Abstract
Some biotechnological inventions involve expensive, sophisticated machines. Others are relatively simple innovations that nevertheless address, and solve difficult problems. Synthesis and purification of highly hydrophobic peptides can be a difficult and challenging task, particularly when these peptides have low solubility in both aqueous and organic solvents. Here we describe the synthesis and purification of a series of peptides derived from the hydrophobic C-terminus of the 42-residue form of amyloid β-protein (Aβ42), a peptide believed to be the primary cause for Alzheimer’s disease (AD). The series of C-terminal fragments (CTFs) had the general formula Aβ(x-42), x=28–39, which potentially can be used as inhibitors of Aβ42 assembly and neurotoxicity. Synthesis and purification of peptides containing 8-residues or less were straightforward. However, HPLC purification of longer peptides was problematic and provided <1% yield in particularly difficult cases due to very poor solubility in the solvent systems used both in reverse- and in normal phase chromatography. Modification of the purification protocol using water precipitation followed by removal of scavengers by washing with diethyl ether circumvented the need for HPLC purification and provided these peptides with purity as high as HPLC-purified peptides and substantially increased yield.
INTRODUCTION
Amyloid β-protein (Aβ) is a small protein of unknown function whose accumulation and self-assembly are believed to be the seminal events causing Alzheimer’s disease (AD) [1, 2]. Aβ is produced from the amyloid β-protein precursor (APP), a type 1 transmembrane protein, in two predominant forms comprising 40 or 42 amino acids, and termed Aβ40 and Aβ42, respectively. Aging-related imbalance between production and clearance of Aβ leads to elevation in its concentration, which in turn causes self-assembly of Aβ into neurotoxic oligomers. The oligomers injure susceptible neurons by mechanisms that are not fully understood, aggregate further into larger assemblies, and eventually form polymers that deposit in the brain in the form of amyloid plaques, one of the pathological hallmarks of AD. Thus, inhibition of Aβ assembly is a promising strategy for prevention of, and therapy for AD.
Multiple lines of evidence point at Aβ42 as the form of Aβ that predominantly causes AD. Aβ40 and Aβ42 exist in the plasma and cerebrospinal fluid (CSF) at a concentration ratio of ~10:1, respectively, yet Aβ42 is deposited first during the development of AD [3], is the predominant component in parenchymal plaques [4], and is more neurotoxic than Aβ40 [5]. An increase in the Aβ42/Aβ40 concentration ratio is associated with early onset familial AD [6], whereas treatments that decrease this ratio reduce the risk for AD [7].
Aβ40 and Aβ42 form distinct oligomer populations in vitro [8]. Several kinds of oligomers formed only by Aβ42 have been shown to be highly neurotoxic [9–11]. Direct comparison of Aβ40 and Aβ42 oligomers formed under similar conditions demonstrate substantially higher toxicity of the Aβ42 oligomers [12, 13]. Because the only structural difference between Aβ40 and Aβ42 is the absence or presence of the last two amino acids, Ile41-Ala42, respectively, it is reasonable to hypothesize that the C-terminus plays an important role in the assembly and/or toxicity of Aβ42. In support of this hypothesis, increased toxicity of Aβ42 relative to Aβ40 correlates not only with distinct oligomer populations, but also with increased conformational stability [14–17] and a putative quasi-stable turn conformation [18–20] in the C-terminal region of Aβ42 but not Aβ40.
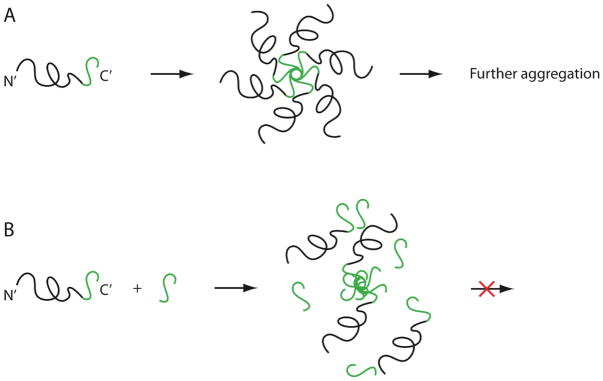
The C-terminal region (last 14 residues) of Aβ42 is highly hydrophobic and believed to reside within the membrane before Aβ is cleaved from APP. Thus, we hypothesized that intermolecular association of the C-termini of several Aβ42 monomers, leading to formation of a core in which hydrophobic residues are sequestered from the aqueous environment, may be a major driving force for formation of Aβ oligomers. If that is the case, we reasoned that C-terminal fragments (CTFs) of Aβ42 might compete with the C-terminus of full-length Aβ42 and disrupt oligomer formation (Fig. 1). To test this hypothesis, we set up to synthesize a series of Aβ42-derived CTFs [Aβ(x-42), x=28–39] and test their ability to inhibit Aβ42 self-assembly and toxicity. Here we report on the synthetic challenges associated with obtaining long CTFs and ways to overcome these challenges. Results of biological and structural characterization of the CTFs will be reported elsewhere.
Fig. 1.
Schematic representation of putative mechanism of action of Aβ42-derived CTF. A) The C-termini of several Aβ42 molecules are hypothesized to form the hydrophobic core of the oligomer. B) CTFs derived from the C-terminus of Aβ42 may displace the C-terminus of the full-length peptide, leading to disruption of oligomerization.
Synthesis and purification of peptides comprising mainly hydrophobic residues is challenging because of the low solubility of these peptides in both hydrophilic and hydrophobic solvents and their high tendency to aggregate [21–23]. Aβ and its derivatives are known to be difficult to synthesize and obtain in high purity [24, 25]. In fact, an early solid-state NMR investigation of Aβ(34–42) has reported that this peptide readily aggregated into amyloid fibrils [26]. Nevertheless, the potential of Aβ42-derived CTFs as inhibitors of Aβ42 toxicity made them attractive for us to synthesize and characterize.
METHODS AND MATERIALS
Reagents and Equipment
Protected amino acids were purchased from Novabiochem (San Diego, CA). N-methylpyrrolidone (NMP), dichloromethane (DCM), acetonitrile (ACN), acetic acid, and trifluoroacetic acid (TFA) were from Fisher Scientific (Pittsburgh, PA). Piperidine, 1-hydroxybenzotriazole, and O-ben- zotriazole-N,N,N′,N′-tetramethyluronium hexafluorophos- phate (HBTU) were from Anaspec (San Jose, CA) or Applied Biosystems (Foster City, CA). N,N-diisopropylethyamine (DIPEA) was from Applied Biosystems. Diethyl ether, etha- nedithiol (EDT), thioanisole, and triisopropylsilane (TIPS) were from Sigma/Aldrich (St. Louis, MO). All the chemicals used were of analytical grade or higher. Deionized water was produced using a Milli-Q A10 system (Millipore, Billerica, MA). Solid-phase peptide synthesis resins were all substituted with Fmoc-Ala and included Wang resin (200–400 mesh, 0.7 mmol/g from Bachem, Torrance, CA or 100–200 mesh, 0.41 mmol/g from Novabiochem, San Diego, CA), TGA resin (100–200 mesh, 0.21 or 0.23 mmol/g, Novabiochem), or 2-chlorotrityl resin (100–200 mesh, 0.6 mmol/g, Novabiochem). Analytical HPLC analysis was done using a 510 system (Waters, Milford, MA) with 250 × 5 mm diphenyl or C4 columns (Vydac, Hesperia, CA). HPLC coupled with mass spectrometry (MS) analysis was done using a LCQ Deca mass spectrometer with electrospray ionization probe coupled to a Surveyor HPLC system (ThermoScientific, Waltham, MA) with 150 × 1 mm diphenyl or C4 columns (Vydac). The mass spectrometer was tuned and optimized on a regular basis at least once a month and calibrated periodically using molecular weight standards according to the manufacturer’s instructions. Semi-preparative HPLC purification was performed using a Dynamax system (Rainin, Oakland, CA) with 250 × 20 mm C4 or diphenyl columns (Vydac). Amino acid analysis (AAA) was performed using an Empower AccQTag system (Waters).
Peptide Synthesis
All the peptides were synthesized by 9-fluorenylmethoxy- carbonyl (FMOC) chemistry using automated Applied Biosystems 433A synthesizers. The synthesis scale was between 0.20–0.25 mmol. The coupling and deprotection cycles were extended from the manufacturer recommended times, 30 and 10 min, respectively, to 60 and 30 min, respectively. Coupling cycles were performed using 4 eq. of incoming amino acid, HBTU, and DIPEA in NMP. FMOC deprotection was done using 20% piperidine in NMP. The εNH2 group of Lys was protected by tert-butoxycarbonyl (BOC). Cleavage of the peptide from the resin and side-chain deprotection (where appropriate) was performed using 10 mL of the following mixtures: A) 9.5:0.5 - TFA:H2O; B) 9.25:0.5:0.25 - TFA:H2O:EDT; C) 8.75:0.5:0.5:0.25 - TFA:H2O:thioanisole: EDT; or D) 9.5:0.25:0.25 - TFA:EDT:TIPS. After filtration of the resin, several methods were used for isolation of the crude peptide from the cleavage mixture (see Results). The integrity of the final products was verified by MS and AAA (Table 1).
Table 1.
| Peptide | Sequence | Yield (%) | Purity (%) | Calculated Mass (amu) | Observed Mass (amu) | Average AAA Error (%)a |
|---|---|---|---|---|---|---|
| Aβ(39–42) | VVIA | 8 | 95 | 401.28 | 401.1 | 9 |
| Aβ(38–42) | GVVIA | 13 | 98 | 458.30 | 458.2 | 4 |
| Aβ(37–42) | GGVVIA | 10 | 97 | 515.32 | 515.2 | 2 |
| Aβ(36–42) | VGGVVIA | 9 | 98 | 614.39 | 614.4 | 12 |
| Aβ(35–42) | MVGGVVIA | 6 | 95 | 745.43 | 745.4 | 4 |
| Aβ(34–42) | LMVGGVVIA | 4 | 97 | 858.51 | 858.3 | 9 |
| Aβ(33–42) | GLMVGGVVIA | 9 | 98 | 915.54 | 915.3 | 14 |
| Aβ(32–42) | IGLMVGGVVIA | 2b | 96d | 1028.62 | 1028.5 | 9 |
| 18c | 95d | 1028.5 | 10 | |||
| Aβ(31–42) | IIGLMVGGVVIA | 3b | 95d | 1141.70 | 1141.3 | 12 |
| 23c | 95d | 1141.4 | 12 | |||
| Aβ(30–42) | AIIGLMVGGVVIA | 0.7 | 97d | 1212.74 | 1213.6 | 9 |
| Aβ(29–42) | GAIIGLMVGGVVIA | 0.7 | 95d | 1269.76 | 1269.5 | 9 |
| Aβ(28–42) | KGAIIGLMVGGVVIA | 3 | 96 | 1397.86 | 1397.9 | 13 |
Average % error for each amino acid type divided by number of amino acids.
Purified by semi-preparative HPLC.
Precipitated in water and washed with diethyl ether.
For these peptides the purity could not be determined using UV monitoring of HPLC. Therefore, the assessment is based on a chromatogram for which the total ion current was measured continuously by a mass-spectrometer.
RESULTS
With exception of the longest CTF we prepared, Aβ(28–42) (Lys-Gly-Ala30-Ile-Ile-Gly-Leu-Met-Val-Gly-Gly-Val-Val40-Ile-Ala, full-length Aβ numbering is used throughout the manuscript), all other CTFs comprised only amino acids with non-charged side-chains. The Gly residues in positions 29, 33, 37, and 38 were the only hydrophilic residues in the sequence, whereas all other residues were hydrophobic. Previous work suggested that Aβ(34–42) readily formed β-sheet-rich fibrillar aggregates [26]. Therefore, we anticipated that the synthesis and purification of the CTFs would become difficult above a certain length. Encouragingly, using the protocol we routinely use for the synthesis of full-length Aβ42, which includes extended coupling and deprotection times (see Methods and Materials) we could synthesize all peptide sequences and did not encounter special problems in any coupling/deprotection cycle as determined by the synthesizer’s online UV monitoring of deprotection products.
The type of resin used was an important determinant of synthesis success. Initially, peptides were synthesized using a 200–400 mesh, 0.4–0.6 mmol/g Wang resin from Novabiochem. This resin worked well in all the cases it was used, but unfortunately, during the period of the project the company discontinued this particular resin. We therefore switched to a 100–200 mesh Wang resin from Novabiochem and found out that with this resin, substitution level had to be kept under 0.5 mmol/g for the synthesis to be successful. Aβ(39–42) through Aβ(33–42) were synthesized successfully with this resin but longer CTFs produced negligible yields. Therefore, Aβ(32–42) and longer peptides were synthesized using a 100–200 mesh TGA resin (Novabiochem), for which we also had to use low substitution levels. Towards the end of the project period, we also have used successfully a 200–400 mesh, 0.7 mmol/g Wang resin from Bachem.
Isolation of the CTFs from the crude cleavage mixture was relatively straightforward for Aβ(39–42) through Aβ(35–42) but became difficult for Aβ(34–42) and longer peptides. In initial experiments, CTFs were cleaved from the resin in 10 mL cleavage mixture B for 120 min. The resin was filtered out and the filtrate volume reduced to 1–2 mL gently under low vacuum. The crude peptide was then precipitated by dilution with 50 mL of ice-cold diethyl ether and collected by filtration. The crude peptide was re-dissolved in milli-Q water and purified by semi-preparative HPLC with a gradient of 20–100% solvent B over 40 min (solvent A = 2% ACN, 0.1% AcOH, 0.02% TFA in milli-Q water, solvent B = 98% ACN, 0.1% AcOH, 0.02% TFA in milli-Q water). All HPLC separations were performed at room temperature with UV monitoring at 214 nm. Flow rates were 5 mL/min for semi-preparative HPLC, 1 mL/min for analytical HPLC, and 0.1 mL/min for LC-MS. Initially, both diphenyl and C4 columns (both analytical and semi-preparative) were used. C4 columns were found to have somewhat better resolution relative to the diphenyl columns and were used in subsequent experiments. This protocol yielded Aβ(39–42) through Aβ(35–42), and Aβ(28–42) in high purity and moderate yield (Table 1).
When attempting to use the same purification protocol for Aβ(34–42) through Aβ(29–42), we realized that these peptides did not precipitate by dilution of the concentrated cleavage mixture in ice-cold ether. Therefore, we tried to purify the crude peptides by loading the cleavage mixture directly onto an HPLC column. To simplify the cleavage mixture, we attempted excluding all the scavengers except water, taking into account that none of the amino acids used for the synthesis contained a side-chain protecting group and that the only moiety predicted to be sensitive to carbocations was the thioether in the side-chain of Met35. Ten mL cleavage mixture A were used to cleave each peptide from the resin for 120 min. After filtration of the resin, the volume was reduced to 1–2 mL and loaded directly onto an HPLC column. Because the mixture contained high concentration of TFA, which could partially dissolve the resin in silica-based columns, we used in these experiments a polymer-based, 250 × 21 mm polyhydroxyethyl A column (The Nest Group, Southborough, MA). However, we could not recover meaningful quantities of peptide from the column using this protocol.
In subsequent experiments, we therefore attempted to go back to the silica-based C4 column used for purification of shorter CTFs. In order to avoid harming the silica-based resin, after reducing the volume of the cleavage mixture to 1–2 mL, it was diluted in a mixture containing milli-Q water, ACN, and in some cases 2,2,2-trifluoroethanol (TFE) or 1,1,1,3,3,3-hexafluoroisopropanol (HFIP). These fluorinated alcohols are known to induce α-helical conformation and help disaggregate β-sheet-rich amyloid protein aggregates. This step was adjusted empirically for each peptide and each synthesis batch with the goal to keep most of the crude peptide in solution. It was impossible to measure precisely the relative quantity of each solvent in each experiment. The final volume loaded onto the C4 column was ~10 mL. Using this procedure, small amount of product could be purified (see Table 1). However, all the peptides contained a side-product that eluted with highly similar retention time to the desired product and displayed a molecular mass increase of 164 mass units. We interpreted this result to signify that during the cleavage reaction, some of the 4-hydroxymethylphe- noxyacetamide linker connecting the peptide to the TGA resin, got cleaved and formed this adduct, presumably by reaction with the thioether side-chain of Met. The close proximity in retention time of the adduct and the pure peptide compromised the yield substantially, in some cases to <1%. In addition, the absorbance peak of the adduct was considerably larger than that of the pure peptide (Fig. 2), either because of higher extinction coefficient at 214 nm or due to higher solubility of the side product containing the adduct relative to the desired peptide that did not contain the adduct. To reduce this side product, we attempted using cleavage mixtures 3 or 4. However, although the scavengers reduced the amount of the side product, its apparent relative abundance in HPLC analysis did not change significantly because of the difficulty to detect the desired product. We also attempted to use 2-chlorotrityl resin instead of TGA resin, to eliminate the presence of the 4-hydroxymethylphe- noxyacetamide linker altogether. However, these experiments yielded negligible amounts of desired products.
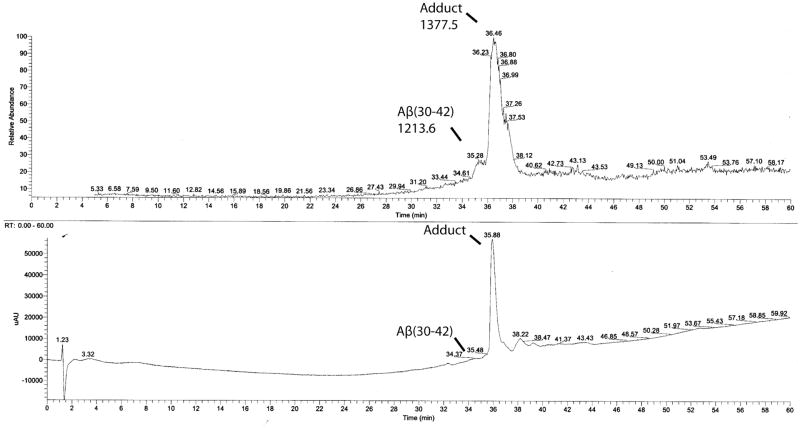
Fig. 2.
HPLC analysis of partially purified Aβ(30–42). Collected fractions from semi-preparative purification of Aβ(30–42) were pooled together, concentrated, and an aliquot injected onto a microbore C4 column interfaced with UV and MS detectors in tandem. Elution was done using a gradient of 20–80% solvent B during 60 min at a flow rate of 100 μL/min. At this flow rate, the delay between the UV and MS detectors is 0.6 min. The upper panel shows the TIC trace, in which a broad peak at 35.28 min is the desired product, followed by the +164 amu adduct at 36.46 min. The lower panel shows the UV trace (at 214 nm), in which the adduct is a large peak at 35.88 min preceded by a small shoulder, which is the desired product.
The low solubility of the CTFs, Aβ(34–42) through Aβ (29–42), in the HPLC solvent system and the need to separate them from closely migrating impurities caused very low recovery of the peptides from the HPLC columns used. Although some of the material loaded onto the column was eluted, during the course of the work we realized that the bulk of these peptides could not be recovered, likely because they precipitated out of solution during the early steps of the elution and did not re-dissolve in later stages or during wash steps. The recovery decreased with increasing peptide length. Peptides longer than Aβ(33–42) necessitated analyzing fractions collected during semi-preparative HPLC purification by MS because the amount of peptide soluble in the mobile phase was too low to detect by UV absorbance (Fig. 2).
To overcome these difficulties, eventually, we modified the purification protocol and found out that CTFs that did not precipitate by addition of diethyl ether to the crude cleavage mixture could be isolated by water precipitation. This procedure was applied only to Aβ(31–42) and Aβ(32–42) but likely is applicable to Aβ(34–42) through Aβ(29–42). The best results were obtained by using cleavage mixture 4 for 120 min. Following this step, Aβ(31–42) or Aβ(32–42) were precipitated by dilution with 25 mL of ice-cold milli-Q water. The precipitate was filtered, reconstituted in 60% ACN and lyophilized. The lyophilizate was washed with diethyl ether to remove scavengers and re-precipitated by centrifugation. The ether was discarded and the peptide dried in an RT100 SpeedVac concentrator (Savant, Ramsey, MN). The dry peptide was reconstituted in 60% ACN and lyophilized. This protocol yielded Aβ(31–42) and Aβ(32–42) at 23% and 18% yield, respectively. Because HPLC analysis could not be used for reliable estimation of peptide purity, the purity was assessed by HPLC-MS and AAA (Table 1).
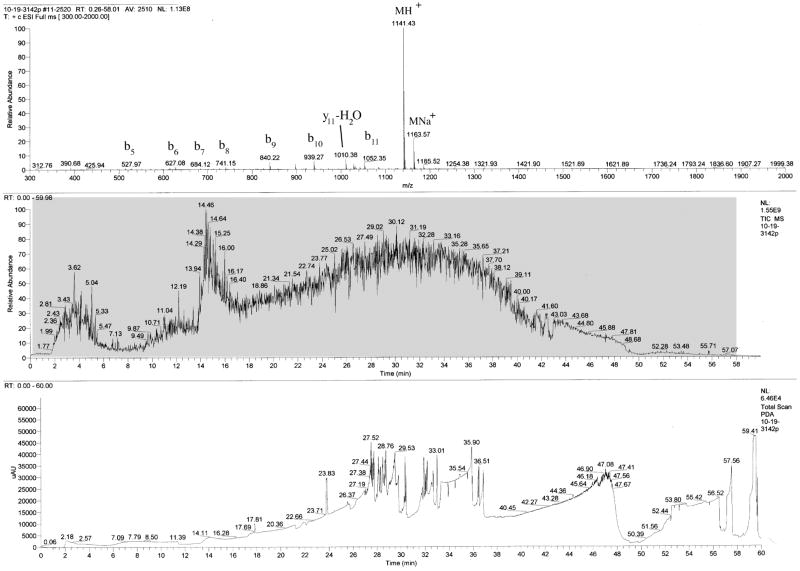
Fig. (3) shows an example of HPLC-MS analysis of Aβ(31–42). The peptide appears as a small broad peak in the UV trace followed by multiple narrow peaks that probably represent negligible impurities and/or system “noise.” The total ion current (TIC) trace of the MS detector shows a ~3 min broad peak followed by a ~30 min broad peak, both likely corresponding to the desired product. The combined mass-spectrum of the entire chromatogram (Fig. 3, gray box) shows Aβ(31–42) as the only product. In addition, the average AAA error (Table 1) is similar to that of the CTFs purified by HPLC, suggesting similar degree of purity.
Fig. 3.
HPLC-MS analysis of water-precipitated Aβ(31–42). Aβ(31–42) was purified by water precipitation and washing with diethyl ether. An aliquot was dissolved in 60% ACN and fractionated on a microbore C4 column as described in the legend of Fig. (2). The lower panel shows the UV trace at 214 nm. A small and wide peak at 14.11 min is a fast migrating fraction of Aβ(31–42). The middle panel shows the TIC trace. The upper panel shows the combined spectra of all the time points between 0.26 and 58.01 min (gray box in middle panel). A main peak of Aβ(31–42) and minor peaks of its gas phase-induced fragments are the only masses observed.
DISCUSSION
Despite decades of research, AD is a disease that currently cannot be cured or prevented. Currently approved drugs for AD treat the symptoms, rather than the causes of the disease and provide only moderate and temporary relief [27]. AD is the leading cause of dementia and one of the leading causes of death among elderly people [28]. A recent report by the Alzheimer Association indicated that in 2007, the prevalence of AD in the US has exceeded 5 million and may increase up to 16 million by the middle of the century if no cure is found [29]. As the population ages, this situation may lead to an epidemic. Current estimates of cost of care for patients with AD in the US are over $148 billion a year [29]. Numbers in other countries also are highly alarming [30–32]. Thus, there is an urgent need for disease-modifying therapeutic strategies for AD. Following the modified Amyloid Cascade Hypothesis [2], leading strategies are focusing on Aβ as a primary cause of AD and therefore target inhibition of Aβ production, enhancement of Aβ clearance, or disruption of Aβ assembly [33]. The normal physiologic function of Aβ is unknown, thus inhibiting its production or increasing its clearance may lead to adverse side effects. In contrast, Aβ self-association into oligomers and polymers is purely a pathologic phenomenon and therefore is an attractive target for development of inhibitors.
Multiple examples of small molecule inhibitors of Aβ fibrillization exist in the literature [34]. Recently, several groups reported inhibitors of Aβ oligomer formation [35–38], following a paradigm shift in the amyloid field that identified pre-fibrillar oligomers as the primary cause of cytotoxicity [39, 40]. Inhibitor selection in these studies was based on empirical findings, rather than on systematic rational design. Therefore, little can be deduced about their mechanism of action. In contrast, structure-based inhibitor design approaches can provide mechanistic data that can be used to improve inhibitor efficacy and pharmacokinetics.
The main driving force for Aβ self-assembly likely is intermolecular hydrophobic interactions that lead to sequestering hydrophobic patches from the aqueous environment [18]. Thus, a reasonable rational design approach to inhibiting the assembly is to use short peptides derived from hydrophobic regions in Aβ itself, which can compete with the same region in the full-length Aβ. Following this rationale, a large body of work has focused on peptides derived from the central hydrophobic cluster (Aβ(17–21)) [41, 42], which had been shown to be important for Aβ fibrillogenesis [43], though not necessarily for the formation of early oligomers. Because oligomers of Aβ42 likely are the main culprit causing neurotoxicity in AD, we decided to use peptides derived from the C-terminus of Aβ42 as potential inhibitors of oligomerization and toxicity of full-length Aβ42. Although this seems like an attractive strategy, presumably it has not been attempted previously because of the difficulty in preparing and solubilizing these highly hydrophobic peptides, particularly of longer CTFs.
The systematic study reported here shows that the synthesis and purification of Aβ42 CTFs longer than 8-amino acids indeed are difficult. Although no particular problems were encountered during the synthesis itself, the low solubility of these CTFs in both aqueous and organic solvents made their purification by common RP-HPLC techniques difficult. Although the bulk of the crude preparation presumably contained the desired peptides, RP-HPLC analysis, particularly of the long CTFs, was substantially skewed, over-represent- ing impurities with higher solubility and/or higher absorbance at the detection wavelength (Figs. 2 and 3). For the same reasons, recovery of the pure peptides following preparative or semi-preparative HPLC purification was very low. To solve this difficulty, we adopted a protocol that circumvented the need for HPLC purification by taking advantage of the same properties that make HPLC purification difficult - low solubility in both hydrophilic and hydrophobic solvents. By precipitating the peptides in water followed by resuspending and washing with diethyl ether, we obtained some of the most difficult sequences in the CTF series with high purity and moderate yield (Fig. 3 and Table 1).
The solubility of the purified CTFs in aqueous buffers ranged from a few micromolar to a few hundred micromolar [44]. Somewhat surprisingly, although Aβ(28–42) could be purified by HPLC at acidic pH with reasonable yield, it was the least soluble CTF at pH 7.4 [44]. The solubility of all other CTFs was sufficient for biological and biophysical studies, the details of which will be published elsewhere. These studies show that the CTFs are capable of inhibiting Aβ42-induced neurotoxicity and therefore are good lead candidates for developing peptidomimetic inhibitors with improved pharmacokinetic characteristics, for prevention and treatment of AD.
Acknowledgments
This study was supported by grants AG027818 from the NIH/NIA and 2005/2E from the Larry L. Hillblom Foundation, and by a generous gift from the Turken family.
References
- 1.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–85. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 2.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–56. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki N, Cheung TT, Cai XD, et al. An increased percentage of long amyloid β protein secreted by familial amyloid β protein precursor (βAPP717) mutants. Science. 1994;264(5163):1336–40. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 4.Suo ZM, Humphrey J, Kundtz A, et al. Soluble Alzheimers β-amyloid constricts the cerebral vasculature in vivo. Neurosci Lett. 1998;257(2):77–80. doi: 10.1016/s0304-3940(98)00814-3. [DOI] [PubMed] [Google Scholar]
- 5.Younkin SG. Evidence that Aβ42 is the real culprit in Alzheimer’s disease. Ann Neurol. 1995;37(3):287–88. doi: 10.1002/ana.410370303. [DOI] [PubMed] [Google Scholar]
- 6.Scheuner D, Eckman C, Jensen M, et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the Presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2(8):864–70. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 7.Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature. 2001;414(6860):212–16. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 8.Bitan G, Kirkitadze MD, Lomakin A, et al. Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc Natl Acad Sci USA. 2003;100(1):330–35. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert MP, Barlow AK, Chromy BA, et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95(11):6448–53. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fradinger EA, Spring SM, Condron MM, et al. Structural stabilization of oligomers increases neurotoxicity of the amyloid β-protein (Aβ). Paper presented at the 34th Annual Meeting of the Society for Neuroscience; Washington, DC. 2005. Abstract number 587.14. [Google Scholar]
- 11.Barghorn S, Nimmrich V, Striebinger A, et al. Globular amyloid β-peptide oligomer - a homogenous and stable neuropathological protein in Alzheimer’s disease. J Neurochem. 2005;95(3):834–47. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- 12.Dahlgren KN, Manelli AM, Stine WB, et al. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem. 2002;277(35):32046–53. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 13.Hoshi M, Sato M, Matsumoto S, et al. Spherical aggregates of β-amyloid (amylospheroid) show high neurotoxicity and activate tau protein kinase I/glycogen synthase kinase-3β. Proc Natl Acad Sci USA. 2003;100(11):6370–75. doi: 10.1073/pnas.1237107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murakami K, Irie K, Ohigashi H, et al. Formation and stabilization model of the 42-mer Aβ radical: implications for the long-lasting oxidative stress in Alzheimer’s disease. J Am Chem Soc. 2005;127(43):15168–74. doi: 10.1021/ja054041c. [DOI] [PubMed] [Google Scholar]
- 15.Riek R, Guntert P, Döbeli H, et al. NMR studies in aqueous solution fail to identify significant conformational differences between the monomeric forms of two Alzheimer peptides with widely different plaque-competence, Aβ(1–40)ox and Aβ(1–42)ox. Eur J Biochem. 2001;268(22):5930–36. doi: 10.1046/j.0014-2956.2001.02537.x. [DOI] [PubMed] [Google Scholar]
- 16.Yan Y, Liu J, McCallum SA, et al. Methyl dynamics of the amyloid-β peptides Aβ40 and Aβ42. Biochem Biophys Res Commun. 2007;362(2):410–14. doi: 10.1016/j.bbrc.2007.07.198. [DOI] [PubMed] [Google Scholar]
- 17.Lazo ND, Grant MA, Condron MC, et al. On the nucleation of amyloid β-protein monomer folding. Protein Sci. 2005;14(6):1581–96. doi: 10.1110/ps.041292205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urbanc B, Cruz L, Yun S, et al. In silico study of amyloid β-protein folding and oligomerization. Proc Natl Acad Sci USA. 2004;101(50):17345–50. doi: 10.1073/pnas.0408153101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yun S, Urbanc B, Cruz L, et al. Role of Electrostatic Interactions in Amyloid β-Protein (Aβ) Oligomer Formation: A Discrete Molecular Dynamics Study. Biophys J. 2007;92(11):4064–77. doi: 10.1529/biophysj.106.097766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krafft GA, Joyce J, Jerecic J, et al. Design of arrested-assembly Aβ1–42 peptide variants to elucidate the ADDL oligomerization pathway and conformational specificity of anti-ADDL antibodies. Paper presented at the 35th Annual Meeting of the Society for Neuroscience; Atlanta, GA. 2006. Abstract number 509.5. [Google Scholar]
- 21.Sampson WR, Patsiouras H, Ede NJ. The synthesis of ‘difficult’ peptides using 2-hydroxy-4-methoxybenzyl or pseudoproline amino acid building blocks: a comparative study. J Pept Sci. 1999;5(9):403–09. doi: 10.1002/(SICI)1099-1387(199909)5:9<403::AID-PSC213>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 22.Ajikumar PK, Devaky KS. Solid phase synthesis of hydrophobic difficult sequence peptides on BDDMA-PS support. J Pept Sci. 2001;7(12):641–49. doi: 10.1002/psc.355. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson MR, Nguyen LL, Raleigh DP. Synthesis and purification of amyloidogenic peptides. Anal Biochem. 2001;288(1):76–82. doi: 10.1006/abio.2000.4887. [DOI] [PubMed] [Google Scholar]
- 24.Tickler AK, Clippingdale AB, Wade JD. Amyloid-β as a “difficult sequence” in solid phase peptide synthesis. Protein Pept Lett. 2004;11(4):377–84. doi: 10.2174/0929866043406986. [DOI] [PubMed] [Google Scholar]
- 25.Sohma Y, Chiyomori Y, Kimura M, et al. ‘O-Acyl isopeptide method’ for the efficient preparation of amyloid β peptide 1–42 mutants. Bioorg Med Chem. 2005;13(22):6167–74. doi: 10.1016/j.bmc.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 26.Lansbury PT, Costa PR, Griffiths JM, et al. Structural model for the β-amyloid fibril based on interstrand alignment of an antiparallel-sheet comprising a C-terminal peptide. Nat Struct Biol. 1995;2(11):990–98. doi: 10.1038/nsb1195-990. [DOI] [PubMed] [Google Scholar]
- 27.Monien BH, Apostolova LG, Bitan G. Early diagnostics and therapeutics for Alzheimer’s disease-how early can we get there? Expert Rev Neurother. 2006;6(9):1293–306. doi: 10.1586/14737175.6.9.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ewbank DC. Deaths attributable to Alzheimer’s disease in the United States. Am J Publ Health. 1999;89(1):90–92. doi: 10.2105/ajph.89.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alz.org [hompage on the Internet] Washington, DC: The Alzheimer Association; Every 72 seconds someone in America develops Alzheimer’s. Available from http://www.alz.org/news_and_events_rates_rise.asp. [Google Scholar]
- 30.Leung GM, Yeung RY, Chi I, et al. The economics of Alzheimer disease. Dement Geriatr Cogn Disord. 2003;15(1):34–43. doi: 10.1159/000066675. [DOI] [PubMed] [Google Scholar]
- 31.Beeri MS, Werner P, Adar Z, et al. Economic cost of Alzheimer disease in Israel. Alzheimer Dis Assoc Disord. 2002;16(2):73–80. doi: 10.1097/00002093-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Zencir M, Kuzu N, Beser NG, et al. Cost of Alzheimer’s disease in a developing country setting. Int J Geriatr Psychiatry. 2005;20(7):616–22. doi: 10.1002/gps.1332. [DOI] [PubMed] [Google Scholar]
- 33.Shanmugam A, Monien BH, Bitan G. Development in Diagnostic and Therapeutic Strategies for Alzheimer’s Disease. In: Sun M-K, editor. Research Progress in Alzheimer’s Disease and Dementia. Nova Science Publishers, Inc; In press. [Google Scholar]
- 34.Doig AJ, Stott K, Treherne JM. Inhibitors of amyloid aggregation: technologies for the discovery of novel lead compounds. Biotech Genet Eng Rev. 2004;21:197–212. doi: 10.1080/02648725.2004.10648055. [DOI] [PubMed] [Google Scholar]
- 35.Wang Z, Chang L, Klein WL, et al. Per-6-substituted-per-6-deoxy β-cyclodextrins inhibit the formation of β-amyloid peptide derived soluble oligomers. J Med Chem. 2004;47(13):3329–33. doi: 10.1021/jm034224e. [DOI] [PubMed] [Google Scholar]
- 36.Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280(7):5892–901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 37.Walsh DM, Townsend M, Podlisny MB, et al. Certain inhibitors of synthetic amyloid β-peptide (Aβ) fibrillogenesis block oligomerization of natural Aβ and thereby rescue long-term potentiation. J Neurosci. 2005;25(10):2455–62. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Necula M, Kayed R, Milton S, et al. Small molecule inhibitors of aggregation indicate that amyloid β oligomerization and fibrillization pathways are independent and distinct. J Biol Chem. 2007;282(14):10311–24. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- 39.Kirkitadze MD, Bitan G, Teplow DB. Paradigm shifts in Alzheimer’s disease and other neurodegenerative disorders: The emerging role of oligomeric assemblies. J Neurosci Res. 2002;69(5):567–77. doi: 10.1002/jnr.10328. [DOI] [PubMed] [Google Scholar]
- 40.Klein WL, Stine WB, Jr, Teplow DB. Small assemblies of unmodified amyloid β-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiol Aging. 2004;25(5):569–80. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 41.Bieler S, Soto C. β-sheet breakers for Alzheimer’s disease therapy. Curr Drug Targets. 2004;5(6):553–8. doi: 10.2174/1389450043345290. [DOI] [PubMed] [Google Scholar]
- 42.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4(1):49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 43.Hilbich C, Kisters-Woike B, Reed J, et al. Substitutions of hydrophobic amino acids reduce the amyloidogenicity of Alzheimer’s disease βA4 peptides. J Mol Biol. 1992;228(2):460–73. doi: 10.1016/0022-2836(92)90835-8. [DOI] [PubMed] [Google Scholar]
- 44.Monien BH, Lomakin A, Li H, et al. Mechanistic Insight into the Inhibition of Aβ42 Neurotoxicity by Aβ42 C-terminal Fragments. Manuscript in preparation. [Google Scholar]