Abstract
Epithelia form physical barriers that separate the internal milieu of the body from its external environment. The biogenesis of functional epithelia requires the precise coordination of many cellular processes. One of the key events in epithelial biogenesis is the establishment of cadherin-dependent cell–cell contacts, which initiate morphological changes and the formation of other adhesive structures. Cadherin-mediated adhesions generate intracellular signals that control cytoskeletal reorganization, polarity, and vesicle trafficking. Among such signaling pathways, those involving small GTPases play critical roles in epithelial biogenesis. Assembly of E-cadherin activates several small GTPases and, in turn, the activated small GTPases control the effects of E-cadherin-mediated adhesions on epithelial biogenesis. Here, we focus on small GTPase signaling at E-cadherin-mediated epithelial junctions.
Small GTPases play essential roles in formation of epithelia, activating feedback mechanisms that regulate the strength of cadherin-based cell contacts.
Cell–cell adhesions are involved in a diverse range of physiological processes, including morphological changes during tissue development, cell scattering, wound healing, and synaptogenesis (Adams and Nelson 1998; Gumbiner 2000; Halbleib and Nelson 2006; Takeichi 1995; Tepass et al. 2000). In epithelial cells, cell–cell adhesions are classified into three kinds of adhesions: adherens junction, tight junction, and desmosome (for more details, see Meng and Takeichi 2009, Furuse 2009, and Delva et al. 2009, respectively). A key event in epithelial polarization and biogenesis is the establishment of cadherin-dependent cell–cell contacts. Cadherins belong to a large family of adhesion molecules that require Ca2+ for their homophilic interactions (Adams and Nelson 1998; Blanpain and Fuchs 2009; Gumbiner 2000; Hartsock and Nelson 2008; Takeichi 1995; Tepass et al. 2000). Cadherins form transinteraction on the surface of neighboring cells (for details, see Shapiro and Weis 2009). For the development of strong and rigid adhesions, cadherins are clustered concomitantly with changes in the organization of the actin cytoskeleton (Tsukita et al. 1992). Classical cadherins are required, but not sufficient, to initiate cell–cell contacts, and other adhesion protein complexes subsequently assemble (for details, see Green et al. 2009). These complexes include the tight junction, which controls paracellular permeability, and desmosomes, which support the structural continuum of epithelial cells. A fundamental problem is to understand how these diverse cellular processes are regulated and coordinated. Intracellular signals, generated when cells attach with one another, mediate these complicated processes.
Several signaling pathways upstream or downstream of cadherin-mediated cell–cell adhesions have been identified (Perez-Moreno et al. 2003) (see also McCrea et al. 2009). Among these pathways, small GTPases including the Rho and Ras family GTPases play critical roles in epithelial biogenesis and have been studied extensively. Many key morphological and functional changes are induced when these small GTPases act at epithelial junctions, where they mediate an interplay between cell–cell adhesion molecules and fundamental cellular processes including cytoskeletal activity, polarity, and vesicle trafficking. In addition to these small GTPases, Ca2+ signaling and phosphorylation of cadherin complexes also play pivotal roles in the formation and maintenance of cadherin-mediated adhesions. Here, we focus on signaling pathways involving the small GTPases in E-cadherin-mediated cell–cell adhesions. Other signaling pathways are described in recent reviews (Braga 2002; Fukata and Kaibuchi 2001; Goldstein and Macara 2007; McLachlan et al. 2007; Tsukita et al. 2008; Yap and Kovacs 2003; see also McCrea et al. 2009).
MODE OF ACTION OF THE SMALL GTPases
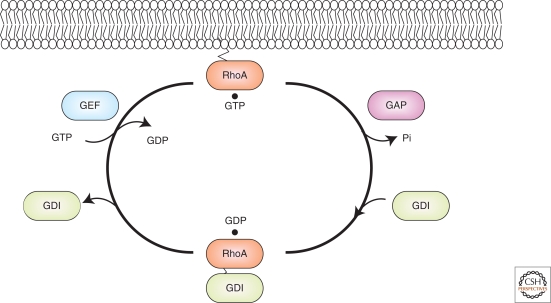
Small GTPases have GDP/GTP binding and GTPase activity. They cycle between a GTP-bound active state and a GDP-bound inactive state, thus functioning as molecular switches in cells (Fig. 1). The nucleotide state of the small GTPases is generally controlled by three classes of key regulators: Guanine nucleotide exchange factors (GEFs), which promote the exchange of GDP for GTP; GDP dissociation inhibitors (GDIs), which interact with GDP-bound small GTPases, inhibit the exchange of GDP for GTP, and sequester the small GTPases into the cytosol (note that a GDI for the Ras family has not been identified); and GTPase-activating proteins (GAPs), which enhance the intrinsic GTPase activity of small GTPases. These regulators ensure that activation and inactivation of small GTPases is tightly regulated both spatially and temporally in order to generate specific and localized effects (Gulli and Peter 2001; Jaffe and Hall 2005; Kaibuchi et al. 1999; Van Aelst and D’souza-Schorey 1997). The modes of action of small GTPases have been elucidated by the identification and characterization of specific effectors. Such effector molecules interact with small GTPases only in their GTP-bound state to transmit signals downstream and exert physiological functions (Gulli and Peter 2001; Jaffe and Hall 2005; Kaibuchi et al. 1999; Van Aelst and D’souza-Schorey 1997).
Figure 1.
Regulation of the small GTPases. In this figure, RhoA is depicted. In resting cells, Rho exists mostly in the GDP-bound form (GDP · Rho) and in complexes with Rho GDIs in the cytosol. On stimulation with extracellular signals, Rho is likely to be dissociated from Rho GDIs and targeted to specific membranes by its carboxy-terminal prenyl group. At the membrane, specific GEFs for Rho are activated: GDP · Rho is then converted to GTP · Rho. GTP · Rho interacts with its specific effectors and exerts its functions. GAPs enhance the GTPase activity of Rho and reconvert Rho to its inactive GDP-bound form. Rho GDI can then form a complex with GDP · Rho and extract it from the membrane back into the cytosol.
The Rho family GTPases are believed to shuttle between the cytosol and specific membrane sites after extracellular stimulation (Fleming et al. 1996; Kranenburg et al. 1997). In epithelial cells, Rac1 and Cdc42, which are members of the Rho family GTPases, localize to sites of cell–cell junctions (Jou and Nelson 1998; Kuroda et al. 1998; Nakagawa et al. 2001; Takaishi et al. 1997). In Madin-Darby canine kidney II (MDCKII) cells, Rac1 is colocalized with E-cadherin at sites of cell–cell contact and is translocated to the cytosol during disruption of cell–cell adhesion by Ca2+ chelation with EGTA (Nakagawa et al. 2001). Furthermore, studies using green fluorescent protein (GFP) show that an anti-E-cadherin antibody or the functional extracellular domain of E-cadherin fused to Fc region can recruit Rac1-GFP immediately at contact sites (Kovacs et al. 2002; Nakagawa et al. 2001; Niessen and Gumbiner 2002; Perez et al. 2008). Rac1 is also colocalized to E-cadherin-containing vesicles in keratinocytes (Akhtar and Hotchin 2001). Cdc42 is found at sites of cell–cell contact as well as the Golgi apparatus (Kroschewski et al. 1999), where it is thought to regulate the establishment of epithelial polarity during secretory and endocytic vesicle transport. On the other hand, RhoA, another member of the Rho family GTPases, localizes diffusely in the cytosol (Takaishi et al. 1997). Rap1, a member of the Ras family GTPases, accumulates at sites of cell–cell contact in an activity-independent manner. Similar to the case of Rac1, E-cadherin-Fc can recruit Rap1 to the plasma membrane (Hogan et al. 2004).
Several regulators of small GTPases accumulate at the site of cell–cell contacts through PDZ (PSD-95, Dlg, and ZO-1) domain-mediated interactions. β-catenin possesses a PDZ-binding consensus sequence at its carboxyl terminus and can recruit several PDZ domain-containing scaffolding and signaling molecules (Dobrosotskaya and James 2000; Ide et al. 1999; Kawajiri et al. 2000; Perego et al. 2000). PDZ domain-containing GEFs for small GTPases have also been shown to localize to intercellular adhesion sites. These GEFs include Tiam1 (Michiels et al. 1995), STEF (Hoshino et al. 1999), PDZ-RhoGEF (Fukuhara et al. 1999), and LARG (Kourlas et al. 2000; Taya et al. 2001). This localization of GEFs may account for the activation of the Rho family GTPases at intercellular adhesion sites (see the following).
The function of small GTPases is investigated with the use of three kinds of tools: point mutants of small GTPases to serve as constitutively active and dominant negative forms, affinity precipitation with a specific effector to monitor the level of activated small GTPases, and FRET (fluorescence resonance energy transfer)-based imaging techniques to show the spatiotemporal activation states of small GTPases. These biosensors provide valuable insights because small GTPases are activated separately in space and time within a single epithelial cell. Recent RNAi-based methods and the availability of knockout mice provide additional approaches to examine effects of the loss of function of small GTPases. These tools have increased our understanding of the roles of small GTPases in epithelial as well as nonepithelial cells, such as fibroblasts and neuronal cells (Braga and Yap 2005; Fukata and Kaibuchi 2001; Kiyokawa et al. 2006; Pertz and Hahn 2004; Van Aelst and Symons 2002).
DE NOVO CADHERIN-MEDIATED ADHESIONS CONTROL THE ACTIVITY OF SMALL GTPases
Like stimulation by growth factors, the engagement of integrins with fibronectin induces the activation of small GTPases (Price et al. 1998). Analogies with integrin-mediated cell–substratum adhesions lead to the speculation that cadherins might also function as receptors that transduce signals to small GTPases (outside-in-signal) (Table 1). Indeed, E-cadherin-mediated cell–cell interactions result in the rapid activation of Cdc42 in MCF-7 epithelial cells (Kim et al. 2000). Homophilic E-cadherin ligations recruit Rac1 at adhesion sites and induce its activation in MDCKII cells, and basal levels of active Rac1 are still maintained even when cells become confluent (Betson et al. 2002; Braga et al. 1997; Nakagawa et al. 2001; Noren et al. 2001). These findings raise the question of how cadherin-mediated cell–cell adhesions modulate the activity of Rho family GTPases. It has been reported that E-cadherin-mediated cell–cell adhesions stimulate phosphatidylinositol 3-kinase (PI3K) activity in MDCKII cells (Pece et al. 1999). Moreover, PI3K has been shown to interact with E-cadherin (Pece et al. 1999) and β-catenin (Espada et al. 1999). Because PI3K is thought to function upstream of Rac1 (Kotani et al. 1994), these observations indicate the possible involvement of PI3K in E-cadherin-dependent Rac1 activation. Indeed, wortmannin—an inhibitor of PI3K—inhibits E-cadherin-induced Rac1 activation but does not affect the localization of Rac1 or E-cadherin (Nakagawa et al. 2001).
Table 1.
Activation of small GTPases by cadherin
| Small GTPase | E-cadherin | N-cadherin | VE-cadherin | R-cadherin | C-cadherin |
|---|---|---|---|---|---|
| Cdc42 | + (MCF-7) | – | + | −/+ | – |
| Rac1 | + | – | + | + | + |
| RhoA | + | + | – | – | – |
| Rap1 | + | ND | + | ND | ND |
Activation of small GTPases by various cadherins. Increased levels of activated Cdc42, Rac1, RhoA, and Rap1 are assessed by biochemical or FRET-based methods. (ND) not determined; (+) activated; (–) not activated.
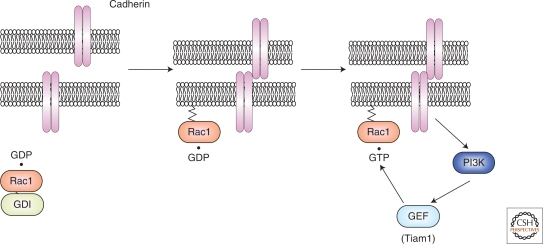
Activation of Rac1 through PI3K by E-cadherin-mediated cell–cell adhesions seems to require at least two steps (Fig. 2): (1) Rac1 recruitment to sites of cell–cell contacts, and (2) Rac1 activation by a GEF that responds to PI3K products. Consistently, Rac1 rapidly accumulates at the sites of E-cadherin engagement (1–3 min), and this accumulation is independent of Rac1 GTP binding/hydrolysis (Perez et al. 2008). Considering that Tiam1 is localized at sites of cell–cell contacts and functions downstream of PI3K, Tiam1 appears to act as a Rac1 GEF that functions downstream of E-cadherin engagement. Another possible mechanism for the regulation of Rho family GTPases involves p120ctn, which binds directly to the juxtamembrane domain of E-cadherin. p120ctn likely activates Rac1 and Cdc42 and inhibits RhoA. Vav2, a RhoGEF, directly binds to p120ctn, which may account for the ability of p120ctn to activate Rac1 and Cdc42 (Noren et al. 2000). Because Rac1 antagonizes RhoA through a mechanism that involves p190 RhoGAP, it is possible that the inhibition of RhoA by p120ctn occurs indirectly through the activation of Rac1 (Bustos et al. 2008; Nimnual et al. 2003). However, it remains controversial how p120ctn regulates Rho family GTPases (Anastasiadis 2007; Reynolds 2007; Wildenberg et al. 2006).
Figure 2.
Mode of activation of Rac1 by the formation of E-cadherin-mediaTed cell–cell adhesions. Before the establishment of E-cadherin-mediated cell–cell adhesions, GDP · Rac1 is sequestered in the cytosol by Rho GDI. When cadherin-mediated homophilic interactions occur, GDP · Rac1 is dissociated from Rho GDI by an unknown mechanism and is targeted to the plasma membrane. GDP · Rac1 is converted to GTP · Rac1 through the action of a GEF (e.g., Tiam1) downstream of PI3k. Activated Rac1 then positively regulates E-cadherin-mediated cell–cell adhesions.
RhoA is also activated during calcium-induced keratinocyte differentiation (Calautti et al. 2002). Localization of Rac1 and RhoA activity using FRET biosensors shows spatiotemporal activation during the initiation and expansion of epithelial cell–cell junctions (Yamada and Nelson 2007). The zones of Rac1 and lamellipodia activity as well as those of RhoA and actomyosin contractility are restricted to the periphery of the contacting membranes and together drive the initiation, expansion, and completion of cell–cell adhesions. These observations are consistent with the proposal that Rac1/Cdc42 and RhoA exert mutually antagonistic effects at some point during contact initiation, maturation, and reorganization, as in the case of the formation of focal adhesions (Rottner et al. 1999) and neurite extension (Kozma et al. 1997). It has also been shown that Tiam1 or constitutively active Rac1 (Rac1V12) can down-regulate RhoA activity and revert the mesenchymal phenotype of RasV12-transformed MDCKII cells to an epithelial phenotype (Sander et al. 1999). Thus, E-cadherin-mediated adhesions activate Rac1 and Cdc42, and active Rac1 down-regulates RhoA spatially, possibly through certain types of Rho GAPs, such as p190 RhoGAP (Bustos et al. 2008; Nimnual et al. 2003; Wildenberg et al. 2006).
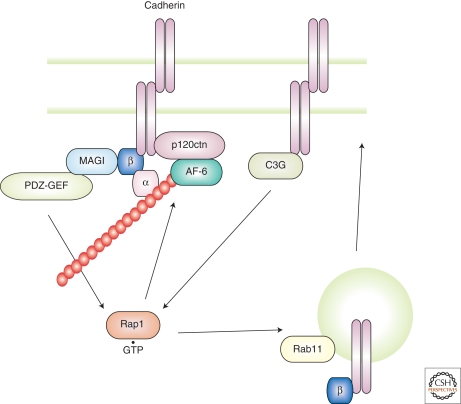
Rap1 is activated by many extracellular stimuli and is strongly implicated in the control of integrin-mediated cell adhesions. Recent evidence indicates that Rap1 also plays key roles in the formation of cadherin-based cell–cell adhesions (Fig. 3) (Kooistra et al. 2007; Retta et al. 2006). Rap1 is activated by homophilic E-cadherin ligation (Hogan et al. 2004). This Rap1 activation is required for subsequent Cdc42 activation. Interestingly, Rap1 GEFs such as PDZ-GEF1 and C3G are linked to cadherin directly and indirectly, respectively, and contribute to Rap1 activation (Hogan et al. 2004; Kawajiri et al. 2000; Sakurai et al. 2006). Hogan and colleagues (2004) found that C3G binds to the cadherin cytoplasmic tail, providing a potential mechanism for E-cadherin to locally activate Rap1. Furthermore, C3G has been implicated in activating Rap1 downstream of the junctional protein nectin. In this case, C3G requires activation of Src after nectin clustering. It should be noted that nectin-based adhesions sequentially activate Rap1, Cdc42, and Rac1 (Fukuhara et al. 2004; Fukuyama et al. 2005; Kawakatsu et al. 2005; Kawakatsu et al. 2002). Additionally, cAMP analogues can influence cadherin-mediated adhesions in a Rap1-dependent manner, suggesting that a cAMP-responsible Rap1GEF, EPAC, is involved in signaling from cadherins (Price et al. 2004; Wittchen et al. 2005).
Figure 3.
Model for the mode of action of Rap1 at cell–cell junctions. At initial cell–cell contacts, C3G bound to E-cadherin activates Rap1. Alternatively, PDZ-GEF linked to E-cadherin through MAGI and β-catenin can activate Rap1. Rap1 is also activated during E-cadherin endocytosis and locates to the E-cadherin/Rab11-containing vesicles. This population is likely involved in E-cadherin trafficking.
Thus, E-cadherin-mediated cell–cell contacts activate small GTPases including Rac1, Cdc42, and Rap1. However, it remains elusive how E-cadherin spatially and temporally regulates these small GTPases.
SMALL GTPases REGULATE ESTABLISHED ADHERENS JUNCTIONS
When small GTPases are activated by E-cadherin-mediated cell–cell contacts, they control existing E-cadherin-mediated adhesions (inside-out-signal) (Fig. 4). Braga and colleagues (Braga et al. 1997) found that the Rho family GTPases affect the formation of cell–cell junctions. When dominant–negative Rac1 (Rac1N17) or C3 botulinum toxin (an inhibitor of RhoA by ADP-ribosylation) is microinjected into keratinocytes, cadherin accumulation is inhibited at sites of cell–cell contacts. Subsequent studies from Takaishi and colleagues (1997) revealed that overexpression of constitutively active Rac1 (Rac1V12) in MDCKII cells promotes basal accumulation of E-cadherin, β-catenin, and actin filaments at sites of cell–cell contacts, whereas overexpression of Rac1N17 reduces their accumulation. Moreover, Cdc42, as well as Rac1, is required for E-cadherin-mediated cell–cell adhesions in MDCKII cells.
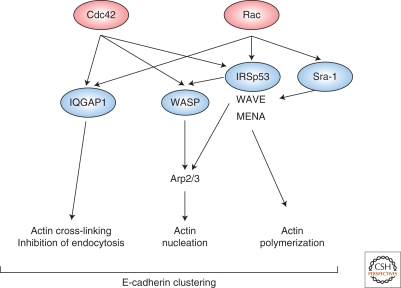
Figure 4.
Possible mode of action of activated Rac1 and Cdc42 for E-cadherin-mediated adhesions. Activated Cdc42 induces actin nucleation through WASP and the Arp2/3 complex. Acting through WAVEs, Rac1 enhances actin polymerization locally at sites of E-cadherin-mediated adhesions. This results in the local remodeling of actin filaments near the Rac1/Cdc42-activated membrane. In addition, IQGAP1, an effector of Rac1 and Cdc42, positively regulates E-cadherin-dependent intercellular adhesions through cross-linking actin filaments as well as inhibiting of E-cadherin endocytosis.
Those studies indicate that Rho family GTPases control cadherin-mediated cell–cell adhesions. More direct evidence was obtained using a quantitative, cell-dissociation assay for E-cadherin activity. These experiments sought to determine whether Rho family GTPases regulate cadherin-mediated cell–cell adhesions by acting on either the cadherin–catenin complex or the actin cytoskeleton and other components. Two stable cell lines derived from mouse L fibroblasts, which lack cadherin, are used: (1) EL cells express wild-type E-cadherin; (2) nEαCL cells express an E-cadherin mutant in which the distal β-catenin binding is replaced by the carboxy-terminal domain of α-catenin, thereby preventing remodeling of the cadherin–catenin complex. Although both cell lines adhere to each other in an E-cadherin-dependent manner, cell–cell adhesions in nEaCL cells do not require the distal β-catenin-binding domain of E-cadherin, β-catenin, or the amino terminus of α-catenin. The cadherin–catenin complex in nEαCL cells is not remodeled (Nagafuchi et al. 1994). The expression of Rac1N17 or dominant–negative Cdc42 (Cdc42N17) in EL cells markedly reduces E-cadherin activity, whereas expression of Rac1N17 or Cdc42N17 in nEαCL cells hardly reduces mutant E-cadherin activity (Fukata et al. 1999). This observation indicates that Rac1 and Cdc42 regulate E-cadherin activity through the cadherin–catenin complex. On the other hand, the expression of dominant–negative RhoA (RhoAN19) slightly reduces E-cadherin activity in both EL cells and nEαCL cells, suggesting that RhoA affects E-cadherin-mediated adhesive activity (presumably through the actin cytoskeleton or other components) but does not affect the cadherin–catenin complex.
How Rho family GTPases control E-cadherin-mediated intercellular adhesions remains an interesting question. One of the explanations involves the coordination of downstream effectors (Fig. 4). IQGAP1, an effector of Rac1 and Cdc42, has been implicated in E-cadherin-mediated intercellular adhesions. IQGAP1 has actin-cross-linking activity that is enhanced by activated Rac1/Cdc42. IQGAP1 localizes to sites of cell–cell contact in epithelial cells and can associate with β-catenin in vivo and in vitro (Kuroda et al. 1998). When overexpressed in EL cells, IQGAP1 interacts with β-catenin and induces the dissociation of α-catenin from β-catenin. This, in turn, weakens E-cadherin-mediated intercellular adhesions. When the amount of activated Rac1 increases, Rac1 interacts with IQGAP1, thereby stabilizing actin filaments. Under these conditions, IQGAP1 does not bind to β-catenin and cannot dissociate α-catenin from the cadherin–catenin complex, leading to strong adhesions. In contrast, when the amount of inactivated Rac1 increases, IQGAP1 is freed from Rac1 and interacts with β-catenin to dissociate α-catenin from the cadherin–catenin complex, resulting in weak adhesions (Noritake et al. 2005). Because IQGAP1 has anti-GTPase activity (Hart et al. 1996), it is possible that IQGAP1 controls the amount of GTP-bound Rac1 at sites of cell–cell contacts and leads to stable adhesions (Noritake et al. 2005). Furthermore, it has been shown that the inhibition of transinteraction E-cadherin endocytosis is mediated by the reorganization of the actin cytoskeleton by the IQGAP1-Rac/Cdc42 complex (Izumi et al. 2004). Thus, IQGAP1 behaves as a positive and negative regulator of cell–cell adhesion downstream of Rac1.
The actin polymerization machinery is another mediator between Rac1 activation and E-cadherin-mediated cell–cell adhesion (Fig. 4). Recruitment and activation of Rac1 and Cdc42 by homophilic E-cadherin ligations can induce actin polymerization through the Arp2/3 complex and WAVEs or WASP. Together with other actin-binding proteins such as cortactin, polymerized actin filaments reinforce cadherin-mediated adhesions (Gates and Peifer 2005; Scott and Yap 2006; Vasioukhin and Fuchs 2001). Significantly, inhibition of Rac1 signaling blocks actin assembly at sites of cell–cell adhesion (Braga et al. 1997; Takaishi et al. 1997). It is interesting to note that Rac1 also functions in the clustering of integrin receptors, suggesting that it could play a similar role in the clustering of E-cadherin. However, the effect of constitutively active Rac1 on adherens junctions depends on the cell type. In keratinocytes, constitutively active Rac1 (Rac1L61) causes the disassembly of adherens junctions (Braga et al. 2000), which is opposite to what is observed in MDCKII cells (see previous). The molecular mechanisms underlying these cell type-dependent effects need to be clarified.
Downstream signaling pathways that mediate the effects of RhoA on adherens junctions remain largely unknown. In keratinocytes, adherens junction formation has been shown to depend on the activity of the tyrosine kinase Fyn (Calautti et al. 2002). Constitutively active RhoA (RhoAV14) stimulates Fyn-mediated tyrosine phosphorylation of catenins and cell–cell adhesions, indicating that Fyn and possibly other Src family kinases can function downstream of RhoA in the establishment of adherens junctions. As described previously, during the establishment of cell–cell adhesions, RhoA is activated at the most distal edges of the expanding cell–cell contacts along with phosphorylated myosin II (Yamada and Nelson 2007). Rho-kinase, an effector of RhoA, directly phosphorylates myosin light chain and myosin phosphatase target subunit-1 and inhibits myosin phosphatase activity (Kaibuchi et al. 1999). This results in an increase in myosin light chain phosphorylation and subsequent actomyosin contraction (Kaibuchi et al. 1999). E-cadherin-mediated activation of RhoA is likely involved in the expansion of cell–cell adhesions through Rho-kinase.
Activated Rho family GTPases affect the surface level of E-cadherin expression, presumably by regulating endocytic transport (Mosesson et al. 2008). Constitutively active Rac1 (Rac1L61) and RhoA (RhoAL63) inhibit clathrin-dependent endocytosis in fibroblasts (Lamaze et al. 1996). This raises the possibility that Rho family GTPases could regulate cadherin-mediated cell–cell adhesions by controlling cadherin transport. Indeed, Rac1V12 and RhoAV14 inhibit HGF- and TPA-induced endocytosis of cadherin in MDCKII cells (Kamei et al. 1999). However, microinjection of Rac1L61 in keratinocytes causes E-cadherin to relocalize in large intracellular vesicles via a clathrin-independent pathway and disrupts cell–cell contacts. This discrepancy may be because of differences in cell types or levels of Rac1 activity (Braga et al. 2000; Braga et al. 1999). Regardless, the molecular mechanisms by which Rac1 and RhoA regulate the endocytosis of E-cadherin remains to be clarified.
The first evidence indicating that Rap1 is required for cell–cell junctions came from genetic studies in Drosophila melanogaster (Knox and Brown 2002). In MDCKII cells, Rap1 activity is required not only for Cdc42 activation during cell–cell junction formation but also for maintaining E-cadherin-mediated cell–cell adhesions (Fukuyama et al. 2005; Hogan et al. 2004). “Fast cycling” constitutively active Cdc42 (Cdc42L28) can rescue the effects of Rap1GAP on cell–cell junction formation. Furthermore, as described previously, activation of Rap1 by nectins is required for the subsequent activation of Cdc42 and Rac1. Thus, one of the functions of Rap1 activation may be the recruitment of Rac1 and Cdc42 GEFs to the site of initial cell–cell contacts to provide a link with the actin cytoskeleton. In addition, Rap1 may also be involved in the recruitment of junctional proteins. One protein heavily involved in this process is afadin/AF6, an effector of Rap1. In vitro studies show that afadin/AF6 in the presence of Rap1 inhibits the endocytosis of E-cadherins that are not engaged in homophilic transinteractions (Hoshino et al. 2005). Furthermore, strong activation of Rap1 occurs on adherens junction disassembly triggered by E-cadherin internalization and trafficking in the endocytic pathway (Retta et al. 2006). Such internalized E-cadherin is colocalized with Rap1 at the perinuclear Rab11-positive recycling endosome compartment, and Rap1 associates with a subset of E-cadherin–catenin complexes lacking p120ctn. Although the mode of action of Rap1 at the cell–cell adhesions remains largely unknown, Rap1 appears to act as a critical regulator of E-cadherin-mediated intercellular adhesion.
Recent evidence indicates that the Rab and Arf family of small GTPases are critical regulators that control the surface level of E-cadherin. Even in confluent cells, E-cadherin undergoes continuous endocytosis and exocytosis. The majority of studies implicates a clathrin-dependent route of E-cadherin internalization (Kimura et al. 2006), whereas some suggest the existence of non-clathrin-dependent pathways (e.g., caveola-mediated endocytosis) (Lu et al. 2003) and an EGF-induced macropinocytosis pathway (Bryant et al. 2007). Important mechanisms controlling E-cadherin endocytosis involve binding of p120ctn to the juxtamembrane domain of E-cadherin, and the tyrosine phosphorylation of E-cadherin by receptor tyrosine kinases and Src family kinases (Mellman and Nelson 2008; Mosesson et al. 2008).
The intracellular fate of internalized E-cadherin (i.e., sorting for degradation or recycling) is crucial, as it eliminates existing junctions or re-deploys E-cadherin to new junctions. Along with constitutive clathrin-mediated endocytosis, cadherin internalization is selectively induced by growth factors. The recycling endosome serves as a major location of cadherin for sorting back to the plasma membrane. Arf6, an endosomal GTP-binding protein, regulates cadherin endocytosis in response to EGF. Rab4 and Rab11 on the recycling endosomes also participate in E-cadherin endocytosis. Activated Rab4 interacts within the trans-Golgi network, whereas Rab11 seems to regulate not only the delivery of nascent E-cadherin from the Golgi to the basolateral surface but also the ability of internalized E-cadherin to activate Rap1 while residing in recycling endosomes (Balzac et al. 2005). Mechanisms that regulate the surface level of E-cadherin expression have begun to be uncovered. It will be worthwhile for future studies to reveal the functional cross talk among small GTPases in this process.
CONCLUDING REMARKS
Small GTPases have emerged as key players mediating the effects of signals, from cadherin engagement to downstream cellular machines that control the organization of the actin cytoskeleton, contractility, and vesicle trafficking. The identification and characterization of effectors for small GTPases have unraveled the action of small GTPases, especially those with relevance to the actin cytoskeleton, in E-cadherin-mediated intercellular adhesions. It is worthwhile in the future to examine in more detail the molecular mechanisms that control E-cadherin trafficking in the maintenance of intercellular adhesions. In contrast, much less is known about the signaling mechanisms that translate cadherin-mediated cues into spatio-temporal regulation of the small GTPases. It is likely, however, that the concerted action of GEFs and GAPs is critical for the precisely timing and localized activation of small GTPases.
Footnotes
Editors: W. James Nelson and Elaine Fuchs
Additional Perspectives on Cell Junctions available at www.cshperspectives.org
REFERENCES
- Adams CL, Nelson WJ 1998. Cytomechanics of cadherin-mediated cell–cell adhesion. Current Opinion Cell Biol 10:572–577 [DOI] [PubMed] [Google Scholar]
- Akhtar N, Hotchin NA 2001. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol Biol Cell 12:847–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadis PZ 2007. p120-ctn: A nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta 1773:34–46 [DOI] [PubMed] [Google Scholar]
- Balzac F, Avolio M, Degani S, Kaverina I, Torti M, Silengo L, Small JV, Retta SF 2005. E-cadherin endocytosis regulates the activity of Rap1: A traffic light GTPase at the crossroads between cadherin and integrin function. J Cell Sci 118:4765–4783 [DOI] [PubMed] [Google Scholar]
- Betson M, Lozano E, Zhang J, Braga VM 2002. Rac activation upon cell–cell contact formation is dependent on signaling from the epidermal growth factor receptor. J Biol Chem 277:36962–36969 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E 2009. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10:207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM 2002. Cell–cell adhesion and signalling. Current Opinion Cell Biol 14:546–556 [DOI] [PubMed] [Google Scholar]
- Braga VM, Yap AS 2005. The challenges of abundance: Epithelial junctions and small GTPase signalling. Current Opinion Cell Biol 17:466–474 [DOI] [PubMed] [Google Scholar]
- Braga VM, Betson M, Li X, Lamarche-Vane N 2000. Activation of the small GTPase Rac is sufficient to disrupt cadherin-dependent cell–cell adhesion in normal human keratinocytes. Mol Biol Cell 11:3703–3721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Del Maschio A, Machesky L, Dejana E 1999. Regulation of cadherin function by Rho and Rac: Modulation by junction maturation and cellular context. Mol Biol Cell 10:9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA 1997. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell–cell contacts. J Cell Biol 137:1421–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Kerr MC, Hammond LA, Joseph SR, Mostov KE, Teasdale RD, Stow JL 2007. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J Cell Sci 120:1818–1828 [DOI] [PubMed] [Google Scholar]
- Bustos RI, Forget MA, Settleman JE, Hansen SH 2008. Coordination of Rho and Rac GTPase function via p190B RhoGAP. Current Biol 18:1606–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calautti E, Grossi M, Mammucari C, Aoyama Y, Pirro M, Ono Y, Li J, Dotto GP 2002. Fyn tyrosine kinase is a downstream mediator of Rho/PRK2 function in keratinocyte cell–cell adhesion. J Cell Biol 156:137–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delva E, Tucker DK, Kowalczyk AP 2009. The desmosome. Cold Spring Harb Perspect Biol 1:a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrosotskaya IY, James GL 2000. MAGI-1 interacts with β-catenin and is associated with cell–cell adhesion structures. Biochem Biophys Res Comm 270:903–909 [DOI] [PubMed] [Google Scholar]
- Espada J, Perez-Moreno M, Braga VM, Rodriguez-Viciana P, Cano A 1999. H-Ras activation promotes cytoplasmic accumulation and phosphoinositide 3-OH kinase association of β-catenin in epidermal keratinocytes. J Cell Biol 146:967–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming IN, Elliott CM, Exton JH 1996. Differential translocation of rho family GTPases by lysophosphatidic acid, endothelin-1, and platelet-derived growth factor. J Biol Chem 271:33067–33073 [DOI] [PubMed] [Google Scholar]
- Fukata M, Kaibuchi K 2001. Rho-family GTPases in cadherin-mediated cell–cell adhesion. Nat Rev Mol Cell Biol 2:887–897 [DOI] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, Matsuura Y, Yonehara S, Fujisawa H, Kikuchi A, et al. 1999. Cdc42 and Rac1 regulate the interaction of IQGAP1 with β-catenin. J Biol Chem 274:26044–26050 [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS 1999. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem 274:5868–5879 [DOI] [PubMed] [Google Scholar]
- Fukuhara T, Shimizu K, Kawakatsu T, Fukuyama T, Minami Y, Honda T, Hoshino T, Yamada T, Ogita H, Okada M, et al. 2004. Activation of Cdc42 by trans interactions of the cell adhesion molecules nectins through c-Src and Cdc42-GEF FRG. Journal of Cell Biology 166:393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama T, Ogita H, Kawakatsu T, Fukuhara T, Yamada T, Sato T, Shimizu K, Nakamura T, Matsuda M, Takai Y 2005. Involvement of the c-Src-Crk-C3G-Rap1 signaling in the nectin-induced activation of Cdc42 and formation of adherens junctions. J Biol Chem 280:815–825 [DOI] [PubMed] [Google Scholar]
- Furuse M 2009. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol 2:a002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates J, Peifer M 2005. Can 1000 reviews be wrong? Actin, α-Catenin, and adherens junctions. Cell 123:769–772 [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG 2007. The PAR proteins: Fundamental players in animal cell polarization. Develop Cell 13:609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Getsios S, Troyanovsky S, Godsel LM 2009. Intercellular junction assembly, dynamics and homeostasis. Cold Spring Harb Perspect Biol 2:a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulli MP, Peter M 2001. Temporal and spatial regulation of Rho-type guanine-nucleotide exchange factors: The yeast perspective. Genes Develop 15:365–379 [DOI] [PubMed] [Google Scholar]
- Gumbiner BM 2000. Regulation of cadherin adhesive activity. J Cell Biol 148:399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ 2006. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Develop 20:3199–3214 [DOI] [PubMed] [Google Scholar]
- Hart MJ, Callow MG, Souza B, Polakis P 1996. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J 15:2997–3005 [PMC free article] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ 2008. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778:660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y 2004. Rap1 regulates the formation of E-cadherin-based cell–cell contacts. Mol Cell Biol 24:6690–6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y 2005. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem 280:24095–24103 [DOI] [PubMed] [Google Scholar]
- Hoshino M, Sone M, Fukata M, Kuroda S, Kaibuchi K, Nabeshima Y, Hama C 1999. Identification of the stef gene that encodes a novel guanine nucleotide exchange factor specific for Rac1. J Biol Chem 274:17837–17844 [DOI] [PubMed] [Google Scholar]
- Ide N, Hata Y, Deguchi M, Hirao K, Yao I, Takai Y 1999. Interaction of S-SCAM with neural plakophilin-related Armadillo-repeat protein/delta-catenin. Biochem Biophys Res Comm 256:456–461 [DOI] [PubMed] [Google Scholar]
- Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y 2004. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J Cell Biol 166:237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A 2005. Rho GTPases: Biochemistry and biology. Ann Rev Cell Develop Biol 21:247–269 [DOI] [PubMed] [Google Scholar]
- Jou TS, Nelson WJ 1998. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol 142:85–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M 1999. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Ann Rev Biochem 68:459–486 [DOI] [PubMed] [Google Scholar]
- Kamei T, Matozaki T, Sakisaka T, Kodama A, Yokoyama S, Peng YF, Nakano K, Takaishi K, Takai Y 1999. Coendocytosis of cadherin and c-Met coupled to disruption of cell–cell adhesion in MDCK cells–regulation by Rho, Rac and Rab small G proteins. Oncogene 18:6776–6784 [DOI] [PubMed] [Google Scholar]
- Kawajiri A, Itoh N, Fukata M, Nakagawa M, Yamaga M, Iwamatsu A, Kaibuchi K 2000. Identification of a novel β-catenin-interacting protein. Biochem Biophys Res Commun 273:712–717 [DOI] [PubMed] [Google Scholar]
- Kawakatsu T, Ogita H, Fukuhara T, Fukuyama T, Minami Y, Shimizu K, Takai Y 2005. Vav2 as a Rac-GDP/GTP exchange factor responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J Biol Chem 280:4940–4947 [DOI] [PubMed] [Google Scholar]
- Kawakatsu T, Shimizu K, Honda T, Fukuhara T, Hoshino T, Takai Y 2002. Trans-interactions of nectins induce formation of filopodia and Lamellipodia through the respective activation of Cdc42 and Rac small G proteins. J Biol Chem 277:50749–50755 [DOI] [PubMed] [Google Scholar]
- Kim SH, Li Z, Sacks DB 2000. E-cadherin-mediated cell–cell attachment activates Cdc42. J Biol Chem 275:36999–37005 [DOI] [PubMed] [Google Scholar]
- Kimura T, Sakisaka T, Baba T, Yamada T, Takai Y 2006. Involvement of the Ras-Ras-activated Rab5 guanine nucleotide exchange factor RIN2-Rab5 pathway in the hepatocyte growth factor-induced endocytosis of E-cadherin. J Biol Chem 281:10598–10609 [DOI] [PubMed] [Google Scholar]
- Kiyokawa E, Hara S, Nakamura T, Matsuda M 2006. Fluorescence (Forster) resonance energy transfer imaging of oncogene activity in living cells. Cancer Sci 97:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox AL, Brown NH 2002. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science 295:1285–1288 [DOI] [PubMed] [Google Scholar]
- Kooistra MR, Dube N, Bos JL 2007. Rap1: A key regulator in cell–cell junction formation. J Cell Sci 120:17–22 [DOI] [PubMed] [Google Scholar]
- Kotani K, Yonezawa K, Hara K, Ueda H, Kitamura Y, Sakaue H, Ando A, Chavanieu A, Calas B, Grigorescu F, et al. 1994. Involvement of phosphoinositide 3-kinase in insulin- or IGF-1-induced membrane ruffling. EMBO J 13:2313–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourlas PJ, Strout MP, Becknell B, Veronese ML, Croce CM, Theil KS, Krahe R, Ruutu T, Knuutila S, Bloomfield CD, et al. 2000. Identification of a gene at 11q23 encoding a guanine nucleotide exchange factor: Evidence for its fusion with MLL in acute myeloid leukemia. Proc Natl Acad Sci 97:2145–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EM, Ali RG, McCormack AJ, Yap AS 2002. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J Biol Chem 277:6708–6718 [DOI] [PubMed] [Google Scholar]
- Kozma R, Sarner S, Ahmed S, Lim L 1997. Rho family GTPases and neuronal growth cone remodelling: Relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol Cell Biol 17:1201–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenburg O, Poland M, Gebbink M, Oomen L, Moolenaar WH 1997. Dissociation of LPA-induced cytoskeletal contraction from stress fiber formation by differential localization of RhoA. J Cell Sci 110:2417–2427 [DOI] [PubMed] [Google Scholar]
- Kroschewski R, Hall A, Mellman I 1999. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol 1:8–13 [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, et al. 1998. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell–cell adhesion. Science 281:832–835 [DOI] [PubMed] [Google Scholar]
- Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL 1996. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature 382:177–179 [DOI] [PubMed] [Google Scholar]
- Lu Z, Ghosh S, Wang Z, Hunter T 2003. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of β-catenin, and enhanced tumor cell invasion. Cancer Cell 4:499–515 [DOI] [PubMed] [Google Scholar]
- McCrea PD, Gu D, Balda M 2009. Junctional music that the nucleus hears: Cell-cell junction signaling and the modulation of gene activity. Cold Spring Harb Perspect Biol 1:a002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan RW, Kraemer A, Helwani FM, Kovacs EM, Yap AS 2007. E-cadherin adhesion activates c-Src signaling at cell–cell contacts. Mol Biol Cell 18:3214–3223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ 2008. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol 9:833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Takeichi M 2009. Adherens junction: Molecular architecture and regulation. Cold Spring Harb Perspect Biol 1:a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels F, Habets GG, Stam JC, van der Kammen RA, Collard JG 1995. A role for Rac in Tiam1-induced membrane ruffling and invasion. Nature 375:338–340 [DOI] [PubMed] [Google Scholar]
- Mosesson Y, Mills GB, Yarden Y 2008. Derailed endocytosis: An emerging feature of cancer. Nat Rev Cancer 8:835–850 [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Ishihara S, Tsukita S 1994. The roles of catenins in the cadherin-mediated cell adhesion: Functional analysis of E-cadherin-α catenin fusion molecules. J Cell Biol 127:235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K 2001. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell–cell adhesion sites. J Cell Sci 114:1829–1838 [DOI] [PubMed] [Google Scholar]
- Niessen CM, Gumbiner BM 2002. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J Cell Biol 156:389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimnual AS, Taylor LJ, Bar-Sagi D 2003. Redox-dependent downregulation of Rho by Rac. Nat Cell Biol 5:236–241 [DOI] [PubMed] [Google Scholar]
- Noren NK, Liu BP, Burridge K, Kreft B 2000. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J Cell Biol 150:567–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren NK, Niessen CM, Gumbiner BM, Burridge K 2001. Cadherin engagement regulates Rho family GTPases. J Biol Chem 276:33305–33308 [DOI] [PubMed] [Google Scholar]
- Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K 2005. IQGAP1: A key regulator of adhesion and migration. J Cell Sci 118:2085–2092 [DOI] [PubMed] [Google Scholar]
- Pece S, Chiariello M, Murga C, Gutkind JS 1999. Activation of the protein kinase Akt/PKB by the formation of E-cadherin-mediated cell–cell junctions. Evidence for the association of phosphatidylinositol 3-kinase with the E-cadherin adhesion complex. J Biol Chem 274:19347–19351 [DOI] [PubMed] [Google Scholar]
- Perego C, Vanoni C, Massari S, Longhi R, Pietrini G 2000. Mammalian LIN-7 PDZ proteins associate with β-catenin at the cell- cell junctions of epithelia and neurons. EMBO J 19:3978–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez TD, Tamada M, Sheetz MP, Nelson WJ 2008. Immediate-early signaling induced by E-cadherin engagement and adhesion. J Biol Chem 283:5014–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Moreno M, Jamora C, Fuchs E 2003. Sticky business: Orchestrating cellular signals at adherens junctions. Cell 112:535–548 [DOI] [PubMed] [Google Scholar]
- Pertz O, Hahn KM 2004. Designing biosensors for Rho family proteins–deciphering the dynamics of Rho family GTPase activation in living cells. J Cell Sci 117:1313–1318 [DOI] [PubMed] [Google Scholar]
- Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL 2004. Rap1 regulates E-cadherin-mediated cell–cell adhesion. J Biol Chem 279:35127–35132 [DOI] [PubMed] [Google Scholar]
- Price LS, Leng J, Schwartz MA, Bokoch GM 1998. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell 9:1863–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retta SF, Balzac F, Avolio M 2006. Rap1: A turnabout for the crosstalk between cadherins and integrins. Eur J Cell Biol 85:283–293 [DOI] [PubMed] [Google Scholar]
- Reynolds AB 2007. p120-catenin: Past and present. Biochim Biophys Acta 1773:2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV 1999. Interplay between Rac and Rho in the control of substrate contact dynamics. Current Biol 9:640–648 [DOI] [PubMed] [Google Scholar]
- Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, Nakaoka Y, Mochizuki N 2006. MAGI-1 is required for Rap1 activation upon cell–cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell 17:966–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG 1999. Rac downregulates Rho activity: Reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol 147:1009–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA, Yap AS 2006. Cinderella no longer: α-catenin steps out of cadherin’s shadow. J Cell Sci 119:4599–4605 [DOI] [PubMed] [Google Scholar]
- Shapiro L, Weis WI 2009. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol 1:a003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y 1997. Regulation of cell–cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol 139:1047–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M 1995. Morphogenetic roles of classic cadherins. Current Opinion Cell Biol 7:619–627 [DOI] [PubMed] [Google Scholar]
- Taya S, Inagaki N, Sengiku H, Makino H, Iwamatsu A, Urakawa I, Nagao K, Kataoka S, Kaibuchi K 2001. Direct interaction of insulin-like growth factor-1 receptor with leukemia-associated RhoGEF. J Cell Biol 155:809–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U, Truong K, Godt D, Ikura M, Peifer M 2000. Cadherins in embryonic and neural morphogenesis. Nat Rev Mol Cell Biol 1:91–100 [DOI] [PubMed] [Google Scholar]
- Tsukita S, Tsukita S, Nagafuchi A, Yonemura S 1992. Molecular linkage between cadherins and actin filaments in cell–cell adherens junctions. Current Opinion Cell Biol 4:834–839 [DOI] [PubMed] [Google Scholar]
- Tsukita S, Yamazaki Y, Katsuno T, Tamura A 2008. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene 27:6930–6938 [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D’souza-Schorey C 1997. Rho GTPases and signaling networks. Genes Develop 11:2295–2322 [DOI] [PubMed] [Google Scholar]
- Van Aelst L, Symons M 2002. Role of Rho family GTPases in epithelial morphogenesis. Genes Develop 16:1032–1054 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Fuchs E 2001. Actin dynamics and cell–cell adhesion in epithelia. Current Opinion Cell Biol 13:76–84 [DOI] [PubMed] [Google Scholar]
- Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB 2006. p120-catenin and p190RhoGAP regulate cell–cell adhesion by coordinating antagonism between Rac and Rho. Cell 127:1027–1039 [DOI] [PubMed] [Google Scholar]
- Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K 2005. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem 280:11675–11682 [DOI] [PubMed] [Google Scholar]
- Yamada S, Nelson WJ 2007. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J Cell Biol 178:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Kovacs EM 2003. Direct cadherin-activated cell signaling: A view from the plasma membrane. J Cell Biol 160:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]