Abstract
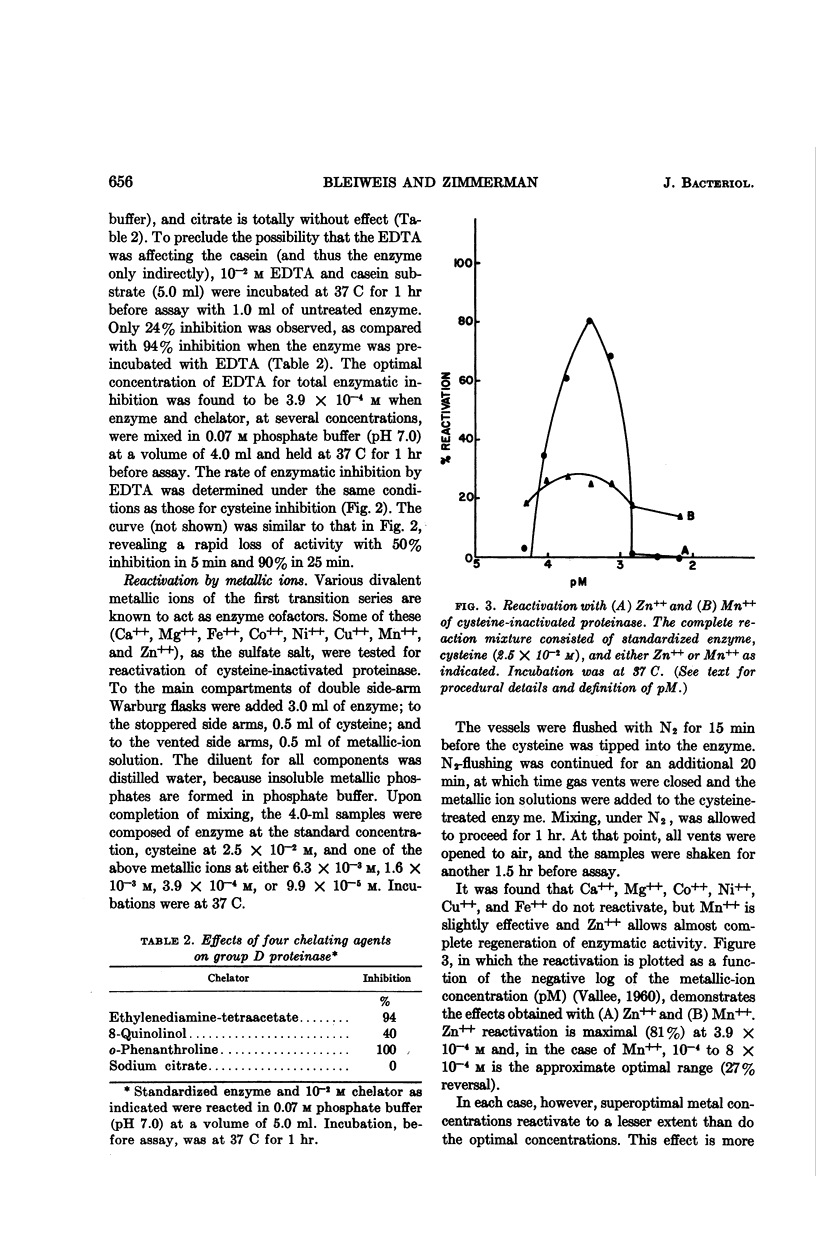
Bleiweis, Arnold S. (The Pennsylvania State University, University Park), and Leonard N. Zimmerman. Properties of proteinase from Streptococcus faecalis var. liquefaciens. J. Bacteriol. 88:653–659. 1964.—The extracellular group D streptococcal proteinase is inactivated by chelating agents [ethylenediamine-tetraacetate (EDTA), o-phenanthroline, and 8-quinolinol] and mercaptans (cysteine, mercaptoethanol, and thioglycolate). The optimal inhibitory concentrations of EDTA (4 × 10−4m) and cysteine (2.5 × 10−2m) promote rapid loss of activity with 50% inactivation after 4 to 5 min. Enzyme inactivated by either EDTA or cysteine is reactivated about 80% by 4 × 10−4m Zn++. Such reactivation of EDTA-treated enzyme is prevented completely by iodoacetate (5 × 10−2m) and of cysteine-treated enzyme by oxidizing conditions, which suggests that the zinc binding-site may be a thiol. High levels of zinc (10−3m) do not allow reactivation in either case, and actually inhibit native proteinase. Ca++, Mg++, Co++, Fe++, Cu++, and Ni++ do not reactivate cysteine-treated enzyme, but Mn++ (10−4 to 8 × 10−4m) allows 27% reversal. N2-held, cysteine-treated enzyme can be spontaneously reactivated if the substrate is flushed with O2 during the assay; leakage of air or O2 into the samples before assay leads to loss of reactivatability. Native proteinase does not lose activity after dialysis for 43 hr against 0.07 m phosphate buffer. It is concluded that the group D proteinase obtained from Streptococcus faecalis var. liquefaciens is probably a zinc metalloenzyme that is unlike the thiol-activated, group A streptococcal proteinase, but similar to the mammalian carboxypeptidase A.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLEMAN J. E., VALLEE B. L. Metallocarboxypeptidases: stability constants and enzymatic characteristics. J Biol Chem. 1961 Aug;236:2244–2249. [PubMed] [Google Scholar]
- COOMBS T. L., FELBER J. P., VALLEE B. L. Metallocarboxpeptidases: mechanism of inhibition by chelating agents, mercaptans, and metal ions. Biochemistry. 1962 Sep;1:899–905. doi: 10.1021/bi00911a024. [DOI] [PubMed] [Google Scholar]
- ELLIOTT S. D. The crystallization and serological differentiation of a streptococcal proteinase and its precursor. J Exp Med. 1950 Sep;92(3):201–218. doi: 10.1084/jem.92.3.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELBER J. P., COOMBS T. L., VALLEE B. L. The mechanism of inhibition of carboxypeptidase A by 1,10-phenanthroline. Biochemistry. 1962 Mar;1:231–238. doi: 10.1021/bi00908a006. [DOI] [PubMed] [Google Scholar]
- GRUTTER F. H., ZIMMERMAN L. N. A proteolytic enzyme of Streptococcus zymogenes. J Bacteriol. 1955 Jun;69(6):728–732. doi: 10.1128/jb.69.6.728-732.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMAN R. E., ZIMMERMAN L. N. Effect of the arginine dihydrolase enzyme system on proteinase biosynthesis by Streptococcus faecalis var. liquefaciens. J Bacteriol. 1960 Dec;80:753–761. doi: 10.1128/jb.80.6.753-761.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMAN R. E., ZIMMERMAN L. N., RABIN R. Proteinase biosynthesis by Streptococcus liquefaciens. II. Purine, pyrimidine, and vitamin requirements. Can J Microbiol. 1957 Jun;3(4):553–558. doi: 10.1139/m57-060. [DOI] [PubMed] [Google Scholar]
- LIU T. Y., NEUMANN N. P., ELLIOTT S. D., MOORE S., STEIN W. H. Chemical properties of streptococcal proteinase and its zymogen. J Biol Chem. 1963 Jan;238:251–256. [PubMed] [Google Scholar]
- SHEDLOVSKY T., ELLIOTT S. D. An electrophoretic study of a streptococcal proteinase and its precursor. J Exp Med. 1951 Nov;94(5):363–372. doi: 10.1084/jem.94.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]



