi: 10.1161/01.RES.0000218272.18669.6e. [DOI] [PubMed] [Google Scholar]
- 2.Wollert KC, Drexler H. Clinical Applications of Stem Cells for the Heart. Circ Res. 2005;96:151–163. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 3.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. Journal of Molecular and Cellular Cardiology. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, Kangawa K, Kitamura S. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovascular Research. 2005;66:543–551. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Campbell GR, Campbell JH. Development of tissue engineered vascular grafts. Curr Pharm Biotechnol. 2007;8:43–50. doi: 10.2174/138920107779941426. [DOI] [PubMed] [Google Scholar]
- 6.L’Heureux N, Dusserre N, Marini A, Garrido S, de la Fuente L, McAllister T. Technology insight: the evolution of tissue-engineered vascular grafts—from research to clinical practice. Nat Clin Pract Cardiovasc Med. 2007;4:389–95. doi: 10.1038/ncpcardio0930. [DOI] [PubMed] [Google Scholar]
- 7.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic Potential of Endothelial Progenitor Cells in Cardiovascular Diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 8.DiMatteo C, Golesorkhi N, Fischer L, Wrigley C, McIlhenny S, Tulenko T, Shapiro I, Carabasi R, Lombardi J, Larson R, DiMuzio P. Isolation of Adipose-Derived Stem Cells in Patients With Vascular Disease. Circulation. 2006;114:SII446. (abstract) [Google Scholar]
- 9.Terramani T, Eton D, Bui P, Wang Y, Weaver F, Yu H. Human macrovascular endothelial cells: optimization of culture conditions. In vitro cell dev biol anim. 2000;36:125–132. doi: 10.1290/1071-2690(2000)036<0125:HMECOO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 10.Maciag T, Hoover G, Stemerman M, Weinstein R. Serial propagation of human endothelial cells in vitro. J Cell Biol. 1981;91:420–426. doi: 10.1083/jcb.91.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siasos G, Tousoulis D, Siasou A, Stefanadis C, Papavassiliou A. Shear force, protein kinases, and atherosclerosis. Curr Med Chem. 2007;14:1567–1572. doi: 10.2174/092986707780831087. [DOI] [PubMed] [Google Scholar]
- 12.Davies PF, Zilberberg J, Helmke BP. Spatial Microstimuli in Endothelial Mechanosignaling. Circ Res. 2003;92:359–370. doi: 10.1161/01.RES.0000060201.41923.88. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Cardena G, Gimbrone MA., Jr Biomechanical modulation of endothelial phenotype: implications for health and disease. Handb Exp Pharmacol. 2006;176:79–95. doi: 10.1007/3-540-36028-x_3. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Yan S, Chai H, Riha GM, Li M, Yao Q, Chen C. Shear force induces endothelial transdifferentiation from mouse smooth muscle cells. Biochemical and Biophysical Research Communications. 2006;346:860–865. doi: 10.1016/j.bbrc.2006.05.196. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Riha GM, Yan S, Li M, Chai H, Yang H, Yao Q, Chen C. Shear Force Induces Endothelial Differentiation From a Murine Embryonic Mesenchymal Progenitor Cell Line. Arterioscler Thromb Vasc Biol. 2005;25:1817–1823. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, Ohura N, Matsushita A, Kamiya A, Ando J. Fluid shear force induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H1915–1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 17.Institute of Laboratory Animal Resources. Guide for the Care and Use of Laboratory Animals/Institute of Laboratory Animal Resources, National Research Council. Washington, DC: National Academy Press; 1996. [Google Scholar]; Guide for the Care and Use of Laboratory Animals/Institute of Laboratory Animal Resources, Commission of Life Sciences, National Research Council. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 18.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, Di Halvorsen Y, Storms RW, Goh B, Kilroy G, Wu X, Gimble JM. Immunophenotype of Human Adipose-Derived Cells: Temporal Changes in Stromal-Associated and Stem Cell-Associated Markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 19.Dardik A, Chen L, Frattini J, Asada H, Aziz F, Kudo FA, Sumpio BE. Differential effects of orbital and laminar shear force on endothelial cells. Journal of Vascular Surgery. 2005;41:869–880. doi: 10.1016/j.jvs.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Reneman R, Arts T, Hoeks A. Wall Shear Force - an Important Determinant of Endothelial Cell Function and Structure - in the Arterial System in vivo. J Vasc Res. 2006;43:251–269. doi: 10.1159/000091648. [DOI] [PubMed] [Google Scholar]
- 21.Stroev PV, Hoskins PR, Easson WJ. Distribution of wall shear rate throughout the arterial tree: A case study. Atherosclerosis. 2007;191:276–280. doi: 10.1016/j.atherosclerosis.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola NA, Leichter RF, Carabasi RA, DiMuzio PJ. Decellularized vein as a potential scaffold for vascular tissue engineering. Journal of Vascular Surgery. 2004;40:146–153. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human Adipose Tissue Is a Source of Multipotent Stem Cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strem B, Hicok K, Zhu M, Wulur I, Alfonso ZC, Schreiber R, Fraser JK, Hedrick MH. Mulitpotential differentiation of adipose-derived stem cells. Keio J Med. 2005;54:132–41. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 25.Zannettino A, Paton S, Arthur A, Khor F, Itescu S, Gimble J, Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. Journal of Cellular Physiology. 2007;9999:n/a. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 26.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of Postnatal Neovascularization by Human Adipose Tissue-Derived Stem Cells. Circulation. 2004;110:349–355. doi: 10.1161/01.CIR.0000135466.16823.D0. [DOI] [PubMed] [Google Scholar]
- 27.Planat-Benard V, Silvestre J-S, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of Human Adipose Lineage Cells Toward Endothelial Cells: Physiological and Therapeutic Perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Sun Z, Liao L, Meng Y, Han Q, Zhao RC. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochemical and Biophysical Research Communications. 2005;332:370–379. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 29.Wosnitza M, Hemmrich K, Groger A, Graber S, Pallua N. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation. 2007;75:12–23. doi: 10.1111/j.1432-0436.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- 30.Hinsbergh Vv, Mommaas-Kienhuis A, Weinstein R, Maciag T. Propagation and morphologic phenotypes of human umbilical cord artery endothelial cells. Eur J Cell Biol. 1986;42:101–10. [PubMed] [Google Scholar]
- 31.Barbee K, Davies P, Lal R. Shear force-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circulation Research. 1994;74:163–71. doi: 10.1161/01.res.74.1.163. [DOI] [PubMed] [Google Scholar]
- 32.Helmlinger G, Geiger R, Shreck S, Nerem R. Effects of pulsatile flow on cultured vascular endothelial cell morphology. J Biomech Eng. 1991;113:123–131. doi: 10.1115/1.2891226. [DOI] [PubMed] [Google Scholar]
- 33.Shyy JY-J, Chien S. Role of Integrins in Endothelial Mechanosensing of Shear Force. Circulation Research. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 34.Gnasso A, Carallo C, Irace C, De Franceschi MS, Mattioli PL, Motti C, Cortese C. Association between wall shear force and flow-mediated vasodilation in healthy men. Atherosclerosis. 2001;156:171–176. doi: 10.1016/s0021-9150(00)00617-1. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Takahashi T, Asahara T, Ohura N, Sokabe T, Kamiya A, Ando J. Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear force. J Appl Physiol. 2003;95:2081–2088. doi: 10.1152/japplphysiol.00232.2003. [DOI] [PubMed] [Google Scholar]
- 36.Newton JP, Hunter AP, Simmons DL, Buckley CD, Harvey DJ. CD31 (PECAM-1) Exists as a Dimer and Is Heavily N-Glycosylated. Biochemical and Biophysical Research Communications. 1999;261:283–291. doi: 10.1006/bbrc.1999.1018. [DOI] [PubMed] [Google Scholar]
- 37.Osawa M, Masuda M, Kusano K-i, Fujiwara K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J Cell Biol. 2002;158:773–785. doi: 10.1083/jcb.200205049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niwa K, Kado T, Sakai J, Karino T. The effects of a shear flow on the uptake of LDL and acetylated LDL by an EC monoculture and an EC-SMC coculture. Ann Biomed Eng. 2004;32:537–43. doi: 10.1023/b:abme.0000019173.79939.54. [DOI] [PubMed] [Google Scholar]
- 39.Hashi CK, Zhu Y, Yang GY, Young WL, Hsiao BS, Wang K, Chu B, Li S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci USA. 2007;104:11915–20. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fleser PS, Nuthakki VK, Malinzak LE, Callahan RE, Seymour ML, Reynolds MM, Merz SI, Meyerhoff ME, Bendick PJ, Zelenock GB, Shanley CJ. Nitric oxide-releasing biopolymers inhibit thrombus formation in a sheep model of arteriovenous bridge grafts. J Vasc Surg. 2004;40:803–811. doi: 10.1016/j.jvs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 42.Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, Katsumata T. Synthetic vascular prosthesis impregnated with mesenchymal stem cells overexpressing endothelial nitric oxide synthase. Circulation. 2006;114:I327–30. doi: 10.1161/CIRCULATIONAHA.105.001586. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Qi H, Wang H, Hu P, Ou L, Guo S, Li J, Che Y, Yu Y, Kong D. Engineering of vascular grafts with genetically modified bone marrow mesenchymal stem cells on poly (propylene carbonate) graft. Artif Organs. 2006;30:898–905. doi: 10.1111/j.1525-1594.2006.00322.x. [DOI] [PubMed] [Google Scholar]
- 44.L’Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med. 2007;357:1451–3. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- 45.Martin ND, Schaner PJ, Tulenko TN, Shapiro IM, DiMatteo CA, Williams TK, Hager ES, DiMuzio P. In vivo behavior of decellularized vein allograft. J Surg Res. 2005;129:17–23. doi: 10.1016/j.jss.2005.06.037. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.