INTRODUCTION
Cerebral palsy is common, affecting about 2–3 per 1000 children1. These children may have a motor disorder characterised by spasticity, dystonia or both. This can result in significant difficulty with activities of daily living, pain and long term joint deformity2. There are a number of treatments available for the management of spasticity and dystonia. This review will examine indications and practical issues for some of the common treatment options used in the paediatric population such as botulinum toxin and intrathecal baclofen and the newer therapy for dystonia management, deep brain stimulation.
Spasticity management in childhood cerebral palsy
A definition of spasticity was provided by Lance in 1980, ‘Spasticity is a motor disorder characterized by a velocity-dependent increase in tonic stretch reflexes (“muscle tone”) with exaggerated tendon jerks, resulting from hyper excitability of the stretch reflex, as one component of the upper motor neurone syndrome’.
Spasticity results from a lack of descending impulses that normally stimulate the release of the inhibitory neurotransmitter GABA which acts presynaptically to inhibit the release of excitatory neurotransmitters. Two of the most common causes of spasticity are cerebral palsy and acquired brain injury. Spasticity can result in functional problems with daily activities such as gait, feeding, washing and dressing. Over time it may result in joint contracture and hip dislocation in more severely affected individuals. It is recognised that a painful hip joint can result in poor tolerance of the seated position and may result in the need for frequent turning at night. The discomfort and lack of sleep can exacerbate seizures, dystonic posturing and spasms, the child can present as irritable and unsettled, in some the manifestations of pain include teeth grinding, biting and head banging. Identifying the source of pain and treating it can reduce the need for changes in anticonvulsants and tone modifying agents. However spasticity can also be helping the child to maintain posture and function as underlying muscle strength may be low. Therefore it is important for any treatment to be titratable in order to maintain functional benefits whilst reducing spasticity.
Treatment options for spasticity
Baclofen is a GABA agonist that is used to reduce muscle tone. Baclofen crosses the blood-brain barrier and binds at the GABA-B receptors of the laminae I-IV of the spinal cord, where primary sensory fibres end. It may be used for both oral and intrathecal administration. Baclofen can produce sedation when administered orally (that is, dose-related – table I); this may be minimized by initiating treatment at a low dose. This drug may also cause impairments of cognitive functions, hypotonia and may lower the seizure threshold. Maintenance doses are titrated to individual need and the titration should be done slowly, ideally incremental increases should be three days apart.
Table I.
Guide to the oral baclofen dose range in childhood:
| 1 month – 2 years | 2 - 12 years | 12 - 18 years | Notes |
|---|---|---|---|
| 1–2mg | 2mg | 5mg | Initial dose gradually increasing every three days |
| > 1year 5mg | 2–6 years 10mg | 20–30mg. Max daily dose 100mg | Maintenance three times daily |
| 6–12 years 15mg | |||
INTRATHECAL BACLOFEN
Intrathecal baclofen is a treatment used in adults and children since the mid 1980's. It can be considered in two groups: older ambulatory children or patients with severe spasticity in the upper and lower extremities. It has advantages in that it is titratable and reversible.
Mechanism
Intrathecal baclofen is delivered via an implantable pump. By infusing baclofen directly into the subarachnoid space around the spinal cord, potentiation of GABA-mediated inhibition of spasticity can be achieved while minimizing side effects secondary to high levels of baclofen in the brain. Intrathecal infusion of baclofen may be varied through the day to accommodate the patient's activities3. Many studies have demonstrated that intrathecal baclofen therapy is useful in the management of spasticity with cerebral palsy and spinal origin4,5. In patients with spasticity of cerebral origin reduction in spasticity in the upper and lower extremities after six months of therapy has been reported5. ITB produces levels greater than four times the oral dose facilitating the use of much smaller doses for example 0.05–1.2mg per day
Patient management generally takes place in three phases: patient selection, patients and family education, and screening.
Patient Selection
Clinically severe spasticity which impairs function in:
Older ambulatory children
Patients with severe spasticity in upper and lower extremities
Patients with a combination of spasticity + dystonia
Spinal injury
Ideally patients should have been tried on oral tone modifying medications first and have had some response to baclofen and no adverse reaction.
Patient and family education
It is imperative that families have a clear understanding not only of the surgical procedure required but of the long term commitment in terms of pump maintenance and refilling. Patients should undergo functional assessments during the process of consideration for a baclofen pump. This facilitates a greater understanding of the level of ability and allows a clearer explanation of what might be improved using intrathecal baclofen. Local therapy teams can provide useful background information which assists the process. Written information should be provided explaining the assessment process, surgical procedure and follow up arrangements. Families and local teams should have access to protocols for emergency management. All of the information should be available in a child friendly format and accessible for young people with a learning disability.
Baclofen screening trial
A dose of baclofen is delivered via an intra thecal bolus using the standard lumbar puncture technique. Generally a dose of 50μg of baclofen is given in this intrathecal injection. The aim is to observe a demonstrable reduction in spasticity. There are a variety of techniques used in different centres to reliably assess this change in muscle tone, examples include a pre and post comparison of Ashworth scores, and change in range of hip abduction. It is important to explain to patients and families that the effect produced by the trial dose is not predictive of functional changes or the impact on upper limb tone which may be experienced following pump insertion.
Practical issues
Insertion of the baclofen pump is undertaken by the neurosurgical team. The procedure for insertion of an intrathecal baclofen pump lasts 1–1.5 hours. The pump is inserted under the covering of the abdominal muscles while the patient is under a general anaesthetic. A small catheter is inserted through a needle into the spinal fluid and is threaded upward toward the neck. The catheter is tunnelled under the skin to the abdomen and is connected to the pump. The pump is filled with the drug baclofen and is programmed by a computer to continuously release a specified dose. Patients usually remain in hospital for five days after pump insertion. During that time, their baclofen dose is increased every day as needed. The pump needs to be refilled every two to six months, depending on the pump size, concentration and dose. Refills are done using a syringe and needle and take approximately thirty minutes to complete. At that time, baclofen doses are adjusted depending on the effects that are being seen. Doses typically increase slowly during the first year, and then remain at that level for years thereafter. The drug dose is not related to age or weight, and a gradual increase during the first six to nine months is required. Generally, patients with ventriculoperitoneal shunts require lower doses. The battery in the pump lasts up to eight years at which time the pump needs to be replaced6.
Problems with baclofen pumps
Empty pump reservoir, catheter leaks or displacement, pump malfunction, programming error and refill of pump with improper drug concentration are the possible mechanisms which could lead to an ITB withdrawal syndrome (table II) which can lead to muscle breakdown and multi system failure. Management includes an early recognition of syndrome, proper intensive care management, high-dose benzodiazepines and prompt analysis of intrathecal pump with reinstitution of baclofen.
Table II.
ITB withdrawal.
| Clinical signs of ITB withdrawal |
|---|
| Increased spasticity |
| Agitation |
| Hallucinations |
| Severe itching |
| Hyperthermia |
| Seizures |
Table III.
ITB overdose.
| Signs of ITB overdose |
|---|
| Excessive hypotonia |
| Drowsiness/reduced arousal |
| Respiratory depression requiring ventilation |
Outcomes following intrathecal baclofen therapy
Reduction in use of oral medication for children with spasticity has been described, with improvements in comfort and function; decreased tone and spasms also observed5. Some aspects of speech, communication, and saliva control seem to have improved, with bowel movement frequency decreased in some children receiving intrathecal baclofen. Few changes in feeding and nutritional status have been reported. The majority of patients will sustain improved range of motion, decreased painful muscle spasm, and improvement in measures of independent function3–5. Finally, this treatment may be associated with possible complications, some studies suggest that children of younger age, as well as those with gastrostomy tubes and non-ambulatory status, were more likely to encounter complications necessitating removal of the pump such as pump pocket collections and infections7,8. Infection may remain isolated to the pump pocket or may track along the catheter, with consequent meningitis5.
Vloeberghs group demonstrated that ITB has a role in children with a combination of spasticity and dystonia. Interestingly they concluded that it was safe and effective to have both ITB and DBS together in selected patients with primary dystonias9. The American Academy reviewed all the trials for ITB available by 2000 and concluded that in a total of twenty-five trials seventeen had shown clear statistically significant improvements in areas such as moving and handling, pain, spasms, social participation and reducing the need for orthopaedic procedures. They commented that the other smaller trials had no power calculations therefore they could only be termed inconclusive rather than negative10. Other potential benefits that have been less extensively studied are benefits to sleep and respiratory function in patients with spasticity (table IV). One trial demonstrated significant improvements in these areas; however this clearly needs more investigation11.
Table IV.
Benefits of ITB.
| Positive results with Intrathecal baclofen |
|---|
| Reduced pain |
| Improved QOL |
| Improved seating tolerance |
| Improved transfers |
| Ambulatory patients improving capacity to walk |
| Ease of care / level of support |
| Reduction in orthopaedic surgery |
BOTULINUM TOXIN THERAPY IN THE MANAGEMENT OF CHILDHOOD SPASTICITY
Prior to the late 1990's we were extremely limited in the focal medical management of movement disorders in children. A so called ‘birthday syndrome’ of almost annual Orthopaedic interventions overcame the functional difficulties associated with tightening at the ankle dorsiflexors, knee and hip flexors. In 1998 Botulinum Toxin type A became licensed for use in children over the age of two for management of dynamic equinus foot deformity caused by spasticity in ambulant children with cerebral palsy. This remains the only on license indication, however there is a wide range of accepted ‘off license’ use for children, as outlined in summary documents such as the European Consensus Statements. Targeted injections to skeletal muscles (table V) in areas including the neck, upper limb, spine, hip and lower limb have been shown to reduce problems associated with increased muscle tone as part of the upper motor neurone syndrome12–15.
Table V.
Author centre uses of Botulinum Toxin type A in movement disorders
| Area | Problems |
|---|---|
| Head and neck | Sialorrhoea, Retro, lateral or rotatory torticollis |
| Shoulder | Axillary hygiene, retraction, protraction, abduction, focal dystonia |
| Elbow | Flexion, Extension |
| Wrist, thumb and hand | Flexion, hand hygiene/hyperhydrosis, thumb adduction and flexion |
| Back | Opisthotonus, extension, pain |
| Hip | Pain secondary to spasm, flexion, adduction/scissoring, activities of daily living (toileting, dressing, changing), postural adaptation – comfort in sitting |
| Knee | Pain, quadriceps spasticity, flexion, crouch/jump gait patterns |
| Ankle | Equinus gait, inversion, pain |
| Foot, toe | Claw toe, hitchhiker toe, dynamic toe and foot deformities |
There are seven different naturally occurring serotypes of Botulinum Toxin, each acting at the nerve terminal end plate to block the release of Acetylcholine at the neuro-muscular junction, abolishing motor end plate potential. Botulinum Toxin type A (e.g. Botox, Dysport, Xeomin) has the widest international clinical use as it causes the longest duration of muscle relaxation. It is taken up into the end plate via the high affinity synaptic vesicle protein 2 (SV2) which is up regulated in active neurons. It then irreversibly stops exocytosis of Acetylcholine by targeting the SNAP-25 protein element of the synaptic vesicle release complex. This is clinically vital as active toxin works on active synapses, those muscles with over activation causing clinical problems are specifically targeted. The terminal end plate ceases to function and regresses over the next couple of months to the end of the axonal sheath. Resprouting then occurs from this point with the growth of a new neuro-muscular junction. This leads to the duration of action within the targeted nerve-muscle of between three to four months. Clinical gain is often seen for far longer than this however with functional benefits lasting up to twelve to eighteen months16,17.
Different preparations have different pharmacokinetic and pharmacodynamic properties. Dose ranges are provided in Consensus documents and it is vital to remember to adhere to them as Botulinum Toxin type A is the most potent neurotoxin known to man. There is no direct correlation between doses of the preparations. Maximum doses of 12–14iu/kg of Botox or 25–30iu/kg of Dysport are recommended18.
The core of successful focal or global management in movement therapy is a multidisciplinary team assessment that works out functional goals for interventional therapy. Specific evaluation tools are necessary dependent on the possible site of intervention, the individual's level of functional ability and age. A range of treatments are appropriate at different times of growth and development. As with Orthopaedic interventions it is important to consider a multi-level functional approach rather than injections to a single muscle body19.
Careful explanation of effects and side effects of the Botulinum Toxin injections should be given and informed written consent obtained. Rare local haematoma is the most common observed effect. There is no published report of localized infection or inflammation, though the authors have observed this twice in over 8,000 courses. More serious adverse events have been reported if dosing and/or dilution guidelines are not respected. These include excessive muscle weakness and local spread to other muscle groups. If the injections occur at the hip level then transient bowel or bladder incontinence has been observed, generally at the peak of effect one month following administration. There are two reported international fatalities over the last twelve years, potentially linked to Botulinum Toxin type A injections. Both of these had considerable confounding factors and though dilution and dosing guidelines were vastly exceeded in both cases neither had proven systemic spread of toxin on immunoassay of distal neuro-muscular junctions.
The Botulinum Toxin injections should be administered by an experienced team in a child friendly setting. The injections themselves should take place with care to minimize trauma for the child and family. Local anaesthetic and inhaled or oral sedation or even general anaesthetic should be considered. It is generally accepted now that guidance beyond clinical palpation is needed for needle placement. In children the use of ultrasonography to localize muscles is widely used, though some centres use electrical stimulation or EMG.
Re-evaluation is necessary at the peak of potential benefit and at the time of terminal re-sprouting. Botulinum Toxin injections are not a stand alone treatment modality and once again it should be stressed that it they are used as part of an integrated therapeutic approach in Paediatric Neurodisability. A post injection management programme is necessary. It may be necessary to repeat the course of injections and continuation or discontinuation as a therapeutic modality is dependent on functional gains, improved posture and/or improved comfort in the individual child. Secondary non-response due to muscular fibrosis or the formation of neutralizing antibodies against Botulinum Toxin A is rarely seen. When benefits are no longer observed onward referral to other elements of movement therapy services may be necessary.
DYSTONIA IN CHILDREN WITH CEREBRAL PALSY
Dystonia is physiologically characterised by the involuntary co-contraction of agonist and antagonist muscle groups at a joint and an overflow flow into muscles or limbs not normally involved with intended movement, resulting in abnormalities in posture and twisting movements. Dyskinetic cerebral palsy has an estimated prevalence of 0.27 per 1000 live births20. In dystonia muscle tone typically fluctuates, varying from normal or low to extreme hypertonia. It can be precipitated or worsened by attempts to move and can vary according to emotional state. Dystonia typically diminishes or disappears with distraction and sleep
There are numerous systems for the classification of dystonia. Considering the underlying pathological process, dystonia may be defined as (i) Primary, (ii) Secondary or (iii) Heredodegenerative. Primary dystonias are unaccompanied by other neurologic abnormalities, except tremor and occasionally myoclonus. Primary dystonias are often attributable to a genetic cause21. To date over 20 such dystonic syndromes have been identified, with mutations denoted DYT21–24. Secondary dystonia can be considered symptomatic, due to identifiable brain lesions or metabolic abnormalities. Dyskinetic cerebral palsies fall into this category. Dystonia may also be classified as focal, segmental or generalised depending upon the extent and distribution of muscle involvement.
The basal ganglia have long been implicated in the pathophysiology of dystonia25,26. Considerable evidence from electrophysiological studies in patients with primary dystonia suggests that abnormalities in the central nervous system are more widespread. These abnormalities include a loss of inhibition at various levels of the nervous system, abnormalities in sensory-motor integration and abnormal neuroplasticity27. The basal ganglia connect to the cortex and thalamus and organize muscle-driven “motor” movements of the body. The major divisions of the basal ganglia are the caudate nucleus, putamen, globus pallidus and substantia nigra. The globus pallidus can be further divided into two subsections, the globus pallidus interna (GPi) and globus pallidus externa (GPe). Microelectrode recordings of GPi neurons taken during neurosurgical procedures on patients with primary and secondary dystonias have demonstrated low discharge rates, with irregular firing patterns6,28,29. Local field potentials recorded from the GPi have also demonstrated a prominent and abnormal oscillation in the 3–12 Hz band, synchronising with discharges from local neurons30. Further studies have demonstrated correlation between these abnormal local field potential oscillations and oscillations in EMG recordings in dystonic patients31. This coupling is bi-directional, though the drive from the GPi to peripheral muscle outweighs that in the reverse direction32. Secondary Dystonia has been hypothesised as deriving from a reduced and disordered GPi output, with a pathological low frequency oscillation.
TREATMENT OF DYSTONIA
Treatment options in dystonia include oral medications, botulinum toxin for focal dystonia, intrathecal baclofen and neurosurgical interventions. In this latter category, surgical pallidotomy has been largely replaced with DBS to the GPi.
Oral medications
Numerous medications have been used in the management of dystonia, including anti-cholinergic medications (e.g. trihexiphenidyl), tetrabenazine, benzodiazepines (e.g. diazepam) and baclofen.
Trihexyphenidyl has an anticholinergic effect, reducing striated muscle contractions and parasympathetic activity. It has been widely used in the treatment of both primary and secondary dystonias for more then 20 years33. Retrospective data has suggested a beneficial effect in the management of dystonic cerebral palsy34. A more recent open-labelled study in children with CP suggested an improvement in arm function with its use35, though robust prospective data is lacking. A starting dose of 0.5 mg (infant), 1 mg (child) or 2 mg (adolescent) three times a day is generally used by the authors, increasing by 0.5, 1 or 2mg per day each week until either an effect is seen. In children and young adults doses can be increased until side effects occur or a total daily dose of 9, 30 or 90 mg is reached. The main side effects of trihexyphenidyl include dry eyes and mouth, gastrointestinal disturbances, urinary retention and behavioural disturbances. Pupil dilatation should not be considered a dose limiting side effect. These doses are in excess of the dose guidance in the British National Formulary. Use of these medications should only be considered in the context of ongoing specialist neurodisability follow up.
Tetrabenazine is a benzoquinolone that depletes synaptic dopamine transmission by a dual action, reducing vesicular monoamine uptake by binding to vesicular monoamine transporters, and also by acting as a post-synaptic dopamine receptor antagonist. Tetrabenazine is more widely used in the treatment of chorea in adult Huntington's disease. Its use in children is less common, though a number of case reports have suggested efficacy. A retrospective study of 31 paediatric patients with hyperkinetic movement disorders refractory to other medications suggested a symptomatic improvement in 24 (77%) patients36. In this study relatively high doses of tetrabenazine were required, over 100mg/day in three divided doses compared to an average of 70mg/day reported in adult studies.
Parkinsonism is recognised as a common side effect of tetrabenazine use in the adult population37. It is not possible to estimate the incidence in the paediatric population, but it has been hypothesised that this should be seen less frequently given the decline in dopamine function seen with age38.
DEEP BRAIN STIMULATION
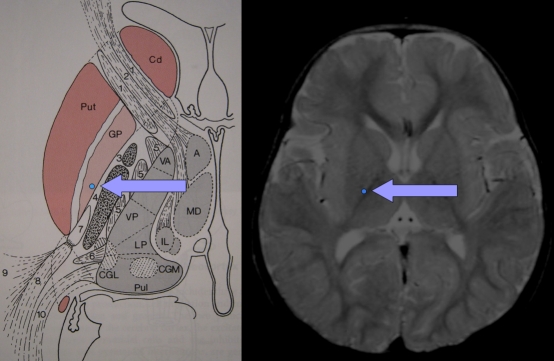
In recent years Deep Brain Stimulation (DBS)39 has increasingly been used in the treatment of dystonia in both the paediatric and adult age group40–44. The technique utilises quadripolar electrodes implanted within the deep nuclei of the brain to deliver a continuous electrical signal. The GPi has become the established target in the treatment of dystonia (figure 1). The implanted electrodes are linked by an extension lead to a remote stimulator unit that is implanted in a similar fashion to a cardiac pacemaker into the subcutaneous tissue in the supraclavicular region or the abdomen. This unit may control either one or both of the implanted electrodes. Medtronic are currently the only manufacturer of approved devices. In paediatric patients, the contacts on the implanted electrodes are separated by 0.5 mm.
Fig 1.
Anatomical location of the Globus Pallidus Internus (GPi)
The Complex Motor Disorder Service (CMDS) at the Evelina Children's Hospital, London, in conjunction with the Functional Neurosurgery service at King's College Hospital, London, currently provides the only dedicated paediatric DBS service in the UK. Patients with dystonia would be considered suitable for referral where conventional therapies (i.e. medications) do not adequately control symptoms or where side effects can not be tolerated by patients (figure 2).
Fig 2.
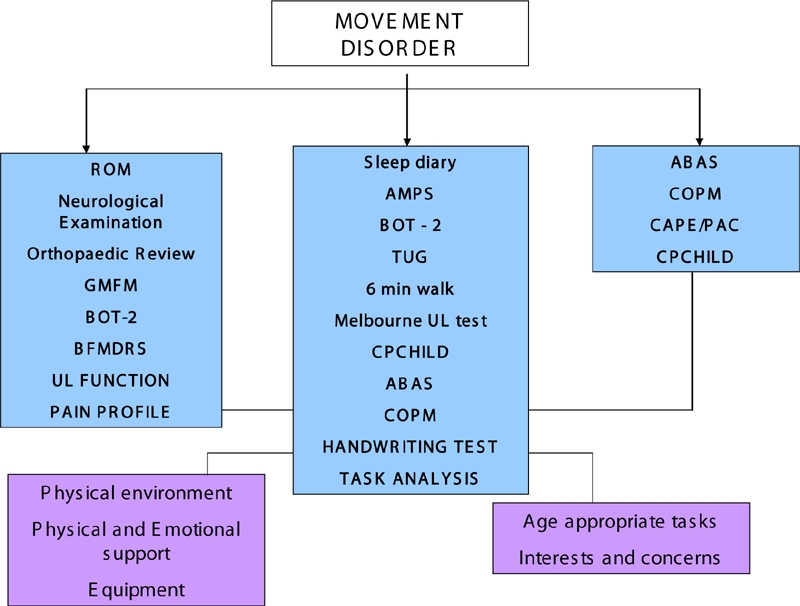
Assessment techniques in children with movement disorders
Pre-Operative Assessment
Following referral to the CMDS, patients receive a comprehensive baseline assessment by the multi-disciplinary therapy team. MRI of the brain allows detailed anatomical pictures of structures of interest, which is useful in determining diagnosis, location and extent of visible lesions, presence of co-morbidities or other abnormalities. This is particularly important in determining whether there is an intact GPi target site for DBS. Transcranial Magnetic Stimulation (TMS), also termed “Magstim” testing, is a non invasive and painless method of exciting neurons using strong magnetic fields held over the cortex. Electromyogram (EMG) recorded over hands and feet are used to measure nerve conduction and velocity to ensure the corticospinal tract is intact. Extensive corticospinal damage would be considered a relative contraindication to DBS. Such patients would instead be considered for ITB.
DBS Surgery
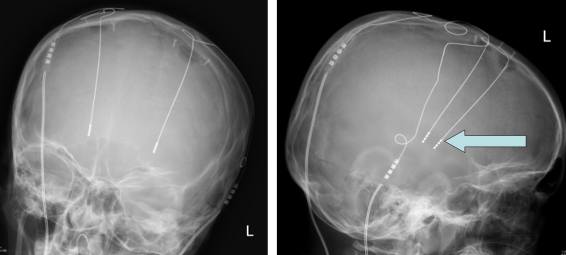
Pallidal DBS should be considered a reversible procedure, in contrast to the pallidotomy it has largely superseded. The implanted neurostimulator is often described as a brain “pace maker” (figure 3). The surgery is performed in awake adults, but in children a general anaesthetic is used.
Fig 3.
X-rays demonstrating the position of DBS wires
Mechanism and efficacy of DBS
The precise mechanism of action of DBS is far from clear. In broad terms, stimulation either creates a “functional lesion” in the target nuclei or replaces an aberrant output from the target nuclei with a more physiological signal45–47. A functional lesioning mechanism would be consistent with the comparable effect of pallidal DBS and surgical pallidotomy.
Most studies of DBS in the treatment of dystonia have used the Burke-Fahn-Marsden Dystonia Rating scale as a measure of the severity of dystonic symptoms48. Primary dystonia is more responsive to Pallidal DBS then secondary dystonia49. In Secondary dystonia, improvements in BFMDRS compared to baseline of 5–40% have been reported. This contrasts with the up to 80% improvement seen in Primary dystonia. A minimum response of 25% is often defined as a “success”. It important to note that reduction in dystonia does not always equate to improvements in function, but quality of life, comfort and carer burden issues can be much improved both for the child and their carers.
The range of treatments available for children with motor disorders has expanded considerably in recent years. The best outcomes are obtained in the setting of an experienced multiprofessional team where children and families have the opportunity to learn about treatment options and have access to the right intervention at the right time. Treatment is more likely to be successful in the context of a child who is not in pain, has comfortable and appropriate equipment and is able to communicate.
Acknowledgments
Jean-Pierre Lin, Kylee Tustin and Hortensia Gimeno.
The authors have no conflict of interest.
REFERENCES
- 1.Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE) Dev Med Child Neurol. 2000;42(12):816–24. doi: 10.1017/s0012162200001511. [DOI] [PubMed] [Google Scholar]
- 2.Beckung E, White-Koning M, Marcelli M, McManus V, Michelsen S, Parkes J, et al. Health status of children with cerebral palsy living in Europe: a multi-centre study. Child Care Health Dev. 2008;34(6):806–14. doi: 10.1111/j.1365-2214.2008.00877.x. [DOI] [PubMed] [Google Scholar]
- 3.Gilmartin R, Bruce D, Storrs BB, Abbott R, Krach L, Ward J, et al. Intrathecal baclofen for management of spastic cerebral palsy: multicenter trial. J Child Neurol. 2000;15(2):71–7. doi: 10.1177/088307380001500201. [DOI] [PubMed] [Google Scholar]
- 4.Albright AL. Neurosurgical treatment of spasticity and other pediatric movement disorders. J Child Neurol. 2003;18(Suppl 1):S67–S78. doi: 10.1177/0883073803018001S0801. [DOI] [PubMed] [Google Scholar]
- 5.Verrotti A, Greco R, Spalice A, Chiarelli F, Iannetti P. Pharmacotherapy of spasticity in children with cerebral palsy. Pediatr Neurol. 2006;34(1):1–6. doi: 10.1016/j.pediatrneurol.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Zhuang P, Li Y, Hallett M. Neuronal activity in the basal ganglia and thalamus in patients with dystonia. Clin Neurophysiol. 2004;115(11):2542–57. doi: 10.1016/j.clinph.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Coffey JR, Cahill D, Steers W, Park TS, Ordia J, Meythaler J, et al. Intrathecal baclofen for intractable spasticity of spinal origresults of a long-term multicenter study. J Neurosurg. 1993;78(2):226–32. doi: 10.3171/jns.1993.78.2.0226. [DOI] [PubMed] [Google Scholar]
- 8.Levin AB, Sperling KB. Complications associated with infusion pumps implanted for spasticity. Stereotact Funct Neurosurg. 1995;65(1–4):147–51. doi: 10.1159/000098887. [DOI] [PubMed] [Google Scholar]
- 9.Woon K, Tsegaye M, Vloeberghs MH. The role of intrathecal baclofen in the management of primary and secondary dystonia in children. Br J Neurosurg. 2007;21(4):355–8. doi: 10.1080/02688690701392899. [DOI] [PubMed] [Google Scholar]
- 10.Butler C, Campbell S. Evidence of the effects of intrathecal baclofen for spastic and dystonic cerebral palsy. AACPDM Treatment Outcomes Committee Review Panel. Dev Med Child Neurol. 2000;42(9):634–45. doi: 10.1017/s0012162200001183. [DOI] [PubMed] [Google Scholar]
- 11.Bensmail D, Quera Salva MA, Roche N, Benyahia S, Bohic M, Denys P, et al. Effect of intrathecal baclofen on sleep and respiratory function in patients with spasticity. Neurology. 2006;67(8):1432–6. doi: 10.1212/01.wnl.0000239827.38036.23. [DOI] [PubMed] [Google Scholar]
- 12.Baker R, Jasinski M, Maciag-Tymecka I, Michalowska-Mrozek J, Bonikowski M, Carr L, et al. Botulinum toxin treatment of spasticity in diplegic cerebral palsy: a randomized, double-blind, placebo-controlled, dose-ranging study. Dev Med Child Neurol. 2002;44(10):666–75. doi: 10.1017/s0012162201002730. [DOI] [PubMed] [Google Scholar]
- 13.Carr LJ, Cosgrove AP, Gringras P, Neville BG. Position paper on the use of botulinum toxin in cerebral palsy. UK Botulinum Toxin and Cerebral Palsy Working Party. Arch Dis Child. 1998;79(3):271–3. doi: 10.1136/adc.79.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cosgrove AP, Corry IS, Graham HK. Botulinum toxin in the management of the lower limb in cerebral palsy. Dev Med Child Neurol. 1994;36(5):386–96. doi: 10.1111/j.1469-8749.1994.tb11864.x. [DOI] [PubMed] [Google Scholar]
- 15.Lundy CT, Doherty GM, Fairhurst CB. Botulinum toxin type A injections can be an effective treatment for pain in children with hip spasms and cerebral palsy. Dev Med Child Neurol. doi: 10.1111/j.1469-8749.2009.03315.x. Epub 2009. Apr 21. [DOI] [PubMed] [Google Scholar]
- 16.Dolly JO, Aoki KR. The structure and mode of action of different botulinum toxins. Eur J Neurol. 2006;13(Suppl 4):1–9. doi: 10.1111/j.1468-1331.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 17.Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, et al. SV2 is the protein receptor for botulinum neurotoxin A. Science. 2006;312(5773):592–6. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- 18.Naumann M, Jankovic J. Safety of botulinum toxin type A: a systematic review and meta-analysis. Curr Med Res Opin. 2004;20(7):981–90. doi: 10.1185/030079904125003962. [DOI] [PubMed] [Google Scholar]
- 19.Association of Paediatric Chartered Physiotherapists. Evidence based guidance for physiotherapists: the use of Botulinum Toxin in children with neurological conditions. Huntingdon: APCP; 2008. [Google Scholar]
- 20.Himmelmann K, Hagberg G, Wiklund LM, Eek MN, Uvebrant P. Dyskinetic cerebral palsy: a population-based study of children born between 1991 and 1998. Dev Med Child Neurol. 2007;49(4):246–51. doi: 10.1111/j.1469-8749.2007.00246.x. [DOI] [PubMed] [Google Scholar]
- 21.de Carvalho Aguiar PM, Ozelius LJ. Classification and genetics of dystonia. Lancet Neurol. 2002;1(5):316–25. doi: 10.1016/s1474-4422(02)00137-0. [DOI] [PubMed] [Google Scholar]
- 22.Han F, Racacho L, Lang AE, Bulman DE, Grimes DA. Refinement of the DYT15 locus in myoclonus dystonia. Mov Disord. 2007;22(6):888–92. doi: 10.1002/mds.21400. [DOI] [PubMed] [Google Scholar]
- 23.Grotzsch H, Pizzolato GP, Ghika J, Schorderet D, Vingerhoets FJ, Landis T, et al. Neuropathology of a case of dopa-responsive dystonia associated with a new genetic locus, DYT14. Neurology. 2002;58(12):1839–42. doi: 10.1212/wnl.58.12.1839. [DOI] [PubMed] [Google Scholar]
- 24.Klein C, Breakefield XO, Ozelius LJ. Genetics of primary dystonia. Semin Neurol. 1999;19(3):271–80. doi: 10.1055/s-2008-1040843. [DOI] [PubMed] [Google Scholar]
- 25.Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain. 1985;108(2):463–83. doi: 10.1093/brain/108.2.463. [DOI] [PubMed] [Google Scholar]
- 26.Pettigrew LC, Jankovic J. Hemidystonia: a report of 22 patients and a review of the literature. J Neurol Neurosurg Psychiatry. 1985;48(7):650–7. doi: 10.1136/jnnp.48.7.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quartarone A, Rizzo V, Morgante F. Clinical features of dystonia: a pathophysiological revisitation. Curr Opin Neurol. 2008;21(4):484–90. doi: 10.1097/WCO.0b013e328307bf07. [DOI] [PubMed] [Google Scholar]
- 28.Sanghera MK, Grossman RG, Kalhorn CG, Hamilton WJ, Ondo WG, Jankovic J. Basal ganglia neuronal discharge in primary and secondary dystonia in patients undergoing pallidotomy. Neurosurgery. 2003;52(6):1358–70. doi: 10.1227/01.neu.0000064805.91249.f5. [DOI] [PubMed] [Google Scholar]
- 29.Vitek JL, Chockkan V, Zhang JY, Kaneoke Y, Evatt M, DeLong MR, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46(1):22–35. doi: 10.1002/1531-8249(199907)46:1<22::aid-ana6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 30.Chen CC, Kuhn AA, Trottenberg T, Kupsch A, Schneider GH, Brown P. Neuronal activity in globus pallidus interna can be synchronized to local field potential activity over 3–12 Hz in patients with dystonia. Exp Neurol. 2006;202(2):480–6. doi: 10.1016/j.expneurol.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Chen CC, Kuhn AA, Hoffmann KT, Kupsch A, Schneider GH, Trottenberg T, et al. Oscillatory pallidal local field potential activity correlates with involuntary EMG in dystonia. Neurology. 2006;66(3):418–20. doi: 10.1212/01.wnl.0000196470.00165.7d. [DOI] [PubMed] [Google Scholar]
- 32.Sharott A, Grosse P, Kuhn AA, Salih F, Engel AK, Kupsch A, et al. Is the synchronization between pallidal and muscle activity in primary dystonia due to peripheral afferance or a motor drive? Brain. 2008;131(2):473–84. doi: 10.1093/brain/awm324. [DOI] [PubMed] [Google Scholar]
- 33.Fahn S. High-dosage anticholinergic therapy in dystonia. Adv Neurol. 1983;37:177–88. [PubMed] [Google Scholar]
- 34.Hoon AH, Jr., Freese PO, Reinhardt EM, Wilson MA, Lawrie WT, Jr., et al. Age-dependent effects of trihexyphenidyl in extrapyramidal cerebral palsy. Pediatr Neurol. 2001;25(1):55–8. doi: 10.1016/s0887-8994(01)00287-9. [DOI] [PubMed] [Google Scholar]
- 35.Sanger TD, Bastian A, Brunstrom J, Damiano D, Delgado M, Dure L, et al. Prospective open-label clinical trial of trihexyphenidyl in children with secondary dystonia due to cerebral palsy. J Child Neurol. 2007;22(5):530–7. doi: 10.1177/0883073807302601. [DOI] [PubMed] [Google Scholar]
- 36.Jain S, Greene PE, Frucht SJ. Tetrabenazine therapy of pediatric hyperkinetic movement disorders. Mov Disord. 2006;21(11):1966–72. doi: 10.1002/mds.21063. [DOI] [PubMed] [Google Scholar]
- 37.Jankovic J, Beach J. Long-term effects of tetrabenazine in hyperkinetic movement disorders. Neurology. 1997;48(2):358–62. doi: 10.1212/wnl.48.2.358. [DOI] [PubMed] [Google Scholar]
- 38.Paleacu D, Giladi N, Moore O, Stern A, Honigman S, Badarny S. Tetrabenazine treatment in movement disorders. Clin Neuropharmacol. 2004;27(5):230–3. doi: 10.1097/01.wnf.0000136892.24629.96. [DOI] [PubMed] [Google Scholar]
- 39.Krause M, Fogel W, Kloss M, Rasche D, Volkmann J, Tronnier V. Pallidal stimulation for dystonia. Neurosurgery. 2004;55(6):1361–8. doi: 10.1227/01.neu.0000143331.86101.5e. [DOI] [PubMed] [Google Scholar]
- 40.Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med. 2005;352(5):459–67. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- 41.Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Lagrange C, Yelnik J, et al. Bilateral, pallidal, deep-brain stimulation in primary generalised dystonia: a prospective 3 year follow-up study. Lancet Neurol. 2007;6(3):223–9. doi: 10.1016/S1474-4422(07)70035-2. [DOI] [PubMed] [Google Scholar]
- 42.Coubes P, Cif L, El FH, Hemm S, Vayssiere N, Serrat S, et al. Electrical stimulation of the globus pallidus internus in patients with primary generalized dystonia: long-term results. J Neurosurg. 2004;101(2):189–94. doi: 10.3171/jns.2004.101.2.0189. [DOI] [PubMed] [Google Scholar]
- 43.Bittar RG, Yianni J, Wang S, Liu X, Nandi D, Joint C, et al. Deep brain stimulation for generalised dystonia and spasmodic torticollis. J Clin Neurosci. 2005;12(1):12–6. doi: 10.1016/j.jocn.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 44.Castelnau P, Cif L, Valente EM, Vayssiere N, Hemm S, Gannau A, et al. Pallidal stimulation improves pantothenate kinase-associated neurodegeneration. Ann Neurol. 2005;57(5):738–41. doi: 10.1002/ana.20457. [DOI] [PubMed] [Google Scholar]
- 45.Dostrovsky JO, Lozano AM. Mechanisms of deep brain stimulation. Mov Disord. 2002;17(Suppl 3):S63–S68. doi: 10.1002/mds.10143. [DOI] [PubMed] [Google Scholar]
- 46.Kuncel AM, Grill WM. Selection of stimulus parameters for deep brain stimulation. Clin Neurophysiol. 2004;115(11):2431–41. doi: 10.1016/j.clinph.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 47.Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002;17(Suppl 3):S69–S72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- 48.Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology. 1985;35(1):73–7. doi: 10.1212/wnl.35.1.73. [DOI] [PubMed] [Google Scholar]
- 49.Eltahawy HA, Saint-Cyr J, Giladi N, Lang AE, Lozano AM. Primary dystonia is more responsive than secondary dystonia to pallidal interventions: outcome after pallidotomy or pallidal deep brain stimulation. Neurosurgery. 2004;54(3):613–9. doi: 10.1227/01.neu.0000108643.94730.21. [DOI] [PubMed] [Google Scholar]