Abstract
Cell–cell junctions continue to capture the interest of cell and developmental biologists, with an emerging area being the molecular means by which junctional signals relate to gene activity in the nucleus. Although complexities often arise in determining the direct versus indirect nature of such signal transduction, it is clear that such pathways are essential for the function of tissues and that alterations may contribute to many pathological outcomes. This review assesses a variety of cell–cell junction-to-nuclear signaling pathways, and outlines interesting areas for further study.
Cell junctions do more than rivet cells together. They also signal to the nucleus, acting via catenins, small GTPases, and cross talk with growth factor signaling.
The evolution of multicellular life forms has to a significant extent involved refinements of each cell's capacity to sense the state of its directly contacting neighbors. This exchange of information often occurs within tissues, with the result that gene activity in the nucleus is altered or maintained accordingly. In this article, we focus on how signals arise at cell–cell junctions and are transduced to the nucleus; we do not include discussion of mechanical/cytoskeletal signals influencing nuclear decisions, and the reader is directed to a recent review of this topic (Ingber 2008).
An issue that arises when addressing cell–cell junction(s), referred to as CCJ(s), -to-nuclear signals, is that homotypic or heterotypic junctional proteins responsible for conferring adhesive activity are often in a much larger complex of proteins. These interactions may be either in cis (interacting within the plasma membrane of the cell) or trans orientations (interacting through ectodomain contacts extended between cells). Most of these transmembrane proteins are likely to have the potential to contribute to downstream signaling events, and many may associate with one another only under specific physiological conditions. For example, certain receptor tyrosine kinases (RTKs) associate with particular cadherins, and when associated are relevant to that cadherin's functions (Wheelock and Johnson 2003; Andl and Rustgi 2005). In this article, we discuss relationships such as these in the context of CCJ-nuclear signaling. A topic not represented here is the CCJ signaling of immune surveillance cells, for example, pathways activated following leukocyte–endothelia contact. This area is of great basic and biomedical interest, but is addressed elsewhere (Dustin 2007).
We focus on signaling by a select number of junction types, including adherens, desmosomal, and tight junctions, and to a lesser extent, gap junctions. Details of the structure and function of each of these junctions are presented in other articles (see Meng and Takeichi 2009, Delva et al. 2009, Furuse 2009, and Goodenough and Paul 2009, respectively). These junctions are often represented in textbooks as distinct entities in the context of epithelial tissues, but their structures and how they respond to or generate signaling cues vary according to cellular context. Select components within these junctions may be shared, for example between desmosomal, adherens, and tight junctions, and in some instances, intimate physical proximities are likely to advance these junctions' functional interrelation. Further, different cell types show less common junctional organizations (Straub et al. 2003; Wuchter et al. 2007), such that the total spectrum of CCJ signals is likely to be impressive, and far beyond what is currently known or understood. Given the interdependence of cell neighbors in forming and maintaining cell groupings, high diversity and sophistication arose in complex organisms, both in CCJ structures themselves and their associated nuclear signaling pathways. Compared with the knowledge accumulated over the past two decades on cell–extracellular matrix signaling via integrins (Abram and Lowell 2009), we know less about signals initiated from forming or mature cell–cell contacts in epithelial, neural, or endothelial tissues. Thus, as the field moves forward, there is the potential to achieve a deepened understanding of how the cell–extracellular matrix and cell–cell adhesion systems are coupled in a signaling context, and how they collectively relate to the adhesion, motility, and differentiation of cells and tissues.
ADHERENS AND DESMOSOMAL JUNCTIONS
Adherens junctions are comprised of a number of transmembrane proteins, including the classic cadherins (see Meng and Takeichi 2009) and nectins, which are members of the distinct Ig (immunoglobulin) family of adhesion proteins/receptors (Fig. 1) (Takai et al. 2008a; Takai et al. 2008b). In some cases, cadherins and nectins associate through intracellular proteins, including p120-catenin bound to the juxtamembrane domain of cadherin and afadin bound to nectin (Ogita and Takai 2008). RTKs are also present at adherens junctions, although unlike cadherins and nectins, they are not viewed as adhesion proteins, but rather as signaling entities instructing, responding, or working in parallel with other signal generators within the cadherin/nectin macromolecular complex.
Figure 1.
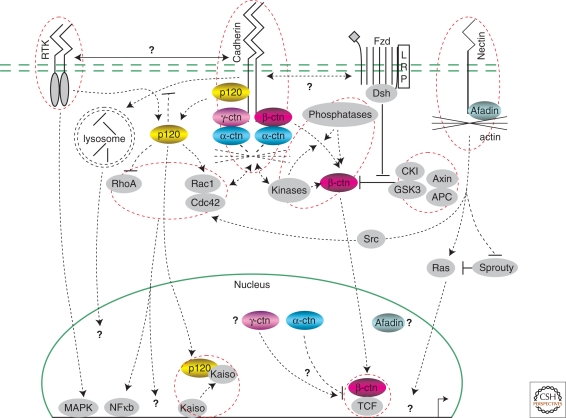
Adherens junction signaling to the nucleus. Proteins binding to the intracellular regions of cadherins include catenins, with β-catenin further associating with α-catenin and perhaps indirectly/dynamically with actin microfilaments. The structurally related p120-catenin subfamily is proposed to modulate cadherin lateral clustering, cadherin endocytosis, and activities of Rho-family GTPases. β-catenin acts in various ways within the nucleus, including as a transcriptional coactivator in conjunction with LEF/TCF, whereas p120-catenin relieves Kaiso-mediated gene repression. Other catenins have also been observed in the nucleus, where they have putative or shared roles in transcriptional regulation. Signaling from the nectin/afadin complex to the nucleus may occur indirectly through Ras or Rho, or more directly through afadin's potential association with chromatin or transcriptional modulators. Some evidence has indicated that receptor tyrosine kinases/growth factor receptors, or the frizzled/LRP complex, modulate signals initiated from the adherens junction and vice versa. Dotted circles highlight select signaling entities where research has been focused over the past decade.
Desmosomal junctions contain additional members of the cadherin superfamily, represented in the transmembrane polypeptides of the desmocollin and desmoglein subfamilies (Fig. 2) (Green and Simpson 2007; Holthofer et al. 2007; Garrod and Chidgey 2008) (see also Delva et al. 2009). Desmosomal cadherins bind catenins that are different from those found in complex with classic cadherins at adherens junctions (Hatzfeld 2007). There are, however, catenins such as plakoglobin that are found at more than one junction (Zhurinsky et al. 2000). This may occur more often than realized as β-catenin, which is generally thought to bind only cadherins at adherens junctions, is also found at desmosomes under certain conditions (Bierkamp et al. 1999). In addition, p120-catenin has been identified at desmosomal junctions (Kanno et al. 2008), and ZO-1, generally found at tight junctions, associates with adherens junctions during their assembly (Itoh et al. 1993; Muller et al. 2005). Finally, p0071-catenin was first characterized in association with desmosomal cadherins but also localizes to adherens junction (Hatzfeld et al. 2003; Calkins et al. 2003). Such examples of catenins being shared across junctional types may reflect a means to coordinately regulate distinct cell–cell contacts, to enhance cross talk between junctions, or to provide additive junctional signals directed to other cellular compartments such as the nucleus.
Figure 2.
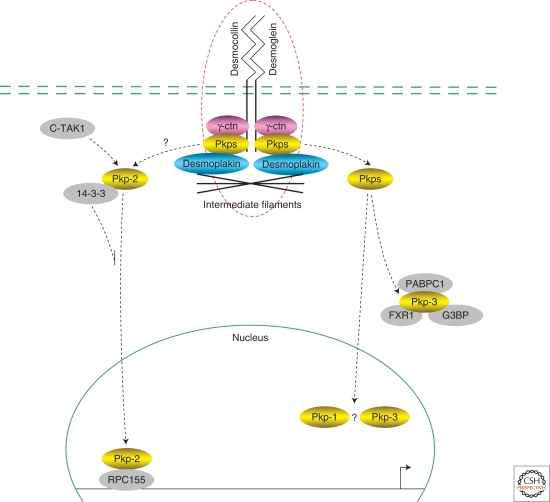
Desmosome junction signaling to the nucleus. Desmosomes are adhesive plaques containing the transmembrane cadherin superfamily members desmocollin and desmoglein, as well as intracellular binding partners such as plakoglobin (γ-catenin), plakophilins (Pkps), and desmoplakin. A number of desmosomal catenins have been localized to the nucleus although the functional relevance remains to be elucidated. Plakophilin-2, for example, has been found to reside within the PolIII complex and to associate with RPC155 and the transcription factor TFIIIB.
How signals cross the membrane following trans-cadherin ectodomain contact is not well understood. The relevance of cadherin cis-dimerization to subsequent trans interactions is proposed in some but not all models (Brieher et al. 1996; Yap et al. 1997; Yap et al. 1998; Patel et al. 2003; Yap and Kovacs 2003; Troyanovsky 2005; Troyanovsky et al. 2007; Nelson 2008), with higher order clustering possibly correlating with raised levels of interacting phosphatases, kinases, or small GTPases (see also Watenabe et al. 2009). This may be similar to models of RTK clustering and activation in which cytoplasmic kinase domains come in closer proximity to initiate signaling. Following ectodomain engagement, transmembrane signaling might occur through displacement of adhesion molecules relative to the plasma membrane, perhaps exposing or removing an intracellular binding site, or displacements relative to other transmembrane polypeptides, analogous to that occurring between the α- and β-integrin subunits on binding extracellular matrix.
Our focus here is on the nuclear signaling pathways that are initiated at cell–cell junctions. From a conceptual perspective, it seems that evolution would favor signals passing in both directions, thereby informing nuclear as well as cell–cell contact decision-making in a reciprocal manner. This would include still incompletely understood concepts such as contact inhibition, where chromosomal replication/cell division slows with increased cell–cell association or, conversely, the lessening or remodeling of cell–cell contacts in cells initiating gene programs that favor a migratory phase or epithelial-to-mesenchymal transitions.
CATENIN SIGNALING
A signaling trajectory that might at first seem obvious between cell–cell junctions and downstream nuclear events is that involving β-catenin. β-catenin binds the distal (carboxy-terminal) domain of cadherin cytoplasmic tails via β-catenin's central Armadillo domain (Huber and Weis 2001; Xu and Kimelman 2007). Concomitantly, β-catenin binds to α-catenin via its amino-terminal domain (Pokutta and Weis 2000). A recent model proposes that the cadherin–β-catenin complex may locally enrich α-catenin (Yamada et al. 2005; Drees et al. 2005; Weis and Nelson 2006), which when released forms homo-dimers capable of lowering Arp 2/3 activity (Yamada and Nelson 2007). This is then expected to reduce actin branching and cellular protrusive activity within the microenvironment, facilitating contact formation and stability.
Another major role of β-catenin is in the canonical Wnt signaling pathway (Clevers 2006; Gavert and Ben-Ze'ev 2007; Huang and He 2008; Barker 2008; Mosimann et al. 2009) (see also Heuberger and Birchmeier 2009; Cadigan and Peifer 2009). Following Wnt-ligand association with the plasma membrane receptor–coreceptor complex of Frizzled–Lrp, a signaling pool of β-catenin is formed that is not bound to cadherin. This process involves Wnt-ligand-directed inactivation of an intracellular β-catenin destruction complex (involving components such as GSK3β, CK1α, Axin, and APC), that in the absence of Wnt ligand would otherwise basally suppress the Wnt pathway (see Cadigan and Peifer 2009 for details). Once stabilized, a number of poorly understood events allow β-catenin to enter the nucleus to activate Wnt target genes important in many developmental and pathological contexts (Suh and Gumbiner 2003; Gottardi and Gumbiner 2004; Mosimann et al. 2009). Activation occurs on β-catenin's association with TCF/LEF transcription factors resident at TCF/LEF consensus binding sites within Wnt target gene promoters.
It was envisaged that β-catenin released from the cadherin complex, perhaps in response to specific phosphorylation events (Daniel and Reynolds 1997; Potter et al. 2005; Sallee et al. 2006; Alema and Salvatore 2007), might also enter a signaling pool and activate Wnt target genes (Perez-Moreno et al. 2003; Nelson and Nusse 2004; Bienz 2005; Perez-Moreno and Fuchs 2006). However, unless Wnt signals are already active, it remains reasonable to ask how β-catenin released from cadherin escapes the destruction complex. Indeed, in a number of contexts, it appears that cadherin loss alone may not be sufficient to activate β-catenin signaling (Caca et al. 1999; van de Wetering et al. 2001; Herzig et al. 2007), even as cadherin loss increases Wnt signals activated by other means (Gottardi et al. 2001; Kuphal and Behrens 2006).
Evidence has accumulated over the past decade that β-catenin released from cadherin is associated with increased Wnt pathway activity in some experimental contexts, such as in response to RTK or Src activity (Nelson and Nusse 2004; Bienz 2005; Lilien and Balsamo 2005; Brembeck et al. 2006; Gavert and Ben-Ze'ev 2007; McLachlan and Yap 2007). Src is capable of phosphorylating β-catenin at tyrosine 654, and reducing its association with cadherin (Roura et al. 1999). Most studies have been correlative, with the dissociation of β-catenin from cadherin occurring concurrent with increased transcriptional activity from endogenous Wnt gene targets or luciferase reporters. However, the kinases examined may well have produced effects beyond β-catenin release from cadherin. For example, activation of Akt and/or inhibition of GSK3β could result in β-catenin stabilization and modulation of the Wnt endogenous genes or reporter constructs by an alternative upstream path (Larue and Bellacosa 2005). Just as kinases bind and act on the cadherin–catenin complex, so too do a variety of phosphatases that dephosphorylate both cadherins and catenins (Lilien et al. 2002; Sallee et al. 2006; McLachlan and Yap 2007). The phosphatases involved are numerous and include membrane-spanning receptor-type phosphatases such as DEP-1, PTPmu, LAR, and VE-PTP, as well as cytoplasmic phosphatases including PTP1B and Shp-2. In addition to the opposing impact of kinases and phosphatases on catenin signaling, a further means of promoting β-catenin's displacement from the larger cadherin–catenin complex toward a nuclear signaling pool involves the β-catenin-binding protein BCL9-2, a homolog of the human protooncogene product BCL9 (Brembeck et al. 2004; Brembeck et al. 2006). In response to RTK activation, direct or indirect β-catenin phosphorylation at tyrosine 142 occurs (e.g., via the RTK Met versus the cytoplasmic tyrosine kinases fer or fyn), decreasing β-catenin's association with α-catenin in favor of BCL9, and promoting β-catenin nuclear entry and activity. Such examples have brought attention to the likely interrelation of adherens junction states, inclusive of secondary protein modifications, with transcriptional outcomes (see also Heuberger and Birchmeier 2009).
Because the Wnt pathway impinges directly or indirectly on multiple classes of genes and produces various cellular responses (proliferation, differentiation, stem-cell maintenance), the impact of increased β-catenin signaling cannot be predicted without knowing the molecular details of the tissue or larger developmental programs involved (Clevers 2006; Gavert and Ben-Ze'ev 2007; Huang and He 2008; Barker 2008; Mosimann et al. 2009). However, in the case of RTK effects on the cadherin–catenin complex in particular, assessments have generally been made of endogenous β-catenin gene targets such as cyclin-D1 and c-myc, whose increased expression in many cases correlates with increased cell-cycle progression under normal and pathological conditions. Even though a large number of factors are contributory, decreased E-cadherin levels or function in many carcinomas is correlated with increased proliferation. Thus, the field will continue to be interested in the biological roles of catenin nuclear signals originating from the cadherin–catenin macromolecular complex in future studies of normal or diseased cells and tissues.
OTHER CATENINS
Cadherins bind a number of other catenins distinct from β-catenin that may be involved in CCJ signaling to the nucleus. One of these is plakoglobin (γ-catenin), which being very similar in structure to β-catenin, competes with β-catenin for binding to the membrane-distal region of cadherin tails, and can associate with α-catenin (Zhurinsky et al. 2000). In common with β-catenin, signaling pools of plakoglobin have been found to respond to signals initiated by Wnt ligands (thereby escaping destruction), although plakoglobin's nuclear role has been characterized as either activating, repressive, or relatively neutral in nature probably because of context issues (Merriam et al. 1997; Kodama et al. 1999; Kolligs et al. 2000; Williams et al. 2000; Charpentier et al. 2000; Shtutman et al. 2002; Maeda et al. 2004; Garcia-Gras et al. 2006; Dusek et al. 2007; Shimizu et al. 2008; Martin et al. 2009). Although less studied than β-catenin, it is possible that plakoglobin once dissociated from the cadherin complex might also represent a signaling entity arising from cell–cell junctions. Interestingly, plakoglobin is further present in association with the desmoglein and desmocollin cadherin family members (Garrod and Chidgey 2008), the principal transmembrane proteins conferring adhesive activity at desmosomal junctions. Thus, a signaling pool of plakoglobin could conceivably arise from either of these cell–cell junctions, or perhaps coordinately from both.
Loss of desmosome-associated desmoplakin in mouse cardiomyocytes was associated with increased levels of plakoglobin in the nucleus and a reduction in β-catenin-mediated Wnt signaling. This led to fibroadipocytic replacement of cardiac myocytes that mimics the human genetic condition arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVC) (Garcia-Gras et al. 2006). Additional evidence showed that plakoglobin entered the nucleus to modulate some Wnt/β-catenin target genes (Maeda et al. 2004), and that it might modulate cellular Src activity (Yin et al. 2005). Correlative links have also been made between misdirected desmocollin (3a and 3b) expression in transgenic mice and altered keratinocyte differentiation, with altered β-catenin stability and, thereby, Wnt activity being a possible basis of the phenotype (Hardman et al. 2005). Thus, desmosome-to-nuclear communication occurs in pathologic/experimental contexts, and presumably also under physiologic conditions.
α-Catenin, which is structurally unrelated to the other catenins (Kobielak and Fuchs 2004; Pokutta et al. 2008), binds to β-catenin within the cadherin–catenin complex, and independently modulates, and associates with the actin cytoskeleton (Yamada et al. 2005; Drees et al. 2005). Phosphorylation of α-catenin at tyrosine 148 increases its affinity for β-catenin (Burks and Agazie 2006), whereas β-catenin phosphorylation at tyrosine 142 lowers its affinity for α-catenin (Piedra et al. 2003). Just as the absence or presence of these secondary modifications represent points that might assist β-catenin release into a signaling pool, so might α-catenin be released in response to upstream kinases/phosphatases to modulate cytoskeletal or perhaps nuclear outcomes. α-Catenin has been observed in the nucleus (Giannini et al. 2004; El-Bahrawy et al. 2002), and reports suggest it may modulate β-catenin nuclear signaling (Giannini et al. 2004). Although the mechanisms are uncertain, genetic ablation of α-catenin alters NFκB, HH, MAPK, and Wnt signaling pathways (Kobielak and Fuchs 2006; Lien et al. 2006; Vasioukhin et al. 2001; Merdek et al. 2004; Hwang et al. 2005). Further studies may reveal activities of α-catenin that strengthen its position as a nuclear signaling entity, whereas additional work will be needed to determine if α-catenin originating from the cadherin–catenin complex contributes to CCJ-nuclear signaling.
p120 Subfamily
The p120-catenin subfamily includes p120, and the ARVCF-, δ-, and p0071-catenins (Hatzfeld 2005; Reynolds 2007; McCrea and Park 2007). Each binds weakly to the membrane proximal tail region of classic cadherins, and some members, such as p120, also interact with desmosomal cadherins (desmoglein 3) (Kanno et al. 2008). Interestingly, p120 subfamily members have a prominent role in determining the rate of cadherin endocytosis (Kowalczyk and Reynolds 2004; Xiao et al. 2007), and thus in determining the state of cadherin-mediated contacts.
p120 subfamily members directly or indirectly modulate small GTPases such as Rac1 and RhoA (Anastasiadis et al. 2000; Noren et al. 2001; Yap and Kovacs 2003; Wolf et al. 2006; Anastasiadis 2007) (see also Watanabe et al. 2009). This activity functionally distinguishes p120 family members from β-catenin and plakoglobin. An open question is the extent to which p120 members modulate small GTPases in the immediate molecular vicinity of the cadherin complex (Braga 2002). Although cadherin association appears to lower their capacity to modulate Rac1 or RhoA, p120 subfamily members may cycle on-and-off cadherins to modulate local GTPases and thereby cytoskeletal structures. Once fully dissociated from cadherin, p120 subfamily members could diffuse elsewhere to activate small GTPases whose downstream impact may include the modulation of gene activity via effects on cell-cycle progression (Villalonga and Ridley 2006; Wolf et al. 2006).
The most established outcome of p120 entry into the nucleus is loss of gene repression conferred by Kaiso, a POZ/zinc-finger family member. This outcome is conceptually analogous to β-catenin relieving TCF/LEF mediated repression, although the underlying mechanism involving p120 is different (Kelly et al. 2004; Daniel 2007). Although results relating to Kaiso's functions in Wnt signaling and TCF/LEF differ (Ruzov et al. 2009a; Ruzov et al. 2009b; Iioka et al. 2009), other evidence suggests that p120-catenin assists in de-repressing select Wnt/β-catenin target genes whose promoters have both TCF/LEF and Kaiso binding sites (Kim et al. 2004; Park et al. 2005; Spring et al. 2005; van Roy and McCrea 2005; Park et al. 2006). In addition to Kaiso, recent work revealed that the transcriptional repressor Glis2 associates with p120 (Hosking et al. 2007). Glis2 and Src promote p120's localization to the nucleus, and functional studies suggest that Glis2 participates in neuronal differentiation. Thus, as for β-catenin, we should consider the possibility that p120 has a nuclear function on its dissociation from cadherin. A recent report documented the nuclear- and p120-catenin-dependent entry of a cadherin cyto-domain fragment generated by presenilin, with consequent effects on Kaiso-mediated gene repression (Ferber et al. 2008). Recent results further indicate that, in common with β-catenin and plakoglobin, p120 subfamily members are stabilized in response to upstream Wnt signals (Park et al. 2006; JY Hong and PDM, unpublished results). This suggests an integrated network of catenin downstream effects, including those involving gene regulation. Such integrated responses could arise on Wnt-ligand signaling (Wnt-ligand–Frizzled–LRP complex formation), or in response to local kinase or other activities at CCJs. More work is required to address the validity of this latter possibility, as the graphic dissociation of multiple catenins into signaling pools is not often reported in response to stimuli.
It is intriguing to consider that activities of the cadherin and Wnt pathways may be more closely linked than presently recognized. For example, in addition to reports of Wnt pathway activation having effects on cadherin function (Bradley et al. 1993; Hinck et al. 1994b; Hinck et al. 1994a; Yanagawa et al. 1997; Shariatmadari et al. 2005; Ulrich et al. 2005; Wodarz et al. 2006), there are indications of physical associations between Wnt and cadherin/protocadherin pathway components (Medina et al. 2004; Qin et al. 2005; Hay et al. 2009), suggesting that cadherins are likely to modulate canonical (β-catenin mediated) or noncanonical (alternative) Wnt pathways. As cadherin-mediated adhesion is required for neighboring cells to engage in multiple ligand–receptor interactions, for example those occurring at varied cell synapses or contacts, cadherins contribute to quite indirect yet essential effects on downstream/nuclear signaling. Although not addressed in this article in detail, it is also important to note that the cadherin superfamily is diverse in structure and thus function, with the unifying element being the presence of cadherin-like repeats within the extracellular domain (Hulpiau and van Roy 2009). Some members appear to act more as signaling receptors (or ligands), as opposed to combined adhesion/signaling receptors. Interesting examples include the Dachsous and FAT cadherins (see also McNeill et al. 2009), the putative ligand and receptor of the newly characterized Hippo pathway, which shows a central and conserved role in tissue growth and size control in both vertebrates and invertebrates (Zhao et al. 2008). A further interesting subgroup of cadherins covered elsewhere is the protocadherins (Morishita and Yagi 2007). Protocadherins are expressed in multiple cell types but most prominently in neural tissues, and appear to regulate a number of signaling pathways relevant to cytoskeletal functions or gene activity (Medina et al. 2004; Unterseher et al. 2004; Yang et al. 2005; Chen and Gumbiner 2006; Wang et al. 2008).
Plakophilin Subfamily
A third class of catenins are the plakophilins (PKPs) (Hatzfeld 2007). Plakophilins contain a central Armadillo domain, similar to the β-catenin and p120-catenin subfamilies. In the case of plakophilins, however, the amino-terminal domains mediate interactions with desmogleins or desmocollins. A number of plakophilins have been localized to the nucleus (Mertens et al. 1996; Schmidt et al. 1997). For example, PKP2 associates with desmosomal cadherins and components of the PolIII transcriptional complex, such as RPC155 and the transcription factor TFIIIB (Mertens et al. 2001), and has been found to influence β-catenin-mediated Wnt signaling (Chen et al. 2002). The intracellular localization of PKP2 is modulated through phosphorylation by the serine kinase Cdc25C-associated kinase 1, which promotes the association of PKP2 with 14-3-3 proteins and localizes PKP2 to junctions rather than the nucleus (Muller et al. 2003). Another PKP family member, PKP1b, is mostly localized to the nucleus, whereas the alternatively spliced isoform PKP1a is mostly at junctions, suggesting that RNA processing of PKP family members affects PKP nuclear functions (Schmidt et al. 1997). Thus, plakophilins appear to function at both the desmosome and nucleus, although it remains unknown if plakophilins enter a signaling pool as a consequence of dissociating from desmosomal cadherins (bona fide junctional–nuclear signaling).
Another Perspective Relating to Junctional–Nuclear Catenin Signaling
As presented above, catenins may transduce signals to the nucleus on their dissociation from cell–cell junctions. An alternative view, not incompatible with the first, is that cell–cell junctions and nuclear components compete for catenin binding. Thus, when the level of cadherin is relatively high, catenins may be sequestered away from nuclear complexes (Choi et al. 2006). In experimental contexts, this has been shown to occur for β-catenin in which downstream Wnt pathway activity (e.g., transcription from endogenous Wnt gene targets or luciferase reporters) is suppressed upon cadherin cyto-domain sequestration of β-catenin (Heasman et al. 1994; Sanson et al. 1996; Orsulic et al. 1999). In such a case, the degree of catenin nuclear signaling might be modulated by the expression of cadherin, or through changes in cadherin–catenin affinity states or physical access. For example, in epithelial-to-mesenchymal transitions, the transcriptional repressors Snail and Slug repress E-cadherin expression (Peinado et al. 2007; Alves et al. 2009). If another cadherin does not sequester the population of catenin that would become available, and this population escapes metabolic destruction, effects on gene expression or small GTPase activity may result. Whether increased junctional–nuclear signaling results from catenin dissociation from cadherins (initial control at the protein level) and/or from increased catenin signaling pools that arise because of reduced cadherin expression (initial control at the transcriptional level), nuclear decisions are sensitive to the state of cadherin–catenin cell–cell contacts.
Receptor Tyrosine Kinases (RTKs)/Growth Factor Receptors
Cadherins and Ig-family adhesion receptors such as nectins (see the following) have been linked to the functions of growth factor receptors/RTKs (Comoglio et al. 2003). Examples include the roles of N-cadherin in neurite outgrowth (Williams et al. 1994; Sanchez-Heras et al. 2006) and of the Ig-family member N-CAM in tumor cell adhesion (Cavallaro et al. 2001). In the context of neural cells, N-caherin is thought to facilitate FGF receptor dimerization and signaling in a manner that is independent of FGF ligand (Doherty et al. 2000; Skaper et al. 2001). This relationship also appears to be independent of N-cadherin adhesive activity (Utton et al. 2001) and to involve N-cadherin and FGF receptor clustering (Williams et al. 2002). Work in epithelial and endothelial cells has indicated physical and functional interactions between N-cadherin or another classic cadherin, with the FGF receptor (Nieman et al. 1999; Kim et al. 2000; Suyama et al. 2002; Erez et al. 2004). N-cadherin–FGF-receptor interactions appear to involve interactions through their ectodomains (Kim et al. 2000; Suyama et al. 2002; Sanchez-Heras et al. 2006), although in some contexts this association is difficult to detect at the biochemical level (Kim et al. 2005). Enhanced MAPK-ERK signaling correlates with N-cadherin-mediated cell surface retention and clustering of the FGF receptor, as well as an increase in FGF ligand responsiveness (Suyama et al. 2002). Regardless of the specific molecular underpinnings, N-cadherin's interaction with RTKs is likely relevant to its functional association with more dynamic/motile cell states in physiologic and pathologic contexts (Wheelock et al. 2008), and shows the role of cadherin signaling both in the context of localized phenomena such as cytoskeletal rearrangement and proliferative signaling by gene activity.
Cell–cell contact, presumably mediated by cadherins, has been shown to reduce cell proliferation promoted by the EGF ligand (Qian et al. 2004). E-cadherin has been found in complex with the EGF, FGF, and cErbB2 receptors (Ochiai et al. 1994; Fedor-Chaiken et al. 2003; Qian et al. 2004; Bryant et al. 2005). E-cadherin ligation reduces Stat 5b proliferative signaling downstream of the EGF receptor (Perrais et al. 2007), and FGF-induced MAPK signaling and receptor internalization (Bryant et al. 2005). In keratinocytes and mammary epithelial cells, however, E-cadherin was found to activate the EGF receptor (Fedor-Chaiken et al. 2003), which on cell–cell contact occurred even in the absence of EGF ligand (Pece and Gutkind 2000). In endothelial cells, VE-cadherin associates with, and reduces VEGF receptor activity, an effect that may occur with the cell-density dependent phosphatase PTP1 (Grazia Lampugnani et al. 2003). This VE-cadherin–RTK interaction appears to occur indirectly via cytoplasmic domain interactions, requiring β-catenin as reported also for the E-cadherin–EGF-receptor interaction (Hoschuetzky et al. 1994). Analogous to some cadherin–RTK contexts, VE-cadherin limits VEGF-receptor internalization, thereby lowering PLCγ/MAPK downstream signaling in response to growth signals (Lampugnani et al. 2006). This is similar to the effects of the E-cadherin–FGF-receptor complex, but not the N-cadherin–FGF-receptor complex. Several possibilities have been proposed for how cadherins might alter RTK (or other receptor) signaling, such as lateral receptor clustering to facilitate RTK auto-activation, alteration of ligand–RTK interactions, or modulation of RTK endocytosis.
In summary, although cadherin and Ig-family adhesion protein interactions with RTKs (as well as phosphatases) are incompletely understood, they represent a link between adhesive junction components and signaling pathways that modulate cell growth and a host of other processes. Further, although some of these relationships may not be dependent on cell–cell adhesion per se, others appear adhesion- or contact-responsive, such as E-cadherin-mediated growth arrest via effects on EGF-receptor interactions (Perrais et al. 2007). Interestingly, the FGF receptor appears to associate more with nonjunctional cadherins or Ig-family (N-CAM) adhesion proteins in cell lines derived from a number of sources (e.g., epithelial, myoblastic, and fibroblastic) (Sanchez-Heras et al. 2006). Further, N-cadherin and the PDGF receptor associate to promote cell movements at the leading edge of cells, with their cytoplasmic domain interactions occurring indirectly via β-catenin and NHERF (Theisen et al. 2007). These considerations indicate that much remains to be learned about how adhesive-protein–RTK signaling is engineered within cells, while already suggesting an intimate cell-surface interplay that ultimately modulates gene activity.
Small GTPases
We will discuss briefly junction-mediated effects on small GTPases (see by Watanabe et al). Cadherin-mediated adhesion has been shown to modulate Rac and less directly Rho and Cdc42, with these GTPases reciprocally contributing to determining cell–cell junction states (Anastasiadis 2007; Yap and Kovacs 2003; Braga and Yap 2005; Yap et al. 2007). Junctional effects on small GTPases (or the reverse) are complex, as differences are apparent as a function of cell type and context. Most of the effects studied have been within the junctional area itself, or cytoplasmic effects that largely relate to cytoskeletal structure–function.
Although GTPase effects are thought to be compartmentalized, including a proportion of those acting at cell–cell contact sites, it is likely that some GTPase signals contribute directly to longer-range signaling to the nucleus. Future studies might address, for example, the impact of junctionally activated (or inhibited) small GTPases on cell cycle progression. In such cases, it is likely that p120-catenin subfamily members (Anastasiadis 2007), or other molecular players such as PI3 kinase or Akt (Yap and Kovacs 2003; Yap et al. 2007) (see the following), would act as intermediates. Importantly, Rho, Rac, and Cdc42 are known to play roles in cell cycle progression (Coleman et al. 2004; Villalonga and Ridley 2006), and each is responsive to cadherin-mediated cell–cell contact in context-dependent manners (Yap and Kovacs 2003; Burridge and Wennerberg 2004; Yap et al. 2007; Anastasiadis 2007). Thus, as we learn more from future studies, small GTPases are likely to become recognized players in CCJ-nuclear signaling.
PI3 Kinase and Akt
In addition to small GTPase signals arising from CCJs, other signaling mediators such as PI3K and Akt have attracted attention (Larue and Bellacosa 2005; Giehl and Menke 2008). For example, the survival of melanoma cells is enhanced by N-cadherin through an Akt-dependent mechanism (Li et al. 2001), whereas reduced N- or VE-cadherin function in endothelial, granulose, or hepatocellular carcinoma cells results in apoptosis (Peluso et al. 1996; Makrigiannakis et al. 1999; Erez et al. 2004; Gwak et al. 2006; Jiang et al. 2007). E-cadherin also promotes cell survival, inclusive of cancer contexts (St Croix and Kerbel 1997; Kantak and Kramer 1998; Kang et al. 2007). As junctional molecules such as cadherins allow for multiple ligand–receptor interactions, it is not possible in many cases to conclude that cell survival effects are mediated as a direct consequence of cadherin cytoplasmic domain interactions. However, the involvement of cadherin–catenin complexes in producing intracellular signals suggest that direct cadherin effects are contributory and perhaps even predominant in some instances. In this regard, strong evidence has accumulated that PI3 kinase and Rac activation results following cadherin–cadherin trans interactions, and that the cadherin cytoplasmic domain is involved (Kovacs et al. 2002; Goodwin et al. 2003). Future work will be required to determine the relative impact of such signals locally, relative to those that might quickly reach the nucleus to modulate gene programs.
NECTIN BASED SIGNALING
Thus far, our discussion has largely taken place from a cadherin-centric perspective. However, it is clear that other adhesive transmembrane polypeptides, such as the Ig-family member N-CAM, also modulate signaling pathways reaching the nucleus (Williams et al. 1994; Choi et al. 2001; Little et al. 2001). Although we will not discuss N-CAM further, we will summarize evidence that the nectin Ig-family of adhesion receptors have important roles in junction formation in epithelia, fibroblasts, and neurons (Takai and Nakanishi 2003; Takai et al. 2008a; Sakisaka et al. 2007). Heterophilic trans interactions between nectin1 and nectin3 occur at synaptic junctions (Mizoguchi et al. 2002; Togashi et al. 2006), whereas homophilic N-cadherin trans interactions are from both sides of the synapse. Nectins bind several cytoplasmic proteins including afadin, which binds actin, and the cell polarity protein Par3. Indirectly, nectins associate with α-catenin, annexin II, IQGAP1, and ZO1. This latter ZO1 interaction is relevant to nectin's roles in tight junction formation (Ooshio et al. 2007). With respect to signaling, nectin3 associates in cis with the integrin α(v)β(3), which appears important for peripheral cytoskeletal organization and activity, and formation of adherens and tight junctions (Sakamoto et al. 2006; Ozaki et al. 2007; Sakamoto et al. 2008). PKC, FAK, and c-Src participate in nectin function downstream of α(v)β(3) (Ozaki et al. 2007; Sakamoto et al. 2008). The fact that nectins interact with components of both the adherens junction (cadherins through α-catenin–afadin associations), tight junctions (ZO1), and α(v)β(3) integrin, appears to provide an example of junctional cross talk. Nectin3 has also been found in cis association with the PDGF, but not the EGF receptor, raising the question whether nectin modulates RTK signaling or vice-versa, and if this nectin complex might also include cadherin or α(v)β(3) integrin (unpublished in Sakisaka et al. 2007). A number of studies have further established functional links between nectins and the activation of the small G proteins Rap1, Cdc42, and Rac through c-Src (Ogita and Takai 2006; Kawakatsu et al. 2005).
It has been proposed that distinct nectin-like (Necl) transmembrane molecules link nectin pathways directly with the nucleus. Much of the focus has been on Necl5, which appears to contribute to contact-mediated inhibition of cell growth, and is capable of binding in trans to nectin3 in confluent cell culture. In nonconfluent cells, however, Necl5 associates in cis with the PDGF receptor, and α(v)β(3) integrin. These binary associations appear to influence Rap1 and Rac signals involved in cell movements, and Ras signals that induce cell proliferation (H. Amano et al. unpublished; Sakisaka et al. 2007; Minami et al. 2007; Kakunaga et al. 2004). Once cells reach higher densities, Necl5 is thought to engage in heterotypic interactions with nectin3, reducing Ras‐mediated proliferative signaling and enhancing signals that strengthen junctions through cadherin and cytoskeletal reorganization. Necl5 is also associated with Sprouty, a negative regulator of growth factor signaling through inhibition of Ras (Kajita et al. 2007). In subconfluent cells, Necl5 appears to block Sprouty inhibition of Ras, favoring a proliferative cell state. In confluent cells, Necl5 is endocytosed more rapidly, and this reduction in Necl5 levels is proposed to allow Sprouty to inhibit Ras, leading to slowed cell proliferation. Finally, recent work indicates that the nectin–afadin complex contributes to PDGF-induced cell survival through the PI3K-Akt signaling pathway (Kanzaki et al. 2008). Cadherins have also been linked to cell survival signaling (Peluso et al. 1996; Kantak and Kramer 1998; St Croix and Kerbel 1997; Makrigiannakis et al. 1999; Gwak et al. 2006; Jiang et al. 2007; Wheelock et al. 2008), and as above noted, associate with RTKs. Prosurvival effects arise from Akt and β-catenin signaling in the case of N-cadherin (Li et al. 2001), and Akt signaling upon E-cadherin association with ErbB4 (Kang et al. 2007). Given the previously noted associations that have been resolved between cadherins and nectins, it may well be that their respective downstream signals are coordinated or interdependent.
GAP JUNCTIONS
Among CCJs, gap junctions represent a unique nonadhesive connection formed from the docking of two hemichannels (or connexon, a hexameric cluster of various connexin isoforms), allowing direct intercellular communications between adjacent cells (Fig. 3) (see Goodenough and Paul 2009). Such an exchange of chemical or electrical signals has been implicated in a broad spectrum of biological events, including metabolic and ion homeostasis, synchronization of muscle contraction, and cell proliferation, survival, differentiation, and migration (Wong et al. 2008; Harris 2007; Mese et al. 2007).
Figure 3.
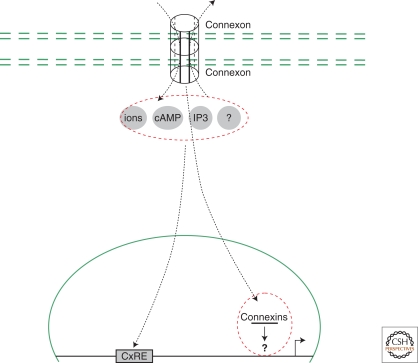
Gap junction signaling to the nucleus. Gap junctions are nonadhesive channels that allow regulated intercellular transmission of electrical or small molecule signals. Connexin 43, a gap junction structural component, has been observed in the nuclei of certain cells and associated with decreased cell proliferation.
Unlike many classical outside-in signaling pathways, in which ligands bind receptors to trigger events such as phosphorylation or oligomerization and ultimately alter gene expression, gap junctions or hemichannels function as gate keepers to permit molecular diffusion in their opened conformation and prevent such diffusion in closed states (Harris 2007). Entering signaling entities include calcium and other critical metabolites or second messengers that may alter gene expression through known (e.g., CxRE dependent) or unknown mechanisms (Rodriguez-Sinovas et al. 2007).
A number of connexins appear to localize to the nucleus. For example, on immunostaining, connexin 43 has been observed in the nuclei of magnocellular neurons (Eisner and Colombo 2002), whereas its carboxy-terminal fragment localizes to cardiomyocyte and HeLa cell nuclei and correlates with decreased cell proliferation (Dang et al. 2003). Further experimentation is needed, however, to determine if physiologic gap junction–nuclear signaling occurs as a function of connexin or connexin-fragment entry to the nucleus, or through other proteins originally associated with plasma membrane-associated gap junctions.
TIGHT JUNCTIONS
Tight junctions (TJs) are areas of close contact between neighboring plasma membranes, where intramembrane strands at the contact sites can be visualized by freeze-fracture electron microscopy (Tsukita et al. 2001; see Furuse 2009). Tight junctions act as barriers that show both gating and fencing functions: Gating determines the paracellular diffusion of molecules according to size and charge, whereas fencing restricts lipid diffusion between the apical and basolateral intramembrane domains (Balda and Matter 2008; Cereijido et al. 2008; Paris et al. 2008; see Anderson and Van Itallie 2009). Certain proteins of the epithelial TJ show cellular localizations other than the TJ. These proteins localize to both nuclei and TJ, and tend to be involved in the regulation of cell proliferation, gene expression, and cell differentiation (Fig. 4) (Balda and Matter 2003; Matter and Balda 2007). Here, we discuss TJ proteins having both junctional and nuclear localizations, and summarize what is known about their functions, regulation, and expression.
Figure 4.
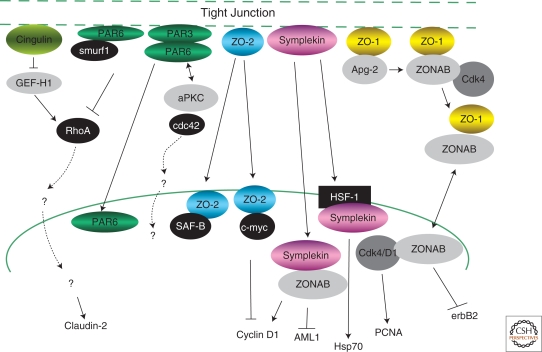
Tight junction signaling to the nucleus. Displayed are pathways associated with tight junctions and suggested or showed to regulate gene expression. Arrows refer to positive functional interactions that could be because of either indirect positive effects or direct physical interactions. Conversely, lines ending with a “T” are negative functional interactions reflecting indirect effects or direct physical interactions. Although most of the junctional proteins depicted associate with junctional membrane proteins, many of the transmembrane entities relevant to the indicated pathways are not known and therefore not indicated.
Six TJ-associated transmembrane proteins have been identified: occludin, claudins, tricellulin, JAMs (Junction Adhesion Molecules), CRB-3 (a human homolog of Drosophila Crumbs), and Bves (blood vessel/epicardial substance) (Lemmers et al. 2004; Osler et al. 2005; Furuse and Tsukita 2006; Wang and Margolis 2007; Chiba et al. 2008). It remains unclear how each of these proteins interacts with components of the TJ cytoplasmic plaque and how these interactions result in a protein network that controls paracellular permeability, gene expression, junctional dynamics, proliferation, and polarity. The TJ cytoplasmic plaque is formed by multiple adaptor and scaffolding proteins (e.g., ZO-1/2/3, PATJ, Pals1, PAR-3, and PAR-6), different types of signaling components such as GTP-binding proteins, protein kinases, and phosphatases, as well as transcriptional and post-transcriptional regulators (Matter and Balda 2007; Paris et al. 2008). Several proteins have dual localizations at TJs and the nucleus, including ZONAB, cdk4, symplekin, ZO-2, PAR-6, huASH1, and ubinuclein. Most of these proteins have been linked to cell proliferation, gene expression, and/or cell differentiation, and interact with and regulate transcription factors.
ZO-1 AND ZONAB IN CELL PROLIFERATION AND GENE EXPRESSION
ZO-1, the first TJ protein identified (Stevenson et al. 1986), contains distinct protein–protein interaction domains including three PDZ, an SH3 domain, and a domain homologous to yeast guanylate kinase (GUK domain) (Willott et al. 1993). ZO-1 belongs to the same protein family as DlgA (Discs large A), a Drosophila tumor suppressor (Tsukita et al. 1993; Woods and Bryant 1993; Fanning et al. 2007). ZO-1 is present not only in epithelial TJs, but also in fibroblasts, neurons, and astrocytes (Howarth et al. 1992), suggesting it has more general functions in cells that do not have tight junctions. Although ZO-1 has been observed in the nucleus of certain cells and conditions, some of the data is conflicting (Gottardi et al. 1996; Balda and Matter 2000; Reichert et al. 2000; Glaunsinger et al. 2001; Matter and Balda 2003; Traweger et al. 2003). Moreover, the known signaling functions of ZO-1 do not require a nuclear pool of ZO-1, but involve interactions that occur in the cytoplasm and lead to the cytoplasmic sequestration of the dual localization protein ZONAB.
ZONAB is a Y-box transcription factor that belongs to a small family of multifunctional regulators of transcription and post-transcriptional processes (Gallia et al. 2000; Kohno et al. 2003). The Y-box factor family includes DbpA/ZONAB, DbpB/YB-1, and DbpC/contrin (Sakura et al. 1988; Lloberas et al. 1995). ZONAB controls expression of the growth factor coreceptor erbB2, cell cycle regulators such as cyclin D1 and PCNA, and regulates epithelial morphogenesis (Balda and Matter 2000; Balda et al. 2003; Sourisseau et al. 2006). Knockout experiments in mice have shown that mice deficient in DbpA/ZONAB (also called MSY3 in mice) develop normally. However, functional redundancy is indicated as DbpA/ZONAB and DbpB/YB-1 double knockout mice die earlier than animals knocked out for DbpB/YB-1 alone (Lu et al. 2006).
ZONAB binds to the SH3 domain of ZO-1, which contributes to ZONAB's regulation (Balda and Matter 2000; Balda et al. 2003). In MDCK cells, ZO-1 and ZONAB expression are inversely regulated by cell density, and results from different experimental approaches indicate that ZO-1 suppresses proliferation by inhibiting ZONAB's nuclear accumulation (Balda and Matter 2000; Balda et al. 2003). Indeed, the SH3 domain of ZO-1 that binds to ZONAB is necessary and sufficient to sequester ZONAB in the cytoplasm and reduce proliferation (Balda et al. 2003). ZONAB associates with the cell division kinase (CDK) 4, which also has a TJ-nuclear localization; thus, reduction of nuclear ZONAB levels results in reduced nuclear CDK4. Hence, the cell-density dependent accumulation of ZO-1 at TJs works to inhibit the proliferation-promoting ZONAB and CDK4 pathways.
The relative expression levels of ZONAB and ZO-1 are tightly regulated. ZO-1 is expressed at low levels in sparse cultures of MDCK cells, and its levels rises as a function of increased cell density (Balda and Matter 2000). As ZO-1 interacts with many junctional proteins and its half-life increases with greater cell–cell contact, its up-regulation seems to involve junctional stabilization (Gumbiner et al. 1991). Transcriptional mechanisms also affect ZO-1 expression. ZO-1 levels are decreased during corneal wound repair (Cao et al. 2002), in response to β–catenin (Mann et al. 1999), on experimental cell transformation and in a variety of cancers (Miettinen et al. 1994; Chen et al. 2000; Tian and Phillips 2002; Eger et al. 2004; Harten et al. 2009). Analysis of the ZO-1 promoter revealed response elements targeted during EMT (Venkov et al. 2007), as well as by signaling networks activated in neoplastic, metabolic, and viral diseases (Chen et al. 2008). In contrast to ZO-1, ZONAB/DbpA expression increases in contexts that favor proliferation, such as transcriptional regulation by E2F1, and thus is a prognostic marker for hepatocellular carcinomas (Yasen et al. 2005).
ZONAB activity is also regulated by other proteins that control its interaction with ZO-1. One such protein is Apg-2, a heat shock protein that like ZONAB binds the SH3 domain of ZO-1 (Tsapara et al. 2006). Apg-2 is a member of the Hsp110 family of chaperones in the cytosol, the nucleus, and to a lesser extent at the cell–cell junctions in MDCK cells (Tsapara et al. 2006). In response to heat shock, Apg-2 is recruited to junctions and binds ZO-1, leading to ZONAB dissociation, nuclear localization, and activation. Apg-2 depletion retards TJ assembly in calcium switch experiments using two-dimensional cultures, and affects the development of well-organized polarized cysts in three-dimensional cultures (Aijaz et al. 2007), similar to the effects of ZO-1 depletion (Aijaz et al. 2006; Sourisseau et al. 2006). However, whether this effect is due solely to Apg-2's relationship with ZO-1 and ZONAB is not clear. Nevertheless, a functional interaction of these components is further suggested by Apg-2's over-expression in hepatocellular carcinomas (Gotoh et al. 2004), where ZONAB/DbpA is a prognostic marker (Yasen et al. 2005), and ZO-1 is down-regulated (Orban et al. 2008). Thus, it seems that liver cancer correlates with increased ZONAB activity either because of increased expression of ZONAB itself or of Apg-2, or because of down-regulation of ZO-1.
SYMPLEKIN IN mRNA PROCESSING AND CELL PROLIFERATION
Symplekin, a nuclear protein that can associate with TJs, has been linked to the machinery involved in 3′-end processing of pre-mRNA and polyadenylation (Keon et al. 1996; Takagaki and Manley 2000; Hofmann et al. 2002). In stressed cells, symplekin interacts with HSF-1 (heat shock inducible transcription factor one) to regulate hsp70 mRNA polyadenylation (Xing et al. 2004). In Xenopus, symplekin and an unusual poly(A) polymerase, GLD-2, are required for cytoplasmic polyadenylation (Barnard et al. 2004). Furthermore, symplekin and the polyadenylation factor CPSF-73 are targets of the small ubiquitin-like modifier SUMO (Vethantham et al. 2007). Thus, symplekin has a role in mRNA processing.
Whether symplekin's junctional localization reflects a regulatory role of cell–cell adhesion in polyadenylation is not known. As symplekin appears to bind transcription factors, it may localize to sites where those interacting transcription factors are found. This possibility is supported by the finding that symplekin interacts with ZONAB. Symplekin depletion by RNA interference reduces ZONAB nuclear accumulation, transcriptional activity, cell proliferation and expression of cyclin D1 in a ZONAB-dependent manner (Kavanagh et al. 2006). Furthermore, symplekin cooperates with ZONAB to negatively regulate intestinal goblet cell differentiation, acting via repression of the AML1 transcription factor (Buchert et al. 2009). As ZONAB is a Y-box factor, a family of multifunctional proteins that can interact with DNA as well as RNA (Kohno et al. 2003), it is tempting to speculate that the interaction between symplekin and ZONAB is not only functionally important for transcription but also for RNA processing, stability, and/or localization.
ZO-2 IN CELL PROLIFERATION AND GENE EXPRESSION
ZO-2 has the same domain structure as ZO-1 and was identified because of its interaction with ZO-1 (Gumbiner et al. 1991; Jesaitis and Goodenough 1994). ZO-2 shuttles from the cell periphery to the nucleus (Islas et al. 2002; Traweger et al. 2003; Kausalya et al. 2004), interacts with the nuclear ribonucleoprotein scaffold attachment factor-B (SAF-B) (Traweger et al. 2003) and several transcription factors (Betanzos et al. 2004; Huerta et al. 2007). ZO-2 inhibits cell proliferation by affecting transcription, translation, and degradation of cyclin D1 (Huerta et al. 2007; Tapia et al. 2009), coincident with observations that its expression is reduced in certain tumors (Chlenski et al. 2000; Fink et al. 2006; Paschoud et al. 2007). ZO-2 binds and inactivates oncogenic viral proteins (Glaunsinger et al. 2000; Lee et al. 2000; Glaunsinger et al. 2001), and its degradation is induced by HIV type 1 gp120 (Nakamuta et al. 2008). Conversely, in some epithelial and endothelial cells, ZO-2 has been reported to increase proliferation and regulate the expression of M2 pyruvate kinase (Traweger et al. 2008).
In mice, ZO-2 deficiency is embryonic lethal; embryos show decreased proliferation at embryonic day 6.5, and increased apoptosis at embryonic day 7.5, indicating an arrest in early gastrulation (Xu et al. 2008). Depletion of ZO-2 protein by RNA interference in two-cell embryos delays blastocoel cavity formation with normal cell proliferation and morphogenesis. In contrast, ZO-1 knockdown, or combined ZO-1 and ZO-2 knockdown, produces a more severe inhibition of blastocoel formation, indicating distinct but possibly overlapping roles for ZO proteins in blastocyst morphogenesis (Sheth et al. 2008). Further studies are needed to address these contradictions in ZO-2's role in cell proliferation, possibly because of different experimental systems/contexts, and to identify genes regulated by ZO-2 in association with SAF-B or other transcription factors.
HuASH1 AND UBINUCLEIN IN CHROMATIN REMODELING AND GENE EXPRESSION
HuASH1, the human homolog of Drosophila ASH1 (absent, small, or homeotic discs 1), belongs to the trithorax group of transcription factors. It was found to colocalize with TJ markers by immunofluorescence (Nakamura et al. 2000). HuASH1 functions as a histone methyltransferase, an enzymatic activity involved in chromatin remodeling and gene expression (Beisel et al. 2002; Gregory et al. 2007), and interacts with HDAC1 repression complexes (Tanaka et al. 2008). It remains unknown how huASH1 is recruited to intercellular junctions, or the functional relevance of its junctional association in chromatin remodeling and gene expression.
Ubinuclein belongs to a novel family of histone chaperones conserved throughout eukaryotes (Balaji et al. 2009), and has been found to interact with viral transcription factors in the nucleus and ZO-1 at epithelial TJs (Aho et al. 2008). When overexpressed, ubinuclein prevents MDCK cells from going through cytokinesis (Aho et al. 2008). Whether and how ZO-1 might regulate the function and localization of ubinuclein, or the specifics of ubinuclein's interactions with other proteins in the nucleus, remain open questions.
TIGHT JUNCTION-ASSOCIATED REGULATION OF SMALL GTPases
TJs also participate in the regulation of small GTPases (see also Watanabe et al. 2009). RhoA activation affects actin organization and stimulates epithelial cell proliferation, gene expression, and differentiation (Jaffe and Hall 2002; Sahai and Marshall 2002; Nelson 2008). RhoA is down-regulated when epithelial cells reach confluence, resulting in inhibition of signaling pathways that stimulate proliferation. GEF-H1/Lfc, a guanine nucleotide exchange factor specific for RhoA, is involved in the regulation of paracellular permeability and cell proliferation (Benais-Pont et al. 2003; Aijaz et al. 2005). GEF-H1/Lfc directly interacts with cingulin, a junctional adaptor, resulting in its inhibition and thus reduced RhoA activity. In MDCK cells with reduced cingulin levels, increased claudin-2 expression is RhoA-dependent, suggesting it might be mediated by GEF-H1 signaling (Guillemot and Citi 2006).
JACOP/paracingulin, a TJ plaque protein with sequence similarity to cingulin (Ohnishi et al. 2004), interacts with GEF-H1 as well as the Rac GEF Tiam1, thereby influencing junction assembly (Guillemot et al. 2008). Tiam1 and Cdc42 have been linked to the assembly of the TJ-associated, evolutionarily conserved cell polarity signaling pathway that includes the PAR3/PAR6/aPKC complex, and is required for the formation of distinct tight and adherens junctions (Ebnet et al. 2004; Assemat et al. 2008; Iden and Collard 2008; Macara and Mili 2008).
PAR6 itself is a protein with multiple interaction partners and also shows dual localization. In different experimental systems, PAR6 was shown to interact with small GTPases such as cdc42, Rac2, Rin, Rit, and Wrch-1 (Johansson et al. 2000; Hoshino et al. 2005; Macara and Mili 2008; Brady et al. 2009), as well as p190RhoGAP (Zhang and Macara 2008). PAR6 is also part of the TGF-β-activated pathway leading to ubiquitination and degradation of RhoA (Wang and Margolis 2007). PAR6 localizes to nuclear speckles and colocalizes with Tax, a transcriptional activator of the human T-cell leukemia virus type 1 long terminal repeat (Cline and Nelson 2007). Although its nuclear functions are not clear, PAR6 does not seem to be directly involved in transcription but likely instead plays an architectural role.
TIGHT JUNCTION CROSS TALK WITH GROWTH FACTORS AND EXTRACELLULAR MATRIX PATHWAYS
The relationship of TJs with growth factor and matrix signaling is an emerging area of research. Occludin regulates localization of the TGF-β type I receptor and thus plays a role in TGF-β-dependent dissolution of TJs during epithelial-to-mesenchymal transitions (Bose and Wrana 2006). FGF-2 fails to induce angiogenesis in JAM-A-deficient mice (Cooke et al. 2006), suggesting a role of JAM-A in FGF-2 signaling, which might be mediated by the PAR3/PAR6/aPKC complex (Ebnet et al. 2004). Additionally, EGF signaling seems to affect the expression and localization of several TJ proteins that regulate TJ assembly and barrier functions (Van Itallie et al. 1995; Basuroy et al. 2006; Wang et al. 2006; Flores-Benitez et al. 2007; Singh et al. 2007), suggesting that EGF signaling could have an indirect role in regulating TJ-nuclear signaling by modulating the activities of the PAR3/PAR6/aPKC and/or ZO-1/ZONAB pathways.
It is generally accepted that integrin signaling pathways regulate cell migration, junctional complex stability, and cell–cell interactions including those taking place at TJs (Ojakian et al. 2001). There are also examples linking TJ-ssociated proteins with integrins. For example, claudin-11 forms a complex with OAP-1, a tetraspan family protein, and with β1 integrin to regulate proliferation and migration of oligodendrocytes (Tiwari-Woodruff et al. 2001). JAMs regulate cell migration through effects on β(1) integrin or, depending on the cellular context, on α(v)β(3) integrin (Mandell et al. 2005; Naik and Naik 2006; Mandicourt et al. 2007). These results suggest that transmembrane TJ proteins may influence nuclear events through their effects on integrins. It is tempting to speculate that such relationships exist because down-regulation of ZO-1 or manipulation of ZO-1 interactions with ZONAB produce mild phenotypes in monolayers but inhibit cyst formation in three dimensional (collagen/matrigel) cultures (Umeda et al. 2004; Aijaz et al. 2006; McNeil et al. 2006; Sourisseau et al. 2006; Aijaz et al. 2007). Future studies will need to address the specific molecular mechanisms involved; for example, if/how the TJ affects gene expression profiles relevant to extracellular matrix adhesion and signaling.
ADHERENS AND TIGHT JUNCTION CROSS TALK
In revising earlier ideas in which junctions may have been viewed as rather distinct functional entities, it seems likely that in many cases shared functions occur including those involving signals directed to the nucleus. For example, the p120- and ARVCF-catenins, traditionally viewed as components of adherens junctions, also interact with ZO-1 and ZO-2 (Kausalya et al. 2004). In polarized epithelial MDCK cells, ARVCF and ZO-1 partially colocalize in the vicinity of the apical adhesion complex. Disruption of cell–cell adhesion releases ARVCF from the plasma membrane, with an increased ARVCF fraction localizing to the nucleus where it binds ZO-2 (Kausalya et al. 2004). Thus, even as the nuclear roles of ARVCF are not known, the interaction of TJ and adherens junction proteins may allow cooperation between junctions in regulating gene expression.
Cyclin D1 is an example of a gene target responsive to both TJ and adherens junction function, and which is up-regulated in many tumors (Coqueret 2002; Fu et al. 2004). Several signaling pathways that regulate cell–cell and cell–extracellular matrix adhesion have an impact on cyclin D1 expression (Shtutman et al. 1999; Tetsu and McCormick 1999; D'Amico et al. 2000; Amanatullah et al. 2001; Zhao et al. 2001; Balda and Matter 2003; Zhao et al. 2003). The identification of other genes regulated by both adherens and tight junctions should help us understand downstream cross talk occurring between adherens and tight junctions, as well as appreciate how these pathways interact with those activated by growth factors or the extracellular matrix (Balda and Matter 2003).
Junctional cross talk involving nuclear signals is further reflected in roles of the adherens junction in transcriptional regulation of TJ gene products. In endothelia, for example, the level of VE-cadherin affects that of the TJ protein claudin-5. This effect appears to occur through Akt, which phosphorylates the forkhead box factor FoxO1, and regulates the TCF4–β-catenin transcriptional repressor complex (Taddei et al. 2008). It has been suggested that potential TCF/LEF binding sites present in the promoter of claudin-3 may account for its down-regulation in response to reduced β-catenin activity (Liebner et al. 2008). In the future, it will be interesting to further examine whether signaling pathways downstream of one junction have an impact on the expression of gene products comprising another junction.
CONCLUDING REMARKS
Although it is clear that CCJ-nuclear signaling takes place in cells and tissues, it is also apparent that our present knowledge is not satisfactory in understanding the larger roles of CCJs in transcriptional modulation. Although we are able to list a number of intriguing examples for each junction type, we are far from having a coherent picture of the networks involved at the plasma membrane, cytoplasmic, or nuclear levels. An inherent difficulty in identifying the network and its details is that downstream signals originating from cell–cell contacts are both direct and indirect, and are likely to be concurrent. For example, signals may be blocked or promoted by a component being junctionally sequestered or released from the adhesion complex to the nucleus (direct) or, alternatively, mediated through associated signaling molecules such as an RTKs, or other ligand–receptor or adaptor/signaling pairs dependent on the presence of the junction (each indirect).
One approach that has been helpful is the use of engineered contacts bearing purified adhesion receptors (coated beads, etc.). Although this is not physiologic, it assure that the physical interaction presented in trans is defined and, if desired, singular. Such methods, combined with the use of reagents to report pathway activation in real time, can also be useful in working out the temporal sequence of pathway activation following contact. In time, it may be that single molecule tracking methods will be used to more firmly establish the fate of individual molecules putatively traveling between junctions and the nucleus. This could assist, for example, in ascertaining if a specific catenin molecule dissociating from a cadherin complex is actually capable of reaching transcriptional regulatory regions in the nucleus. Although much work remains, we can appreciate the findings that have already been made, as they tell us of two fascinating worlds that listen to one another, one at the cell surface where cells meet and touch, and the other internal where transcriptional and proliferative/differentiation outcomes are further processed.
ACKNOWLEDGMENTS
This article is dedicated to Margaret Jean (Peggy) Wheelock, PhD (1945–2009). We thank W.J. Nelson for his critical reading, helpful comments, and patience during the review process. The laboratory of PDM is supported through funding from NIH (RO1GM52112), Texas ARP (003657-0008-2006), and UT MDACC NCI Core Grants (CA-16672). The laboratory of M.S.B. is supported through funding from the Wellcome Trust, MRC, BBSRC, AICR, and Fight for Sight.
Footnotes
Editors: W. James Nelson and Elaine Fuchs
Additional Perspectives on Cell Junctions available at www.cshperspectives.org
REFERENCES
- Abram CL, Lowell CA 2009. The ins and outs of leukocyte integrin signaling. Annu Rev Immunol 27:339–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho S, Lupo J, Coly PA, Sabine A, Castellazzi M, Morand P, Sergeant A, Manet E, Boyer V, Gruffat H 2008. Characterization of the ubinuclein protein as a new member of the nuclear and adhesion complex components (NaCos). Biol Cell 101:319–334 [DOI] [PubMed] [Google Scholar]
- Aijaz S, D'Atri F, Citi S, Balda MS, Matter K 2005. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell 8:777–786 [DOI] [PubMed] [Google Scholar]
- Aijaz S, Balda MS, Matter K 2006. Tight junctions: Molecular architecture and function. Int Rev Cytol 248:261–298 [DOI] [PubMed] [Google Scholar]
- Aijaz S, Sanchez-Heras E, Balda MS, Matter K 2007. Regulation of tight junction assembly and epithelial morphogenesis by the heat shock protein Apg-2. BMC Cell Biol 8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alema S, Salvatore AM 2007. 120 catenin and phosphorylation: Mechanisms and traits of an unresolved issue. Biochim Biophys Acta 1773:47–58 [DOI] [PubMed] [Google Scholar]
- Alves CC, Carneiro F, Hoefler H, Becker KF 2009. Role of the epithelial-mesenchymal transition regulator Slug in primary human cancers. Front Biosci 14:3035–3050 [DOI] [PubMed] [Google Scholar]
- Amanatullah DF, Zafonte BT, Albanese C, Fu M, Messiers C, Hassell J, Pestell RG 2001. Ras regulation of cyclin D1 promoter. Methods Enzymol 333:116–127 [DOI] [PubMed] [Google Scholar]
- Anastasiadis PZ 2007. 120-ctn: A nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta 1773:34–46 [DOI] [PubMed] [Google Scholar]
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB 2000. Inhibition of RhoA by p120 catenin. Nat Cell Biol 2:637–644 [DOI] [PubMed] [Google Scholar]
- Anderson JM, Van Itallie CM 2009. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1:a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andl CD, Rustgi AK 2005. No one-way street: Cross-talk between e-cadherin and receptor tyrosine kinase (RTK) signaling: A mechanism to regulate RTK activity. Cancer Biol Ther 4:28–31 [DOI] [PubMed] [Google Scholar]
- Assemat E, Bazellieres E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D 2008. Polarity complex proteins. Biochim Biophys Acta 1778:614–630 [DOI] [PubMed] [Google Scholar]
- Balaji S, Iyer LM, Aravind L 2009. HPC2 and ubinuclein define a novel family of histone chaperones conserved throughout eukaryotes. Mol Biosyst 5:269–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Matter K 2000. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. Embo J 19:2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Matter K 2003. Epithelial cell adhesion and the regulation of gene expression. Trends Cell Biol 13:310–318 [DOI] [PubMed] [Google Scholar]
- Balda MS, Matter K 2008. Tight junctions at a glance. J Cell Sci 121:3677–3682 [DOI] [PubMed] [Google Scholar]
- Balda MS, Garrett MD, Matter K 2003. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol 160:423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N 2008. The canonical Wnt/β-catenin signalling pathway. Methods Mol Biol 468:5–15 [DOI] [PubMed] [Google Scholar]
- Barnard DC, Ryan K, Manley JL, Richter JD 2004. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell 119:641–651 [DOI] [PubMed] [Google Scholar]
- Basuroy S, Seth A, Elias B, Naren AP, Rao R 2006. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J 393:69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel C, Imhof A, Greene J, Kremmer E, Sauer F 2002. Histone methylation by the Drosophila epigenetic transcriptional regulator Ash1. Nature 419:857–862 [DOI] [PubMed] [Google Scholar]
- Benais-Pont G, Punn A, Flores-Maldonado C, Eckert J, Raposo G, Fleming TP, Cereijido M, Balda MS, Matter K 2003. Identification of a tight junction-associated guanine nucleotide exchange factor that activates Rho and regulates paracellular permeability. J Cell Biol 160:729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, Gonzalez-Mariscal L 2004. The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp Cell Res 292:51–66 [DOI] [PubMed] [Google Scholar]
- Bienz M 2005. β-Catenin: A pivot between cell adhesion and Wnt signalling. Curr Biol 15:R64–67 [DOI] [PubMed] [Google Scholar]
- Bierkamp C, Schwarz H, Huber O, Kemler R 1999. Desmosomal localization of β-catenin in the skin of plakoglobin null-mutant mice. Development 126:371–381 [DOI] [PubMed] [Google Scholar]
- Bose R, Wrana JL 2006. Regulation of Par6 by extracellular signals. Curr Opin Cell Biol 18:206–212 [DOI] [PubMed] [Google Scholar]
- Bradley RS, Cowin P, Brown AM 1993. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J Cell Biol 123:1857–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady DC, Alan JK, Madigan JP, Fanning AS, Cox AD 2009. The transforming Rho family GTPase Wrch-1 disrupts epithelial cell tight junctions and epithelial morphogenesis. Mol Cell Biol 29:1035–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM 2002. Cell–cell adhesion and signalling. Curr Opin Cell Biol 14:546–556 [DOI] [PubMed] [Google Scholar]
- Braga VM, Yap AS 2005. The challenges of abundance: Epithelial junctions and small GTPase signalling. Curr Opin Cell Biol 17:466–474 [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Rosario M, Birchmeier W 2006. Balancing cell adhesion and Wnt signaling, the key role of β-catenin. Curr Opin Genet Dev 16:51–59 [DOI] [PubMed] [Google Scholar]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W 2004. Essential role of BCL9-2 in the switch between β-catenin's adhesive and transcriptional functions. Genes Dev 18:2225–2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher WM, Yap AS, Gumbiner BM 1996. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J Cell Biol 135:487–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Wylie FG, Stow JL 2005. Regulation of endocytosis, nuclear translocation, and signaling of fibroblast growth factor receptor 1 by E-cadherin. Mol Biol Cell 16:14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchert M, Darido C, Lagerqvist E, Sedello A, Cazevieille C, Buchholz F, Pannequin J, Joubert D, Hollande F 2009. The symplekin/ZONAB complex inhibits intestinal cell differentiation via the repression of AML1/Runx1. Gastroenterology doi: 10.1053/j.gastro.2009.03.037 [DOI] [PubMed] [Google Scholar]
- Burks J, Agazie YM 2006. Modulation of α-catenin Tyr phosphorylation by SHP2 positively effects cell transformation induced by the constitutively active FGFR3. Oncogene 25:7166–7179 [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K 2004. Rho and Rac take center stage. Cell 116:167–179 [DOI] [PubMed] [Google Scholar]
- Caca K, Kolligs FT, Ji X, Hayes M, Qian J, Yahanda A, Rimm DL, Costa J, Fearon ER 1999. β- and γ-catenin mutations, but not E-cadherin inactivation, underlie T-cell factor/lymphoid enhancer factor transcriptional deregulation in gastric and pancreatic cancer. Cell Growth Differ 10:369–376 [PubMed] [Google Scholar]
- Cadigan KM, Peifer M 2009. Wnt signaling from development to disease: Insights from model systems. Cold Spring Harb Perspect Biol 1:a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins CC, Hoepner BL, Law CM, Novak MR, Setzer SV, Hatzfeld M, Kowalczyk AP 2003. The Armadillo family protein p0071 is a VE-cadherin- and desmoplakin-binding protein. J Biol Chem 278:1774–1783 [DOI] [PubMed] [Google Scholar]
- Cao Z, Wu HK, Bruce A, Wollenberg K, Panjwani N 2002. Detection of differentially expressed genes in healing mouse corneas, using cDNA microarrays. Invest Ophthalmol Vis Sci 43:2897–2904 [PubMed] [Google Scholar]
- Cavallaro U, Niedermeyer J, Fuxa M, Christofori G 2001. N-CAM modulates tumour-cell adhesion to matrix by inducing FGF-receptor signalling. Nat Cell Biol 3:650–657 [DOI] [PubMed] [Google Scholar]
- Cereijido M, Contreras RG, Shoshani L, Flores-Benitez D, Larre I 2008. Tight junction and polarity interaction in the transporting epithelial phenotype. Biochim Biophys Acta 1778:770–793 [DOI] [PubMed] [Google Scholar]
- Charpentier E, Lavker RM, Acquista E, Cowin P 2000. Plakoglobin suppresses epithelial proliferation and hair growth in vivo. J Cell Biol 149:503–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gumbiner BM 2006. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J Cell Biol 174:301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xiao L, Rao JN, Zou T, Liu L, Bellavance E, Gorospe M, Wang JY 2008. JunD represses transcription and translation of the tight junction protein zona occludens-1 modulating intestinal epithelial barrier function. Mol Biol Cell 19:3701–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Bonne S, Hatzfeld M, van Roy F, Green KJ 2002. Protein binding and functional characterization of plakophilin 2. Evidence for its diverse roles in desmosomes and β-catenin signaling. J Biol Chem 277:10512–10522 [DOI] [PubMed] [Google Scholar]
- Chen Y, Lu Q, Schneeberger EE, Goodenough DA 2000. Restoration of tight junction structure and barrier function by down-regulation of the mitogen-activated protein kinase pathway in ras-transformed Madin-Darby canine kidney cells. Mol Biol Cell 11:849–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba H, Osanai M, Murata M, Kojima T, Sawada N 2008. Transmembrane proteins of tight junctions. Biochim Biophys Acta 1778:588–600 [DOI] [PubMed] [Google Scholar]
- Chlenski A, Ketels KV, Korovaitseva GI, Talamonti MS, Oyasu R, Scarpelli DG 2000. Organization and expression of the human zo-2 gene (tjp-2) in normal and neoplastic tissues. Biochim Biophys Acta 1493:319–324 [DOI] [PubMed] [Google Scholar]
- Choi HJ, Huber AH, Weis WI 2006. Thermodynamics of β-catenin-ligand interactions: The roles of the N- and C-terminal tails in modulating binding affinity. J Biol Chem 281:1027–1038 [DOI] [PubMed] [Google Scholar]
- Choi J, Krushel LA, Crossin KL 2001. NF-kappaB activation by N-CAM and cytokines in astrocytes is regulated by multiple protein kinases and redox modulation. Glia 33:45–56 [DOI] [PubMed] [Google Scholar]
- Clevers H 2006. Wnt/β-catenin signaling in development and disease. Cell 127:469–480 [DOI] [PubMed] [Google Scholar]
- Cline EG, Nelson WJ 2007. Characterization of mammalian Par 6 as a dual-location protein. Mol Cell Biol 27:4431–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ML, Marshall CJ, Olson MF 2004. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat Rev Mol Cell Biol 5:355–366 [DOI] [PubMed] [Google Scholar]
- Comoglio PM, Boccaccio C, Trusolino L 2003. Interactions between growth factor receptors and adhesion molecules: Breaking the rules. Curr Opin Cell Biol 15:565–571 [DOI] [PubMed] [Google Scholar]
- Cooke VG, Naik MU, Naik UP 2006. Fibroblast growth factor-2 failed to induce angiogenesis in junctional adhesion molecule-A-deficient mice. Arterioscler Thromb Vasc Biol 26:2005–2011 [DOI] [PubMed] [Google Scholar]
- Coqueret O 2002. Linking cyclins to transcriptional control. Gene 299:35–55 [DOI] [PubMed] [Google Scholar]
- D'Amico M, Hulit J, Amanatullah DF, Zafonte BT, Albanese C, Bouzahzah B, Fu M, Augenlicht LH, Donehower LA, Takemaru KI, et al. 2000. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3β and cAMP-responsive element-binding protein-dependent pathways. J Biol Chem 275:32649–32657 [DOI] [PubMed] [Google Scholar]
- Dang X, Doble BW, Kardami E 2003. The carboxy-tail of connexin-43 localizes to the nucleus and inhibits cell growth. Mol Cell Biochem 242:35–38 [PubMed] [Google Scholar]
- Daniel JM 2007. Dancing in and out of the nucleus: p120(ctn) and the transcription factor Kaiso. Biochim Biophys Acta 1773:59–68 [DOI] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB 1997. Tyrosine phosphorylation and cadherin/catenin function. Bioessays 19:883–891 [DOI] [PubMed] [Google Scholar]
- Delva E, Tucker DK, Kowalczyk AP 2009. The Desmosome. Cold Spring Harb Perspect Biol 1:a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P, Williams G, Williams EJ 2000. CAMs and axonal growth: A critical evaluation of the role of calcium and the MAPK cascade. Mol Cell Neurosci 16:283–295 [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI 2005. α-catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly. Cell 123:903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek RL, Godsel LM, Chen F, Strohecker AM, Getsios S, Harmon R, Muller EJ, Caldelari R, Cryns VL, Green KJ 2007. Plakoglobin deficiency protects keratinocytes from apoptosis. J Invest Dermatol 127:792–801 [DOI] [PubMed] [Google Scholar]
- Dustin ML 2007. Cell adhesion molecules and actin cytoskeleton at immune synapses and kinapses. Curr Opin Cell Biol 19:529–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Ohno S, Vestweber D 2004. Junctional adhesion molecules (JAMs): More molecules with dual functions? J Cell Sci 117:19–29 [DOI] [PubMed] [Google Scholar]
- Eger A, Stockinger A, Park J, Langkopf E, Mikula M, Gotzmann J, Mikulits W, Beug H, Foisner R 2004. β-Catenin and TGFβ signalling cooperate to maintain a mesenchymal phenotype after FosER-induced epithelial to mesenchymal transition. Oncogene 23:2672–2680 [DOI] [PubMed] [Google Scholar]
- Eisner I, Colombo JA 2002. Detection of a novel pattern of connexin 43 immunoreactivity responsive to dehydration in rat hypothalamic magnocellular nuclei. Exp Neurol 177:321–325 [DOI] [PubMed] [Google Scholar]
- El-Bahrawy M, Talbot I, Poulsom R, Alison M 2002. Variable nuclear localization of α-catenin in colorectal carcinoma. Lab Invest 82:1167–1174 [DOI] [PubMed] [Google Scholar]
- Erez N, Zamir E, Gour BJ, Blaschuk OW, Geiger B 2004. Induction of apoptosis in cultured endothelial cells by a cadherin antagonist peptide: Involvement of fibroblast growth factor receptor-mediated signalling. Exp Cell Res 294:366–378 [DOI] [PubMed] [Google Scholar]
- Fanning AS, Little BP, Rahner C, Utepbergenov D, Walther Z, Anderson JM 2007. The unique-5 and -6 motifs of ZO-1 regulate tight junction strand localization and scaffolding properties. Mol Biol Cell 18:721–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor-Chaiken M, Hein PW, Stewart JC, Brackenbury R, Kinch MS 2003. E-cadherin binding modulates EGF receptor activation. Cell Commun Adhes 10:105–118 [PubMed] [Google Scholar]
- Ferber EC, Kajita M, Wadlow A, Tobiansky L, Niessen C, Ariga H, Daniel J, Fujita Y 2008. A role for the cleaved cytoplasmic domain of E-cadherin in the nucleus. J Biol Chem 283:12691–12700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink C, Weigel R, Hembes T, Lauke-Wettwer H, Kliesch S, Bergmann M, Brehm RH 2006. Altered expression of ZO-1 and ZO-2 in Sertoli cells and loss of blood-testis barrier integrity in testicular carcinoma in situ. Neoplasia 8:1019–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Benitez D, Ruiz-Cabrera A, Flores-Maldonado C, Shoshani L, Cereijido M, Contreras RG 2007. Control of tight junctional sealing: Role of epidermal growth factor. Am J Physiol Renal Physiol 292:F828–836 [DOI] [PubMed] [Google Scholar]
- Fu M, Wang C, Li Z, Sakamaki T, Pestell RG 2004. Minireview: Cyclin D1: Normal and abnormal functions. Endocrinology 145:5439–5447 [DOI] [PubMed] [Google Scholar]
- Furuse M 2009. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol 2:a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Tsukita S 2006. Claudins in occluding junctions of humans and flies. Trends Cell Biol 16:181–188 [DOI] [PubMed] [Google Scholar]
- Gallia GL, Johnson EM, Khalili K 2000. Purα: A multifunctional single-stranded DNA- and RNA-binding protein. Nucleic Acids Res 28:3197–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, Marian AJ 2006. Suppression of canonical Wnt/β-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest 116:2012–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D, Chidgey M 2008. Desmosome structure, composition and function. Biochim Biophys Acta 1778:572–587 [DOI] [PubMed] [Google Scholar]
- Gavert N, Ben-Ze'ev A 2007. β-Catenin signaling in biological control and cancer. J Cell Biochem 102:820–828 [DOI] [PubMed] [Google Scholar]
- Giannini A, Mazor M, Orme M, Vivanco M, Waxman J, Kypta R 2004. Nuclear export of α-catenin: Overlap between nuclear export signal sequences and the β-catenin binding site. Exp Cell Res 295:150–160 [DOI] [PubMed] [Google Scholar]
- Giehl K, Menke A 2008. Microenvironmental regulation of E-cadherin-mediated adherens junctions. Front Biosci 13:3975–3985 [DOI] [PubMed] [Google Scholar]
- Glaunsinger BA, Lee SS, Thomas M, Banks L, Javier R 2000. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene 19:5270–5280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaunsinger BA, Weiss RS, Lee SS, Javier R 2001. Link of the unique oncogenic properties of adenovirus type 9 E4-ORF1 to a select interaction with the candidate tumor suppressor protein ZO-2. EMBO J 20:5578–5586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL 2009. Gap Junctions. Cold Spring Harb Perspect Biol 1:a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin M, Kovacs EM, Thoreson MA, Reynolds AB, Yap AS 2003. Minimal mutation of the cytoplasmic tail inhibits the ability of E-cadherin to activate Rac but not phosphatidylinositol 3-kinase: Direct evidence of a role for cadherin-activated Rac signaling in adhesion and contact formation. J Biol Chem 278:20533–20539 [DOI] [PubMed] [Google Scholar]
- Gotoh K, Nonoguchi K, Higashitsuji H, Kaneko Y, Sakurai T, Sumitomo Y, Itoh K, Subjeck JR, Fujita J 2004. Apg-2 has a chaperone-like activity similar to Hsp110 and is overexpressed in hepatocellular carcinomas. FEBS Lett 560:19–24 [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM 2004. Distinct molecular forms of β-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol 167:339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Wong E, Gumbiner BM 2001. E-cadherin suppresses cellular transformation by inhibiting β-catenin signaling in an adhesion-independent manner. J Cell Biol 153:1049–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Arpin M, Fanning AS, Louvard D 1996. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell-cell contacts. Proc Natl Acad Sci 93:10779–10784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grazia Lampugnani M, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, et al. 2003. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, β-catenin, and the phosphatase DEP-1/CD148. J Cell Biol 161:793–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Simpson CL 2007. Desmosomes: New perspectives on a classic. J Invest Dermatol 127:2499–2515 [DOI] [PubMed] [Google Scholar]
- Gregory GD, Vakoc CR, Rozovskaia T, Zheng X, Patel S, Nakamura T, Canaani E, Blobel GA 2007. Mammalian ASH1L is a histone methyltransferase that occupies the transcribed region of active genes. Mol Cell Biol 27:8466–8479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot L, Citi S 2006. Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase RhoA. Mol Biol Cell 17:3569–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot L, Paschoud S, Jond L, Foglia A, Citi S 2008. Paracingulin regulates the activity of Rac1 and RhoA GTPases by recruiting Tiam1 and GEF-H1 to epithelial junctions. Mol Biol Cell 19:4442–4453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, Lowenkopf T, Apatira D 1991. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci 88:3460–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwak GY, Yoon JH, Yu SJ, Park SC, Jang JJ, Lee KB, Lee SH, Lee SM, Shin CS, Suh KS, Lee HS 2006. Anti-apoptotic N-cadherin signaling and its prognostic implication in human hepatocellular carcinomas. Oncol Rep 15:1117–1123 [PubMed] [Google Scholar]
- Hardman MJ, Liu K, Avilion AA, Merritt A, Brennan K, Garrod DR, Byrne C 2005. Desmosomal cadherin misexpression alters β-catenin stability and epidermal differentiation. Mol Cell Biol 25:969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AL 2007. Connexin channel permeability to cytoplasmic molecules. Prog Biophys Mol Biol 94:120–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harten SK, Shukla D, Barod R, Hergovich A, Balda MS, Matter K, Esteban MA, Maxwell PH 2009. Regulation of renal epithelial tight junctions by the von Hippel-Lindau tumor suppressor gene involves occludin and claudin 1 and is independent of E-cadherin. Mol Biol Cell 20:1089–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M 2005. The p120 family of cell adhesion molecules. Eur J Cell Biol 84:205–214 [DOI] [PubMed] [Google Scholar]
- Hatzfeld M 2007. Plakophilins: Multifunctional proteins or just regulators of desmosomal adhesion? Biochim Biophys Acta 1773:69–77 [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Green KJ, Sauter H 2003. Targeting of 0071 to desmosomes and adherens junctions is mediated by different protein domains. J Cell Sci 116:1219–1233 [DOI] [PubMed] [Google Scholar]
- Hay E, Laplantine E, Geoffroy V, Frain M, Kohler T, Muller R, Marie PJ 2009. N-cadherin interacts with axin and LRP5 to negatively regulate Wnt/β-catenin signaling, osteoblast function, and bone formation. Mol Cell Biol 29:953–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C 1994. Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell 79:791–803 [DOI] [PubMed] [Google Scholar]
- Herzig M, Savarese F, Novatchkova M, Semb H, Christofori G 2007. Tumor progression induced by the loss of E-cadherin independent of β-catenin/Tcf-mediated Wnt signaling. Oncogene 26:2290–2298 [DOI] [PubMed] [Google Scholar]
- Heuberger J, Birchmeier W 2009. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol 2:a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Nelson WJ, Papkoff J 1994b. Wnt-1 modulates cell-cell adhesion in mammalian cells by stabilizing β-catenin binding to the cell adhesion protein cadherin. J Cell Biol 124:729–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Nathke IS, Papkoff J, Nelson WJ 1994a. β-catenin: A common target for the regulation of cell adhesion by Wnt-1 and Src signaling pathways. Trends Biochem Sci 19:538–542 [DOI] [PubMed] [Google Scholar]
- Hofmann I, Schnolzer M, Kaufmann I, Franke WW 2002. Symplekin, a Constitutive Protein of Karyo- and Cytoplasmic Particles Involved in mRNA Biogenesis in Xenopus laevis Oocytes. Mol Biol Cell 13:1665–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthofer B, Windoffer R, Troyanovsky S, Leube RE 2007. Structure and function of desmosomes. Int Rev Cytol 264:65–163 [DOI] [PubMed] [Google Scholar]
- Hoschuetzky H, Aberle H, Kemler R 1994. β-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol 127:1375–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino M, Yoshimori T, Nakamura S 2005. Small GTPase proteins Rin and Rit Bind to PAR6 GTP-dependently and regulate cell transformation. J Biol Chem 280:22868–22874 [DOI] [PubMed] [Google Scholar]
- Hosking CR, Ulloa F, Hogan C, Ferber EC, Figueroa A, Gevaert K, Birchmeier W, Briscoe J, Fujita Y 2007. The transcriptional repressor Glis2 is a novel binding partner for pp120 catenin. Mol Biol Cell 18:1918–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howarth AG, Hughes MR, Stevenson BR 1992. Detection of the tight junction-associated protein ZO-1 in astrocytes and other nonepithelilal cell types. Am J Physiol 262:C461–C469 [DOI] [PubMed] [Google Scholar]
- Huang H, He X 2008. Wnt/β-catenin signaling: New (and old) players and new insights. Curr Opin Cell Biol 20:119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AH, Weis WI 2001. The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell 105:391–402 [DOI] [PubMed] [Google Scholar]
- Huerta M, Munoz R, Tapia R, Soto-Reyes E, Ramirez L, Recillas-Targa F, Gonzalez-Mariscal L, Lopez-Bayghen E 2007. Cyclin D1 Is Transcriptionally Down-Regulated by ZO-2 via an E Box and the Transcription Factor c-Myc. Mol Biol Cell 18:4826–4836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulpiau P, van Roy F 2009. Molecular evolution of the cadherin superfamily. Int J Biochem Cell Biol 41:349–369 [DOI] [PubMed] [Google Scholar]
- Hwang SG, Yu SS, Ryu JH, Jeon HB, Yoo YJ, Eom SH, Chun JS 2005. Regulation of β-catenin signaling and maintenance of chondrocyte differentiation by ubiquitin-independent proteasomal degradation of α-catenin. J Biol Chem 280:12758–12765 [DOI] [PubMed] [Google Scholar]
- Iden S, Collard JG 2008. Crosstalk between small GTPases and polarity proteins in cell polarization. Nat Rev Mol Cell Biol 9:846–859 [DOI] [PubMed] [Google Scholar]
- Iioka H, Doerner SK, Tamai K 2009. Kaiso is a bimodal modulator for Wnt/β-catenin signaling. FEBS Lett 583:627–632 [DOI] [PubMed] [Google Scholar]
- Ingber DE 2008. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol 97:163–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas S, Vega J, Ponce L, Gonzalez-Mariscal L 2002. Nuclear Localization of the Tight Junction Protein ZO-2 in Epithelial Cells. Exp Cell Res 274:138–148 [DOI] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S 1993. The 220-kD protein colocalizing with cadherins in nonepithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol 121:491–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A 2002. Rho GTPases in transformation and metastasis. Adv Cancer Res 84:57–80 [DOI] [PubMed] [Google Scholar]
- Jesaitis LA, Goodenough DA 1994. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and Drosophila tumor suppressor gene dlg-A. J Cell Biol 124:949–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Pecha J, Hoshino I, Ankrapp D, Xiao H 2007. TIP30 mutant derived from hepatocellular carcinoma specimens promotes growth of HepG2 cells through up-regulation of N-cadherin. Cancer Res 67:3574–3582 [DOI] [PubMed] [Google Scholar]
- Johansson A, Driessens M, Aspenstrom P 2000. The mammalian homologue of the Caenorhabditis elegans polarity protein PAR-6 is a binding partner for the Rho GTPases Cdc42 and Rac1. J Cell Sci 113:3267–3275 [DOI] [PubMed] [Google Scholar]
- Kajita M, Ikeda W, Tamaru Y, Takai Y 2007. Regulation of platelet-derived growth factor-induced Ras signaling by poliovirus receptor Necl-5 and negative growth regulator Sprouty2. Genes Cells 12:345–357 [DOI] [PubMed] [Google Scholar]
- Kakunaga S, Ikeda W, Shingai T, Fujito T, Yamada A, Minami Y, Imai T, Takai Y 2004. Enhancement of serum- and platelet-derived growth factor-induced cell proliferation by Necl-5/Tage4/poliovirus receptor/CD155 through the Ras-Raf-MEK-ERK signaling. J Biol Chem 279:36419–36425 [DOI] [PubMed] [Google Scholar]
- Kang HG, Jenabi JM, Zhang J, Keshelava N, Shimada H, May WA, Ng T, Reynolds CP, Triche TJ, Sorensen PH 2007. E-cadherin cell-cell adhesion in ewing tumor cells mediates suppression of anoikis through activation of the ErbB4 tyrosine kinase. Cancer Res 67:3094–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno M, Isa Y, Aoyama Y, Yamamoto Y, Nagai M, Ozawa M, Kitajima Y 2008. P120-catenin is a novel desmoglein 3 interacting partner: Identification of the p120-catenin association site of desmoglein 3. Exp Cell Res 314:1683–1692 [DOI] [PubMed] [Google Scholar]
- Kantak SS, Kramer RH 1998. E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. J Biol Chem 273:16953–16961 [DOI] [PubMed] [Google Scholar]
- Kanzaki N, Ogita H, Komura H, Ozaki M, Sakamoto Y, Majima T, Ijuin T, Takenawa T, Takai Y 2008. Involvement of the nectin-afadin complex in PDGF-induced cell survival. J Cell Sci 121:2008–2017 [DOI] [PubMed] [Google Scholar]
- Kausalya PJ, Phua DC, Hunziker W 2004. Association of ARVCF with zonula occludens (ZO)-1 and ZO-2: Binding to PDZ-domain proteins and cell-cell adhesion regulate plasma membrane and nuclear localization of ARVCF. Mol Biol Cell 15:5503–5515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh E, Buchert M, Tsapara A, Choquet A, Balda MS, Hollande F, Matter K 2006. Functional interaction between the ZO-1-interacting transcription factor ZONAB/DbpA and the RNA processing factor symplekin. J Cell Sci 119:5098–5105 [DOI] [PubMed] [Google Scholar]
- Kawakatsu T, Ogita H, Fukuhara T, Fukuyama T, Minami Y, Shimizu K, Takai Y 2005. Vav2 as a Rac-GDP/GTP exchange factor responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J Biol Chem 280:4940–4947 [DOI] [PubMed] [Google Scholar]
- Kelly KF, Spring CM, Otchere AA, Daniel JM 2004. NLS-dependent nuclear localization of p120ctn is necessary to relieve Kaiso-mediated transcriptional repression. J Cell Sci 117:2675–2686 [DOI] [PubMed] [Google Scholar]
- Keon BH, Schäfer S, Kuhn C, Grund C, Franke WW 1996. Symplekin, a novel type of tight junction plaque protein. J Cell Biol 134:1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Johnson KR, Wheelock MJ 2005. N-cadherin-mediated cell motility requires cis dimers. Cell Commun Adhes 12:23–39 [DOI] [PubMed] [Google Scholar]
- Kim JB, Islam S, Kim YJ, Prudoff RS, Sass KM, Wheelock MJ, Johnson KR 2000. N-Cadherin extracellular repeat 4 mediates epithelial to mesenchymal transition and increased motility. J Cell Biol 151:1193–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Park JI, Spring CM, Sater AK, Ji H, Otchere AA, Daniel JM, McCrea PD 2004. Non-canonical Wnt signals are modulated by the Kaiso transcriptional repressor and p120-catenin. Nat Cell Biol 6:1212–1220 [DOI] [PubMed] [Google Scholar]
- Kobielak A, Fuchs E 2004. α-catenin: At the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol 5:614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A, Fuchs E 2006. Links between α-catenin, NF-kappaB, and squamous cell carcinoma in skin. Proc Natl Acad Sci 103:2322–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama S, Ikeda S, Asahara T, Kishida M, Kikuchi A 1999. Axin directly interacts with plakoglobin and regulates its stability. J Biol Chem 274:27682–27688 [DOI] [PubMed] [Google Scholar]
- Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M 2003. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays 25:691–698 [DOI] [PubMed] [Google Scholar]
- Kolligs FT, Kolligs B, Hajra KM, Hu G, Tani M, Cho KR, Fearon ER 2000. γ-catenin is regulated by the APC tumor suppressor and its oncogenic activity is distinct from that of β-catenin. Genes Dev 14:1319–1331 [PMC free article] [PubMed] [Google Scholar]
- Kovacs EM, Ali RG, McCormack AJ, Yap AS 2002. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J Biol Chem 277:6708–6718 [DOI] [PubMed] [Google Scholar]
- Kowalczyk AP, Reynolds AB 2004. Protecting your tail: Regulation of cadherin degradation by p120-catenin. Curr Opin Cell Biol 16:522–527 [DOI] [PubMed] [Google Scholar]
- Kuphal F, Behrens J 2006. E-cadherin modulates Wnt-dependent transcription in colorectal cancer cells but does not alter Wnt-independent gene expression in fibroblasts. Exp Cell Res 312:457–467 [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E 2006. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol 174:593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Bellacosa A 2005. Epithelial-mesenchymal transition in development and cancer: Role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24:7443–7454 [DOI] [PubMed] [Google Scholar]
- Lee SS, Glaunsinger B, Mantovani F, Banks L, Javier RT 2000. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J Virol 74:9680–9693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi MH, Medina E, Arsanto JP, Le Bivic A 2004. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell 15:1324–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Satyamoorthy K, Herlyn M 2001. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res 61:3819–3825 [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. 2008. Wnt/β-catenin signaling controls development of the blood-brain barrier. J Cell Biol 183:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien WH, Klezovitch O, Fernandez TE, Delrow J, Vasioukhin V 2006. αE-catenin controls cerebral cortical size by regulating the hedgehog signaling pathway. Science 311:1609–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilien J, Balsamo J 2005. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of β-catenin. Curr Opin Cell Biol 17:459–465 [DOI] [PubMed] [Google Scholar]
- Lilien J, Balsamo J, Arregui C, Xu G 2002. Turn-off, drop-out: Functional state switching of cadherins. Dev Dyn 224:18–29 [DOI] [PubMed] [Google Scholar]
- Little EB, Crossin KL, Krushel LA, Edelman GM, Cunningham BA 2001. A short segment within the cytoplasmic domain of the neural cell adhesion molecule (N-CAM) is essential for N-CAM-induced NF-kappa B activity in astrocytes. Proc Natl Acad Sci 98:2238–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloberas J, Maki RA, Celada A 1995. Repression of major histocompatibility complex I-A β gene expression by dbpA and dbpB (mYB-1) proteins. Mol Cell Biol 15:5092–5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZH, Books JT, Ley TJ 2006. Cold shock domain family members YB-1 and MSY4 share essential functions during murine embryogenesis. Mol Cell Biol 26:8410–8417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macara IG, Mili S 2008. Polarity and differential inheritance–universal attributes of life? Cell 135:801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda O, Usami N, Kondo M, Takahashi M, Goto H, Shimokata K, Kusugami K, Sekido Y 2004. Plakoglobin (γ-catenin) has TCF/LEF family-dependent transcriptional activity in β-catenin-deficient cell line. Oncogene 23:964–972 [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Coukos G, Christofidou-Solomidou M, Gour BJ, Radice GL, Blaschuk O, Coutifaris C 1999. N-cadherin-mediated human granulosa cell adhesion prevents apoptosis: A role in follicular atresia and luteolysis? Am J Pathol 154:1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell KJ, Babbin BA, Nusrat A, Parkos CA 2005. Junctional adhesion molecule 1 regulates epithelial cell morphology through effects on β1 integrins and Rap1 activity. J Biol Chem 280:11665–11674 [DOI] [PubMed] [Google Scholar]
- Mandicourt G, Iden S, Ebnet K, Aurrand-Lions M, Imhof BA 2007. JAM-C regulates tight junctions and integrin-mediated cell adhesion and migration. J Biol Chem 282:1830–1837 [DOI] [PubMed] [Google Scholar]
- Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, Bodmer WF, Moyer MP, Riecken EO, Buhr HJ, et al. 1999. Target genes of β-catenin-T cell-factor/lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci 96:1603–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ED, Moriarty MA, Byrnes L, Grealy M 2009. Plakoglobin has both structural and signalling roles in zebrafish development. Dev Biol 327:83–96 [DOI] [PubMed] [Google Scholar]
- Matter K, Balda MS 2003. Signalling to and from tight junctions. Nat Rev Mol Cell Biol 4:225–236 [DOI] [PubMed] [Google Scholar]
- Matter K, Balda MS 2007. Epithelial tight junctions, gene expression and nucleo-junctional interplay. J Cell Sci 120:1505–1511 [DOI] [PubMed] [Google Scholar]
- McCrea PD, Park JI 2007. Developmental functions of the P120-catenin sub-family. Biochim Biophys Acta 1773:17–33 [DOI] [PubMed] [Google Scholar]
- McLachlan RW, Yap AS 2007. Not so simple: The complexity of phosphotyrosine signaling at cadherin adhesive contacts. J Mol Med 85:545–554 [DOI] [PubMed] [Google Scholar]
- McNeil E, Capaldo CT, Macara IG 2006. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell 17:1922–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H 2009. Planar cell polarity: keeping hairs straight is not so simple. Cold Spring Harb Perspect Biol 2:a003376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina A, Swain RK, Kuerner KM, Steinbeisser H 2004. Xenopus paraxial protocadherin has signaling functions and is involved in tissue separation. Embo J 23:3249–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Takeichi M 2009. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol 1:a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdek KD, Nguyen NT, Toksoz D 2004. Distinct activities of the α-catenin family, α-catulin and α-catenin, on β-catenin-mediated signaling. Mol Cell Biol 24:2410–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam JM, Rubenstein AB, Klymkowsky MW 1997. Cytoplasmically anchored plakoglobin induces a WNT-like phenotype in Xenopus. Dev Biol 185:67–81 [DOI] [PubMed] [Google Scholar]
- Mertens C, Kuhn C, Franke WW 1996. Plakophilins 2a and 2b: Constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol 135:1009–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens C, Hofmann I, Wang Z, Teichmann M, Sepehri Chong S, Schnolzer M, Franke WW 2001. Nuclear particles containing RNA polymerase III complexes associated with the junctional plaque protein plakophilin 2. Proc Natl Acad Sci 98:7795–7800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mese G, Richard G, White TW 2007. Gap junctions: Basic structure and function. J Invest Dermatol 127:2516–2524 [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R 1994. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: Involvement of type I receptors. J Cell Biol 127:2021–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y, Ikeda W, Kajita M, Fujito T, Amano H, Tamaru Y, Kuramitsu K, Sakamoto Y, Monden M, Takai Y 2007. Necl-5/poliovirus receptor interacts in cis with integrin αVβ3 and regulates its clustering and focal complex formation. J Biol Chem 282:18481–18496 [DOI] [PubMed] [Google Scholar]
- Mizoguchi A, Nakanishi H, Kimura K, Matsubara K, Ozaki-Kuroda K, Katata T, Honda T, Kiyohara Y, Heo K, Higashi M, et al. 2002. Nectin: An adhesion molecule involved in formation of synapses. J Cell Biol 156:555–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Yagi T 2007. Protocadherin family: Diversity, structure, and function. Curr Opin Cell Biol 19:584–592 [DOI] [PubMed] [Google Scholar]
- Mosimann C, Hausmann G, Basler K 2009. β-catenin hits chromatin: Regulation of Wnt target gene activation. Nat Rev Mol Cell Biol 10:276–286 [DOI] [PubMed] [Google Scholar]
- Muller SL, Portwich M, Schmidt A, Utepbergenov DI, Huber O, Blasig IE, Krause G 2005. The tight junction protein occludin and the adherens junction protein α-catenin share a common interaction mechanism with ZO-1. J Biol Chem 280:3747–3756 [DOI] [PubMed] [Google Scholar]
- Muller J, Ritt DA, Copeland TD, Morrison DK 2003. Functional analysis of C-TAK1 substrate binding and identification of PKP2 as a new C-TAK1 substrate. Embo J 22:4431–4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik MU, Naik UP 2006. Junctional adhesion molecule-A-induced endothelial cell migration on vitronectin is integrin α v β 3 specific. J Cell Sci 119:490–499 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Blechman J, Tada S, Rozovskaia T, Itoyama T, Bullrich F, Mazo A, Croce CM, Geiger B, Canaani E 2000. huASH1 protein, a putative transcription factor encoded by a human homologue of the Drosophila ash1 gene, localizes to both nuclei and cell-cell tight junctions. Proc Natl Acad Sci 97:7284–7289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamuta S, Endo H, Higashi Y, Kousaka A, Yamada H, Yano M, Kido H 2008. Human immunodeficiency virus type 1 gp120-mediated disruption of tight junction proteins by induction of proteasome-mediated degradation of zonula occludens-1 and -2 in human brain microvascular endothelial cells. J Neurovirol 14:186–195 [DOI] [PubMed] [Google Scholar]
- Nelson WJ 2008. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans 36:149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R 2004. Convergence of Wnt, β-catenin, and cadherin pathways. Science 303:1483–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ 1999. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol 147:631–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren NK, Niessen CM, Gumbiner BM, Burridge K 2001. Cadherin engagement regulates Rho family GTPases. J Biol Chem 276:33305–33308 [DOI] [PubMed] [Google Scholar]
- Ochiai A, Akimoto S, Kanai Y, Shibata T, Oyama T, Hirohashi S 1994. c-erbB-2 gene product associates with catenins in human cancer cells. Biochem Biophys Res Commun 205:73–78 [DOI] [PubMed] [Google Scholar]
- Ogita H, Takai Y 2006. Activation of Rap1, Cdc42, and rac by nectin adhesion system. Methods Enzymol 406:415–424 [DOI] [PubMed] [Google Scholar]
- Ogita H, Takai Y 2008. Cross-talk among integrin, cadherin, and growth factor receptor: Roles of nectin and nectin-like molecule. Int Rev Cytol 265:1–54 [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Nakahara T, Furuse K, Sasaki H, Tsukita S, Furuse M 2004. JACOP, a novel plaque protein localizing at the apical junctional complex with sequence similarity to cingulin. J Biol Chem 279:46014–46022 [DOI] [PubMed] [Google Scholar]
- Ojakian GK, Ratcliffe DR, Schwimmer R 2001. Integrin regulation of cell-cell adhesion during epithelial tubule formation. J Cell Sci 114:941–952 [DOI] [PubMed] [Google Scholar]
- Ooshio T, Fujita N, Yamada A, Sato T, Kitagawa Y, Okamoto R, Nakata S, Miki A, Irie K, Takai Y 2007. Cooperative roles of Par-3 and afadin in the formation of adherens and tight junctions. J Cell Sci 120:2352–2365 [DOI] [PubMed] [Google Scholar]
- Orban E, Szabo E, Lotz G, Kupcsulik P, Paska C, Schaff Z, Kiss A 2008. Different expression of occludin and ZO-1 in primary and metastatic liver tumors. Pathol Oncol Res 14:299–306 [DOI] [PubMed] [Google Scholar]
- Orsulic S, Huber O, Aberle H, Arnold S, Kemler R 1999. E-cadherin binding prevents β-catenin nuclear localization and β-catenin/LEF-1-mediated transactivation. J Cell Sci 112:1237–1245 [DOI] [PubMed] [Google Scholar]
- Osler ME, Chang MS, Bader DM 2005. Bves modulates epithelial integrity through an interaction at the tight junction. J Cell Sci 118:4667–4678 [DOI] [PubMed] [Google Scholar]
- Ozaki M, Ogita H, Takai Y 2007. Involvement of integrin-induced activation of protein kinase C in the formation of adherens junctions. Genes Cells 12:651–662 [DOI] [PubMed] [Google Scholar]
- Paris L, Tonutti L, Vannini C, Bazzoni G 2008. Structural organization of the tight junctions. Biochim Biophys Acta 1778:646–659 [DOI] [PubMed] [Google Scholar]
- Park JI, Ji H, Jun S, Gu D, Hikasa H, Li L, Sokol SY, McCrea PD 2006. Frodo links Dishevelled to the p120-catenin/Kaiso pathway: Distinct catenin subfamilies promote Wnt signals. Dev Cell 11:683–695 [DOI] [PubMed] [Google Scholar]
- Park JI, Kim SW, Lyons JP, Ji H, Nguyen TT, Cho K, Barton MC, Deroo T, Vleminckx K, Moon RT, et al. 2005. Kaiso/p120-catenin and TCF/β-catenin complexes coordinately regulate canonical Wnt gene targets. Dev Cell 8:843–854 [DOI] [PubMed] [Google Scholar]
- Paschoud S, Bongiovanni M, Pache JC, Citi S 2007. Claudin-1 and claudin-5 expression patterns differentiate lung squamous cell carcinomas from adenocarcinomas. Mod Pathol 20:947–954 [DOI] [PubMed] [Google Scholar]
- Patel SD, Chen CP, Bahna F, Honig B, Shapiro L 2003. Cadherin-mediated cell-cell adhesion: Sticking together as a family. Curr Opin Struct Biol 13:690–698 [DOI] [PubMed] [Google Scholar]
- Pece S, Gutkind JS 2000. Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem 275:41227–41233 [DOI] [PubMed] [Google Scholar]
- Peinado H, Olmeda D, Cano A 2007. Snail, Zeb and bHLH factors in tumour progression: An alliance against the epithelial phenotype? Nat Rev Cancer 7:415–428 [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Trolice MP 1996. N-cadherin-mediated cell contact inhibits granulosa cell apoptosis in a progesterone-independent manner. Endocrinology 137:1196–1203 [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E 2006. Catenins: keeping cells from getting their signals crossed. Dev Cell 11:601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Jamora C, Fuchs E 2003. Sticky business: Orchestrating cellular signals at adherens junctions. Cell 112:535–548 [DOI] [PubMed] [Google Scholar]
- Perrais M, Chen X, Perez-Moreno M, Gumbiner BM 2007. E-cadherin homophilic ligation inhibits cell growth and epidermal growth factor receptor signaling independently of other cell interactions. Mol Biol Cell 18:2013–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra J, Miravet S, Castano J, Palmer HG, Heisterkamp N, Garcia de Herreros A, Dunach M 2003. p120 Catenin-associated Fer and Fyn tyrosine kinases regulate β-catenin Tyr-142 phosphorylation and β-catenin-α-catenin Interaction. Mol Cell Biol 23:2287–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Weis WI 2000. Structure of the dimerization and β-catenin-binding region of α-catenin. Mol Cell 5:533–543 [DOI] [PubMed] [Google Scholar]
- Pokutta S, Drees F, Yamada S, Nelson WJ, Weis WI 2008. Biochemical and structural analysis of α-catenin in cell-cell contacts. Biochem Soc Trans 36:141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter MD, Barbero S, Cheresh DA 2005. Tyrosine phosphorylation of VE-cadherin prevents binding of pp120- and β-catenin and maintains the cellular mesenchymal state. J Biol Chem 280:31906–31912 [DOI] [PubMed] [Google Scholar]
- Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR 2004. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. Embo J 23:1739–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Capaldo C, Gumbiner BM, Macara IG 2005. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol 171:1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert M, Muller T, Hunziker W 2000. The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells. Evidence for a role of β-catenin/Tcf/Lef signaling. J Biol Chem 275:9492–9500 [DOI] [PubMed] [Google Scholar]
- Reynolds AB 2007. 120-catenin: Past and present. Biochim Biophys Acta 1773:2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Sinovas A, Cabestrero A, Lopez D, Torre I, Morente M, Abellan A, Miro E, Ruiz-Meana M, Garcia-Dorado D 2007. The modulatory effects of connexin 43 on cell death/survival beyond cell coupling. Prog Biophys Mol Biol 94:219–232 [DOI] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M 1999. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem 274:36734–36740 [DOI] [PubMed] [Google Scholar]
- Ruzov A, Hackett JA, Prokhortchouk A, Reddington JP, Madej MJ, Dunican DS, Prokhortchouk E, Pennings S, Meehan RR 2009a. The interaction of xKaiso with xTcf3: A revised model for integration of epigenetic and Wnt signalling pathways. Development 136:723–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzov A, Savitskaya E, Hackett JA, Reddington JP, Prokhortchouk A, Madej MJ, Chekanov N, Li M, Dunican DS, Prokhortchouk E, et al. 2009b. The non-methylated DNA-binding function of Kaiso is not required in early Xenopus laevis development. Development 136:729–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ 2002. RHO-GTPases and cancer. Nat Rev Cancer 2:133–142 [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Ogita H, Hirota T, Kawakatsu T, Fukuyama T, Yasumi M, Kanzaki N, Ozaki M, Takai Y 2006. Interaction of integrin α(v)β3 with nectin. Implication in cross-talk between cell-matrix and cell-cell junctions. J Biol Chem 281:19631–19644 [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Ogita H, Komura H, Takai Y 2008. Involvement of nectin in inactivation of integrin α(v)β(3) after the establishment of cell-cell adhesion. J Biol Chem 283:496–505 [DOI] [PubMed] [Google Scholar]
- Sakisaka T, Ikeda W, Ogita H, Fujita N, Takai Y 2007. The roles of nectins in cell adhesions: Cooperation with other cell adhesion molecules and growth factor receptors. Curr Opin Cell Biol 19:593–602 [DOI] [PubMed] [Google Scholar]
- Sakura H, Maekawa T, Imamoto F, Yasuda K, Ishii S 1988. Two human genes isolated by a novel method encode DNA-binding proteins containing a common region of homology. Gene 73:499–507 [DOI] [PubMed] [Google Scholar]
- Sallee JL, Wittchen ES, Burridge K 2006. Regulation of cell adhesion by protein-tyrosine phosphatases: II. Cell-cell adhesion. J Biol Chem 281:16189–16192 [DOI] [PubMed] [Google Scholar]
- Sanchez-Heras E, Howell FV, Williams G, Doherty P 2006. The fibroblast growth factor receptor acid box is essential for interactions with N-cadherin and all of the major isoforms of neural cell adhesion molecule. J Biol Chem 281:35208–35216 [DOI] [PubMed] [Google Scholar]
- Sanson B, White P, Vincent JP 1996. Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature 383:627–630 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Langbein L, Rode M, Pratzel S, Zimbelmann R, Franke WW 1997. Plakophilins 1a and 1b: Widespread nuclear proteins recruited in specific epithelial cells as desmosomal plaque components. Cell Tissue Res 290:481–499 [DOI] [PubMed] [Google Scholar]
- Shariatmadari M, Peyronnet J, Papachristou P, Horn Z, Sousa KM, Arenas E, Ringstedt T 2005. Increased Wnt levels in the neural tube impair the function of adherens junctions during neurulation. Mol Cell Neurosci 30:437–451 [DOI] [PubMed] [Google Scholar]
- Sheth B, Nowak RL, Anderson R, Kwong WY, Papenbrock T, Fleming TP 2008. Tight junction protein ZO-2 expression and relative function of ZO-1 and ZO-2 during mouse blastocyst formation. Exp Cell Res 314:3356–3368 [DOI] [PubMed] [Google Scholar]
- Shimizu M, Fukunaga Y, Ikenouchi J, Nagafuchi A 2008. Defining the roles of β-catenin and plakoglobin in LEF/T-cell factor-dependent transcription using β-catenin/plakoglobin-null F9 cells. Mol Cell Biol 28:825–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Oren M, Levina E, Ben-Ze'ev A 2002. PML is a target gene of β-catenin and plakoglobin, and coactivates β-catenin-mediated transcription. Cancer Res 62:5947–5954 [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A 1999. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc Natl Acad Sci 96:5522–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AB, Sugimoto K, Dhawan P, Harris RC 2007. Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am J Physiol Cell Physiol 293:C1660–1668 [DOI] [PubMed] [Google Scholar]
- Skaper SD, Moore SE, Walsh FS 2001. Cell signalling cascades regulating neuronal growth-promoting and inhibitory cues. Prog Neurobiol 65:593–608 [DOI] [PubMed] [Google Scholar]
- Sourisseau T, Georgiadis A, Tsapara A, Ali RR, Pestell R, Matter K, Balda MS 2006. Regulation of PCNA and cyclin D1 expression and epithelial morphogenesis by the ZO-1-regulated transcription factor ZONAB/DbpA. Mol Cell Biol 26:2387–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring CM, Kelly KF, O'Kelly I, Graham M, Crawford HC, Daniel JM 2005. The catenin p120ctn inhibits Kaiso-mediated transcriptional repression of the β-catenin/TCF target gene matrilysin. Exp Cell Res 305:253–265 [DOI] [PubMed] [Google Scholar]
- St Croix B, Kerbel RS 1997. Cell adhesion and drug resistance in cancer. Curr Opin Oncol 9:549–556 [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA 1986. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103:755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub BK, Boda J, Kuhn C, Schnoelzer M, Korf U, Kempf T, Spring H, Hatzfeld M, Franke WW 2003. A novel cell-cell junction system: The cortex adhaerens mosaic of lens fiber cells. J Cell Sci 116:4985–4995 [DOI] [PubMed] [Google Scholar]
- Suh EK, Gumbiner BM 2003. Translocation of β-catenin into the nucleus independent of interactions with FG-rich nucleoporins. Exp Cell Res 290:447–456 [DOI] [PubMed] [Google Scholar]
- Suyama K, Shapiro I, Guttman M, Hazan RB 2002. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2:301–314 [DOI] [PubMed] [Google Scholar]
- Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, Potente M, Daly C, Dimmeler S, Dejana E 2008. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol 10:923–934 [DOI] [PubMed] [Google Scholar]
- Takagaki Y, Manley JL 2000. Complex protein interactions within the human polyadenylation machinery identify a novel component. Mol Cell Biol 20:1515–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Ikeda W, Ogita H, Rikitake Y 2008a. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol 24:309–342 [DOI] [PubMed] [Google Scholar]
- Takai Y, Miyoshi J, Ikeda W, Ogita H 2008b. Nectins and nectin-like molecules: Roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol 9:603–615 [DOI] [PubMed] [Google Scholar]
- Takai Y, Nakanishi H 2003. Nectin and afadin: Novel organizers of intercellular junctions. J Cell Sci 116:17–27 [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nakayama Y, Taniguchi M, Kioussis D 2008. Regulation of early T cell development by the PHD finger of histone lysine methyltransferase ASH1. Biochem Biophys Res Commun 365:589–594 [DOI] [PubMed] [Google Scholar]
- Tapia R, Huerta M, Islas S, Avila-Flores A, Lopez-Bayghen E, Weiske J, Huber O, Gonzalez-Mariscal L 2009. Zona occludens-2 inhibits cyclin D1 expression and cell proliferation and exhibits changes in localization along the cell cycle. Mol Biol Cell 20:1102–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F 1999. β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422–426 [DOI] [PubMed] [Google Scholar]
- Theisen CS, Wahl JK 3rd, Johnson KR, Wheelock MJ 2007. NHERF links the N-cadherin/catenin complex to the platelet-derived growth factor receptor to modulate the actin cytoskeleton and regulate cell motility. Mol Biol Cell 18:1220–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian YC, Phillips AO 2002. Interaction between the transforming growth factor-β type II receptor/Smad pathway and β-catenin during transforming growth factor-β1-mediated adherens junction disassembly. Am J Pathol 160:1619–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff SK, Buznikov AG, Vu TQ, Micevych PE, Chen K, Kornblum HI, Bronstein JM 2001. OSP/claudin-11 forms a complex with a novel member of the tetraspanin super family and β1 integrin and regulates proliferation and migration of oligodendrocytes. J Cell Biol 153:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi H, Miyoshi J, Honda T, Sakisaka T, Takai Y, Takeichi M 2006. Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J Cell Biol 174:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traweger A, Fuchs R, Krizbai IA, Weiger TM, Bauer HC, Bauer H 2003. The Tight Junction Protein ZO-2 Localizes to the Nucleus and Interacts with the Heterogeneous Nuclear Ribonucleoprotein Scaffold Attachment Factor-B. J Biol Chem 278:2692–2700 [DOI] [PubMed] [Google Scholar]
- Traweger A, Lehner C, Farkas A, Krizbai IA, Tempfer H, Klement E, Guenther B, Bauer HC, Bauer H 2008. Nuclear Zonula occludens-2 alters gene expression and junctional stability in epithelial and endothelial cells. Differentiation 76:99–106 [DOI] [PubMed] [Google Scholar]
- Troyanovsky S 2005. Cadherin dimers in cell-cell adhesion. Eur J Cell Biol 84:225–233 [DOI] [PubMed] [Google Scholar]
- Troyanovsky RB, Laur O, Troyanovsky SM 2007. Stable and unstable cadherin dimers: Mechanisms of formation and roles in cell adhesion. Mol Biol Cell 18:4343–4352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapara A, Matter K, Balda MS 2006. The heat-shock protein Apg-2 binds to the tight junction protein ZO-1 and regulates transcriptional activity of ZONAB. Mol Biol Cell 17:1322–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M 2001. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2:286–293 [DOI] [PubMed] [Google Scholar]
- Tsukita S, Itoh M, Nagafuchi A, Yonemura S, Tsukita S 1993. Submembranous junctional plaque proteins include potential tumor suppressor molecules. J Cell Biol 123:1049–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP 2005. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev Cell 9:555–564 [DOI] [PubMed] [Google Scholar]
- Umeda K, Matsui T, Nakayama M, Furuse K, Sasaki H, Furuse M, Tsukita S 2004. Establishment and characterization of cultured epithelial cells lacking expression of ZO-1. J Biol Chem 279:44785–44794 [DOI] [PubMed] [Google Scholar]
- Unterseher F, Hefele JA, Giehl K, De Robertis EM, Wedlich D, Schambony A 2004. Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK. Embo J 23:3259–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utton MA, Eickholt B, Howell FV, Wallis J, Doherty P 2001. Soluble N-cadherin stimulates fibroblast growth factor receptor dependent neurite outgrowth and N-cadherin and the fibroblast growth factor receptor co-cluster in cells. J Neurochem 76:1421–1430 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Barker N, Harkes IC, van der Heyden M, Dijk NJ, Hollestelle A, Klijn JG, Clevers H, Schutte M 2001. Mutant E-cadherin breast cancer cells do not display constitutive Wnt signaling. Cancer Res 61:278–284 [PubMed] [Google Scholar]
- Van Itallie CM, Balda MS, Anderson JM 1995. Epidermal growth factor induces tyrosine phosphorylation and reorganization of the tight junction protein ZO-1 in A431 cells. J Cell Sci 108:1735–1742 [DOI] [PubMed] [Google Scholar]
- van Roy FM, McCrea PD 2005. A role for Kaiso-p120ctn complexes in cancer? Nat Rev Cancer 5:956–964 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E 2001. Hyperproliferation and defects in epithelial polarity upon conditional ablation of α-catenin in skin. Cell 104:605–617 [DOI] [PubMed] [Google Scholar]
- Venkov CD, Link AJ, Jennings JL, Plieth D, Inoue T, Nagai K, Xu C, Dimitrova YN, Rauscher FJ, Neilson EG 2007. A proximal activator of transcription in epithelial-mesenchymal transition. J Clin Invest 117:482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vethantham V, Rao N, Manley JL 2007. Sumoylation modulates the assembly and activity of the pre-mRNA 3′ processing complex. Mol Cell Biol 27:8848–8858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalonga P, Ridley AJ 2006. Rho GTPases and cell cycle control. Growth Factors 24:159–164 [DOI] [PubMed] [Google Scholar]
- Wang Q, Margolis B 2007. Apical junctional complexes and cell polarity. Kidney Int 72:1448–1458 [DOI] [PubMed] [Google Scholar]
- Wang Y, Du D, Fang L, Yang G, Zhang C, Zeng R, Ullrich A, Lottspeich F, Chen Z 2006. Tyrosine phosphorylated Par3 regulates epithelial tight junction assembly promoted by EGFR signaling. Embo J 25:5058–5070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Janicki P, Koster I, Berger CD, Wenzl C, Grosshans J, Steinbeisser H 2008. Xenopus Paraxial Protocadherin regulates morphogenesis by antagonizing Sprouty. Genes Dev 22:878–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Sato K, Kaibuchi K 2009. Cadherin-mediated intercellular adhesions and signaling cascades involving in small GTPases. Cold Spring Harb Perspect Biol 1:a003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis WI, Nelson WJ 2006. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem 281:35593–35597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR 2003. Cadherin-mediated cellular signaling. Curr Opin Cell Biol 15:509–514 [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR 2008. Cadherin switching. J Cell Sci 121:727–735 [DOI] [PubMed] [Google Scholar]
- Williams G, Williams EJ, Doherty P 2002. Dimeric versions of two short N-cadherin binding motifs (HAVDI and INPISG) function as N-cadherin agonists. J Biol Chem 277:4361–4367 [DOI] [PubMed] [Google Scholar]
- Williams BO, Barish GD, Klymkowsky MW, Varmus HE 2000. A comparative evaluation of β-catenin and plakoglobin signaling activity. Oncogene 19:5720–5728 [DOI] [PubMed] [Google Scholar]
- Williams EJ, Furness J, Walsh FS, Doherty P 1994. Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron 13:583–594 [DOI] [PubMed] [Google Scholar]
- Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM 1993. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci 90:7834–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Stewart DB, Nelson WJ, Nusse R 2006. Wingless signaling modulates cadherin-mediated cell adhesion in Drosophila imaginal disc cells. J Cell Sci 119:2425–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A, Keil R, Gotzl O, Mun A, Schwarze K, Lederer M, Huttelmaier S, Hatzfeld M 2006. The armadillo protein p0071 regulates Rho signalling during cytokinesis. Nat Cell Biol 8:1432–1440 [DOI] [PubMed] [Google Scholar]
- Wong RC, Pera MF, Pebay A 2008. Role of gap junctions in embryonic and somatic stem cells. Stem Cell Rev 4:283–292 [DOI] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ 1993. ZO-1, DlgA and PSD-95/SAP90: Homologous proteins in tight, septate and synaptic junctions. Mech Dev 44:85–89 [DOI] [PubMed] [Google Scholar]
- Wuchter P, Boda-Heggemann J, Straub BK, Grund C, Kuhn C, Krause U, Seckinger A, Peitsch WK, Spring H, Ho AD, Franke WW 2007. Processus and recessus adhaerentes: Giant adherens cell junction systems connect and attract human mesenchymal stem cells. Cell Tissue Res 328:499–514 [DOI] [PubMed] [Google Scholar]
- Xiao K, Oas RG, Chiasson CM, Kowalczyk AP 2007. Role of p120-catenin in cadherin trafficking. Biochim Biophys Acta 1773:8–16 [DOI] [PubMed] [Google Scholar]
- Xing H, Mayhew CN, Cullen KE, Park-Sarge OK, Sarge KD 2004. HSF1 modulation of Hsp70 mRNA polyadenylation via interaction with symplekin. J Biol Chem 279:10551–10555 [DOI] [PubMed] [Google Scholar]
- Xu J, Kausalya PJ, Phua DC, Ali SM, Hossain Z, Hunziker W 2008. Early embryonic lethality of mice lacking ZO-2, but Not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol Cell Biol 28:1669–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Kimelman D 2007. Mechanistic insights from structural studies of β-catenin and its binding partners. J Cell Sci 120:3337–3344 [DOI] [PubMed] [Google Scholar]
- Yamada S, Nelson WJ 2007. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol 178:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ 2005. Deconstructing the cadherin-catenin-actin complex. Cell 123:889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa S, Lee JS, Haruna T, Oda H, Uemura T, Takeichi M, Ishimoto A 1997. Accumulation of Armadillo induced by Wingless, Dishevelled, and dominant-negative Zeste-White 3 leads to elevated DE-cadherin in Drosophila clone 8 wing disc cells. J Biol Chem 272:25243–25251 [DOI] [PubMed] [Google Scholar]
- Yang X, Chen MW, Terry S, Vacherot F, Chopin DK, Bemis DL, Kitajewski J, Benson MC, Guo Y, Buttyan R 2005. A human- and male-specific protocadherin that acts through the wnt signaling pathway to induce neuroendocrine transdifferentiation of prostate cancer cells. Cancer Res 65:5263–5271 [DOI] [PubMed] [Google Scholar]
- Yap AS, Kovacs EM 2003. Direct cadherin-activated cell signaling: A view from the plasma membrane. J Cell Biol 160:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM 1997. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol 13:119–146 [DOI] [PubMed] [Google Scholar]
- Yap AS, Crampton MS, Hardin J 2007. Making and breaking contacts: The cellular biology of cadherin regulation. Curr Opin Cell Biol 19:508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Niessen CM, Gumbiner BM 1998. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol 141:779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasen M, Kajino K, Kano S, Tobita H, Yamamoto J, Uchiumi T, Kon S, Maeda M, Obulhasim G, Arii S, et al. 2005. The up-regulation of Y-box binding proteins (DNA binding protein A and Y-box binding protein-1) as prognostic markers of hepatocellular carcinoma. Clin Cancer Res 11:7354–7361 [DOI] [PubMed] [Google Scholar]
- Yin T, Getsios S, Caldelari R, Kowalczyk AP, Muller EJ, Jones JC, Green KJ 2005. Plakoglobin suppresses keratinocyte motility through both cell-cell adhesion-dependent and -independent mechanisms. Proc Natl Acad Sci 102:5420–5425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Macara IG 2008. The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev Cell 14:216–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Lei QY, Guan KL 2008. The Hippo-YAP pathway: New connections between regulation of organ size and cancer. Curr Opin Cell Biol 20:638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Pestell R, Guan JL 2001. Transcriptional activation of cyclin D1 promoter by FAK contributes to cell cycle progression. Mol Biol Cell 12:4066–4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bian ZC, Yee K, Chen BP, Chien S, Guan JL 2003. Identification of transcription factor KLF8 as a downstream target of focal adhesion kinase in its regulation of cyclin D1 and cell cycle progression. Mol Cell 11:1503–1515 [DOI] [PubMed] [Google Scholar]
- Zhurinsky J, Shtutman M, Ben-Ze'ev A 2000. Plakoglobin and β-catenin: Protein interactions, regulation and biological roles. J Cell Sci 113:3127–3139 [DOI] [PubMed] [Google Scholar]