Abstract
Glutamate is a key neurotransmitter and its levels in the synaptic cleft are tightly regulated by reuptake mechanisms that primarily involve transporters in astrocytes. This requires that the glutamate transporters be spatially constrained to effect maximum glutamate transport. GLAST (EAAT1) is the predominant astroglial transporter and contains a class I PDZ-binding consensus (ETKM) in its C-terminus. The epithelial Na+/H+ exchanger regulatory factors NHERF1 and NHERF2 are PDZ proteins that contain two tandem PDZ domains and a C-terminal domain that binds members of the ERM (ezrin–radixin–moesin) family of membrane-cytoskeletal adaptors. NHERF proteins have been extensively characterized in renal epithelia and their expression in brain has recently been reported; however, their function in the brain remains unknown. The aims of the current study were to (1) determine the distribution of NHERF1/2 in the rodent brain and (2) investigate whether GLAST was a physiological ligand for NHERF1/2. Immunohistochemistry revealed that NHERF1 expression was widespread in rat brain (abundant in cerebellum, cerebral cortex, hippocampus, and thalamus) and primarily restricted to astrocytes whereas NHERF2 expression was primarily restricted to endothelial cells of blood vessels and capillaries. Importantly, NHERF1 distribution closely matched that of GLAST and confocal imaging demonstrated co-localization of the two proteins. Co-immunoprecipitation demonstrated that GLAST, NHERF1, and ezrin associate in vivo. In vitro binding assays showed that GLAST bound directly to the PDZ1 domain of NHERF1 via the C-terminal ETKM motif of GLAST. These findings implicate the GLAST–NHERF1 complex in the regulation of glutamate homeostasis in astrocytes.
Keywords: NHERF1, NHERF2, GLAST, astrocytes, glutamate transport
INTRODUCTION
Glutamate is a key excitatory neurotransmitter of the vertebrate central nervous system (CNS) (Fonnum, 1984) and is released into the synaptic cleft during neurotransmission, where it activates glutamate receptors. Marcaggi and Attwell (2004) in reviewing the role of glial cells in the modulation of glutamatergic signaling noted that synapses are partly or wholly wrapped by glial processes expressing a high density of glial glutamate transporters (Lehre and Danbolt, 1998). At high extracellular levels, glutamate acts as a potent neurotoxin and alterations in glutamate transporter expression/activity have been implicated in a number of neurological disorders, including stroke, epilepsy, Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and Huntington’s disease (Danbolt, 2001). Thus, glutamate levels in the extracellular space must be subject to tight temporal and spatial constraint and this must in term be reflected by spatial constraint of the glutamate transporters. Indeed, these transporters have been shown to have differential distribution patterns, are known to be enriched in specific membrane domains, and exist in puncta in astrocyte processes (Danbolt, 2001). However, the identities of the proteins and the interactions that mediate these spatial constraints remain to be elucidated.
To date, five different members of the Na+-dependent glutamate transporter family have been identified (EAAT1–5), and it is generally believed that GLAST (EAAT1) and GLT-1 (EAAT2) play the major roles in maintaining low extracellular concentrations of glutamate at the synapse (Danbolt, 2001). GLAST is primarily an astroglial transporter and the principle transporter protein present during CNS development (Furuta et al., 1997). In adult tissue, GLAST is predominantly expressed in the Bergman glia of the cerebellum, although this transporter is also expressed in other areas of the brain. In contrast, GLT-1, which is also an astroglial transporter, is expressed most notably in the hippocampus, lateral septum, cerebral cortex, and striatum (Lehre et al., 1995). The comparative distributions of GLT-1 including its splice variants have recently been described for a variety of species including humans (Williams et al., 2005). This and most other studies on brain tissues have described GLT-1 as being predominantly glial in localization, though some studies have described a restricted neuronal localization for GLT1a or GLT-1b, based on immunohistochemical and in situ hybridization data (Berger et al., 2005; Chen et al., 2004).
All of these glutamate transporters have intracellular C-termini that may interact with proteins that direct the formation of functional complexes at the plasma membrane. One of the most common protein modules involved in scaffolding interactions are the PSD-95/Disc-large/ZO-1 (PDZ) domains, which are responsible for a wide array of protein–protein interactions in the CNS and other tissues. These modules bind to short sequences at the extreme C-terminus of target proteins, thereby nucleating the formation of specific functional complexes at the membrane. For example, the PDZ module of PICK1 interacts with the C-terminus of the dopamine transporter DAT, which results in clustering of DAT on the cell surface of dopaminergic neurons and a concomitant increase in DAT uptake (Torres et al., 2001). In astrocytes, the best characterized PDZ scaffold is α-syntrophin. This protein links the water channel AQP4 to the K+ channel Kir4.1 and thereby spatially constraining the complexes at end feet processes of astrocytes where they regulate water and K+ levels (Neely et al., 2001). PDZ-protein-mediated regulation of glutamate transporters has been demonstrated for the neuronal EAAT4, which associates with GTRAP48 (for glutamate transporter-4 associated protein), a PDZ domain containing protein expressed predominantly in the cerebellum (Jackson et al., 2001). GTRAP48 specifically interacts with the C-terminus of EAAT4 and positively modulates its glutamate transport activity by enhancing the cell surface expression of EAAT4 (Jackson et al., 2001). The retinal EAAT5 has also been reported to contain a PDZ binding motif at its C-terminus, based on findings from yeast two-hybrid screening, making it likely that the EAAT5 C-terminus may be involved in EAAT5-PDZ interactions (Arriza et al., 1997). The astroglial EAAT1 (GLAST) and EAAT2 splice variant (GLT1b) and the neuronal EAAT3 also contain putative PDZ binding motifs in their extreme C-termini; however, there are to date no reports of PDZ proteins interacting with these transporters.
One class of PDZ proteins that is being found to have an increasingly diverse range of functions are the Na+/H+ exchanger regulatory factors, NHERF1 and NHERF2 (Brone and Eggermont, 2005; Shenolikar et al., 2004). These proteins were the first PDZ adaptors found in epithelial tissue, initially characterized as facilitating the formation of a multiprotein complex that mediated PKA phosphorylation of the Na+/H+ exchanger 3 (NHE3) and down-regulation of its activity (Lamprecht et al., 1998; Weinman et al., 1995; Yun et al., 1997). NHERF1/2 contains two PDZ modules (PDZ1 and PDZ2) that can bind to the C-terminal tails of ion channels, transporters, and receptors (Brone and Eggermont, 2005). NHERF1/2 also contain at their C-terminus, an ezrin–radixin–moesin (ERM)-binding domain that interacts with ezrin, a widely expressed protein found predominantly in the apical aspect of many polarized epithelial cells where it functions as a microfilament membrane linker that participates in, and is regulated by signal transduction pathways (Ramesh, 2004). NHERF1/2 nucleates the formation of macromolecular complexes by physically linking plasma membrane proteins to the cytoskeleton and recruiting signaling molecules such as PKA (Yun et al., 1997), PKC (Liedtke et al., 2002), and PLCβ (Tang et al., 2002) to the complex. Although it was first thought to be restricted to transporting epithelia, NHERF1/2 have since been reported in cochlea (Kanjhan et al., 2006), retina (Nawrot et al., 2004), dorsal root ganglia (Deval et al., 2006), vascular smooth muscle, lymphocytes, and endometrium (Stemmer-Rachamimov et al., 2001).
There have been some reports of NHERF1/2 in the brain. Initially, Northern blotting demonstrated that NHERF1 mRNA was present in many regions of the mammalian brain (Ediger et al., 1999; Weinman et al., 1995). More recently, NHERF1 protein expression was reported in polarized Schwann cell processes with an implicated role in actin remodeling (Gatto et al., 2003). NHERF1 protein expression was also found in cultured astrocytes where it associates with TRPC4 (Song et al., 2005). As for NHERF2, this protein was recently found to be expressed in perivascular glial cells and interacts with P2Y1R (Fam et al., 2005). Of particular interest is the observation that ERM proteins that bind NHERF1 (Bretscher et al., 2000) show a similar distribution pattern to GLAST and GLT-1b in cultured astrocytes. Furthermore, Marie and Attwell (1999) have also shown that whole cell patch-clamped retinal glia dialyzed with peptides identical to the GLAST C-terminal eight amino acids, which contains the putative PDZ binding motif significantly altered GLAST glutamate affinity. This suggests that the endogenous protein being displaced by dialysis with the GLAST C-terminus peptide is likely to be a PDZ domain containing protein (Marie and Attwell, 1999). These observations combined with the fact that both GLAST and GLT-1b possess putative PDZ binding motifs (ETKM and ETCI, respectively) raises the intriguing possibility that NHERF1 may be a scaffold for glutamate transporter complexes in astrocytes. The current study was undertaken to determine the broad distribution of NHERF1/2 in the rat brain and investigate the interaction between these glutamate transporters and NHERF1/2.
MATERIALS AND METHODS
Antibodies
Antibodies against NHERF1 and NHERF2 (Kanjhan et al., 2006), GLAST (Pow and Barnett, 1999), and GLT1b (Reye et al., 2002) have been described previously. Antibody specific for ezrin (residues 362–585 of human ezrin, GenBank™ accession number NM_003379) was purchased from Zymed Laboratories (San Francisco, CA, USA).
Tissue Preparation and Immunohistochemistry
Adult rats (n = 6) were euthanized by an overdose of sodium pentobarbital (100 mg/kg, administered i.p.). Animals were fixed initially by perfusion via the heart with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.2) and then fixed by immersion for a further 1 h in the same fixative. Coronal and sagittal sections of brains (40-μm thick) were cut using a Vibratome. Immunohistochemistry was performed on free-floating sections, using standard immunocytochemical techniques as previously described (Pow and Hendrickson, 1999; Williams et al., 2005). For controls, sections were routinely labeled using preimmune serum in place of immune serum. Preabsorption control experiments were routinely included in which the dilute immune serum was preabsorbed overnight at 4°C with ~50 μg of immunizing peptide per milliliter of diluted antiserum. Peroxidase-labeled sections were photographed using a Nikon DXM 1200 Digital Camera. All digital files were imported into Adobe Photoshop 7.
Immunofluorescence and Confocal Microscopy
Dual immunofluorescence labeling was performed essentially as above, using a GLAST antibody raised in guinea pig and the NHERF1 antibody raised in rabbit. Detection of labeling was by means of species-specific secondary antibodies labeled with Texas Red (for GLAST) or FITC (for NHERF1). Confocal imaging of immunofluorescently labeled sections was performed using a Nikon C1 confocal microscope equipped with solid-state lasers emitting at 488 and 594 nm to excite FITC and Texas Red, respectively. Images were sequentially acquired, the red and green channels combined and saved as tiff files.
Cloning of Rat GLAST cDNA, Plasmid Constructions, and Fusion Protein Expression
GLAST cDNA was cloned from rat astroglial-rich primary cultures by high fidelity PCR with primers corresponding to sequence from GenBank™ accession number NM_019225. cDNA encoding the C-terminal tail of GLAST was inserted into the glutathione S-transferase (GST) fusion vector, pGEX6P-1 (Amersham Pharmacia Biotech, NJ, USA) to produce GLAST-CT construct. The C-terminal deletion construct (GLAST Δ-PDZ) was generated using the Quick Change Site-Directed Mutagenesis kit (Strategene, CA, USA) from the GLAST-CT construct with sense primer 5′-CCCGTGGCAGACAGCTAAACCAAGATGTAGTCG-3′ and antisense primer 5′-CGACTACATCTTGGTTTAGCTGTCTGCCACGGG-3′. Fidelity of the plasmid constructs was confirmed by DNA sequencing. Full-length NHERF1 and individual domains of NHERF1 (PDZ1, PDZ2, and COOH), each in a pGEX4T-1 vector, have been described previously (Fouassier et al., 2000). GST fusion proteins were expressed in the Escherichia coli strain BL21 (Strategene). Optimal expression of fusion proteins was achieved by adding a final concentration of 2 mM isopropyl-β-D-thiogalactopyranoside to the bacterial culture, with overnight incubation at 20°C. Bacteria were harvested by centrifugation and resuspended in phosphate-buffered saline (PBS). After sonication, GST-fusion proteins were purified using glutathione–sepharose 4B beads, following the manufacturer’s instructions (Amersham Pharmacia Biotech).
Cross-linking of Antibodies to Protein A Agarose
Anti-GLAST/GLT1b/NHERF1 antibodies were incubated with protein A agarose (Roche Diagnostics GmbH, Penzberg, Germany) for 4 h at 4°C. Antibody-bound protein A agarose was washed three times in PBS and resuspended in PBS containing freshly prepared disuccinimidyl suberate (DSS) cross-linker (~3 mg/mL; Sigma-Aldrich, NSW, Australia) and incubated for 1.5 h at room temperature. Excess DSS was removed by washing the antibody-bound protein A agarose four times with Tris-buffered saline (TBS; 25 mM Tris and 150 mM NaCl, pH 7.6), four times with 0.1 M glycine (pH 2.8) to remove free antibody and finally four times with TBS.
Co-immunoprecipitation and Western Blotting
Brain tissues (from adult rats) were homogenized in IP-lysis buffer containing 50 mM Tris–HCl (pH 8.0), 150 mM NaCl, 1% Triton X-100, and protease inhibitor cocktail (Roche diagnostics GmbH). After gentle rotation for 2 h at 4°C, homogenates were centrifuged at 100,000g for 60 min at 4°C and the supernatant collected. Brain lysate was precleared with protein A agarose for 2 h at 4°C and incubated with the antibody-protein A agarose (prepared as described above) overnight at 4°C. The beads were washed three times with TBS and the protein complexes were eluted three times with 0.1 M glycine (pH 2.8). Elutions were pooled and concentrated using a rotary vacuum concentrator. Proteins were separated on a 7% SDS–polyacrylamide gel and transferred to nitrocellulose membrane (Bio-Rad Laboratories, CA, USA) by electroblotting. Blots were incubated with antibodies against GLAST, NHERF1/2, or ezrin overnight at 4°C in 5% nonfat milk, 100 mM Tris (pH 7.5), 306 mM NaCl, and 0.1% Tween-20. Blots were washed four times with Tris–NaCl–Tween buffer, incubated for 1 h with horseradish peroxidase-conjugated secondary antibody (Pierce Biotechnology, Illinois, USA), and washed again. Immunoreactive proteins were detected by enhance chemiluminescence, using the SuperSignal kit (Pierce Biotechnology).
In Vitro Binding Assays
GST-GLASTct, GST-GLAST Δ-PDZ, and GST (10 μg) were resolved by SDS–polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane and blocked with blocking buffer (5% skim milk, 10 mM Tris (pH 8.0), 150 mM NaCl) containing 1% BSA at room temperature for 3 h. The membrane was overlaid with [35S]-methionine-labeled full-length NHERF1 [generated using the TNT in vitro transcription–translation system (Promega)] in blocking buffer containing 0.1% BSA for 4 h. The membrane was washed three times in wash buffer [10 mM Tris (pH 8.0), 150 mM NaCl, 0.05% Tween-20] and the label visualized by autoradiography.
GST Pull-down Assays
Brain tissues were homogenized in HNT buffer containing 20 mM Hepes–Tris (pH 7.5), 120 mM NaCl, 0.6% Triton X-100, 5 mM EDTA, and protease inhibitor cocktail (Roche Diagnostics GmbH). After gentle rotation for 2 h at 4°C, homogenates were centrifuged at 27,000g for 30 min at 4°C and the supernatant was collected. Brain lysate (5–10 mg) was then incubated with 10 μg GST fusion protein beads in HNT buffer at 4°C overnight with gentle rotation. Bound proteins were washed four times with HNT buffer and resuspended in SDS sample buffer. The samples were resolved by SDS–polyacrylamide gel electrophoresis and Western blotted (as described above) with appropriate antibodies.
RESULTS
NHERF1 and NHERF2 Expression in Brain Lysates
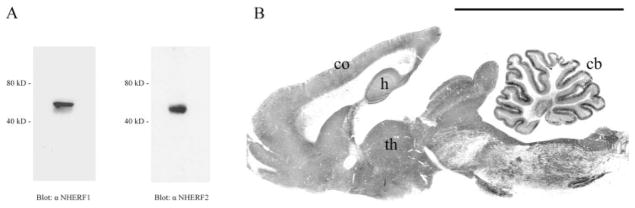
The specificities of the NHERF1 and NHERF2 antibodies for their targets in immunocytochemistry have been demonstrated previously (Kanjhan et al., 2006). We also tested the specificity of the NHERF1 and NHERF2 antibodies on immunoblots of electrophorectically separated SDS-solubilized brain lysate. Both NHERF1 and NHERF2 antibodies labeled a single band of ~48–52 kDa (Fig. 1A), consistent with the molecular mass of the corresponding NHERF proteins in kidney (Yun et al., 1997, 2002).
Fig. 1.
NHERF protein expression in brain. (A) Immunoblot analysis of homogenized brain lysate using anti-NHERF1 and anti-NHERF2 antibodies. (B) Low-magnification view of rat brain sagittal section immunolabeled for NHERF1. NHERF1 was highest in the cerebellum (cb) and moderately strong labeling was also detected in hippocampus (h), cerebral cortex (co), and thalamus (t). Scale bar = 1 cm.
Expression of NHERF1 in Rat Brain
Low-magnification image of entire sagittal section revealed that NHERF1 occur in many regions of the rodent brain, but at varying concentrations. NHERF1 labeling was extremely abundant in cerebellum and moderately strong labeling was also detected in regions such as cerebral cortex, hippocampus, and thalamus (Fig. 1B).
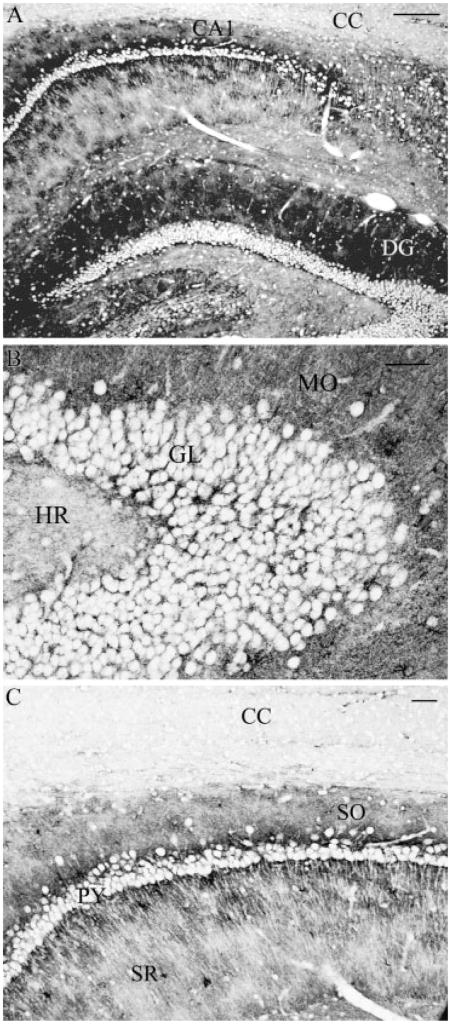
In brain regions such as hippocampus, strong immunolabeling was evident in the grey matter regions, including CA1 and dentate gyrus (Fig. 2A). Higher magnification of these regions revealed that neuronal elements were not labeled, in contrast to the dense labeling of astrocytes surrounding such neurons (Figs. 2B,C). For example, neuronal cell bodies were visible in the granular layer of the dentate gyrus and the pyramidal cell bodies in the stratum pyramidale in the CA1 region of the hippocampus. These structures were visible due to the staining for NHERF1 in surrounding astrocytes, as no staining occurs in the neuronal somata. In contrast, regions surrounding neuronal cell bodies were intensely stained, like the dendritic-rich regions of the molecular layer in the dentate gyrus (Fig. 2B) and the stratum oriens and stratum radiatum of the CA1 region. At higher magnification, dendritic striations can be indirectly observed in the stratum radiatum, again, due to NHERF1 labeling in surrounding astrocytes (Fig. 2C). A difference in labeling intensities can be seen when comparing the grey matter areas of the CA1 region of the hippocampus with the adjacent white matter region of the corpus callosum (Fig. 2A).
Fig. 2.
Sagittal section of rat brain hippocampus immunolabeled for NHERF1. (A) Strong labeling was observed in grey matter regions, including dentate gyrus (DG) and CA1 region (CA1), while negligible labeling was observed in white matter regions such as corpus callosum (CC). (B) In dentate gyrus, strongest labeling was observed in the molecular layer (MO), whereas weak labeling was observed in the hilar region (HR). In granule cell layer (GL), significant labeling was associated with astrocytes surrounding unlabeled neuronal elements. (C) In CA1 region, the strata oriens (SO) was strongly labeled, while less staining was observed for the strata radiatum (SR). Significant labeling was associated with astrocytes surrounding unlabeled neuronal elements such as pyramidal cells (py). Scale bars: (A) 100 μm, (B, C) 25 μm.
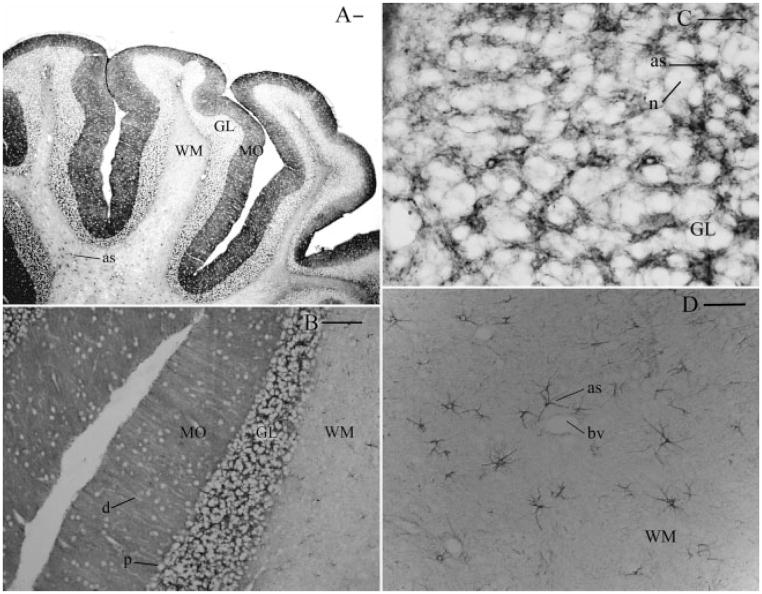
The cerebellum was another region that showed strong NHERF1 staining, with the vast majority of labeling associated with Bergmann glial cells in the molecular layer of the cerebellar cortex (Figs. 3A,B). Under higher magnification, radial striations that represented the nonstaining dendritic branches of Purkinje cells were easily seen (Fig. 3B), while NHERF1 staining was found in the surrounding Bergmann glial cells. NHERF1 was also expressed at high levels in puncta within astrocytes of the granule cell layer (Fig. 3C), while modest labeling was observed in the white matter tracts (Fig. 3A). The granule cell layer showed a large number of heavily stained astrocytes juxtaposed with non-NHERF1 staining granule cell somata (Fig. 3C). Similarly, the cerebellar white matter tracts showed that NHERF1 labeling was readily detected in processes of fibrous astrocytes. Such NHERF1 immunoreactive processes were often observed to be clustered around blood vessels (Fig. 3D). Under higher magnification, NHERF1 showed a striking punctate labeling pattern in individual astrocytes, which was similar to the punctate distribution of GLAST that we also observed in astrocytic processes (data not shown). The specificity of NHERF1 immunohistochemistry was confirmed by the disappearance of labeling by using antibody preabsorbed by the antigen peptide (~50 μg/mL; data not shown).
Fig. 3.
Sagittal section of rat brain cerebellum immunolabeled for NHERF1. (A) Heavy staining was evident in the molecular layer (MO) and granule cell layer (GL) when compared with the white matter tracts (WM), which featured staining in isolated fibrous astrocytes (as). (B) Vast majority of staining seen in the molecular layer was associated with Bergman glial cells. Areas surrounding medium to Purkinje cell somata (P) and dendritic processes (d) were immunonegative. (C) Higher magnification of the granule cell layer showed extensive labeling of astrocytes (as) surrounding unlabeled neuronal elements (n). (D) In the white matter tracts, strong labeling was readily detected in distinct astrocytes (as), many of which were often clustered around blood vessels (Bv). Scale bars: (A, B) 150 μm, (C) 31 μm, (D) 62 μm.
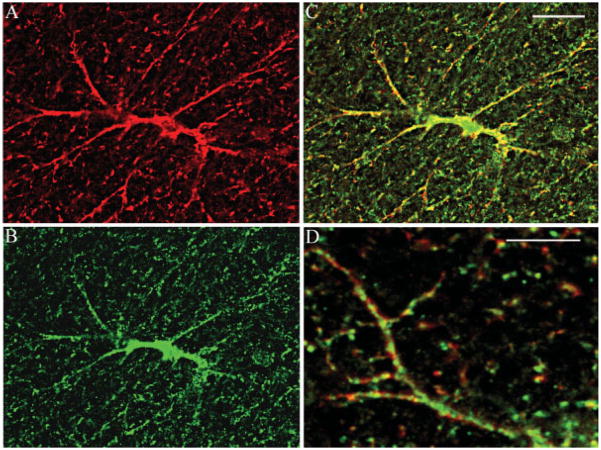
Confocal Imaging for NHERF1 and GLAST
In all brain regions studied, dual fluorescence labeling for GLAST and NHERF1 revealed the presence of each of these markers in cells with an astrocyte-like morphology (Figs. 4A,B). Sequential imaging for red and green fluorescence signals (Fig. 4C) revealed extensive co-localization between GLAST and NHERF1. Although overlap of signals was extensive, there were subtle differences. Both markers showed a punctate distribution and there was a small but identifiable spatial shift between the two signals, and this is clearly seen in single astrocyte processes when viewed under higher magnification (Fig. 4D). This indicated the close spatial association between the two markers, but the disparities confirmed that we were not simply seeing “bleed through” of signals between fluorescence channels.
Fig. 4.
Confocal images of NHERF1 and GLAST co-localization in astrocyte in thalamus. (A) GLAST localization in astrocyte (red); (B) NHERF1 in astrocyte (green); yellow labeling shows co-localization in astrocyte cell body (C), and processes (D). Scale bar = 10 μm.
Expression of NHERF2 in Rat Brain
Immunocytochemistry on rat brain using the NHERF2 antibody revealed a different pattern of labeling when compared with that of NHERF1. In all regions investigated (cortex, thalamus, and cerebellum), labeling for NHERF2 was restricted to blood vessels and capillaries (Figs. 5A–C) and clearly associated with the luminal membrane of endothelial cell bodies (Figs. 5C,D). Addition of recombinant NHERF2 (~50 μg/mL) to the NHERF2 antiserum completely abolished immunostaining (data not shown), confirming specificity of the NHERF2 immunohistochemistry.
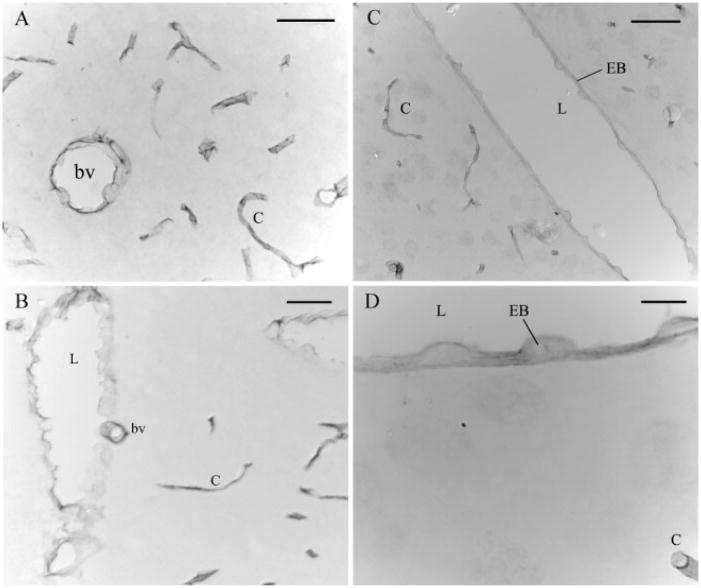
Fig. 5.
Rat brain sagittal sections immunolabeled for NHERF2. In sagittal sections of cerebral cortex (A), cerebellum (B), and thalamus (C, D), NHERF2 labeling was evident in endothelial cells of blood vessels (bv) and capillaries (c) and clearly associated with the luminal membrane (L) of endothelial cell bodies (EB). Scale bars: (A, B) 31.25 μm; (C) 62.5 μm; (D) 12.5 μm.
In Vivo Interaction between NHERF1 and GLAST
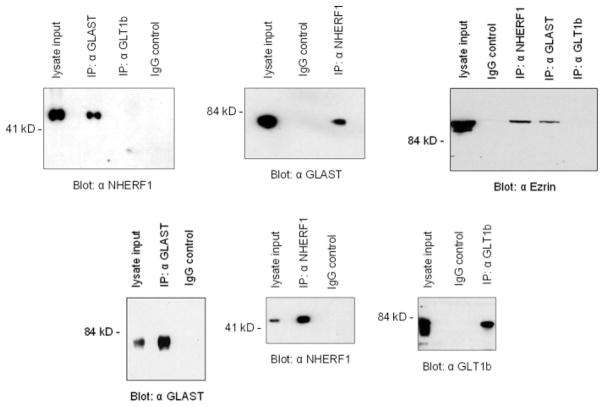
Since NHERF1 was shown to be highly expressed in astrocytes with similar distribution pattern as that of GLAST, we next asked whether these proteins co-associate in brain. We used polyclonal antibodies to immunoprecipitate GLAST from brain lysate, and the recovered immunocomplexes were then subjected to immunoblot analysis. As shown in Fig. 6, the immunoprecipitate obtained using an anti-GLAST antibody (but not anti-GLT1b) contained NHERF1, the band detected at ~52 kDa. No bands were detected when the blot was probed with the anti-NHERF2 antibody (data not shown). To further confirm the GLAST–NHERF1 interaction, we immunoprecipitated total brain lysate with an anti-NHERF1 antibody and detected GLAST (see Fig 6). We also demonstrated an association between endogenous GLAST and ezrin by co-immunoprecipitating ezrin from brain extracts using the anti-GLAST antibody, but not GLT1b (see Fig. 6). These results would indicate that in brain, GLAST, NHERF1, and ezrin co-associate in vivo.
Fig. 6.
Co-immunoprecipitation of GLAST, NHERF1, and ezrin in brain lysate. Total brain lysate was incubated with antibodies (directed against GLAST, GLT1b, or NHERF1) and precipitated using protein A beads. The samples were resolved by SDS-PAGE and Western blotted with appropriate antibodies (as indicated).
NHERF1 Binds Directly to the C-Terminal ETKM Motif of GLAST
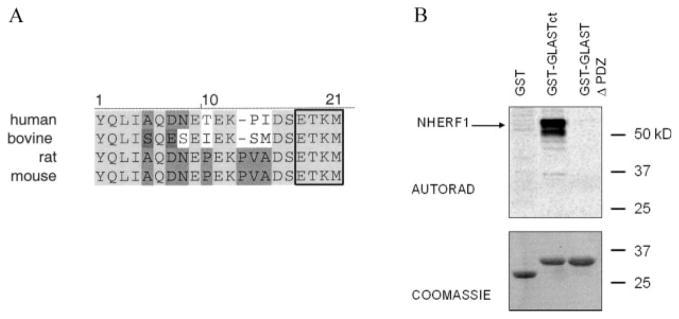
To date, numerous proteins have been found to posses a PDZ binding consensus (X–S/T–X–ϕ; where ϕ = hydrophobic residue) at their C-termini, which enables them to associate with PDZ domain-containing proteins (Hernando et al., 2004). The C-terminus of GLAST also contains such a motif (ETKM) and is highly conserved among mammals (Fig. 7A). To determine whether NHERF1 bind GLAST via its C-terminal ETKM motif, we used in vitro protein overlay assays. We generated two GST fusion proteins: one containing wild-type GLAST C-terminus with the ETKM motif (GST-GLASTct) and the other containing the GLAST C-terminal deletion mutant (without the ETKM motif; GST-GLAST Δ-PDZ). These fusion proteins along with GST were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and probed with [35S]-methionine-labeled NHERF1 generated by in vitro transcription–translation. As shown in Fig. 7B, NHERF1 interacted with the C-terminus of GLAST that contains the intact ETKM motif. No interaction of NHERF1 with GLAST Δ-PDZ or with GST alone was detected. These results demonstrate that NHERF1 interacts with GLAST via its C-terminal ETKM motif. Furthermore, since only labeled NHERF1 were present in the overlay assay, we conclude that the GLAST–NHERF1 interaction is direct.
Fig. 7.
NHERF1 interacts directly with the C-terminal ETKM motif of GLAST. (A) Sequence alignment of the mammalian GLAST C-terminal tails (generated using the Vector NTI program). Identical amino acids are depicted in grey shading and the PDZ binding consensus is boxed. (B) In vitro binding assay. GST fusion proteins containing the GLAST C-terminus (GST–GLASTct) and the GLAST C-terminus deletion mutant (GST–GLAST Δ-PDZ), as well as GST alone were separated on SDS-PAGE, transferred to nitrocellulose membrane and probed with [35S]-methionine-labeled NHERF1. Binding was detected by autoradiography.
GLAST Binds the First PDZ Domain of NHERF1
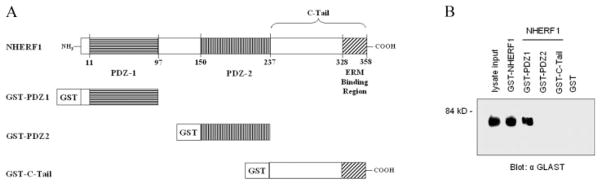
NHERF1 contains two tandem protein interaction domains called PDZ1 and PDZ2 and a C-terminal domain that has been shown to bind members of the ERM family of proteins (see Fig. 8A). Both PDZ1 and PDZ2 are capable of binding the C-terminus of target peptides. To elucidate which domain of NHERF1 interacts with GLAST, brain lysate was incubated with individual domains of NHERF1 (PDZ1, PDZ2, and C-terminus) fused to GST. Bound proteins were resolved by SDS-PAGE and Western blotted with the anti-GLAST antibody. Our results showed that GLAST bound specifically to PDZ1 but not to PDZ2, the C-terminal domain of NHERF1 or to GST itself (Fig. 8B), indicating specificity of the association between GLAST and NHERF1.
Fig. 8.
GLAST preferentially interacts with PDZ1 of NHERF1. (A) Full-length NHERF1, its PDZ domains (PDZ1 and PDZ2) and its C-terminus were expressed as GST-tag fusion proteins. (B) GST-tag fusion proteins as well as GST alone (as indicated) were immobilized on glutathione–sepharose beads and incubated with total brain lysate. Precipitated proteins were resolved on SDS-PAGE and Western blotted with anti-GLAST antibody.
DISCUSSION
In this study, we mapped the distribution of NHERF1 and NHERF2 immunohistochemically in the rat brain. NHERF1 predominantly labeled the astrocytes, while NHERF2 was most prominent in the endothelial cells. NHERF1 displayed a significant degree of co-localization with the glutamate transporter GLAST. Co-immunoprecipitation revealed that NHERF1 is a binding partner of GLAST and this interaction depends on the PDZ binding motif on the C-terminus of GLAST. NHERF1 has a key role in nucleating the formation of functional macromolecular complexes in epithelial cells; our data suggest that this epithelial paradigm for the regulation of transporters can be extended into the brain.
Distribution of NHERF1 and NHERF2 Proteins in the Rat Brain
NHERF1 was more widely distributed in the rodent brain, with an abundance of labeling associated with astrocytes rather than with neurons, in areas such as cortex, hippocampus, and cerebellum. This is of particular interest since astrocytes are a specific subset of glial cells whose function lies in the control of ionic and osmotic composition of the extracellular environment. In addition, astrocytes perform a number of other interrelated tasks, including transport of water, nutrients, and metabolites to and from the endothelium (Benarroch, 2005). Therefore, the presence of NHERF1 in astrocytes supports the role of this PDZ protein as a possible regulator of astroglial membrane transporters/channels. Clearly, areas of high NHERF1 immunoreactivity include the cerebellar molecular layer and the hippocampus CA1 pyrimidal layer, which are regions containing high concentrations of the astroglial glutamate transporter GLAST (Lehre et al., 1995).
Our observation that NHERF2 was present in endothelial cells of blood vessels and capillaries throughout the brain is consistent with recent data published in mouse brain using the same antibody (Paquet et al., 2006). In addition, however, the study in mouse brain showed labeling for NHERF2 in other cell types, including astrocytes and neurons. In the current study, we used bright field immunohistochemistry to examine the broad distribution patterns of NHERF1/2 and found that NHERF2 was predominantly located in the capillaries. Less intense labeling of NHERF2 in other cell types is, however, clearly evident in Fig. 5. Paquet et al. (2006) used higher magnification electron microscopy to study subcellular localization of NHERF2 and were therefore able to detect NHERF2 in the synaptic elements. Interestingly, we found that NHERF2 staining of endothelial cells was prominent at the apical aspect of the cells, which may reflect a role for NHERF2 in the regulation of channels/transporters involved in the movement of ion/nutrients between the blood and brain. We have recently reported that NHERF1/2 are widely distributed in the cochlea. In particular, NHERF2 immunolabeling was also evident in walls of large blood vessels including spiral modiolar artery and vein (Kanjhan et al., 2006). While the precise function(s) of NHERF2 within endothelial cells remains to be determined, it is interesting to note that several members of the previously characterized NHERF2 binding partners, namely P2Y1 receptor (Fam et al., 2005), PTH receptor (Mahon et al., 2002), and β2AR (Hall et al., 1998) are also expressed in endothelial cells of the mammalian brain. Thus, NHERF2 may play important roles in regulating diverse aspects of brain endothelial physiology.
NHERF1 and GLAST Interactions
The GLAST–NHERF1 interaction is dependent upon the C-terminal PDZ binding motif (ETKM) of GLAST and the first PDZ domain of NHERF1. A number of other proteins have been described to interact with PDZ1 of NHERF1. For example, the CFTR–NHERF1 interaction in airway epithelia requires the C-terminal DTRL sequence of CFTR and utilizes PDZ1 of NHERF (Wang et al., 1998). Similarly, PDZ1 of NHERF1 has been shown to interact with the membrane receptors β2AR and PDGFR via their C-terminal PDZ motifs DSLL and DSFL, respectively (Hall et al., 1998; Maudsley et al., 2000). This is of particular relevance as NHERF1 can self-associate to form homo-dimers via preferential binding of its N-terminal PDZ1 domain (i.e., PDZ1 to PDZ1) (Fouassier et al., 2000; Shenolikar et al., 2001). Therefore, our data suggest that the GLAST–NHERF1 complex must bind another protein partner via the PDZ2 domain, in a manner similar to β2AR–NHERF1–NHE3 (Hall et al., 1998) and CFTR–NHERF1–CLC3B (Ogura et al., 2002) macromolecular complexes proposed in transporting epithelia. This interaction between GLAST and NHERF1 occurs endogenously in the brain as demonstrated by co-immunoprecipitation experiments. In contrast, the b-splice variant of another glutamate transporter, GLT-1b, which posses a putative class I PDZ binding motif, does not co-immunoprecipitate with NHERF1 from the brain. Importantly, we show that the NHERF1–GLAST complex also associated with ezrin in the brain. Ezrin is likely to act as a linker to join the NHERF1–GLAST complex to the actin cytoskeleton. Furthermore, astrocytes have been shown to express ERM proteins and GLAST and NHERF1 show pronounced overlap in astrocytes. These observations support the notion that NHERF1 links the GLAST transporter to the actin cytoskeleton through ezrin, thus forming a multiprotein complex. The linkage of GLAST to NHERF1 may thus, serve as an important mechanism for localizing the GLAST transporter to appropriate membrane domains in astrocytes. Indeed, we observed a tight concordance between the distribution of GLAST and the distribution of NHERF1, and that the distribution of both NHERF1 and GLAST was punctate, indicating a spatial constrain of both proteins. We note that whilst the NHERF1 and GLAST puncta show extensive overlap there are small spatial disparities. We suggest these spatial disparities may reflect the probable role of NHERF1 not only in anchoring GLAST but probably in also complexing with other proteins that have PDZ binding motifs, such additional interactions precluding total co-localization at the molecular level and thus at the anatomic level. In light of the recent report that astrocytes in mouse brain show more restricted distribution of NHERF2 (Paquet et al., 2006), it is interesting to note that we find GLAST also binds NHERF2 in vitro (unpublished data); however, GLAST does not bind NHERF2 in vivo (as demonstrated by co-immunoprecipitation experiments).
Functions of NHERF1–GLAST Interaction
What is the physiological significance of the GLAST–NHERF1 complex? Dialysis of Muller glial cells with a peptide identical to the C-terminal eight residues of GLAST was previously shown to significantly increase the glutamate affinity of GLAST (Marie and Attwell, 1999). This demonstrates that disruption of an interaction between an endogenous protein and the last eight residues of the GLAST C-terminus which contains the PDZ binding motif, can produce an alteration in the transporter’s glutamate affinity. Thus, C-terminal interaction with the endogenous protein (conceivably a PDZ protein) could modulate GLAST transporter function (Marie and Attwell, 1999). Modulation of glutamate transport via C-terminal–protein interaction has also been reported for another member of the glutamate transporter family, namely EAAT4 (Jackson et al., 2001). EAAT4 is positively regulated by the glutamate transporter associated proteins GTRAP41 and GTRAP48, which both increase uptake by enhancing cell surface expression of EAAT4. Of particular interest is the finding that GTRAP48 is a PDZ domain containing protein that also appears to couple EAAT4 to the Rho GTPase signal transduction cascade (Jackson et al., 2001). It will be interesting to see whether the NHERF1 interactions characterized in the present study could also modulate GLAST transporter function and whether this interaction in astrocytes involves other structural and regulatory proteins. For instance, protein kinase C (PKC) appears to be a potent signal regulating GLAST activity. In human embryonic kidney cells expressing GLAST, acute exposure to phorbol 12-myristate 13-acetate (PMA) dramatically decreased transport activity (Conradt and Stoffel, 1997) while in cerebellar glial cells, PKC activation was shown to reduce GLAST-mediated transport via decrease in Vmax (Gonzalez and Ortega, 1997). In contrast, Susarla et al. (2004) demonstrated that acute exposure to PMA increased GLAST-mediated transport in primary astrocyte cultures from forebrain. The type IIa Na+/Pi transporter in kidney also binds PDZ1 of NHERF1 (Hernando et al., 2002) and it has recently been shown that PKC dissociates the Na+/Pi transporter–NHERF1 complex (Deliot et al., 2005). Further studies are required to resolve the exact mode of action of PKC on GLAST activity.
It is also evident from our studies that although there is significant co-localization of NHERF1 and GLAST, there are also regions of the cells where they do not associate. This significant level of spatial heterogeneity suggests that these two proteins also exist in separate functional domains where they may assemble into other functional complexes. Given that there are multiple PDZ proteins reported in astrocytes, such as α-syntrophin, which interacts with AQP4 (Neely et al., 2001), or ZO-1, which interacts with TRPC-1 (Song et al., 2005), it is highly likely that the GLAST–NHERF1 complex represents only one of several functional GLAST–PDZ complexes in astrocytes. From this perspective, it is interesting to note that there are no reports of overt brain dysfunction in NHERF1 knockout mice, however, it has been reported that they are susceptible to hydrocephaly (Shenolikar et al., 2002). This is an intriguing observation when taken together with recent findings showing that in addition to glutamate and ions, GLAST also transports a considerable amount of water (MacAulay et al., 2004).
In summary, we demonstrate that both NHERF1 and NHERF2 are highly expressed in specific cell types in the brain. NHERF1 is present primarily in glial cells while NHERF2 is predominantly in endothelia. In the brain, NHERF1 co-localizes and associates with a certain pool of GLAST in a complex that includes ezrin. These data strongly support a key role for NHERF1 in mediating glutamate transport by the astrocytes and represents a new role for this PDZ protein that was originally described in the kidney.
Acknowledgments
National Health and Medical Research Council, Australia.
References
- Arriza J, Eliasof S, Kavanaugh M, Amara S. Excitatory amino acid transporter 5: A retinal glutamate transporter coupled to a chloride conductance. Proc Natl Acad Sci USA. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E. Neuron-astrocyte interactions: Partnership for normal function and disease in the central nervous system. Mayo Clin Proc. 2005;80:1326–1338. doi: 10.4065/80.10.1326. [DOI] [PubMed] [Google Scholar]
- Berger U, DeSilva T, Chen W, Rosenberg P. Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. J Comp Neurol. 2005;492:78–89. doi: 10.1002/cne.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Chambers D, Nguyen R, Reczek D. ERM-Merlin and EBP50 protein families in plasma membrane organization and function. Annu Rev Cell Dev Biol. 2000;16:113–143. doi: 10.1146/annurev.cellbio.16.1.113. [DOI] [PubMed] [Google Scholar]
- Brone B, Eggermont J. PDZ proteins retain and regulate membrane transporters in polarized epithelial cell membranes. Am J Physiol Cell Physiol. 2005;288:C20–29. doi: 10.1152/ajpcell.00368.2004. [DOI] [PubMed] [Google Scholar]
- Chen W, Mahadomrongkul V, Berger U, Bassan M, DeSilva T, Tanaka K, Irwin N, Aoki C, Rosenberg PA. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. J Neurosci. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt M, Stoffel W. Inhibition of the high-affinity brain glutamate transporter GLAST-1 via direct phosphorylation. J Neurochem. 1997;68:1244–1251. doi: 10.1046/j.1471-4159.1997.68031244.x. [DOI] [PubMed] [Google Scholar]
- Danbolt N. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Deliot N, Hernando N, Horst-Liu Z, Gisler S, Capuano P, Wagner C, Bacic D, O’Brien S, Biber J, Murer H. Parathyroid hormone treatment induces dissociation of type IIa Na+-Pi cotransporter-Na+/H+ exchanger regulatory factor-1 complexes. Am J Physiol Cell Physiol. 2005;289:C159–167. doi: 10.1152/ajpcell.00456.2004. [DOI] [PubMed] [Google Scholar]
- Deval E, Friend V, Thirant C, Salinas M, Jodar M, Lazdunski M, Lingueglia E. Regulation of sensory neuron-specific acid-sensing ion channel 3 by the adaptor protein Na+/H+ exchanger regulatory factor-1. J Biol Chem. 2006;281:1796–1807. doi: 10.1074/jbc.M509669200. [DOI] [PubMed] [Google Scholar]
- Ediger T, Kraus W, Weinman E, Katzenellenbogen B. Estrogen receptor regulation of the Na+/H+ exchange regulatory factor. Endocrinology. 1999;140:2976–2982. doi: 10.1210/endo.140.7.6885. [DOI] [PubMed] [Google Scholar]
- Fam S, Paquet M, Castleberry A, Oller H, Lee C, Traynelis S, Smith Y, Yun C, Hall R. P2Y1 receptor signaling is controlled by interaction with the PDZ scaffold NHERF-2. Proc Natl Acad Sci USA. 2005;102:8042–8047. doi: 10.1073/pnas.0408818102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: A neurotransmitter in mammalian brain. J Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Fouassier L, Yun C, Fitz J, Doctor R. Evidence for ezrin–radixin–moesin-binding phosphoprotein 50 (EBP50) self-association through PDZ-PDZ interactions. J Biol Chem. 2000;275:25039–25045. doi: 10.1074/jbc.C000092200. [DOI] [PubMed] [Google Scholar]
- Furuta A, Rothstein J, Martin L. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci. 1997;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatto C, Walker B, Lambert S. Local ERM activation and dynamic growth cones at Schwann cell tips implicated in efficient formation of nodes of Ranvier. J Cell Biol. 2003;162:489–498. doi: 10.1083/jcb.200303039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, Ortega A. Regulation of the Na+-dependent high affinity glutamate/aspartate transporter in cultured Bergmann glia by phorbol esters. J Neurosci Res. 1997;50:585–590. doi: 10.1002/(SICI)1097-4547(19971115)50:4<585::AID-JNR9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hall R, Premont R, Chow C, Blitzer J, Pitcher J, Claing A, Stoffel R, Barak L, Shenolikar S, Weinman EJ, Grinstein S, Lefkowitz RJ. The beta2-adrenergic receptor interacts with the Na+/H+-exchanger regulatory factor to control Na+/H+ exchange. Nature. 1998;392:626–630. doi: 10.1038/33458. [DOI] [PubMed] [Google Scholar]
- Hernando N, Deliot N, Gisler S, Lederer E, Weinman E, Biber J, Murer H. PDZ-domain interactions and apical expression of type IIa Na/Pi cotransporters. Proc Natl Acad Sci USA. 2002;99:11957–11962. doi: 10.1073/pnas.182412699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando N, Wagner C, Gisler S, Biber J, Murer H. PDZ proteins and proximal ion transport. Curr Opin Nephrol Hypertens. 2004;13:569–574. doi: 10.1097/00041552-200409000-00014. [DOI] [PubMed] [Google Scholar]
- Jackson M, Song W, Liu M, Jin L, Dykes-Hoberg M, Lin C, Bowers W, Federoff H, Sternweis P, Rothstein J. Modulation of the neuronal glutamate transporter EAAT4 by two interacting proteins. Nature. 2001;410:89–93. doi: 10.1038/35065091. [DOI] [PubMed] [Google Scholar]
- Kanjhan R, Hryciw D, Yun C, Bellingham M, Poronnik P. Postnatal developmental expression of the PDZ scaffolds Na+/H+ exchanger regulatory factors 1 and 2 in the rat cochlea. Cell Tissue Res. 2006;323:53–70. doi: 10.1007/s00441-005-0051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht G, Weinman E, Yun C. The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J Biol Chem. 1998;273:29972–29978. doi: 10.1074/jbc.273.45.29972. [DOI] [PubMed] [Google Scholar]
- Lehre K, Danbolt N. The number of glutamate transporter subtype molecules at glutamatergic synapses: Chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre K, Levy L, Ottersen O, Storm-Mathisen J, Danbolt N. Differential expression of two glial glutamate transporters in the rat brain: Quantitative and immunocytochemical observations. J Neurosci. 1995;15(3 Pt 1):1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Li J, Straight S, Kershaw D. PDZ domain-mediated interaction of rabbit podocalyxin and Na+/H+ exchange regulatory factor-2. Am J Physiol Renal Physiol. 2002;282:F1129–1139. doi: 10.1152/ajprenal.00131.2001. [DOI] [PubMed] [Google Scholar]
- Liedtke C, Yun C, Kyle N, Wang D. Protein kinase C epsilon-dependent regulation of cystic fibrosis transmembrane regulator involves binding to a receptor for activated C kinase (RACK1) and RACK1 binding to Na+/H+ exchange regulatory factor. J Biol Chem. 2002;277:22925–22933. doi: 10.1074/jbc.M201917200. [DOI] [PubMed] [Google Scholar]
- MacAulay N, Hamann S, Zeuthen T. Water transport in the brain: Role of cotransporters. Neuroscience. 2004;129:1031–1044. doi: 10.1016/j.neuroscience.2004.06.045. [DOI] [PubMed] [Google Scholar]
- Mahon M, Donowitz M, Yun C, Segre G. Na+/H+ exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature. 2002;417:858–861. doi: 10.1038/nature00816. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Role of glial amino acid transporters in synaptic transmission and brain energetics. Glia. 2004;47:217–225. doi: 10.1002/glia.20027. [DOI] [PubMed] [Google Scholar]
- Marie H, Attwell D. C-terminal interactions modulate the affinity of GLAST glutamate transporters in salamander retinal glial cells. J Physiol. 1999;520(Pt 2):393–397. doi: 10.1111/j.1469-7793.1999.00393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maudsley S, Zamah A, Rahman N, Blitzer J, Luttrell L, Lefkowitz R, Hall R. Platelet-derived growth factor receptor association with Na+/H+ exchanger regulatory factor potentiates receptor activity. Mol Cell Biol. 2000;20:8352–8363. doi: 10.1128/mcb.20.22.8352-8363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot M, West K, Huang J, Possin D, Bretscher A, Crabb J, Saari J. Cellular retinaldehyde-binding protein interacts with ERM-binding phosphoprotein 50 in retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2004;45:393–401. doi: 10.1167/iovs.03-0989. [DOI] [PubMed] [Google Scholar]
- Neely J, Amiry-Moghaddam M, Ottersen O, Froehner S, Agre P, Adams M. Syntrophin-dependent expression and localization of Aquaporin-4 water channel protein. Proc Natl Acad Sci USA. 2001;98:14108–14113. doi: 10.1073/pnas.241508198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T, Furukawa T, Toyozaki T, Yamada K, Zheng Y, Katayama Y, Nakaya H, Inagaki N. ClC-3B, a novel ClC-3 splicing variant that interacts with EBP50 and facilitates expression of CFTR-regulated ORCC. FASEB J. 2002;16:863–865. doi: 10.1096/fj.01-0845fje. [DOI] [PubMed] [Google Scholar]
- Paquet M, Kuwajima M, Yun C, Smith Y, Hall R. Astrocytic and neuronal localization of the scaffold protein Na+/H+ exchanger regulatory factor 2 (NHERF-2) in mouse brain. J Comp Neurol. 2006;494:752–762. doi: 10.1002/cne.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pow D, Barnett N. Changing patterns of spatial buffering of glutamate in developing rat retinae are mediated by the Muller cell glutamate transporter GLAST. Cell Tissue Res. 1999;297:57–66. doi: 10.1007/s004410051333. [DOI] [PubMed] [Google Scholar]
- Pow D, Hendrickson A. Distribution of the glycine transporter glyt-1 in mammalian and nonmammalian retinae. Vis Neurosci. 1999;16:231–239. doi: 10.1017/s0952523899162047. [DOI] [PubMed] [Google Scholar]
- Ramesh V. Merlin and the ERM proteins in Schwann cells, neurons and growth cones. Nat Rev Neurosci. 2004;5:462–470. doi: 10.1038/nrn1407. [DOI] [PubMed] [Google Scholar]
- Reye P, Sullivan R, Scott H, Pow D. Distribution of two splice variants of the glutamate transporter GLT-1 in rat brain and pituitary. Glia. 2002;38:246–255. doi: 10.1002/glia.10059. [DOI] [PubMed] [Google Scholar]
- Shenolikar S, Minkoff C, Steplock D, Evangelista C, Liu M, Weinman E. N-terminal PDZ domain is required for NHERF dimerization. FEBS Lett. 2001;489:233–236. doi: 10.1016/s0014-5793(01)02109-3. [DOI] [PubMed] [Google Scholar]
- Shenolikar S, Voltz J, Cunningham R, Weinman E. Regulation of ion transport by the NHERF family of PDZ proteins. Physiology. 2004;19:362–369. doi: 10.1152/physiol.00020.2004. [DOI] [PubMed] [Google Scholar]
- Shenolikar S, Voltz J, Minkoff C, Wade J, Weinman E. Targeted disruption of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci USA. 2002;99:11470–11475. doi: 10.1073/pnas.162232699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Zhao Y, Narcisse L, Duffy H, Kress Y, Lee S, Brosnan C. Canonical transient receptor potential channel 4 (TRPC4) co-localizes with the scaffolding protein ZO-1 in human fetal astrocytes in culture. Glia. 2005;49:418–429. doi: 10.1002/glia.20128. [DOI] [PubMed] [Google Scholar]
- Stemmer-Rachamimov A, Wiederhold T, Nielsen G, James M, Pinney-Michalowski D, Roy J, Cohen W, Ramesh V, Louis D. NHE-RF, a merlin-interacting protein, is primarily expressed in luminal epithelia, proliferative endometrium, and estrogen receptor-positive breast carcinomas. Am J Pathol. 2001;158:57–62. doi: 10.1016/S0002-9440(10)63944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susarla B, Seal R, Zelenaia O, Watson D, Wolfe J, Amara S, Robinson M. Differential regulation of GLAST immunoreactivity and activity by protein kinase C: Evidence for modification of amino and carboxyl termini. J Neurochem. 2004;91:1151–1193. doi: 10.1111/j.1471-4159.2004.02791.x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, Ramesh V, Zhu M. Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J Biol Chem. 2002;275:37559–37564. doi: 10.1074/jbc.M006635200. [DOI] [PubMed] [Google Scholar]
- Torres G, Yao W, Mohn A, Quan H, Kim K, Levey A, Staudinger J, Caron M. Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron. 2001;30:121–134. doi: 10.1016/s0896-6273(01)00267-7. [DOI] [PubMed] [Google Scholar]
- Wang S, Raab R, Schatz P, Guggino W, Li M. Peptide binding consensus of the NHE-RF-PDZ1 domain matches the C-terminal sequence of cystic fibrosis transmembrane conductance regulator (CFTR) FEBS Lett. 1998;427:103–108. doi: 10.1016/s0014-5793(98)00402-5. [DOI] [PubMed] [Google Scholar]
- Weinman E, Steplock D, Wang Y, Shenolikar S. Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na+/H+ exchanger. J Clin Invest. 1995;95:2143–2149. doi: 10.1172/JCI117903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S, Sullivan R, Scott H, Finkelstein D, Colditz P, Lingwood B, Dodd P, Pow D. Glial glutamate transporter expression patterns in brains from multiple mammalian species. Glia. 2005;49:520–541. doi: 10.1002/glia.20139. [DOI] [PubMed] [Google Scholar]
- Yun C, Chen Y, Lang F. Glucocorticoid activation of Na+/H+ exchanger isoform 3 revisited. The roles of SGK1 and NHERF2. J Biol Chem. 2002;277:7676–7683. doi: 10.1074/jbc.M107768200. [DOI] [PubMed] [Google Scholar]
- Yun C, Oh S, Zizak M, Steplock D, Tsao S, Tse C, Weinman E, Donowitz M. cAMP-mediated inhibition of the epithelial brush border Na+/H+ exchanger, NHE3, requires an associated regulatory protein. Proc Natl Acad Sci USA. 1997;94:3010–3015. doi: 10.1073/pnas.94.7.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]