Abstract
The transcription factor NF-κB has diverse functions in the nervous system, depending on the cellular context. NF-κB is constitutively activated in glutamatergic neurons. Knockout of p65 or inhibition of neuronal NF-κB by super-repressor IκB resulted in the loss of neuroprotection and defects in learning and memory. Similarly, p50−/− mice have a lower learning ability and are sensitive to neurotoxins. Activated NF-κB can be transported retrogradely from activated synapses to the nucleus to translate short-term processes to long-term changes such as axon growth, which is important for long-term memory. In glia, NF-κB is inducible and regulates inflammatory processes that exacerbate diseases such as autoimmune encephalomyelitis, ischemia, and Alzheimer's disease. In summary, inhibition of NF-κB in glia might ameliorate disease, whereas activation in neurons might enhance memory. This review focuses on results produced by the analysis of genetic models.
The inflammatory transcription factor NF-κB also functions in the nervous system, where it is important for neuroprotection, learning, and long-term memory.
In vertebrates, the nervous system is composed of the central nervous system (CNS) and the peripheral nervous system (PNS). The CNS comprises the brain and spinal cord, whereas peripheral nerves are part of the PNS. The CNS coordinates different tasks: integration of all stimuli that are presented from outside or inside the organism, coordination of all motor processes, regulation of hormone systems, and organ control. The most fascinating function of the brain is the coordination of learning and memory. This review will focus on the function of NF-κB in the nervous system, especially in learning and memory in rodent genetic models. The most abundant cell types in the vertebrate nervous system are neurons (about 100 billion) and glia (10–50 times more). Typical glial cells are astrocytes, microglia, and the nerve fiber ensheathing cells, such as Schwann cells, which insulate nerves in the PNS and oligodendrocytes in the CNS. Neurons are highly polarized cells. Dendrites and/or soma receive electrochemical signals that are transmitted by axons to other neurons. Synapses are major sites of information input or output. These are formed by the presynaptic boutons derived from axons and the postsynaptic sites mainly localized on dendrites.
In a short introduction to the neurobiology of learning, we present a reductionist view (Dudai 1989). Although it is not easy to formally define learning (see Dudai 1989), here learning is defined simply as the capability of an animal to acquire novel skills. Learning can lead to a lasting modification of the internal representations of the outer world. Memory is thus the retention of the outer world experience-dependent internal representations. Memory retrieval is the use of memory in behavioral tasks. Comparison of learning capabilities in different species has suggested that learning has developed to provide organisms with an improved coping mechanism against adverse environments. Thus the ability to learn is encoded within the genome.
Over the years, a biochemical/cellular concept of memory has emerged that can be summarized as a dialogue between genes and synapses (Kandel 2001). Central to this concept is synaptic transmission, which is the release of, for example, excitatory neurotransmitters (axon potential-inducing) from a presynaptic release site. Transformation of an axon potential to neurotransmitter release takes place at presynaptic sites and is a unidirectional process. Two major transmitter systems in the CNS are predominant: glutamate, released from excitatory glutamatergic neurons, and γ-aminobutyric acid (GABA), released from inhibitory GABAergic neurons. Glutamate can direct the opening of Na+ ion channels at the postsynaptic site, inducing an excitatory neuronal response. In contrast, GABA directs the opening of Cl− channels, thus inducing an inhibitory response. Activity-dependent release of glutamate from presynaptic sites leads to the activation of AMPA receptors and to the depolarization of the postsynaptic neuron. Depolarization can occur by action potentials in the millisecond range locally at the synapse. Depolarization of postsynaptic neurons leads to the removal of NMDA receptor inhibition by Mg++ and then to Ca2+ influx through the receptor. This activates voltage-gated calcium channels (VGCCs), another source of synaptic Ca2+ ions. This short-lived membrane depolarization process can be transformed to changes in gene expression and to long-term morphological changes such as synaptic plasticity, leading to additional or more efficient synapses (Lamprecht and LeDoux 2004). It is thought that by the modification of synapses the internal representations are modified and thus memory is enhanced, retained, or lost.
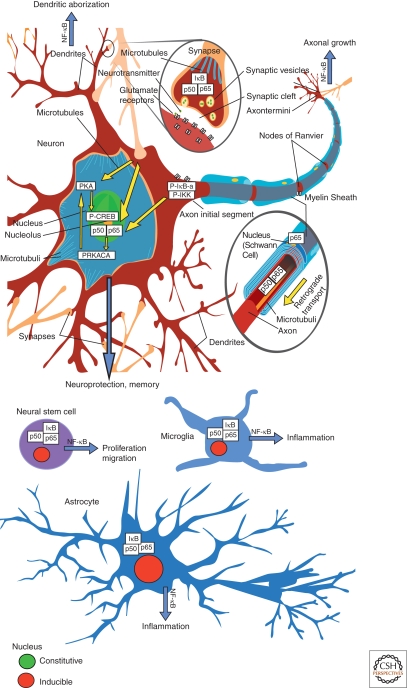
The role of NF-κB in the nervous system has gained interest because of its involvement in synaptic processes, neurotransmission, and neuroprotection. Furthermore, inducible NF-κB plays a crucial role in brain inflammation and neural stem cell proliferation. The role of NF-κB in the cellular context of the nervous system is depicted in Figure 1.
Figure 1.
The role of NF-κB within the cellular context of the nervous system. Constitutively activated NF-κB is detected mostly in glutamatergic neurons (green nuclei), whereas NF-κB in glia has a lower basal activity and is heavily inducible (red nuclei). For details, see text.
All DNA-binding subunits of NF-κB have been detected within the CNS, and in the adult rodent brain the major DNA-binding complexes are p50/p65 (Kaltschmidt et al. 1993; Bakalkin et al. 1993; Schmidt-Ullrich et al. 1996; Meffert et al. 2003). In contrast, in the developing nervous system complexes consisting of cRel/p65, p50/p65, and p50 homodimers were reported (Bakalkin et al. 1993).
NEURONS
Initially, constitutive (high basal) NF-κB activity was found in glutamatergic neurons of the CNS, such as the hippocampus (granule cells and pyramidal neurons of CA1 and CA3) and cerebral cortex (layers 2, 4, and 5), by antibody staining (Kaltschmidt et al. 1993; Kaltschmidt et al. 1994; Kaltschmidt et al. 1995), gelshift assays, and Western blotting. Transgenic reporter mouse models verified these data and also showed constitutive NF-κB activity in several rodent brain regions such as the cerebral cortex, hippocampus, amygdala, olfactory lobes, cerebellum, and hypothalamus (Schmidt-Ullrich et al. 1996; Bhakar et al. 2002). Here, constitutive NF-κB activity shall be defined as activity that makes cells blue in transgenic reporter mice (mainly in neurons, but also endothelial cells). Constitutive NF-κB activity can be suppressed by pharmacological inhibitors such as glutamate antagonists and L-type Ca2+ channel blockers (Lilienbaum and Israel 2003; Meffert et al. 2003). This suggests that constitutive NF-κB results from physiological basal synaptic transmission. Furthermore, constitutive NF-κB activity in endothelial cells, the roof plate (a dorsal signaling center of the developing nervous system), and floor plate (part of the neural tube) is dependent on TRAF6, as shown by crossing lacZ reporter mice with TRAF6−/− mice (Dickson et al. 2004). Inducible NF-κB was detected in biochemically purified synapses (synaptosomes) (Kaltschmidt et al. 1993; Meberg et al. 1996; Meffert et al. 2003). Interestingly, analysis of TNFRI−/−, p65−/− double knockout mice showed no presence of p65, p50, and IκB α and β in synapses. These data suggest that p65 is the driving subunit for synaptic localization and transport processes. It cannot be replaced by alternative subunits such as c-Rel or RelB.
A p65/GFP fusion protein can be retrogradely transported from active synaptic sites back to the nucleus (Wellmann et al. 2001; Meffert et al. 2003) after glutamatergic stimulation. This retrograde transport is dependent on the p65 nuclear localization signal (NLS) and involves a dynein–dynactin motor protein complex gliding on microtubules (Mikenberg et al. 2007; Shrum et al. 2009). p65 NLS is also important for the contact of p65 and the motor protein complexes. In summary, these observations indicate that NF-κB has a central role in translating short-term synaptic events into changes in gene expression.
LEARNING AND MEMORY
Genetic evidence* for the involvement of NF-κB in learning and memory was provided for the first time in TNFRI−/−, p65−/− mice by Meffert et al. 2003. In a radial arm maze, TNFRI−/−, p65−/− mice made significantly more trial errors than control mice. A second, conditional mouse model uses a neuronal promoter-specific ablation of NF-κB in the basal forebrain (CamKII-tTA/ tetOtnIκB-α). In this model, all NF-κB subunits were repressed in vivo as measured by triple transgenic lacZ reporter mice in hippocampal neurons (Fridmacher et al. 2003). These mice showed impairments in spatial learning. The mice had to learn the position of a platform useful for resting that was submersed within a water basin (Morris water maze). Mice expressing the super-repressor (tnIκB-α) showed reduced long-term potentiation (LTP) and long term depression (LTD) (Kaltschmidt et al. 2006). Furthermore, learning during training sessions in a Morris water maze strongly induced NF-κB binding activity in hippocampal extracts of wild-type mice (O’Mahony et al. 2006). To obtain clues about NF-κB-regulated changes in gene expression, transcriptome profiling was performed. The catalytic subunit α of the protein kinase A gene (PRKACA) was identified as a novel NF-κB target gene (Kaltschmidt et al. 2006). Recent research has shown that protein kinase A (PKA)–CREB signaling is an essential pathway for learning and memory (Kandel 2001). Connected with this, repression of NF-κB in transgenic mice resulted in reduced PKA activity, leading to a strong reduction in forskolin-induced CREB phosphorylation (forskolin activates adenylcylase and thus raises intracellular cAMP levels). A functional NF-κB binding element was identified within intron 2 of mouse and human PKA genes (Kaltschmidt et al. 2006). These findings identified a novel transcriptional signaling cascade in neurons in which NF-κB regulates the PKA/CREB pathway function in learning and memory. Synapse density is an important parameter for learning processes. After NF-κB ablation in the hippocampus, the density of synapses was strongly reduced within a hippocampal subfield, the stratum lucidum (Kaltschmidt et al., unpubl.). In addition, in mice with neuronal NF-κB ablation the formation of axonal mossy fiber projections was impaired (Kaltschmidt et al. unpublished). These fibers normally connect granule cells with CA3 pyramidal cells. To our knowledge, this is the first in vivo model showing NF-κB dependent structural plasticity.
Furthermore, dendritic arborization is regulated by NF-κB in peripheral and cortical neurons (Gutierrez et al. 2005). Thus both the receiving structure (dendrite) and the sending structure (axon) seem to be regulated by NF-κB. Current literature suggests that dendritic and axonal outgrowth can be regulated by NF-κB in different ways in different neurons. In cortical neurons, dendritic arborization is repressed by IκB (Gutierrez et al. 2005), whereas in hippocampal granule cells the axonal outgrowth depends on NF-κB activity (Kaltschmidt et al. unpublished). An important region of neuronal information processing is the axon initial segment (AIS). This region is extremely rich in membrane-embedded voltage-gated sodium channels and is the axon potential generating region. Interestingly, the phosphorylated forms of IKK-1, IKK-2, and IκB-α are concentrated within the AIS (Schultz et al. 2006). The node of Ranvier is also enriched in action potential generating sodium channels and also in phosphorylated forms of IKK 1, 2, and IκB-α (Politi et al. 2008). The localization of phosphorylated NF-κB regulators might indicate the major sites of intracellular NF-κB activation. Pharmacological blockade of IKK function in cultured hippocampal neurons interfered with the localization of phosphorylated IκB-α and IKK within the AIS (Sanchez-Ponce et al. 2008). In summary, these data show that NF-κB is an important regulator of neuronal morphology and shapes brain structures that are important for learning and memory.
A critical function of NF-κB in inhibitory GABAergic interneurons was observed in a recent transgenic mouse model (O’Mahony et al. 2006). Cell-type specific expression of super-repressor IκB-α (Prion promoter-driven tTA/tetO super-repressor IκB-α) in both inhibitory GABAergic interneurons (robust expression) and hippocampal excitatory glutamatergic neurons (low level expression) resulted in a phenotype opposite to that resulting from the inhibition of NF-κB in glutamatergic neurons only (Kaltschmidt et al. 2006). Expression of glutamate decarboxylase (GAD65), a rate-limiting enzyme required for the synthesis of the inhibitory neurotransmitter, GABA, was down-regulated in these mice. Super-repressor IκB mice completed a radial maze in less time and made fewer errors than control mice, indicating enhanced spatial learning and memory. Furthermore, LTP was enhanced. These findings might be explained by a model in which excitatory neurons are the motor of learning and GABAergic neurons provide the brake. A blockade of the brake (by expression of the super-repressor) would result in overactivation of the motor (excitatory neurons) and would explain the enhanced learning. Consistent with this is the observation that Baclofen, a pharmacological agonist of GABA, negatively affected learning (McNamara and Skelton 1996). A major advantage of super-repressor expression is the inhibition of all NF-κB subunits. On the other hand, overexpression might have unexpected gain of function effects. The p50–/– mice had defects in novel task acquisition (Kassed et al. 2002), decreased anxiety (Kassed and Herkenham 2004), and reduced short-term memory (Denis-Donini et al. 2008). Homodimers of p50, which lack a transactivation domain, might act as a repressor of gene transcription on a set of specific promoters. Thus, some of the effects observed in p50−/− animals might be in addition to the loss of p50 function, a result of the derepression of NF-κB target genes.
The function of NF-κB was analyzed in fear-conditioning paradigms, a form of learning in which an unconditioned stimulus like a tone is coupled to noxious stimulation such as electric shock. Fear-conditioning seems to depend on the amygdala, a region in which constitutive NF-κB activity has already been reported. Moreover, a c-Rel knockout was analyzed in a cued fear-conditioning paradigm and analyzed 24 h later. No amygdala-dependent changes in long-term memory were reported in c-Rel knockout animals (Levenson et al. 2004). The presentation of landmarks in the fear-conditioning paradigm gave quite different results, with deficits in freezing being observed. In addition, c-Rel−/− mice showed lower activity in an open field test. Bioinformatic analysis suggested an overrepresentation of NF-κB binding sites in the promoters of genes potentially involved in memory consolidation (Levenson et al. 2004), but this was not tested experimentally with, for example, ChIP. Likewise, a recent bioinformatic study has suggested an enrichment of NF-κB and E2F binding sites in genes potentially important for the development of neurons from neural precursors, and thus additional experiments are necessary (Greco et al. 2008).
Recently, a pharmacological study using DDTC (Diethyldithiocarbamate) and SN50 (a cyclic peptide, spanning the NLS of p50) showed impaired memory reconsolidation (Lubin and Sweatt 2007).
Overall, the results of behavioral analyses of mice with reduced NF-κB activity in brain (see Table 1) can be summarized as follows: Deletion of DNA-binding subunits in all cell types, including neurons and glia, resulted in lower performance in different behavioral tests. This phenotype was also seen following repression of NF-κB in glutamatergic neurons (CamKII tTA / tetO super-repressor), suggesting that NF-κB function in glutamatergic neurons is responsible for learning and memory. On the other hand, changing the balance of glutamatergic and GABAergic neurons by the higher expression of super-repressor in GABAergic neurons enhances the learning of spatial clues.
Table 1.
Genetic mouse models interfering with NF-κB activity in the nervous system
| Genotype | Cell type afflicted | Cognitive defect | Additional phenotype | References |
|---|---|---|---|---|
| p50−/− | All | Defect in novel task acquisition; decreased anxiety; reduced short-term memory | Reduced neuroprotection; hearing loss; reduced neurogenesis; reduced ischemic damage; impaired acute and inflammatory nociception | Yu et al. 1999; Yu et al. 2000; Kassed et al. 2002; Kassed and Herkenham 2004; Duckworth et al. 2006; Denis-Donini et al. 2008; Schneider et al. 1999, Niederberger et al. 2007 |
| p65−/− | Isolated sensory neurons | na* | Reduced neuroprotection | Middleton et al. 2000 |
| p65−/− | Isolated Schwann cells | na* | Reduced myelination of peripheral nerves; | Nickols et al. 2003 |
| p65−/− /TnfrI−/− | All | Delayed spatial learning in radial maze | No synaptic NF-κB | Meffert et al. 2003 |
| CamKII tTA/tetO super-repressor IκB-α | Glutamatergic forebrain neurons | Impairments in spatial memory; reduced LTP and LTD | Reduced neuroprotection; decreased PKA expression and P-CREB | Fridmacher et al. 2003; Kaltschmidt et al. 2006 |
| Prion-tTA/tetO super-repressor IκB-α | Glutamatergic and inhibitory neurons | Enhanced spatial learning; enhanced LTD | Reduced GAD65 expression | O’Mahony et al. 2006 |
| GFAP- super-repressor IκB-α | Glia: astrocytes | Deficits in learning only in females: delayed spatial learning, impaired cued fear memory | LTP reduced in females; LTP enhanced in males; reduction of mGluR5 in females; better recovery after spinal cord injury; reduced pain sensitivity | Bracchi-Ricard et al. 2008; Brambilla et al. 2005 |
| c-Rel−/− | All | Impaired late phase LTD; impaired long-term memory; impaired cued fear memory | Reduced neuroprotection | Pizzi et al. 2002; Levenson et al. 2004; Ahn et al. 2008 |
| LysM-Cre/IKK-2FL/FL | Glia: microglia; macrophages | na* | 30% reduction of neuronal death: 10-fold reduced infarct size after MCAO | Cho et al. 2008 |
| Nestin-Cre/IKK-2FL/FL | Neural (glia and neuron) | na* | 25% reduction of infarct size after MCAO; amelioration of EAE | Herrmann et al. 2005; van Loo et al. 2006 |
| Nestin-Cre/Ikk-1FL/FL | Neural (glia and neuron) | na* | No effect on EAE | van Loo et al. 2006 |
| Nestin-Cre/NemoFL/FL | Neural (glia and neuron) | na* | Amelioration of EAE | van Loo et al. 2006 |
| NSE-SR-IκB-α | Neuronal | na* | Improved LPS-induced hypothermia and survival | Juttler et al. 2007 |
na*: not analyzed.
HYPOTHALAMUS
Previous reports analyzing transgenic NF-κB lacZ reporter mice have described constitutive NF-κB activity within the hypothalamus (Schmidt-Ullrich et al. 1996; Bhakar et al. 2002). The hypothalamus contains the dominant neuroendocrine center for the control of food intake and energy expenditure. Recently, it was reported that a high fat chow increased the already activated NF-κB in the hypothalamus neurons two- to fourfold (De Souza et al. 2005). Zhang and coworkers reported high expression of IKK-2 and IκB-α in neurons of the mediobasal hypothalamus, a brain region involved in nutrition sensing. High fat chow, acute administration of glucose, or intraventricular injection of oleic acid hyper-activated NF-κB two- to fourfold (Zhang et al. 2008). Tissue-specific knockout of IKK-2 with Nestin CRE mice or virally transmitted CRE (lentivirus or adenovirus) injected into the hypothalamus reduced food consumption and weight gain in transgenic mice fed with high fat chow. In contrast, the neural expression of a constitutive form of IKK-2 enhanced weight gain after a high-fat diet and impaired hypothalamic sensitivity to insulin and leptin. Ablation of IKK-2 in specific AGRP hypothalamic neurons protected against induction of central insulin and leptin resistance after a high-fat diet. Zhang and coworkers identified SOCS3 (a suppressor of cytokine signaling) as a novel NF-κB target gene and a crucial regulator of diet-induced obesity. Hypothalamic neurons are now also considered to form a key center involved in sleep regulation (Mignot et al. 2002). Sleep-inducing substances include proinflammatory cytokines such as TNF and IL-1. TNFR1 knockout animals cannot sleep in response to TNF-α application (Fang et al. 1997).
A unique population of neurons immunoreactive for the p65 subunit of NF-κB was previously localized within the caudal dorsolateral hypothalamus of rats.
In relation to this, analysis of NF-κB-driven lacZ reporter mice has shown that sleep deprivation increases the number of cells expressing NF-κB-dependent β-galactosidase in the magnocellular lateral hypothalamus and zona incerta dorsal, as well as the adjacent subthalamus in transgenic mice (Brandt et al. 2004). Intracerebral injection of cell permeable NF-κB-inhibiting peptide spanning the NLS of p50 (SN50) inhibited IL-1-induced sleep in rats and rabbits (Kubota et al. 2000). LPS-induced hypothermia activated κB lacZ expression in brain, and neuronal expression of the IkB[α] super-repressor suppressed hypothermia and increased survival (Juttler et al. 2007).
NEUROPROTECTION
Initially observed in cerebellar granule neurons, a subtoxic dose of a neurotoxin (Aβ or Fe++) led to long-lasting NF-κB activation. This was protective against a higher dose of neurotoxins (Kaltschmidt et al. 1999; Kaltschmidt et al. 2002). This process was described by the 16th-century physician Theophrastus Bombastus von Hohenheim (Paracelsus): “All Ding sein Gift allein die Dosis machts” (all things are poisons only the dose is important). Preconditioning or hormesis (Mattson 2008) was dependent on activated NF-κB and might be a subcellular vaccination strategy, as suggested by D. Baltimore (Baltimore 1988). Further in vitro studies provided evidence that activation of NF-κB can protect neurons against amyloid β peptide toxicity (Barger et al. 1995) and excitotoxic or oxidative stress (Goodman and Mattson 1996; Mattson et al. 1997). Adenoviruses expressing super-repressor or dominant-negative NIK reduced survival of cortical neurons, whereas overexpression of p65 protected cortical neurons against apoptotic cell death induced by etopside (Bhakar et al. 2002). In vivo experiments in models of brain preconditioning with kainate or ischemia or linolenic acid showed NF-κB dependent protection against neuronal death (Blondeau et al. 2001). Later, transgenic inhibition of NF-κB by neuronal overexpression of the IkB[α] super-repressor decreased neuroprotection after kainic acid or Fe++ application (Fridmacher et al. 2003). In hippocampal acute slices derived from cRel−/− mice treatment with NMDA and IL-1β increased neurodegeneration (Pizzi et al. 2002). Surprisingly, under wild-type conditions no c-Rel containing DNA binding complexes were detected in adult brain (Kaltschmidt et al. 1993; Schmidt-Ullrich et al. 1996; Bakalkin et al. 1993; Meffert et al. 2003). Only activation of metabotropic glutamate receptors with, for example, 1 mM (R,S)-2-chloro-5-hydroxyphenylglycine (CHPG) uncovered the protective action of c-Rel, whereas RNA against the c-Rel or c-Rel−/− genotype had no cell death enhancing effect in neurons without pharmacological activation of the metabotropic glutamate receptor (Pizzi et al. 2005). Injection of neurotoxins in p50−/− mice such as trimethyltin (Kassed et al. 2004), excitotoxins (Yu et al. 1999), or mitochondrial toxin 3-nitropropionic acid (Yu et al. 2000) increased neuronal damage. Furthermore, p50−/− animals suffered from hearing loss because of degeneration of spiral ganglion neurons (Lang et al. 2006). Ischemia induced in p50−/− mice showed a clear neuroprotective role of NF-κB in the hippocampus and striatum, in which degenerating neurons were detected 4 d after 1 h of ischemia (MCAO paradigm) (Duckworth et al. 2006). Degenerating neurons did not show NF-κB-dependent reporter gene expression, and in addition p50−/− mice experienced a 2.4-fold increase in postoperative death in comparison to controls (Duckworth et al. 2006). At a first glance, these data appear to conflict with reports of reduced infarct volume in p50−/− mice and a 25% reduction in models with neuronal- or brain-specific IKK-2 ablation (Schneider et al. 1999). However, a recent analysis showed a 10-fold reduced infarct size in mice with microglial-specific IKK-2 ablation (Cho et al. 2008). Thus NF-κB-dependent microglia activation might be a crucial contributor to ischemia. Unfortunately, neuroprotective strategies developed in animal models do not work in human stroke (Rother 2008). There could be many reasons for this, such as translation arrest and/or increased vulnerability in human white matter. A summary of the neuroprotective effects observed in genetic models is presented in Table 1.
GLIA
No constitutive NF-κB activity was detected in glial cells of untreated animals (Schmidt-Ullrich et al. 1996; Bhakar et al. 2002), suggesting that inducible NF-κB activity might be linked to pathological events. Initially, it was reported that NF-κB activated in astrocytes via amyloid β peptide (Aβ) led to the production of nitric oxide (Akama et al. 1998). This astroglial neurodegenerative role of Aβ contrasts with the neuroprotective activation of NF-κB in neurons by nanomolar concentrations of Aβ (see previous discussion) (Kaltschmidt et al. 1997; Kaltschmidt et al. 1999). GFAP promoter-driven super-repressor IκB expression was present in astrocytes of brain, spinal cord, and peripheral nerves (Brambilla et al. 2005). Spinal cord contusion injury increased GFAP expression in activated astrocytes at the lesion site. Activated astrocytes release inflammatory mediators such as chemokines and cytokines. Unexpectedly, expression of the NF-κB target gene IL-6 was significantly up-regulated in GFAP super-repressor mice, whereas TNF expression remained unchanged (Brambilla et al. 2005). However, the expression of the chemokines CXCL10 and CCL2 was significantly down-regulated in comparison to wild-type mice after spinal-cord injury in GFAP SR mice. Outcome after spinal-cord injury, as measured by locomoter activity, was significantly ameliorated, suggesting that inducible astrocytic NF-κB does not function in cell survival but exacerbates chemokine-driven defects in spinal-cord recovery. Likewise, NF-κB inhibition resulted in reduced glial scarring, which perhaps allows better axon regeneration (Brambilla et al. 2005). Axotomy of the entorhinal perforant path projection resulted in astrocytic STAT2 up-regulation and phosphorylation and concomitant expression of CCL2. Using the same mouse model (GFAP-SR), it was shown that the lesion-induced expression of CCL2 and STAT2 was significantly blunted (Khorooshi et al. 2008). These results suggest that NF-κB signaling in astrocytes might regulate chemokine-induced infiltration of immune cells into the lesioned brain. Similarly, Nemo and IKK-2 deletion within the CNS resulted in a significant reduction of infiltration of inflammatory cells (van Loo et al. 2006). Experimental autoimmune encephalomyelitis (EAE), a model of human multiple sclerosis, was ameliorated after Nemo or IKK-2 deletion in astrocytes. A 10-fold reduction of nuclear p65 in astrocytes after IL-1 or TNF treatment resulted in a more than 10% reduction in chemokine production (CXCL10). These data underscore the pivotal role of astrocytes in EAE and the recruitment of infiltrating inflammatory cells. The role of microglia in neuroprotection or neurodegeneration is heavily debated. Recently, analysis of LysM-Cre/IKKFL/FL mice showed 36% deletion of the IKK β gene in cultured neonatal microglia (Cho et al. 2008), but only 4% deletion in adult microglia. Kainic acid injection resulted in the deletion of IKK-2 in 73% of microglial cells. Kainic acid-induced cell death was reduced, presumably by decreased activation of NF-κB-regulated microglial proinflammatory genes, such as TNF-α and IL-1β. A remarkable result was the 10-fold reduction of the infarct area after MCAO (Cho et al. 2008). In summary, IKK-2-mediated microglia activation potentiated neuronal excitotoxicity.
PAIN
Recently, a role of glial NF-κB in pain has emerged. Pain can arise from the activation of specific high-threshold PNS neurons (nociceptors) and could serve as a sensing mechanism to prevent further damage. However, clinical pain can arise from damage to the nervous system (neuropathic pain) or chronic inflammation (inflammatory pain). Analysis of p50−/− mice revealed an impairment of acute and inflammatory nociception (Niederberger et al. 2007). Recent data suggest an important role of astroglial NF-κB in pain perception: Inhibition of the expression of pain mediators (TNF-α, IL-6, and iNOS) by lentiviral delivery of IκB-α super-repressor injected into the dorsal horn reduced pain (Meunier et al. 2007). Similarly, GFAP super-repressor mice have decreased formalin pain sensation (Fu et al. 2007).
NEURAL STEM CELLS
During adulthood, neural stem cells continue to proliferate in the subventricular zone (SVZ) and within the hilus of the hippocampus. In adult neural stem cells isolated from the SVZ, p65 regulates proliferation via NF-κB target genes c-myc and cylin D1 (Widera et al. 2006). Furthermore, proliferation was strongly inhibited in neural stem cells prepared from the ganglionic eminence of p50−/− p65−/− embryos (Young et al. 2006). Expression of p65 and p50 persists into adulthood, particularly in subventricular zone astrocyte-like cells and in migrating neuronal precursors, respectively.
In particular, p65 and p50 are expressed in radial glial cells, in migrating neuronal precursors, and in a population belonging to the astrocytic lineage (Shingo et al. 2001). RelB, on the other hand, is only expressed in migrating neuronal precursors, whereas c-Rel is present in a few cells located at the edges of the rostral migratory stream (Denis-Donini et al. 2005).
In p50−/− animals, no defects in proliferation of hippocampal stem cells was detected (Denis-Donini et al. 2008). However, survival of neural progenitors after 21 days post BrdU injection was significantly reduced in p50−/− animals.
Taken together, current literature suggests that NF-κB fulfils very different functions in different cell types. A lot of controversial results were obtained in the last decade by neglecting the cell type-specific effect of NF-κB and the interplay between different neuronal cell types with glia.
In this line, the generation and analysis of sophisticated cell type-specific knockout models might be necessary to unravel all of the mysteries of NF-κB in the nervous system.
ABBREVIATIONS
- PNS:
peripheral nervous system
- CNS:
central nervous system
- MCAO:
middle cerebral artery occlusion
- EAE:
experimental autoimmune encephalomyelitis
- VGCC:
voltage-gated calcium channels
- GABA:
γ-aminobutyric acid
- AIS:
axon initial segment
- ChIP:
chromatin immunoprecipitation
- LTD:
long term depression
- LTP:
long term potentiation
This review focuses on evidence from genetic mouse models. Readers interested in pharmacological approaches to NF-κB function in the nervous system should consult earlier reviews by Kaltschmidt et al. (2005), Meffert and Baltimore (2005), and Romano et al. (2006) for further information.
Editors: Louis M. Staudt and Michael Karin
Additional Perspectives on NF-κB available at www.cshperspectives.org
REFERENCES
- Ahn HJ, Hernandez CM, Levenson JM, Lubin FD, Liou HC, Sweatt JD 2008. c-Rel, an NF-κB family transcription factor, is required for hippocampal long-term synaptic plasticity and memory formation. Learn Mem 15:539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akama KT, Albanese C, Pestell RG, Van Eldik LJ 1998. Amyloid β-peptide stimulates nitric oxide production in astrocytes through an NFκB-dependent mechanism. Proc Natl Acad Sci 95:5795–5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakalkin G, Yakovleva T, Terenius L 1993. NF-κ B-like factors in the murine brain. Developmentally-regulated and tissue-specific expression. Brain Res Mol Brain Res 20:137–146 [DOI] [PubMed] [Google Scholar]
- Baltimore D 1988. Gene therapy. Intracellular immunization. Nature 335:395–396 [DOI] [PubMed] [Google Scholar]
- Barger SW, Horster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP 1995. Tumor necrosis factors α and β protect neurons against amyloid β-peptide toxicity: Evidence for involvement of a κ B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci 92:9328–9332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakar AL, Tannis LL, Zeindler C, Russo MP, Jobin C, Park DS, MacPherson S, Barker PA 2002. Constitutive nuclear factor-κ B activity is required for central neuron survival. J Neurosci 22:8466–8475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau N, Widmann C, Lazdunski M, Heurteaux C 2001. Activation of the nuclear factor-κB is a key event in brain tolerance. J Neurosci 21:4668–4677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracchi-Ricard V, Brambilla R, Levenson J, Hu WH, Bramwell A, Sweatt JD, Green EJ, Bethea JR 2008. Astroglial nuclear factor-κB regulates learning and memory and synaptic plasticity in female mice. J Neurochem 104:611–623 [DOI] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR 2005. Inhibition of astroglial nuclear factor κB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 202:145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt JA, Churchill L, Rehman A, Ellis G, Memet S, Israel A, Krueger JM 2004. Sleep deprivation increases the activation of nuclear factor κ B in lateral hypothalamic cells. Brain Res 1004:91–97 [DOI] [PubMed] [Google Scholar]
- Cho IH, Hong J, Suh EC, Kim JH, Lee H, Lee JE, Lee S, Kim CH, Kim DW, Jo EK, et al. 2008. Role of microglial IKKβ in kainic acid-induced hippocampal neuronal cell death. Brain 131:3019–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA 2005. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146:4192–4199 [DOI] [PubMed] [Google Scholar]
- Denis-Donini S, Caprini A, Frassoni C, Grilli M 2005. Members of the NF-κB family expressed in zones of active neurogenesis in the postnatal and adult mouse brain. Brain Res Dev Brain Res 154:81–89 [DOI] [PubMed] [Google Scholar]
- Denis-Donini S, Dellarole A, Crociara P, Francese MT, Bortolotto V, Quadrato G, Canonico PL, Orsetti M, Ghi P, Memo M, et al. 2008. Impaired adult neurogenesis associated with short-term memory defects in NF-κB p50-deficient mice. J Neurosci 28:3911–3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson KM, Bhakar AL, Barker PA 2004. TRAF6-dependent NF-kB transcriptional activity during mouse development. Dev Dyn 231:122–127 [DOI] [PubMed] [Google Scholar]
- Duckworth EA, Butler T, Collier L, Collier S, Pennypacker KR 2006. NF-κB protects neurons from ischemic injury after middle cerebral artery occlusion in mice. Brain Res 1088:167–175 [DOI] [PubMed] [Google Scholar]
- Dudai Y 1989. The Neurobiology of Memory Oxford University Press, New York [Google Scholar]
- Fang J, Wang Y, Krueger JM 1997. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFα treatment. J Neurosci 17:5949–5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridmacher V, Kaltschmidt B, Goudeau B, Ndiaye D, Rossi FM, Pfeiffer J, Kaltschmidt C, Israel A, Memet S 2003. Forebrain-specific neuronal inhibition of nuclear factor-κB activity leads to loss of neuroprotection. J Neurosci 23:9403–9408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ES, Zhang YP, Sagen J, Yang ZQ, Bethea JR 2007. Transgenic glial nuclear factor-κ B inhibition decreases formalin pain in mice. Neuroreport 18:713–717 [DOI] [PubMed] [Google Scholar]
- Goodman Y, Mattson MP 1996. Ceramide protects hippocampal neurons against excitotoxic and oxidative insults, and amyloid β-peptide toxicity. J Neurochem 66:869–872 [DOI] [PubMed] [Google Scholar]
- Greco D, Somervuo P, Di Lieto A, Raitila T, Nitsch L, Castren E, Auvinen P 2008. Physiology, pathology and relatedness of human tissues from gene expression meta-analysis. PLoS ONE 3:e1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H, Hale VA, Dolcet X, Davies A 2005. NF-κB signalling regulates the growth of neural processes in the developing PNS and CNS. Development 132:1713–1726 [DOI] [PubMed] [Google Scholar]
- Herrmann O, Baumann B, de Lorenzi R, Muhammad S, Zhang W, Kleesiek J, Malfertheiner M, Kohrmann M, Potrovita I, Maegele I, et al. 2005. IKK mediates ischemia-induced neuronal death. Nat Med 11:1322–1329 [DOI] [PubMed] [Google Scholar]
- Juttler E, Inta I, Eigler V, Herrmann O, Maegele I, Maser-Gluth C, Schwaninger M 2007. Neuronal NF-κB influences thermoregulation and survival in a sepsis model. J Neuroimmunol 189:41–49 [DOI] [PubMed] [Google Scholar]
- Kaltschmidt C, Kaltschmidt B, Baeuerle PA 1993. Brain synapses contain inducible forms of the transcription factor NF-κ B. Mech Dev 43:135–147 [DOI] [PubMed] [Google Scholar]
- Kaltschmidt C, Kaltschmidt B, Neumann H, Wekerle H, Baeuerle PA 1994. Constitutive NF-κ B activity in neurons. Mol Cell Biol 14:3981–3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt C, Kaltschmidt B, Baeuerle PA 1995. Stimulation of ionotropic glutamate receptors activates transcription factor NF-κ B in primary neurons. Proc Natl Acad Sci 92:9618–9622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Uherek M, Volk B, Baeuerle PA, Kaltschmidt C 1997. Transcription factor NF-κB is activated in primary neurons by amyloid β peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc Natl Acad Sci 94:2642–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Uherek M, Wellmann H, Volk B, Kaltschmidt C 1999. Inhibition of NF-κB potentiates amyloid β-mediated neuronal apoptosis. Proc Natl Acad Sci 96:9409–9414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Heinrich M, Kaltschmidt C 2002. Stimulus-dependent activation of NF-κB specifies apoptosis or neuroprotection in cerebellar granule cells. Neuromolecular Med 2:299–309 [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, Widera D, Kaltschmidt C 2005. Signaling via NF-κB in the nervous system. Biochim Biopyhs Acta 1745:287–299 [DOI] [PubMed] [Google Scholar]
- Kaltschmidt B, Ndiaye D, Korte M, Pothion S, Arbibe L, Prullage M, Pfeiffer J, Lindecke A, Staiger V, Israel A, et al. 2006. NF-κB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol Cell Biol 26:2936–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER 2001. The molecular biology of memory storage: A dialogue between genes and synapses. Science 294:1030–1038 [DOI] [PubMed] [Google Scholar]
- Kassed CA, Herkenham M 2004. NF-κB p50-deficient mice show reduced anxiety-like behaviors in tests of exploratory drive and anxiety. Behav Brain Res 154:577–584 [DOI] [PubMed] [Google Scholar]
- Kassed CA, Willing AE, Garbuzova-Davis S, Sanberg PR, Pennypacker KR 2002. Lack of NF-κB p50 exacerbates degeneration of hippocampal neurons after chemical exposure and impairs learning. Exp Neurol 176:277–288 [DOI] [PubMed] [Google Scholar]
- Kassed CA, Butler TL, Patton GW, Demesquita DD, Navidomskis MT, Memet S, Israel A, Pennypacker KR 2004. Injury-induced NF-κB activation in the hippocampus: Implications for neuronal survival. FASEB J 18:723–724 [DOI] [PubMed] [Google Scholar]
- Khorooshi R, Babcock AA, Owens T 2008. NF-κB-driven STAT2 and CCL2 expression in astrocytes in response to brain injury. J Immunol 181:7284–7291 [DOI] [PubMed] [Google Scholar]
- Kubota T, Kushikata T, Fang J, Krueger JM 2000. Nuclear factor-κB inhibitor peptide inhibits spontaneous and interleukin-1β-induced sleep. Am J Physiol Regul Integr Comp Physiol 279:R404–413 [DOI] [PubMed] [Google Scholar]
- Lamprecht R, LeDoux J 2004. Structural plasticity and memory. Nat Rev Neurosci 5:45–54 [DOI] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Zhou D, Smythe N, Spicer SS, Schmiedt RA 2006. Nuclear factor κB deficiency is associated with auditory nerve degeneration and increased noise-induced hearing loss. J Neurosci 26:3541–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, Choi S, Lee SY, Cao YA, Ahn HJ, Worley KC, Pizzi M, Liou HC, Sweatt JD 2004. A bioinformatics analysis of memory consolidation reveals involvement of the transcription factor c-rel. J Neurosci 24:3933–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienbaum A, Israel A 2003. From calcium to NF-κ B signaling pathways in neurons. Mol Cell Biol 23:2680–2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Sweatt JD 2007. The IκB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron 55:942–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP 2008. Hormesis defined. Ageing Res Rev 7:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Goodman Y, Luo H, Fu W, Furukawa K 1997. Activation of NF-κB protects hippocampal neurons against oxidative stress-induced apoptosis: Evidence for induction of manganese superoxide dismutase and suppression of peroxynitrite production and protein tyrosine nitration. J Neurosci Res 49:681–697 [DOI] [PubMed] [Google Scholar]
- McNamara RK, Skelton RW 1996. Baclofen, a selective GABAB receptor agonist, dose-dependently impairs spatial learning in rats. Pharmacol Biochem Behav 53:303–308 [DOI] [PubMed] [Google Scholar]
- Meberg PJ, Kinney WR, Valcourt EG, Routtenberg A 1996. Gene expression of the transcription factor NF-κB in hippocampus: Regulation by synaptic activity. Brain Res Mol Brain Res 38:179–190 [DOI] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D 2003. NF-κ B functions in synaptic signaling and behavior. Nat Neurosci 6:1072–1078 [DOI] [PubMed] [Google Scholar]
- Meffert MK, Baltimore D 2005. Physiological functions for brain NF-κB. Trends Neurosci 28:37–43 [DOI] [PubMed] [Google Scholar]
- Meunier A, Latremoliere A, Dominguez E, Mauborgne A, Philippe S, Hamon M, Mallet J, Benoliel JJ, Pohl M 2007. Lentiviral-mediated targeted NF-κB blockade in dorsal spinal cord glia attenuates sciatic nerve injury-induced neuropathic pain in the rat. Mol Ther 15:687–697 [DOI] [PubMed] [Google Scholar]
- Middleton G, Hamanoue M, Enokido Y, Wyatt S, Pennica D, Jaffray E, Hay RT, Davies AM 2000. Cytokine-induced nuclear factor κ B activation promotes the survival of developing neurons. J Cell Biol 148:325–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E, Taheri S, Nishino S 2002. Sleeping with the hypothalamus: Emerging therapeutic targets for sleep disorders. Nat Neurosci 5 Suppl:1071–1075 [DOI] [PubMed] [Google Scholar]
- Mikenberg I, Widera D, Kaus A, Kaltschmidt B, Kaltschmidt C 2007. Transcription factor NF-κB is transported to the nucleus via cytoplasmic dynein/dynactin motor complex in hippocampal neurons. PLoS ONE 2:e589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols JC, Valentine W, Kanwal S, Carter BD 2003. Activation of the transcription factor NF-κB in Schwann cells is required for peripheral myelin formation. Nat Neurosci 6:161–167 [DOI] [PubMed] [Google Scholar]
- Niederberger E, Schmidtko A, Gao W, Kuhlein H, Ehnert C, Geisslinger G 2007. Impaired acute and inflammatory nociception in mice lacking the p50 subunit of NF-κB. Eur J Pharmacol 559:55–60 [DOI] [PubMed] [Google Scholar]
- O’Mahony A, Raber J, Montano M, Foehr E, Han V, Lu SM, Kwon H, LeFevour A, Chakraborty-Sett S, Greene WC 2006. NF-κB/Rel regulates inhibitory and excitatory neuronal function and synaptic plasticity. Mol Cell Biol 26:7283–7298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzi M, Goffi F, Boroni F, Benarese M, Perkins SE, Liou HC, Spano P 2002. Opposing roles for NF-κ B/Rel factors p65 and c-Rel in the modulation of neuron survival elicited by glutamate and interleukin-1β. J Biol Chem 277:20717–20723 [DOI] [PubMed] [Google Scholar]
- Pizzi M, Sarnico I, Boroni F, Benarese M, Steimberg N, Mazzoleni G, Dietz GP, Bahr M, Liou HC, Spano PF 2005. NF-κB factor c-Rel mediates neuroprotection elicited by mGlu5 receptor agonists against amyloid β-peptide toxicity. Cell Death Differ 12:761–772 [DOI] [PubMed] [Google Scholar]
- Politi C, Del Turco D, Sie JM, Golinski PA, Tegeder I, Deller T, Schultz C 2008. Accumulation of phosphorylated I κB α and activated IKK in nodes of Ranvier. Neuropathol Appl Neurobiol 34:357–365 [DOI] [PubMed] [Google Scholar]
- Romano A, Freudenthal R, Merlo E, Routtenberg A 2006. Evolutionarily-conserved role of the NF-kappaB transcription factor in neural plasticity and memory. Eur J Neurosci 24:1507–1516 [DOI] [PubMed] [Google Scholar]
- Rother J 2008. Neuroprotection does not work! Stroke 39:523–524 [DOI] [PubMed] [Google Scholar]
- Sanchez-Ponce D, Tapia M, Munoz A, Garrido JJ 2008. New role of IKK α/β phosphorylated I κ B α in axon outgrowth and axon initial segment development. Mol Cell Neurosci 37:832–844 [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Memet S, Lilienbaum A, Feuillard J, Raphael M, Israel A 1996. NF-κB activity in transgenic mice: Developmental regulation and tissue specificity. Development 122:2117–2128 [DOI] [PubMed] [Google Scholar]
- Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M 1999. NF-κB is activated and promotes cell death in focal cerebral ischemia. Nat Med 5:554–559 [DOI] [PubMed] [Google Scholar]
- Schultz C, Konig HG, Del Turco D, Politi C, Eckert GP, Ghebremedhin E, Prehn JH, Kogel D, Deller T 2006. Coincident enrichment of phosphorylated IκBα, activated IKK, and phosphorylated p65 in the axon initial segment of neurons. Mol Cell Neurosci 33:68–80 [DOI] [PubMed] [Google Scholar]
- Shingo T, Sorokan ST, Shimazaki T, Weiss S 2001. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci 21:9733–9743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrum CK, Defrancisco D, Meffert MK 2009. Stimulated nuclear translocation of NF-κB and shuttling differentially depend on the dynactin complex. PNAS 106:2647–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loo G, De Lorenzi R, Schmidt H, Huth M, Mildner A, Schmidt-Supprian M, Lassmann H, Prinz MR, Pasparakis M 2006. Inhibition of transcription factor NF-κB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol 7:954–961 [DOI] [PubMed] [Google Scholar]
- Wellmann H, Kaltschmidt B, Kaltschmidt C 2001. Retrograde transport of transcription factor NF-κ B in living neurons. J Biol Chem 276:11821–11829 [DOI] [PubMed] [Google Scholar]
- Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B 2006. Tumor necrosis factor α triggers proliferation of adult neural stem cells via IKK/NF-κB signaling. BMC Neurosci 7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KM, Bartlett PF, Coulson EJ 2006. Neural progenitor number is regulated by nuclear factor-κB p65 and p50 subunit-dependent proliferation rather than cell survival. J Neurosci Res 83:39–49 [DOI] [PubMed] [Google Scholar]
- Yu Z, Zhou D, Bruce-Keller AJ, Kindy MS, Mattson MP 1999. Lack of the p50 subunit of nuclear factor-κB increases the vulnerability of hippocampal neurons to excitotoxic injury. J Neurosci 19:8856–8865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Zhou D, Cheng G, Mattson MP 2000. Neuroprotective role for the p50 subunit of NF-κB in an experimental model of Huntington’s disease. J Mol Neurosci 15:31–44 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D 2008. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]