Abstract
Human immunodeficiency virus-1 (HIV-1) infection may produce neurological deficits, such as cognitive decline, that may be worsened by concurrent ethanol (EtOH) abuse. Among the many biochemical cascades likely mediating HIV-1 associated neuronal injury is enhancement of N-methyl-d-aspartate (NMDA) receptor function and progression to excitotoxicity, an effect that may be directly or indirectly related to accumulation in brain of the HIV-1 transcription factor Tat. The present studies were designed to examine the hypothesis that binge-like EtOH pre-exposure would enhance effects of Tat on NMDA receptor function. These studies employed a modified in vivo binge EtOH exposure regimen designed to produce peak blood EtOH levels (B.E.L.) of <200 mg/dl in adult male rats and were designed to examine effects of intra-hippocampal injection of Tat (0.5 µl/500 pM/2 min) on EtOH withdrawal-related behavior, spatial learning, and histological measures. Unilateral cannulae were implanted into the cornu ammonus 1 (CA1) pyramidal cell layer of animals prior to beginning a 4-day binge EtOH regimen. EtOH was administered via intragastric intubation (~3.0–5.0g/kg) with dose determined by behavioral ratings of intoxication daily for four days (at 0800, 1600, and 2400 hrs). EtOH withdrawal behaviors were monitored 12 hr after the last administration of EtOH. Morris water maze learning was assessed during the following 4 days, at which times brains were harvested for autoradiographic measurement of NMDA receptor density and neuroinflammation. Maximal B.E.L.s of 187.69 mg/dl were observed 60 min after EtOH administration on Day 2 of the regimen. In contrast, peak B.E.L.s of approximately 100 mg/dl were observed 60 min after EtOH administration on Day 4 of the regimen, suggesting development of metabolic tolerance. Significant behavioral abnormalities were observed in EtOH withdrawn animals, including tremor and seizures. Intra-CA1 region injection of Tat significantly potentiated EtOH withdrawal behavioral abnormalities, an effect that was reduced by MK-801 pre-exposure. While EtOH withdrawn animals showed learning similar to control animals, EtOH withdrawn animals that received intra-CA1 Tat injection demonstrated persisting deficits in spatial learning on Days 3 and 4 of training, effects that were markedly reduced by administration of the competitive NMDA receptor antagonist MK-801 30 min prior to Tat injection. No changes in [3H]MK-801 binding were observed. Binding density of [3H]PK11195, a ligand for peripheral benzodiazepine receptors expressed on activated microglia, was elevated proximal to cannulae tracts in all animals, but was not altered by EtOH or Tat exposure. These finding suggest that EtOH abuse and/or dependence in HIV-positive individuals may promote HIV-1-associated cognitive deficits by altering NMDA receptor function in the absence of microglial activation or neuroinflammation.
Keywords: Alcoholism, AIDS, Memory, Substance Abuse, HIV-Associated Dementia, Hippocampus
INTRODUCTION
Human immunodeficiency virus 1 (HIV-1) infection is associated with the development of a dementing illness in a significant portion of those infected and has been suggested to be a leading cause of dementia in individuals under the age of 60, with approximately 33% of all seropositive individuals demonstrating some cognitive decline [Janssen et. al 1992; McArthur et. al 1993; Nath et al. 1996]. It has previously been suggested that alcohol dependence and/or abuse may exacerbate HIV-associated neurological decline (Tabakoff 1994; Tyor and Middlebaugh 1999). Examination of this possibility is likely of relevance given estimates that 40% of HIV-positive individuals may meet the diagnostic criteria for alcoholism and/or alcohol abuse [Lefevre et al. 1995]. Further, recent work has suggested that alcoholism and HIV severity may interact to produce synergistic deficits in brain volume (Pfefferbaum et al. 2006). Additional studies have reported that HIV-seropositive men with past alcohol abuse or dependence displayed significant, multi-domain cognitive dysfunction compared to those without an alcohol abuse history [Green et al. 2004; Rothlind et al. 2006]. However, little is known of the biochemical pathways contributing to this putative interaction of alcoholism and HIV-positive status.
Among the many HIV-1 proteins thought to mediate HIV-associated neuronal injury is Tat, an HIV-1 nuclear regulatory protein that functions to stabilize viral gene transcription [Garcia-Martinez et al. 1997; King et al. 2006]. Although Tat does not directly infiltrate neurons, it may be released from infected glia to interact with neurons or induce glia to release inflammatory mediators that influence neuronal function and viability [eg. Ensoli et al. 1993; Pocernich et al. 2005]. It is known that Tat also induces neuronal damage through excitotoxic mechanisms involving excessive Ca2+ influx mediated by glutamatergic receptors [Self et al. 2004; Prendergast et al. 2002; Pocernich et al. 2005a&b]. A postmortem study conducted on human frontal cortex of AIDS patients revealed a significant decrease in NMDA receptor density as compared with control subjects [Sardar et al. 1999], which may suggest the loss of neurons expressing these receptors. Further, Tat exposure in vitro produces a tyrosine kinase-dependent phosphorylation of NMDA receptor NR2 subunits (Haughey et al. 2001). Tat can induce release of the NMDA receptor agonists platelet-activating factor and quinolinic acid from glia, as well as tumor necrosis factor α, which stimulates the release of glutamate from astrocytes and prevents its uptake [Pocernich et al. 2005a&b].
Prolonged ethanol (EtOH) intake has also been widely reported to influence the expression and/or function of NMDA-type glutamate receptors. Findings suggest that acute or prolonged EtOH exposure produces compensatory increases in NMDA receptor function via genomic expression pathways; changes in receptor trafficking; and phosphorylation events involving NR2 subunits (Carpenter-Hyland et al. 2004; Kumari and Anji 2006; Suvarna et al. 2005). Thus, the presence of substances that promote activity of NMDA receptors, such as Tat, may exacerbate EtOH withdrawal behavioral effects and, possibly, microglial activation or neuroinflammation. Indeed, Self et al. (2004) demonstrated that pre-exposure to EtOH in vitro promoted Tat-induced hippocampal injury, in a manner reflecting activation of NMDA receptors. The present studies were designed to examine the effects of binge EtOH exposure and withdrawal in vivo on seizure development, spatial learning and microglial activation or neuroinflammation following intra-hippocampal Tat injection.
EXPERIMENTAL PROCEDURES
Intrahippocampal Cannulation
Ninety adult male Sprague-Dawley rats (N=12–18/group; age 60 days) were obtained (Harlan Laboratories; Indianapolis, IN, USA) and, following habituation, anesthetized with ketamine/xylazine (1 ml/kg i.p.) and placed in a stereotaxic instrument (Kopf; Tujunga, CA). Cannula (26-gauge) with 33-gauge stainless steel obturators (Plastics One; Roanoke, VA) were implanted in the CA1 region of the hippocampus, unilaterally, counterbalancing for side. The CA1 hippocampal region was chosen for cannula placement because this region displays a greater density of NMDA receptors [Martens et al. 1998] as compared to other hippocampal regions. Bilateral cannula implantation was not employed due to mortality concerns as previous studies have found significant mortality using bilateral Tat injections [Wang et al. 1999]. After cannula placement, cannulae were affixed to the skull using super glue, stainless steel skull screws, and dental acrylic. All cannulae supplies from Plastics One, Roanoke, VA.
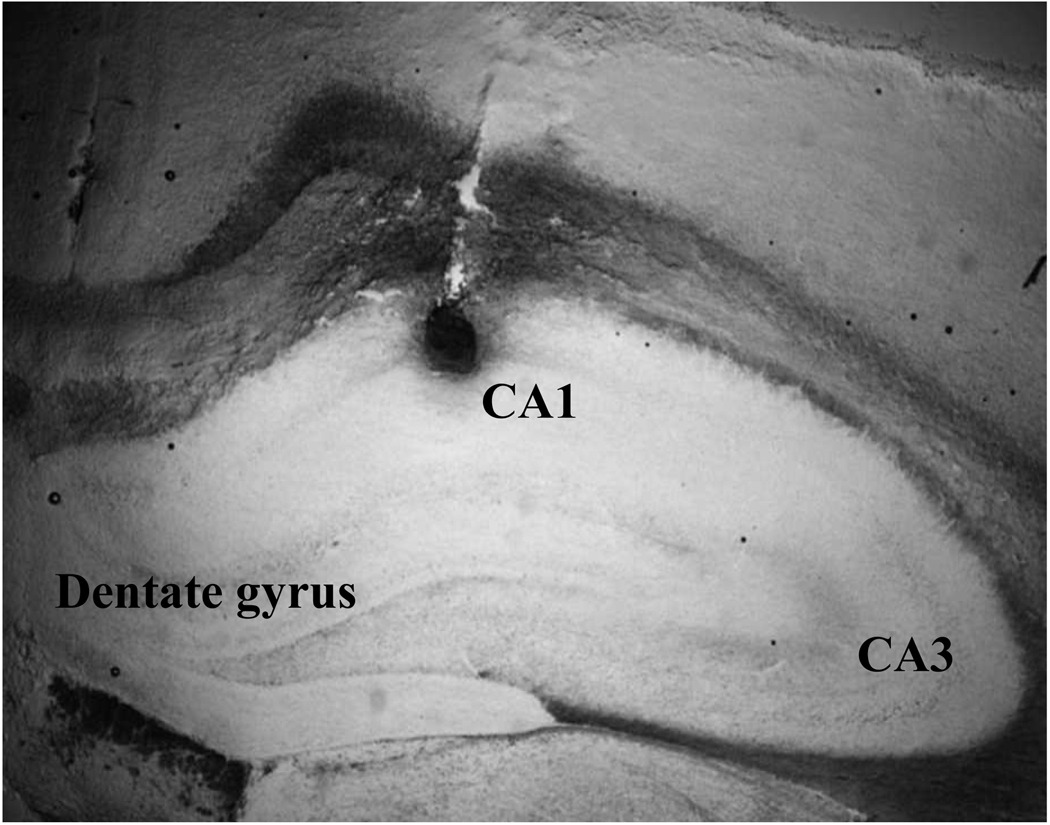
Stereotaxic coordinates used for the right CA1 as described in Paxinos & Watson [1998] were AP: −3.6; DV: 2.8; ML: 2.0 relative to bregma and left CA1 were AP: −3.6; DV: 2.8; ML: −2.0 relative to bregma. Guard caps were applied to guide cannula after surgery and animals were given a minimum of five days to recover before further experimentation, during which time they were monitored for potential infection and/or other complications. All animals were given Rimadyl® (carprofen) pain medication via sub-cutaneous injection 24 hours prior to surgery and 48 hours following surgery (5mg/kg; Pfizer Inc., New York, NY, USA.). A representative image showing injection of 0.2% thionin into the CA1 pyramidal cell somatic and projection layers is shown in Figure 1.
Figure 1.
Representative image of the distribution of thionin (0.2%) injected through an intra-CA1 pyramidal cell layer cannula at coordinates AP: −3.6; DV: 2.8; ML: 2.0/−2.0 relative to bregma and ventral to the juncture of the parietal, retrosplenial, and occipital cortices.
Tat Preparation/Purification
Recombinant HIV-1 Tat 1–72 was prepared by expressing the tat genome encoding the 1–72 amino acid sequence (first full exon) from HIV-1BRU, as a fusion protein with a naturally biotinylated protein at the N-terminus, in E. coli DH5aF’IQ [after Ma & Nath 1997]. The Tat protein was purified to approximately 100% purity as determined by SDS-PAGE followed by silver staining, and examined for any endotoxins and/or bioactivity. The biotinylated Tat protein was purified on a soft release avidin resin, cleaved from the fusion protein using a factor Xa and eluted from the column followed by desalting with a PD10 column and removal of endotoxin with a polymixin B column. Purified Tat contained no deleted or overhanging amino acids. All purification steps contained dithiothreitol to prevent oxidation of the protein. To assure stability and proper folding, each batch of Tat was desalted and stored in a lyophilized form at −80°C in endotoxin-free siliconized microfuge tubes until taken for experimentation. The functional activity of the Tat protein was confirmed via a transactivation assay in HL3TI cells containing an HIV-1 LTR-CAT construct [after Ma & Nath 1997]. Siliconized pipette tips were used for all experiments and whenever possible, single aliquots were used for each experiment due to the susceptibility of Tat to degradation and potential loss of biological activity with each freeze-thaw cycle.
EtOH Administration/Withdrawal
Animal acclimation and handling were conducted during the first two to three weeks after animals arrived at the research facility. During this time the animals were housed in groups of two to three per cage within the animal colony and handled each day. The second week of handling involved animals being transferred to individual cages and trained on the intragastric intubation procedure, during which time they were administered 1 ml of 0.1% saccharin in water each day for at least five days prior to further experimentation. The intubation tube used was a slightly curved 20 gauge stainless steel needle approximately 1 ½ inches long with a 3mm stainless steel feeding ball at the end (Fisher Scientific). EtOH was administered via intragastric intubation (~3.0–5.0g/kg of 40% w/v diluted in vanilla Ensure®/sterile water) with dose determined by behavioral ratings of intoxication thrice daily for four days (0800, 1600, and 2400 hrs), after Majchrowicz (1975), adapted by Acheson et al. [1999], Crews et al. [2000], and Nixon & Crews [2002]. A priming dose of 5.0 g/kg was used at 0800 on Day 1. Control animals received an equivolume isocaloric diet of maltose (40% w/v in vanilla Ensure®/sterile water). All animals had ad libitum access to standard rat chow throughout the experiments.
Determination of Blood EtOH Concentrations
To determine blood EtOH concentrations (B.E.C.), blood was collected via tail nick from all animals (N=12–18/group) at 0900, 0930 and 1500 hrs on the second and fourth days of treatment and centrifuged to collect serum. All serum samples were evaluated on an Analox AM1 apparatus (Analox Instruments; Lunenburg, MA, USA). The Analox AM1 apparatus measures blood alcohol concentrations indirectly through direct measurement of molecular oxygen levels. In the presence of molecular oxygen, EtOH is oxidized by the enzyme alcohol oxidase to form acetaldehyde and hydrogen peroxide. Under the conditions of the assay, oxygen consumption is directly proportional to EtOH concentration in the plasma sample.
Intrahippocampal Tat Injections/Morris Water Maze
Ten hours after the last EtOH gavage, one-half of the animals received a unilateral (counter-balanced) intra-CA1 region injections of saline or Tat 1–72 via a Teflon-treated 10µl Hamilton syringe (.5µl of a 500 pM Tat solution/ 2 minutes). Portions of animals also received an intraperitoneal injection of the non-competitive NMDA receptor antagonist MK-801 (0.075mg/kg; Sigma; St. Louis, MO) 30 minutes prior to microinfusions. Animals were then placed in a 40.64 sq. centimeter plexiglass chamber and withdrawal symptoms monitored for 1 min. Majchrowicz [1975] classified withdrawal symptoms into three categories (i.e., mild, moderate, severe) based on the number of behavioral withdrawal symptoms presented. For instance, animals displaying at least three of the following symptoms were said to be showing the ‘withdrawal syndrome’: tail tremors, tail rigidity, tremors of the caudal region, general rigidity, general tremors, general hyperactivity and convulsions. If an animal demonstrated all these symptoms (with or without violent convulsions) they were classified as being in severe withdrawal. An animal in mild withdrawal was classified as displaying at least three of these symptoms, and an animal in moderate withdrawal displayed more than three symptoms, but not all. Other symptoms were also discussed by Majchrowicz [1975] as being ‘the most salient characteristics’ indicative of the withdrawal syndrome such as wet shakes, teeth chattering, running episodes, stereotyped head and/or body movements, aggressiveness, vocalizations, and bodily excretions and/or secretions. Therefore, in these studies, animals were monitored for all documented withdrawal behaviors, as described in the original Majchrowicz study [1975].
Behavioral Paradigm: Morris Water Maze
Approximately 24 hrs after Tat or saline microinfusions and withdrawal behavior monitoring, animals began a Morris Water Maze (MWM) task to test spatial learning and memory [Morris et al. 1985]. Animals were tested for 5 consecutive days in the MWM apparatus, with acquisition training beginning approximately 37 hours after final EtOH dose and 24 hours after microinfusions. The pool was an open-field circular pool approximately 157.48 cm in diameter X 34.29 cm in height divided into five quadrants (N, S, E, W, and platform). A removable plastic platform (10.16 cm in diameter) was always placed in the North East quadrant approximately 30 cm from the pool wall and 1 cm below the surface of the water. Water in the pool was painted flat black with non-toxic black powdered paint to obscure platform location and water was maintained at 19–21°C.
Each day of testing consisted of four 60 second trials for animals to navigate the pool and locate the hidden platform, with each trial randomized between one of four locations of entry (N, S, E, W). Spatial cues in the testing room remained constant throughout testing and the researcher stepped behind a white screen after placing each animal in the pool to prevent the researcher from serving as a cue to the animals. If an animal found the platform during a given trial they were allowed a brief (~15 seconds) sit on the platform followed by a 5 minute inter-trial interval (ITI) back in their home cages prior to the next trial. If, at the end of the 60 second trial the rat failed to find the platform, they were gently guided through the water to the platform and allowed a 15 second platform sit, again, followed by a 5 minute inter-trial interval in their home cage. Twenty-four hours after the fourth acquisition day and prior to the probe trial, a visual platform test was performed on the animals to ensure that any visual disturbance did not account for any significant differences among treatment groups. In the visual platform test, the animals were allowed 30 seconds to find a visible platform approximately 1 cm above water level located in the south east quadrant of the pool, with animals entering the water from the south quadrant. Following the visual platform test, a probe trial was conducted on all animals consisting of a 30 second swim in the pool with the platform absent. Pool entry for the probe trial was consistently initiated in the center of the South West location for all animals. A video camera was located directly above the pool to record swimming during acquisition and probe trials. A video motion analyzer (EZ Mapper; AccuScan Instrument, Inc.) was utilized to compute swim strategy and speed during acquisition and probe trials. Data were collected using EZ Video DV Version 5.51 software (AccuScan Instruments, Inc., Columbus, OH) for each day of acquisition and probe trials and analyzed as described in the ‘statistical analysis’ section below.
Autoradiographic analysis of [3H]MK-801 and [3H]PK11195 binding
Following EtOH withdrawal, saline, Tat, or Tat + MK-801 injections and/or MWM testing, rats were humanely sacrificed. Brains were flash-frozen in isopentane on dry-ice for histological procedures. Brains were sectioned at 18µm through the rostral hippocampus using a freezing cryostat (Leica) and mounted on gelatin/chromium potassium sulfate-coated slides. Serial hippocampal slices from each animal were placed on different slides for [3H]PK11195 labeling and NMDA receptor density analysis. Tissue was fixed for 30 minutes in 4% phosphate-buffered paraformaldehyde then rinsed in buffer and incubated with 4% hydrogen peroxide for 15 minutes to satiate endogenous peroxidases. Histological examination of hippocampal integrity was conducted via autoradiography using [3H]PK11195 staining to assess glial activation and cannula placement accuracy. Following these procedures, brain sections were blocked in 5% (v/v) horse serum with 1% bovine serum albumin for approximately 30 minutes and then the ligand was applied as described below. In the case of [3H]PK11195 binding, slides were preincubated for 15 minutes at 4°C and washed in 50mM Tris HCl (pH:7.4, 7.88g/L). The slides were then incubated for 2 hours at 4°C with [3H]PK11195 (1nM; 85.5 Ci/mM; 250µCi; 18µl/175ml) radioisotope. Following incubation with the radioisotope, slides were washed three times in the Tris buffer in separate boxes at 4°C for 3 minutes each, then dipped in distilled water at 4°C and allowed to dry thoroughly (overnight) . Tissue slides were then exposed to x-ray radiographic film (3H-Hyperfilm, Amersham, UK) in Wolf X-Ray cassettes that contained calibrated tissue paste 3H standards prepared from whole brain homogenates [Pauly and Collins 1993]. Films were exposed for 4.5 weeks, developed (immersed in Kodak D-19 developer (5min), indicator stop bath (30sec) and Kodak rapid fixer (5min)), and images acquired using NIH image software on an Apple PowerPC connected to a digital camera (Dage CCD100, Michigan City, IN, USA). Following imaging of autoradiograms, all slices were stained with 0.2% thionin to visually confirm the accuracy of cannulae tracts. If cannulae tracts were observed by 2 or more raters to be more than 2 mm divergent from the expected coordinates, as illustrated in Figure 1, those slices were excluded from analyses.
[3H]PK11195 is a isoquinoline carboxamide and a specific ligand for the peripheral benzodiazepine binding site (PBBS) [Pappata et al. 2000]. Previous research has shown that an increase in [3H]PK11195 binding is observed in and around the infarcted tissue after cerebral ischemia in rodent and cat brain, co-localizing with invading cells of mononuclear-phagocytic lineage [Dubois et al. 1988; Myers et al. 1991; Conway et al. 1998]. However, in distant areas that have an intact blood-brain barrier and are free of macrophages, increased PK11195 binding suggests the presence of activated microglia, as opposed to astrocytes which express high levels of PBBSs in cell culture conditions [Itzhak et al. 1993]. Therefore, [3H]PK11195 binding was employed to provide an indication of tissue trauma as a result of cannula placement and Tat/drug administration.
NMDA receptor density was analyzed using coronal sections of brains cut on a cryostat and thaw-mounted onto gelatinized slides. These sections were stored at −80°C until used for autoradiography. Determination of changes in NMDA receptor density was performed using [3H]MK-801 binding. Slide-mounted sections were preincubated in 5 mM Tris HCl buffer + 2.5mM CaCl2, pH 7.4, for 30 min at room temperature. Sections were then transferred to 5mM Tris HCl buffer acetate buffer containing 10nM [3H]MK-801 (Perkin Elmer; Waltham, Massachusetts; specific activity: 1000 Ci/mmol; total activity 10µCi), 5µM spermidine, 100µM glycine, and 5µM glutamic acid and incubated for 90 min at room temperature. After incubation, the sections were washed three times in 5mM Tris HCl (4°C, 20 min each), once in dilute Tris HCl (x 0.1, 4°C, 10 sec) and once in deionized water (ice cold, 10 sec), and then gently air-dried and stored overnight in a desiccator at room temperature. All slides were then exposed to tritium-sensitive Amersham Hyperfilm (3H) in Wolf X-Ray cassettes that contained calibrated tissue paste 3H standards prepared from whole brain homogenates [Pauly and Collins 1993]. Unlabeled MK-801 (10µM) was used for nonspecific binding and this binding signal did not exceed the film background. [3H]PK11195 and [3H]MK-801 films were exposed for 4.5 weeks and processed in Kodak D-19 developer (5min), indicator stop bath (30sec) and Kodak rapid fixer (5min). Images were acquired using NIH image software on an Apple PowerPC connected to a digital camera (Dage CCD100, Michigan City, IN, USA).
Statistical Analysis
A two-way ANOVA was employed to determine effects of time and day on BEC’s, mean intoxication scores, and mean EtOH dose administered. Behavioral ratings of EtOH withdrawal behaviors were converted to a numerical scale based on the severity of withdrawal using the following conversion: mild withdrawal= 1; moderate withdrawal = 2; severe withdrawal= 3; and severe withdrawal with convulsions = 4. These data were analyzed using a one-way ANOVA, as were data for duration of behaviors and latency to initiate behaviors. Two-way repeated measures ANOVAs (treatment X day) were employed to examine treatment effects on water maze behavior, with the exception of the probe trial and visual platform tests, which were analyzed using one-way ANOVAs. Analyses of [3H]PK11195 and [3H]MK801 binding were conducted using two-way ANOVAs (treatment X cannula placement) for each hippocampal region (CA1, CA3, and dentate gyrus). All statistics were conducted using SigmaStat software (Systat, Inc., Chicago, Il).
RESULTS
Binge EtOH dosing
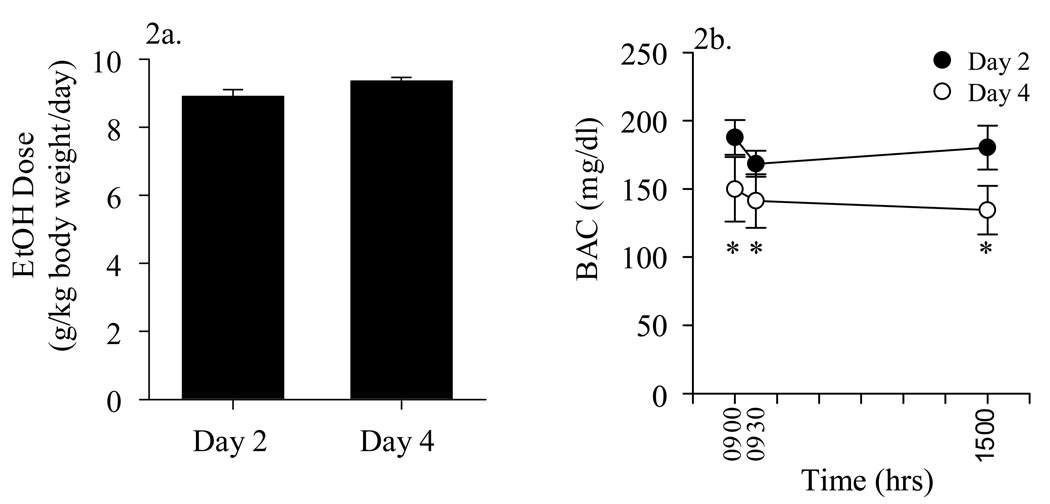
EtOH-treated animals lost a mean of 38.656±2.89 grams, or 11.5% body weight, over the course of the binge treatment. No mortality was observed, on any day of EtOH administration. No significant differences in mean EtOH dose (g/kg/ body weight/day) were observed for Days 1–4 of EtOH administration (Days 2 and 4 illustrated in Figure 2a). In contrast, a two-way ANOVA was conducted to determine effects of day and time on blood EtOH concentrations and indicated that there was a significant effect of day (2 vs 4), but not time [F(1, 60)=5.848, p=0.019]. Blood EtOH levels obtained at 0900, 0930, and 1500 hrs on Day 4 were significantly lower than were those obtained at the same times on Day 2, suggesting the development of metabolic tolerance (Figure 2b). Peak blood EtOH levels reached 187.69 mg/dl ± 12.74 1 hr following the 0800 hr gavage EtOH administration on Day 2 of treatment. In contrast, mean blood EtOH levels reached on 95–100 mg/dl 1 hr following the 0800 hr gavage on Day 4 of treatment. The mean blood EtOH levels in animals eight hours after the final EtOH administration on Day 4 was 103.50 ± 33.79.
Figure 2.
Mean daily EtOH doses and blood EtOH concentrations in EtOH-treated animals at 0900, 0930, and 1500 hrs on Days 2 and 4 of binge EtOH treatment. No significant differences in mean EtOH doses were observed, though blood EtOH levels were significantly reduced by Day 4, as compared to Day 2 of the EtOH dosing regimen. * = P < 0.05 vs measurements taken at same time on Day 2.
Behavioral Testing: EtOH Withdrawal Behaviors
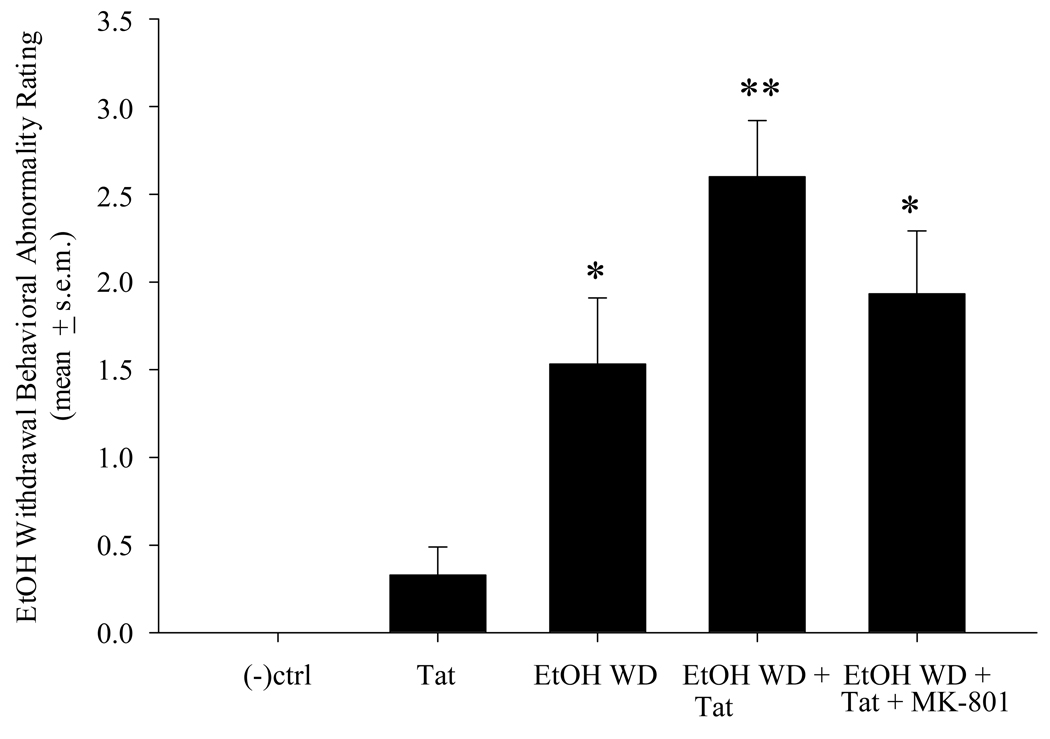
No withdrawal symptoms were evident in any control or MK801-treated animals. Mean withdrawal scores for EtOH-naïve animals injected with Tat were slightly increased above control levels, but this effect did not reach statistical significance. In contrast, EtOH withdrawal produced significant behavioral abnormalities including tremor, splayed paw, tail rigidity and seizure [F(5,84) = 18.49, p < 0.001]. Further, intra-CA1 tat injection immediately prior to monitoring of withdrawal behaviors significantly increased the severity of these behaviors (Figure 3). Intra-CA1 injection of Tat vehicle (0.9% saline) did not alter EtOH withdrawal behavior. Animals that received an ip injection of MK-801 30 min prior to Tat infusion and withdrawal behavioral monitoring displayed EtOH withdrawal behaviors that were not significantly more severe than those observed in EtOH withdrawn Tat-naïve animals
Figure 3.
EtOH withdrawal-related behavioral abnormalities, including tremor, tail rigidity, splayed paw and seizure, were significantly exacerbated by intra-CA1 Tat injection (0.5µl of a 500 pM Tat solution/ 2 minutes) and attenuated by MK-801 pre-treatment (0.075mg/kg, i.p.). *=P<0.05 vs control and Tat-injected animals. **=P<0.05 vs all other groups.
Behavioral Testing: Morris Water Maze
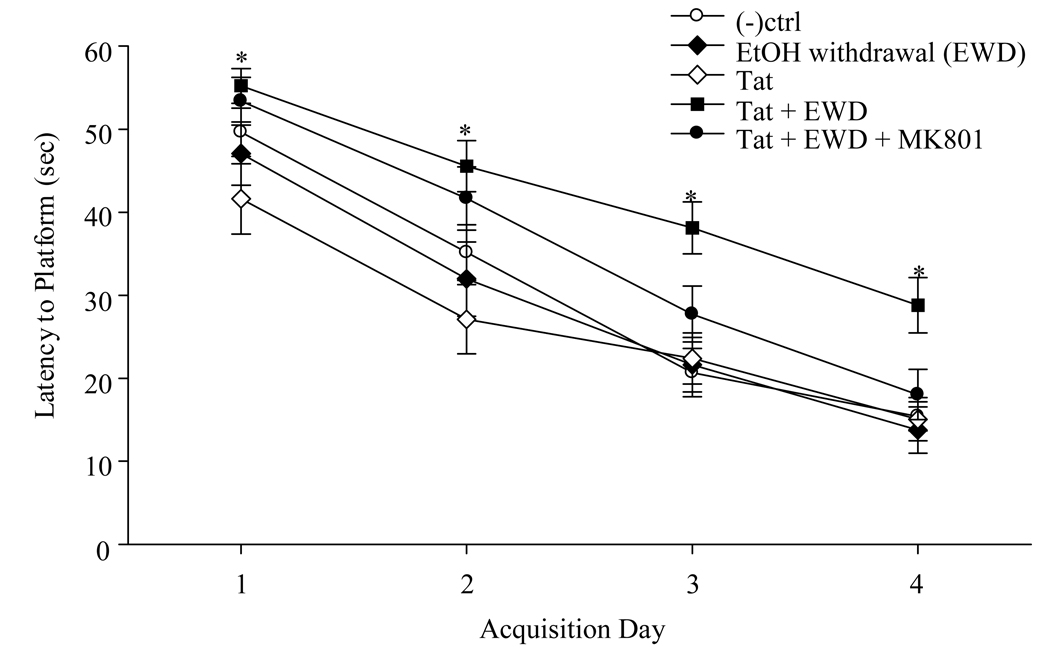
Two-way repeated measures ANOVAs were conducted to examine swim speeds and latencies to find the hidden platform in a Morris water maze tank in animals of different groups across four days of testing. There were no significant group differences in swim speeds or latencies. All animals displayed significant improvement on the MWM task across days of testing, as they were able to locate the platform in less time with each additional day of testing [F(3,142)=112.377, p<0.001]. However, on the third and fourth days of water maze testing it became evident that EtOH withdrawn rats that received Tat injection were performing significantly poorer on the task, as compared to all other experimental groups [F(5,142)=3.748, p=0.006]. These animals were an average of 17.5 and 13.37 sec slower than control animals in finding the hidden platform on Days 3 and 4, respectively. Most importantly, pretreatment with the non-competitive NMDA receptor antagonist MK-801 completely prevented the learning deficits produced by intrahippocampal Tat injection in EtOH withdrawn animals (Figure 4). The mean latency of the MK-801 treated group was not significantly greater than that of all other groups. No group differences in swim speeds, probe trial performance or visual platform behavior were observed.
Figure 4.
Latency to find the hidden platform in the Morris water maze task. Animals that were pre-exposed to the binge EtOH regimen (ending 24 hr prior to Acquisition Day 1) and received intra-CA1 region Tat injection had significantly greater latencies to find the platform than all other groups, most notably, on training days 3 and 4. *= P <0.05 vs all other groups. This learning impairment was prevented by pre-treatment with the non-competitive NMDA receptor antagonist MK-801 (0.075mg/kg, i.p.).
Autoradiographic analysis of [3H]MK-801 and [3H]PK11195 binding
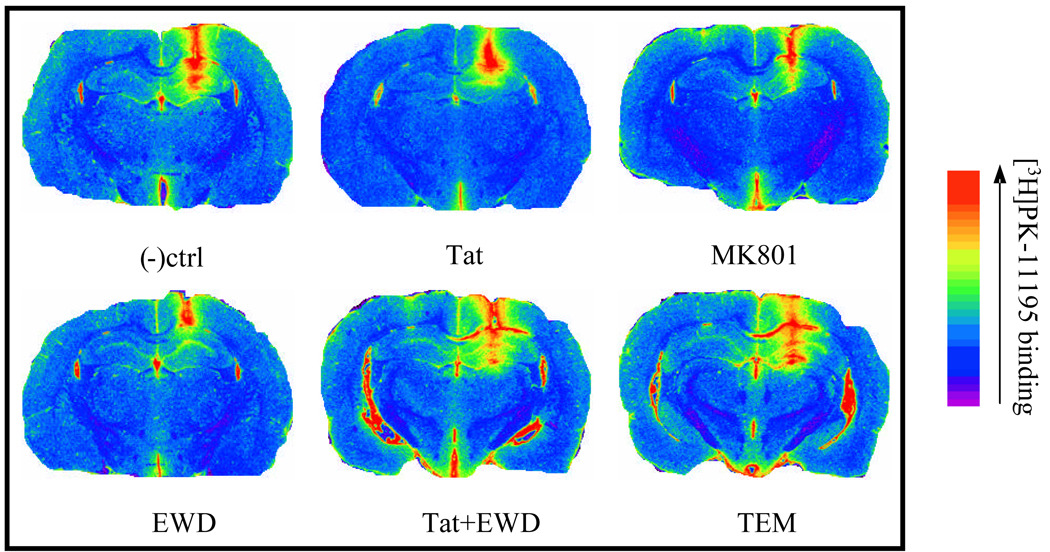
[3H]PK11195 binding was conducted to assess glial infiltration into the hippocampus, as described above in detail. Two-way ANOVAs were conducted for each hippocampal region tested (CA1, CA3, and dentate gyrus) to determine any treatment, cannula side placement, or interactive effects. Hippocampal regions were analyzed separately as there was an a priori hypothesis that regional differences in EtOH and/or Tat effects would be observed, based on previous findings (Collins et al. 1996; Crews et al. 2002; Self et al. 2005). In each hippocampal region tested there was a main effect of cannula side placement, such that the side of the hippocampus that received the cannula implantation displayed significantly more [3H]PK11195 binding than non-cannula side independent of drug treatment (Figure 5). For instance, in the dentate gyrus, the cannula implantation side presented with approximately double the amount of [3H]PK11195 binding as the non-cannula side in every treatment group as evidenced by two-way ANOVA showing a main effect of hemisphere [F(1,136)=162.346, p<0.001]. Further, in the dentate gyrus there were no significant treatment effects found in the non-cannula hemisphere of the hippocampal complex with regard to [3H]PK11195 binding. However, in the cannula side, statistically significant treatment effects were revealed in the dentate gyrus. Similar findings were observed in the CA3 [F(5,136)=3.537, p<0.005] and CA1 [F(1,136)=354.857, p<0.001] regions. Representative color-enhanced images of [3H]PK11195 autoradiograms are provided in Figure 6.
Figure 5.
Effects of intra-CA1 region cannula implantation on binding density of the peripheral benzodiazepine binding site ligand [3H]PK11195 in the hippocampus. [3H]PK11195 binding was markedly increased as a result of cannulae implantation but was not altered by Tat or EtOH treatment. *= P <0.05 vs all other groups.
Figure 6.
Representative false-color images of hippocampal [3H]PK11195 binding in EtOH-naïve and withdrawn (EWD) animals, including those administered MK-801 (0.075mg/kg, i.p.; TEM) following water maze training.
[3H]MK801 binding was assessed to determine NMDA receptor density in brains collected from each treatment group at the end of the EtOH treatment regimen and again after water maze testing was conducted. A two-way ANOVA was initially conducted to determine if there were effects of treatment, cannula placement side, or interactions between the two variables on MK801 binding at the different time points. This test revealed no significant effects of cannula placement side, therefore data from the cannula and non-cannula hippocampal sides were collapsed for subsequent two-way ANOVAs examining treatment effects for each respective hippocampal region, at both time points of tissue collection. No significant group differences in [3H]MK801 binding density were observed at either time of tissue collection.
DISCUSSION
Individuals who are human immunodeficiency virus (HIV) positive abuse alcohol at nearly twice the rate of seronegative individuals [Galvan et al. 2002], with estimates that 40% of the HIV-positive population are alcohol-dependent [LeFevre et al. 1995]. EtOH abuse has been shown to potentiate HIV impairment of immune function; affect viral replication and decrease bioavailability of antiretroviral drugs [Bagby et al. 2003; Samet et al. 2003; Shibata et al. 2003; Witte et al. 1994]. Given these findings and the limited CNS penetration of many antiretroviral medications, it may be expected that combined alcohol abuse and HIV-positive status could compromise neuronal function. Indeed recent studies have reported that seropositive men with past alcohol abuse or dependence displayed significant multi-domain cognitive dysfunction compared to those without an alcohol abuse history [Green et al. 2004; Rothlind et al. 2006].
The present findings demonstrate that 4 days of repeated EtOH intake, which produced blood EtOH levels commonly observed in human binge drinkers [Lange and Voas 2001; Perkins et al. 2001], also produced significant behavioral abnormalities upon EtOH withdrawal that were significantly worsened by intra-hippocampal Tat injection. Whereas only 5 Tat-naïve EtOH withdrawn animals displayed “severe” withdrawal behavioral abnormalities, 10 EtOH withdrawn animals that received intra-CA1 region Tat injection prior to behavioral monitoring displayed severe abnormalities, including 3 animals that displayed seizures. These EtOH withdrawal behavioral abnormalities were modestly, but not entirely, reduced by MK-801 pre-exposure. Further, EtOH exposure and withdrawal sensitized adult male rats to the subsequent and protracted cognitive-impairing effects of intra-hippocampal injection of the HIV-1 transcription factor Tat. Interestingly, while the side of intra-CA1 Tat injection was counterbalanced, side of injection did not moderate the ability of Tat to alter learning, demonstrating the need for bilateral synaptic integrity of hippocampal circuits in the successful formation of spatial memory. EtOH exposure and withdrawal itself did not alter water maze performance or swim speeds, suggesting the absence of significant motoric abnormalities following acute withdrawal. Escape latencies of EtOH withdrawn animals that received a Tat injection 1 day prior to the start of water maze testing were nearly twice those of other animals on Days 3 and 4. All other animals performed the task at rates comparable to controls. Given these findings and evidence that binge ethanol exposure regimens producing higher blood ethanol concentrations have been reported to produce neurodegeneration (Collins et al. 1996; Crews et al. 2002), it will be intriguing to examine the extent to which the spatial learning deficits observed are or are not associated with measurable neuronal injury. Importantly, pharmacological inhibition of NMDA receptor activity, as many as 3 and 4 days prior to behavioral testing, prevented the cognitive impairing effect of combined EtOH withdrawal and intra-hippocampal Tat injection, demonstrating a role for NMDA receptor activity in the cognitive disruption observed.
Previous work has demonstrated that a 1–86 amino acid construct of Tat inhibited formation of long-term potentiation in organotypic slice cultures of both rat and mouse hippocampi (Behnisch et al. 2004; Li et al. 2004). Further, Li et al. (2004) reported that right intracerebroventricular injection of Tat1-86 markedly impaired acquisition of an eight-arm spatial maze task when training was initiated two days after Tat injection. It is possible, if not likely, that Tat effects on long-term potentiation in CA1 region pyramidal cells of EtOH withdrawn animals underlie the disruption of spatial learning that was observed. These findings are consistent with those of the present studies in demonstrating protracted effects of CNS Tat injection on spatial learning. While Li et al. (2004) hypothesized that the learning deficits they observed reflected Tat’s ability to activate extra-synaptic NMDA receptors, they did not employ a means to confirm this possibility.
Examination of [3H]PK11195 hippocampal binding prior to EtOH withdrawal on Day 4 or following 7 days of withdrawal did not provide evidence of microglial activaton and/or neuroinflammation, suggesting that the learning deficits observed were likely independent of acute insult. Our findings that cannulae tracks were associated with significant increases in [3H]PK11195 binding density are consistent with the suggestion that this ligand is a marker of cellular compromise. While use of [3H]PK11195 is often interpreted to reflect influx of activated microglia into areas of tissue injury, it must be noted that the PBBS is also found on mitochondrial membranes and regulates mitochondrial responses to cellular insult. The PBBS has been found to play an instrumental role in regulating the mitochondrial permeability transition pore (MPTP), acting as an oxygen sensor and therefore protecting mitochondria from ROS-mediated damage [Casellas et al. 2002]. Thus, while increases in binding of this ligand are highly suggestive of challenge to cellular survival, it is not clear that the increase observed in the current studies reflect macrophage infiltration and/or upregulation of PBSS on mitochondria of compromised cells.
It is of interest to note that no increases in hippocampal [3H]MK-801 binding density were observed, yet the NMDA receptor system clearly mediated the effect of Tat on learning. A wealth of data demonstrated that prolonged EtOH exposure leads to an upregulation of NMDAr expression and synaptic and/or extra-synaptic sites via changes in genomic expression and NR subunit trafficking in live animal of primary cell cultures [Carpenter-Hyland et al. 2006; Fadda & Rossetti 1998; Suvarna et al. 2005]. Rudolph et al. (1997) also reported a lack of upregulation in binding of [3H]MK-801 in several brain regions following use of a similar 4-day binge EtOH exposure in rats that produce mean blood EtOH levels of 386 mg/dl. Thus, it is not unexpected that changes in binding density were not observed with use of the lower B.E.L. model developed for the current studies. The lack of increases in density of the NMDA receptor channel domain reflecting MK-801 sensitivity does not, however, discount the possibility that MK-801 insensitive binding domains on these receptors were upregulated by binge EtOH exposure (ie. NR2B subunits) or that migration from extra-synaptic to synaptic sites occurred. Alternatively, function of upstream and/or downstream modulators of NMDA receptor activity (eg. mGluR II family receptors, PSD-95) not examined may well have been modified by binge EtOH exposure.
Perhaps the most relevant interpretations of these data are two-fold: first, that protracted intake of EtOH doses producing B.E.L. levels of less than 200 mg/dl results in both significant metabolic-like tolerance and marked EtOH withdrawal abnormalities reflective of excess neuronal excitation. Second, these data support the hypothesis that binge-like EtOH intake may contribute to cognitive decline in HIV-infected individuals and NMDA-type glutamate receptors may be therapeutic targets that may be exploited in the treatment of HIV-associated dementia. Indeed, clinical trials examining effects of the short-acting NMDA channel blocker memantine are being conducted (Schifitto et al. 2007). Further, allosteric modulators of NMDA polyamine sensitive-sites on NR2 subunits are well tolerated in humans [Chizh et al. 2001]. Lastly, with the development of this low blood EtOH level model of binge alcohol intoxication, it is now possible to examine effects of such compounds in an animal model of alcohol and HIV-associated cognitive disturbance.
Acknowledgements
This work was supported by AA015676 to R.L.S. and AA013561 to M.A.P. The authors thank Deanne Hopkins and Layla Ghayoumi for technical assistance. We thank Dr. Mark Fillmore for his aid in statistical analyses.
Abbreviations
- B.E.L.
blood ethanol level
- CA1
cornu ammonus 1
- HIV-1
human immunodeficiency virus-1
- ITI
inter-trial interval
- MWM
Morris Water Maze
- NMDA
N-methyl-d-aspartate
- PBBS
peripheral benzodiazepine binding site
- Tat
trans-activator of transcription
- Tris
tris(hydroxymethyl)aminomethane
- µCi
micro curies
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Acheson SK, Richardson R, Swartzwelder HS. Developmental changes in seizure susceptibility during ethanol withdrawal. Alcohol. 1999;18(1):23–26. doi: 10.1016/s0741-8329(98)00063-9. [DOI] [PubMed] [Google Scholar]
- 2.Bagby GJ, Stoltz DA, Zhang P, Kolls JK, Brown J, Bohm RP, Jr, Rockar R, Purcell J, Murphey-Corb M, Nelson S. The Effect of Chronic Binge Ethanol Consumption on the Primary Stage of SIV Infection in Rhesus Macaques. Alcoholism: Clin & Exp Res. 2003;27(3):495–502. doi: 10.1097/01.ALC.0000057947.57330.BE. [DOI] [PubMed] [Google Scholar]
- 3.Behnisch T, Francesconi W, Sanna P. HIV secreted protein Tat prevents long-term potentiation in the hippocampal CA1 region. Brain Res. 2004;1012:187–189. doi: 10.1016/j.brainres.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic EtOH induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casellas P, Galiegue S, Basile AS. Peripheral benzodiazepine receptors and mitochondrial function. Neurochem Int. 2002;40:478–486. doi: 10.1016/s0197-0186(01)00118-8. [DOI] [PubMed] [Google Scholar]
- 6.Chizh BA, Headley PM, Tzschentke TM. NMDA receptor antagonists as analgesics: focus on the NR2B subtype. Trends Pharmacol Sci. 2001;22:636–642. doi: 10.1016/s0165-6147(00)01863-0. [DOI] [PubMed] [Google Scholar]
- 7.Collins MA, Corso TD, Neafsey EJ. Neuronal degeneration in rat cerebrocortical and olfactory regions during subchronic “binge” intoxication with ethanol: possible explanation for olfactory deficits in alcoholics. Alcohol Clin Exp Res. 1996;20:284–292. doi: 10.1111/j.1530-0277.1996.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 8.Conway EL, Gundlach AL, Craven JA. Temporal changes in glial fibrillary acidic protein messenger RNA and [3H]PK11195 binding in relation to imidazoline-I2-receptor and alpha 2-adrenoceptor binding in the hippocampus following transient global forebrain ischaemia in the rat. Neuroscience. 1998 Feb;82(3):805–817. doi: 10.1016/s0306-4522(97)00321-7. [DOI] [PubMed] [Google Scholar]
- 9.Crews FT, Braun CJ, Hoplight B, Switzer RC, 3rd, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alc Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- 10.Dubois A, Bénavidès J, Peny B, Duverger D, Fage D, Gotti B, MacKenzie ET, Scatton B. Imaging of primary and remote ischaemic and excitotoxic brain lesions. An autoradiographic study of peripheral type benzodiazepine binding sites in the rat and cat. Brain Res. 1988 Mar 29;445(1):77–90. doi: 10.1016/0006-8993(88)91076-1. [DOI] [PubMed] [Google Scholar]
- 11.Ensoli B, Buonaguro L, Barillari G, Fiorelli N, Gendelman R, Morgan RA, Wingfield P, Gallo R. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fada F, Rossetti ZL. Chronic EtOH consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 13.Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J Stud Alcohol. 2002;62:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Martinez LF, Mavankal G, Neveu JM, Lane WS, Ivanov D, Gaynor RB. Purification of a Tat-associated kinase reveals a TFIIH complex that modulates HIV-1 transcription. EMBO J. 1997;16:2836–2850. doi: 10.1093/emboj/16.10.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green JE, Saveanu RV, Bornstein RA. The effect of previous alcohol abuse on cognitive function in HIV infection. Am J Psychiatry. 2004;161:249–254. doi: 10.1176/appi.ajp.161.2.249. [DOI] [PubMed] [Google Scholar]
- 16.Haughey NJ, Nath A, Mattson MP, Slevin JT, Geiger JD. HIV-1 Tat through phosphorylation of NMDA receptors potentiates glutamate excitotoxicity. J Neurochem. 2001;78:457–467. doi: 10.1046/j.1471-4159.2001.00396.x. [DOI] [PubMed] [Google Scholar]
- 17.Itzhak Y, Baker L, Norenberg MD. Characterization of the peripheral-type benzodiazepine receptors in cultured astrocytes: evidence for multiplicity. Glia. 1993;9:211–218. doi: 10.1002/glia.440090306. [DOI] [PubMed] [Google Scholar]
- 18.Janssen ES, Wanyanwn OC, Selik RM, Stehr-Green JK. Epidemiology of human immunodeficiency encephalopathy in the United States. Neurology. 1992;42:1472–1476. doi: 10.1212/wnl.42.8.1472. [DOI] [PubMed] [Google Scholar]
- 19.King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Kumari M, Anji A. A novel RNA binding protein that interacts with NMDA R1 mRNA: regulation by EtOH. Eur J Neurosci. 2006;23:2339–2350. doi: 10.1111/j.1460-9568.2006.04776.x. [DOI] [PubMed] [Google Scholar]
- 21.Lange JE, Voas RB. Defining binge drinking quantities through resulting blood alcohol concentrations. Psychol Addict Behav. 2001;15:310–316. doi: 10.1037//0893-164x.15.4.310. [DOI] [PubMed] [Google Scholar]
- 22.Lefevre F, O’Leary B, Moran M, Mossar M, Yarnold PR, Martin GJ, Glassroth J. Alcohol consumption among HIV-infected patients. J Gen Intern Med. 1995;10:458–460. doi: 10.1007/BF02599920. [DOI] [PubMed] [Google Scholar]
- 23.Li ST, Matsushita M, Moriwaki A, Saheki Y, Lu YF, Tomizawa K, Wu HY, Terada H, Matsui H. HIV-1 Tat inhibits long-term potentiation and attenuates spatial learning. Ann Neurol. 2004;55:362–371. doi: 10.1002/ana.10844. [DOI] [PubMed] [Google Scholar]
- 24.Ma M, Nath A. Molecular determinants for cellular uptake of tat protein of human immunodeficiency virus type 1 in brain cells. J Virology. 1997;71:2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes. Psychopharmocologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- 26.McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP. Dementia in AIDS patients. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 27.Morris RGM. Spatial localisation does not depend on the presence of local cues. Learning Motivation. 1981;12:239–260. [Google Scholar]
- 28.Myers R, Manjil LG, Cullen BM, Price GW, Frackowiak RS, Cremer JE. Macrophage and astrocyte populations in relation to [3H]PK 11195 binding in rat cerebral cortex following a local ischaemic lesion. J Cereb Blood Flow Metab. 1991;11:314–322. doi: 10.1038/jcbfm.1991.64. [DOI] [PubMed] [Google Scholar]
- 29.Nath. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia ;J Infect Dis. 2002;186 Suppl 2:S193–S198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- 30.Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83(5):1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- 31.Pappata S, Levasseur M, Gunn RN, Myers R, Crouzel C, Syrota A, Jones T, Kreutzberg GW, Banati RB. Thalamic microglial activation in ischemic stroke detected in vivo by PET and [11C]PK1195. Neurology. 2000;55:1052–1054. doi: 10.1212/wnl.55.7.1052. [DOI] [PubMed] [Google Scholar]
- 32.Pauly JR, Collins AC. An autoradiographic analysis of nicotinic cholinergic receptors following chronic corticosterone treatment. Neuroendocrinology. 1993;57:262–271. doi: 10.1159/000126368. [DOI] [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. Stereotaxic Coordinates. 4th Edition. San Diego: Academic Press; 1998. The Rat Brain. [Google Scholar]
- 34.Perkins HW, DeJong W, Linkenbach J. Estimated blood alcohol levels reached by “binge” and “nonbinge” drinkers: a survery of young adults in Montana. Psych Addict Behav. 2001;15:317–320. [PubMed] [Google Scholar]
- 35.Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, Sullivan EV. Contribution of alcoholism to brain dysmorphology in HIV infection: effects on the ventricles and corpus callosum. Neuroimage. 2006;33(1):239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 36.Pocernich CB, Boyd-Kimball D, Poon HF, Thongboonkerd V, Lynn BC, Klein JB, Calebrese V, Nath A, Butterfield DA. Proteomics analysis of human astrocytes expressing the HIV protein Tat. Brain Res Mol Brain Res. 2005a;133:307–316. doi: 10.1016/j.molbrainres.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Pocernich CB, Poon HF, Boyd-Kimball D, Lynn BC, Nath A, Klein JB, Butterfield DA. Proteomic analysis of oxidatively modified proteins induced by the mitochondrial toxin 3-nitropropionic acid in human astrocytes expressing the HIV protein tat. Brain Res Mol Brain Res. 2005b;133:299–306. doi: 10.1016/j.molbrainres.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 38.Prendergast MA, Rogers DT, Mulholland PJ, Littleton JM, Wilkins LH, Jr, Self RL, Nath A. Neurotoxic effects of the human immunodeficiency virus type-1 transcription factor Tat require function of a polyamine site on the N-methyl-d-aspartate receptor. Brain Research. 2002;954:300–307. doi: 10.1016/s0006-8993(02)03360-7. [DOI] [PubMed] [Google Scholar]
- 39.Rothlind JC, Greenfield TM, Bruce AV, Meyerhoff DJ, Flenniken DL, Lindgren JA, Weiner MW. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11:70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudolph JG, Walker DW, Imuro Y, Thurman RG, Crews FT. NMDA receptor binding in adult rat brain after several chronic ethanol treatment protocols. Alcohol Clin Exp Res. 1997;21:1508–1519. [PubMed] [Google Scholar]
- 41.Samet JH, Horton NJ, Traphagen ET, Lyon SM, Freedberg KA. Alcohol consumption and HIV disease progression: are they related? Alcohol Clin Exp Res. 2003;27:862–867. doi: 10.1097/01.ALC.0000065438.80967.56. [DOI] [PubMed] [Google Scholar]
- 42.SAMHSA Office of Applied Studies. Drug Abuse Warn Net. 2004. The NSDUH report; pp. 1–3. [Google Scholar]
- 43.Sardar AM, Hutson PH, Reynolds GP. Deficits of NMDA receptors and glutamate uptake sites in the frontal cortex in AIDS. Neuroreport. 1999;10:3513–3515. doi: 10.1097/00001756-199911260-00009. [DOI] [PubMed] [Google Scholar]
- 44.Shibata N, Kageyama M, Kishida T, Kimura K, Yoshikawa Y, Kuwahara T, Toh J, Shirasaka T, Takada K. Pharmacokinetic characterization of a human immunodeficiency virus protease inhibitor, saquinavir, during ethanol intake in rats. Biopharm Drug Dispos. 2003;24(8):335–344. doi: 10.1002/bdd.369. [DOI] [PubMed] [Google Scholar]
- 45.Schiffito G, Navia BA, Yiannoutsos CT, Marra CM, Chang L, Ernst T, Jarvik JG, Miller EN, Singer EJ, Ellis RJ, Kolson DL, Simpson D, Nath A, Berger J, Shriver SL, Millar LL, Colquhoun D, Lenkinksi R, Gonzalez RG, Lipton SA Adult AIDS Clinical Trial Group (ACTG) 301. 700 Teams; HIV MRS Consortium. AIDS. 21:1877–1886. [Google Scholar]
- 46.Self RL, Mulholland PJ, Nath A, Prendergast MA. Cytotoxic effects of exposure to the human immunodeficiency virus type 1 protein Tat in the hippocampus are enhanced by prior EtOH treatment. Alcohol Clin Exp Res. 2004;28:1916–1924. doi: 10.1097/01.alc.0000148108.93782.05. [DOI] [PubMed] [Google Scholar]
- 47.Suvarna N, Borgland SL, Wang J, Phamluong K, Auberson YP, Bonci A, Ron D. EtOH alters trafficking and functional N-methyl-D-aspartate receptor NR2 subunit ration via H-Ras. J Biol Chem. 2005;280:31450–31459. doi: 10.1074/jbc.M504120200. [DOI] [PubMed] [Google Scholar]
- 48.Tabakoff B. Alcohol and AIDS--is the relationship all in our heads? Alcohol Clin Exp Res. 1994;18:415–416. doi: 10.1111/j.1530-0277.1994.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 49.Tyor WR, Middlaugh LD. Do alcohol and cocaine abuse alter the course of HIV-associated dementia complex. J Leuk Biol. 1999;65:475–481. doi: 10.1002/jlb.65.4.475. [DOI] [PubMed] [Google Scholar]
- 50.Wang P, Barks JD, Silverstein FS. Tat, a human immunodeficiency virus-1-derived protein augments excitotoxic hippocampal injury in neonatal rats. Neuroscience. 1999;88:585–597. doi: 10.1016/s0306-4522(98)00242-5. [DOI] [PubMed] [Google Scholar]
- 51.Witte MH, Borgs P, Way DL, Ramirez G, Jr, Witte CL, Bernas MJ. AIDS, alcohol, endothelium, and immunity. Alcohol. 1994;11:91–97. doi: 10.1016/0741-8329(94)90049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]