Abstract
The organization of metazoa is based on the formation of tissues and on tissue-typical functions and these in turn are based on cell–cell connecting structures. In vertebrates, four major forms of cell junctions have been classified and the molecular composition of which has been elucidated in the past three decades: Desmosomes, which connect epithelial and some other cell types, and the almost ubiquitous adherens junctions are based on closely cis-packed glycoproteins, cadherins, which are associated head-to-head with those of the hemi-junction domain of an adjacent cell, whereas their cytoplasmic regions assemble sizable plaques of special proteins anchoring cytoskeletal filaments. In contrast, the tight junctions (TJs) and gap junctions (GJs) are formed by tetraspan proteins (claudins and occludins, or connexins) arranged head-to-head as TJ seal bands or as paracrystalline connexin channels, allowing intercellular exchange of small molecules. The by and large parallel discoveries of the junction protein families are reported.
Werner Franke looks back over more than three decades of research into the structure and function of cell junctions.
In the year of the bicenturial jubilee of Charles Darwin (born 1809) and his 1859 publication of the concept of natural selection as the decisive driving force of evolution, it is perhaps appropriate to begin this review with the notion that the four major kinds of cell–cell junctions are among the oldest and most important structures contributing to the formation and functional diversification of multilayered metazoan organisms. From mere associations of individual cells, whether protozoan or parazoan, it was the cooperation of the molecular ensembles of these junctions to provide the basis for eumetazoan life. Note, however, that some protocadherin glycoproteins and armadillo-type proteins already occur in certain nonmetazoa (e.g., King et al. 2003; Nichols et al. 2006; for refs. Halbleib and Nelson 2006). In particular, diverse cell–cell junction molecules were—and are—needed to assemble and organize metazoan architecture, notably that of the Bilateria, to allow the formation of the epithelial layers of ectoderm and endoderm, mesoderm-derived tissues, and the segregation of diverse kinds of interstitial cells and the organs derived therefrom. So, it is not so surprising that major kinds of cell junctional structures already exist in the lowest divisions of eumetazoa (e.g., Hobmayer et al. 1996, 2000).
In general, metazoan animals possess three intercellular junction systems of the adhering type formed by characteristic transmembrane molecules and proteins that assemble into specific submembranous plaques (Table 1). A fundamentally different type, gap junctions, serves primarily as a system of assemblies of intercellular channels but also contributes to cell–cell adhesion. In summarizing the history of the discoveries of the molecular ensembles forming these junctions, it is striking that remarkably different arts of science and tribes of cell biologists have contributed to the progress in this field. The intercellular junctional systems are:
Desmosomes (maculae adherentes) are by far the most abundant junctions in stratified epithelia and have been studied with special impetus by researchers analyzing the cytoskeleton and tissue architecture, and by dermatologists.
Adherens junctions, including the zonulae and fasciae adherentes, have first attracted the special interest of developmental biologists because of their importance in mammalian embryogenesis.
Tight junctions (zonulae occludentes), and in particular the transmembrane molecules involved, had long been sought as this structure was important in controlling the paracellular transport of molecules and particles. Finally, the centrally important tetraspan proteins, desired by a generation of physiologists and membranologists, have been found as the result of careful cell particle fractionation work and immunoelectron microscopy.
Gap junctions (nexus) had always fascinated electron microscopists and crystallographers because of their esthetic paracrystalline substructural order, as well as physiologists studying lateral direct molecule exchange from cell to cell. Here, the stability of the structure itself helped in its isolation and reconstitution in vitro.
Table 1.
Constitutive molecular components of the major types of symmetrical (homotypic) junctions
| Occurrence | Associated filaments | Transmembrane proteins and glycoproteins | Specific plaque proteins | |
|---|---|---|---|---|
| DesmosomesMaculae adherentes | Epithelial cells, various types of cardiomyocytes, meningothelial cells, dendritic reticulum cells of the thymus and lymph follicles | Intermediate-sized filaments (keratins, vimentin, desmin) | Desmogleins 1–4a desmocollins 1–3a | Plakoglobinb desmoplakin I/II plakophilins 1–3a |
| Adherens junctionsZonulae adherentes Fasciae adherentes Puncta adhaerentia | Epithelial cells, endothelial cells, various types of cardiomyocytes, mesenchymal and neural cells | Microfilaments (actin) | Typical cadherinsa (e.g., E-cadherin, N-cadherin, P-cadherin, VE-cadherin, cadherin-11) | α- and β-Catenin, plakoglobin, protein p120, protein ARVCF, protein p0071, neurojungin (δ-catenin)a, (plakophilin-2c) proteins ZO-1, ZO-2, ZO-3 |
| Tight junctionsZonulae occludentes Fasciae occludentes Puncta adhaerentia | Epithelial cells, endothelial cells | –d | Occludin, claudins 1–24a tricellulin(s)e proteins of the JAMA-group, CARa, ESAMa | Proteins ZO-1, ZO-2, ZO-3, cingulin |
| Gap junctions (nexus) | All kinds of tissue-forming cells | – | Connexins 1–21a | Proteins ZO-1, ZO-2, ZO-3 |
Only established, i.e., repeatedly confirmed, constituent structural molecules are mentioned here; some further regulatory or peripherally associated molecules are not listed here but are discussed in the text.
aOne or combinations of a few representatives, with cell type and cell layer specificities.
bProteins of the so-called armadillo family are in italics.
cOnly in specific proliferatively active cells (Rickelt et al. 2009).
dActin microfilaments are seen near some tight junctions but their specific association is not clear.
eThere are at least two mRNA splice products but only one protein has so far been localized.
Thus, at about the same time in the late 1970s, when the ultrastructural organization and specificities of the diverse kinds of these junctions had been well determined (for reviews, see Farquhar and Palade 1963; Staehelin 1974), the race by cell biologists to elucidate the molecular compositions of these junctions began. Although this analytical research period is not quite over and a few new junction diamonds may still just lie around the corner, the prime interest of the community of cell biological researchers has already moved on to the next research arena, studying the mechanisms of junction formation and the functions of the junctions. Over the last two decades, these major structural elements of our bodies have also become objects of intense medical research, already with some startling results.
In the following, the by and large parallel searches for constituent molecules of the four major categories of cell–cell junctions in vertebrate cells is described. However, only constituents localized and confirmed by different groups and with various methods are mentioned as generally accepted components. This, of course, does not exclude the presence of others for which the available evidence does not yet seem sufficient. Furthermore, certain special types of junctions that do not fit one of the four major categories will be reviewed elsewhere (Franke et al. 2009).
HARD-CORE ANCHORS OF CYTOSKELETAL ELEMENTS: THE DESMOSOMES
The desmosomes represent a category of intercellular junctions that typically reveal a highly distinctive subarchitecture. They are abundant in epithelial and meningothelial tissues and cell cultures, as well as in certain types and stages of cardiomyocyte differentiation, but are also known to connect reticulum cells of the thymus and lymphatic follicles. Because of their relatively good preservation after diverse electron microscopic fixation procedures and their abundance in certain tissues (e.g., in some regions of the stratum spinosum of the epidermis and other stratified squamous epithelia, they occupy 50% or even more of the total cell surface), the ultrastructural principles of desmosome architecture were more or less already clear in the 1970s (see, e.g., Farquhar and Palade 1963; Campbell and Campbell 1971; Staehelin, 1974; for cardiomyocytes see Fawcett and McNutt, 1969).
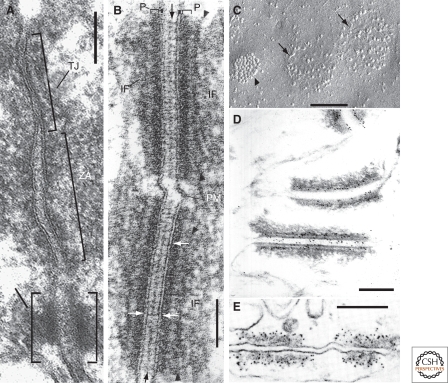
The appearance of desmosomes in electron micrographs of ultrathin sections impresses observers by the parallel alignment of two trilaminar plasma membrane domains, an organized 20–30-nm interface (the “desmogloea”), which often reveals a dense midline structure (the glutinamentum), and periodical cross-striations between this midline and the plasma membranes (Fig. 1A,B). On their cytoplasmic side, the membranes of the desmosomal halves are coated by an electron-dense, 15–25 nm thick plaque, which, in turn, is coated by somewhat less dense material onto which in many, but not in all cells, bundles of intermediate-sized filaments (IFs) insert. Freeze-fractures further reveal the membrane interior of desmosomes as relatively loosely arranged arrays of clustered transmembrane complexes (Fig. 1C) (Leloup et al. 1979).
Figure 1.
Electron microscopic aspects of intercellular junctions, in particular desmosomes. (A) Characteristic subapical trias of tight junction (TJ), zonula adhaerens (ZA), and desmosome (D) of murine intestinal epithelial cells in a longitudinal section. (B) Higher magnification of two adjacent desmosomes in fetal (20 wk) human foot-sole epidermis, showing desmosomal substructures (black arrows: midline structure; white arrows: trilaminar “unit membrane” structure of the plasma membrane domain proper, also pointing to electron dense cross-bridge structures; arrowheads: secondary dense layer of the plaque; P: electron-dense primary plaques; IF; anchoring structures of intermediate-sized filaments). (C) Freeze-fracture image of spinous layer of murine epidermis, showing the intramembranous fracture plane of the plasma membrane with two desmosomes (arrows) and a gap junction (arrowhead). Note the typical paracrystalline packing of the connexin substructures in the gap junction in comparison with the loosely and irregularly arranged transmembrane elements of the desmosomes. (D) Immunoelectron microscopy of an ultrathin section through an epithelium, showing the immunogold decoration (5-nm particles) of desmoplakin at—or near—the desmosomal plaque structures. (E) Immunoelectron microscopy of an ultrathin cross-section through the zonula adhaerens of plasma membranes connecting two endothelial cells of a cardiac capillary, showing the specific 5-nm immunogold decoration of the junctional plaque with plakoglobin antibodies, thus demonstrating that plakoglobin can also occur in the plaques of nondesmosomal adhering junctions. Bars denote 0.1 µm (A, B) and 0.5 µm (C–E). For details, see Cowin et al. (1985a), Franke et al. (1987b), Kapprell et al. (1987), and Schlüter et al. (2007).
The basis for biochemical analyses and the molecular identification of components was laid by a good choice of appropriate tissues, for example bovine muzzle epidermis, for the enrichment of isolated desmosomal structures. The results were markedly dependent on the specific fractionation method used, with more or less preserved plaque structures and “contaminations” by other elements, in particular IF bundle residues (e.g., Skerrow and Matoltsy 1974a,b; Drochmans et al. 1978; Colaco and Evans 1981; Franke et al. 1981; Gorbsky and Steinberg 1981; Mueller and Franke 1983; for review see Skerrow 1986). Although gel electrophoretic analyses of proteins from such fractions allowed some educated guesses about molecular components of desmosomes, it took the direct demonstration of specific proteins by immunofluorescence and immunoelectron microscopy to provide definitive evidence of desmosomal protein localizations. This was first presented at the Cold Spring Harbor Symposium on the “Organization of the Cytoplasm” in the summer of 1981 for the major desmosome-specific plaque protein, desmoplakin, using antibodies that identified desmoplakins I and II and some proteolytic breakdown products thereof (cf. Franke et al. 1982b; see also Franke et al. 1981, 1982a,b) (for an example, see Fig. 1D). Later, K. Green and her group determined that desmoplakins I and II are two splice mRNA forms derived from the same gene and that this large protein presents a very special organization with a near–amino-terminal plakin domain, a central coiled–coil region, and a so-called plakin-repeat domain in the carboxy-terminal portion (Green et al. 1988, 1990; Virata et al. 1992; Godsel et al. 2004), so to say the prototype of what later was termed the “plakin family” of proteins (Ruhrberg and Watt 1997).
The identification of the desmoplakins was soon followed by the localization of epidermal-type desmosomal proteins and glycoproteins in various cell types and species, although not in all cells that were known to contain true desmosomes (cf. Cohen et al. 1983; Cowin and Garrod 1983; Mueller and Franke 1983; Cowin et al. 1984a,b, 1985a,b; Giudice et al. 1984; Gorbsky et al. 1985). As an explanation for cell-type differences, the problem of cell-type specific proteins of complex gene families arose, an experience this research field had just come across with the bewildering complexity of IF proteins.
Two additonal constitutive proteins of desmosomal plaques were identified based on peptide mapping and immunoblotting: They were originally counted as desmosomal bands five- and six-proteins (Franke et al. 1983b) and later named plakoglobin and plakophilin-1 (see also Table 1). Plakoglobin was a particularly challenging component as it turned out also to be a major protein of the plaques of diverse morpho-types of adherens junctions (AJs), including the zonulae adherentes of endothelia (e.g., Fig. 1E) (Cowin et al. 1986; Franke et al. 1987a,b). Plakoglobin was also a very attractive molecule for cDNA- and gene-cloning and soon after the human amino acid sequence had been published (Franke et al. 1989, 1992; Fouquet et al. 1992), Peifer and Wieschaus (1990) recognized that plakoglobin was a close homolog to the armadillo protein encoded by the Drosophila melanogaster segment polarity gene (see also McCrea et al. 1991). This finding resulted in an avalanche of publications leading to the definition of a large and important multigene family, the armadillo (arm) proteins of adhering junctions in general, i.e., AJs and desmosomes. This family includes a number of components (e.g., plakoglobin, β-catenin, proteins p120, p0071, ARVCF, neurojungin, and the plakophilins) that serve more than one function and occur in junction-bound forms as well as in special cytoplasmic and nuclear forms (Peifer et al. 1992, 1994; for reviews, see Hatzfeld 1999; Schmidt and Koch 2008).
In parallel, several laboratories had begun to clone and identify the genes encoding the polypeptide chains of the two prominent desmosomal glycoproteins, desmoglein (Dsg) and desmocollin (Dsc), again starting with RNA isolated from bovine muzzle or related stratified epithelia. Both the Dsg and Dsc molecules revealed amino acid sequences and carbohydrate branch sites that were markedly homologous to those of the “classic” cadherins such as E- and N-cadherin, which had just been identified as the major transmembrane AJ molecules (Koch et al. 1990, 1991a,b; Goodwin et al. 1990; Holton et al. 1990; Amagai et al. 1991; Collins et al. 1991; Mechanic et al. 1991; Nilles et al. 1991; Parker et al. 1991; Wheeler et al. 1991a,b; King et al. 1993a,b; Theis et al. 1993). Remarkably, in less than 3 years, the cDNAs of all six known desmosomal cadherins were cloned and sequenced. Again, however, these amino acid sequences showed that Dsg and Dsc from different cell types differed from each other. Furthermore, the cytoplasmic carboxy-terminal portion of Dsg contained several distinct sequence subdomains (Koch et al. 1990; reviews: Franke et al. 1992; Koch and Franke 1994; Garrod et al. 2002; Godsel et al. 2004) and not only spans the plasma membrane but also extends through the cytoplasmic plaque, as shown by the accessibility to antibodies directly injected into living cultured cells (Schmelz et al. 1986a,b).
Both Dsg and Dsc appeared in cell type- and cell layer-specific subforms (Parrish et al. 1990; Buxton and Magee 1992; Koch et al. 1992; Buxton et al. 1993; King et al. 1993a,b, 1995, 1996, 1997; Koch and Franke 1994). Only one of each group, Dsg2 and Dsc2, was found in all proliferative cells such as one-layered (“simple”) epithelia, basal cell layers of stratified epithelia, meningothelia, cardiomyocytes, and reticulum cells of lymphatic tissues (Schäfer et al. 1994, 1996; Nuber et al. 1995, 1996). By contrast, highly differentiated upper cell layers of several stratified epithelia contained Dsg1 and Dsc1 as predominant desmosomal cadherins, often together with Dsg3 and Dsc3 (Arnemann et al. 1993; King et al. 1993b, 1997; Theis et al. 1993; Legan et al. 1994; Nuber et al. 1996). Dsg4 was a very late addition, synthesized only in the uppermost living cell strata of the epidermis (Kljuic et al. 2003; Bazzi et al. 2006; reviews: Godsel et al. 2004; Schmidt and Koch 2008).
It is striking in desmosomes that both Dsg and Dsc almost always appear together as a pair, probably forming isostoichiometric oligomers that are stabilized by plakoglobin (see, e.g., Troyanovsky et al. 1993, 1994a,b, 1996; Chitaev et al. 1996; Witcher et al. 1996; Chitaev and Troyanovsky 1997; Marcozzi et al. 1998; review: Troyanovsky 2005). Therefore, it came as a surprise when it was recently noted that at least in some nonepithelial cell types (e.g., melanocytes and melanoma cells growing in culture, and melanoma cells in situ), a desmosomal glycoprotein, Dsg2, can occur as an abundant surface component out of any junctional complex (Schmitt et al. 2007; Rickelt et al. 2008).
The last elucidated subfamily of desmosomal components were the plakophilins, apparently located deep in the plaque (see, e.g., Mertens et al. 1996; for differential electron microscopy of desmosomal components, see North et al. 1999; Stokes 2007). Plakophilin was originally numbered as “band 6 protein” in bovine muzzle desmosomes and had been identified as a protein restricted to stratified and complex epithelia (Kapprell et al. 1988). However, it still took several years until the amino acid sequence of this protein, then named plakophilin-1, had been determined as another arm-protein (Schäfer et al. 1993; Hatzfeld et al. 1994; Schmidt et al. 1994). This protein was soon followed by plakophilin-2, the most widespread member of this subgroup of arm-proteins and the only one present in the desmosomes of simple epithelial cells as well as in nonepithelial desmosomes (Mertens et al. 1996, 1999). Finally, plakophilin-3, which coexists with plakophilin-2 in many desmosomes, has also been sequenced and localized (Bonné et al. 1999, 2003; Schmidt et al. 1999). Similar to plakoglobin and β-catenin, plakophilins are frequently found in distinct particles in the nucleus and the cytoplasm, and such particles may include essential nuclear components such as the complexes containing RNA polymerase III (e.g., Mertens et al. 1996, 2001; Schmidt et al. 1997; Bonné et al. 1999).
With remarkable speed, the cell biological insights obtained and the reagents generated for desmosomes were introduced into developmental biology and the medical sciences. Desmosomal molecules could be shown in very different normal and malignantly transformed cell types, either in layers of tightly associated cells or in arrays resembling spinous layer-type growth forms, in which the cells are connected by special bridges containing desmosomes (Fig. 2A,B) as well as AJs of the puncta adherentia type (Vasioukhin et al. 2000). Moreover, the use of subtype-specific antibodies against members of the plakophilin and the cadherin glycoprotein families allowed the determination of not only the cell type but also a certain state of epithelial differentiation in normal tissues and in tumors (Koch et al. 1992; King et al. 1995, 1996, 1997; Nuber et al. 1995, 1996; Schäfer et al. 1996). Consequently, very soon after their first publications in scientific journals, antibodies against cell-type-specific desmosomal proteins were added to the immunodiagnostic armamentarium of pathologists (e.g., Franke et al. 1983a; Schwechheimer et al. 1984; Denk et al. 1985b; Parrish et al. 1986, 1987; Vilela et al. 1987; reviews: Moll et al. 1986; Garrod et al. 1996; Kottke et al. 2006).
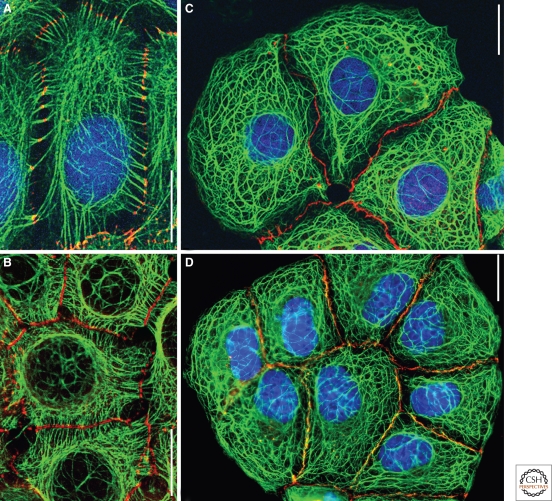
Figure 2.
Double-label immunofluorescence microscopy of monolayer cultures of epithelial cells derived from human multilayered (A, keratinocytes of line HaCaT; B, squamous cell carcinoma-derived line A-431) or one-layered (C, D: liver carcinoma cells of line PLC) tissues. Two forms of attachment of bundles of keratin IFs (green, mouse monoclonal antibody mAb lu-5) to the plaques of desmosomes (A, B: red, desmoplakin, guinea pig antibodies) represent a continuous transcellular cytoskeletal system (the chromatin in the nuclei of A, C, and D is stained blue with DAPI reagent): The cells in A are connected by cell-to-cell bridges with near-centrally located desmosomes, whereas in B the bodies of the cells are directly and tightly associated with each other via numerous, closely spaced desmosomes. In contrast, no specific anchorage of keratin IF bundles is seen at cell junctions of the zonula or punctum adherens type, which are seen by immunoreaction for β-catenin (C, red, guinea pig antibodies) or protein p0071 (D, red, murine mAb). Bars: 20 µm.
Specific probes for desmosomal molecules and their genes soon allowed valuable and pioneering contributions to the elucidation of, for example, autoantibody-caused diseases such as those of the pemphigus kind (Korman et al. 1989; Amagai et al. 1991; reviews: Amagai 1999; Kottke et al. 2006; Holthöfer et al. 2007; Schmidt and Koch 2008; Waschke 2008; see also Delva et al. 2009). Notably, studies of the molecular biology of the genes encoding desmosomal proteins, in particular gene abrogation experiments in mice (e.g., Bierkamp et al. 1996; Ruiz et al. 1996; Gallicano et al. 1998, 2001; Grossmann et al. 2004; Zhou et al. 2004), paved the way for the identification of the causes of a large proportion of human hereditary cardiomyopathies, most strikingly the majority of the forms of arrhythmogenic cardiomyopathies, which often lead to so-called “sudden death” (Gerull et al. 2004; Franke et al. 2009; and Delva et al. 2009).
The definition of the molecular ensembles characteristic of various types of desmosomes and the reagents to distinguish desmosomal and desmosome precursor assemblies from components of AJs (Geiger et al. 1983) also provided the basis for studies of molecular mechanisms involved in the intracellular assembly of desmosomes, their IF anchorage, their exocytotic exposure on the cell surface, and their endocytotic resumption, notably on depletions of Ca2+ ions in the surrounding media (e.g., Kartenbeck et al. 1982, 1991; Watt et al. 1984; Duden and Franke 1988; Pasdar and Nelson 1988a,b; Stappenbeck et al. 1993; Kowalczyk et al. 1994, 1997, 1998; Demlehner et al. 1995; Bornslaeger et al. 1996; Palka and Green 1997; Smith and Fuchs 1998; Hofmann et al. 2000; Chen et al. 2002; Koeser et al. 2003; Goossens et al. 2007; see also Green et al. 2009). Desmosomes had changed from stable anchor structures to highly dynamic cell elements and had finally become subjects of general physiological research.
Aside from the structure-bound states of desmosomal proteins in junction plaques and in nuclear or cytoplasmic particles, it had also become clear that the dynamic basis of all the reactions, the diffusible “regulation-ready” form, must exist somewhere in the cell. This “free” soluble form, however, is one of the most under-researched entities. The major soluble state of only plakoglobin, a 7S dimer, has been determined so far (Kapprell et al. 1987; see also Fouquet et al. 1992).
The molecular definition of true desmosomal components had also allowed the observations that in certain cell types these junctions do not anchor bundles of keratin IFs but rather vimentin IFs (Kartenbeck et al. 1984; Schwechheimer et al. 1984; Moll et al. 1986). Desmin-rich IFs and actin-containing myofilaments have also been shown to insert at the desmosomes and areae compositae of cardiomyocytes (Table 1) (Franke et al. 1982a, 2006; Kartenbeck et al. 1983). Note also that it was shown early on that the formation and maintenance of desmosomes can occur independently of anchorage to IFs (Denk et al. 1985a), indicating that desmosomes have functions other than as anchors and organizers of IF bundles (Green and Gaudry 2000).
THE DECADE OF THE ARMADILLOS AND THE MOLECULAR DIVERSITY OF ADHERENS JUNCTIONS
The discovery of the molecular ensembles forming the plaque-coated AJs has a history very different from that of the research on desmosomes. Here, seminal findings had been made many decades back and almost exclusively in the field of developmental biology. At the turn of the previous century, embryologists and zoologists recognized the importance of certain cell-surface entities for specific cell–cell adhesion and cell sorting in animal embryology as well as in tissue-like cell assemblies in culture dishes, from sponges and polyps to amphibian and chicken embryos, and, ultimately, mammalian organ development and regeneration (reviews to be recommended here: Wilson 1907; Holtfreter 1939; Moscona 1962; Okada 1996).
In the late 1970s, several research groups attempted to identify the molecules and the molecular principles governing cell sorting and adhesion mechanisms essential in embryogenesis. Steinberg (1958) recognized the general importance of Ca2+ ions in these processes. Following the phenomenon of Ca2+-mediated cell–cell assemblies to higher order structures, Takeichi’s group in Kyoto identified a glycoprotein of around 150 kDa mol. wt. (Takeichi, 1977), which a few years later was described—somewhat down-sized to 124 kDa—as a central player in cell–cell adhesion events, in particular in “morula compaction” of early embryogenesis. Consequently, this molecule was named “cadherin” for its Ca2+ dependence and cell adhesion effect (Yoshida-Noro et al. 1984; see also Yoshida-Noro and Takeichi 1982).
The Kyoto group was not alone in their search for the cell surface molecules involved in cell–cell interaction and homophilic sorting in early embryogenesis. Using different experimental approaches, the groups of G. Edelman in New York and F. Jacob in Paris had also embarked on the elucidation of these embryonal cell-adhesion principles and had independently arrived at the same kind of major interaction molecule, which they named uvomorulin, a molecule and concept that was then followed further by R. Kemler’s group (Kemler et al. 1977; Hyafil et al. 1980, 1981; Peyrieras et al. 1983; Vestweber and Kemler 1984, 1985; Boller et al. 1985; Ringwald et al. 1987), or L-CAM (Edelman et al. 1983; Gallin et al. 1983, 1987; Cunningham et al. 1984; see also Thiery et al. 1984). Other groups, using slightly different experimental approaches, called this glycoprotein A-CAM (Volk and Geiger 1984; Geiger et al. 1985a,b) or “glycoprotein Cell-CAM 120/80” (Damsky et al. 1983, 1985). In parallel studies using cell culture approaches, W. Birchmeier and his collaborators (Imhof et al. 1983; Behrens et al. 1985) had also identified this cell-surface glycoprotein, which turned out to be E-cadherin, and the “120/80 kDa” polypeptides were soon identified as a mixture of intact and truncated E-cadherin molecules. So, at a small expert meeting entitled “The Cell in Contact” on the campus of Rockefeller University in 1985, the representatives of these groups recognized that in the previous years they all had worked on the same molecule, the first AJ-specific component identified (for reviews, see Cunningham 1985; Damsky et al. 1985; Edelman 1985; Geiger et al. 1985a; Takeichi et al. 1985; Thiery et al. 1985).
As with the identification of desmosomal proteins, the identification of a cadherin was not the end of a research track but the beginning of a global outburst of hectic and cell biology-complicating work, as it started simultaneously two avalanches of cell type-specific proteins, the “cadherins” and the “armadillos.” Luckily, effective cDNA cloning methods were already at hand so that in only a few years the major cell type-specific cadherins were sequenced and could be localized by effective antibodies: E-cadherin (Gallin et al. 1983; Schuh et al. 1986; Bussemakers et al. 1993), N-cadherin (Hatta and Takeichi 1986; Hatta et al. 1987, 1988; Nose and Takeichi 1986), P-cadherin (Nose and Takeichi 1986; Nose et al. 1987), and the vascular endothelium-characteristic VE-cadherin (Lampugnani et al. 1995; Dejana 1996; Dejana et al. 2000). Finally, more than 50 members of a large “superfamily” in mammals have been identified, including so-called “classic” cadherins, “type II cadherins,” and “protocadherins” (reviews: Takeichi 1988, 1990; Gumbiner 1996; Nollet et al. 2000; Angst et al. 2001; Wheelock and Johnson 2003; Perez and Nelson 2004; Halbleib and Nelson 2006), not to mention the bewildering numbers of cadherins and their splice variants in some other parts of the animal kingdom (for arthropod cadherins, see, e.g., Hsu et al. 2009). Each of these newly discovered cadherins immediately produced “daughter avalanches” of publications dealing with the molecular interactions of the specific cadherins with each other and with AJ plaque proteins, the regulation of assembly or disassembly of AJs, and the functions of different cadherins during development and in the mature tissue, beginning with the study of Matsunaga et al. (1988; see also Meng and Takeichi 2009). In addition, each of the identified cadherins immediately produced questions as to its molecular complex state (for evidence of Ca2+-dependent cis-homodimers, see, e.g., the E-cadherin study by Takeda et al. 1999).
During the early period of cadherin discoveries, a number of researchers noted the biological specificity of the cadherin ensemble of a given cell type but also that there are examples of drastic changes of the type of cadherin produced when cells change their character, for example during wound healing (e.g., Hinz et al. 2004). A very conspicuous change often occurs when epithelial cells turn into mesenchymal cells during development, and when cells transform to malignancy and develop to carcinomas. Increased malignancy often appeared to be associated with the down-regulation of E-cadherin and an up-regulation or even induction of N-cadherin (e.g., Behrens et al. 1989, 1993; Frixen et al. 1991; Mareel et al. 1991, 1994; Vleminckx et al. 1991; Birchmeier et al. 1993, 1995). In addition, an increasing number of cadherin mutations and mRNA splicing errors have been reported that result in, or at least contribute to, carcinogenesis (e.g., Kanai et al. 1994). These principles were in a short period of time confirmed many times and have opened a totally new avenue of cancer research (for recent reviews, see Brabletz 2004; Strumane et al. 2004; Wheelock et al. 2008; see also Berx and van Roy 2009).
In parallel, we all had to watch with care and concern the other avalanche of reports on discoveries of proteins located in the cytoplasmic AJ plaque and directly or indirectly complexed with the cadherins. Soon after the discovery of the prototypic arm-protein, β-catenin, in 1989, this name could then only be used in the plural form (Tables 1 and 2): Six arm-proteins plus the actin-binder and -regulator, α-catenin, were identified in a short time, including some that are restricted to certain cell types and diseases (Ozawa et al. 1989, 1990a,b; Peifer and Wieschaus 1990; Nagafuchi et al. 1991; McCrea and Gumbiner 1991; McCrea et al. 1991; Peifer et al. 1992). Indeed, some of them were so specific that diverse AJ subforms could readily be distinguished not only from desmosomes but also from each other (e.g., Figs. 2C,D and 3).
Table 2.
The armadillo-report avalanche in the 1990s: Some results on arm-repeat and associated actin-interacting proteins in plaques of AJsa
aIncluding some recent review articles.
bHomologous proteins: ezrin, radixin, moesin, and merlin.
cIQ-domain GTPase-activating proteins.
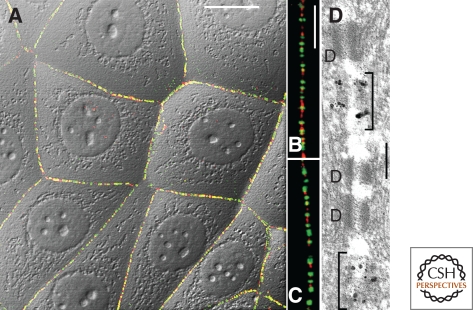
Figure 3.
High-resolution double-label immunofluorescence (A–C) and immunoelectron (D) microscopy of monolayer cell cultures of human breast-carcinoma cells of line MCF-7, as seen after reactions with antibodies to the desmosomal plaque component, desmoplakin (green, guinea pig antibodies), or to the adherens plaque protein, p0071 (red, as in Fig. 2B), as seen on the background of differential interference contrast (DIC) (A), allowing for the most part to distinguish the small puncta adhaerentia from the similarly small desmosomes, located side-by-side. (B, C) Higher magnification micrographs of cells as shown in A, presenting details of cell–cell junctions along close membrane contact regions, allowing to distinguish in many places the alternating pairs of symmetrical plaques of desmosomes (green) and puncta adhaerentia (red). (D) Equivalent comparison at the electron microscopic level, showing an ultrathin section through junctions after immunogold reaction of puncta adhaerentia with antibodies to protein p0071 (brackets, immunogold granules enhanced by secondary silver reaction), in comparison with the negative small desmosomes (D). For details, see Hofmann et al. (2008, 2009). Bars: 20 µm (A), 5 µm (B, C), and 0.1 µm (D).
Perhaps the greatest surprise and an additional attraction for researchers were observations that several of the arm-proteins were detected and seemed to function in very distant and different places in the cell: as structure-integrated rather insoluble components of junctional plaques, and as components of diffusible, relatively small regulatory complexes in the nucleus, involved in diverse processes including gene transcription and other nuclear functions. Thus, it was almost to be expected that an ever increasing number of cell biology groups embarked on research projects trying to unravel the molecular interactions of these proteins, both in the AJ plaques and in nuclei. This led to the founding of several new fields of regulatory cell biology, perhaps best illustrated by the affluence of reports on arm-proteins involved in regulatory pathways, including the Wnt-pathway (Choi and Weis 2004; Brabletz 2004; Drees et al. 2005; Lewis-Tuffin and Anastasiadis 2008; see also Heuberger and Birchmeier 2009, and Cadigan and Peifer 2009). Finally, it should be noted that sizeable portions of arm-proteins generally occur in small diffusible forms in the cytoplasm, such as in the maternal pools of the amphibian ooplasm (e.g., Fouquet et al. 1992).
Some puzzling complications, however, were brought to the field of “cadherinology” by discoveries of a series of special, carboxy-terminally truncated cadherins, i.e., molecules with a significantly longer extracellular portion, but only a very short, e.g., 20 amino acids, cytoplasmic portion. These cadherins included mammalian LI-cadherin found in the polar simple epithelia of liver and/or intestine (Berndorff et al. 1994; Kreft et al. 1997), and Ksp-cadherin of certain kidney epithelia (Thomson et al. 1995). An even further truncated type of cadherin was found in which a normal extracellular element comprising five repeating elements lacked a membrane-anchor but was attached to the plasma membrane by a glycosylphospatidyl inositol residue (for T-cadherin, see Ranscht and Dours-Zimmermann 1991; Tanihara et al. 1994). However, these cadherins are not integrated into an AJ structure but widely scattered over the plasma membrane.
In the mid 1990s, just when the cell biological community thought that it finally knew the composition of AJs, again a disturbing call “halt—not so fast” was heard. The group of Y. Takai had identified yet another cell surface membrane complex, in this case with major transmembrane molecules of the immunoglobulin-like category: The nectins represent a family of four major and several minor (splice-) isoforms that can bind to each other in trans-interactions in a Ca2+-independent mechanism. They are bound to a submembranous plaque-type protein, afadin, which also occurs in several splice variants and in turn is associated with some specific actin-binding proteins, one of which has been named ponsin. This complex was observed close to, and often colocalizing with, AJ structures (Mandai et al. 1997; Takahashi et al. 1999; Satoh-Horikawa et al. 2000; for reviews see Takai and Nakanishi 2003; Irie et al. 2004; Takai et al. 2008a,b). Afadin apparently can also interact with α-catenin, thus providing the possibility of an indirect actin microfilament bridge connection between nectin and cadherin complexes (e.g., Tachibana et al. 2000; see also Takai et al. 2008a). So, why have these “Takai group” molecules still not been “officially” added to the AJ ensemble? One remaining problem is that although they colocalize, even at the electron microscopic level, with some of the typical AJ structures, for example with the zonula adhaerens of polar epithelia, they have not been seen to occur in other AJs such as those of the nearby puncta adhaerentia along the lateral surfaces of the same cells (e.g., Mandai et al. 1997; Takai et al. 2008a,b). Another problem is that components of these two strikingly close structural complexes have not been coimmunoprecipitated or cross-linked by short-distance chemical cross-linkers. However, recent studies indicate that the nectin–afadin transmembrane elements play an important pioneering and dynamic role in AJ assembly because the nectin complex provides the first cell–cell contact structures between two cells whereupon the cadherin-containing AJ elements follow (Takai et al. 2003, 2008a,b; see also Meng and Takeichi 2009).
Finally, a special “border guard” role has been ascribed to the groups of relatively small puncta adhaerentia-type AJs, which in certain brain regions surround neural synapses and seem to be important in the formation and maintained organization of the spatial junction relationship and the proper functioning of these specific synapses (Uchida et al. 1996; see Giagtzoglou et al. 2009).
THE SECRETS OF THE KISSES: THE MEMBRANE MOLECULES FORMING TIGHT JUNCTIONS
The electron microscopic appearance of the subapical TJs of simple epithelial cells (zonulae occludentes) was relatively well-known since the mid 1970s. Transmission electron microscopy of cross sections showed one or a few rather small direct contact sites of the two plasma membranes, commonly called “kisses.” Planar freeze-fractures through the membrane interior revealed, after metal-shadowing, a linear, or ornamentally woven in some tissues, branched relief of one or several ridges (Farquhar and Palade 1963; Goodenough and Revel 1970; Friend and Gilula 1972; Claude and Goodenough 1973; Staehelin 1974; Montesano et al. 1975; Hull and Staehelin 1976; Diamond 1977; reviews: Schneeberger and Lynch 1992; Mitic and Anderson 1998; Förster 2008; see also Goodenough and Paul 2009). Early on, it was also obvious that this structure must be most important in the establishment, maintenance, and functions of epithelia and endothelia, and hence in metazoan life in general. Most convincingly, the barrier role of TJ structures for paracellular diffusion, transport processes, and particle translocations had been directly shown by electrophysiological experiments and by electron microscopy using heavy metal-labeled tracers (see also Anderson and Van Itallie 2009). It had also been suggested that a number of physiological phenomena and diseases, from tissue swellings to liquid losses, were caused by partial or transient interruptions of such a barrier (e.g., Claude 1978; Madara and Dharmsathaphorn 1985; Simons 1990; Matter and Balda 1999; Van Itallie and Anderson 2006). Naturally, this field of research was closely watched by pharmacologists and drug developers awaiting the emergence of principles or molecules that would allow restoration of an interrupted barrier, or to induce a controlled transient permeability of the blood-brain-barrier for drugs of other components.
Molecular candidates and insights, however, arrived only dropwise. The first relevant discovery was made by Stevenson et al. (1986) who isolated and localized a component of the relatively thin plaque on the cytoplasmic face of TJs, therefore named protein ZO-1 (“zonula occludens”-1; identical to the ca. 220-kDa protein described by Itoh et al. 1991, 1993), which was later followed by the identification of ZO-2 (Gumbiner et al. 1991) and ZO-3 (Haskins et al. 1998). These proteins were founding members of the so-called MAGUK (membrane-associated guanylate kinase) family of proteins (for homologous proteins in invertebrates, see the finding in Drosophila by Willott et al. 1993), which soon turned out not to be exclusive for TJs but to be almost as regularly found in AJ- and GJ-associated plaques (Tables 1 and 2) (for reviews, see Anderson 1996; Balda and Matter 2000). These proteins can also occur in the nucleus (Gottardi et al. 1996) and in several other locations and functions, from transcriptional factors to regulators of the behavior of tumor cells (see the anthology edited by Cereijido and Anderson 2001, and the reviews of González-Mariscal et al. 2003; Gottardi and Niessen 2008).
Soon thereafter, Citi et al. (1988, 1989, 1991) discovered and localized another major TJ plaque protein of ∼Mr 140 kDa, which they named cingulin (from the Latin cingulum, i.e., stringlike girdle), which is clearly TJ-specific, albeit seemingly confined to epithelial TJs (e.g., Cordenonsi et al. 1999; Citi 2001; Fanning 2001). Altogether, about a dozen TJ plaque proteins have now been described, but so far their detailed molecular interactions and locations have only partly been elucidated. Although for some of them only molecular complexes with classic TJ components have been reported, others, again, show the phenomenon of dual localization outside of TJs (reviews: Citi 2001; Fanning 2001).
But what was the “core of the barriers,” the “real seal,” the “nature of the kisses,” the “hydrophobic transmembrane stuff?” Here, a first answer came at Christmas 1993 as a present, an unexpected major breakthrough in TJ membranology, a fruit of both “good old” cell fractionation work combined with biologically conceived strategic experimentation. “The Tsukitas,” Sachiko and Shoichiro, already known for the high-quality standard of their plasma membrane fractions from liver tissue (Tsukita and Tsukita 1989) and profiting from the enthusiasm and courage of a young graduate student, M. Furuse, had decided to take the classic route by homogenizing the most suitable tissue and enrich the desired structure by the most suitable techniques. The biological source was cleverly chosen—the bile canalicular front fraction from chicken liver, which is naturally enriched in all major kinds of junctions. Their first “eureka” candidate was a tetraspan transmembrane protein, which they could immunolocalize to the TJ structure, the zonula occludens, of liver and other simple epithelia, and so they named it “occludin” (Furuse et al. 1993; for the appropriate welcome ceremony, see Gumbiner 1993). Particularly satisfying was the result that on transfection of occludin-encoding cDNA expression constructs into TJ-possessing cells, the newly synthesized occludin integrated into pre-existing TJ structures, and when injected into TJ-lacking cells, the gene product was able to form de novo TJ-typical intramembranous “ridges” as well as cytoplasmic stacks of lamellae closely resembling typical TJ membrane structures (Furuse et al. 1994, 1996; Saitou et al. 1997). So, in a wide range of mammals, occludin and its various phosphorylation forms appeared to be the basis of TJ membranes (Fig. 4A) (Ando-Akatsuka et al. 1996; Sahakibara et al. 1997).
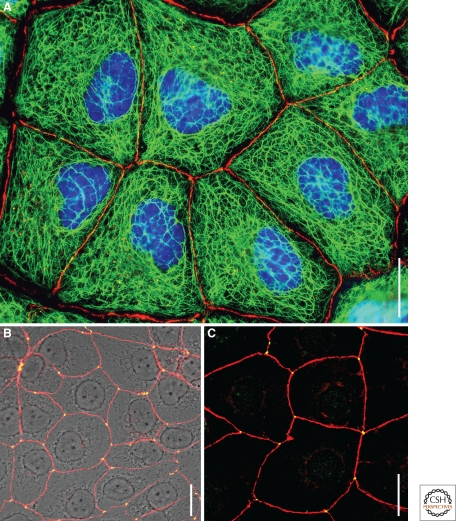
Figure 4.
Double-label immunofluorescence microscopy showing the tight junction (TJ) systems of monolayer cell cultures of human hepatocellular carcinoma-derived cells of line PLC (A) and of cultures of human keratinocytes of line HaCaT beginning to form the first suprabasal cell layer (B, C), using antibodies to keratin (A, green, mAb lu-5), in comparison with the TJ protein, occludin (A, red), or comparing the localization of the protein tricellulin (B, green, rat antibodies) with the TJ plaque protein, ZO-1 (B, red, rabbit antibodies), or the transmembrane TJ protein, occludin (C, red, rabbit antibodies). Note that these cells are totally interconnected by the TJ system (zonula occludens), which is completed at the tricellular corners by the occludin-related, but very specifically located protein, tricellulin, presenting complete colocalization with protein ZO-1 and occludin at the tricellular corners. For details, see also Schlüter et al. 2007. Bars: 20 µm.
Then, suddenly came a deep disappointment—not an uncommon one in modern biology, but in this case fortunately only a short one. Deletion of both alleles of the occludin gene had resulted in occludin-deficient mice, which, however, not only lived but formed typical TJ structures in the typical tissues, combined with plaque proteins of the ZO-1–3 protein family and TJ-typical functions (Saitou et al. 1998; only later, a series of occludin–/– mice with defects in several tissues and functions were reported: Saitou et al. 2000). Consequently, in their 1998 paper, Saitou et al. had to conclude: “These findings indicate that there are as yet unidentified TJ integral membrane protein(s) that can form strand structures, recruit ZO-1, and function as a barrier without occludin.” And so, without much mourning, they went back to square one, the chicken liver bile canalicular membrane fraction, and noticed two further bands of smaller (∼22 kDa) polypeptides that had amino acid sequences also indicative of molecules traversing the TJ membrane four times but without any occludin homology. Nevertheless, the new, very small, kind of protein immunolocalized to TJs, incorporated into TJs of living cells, and formed de novo intramembranous, TJ-typical ridge structures on cDNA transfection, even in TJ-lacking cells. Consequently, our Japanese friends had to expand their Latin vocabulary and named these new TJ proteins “claudins” nos. 1 and 2 (Furuse et al. 1998a,b; Tsukita and Furuse 1998).
But TJ life did not become much easier, and the ups and downs of TJ protein research emotions were not over. Japanese scientists are usually not regarded as hectic, but with the TJ proteins everything was new and different. Shoichiro (Tsukita) had just informed me and a few others about the first claudin amino acid sequence, but then came the stop-fax “hold it” and then the phone call “Sorry: Something strange has happened, these sequences are already in the computer.” Very quickly, however, his good mood returned with the solution: Two claudins were identical to the receptors of a food poisoning enterotoxin of the bacillus Clostridium perfringens, a known cause of gastrointestinal disease (Sonoda et al. 1999). Thus, another medical research path had just been opened: Claudins were recognized as membrane-bound receptors of certain toxins produced by prokaryotic pathogens (for a special review, see, e.g., Hecht 2001).
As we have already experienced with desmosomal molecules, and with cadherin and arm-protein constituents of the AJs, the claudins likewise did not come only as a pair of genes. To the contrary, the Tsukita group had stumbled into another large, novel gene family, the claudins, with very complex and functionally important, cell-type-specific combinations of a few selected representatives (e.g., Furuse et al. 1999; Morita et al. 1999a-c; Tsukita and Furuse 2000). And at the end of that year, coincidentally also the end of the millenium, already 15 different claudins had been discovered and soon thereafter a total number of 24 claudins were found to be encoded in the human genome (Tsukita et al. 2001; Tsukita and Furuse 2002). Some of the claudins came with a list of specific medical research questions, the first example being the Mg++-reabsorption problem protein, just called “paracellin,” now reclassified as claudin-16 (Simon et al. 1990; for a special review see Choate et al. 2001). Even higher claudin gene complexities are found in some other vertebrate species, with a—so far—record number of a total of 56 claudin genes in the—yes, no joke—Japanese puffer fish, Fugu rubripes (Loh et al. 2004; see there for further references). Thus, the smallest vertebrate genome known presents the highest claudin gene number known, an ironical paradox of Mother Nature. And while these sentences are written, a new claudin message comes in, indicating further bewildering complexity: Just one claudin, claudin-10, can occur in six spliced isoforms with different tissue and intracellular localizations and functions (Günzel et al. 2009). Evolution has indeed been merciless to cell biologists!
So the search for claudin functions in the different tissues and for claudin-connected diseases began. But halt once more! When the cell biological world thought that the TJ ensemble was finally complete, it turned out that not all essential facts of TJ structures had been carefully considered. Although complete TJs encircle individual cells of a simple, one-layered epithelium or an endothelium, thus forming a tight-looking barrier (Fig. 4A), in some epithelia, small intercellular “gaps” remain at places where three cells meet each other, a “three corner state” problem, representing gaps through which sizable molecules or even particles might translocate. Again, the Tsukita laboratory came through, identifying, as a very late addition to cell junction biology at the end of the year 2005, the protein “tricellulin,” just in time to let the late Shoichiro be sure about the publication (Ikenouchi et al. 2005). This protein, related by partial sequence homology to occludin, assembles exactly at those “three corner” sites to make the epithelial tight junction system really tight (Fig. 4B,C; Table 1).
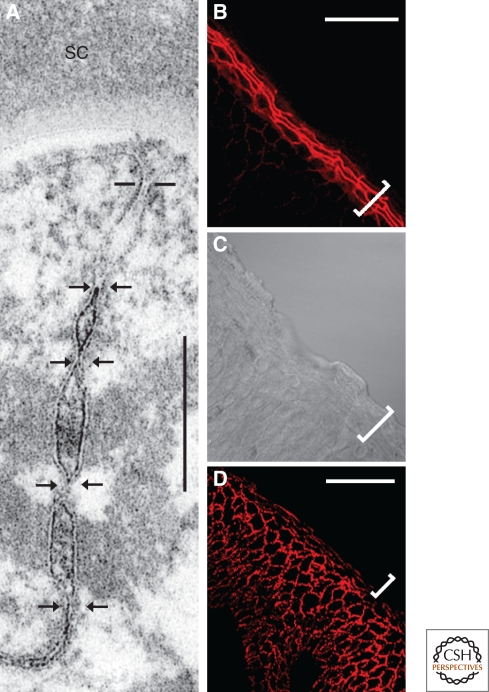
In the meantime, systematic function-oriented studies had tried to elucidate the biological roles as well as the potential importance of specific claudins by gene mutation and deletion experiments. However, it was important to recognize that certain claudins occur in very specific cell types: For example, claudin-11, formerly known as “oligodendrocyte-specific protein” (OSP), was identified in cells as different as Sertoli cells of testis and myelin sheath-forming oligodendrocytes, biological distances and differences difficult to bridge by simple biological hypotheses (e.g., Morita et al. 1999b). The first systematic study of specific claudin gene deletion deficiencies brought both surprises and clarity. Claudin-1-deficient mice died on the first day after birth with wrinkled, dried-out skin, caused by massive transepidermal water loss (Furuse et al. 2002), a finding difficult to explain on the basis of the then prevailing textbook dogma that there are no TJ structures in the upper living layer (stratum granulosum) of the epidermis and other stratified epithelia. However, it was a lucky coincidence that in the summer of 2002, typical TJ structures, positive for claudins and occludin, had also been noted and localized in these uppermost layers of the epidermis and other squamous stratified epithelia (Fig. 5A–C) (Brandner et al. 2002; Furuse et al. 2002; Langbein et al. 2002; Schlüter et al. 2004; see also Morita et al. 1998, 2002; Pummi et al. 2001). These findings have changed the view of how the body surface is sealed from the outside world. Recently, tricellulin was also identified in suprabasal cell layers of keratinocyte cultures (Fig. 5B,C) (Schlüter et al. 2007).
Figure 5.
Electron (A) and immunofluorescence (B–D) microscopy, showing the tight junction (TJ) system in the granular layer (stratum granulosum) of stratified epithelia. (A) The uppermost living layer of human epidermis (the first cornified layer, stratum corneum, is labeled SC) contains a subapical system of TJ structures (horizontal bars and pairs of arrows denote distinct membrane contact and fusion points: “kisses”). Note that the four lowermost TJs (arrows) are interspersed between desmosomes. (B–D) Near-vertical cryostat section through bovine gingiva, showing the TJ system in the uppermost living layers by immunostaining with antibodies to occludin (B, bracket; C, corresponding phase contrast image), whereas the cell–cell junction structures positive for the TJ protein, claudin-1, are not limited to the granular layer but extend basally into a number of stratum spinosum cell layers as well, indicating that there exist further, not yet fully characterized, partly punctate structures containing claudin-1 but not occludin (for methods used, see also Brandner et al. 2002; Schlüter et al. 2007).
Bars: 0.2 µm (A), 50 µm (B–D).
Here, the TJ story could end. There is, however, a remaining fundamental cell biological problem. TJ-type structures, appearing as “kisses” or other close membrane–membrane contacts, can also be seen in interdesmosomal regions of the spinous layer of the epidermis and the equivalent layers of several other stratified epithelia. They appear as small “lamellated” or “sandwich” junctions or even as “stud junctions” (puncta occludentia), exactly in places intensely immunopositive for occludin or certain claudins (Fig. 5B,C) (e.g., Brandner et al. 2002; Langbein et al. 2002, 2003; Schlüter et al. 2007). Could it be that there still exists a so far ignored world of TJ-related junctions? Could it be that discoveries of TJ molecule assemblies are not only history?
GAP JUNCTIONS: THE BEAUTY AND COMPLEXITY OF INTERCELLULAR CHANNELS
In the “good old,” although rather controversial days of molecular discoveries, cell biological discussions of possible functions of a given protein or structure started with educated guesses but often ended with less-educated, semitired jokelike comments. In the case of GJs, intercellular communication and exchange was one of the favorite discussion subjects early on, but non-GJ colleagues said that they were there “only to give pleasure to electron microscopists.” Indeed, these near-ubiquitous tissue structures seemed to have been precipitated out of an electron microscopist’s dreams as they comprised variously sized and shaped, densely packed, planar paracrystalline assemblies of short vertical hollow cylinders (“channels”) made up of symmetrically head-on-head contacting hemichannels. All of these structural details were well-demonstrable in the electron microscope, either in transverse or horizontal ultrathin sections, by freeze fractures (see, e.g., Fig. 1C) or in negatively stained isolated GJs. Thus, it was well known already in the 1960s that the hemichannel interior could be filled, at least partly, with heavy metal molecules but also that the small extracellular spaces in between the channels, the “gaps,” were also penetrable by such agents added from the outside. Moreover, in cell fractionation experiments, GJs were among the more stress-, detergent-, and even alkaline pH-resistant structures (e.g., Dewey and Barr 1964; Goodenough 1974; Henderson et al. 1979; Hertzberg and Gilula 1979; Hertzberg 1984; Stauffer et al. 1993). As a result of these near-optimal conditions, the ultrastructural organization of GJs was essentially known at the end of the 1970s (e.g., Goodenough and Revel 1970, 1971; McNutt and Weinstein 1970; Caspar et al. 1977; Unwin and Zampighi 1980; for reviews see, e.g., Loewenstein 1981; Gilula 1985, 1987, 1990; Revel et al. 1985, 1987; Unwin 1987; Goodenough 1990; Sosinsky 2000; see also Goodenough and Paul 2009). There was indeed no shortage of structural GJ models when the molecular phase began.
The major GJ proteins identified in fractions enriched in isolated junction structures from liver, heart, and eye lens were soon analyzed and characterized, including protein chemistry and reconstitution experiments, and cDNA cloning and sequencing (e.g., Goodenough 1974; Henderson et al. 1979; Hertzberg and Gilula 1979; Hertzberg et al. 1982; Hertzberg and Skibbens 1984; Kumar and Gilula 1986; Paul 1986; for review, see Goodenough 1990). A major protein constitutent was obvious, but it was also recognized early that the molecules representing this major GJ protein in different tissues were related but not identical. “Connexins” was chosen as the family name (Goodenough 1974) and, after some discussion on the molecular relatedness alphabetical nomenclature of the Westcoast and the “connexin plus apparent molecular weight” (e.g., Cx26, etc.) proposal from the Eastcoast, the “Cx plus Mr value” version found more friends. One inherent problem, however, was also noted from the beginning that the Mr value even of the ortholog protein could vary between species. Another problem was the same as encountered with the other cell–cell junctions. Yes, you are right: Connexins are another large junction protein family showing cell-type specificity.
Definitive evidence that a certain protein is indeed part of a GJ channel structure came when antibodies specific for a given type of connexin(s) had been generated. This enabled immunoblot testing for specific reactions with a connexin, or a few related ones, from GJ-enriched fractions, and the identification and exact GJ localization using immunoelectron and immunofluorescence microscopy with isolated gap junction membranes or, most convincing, GJ structures in situ (Willecke et al. 1982; Janssen-Timmen et al. 1983; Dermietzel et al. 1984; Paul 1985; Beyer et al. 1989; Fromaget et al. 1990, 1992, 1993; Gilula 1990; Yeager 1993). As with the transmembrane proteins of the other three major categories of junctions, antibodies that bound to certain epitopes located in the cell–cell bridge domains of connexins were also able to interfere with, or even disrupt, cell–cell coupling (e.g., Warner et al. 1984; Gilula 1990; Hertzberg et al. 1985).
In parallel, an increasing number of connexins had been characterized by cloning and sequencing, using cDNA clones from a wide variety of tissues (Kumar and Gilula 1986; Nicholson et al. 1985; Heynkes et al. 1986; Paul 1986; Beyer et al. 1987, 1990; Traub et al. 1989; Zhang and Nicholson 1989; Kanter et al. 1991; reviews: Beyer et al. 1993; Willecke et al. 1993; Yeager 1993). These studies led to an extremely complex cell-type-specific catalog of mammalian connexins. Twenty-one human Cx gene products have been identified so far, and so one has to recognize the great potential of Cx-type combinations to form GJs (homomeric, heteromeric, homotypic, or heterotypic), and further different properties that may result from phosphorylations and other modifications (reviews: Bruzzone et al. 1996; Goodenough et al. 1996; Kumar and Gilula 1996; Sosinsky 2000; see, e.g., the anthology edited by Peracchia 2000; for a special review integrating recent results and insights, see Goodenough and Paul 2009).
In view of the fundamentally different ultrastructural organization of the GJs, compared to the other three junctional complexes, and the obvious lack of homology between the connexins and the cadherins or claudins, it was finally some surprise, at least to this author, that connexin cytoplasmic domains also specifically associated with certain AJ-typical plaque proteins such as MAGUK proteins of the ZO-1 to ZO-3 group (Giepmans and Molenaar 1998; Toyofuku et al. 1998, 2001; Giepmans et al. 2001; Kausalya et al. 2001; Laing et al. 2001a,b; Nielsen et al. 2001, 2002, 2003; Barker et al. 2002; Jin et al. 2004; Singh et al. 2005; Talhouk et al. 2008). That this interaction reflects direct binding of the MAGUK protein to the connexin partner has perhaps most convincingly been shown using pure ZO-1 protein synthesized by translation in vitro (e.g., Laing et al. 2001a,b). So it is justified to hypothesize that MAGUK proteins are also important components involved in regulatory mechanisms of GJ formation and functions, and that the intracellular GJ membrane surface is a “hot spot” of interactions with diverse cytoplasmic proteins (Table 1) (review: Dbouk et al. 2009). The list of GJ-associated proteins and of interactions of GJs with other cell structures is still growing, although a distinct electron-dense plaque structure has not been demonstrated (for examples and references, see recent reviews: Park et al. 2007; Koval 2008; Prochnow and Dermietzel 2008).
The history of the elucidation of the molecular composition of GJs has been for some time under some strange clouds and question marks. For example, a radically alternative concept of GJ structures in various invertebrates and vertebrates was based on the existence of GJ-like structures assembled from very small (16–27 kDa) polypeptides, later named “ductins” (for references, see Finbow et al. 1985; Finbow and Pitts 1993; Finbow 1997; Ashrafi et al. 2000). Very recently, now such “GJ-mimicking” structures and the ductins could be definitively ascribed to proteins of the vacuolar ATPase proton pump and to be absent from GJs (M. Finbow, personal communication). Another long-standing question mark had already been answered at the turn of the millenium: The so-called major intrinsic polypeptides (MPs or MIPs) of the eye lens, one of the best studied structures in cell biology, were finally identified as true connexins (e.g., MP70 is Cx50; here, the reader is referred to “translations” of MP into Cx language as, e.g., in Graw et al. 2001).
In view of the important cell–cell comunication and exchange functions of GJs, it was expected that studies on the functions of specific GJs and GJ proteins would also bring valuable information about the functions of individual proteins, not only from gene deletion or mutation experiments but also from the growing list of human disorders and diseases that can be clearly related to mutant or disregulated connexins (for examples, see recent reviews: Meşe et al. 2007; Koval 2008). Thus, with connexins as with the constitutive molecules of the other three categories of cell–cell junctions, cell biologists can finally also conclude with satisfaction that basic cell research here has not only revealed the molecular organizations and functions of junction components, but has also made an unexpectedly high number of contributions to medical research and practice.
SOME CONCLUDING REMARKS
Although on a first glance the molecular ensembles and the morphology of the four homotypic cell–cell junctions differ in many aspects, one can also note a series of similarities in structural organization:
All four forms of symmetrical junctions are clusters of densely packed, specific transmembrane molecules.
The transmembrane molecules are either once-spanning glycoproteins (cadherins) or tetraspan proteins.
The cytoplasmically projecting domains of the transmembrane molecules are intimately associated with proteins that form layers or plaques, which widely vary in thickness and density and in certain cases can connect the junction with the cytoskeleton.
The transmembrane molecules of a hemi-junction are connected head-to-head with their counterparts of a hemi-junction of an adjacent cell.
The positions are often not at random but topogenically integrated into the specific cell and tissue architecture.
The types, the sizes, and the topological positions of these junctions are under developmental and functional control. Consequently, disorders often result in disease.
Footnotes
Editors: W. James Nelson and Elaine Fuchs
Additional Perspectives on Cell Junctions available at www.cshperspectives.org
REFERENCES
- Aberle H, Schwartz H, Kemler R 1996. Cadherin-catenin complex: Protein interactions and their implications for cadherin function. J Cell Biochem 61:514–523 [DOI] [PubMed] [Google Scholar]
- Aghib DF, McCrea PD 1995. The E-cadherin complex contains the src substrate p120. Exp Cell Res 218:359–369 [DOI] [PubMed] [Google Scholar]
- Aho S, Rothenberger K, Uitto J 1999. Human p120ctn catenin: Tissue-specific expression of isoforms and molecular interactions with BP180/type XVII collagen. J Cell Biochem 73:390–399 [PubMed] [Google Scholar]
- Amagai M 1999. Autoimmunity against desmosomal cadherins in pemphigus. J Dermatol Sci 20:92–102 [DOI] [PubMed] [Google Scholar]
- Amagai M, Klaus-Kovtun V, Stanley JR 1991. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell 67:869–977 [DOI] [PubMed] [Google Scholar]
- Anastasiadis PZ, Reynolds AB 2000. The 120 catenin family: Complex roles in adhesion, signaling and cancer. J Cell Sci 113:1319–1334 [DOI] [PubMed] [Google Scholar]
- Anastasiadis PZ, Moon SY, Thoreson MA, Mariner DJ, Crawford HC, Zheng Y, Reynolds AB 2000. Inhibition of RhoA by p120 catenin. Nat Cell Biol 2:637–644 [DOI] [PubMed] [Google Scholar]
- Anderson JM 1996. MAGUK magic. Curr Biol 6:326–329 [DOI] [PubMed] [Google Scholar]
- Anderson JM, Van Itallie CM 2009. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1:a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Itoh M, Yonemura S, Furuse M, Tsujita Sh 1996. Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues. J Cell Biol 133:43–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst BD, Marcozzi C, Magee AI 2001. The cadherin superfamily: Diversity in form and function. J Cell Sci 114:629–641 [DOI] [PubMed] [Google Scholar]
- Arnemann J, Sullivan KH, Magee AI, King IA, Buxton RS 1993. Stratification-related expression of isoforms of the desmosomal cadherins in human epidermis. J Cell Sci 104:741–750 [DOI] [PubMed] [Google Scholar]
- Ashrafi GH, Pitts JD, Faccini AM, McLean P, O’Brien V, Finbow ME, Campo MS 2000. Binding of bovine papillomavirus type 4 E8 to ductin (16K proteolipid), down-regulation of gap junction intercellular communication and full cell transformation are independent events. J Gen Virol 81:689–694 [DOI] [PubMed] [Google Scholar]
- Balda MS, Matter K 2000. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J 19:2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RJ, Price RL, Gourdie RG 2002. Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res 90:317–324 [DOI] [PubMed] [Google Scholar]
- Bazzi H, Getz A, Mahoney MG, Ishida-Yamamoto A, Langbein L, Wahl KK III, Christiano AM 2006. Desmoglein 4 is expressed in highly differentiated keratinocytes and trichocytes in human epidermis and hair follicle. Differentiation 74:129–140 [DOI] [PubMed] [Google Scholar]
- Behrens J, Birchmeier W, Goodman SL, Imhof BA 1985. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-arc-1: Mechanistic aspects and identification of the antigen as a component related to uvomorulin. J Cell Biol 101:1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Mareel MM, Van Roy FM, Birchmeier W 1989. Dissecting tumour cell invasion: Epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell–cell adhesion. J Cell Biol 108:2435–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W 1993. Loss of epithelial morphotype and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-src gene. J Cell Biol 120:757–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndorff D, Gessner R, Kreft B, Schnoy N, Lajous-Petter A-M, Loch N, Reutter W, Hortsch M, Tauber R 1994. Liver-intestine cadherin: Molecular cloning and characterization of a novel Ca2+-dependent cell adhesion molecule expressed in liver and intestine. J Cell Biol 125:1353–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berx G, van Roy F 2009. Involvement of members of the cadherin superfamily in cancer. Cold Spring Harb Perspect Biol 1:a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer EC, Goodenough DA, Paul DL 1990. Connexin family of gap-junction proteins. J Membr Biol 116:187–194 [DOI] [PubMed] [Google Scholar]
- Beyer EC, Paul DL, Goodenough DA 1987. Connexin43: A protein from rat heart homologous to a gap junction protein from liver. J Cell Biol 105:2621–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer EC, Kistler J, Paul DL, Goodenough DA 1989. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol 108:595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer EC, Kanter HL, Rup DM, Westphale EM, Reed KE, Larson DM, Saffitz JE 1993. Expression of multiple connexins by cells of the cardiovascular system and lens. Gap junctions (eds. Hall J.E., Zampighi G.A., Davis R.M.), Progr Cell Res. Vol. 3, pp. 171–175 Elsevier, Amsterdam [Google Scholar]
- Bierkamp C, McLaughlin KJ, Schwarz H, Huber O, Kemler R 1996. Embryonic heart and skin defects in mice lacking plakoglobin. Dev Biol 180:780–785 [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Hülsken J, Behrens J 1995. E-cadherin as an invasion suppressor. Junctional complexes of epithelial cells, Ciba Found. Symp.125, pp. 124–141 J. Wiley and Sons, Chichester: [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Weidner KM, Hülsken J, Behrens J 1993. Molecular mechanisms leading to cell junction (cadherin) deficiency in invasive carcinomas. Sem Cancer Biol 4:231–239 [PubMed] [Google Scholar]
- Boller K, Vestweber D, Kemler R 1985. Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol 100:327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonné S, van Hengel J, Nollet F, Kools P, van Roy F 1999. Plakophilin-3, a novel armadillo-like protein present in nuclei and desmosomes of epithelial cells. J Cell Sci 112:2265–2276 [DOI] [PubMed] [Google Scholar]
- Bonné S, Gilbert B, Hatzfeld M, Chen X, Green KJ, van Roy F 2003. Defining desmosomal plakophilin-3 interactions. J Cell Biol 161:403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornslaeger EA, Corcoran CM, Stappenbeck TS, Green KJ 1996. Breaking the connection: Displacement of the desmosomal plaque protein desmoplakin from cell–cell interfaces disrupts anchorage of intermediate filament bundles and alters intercellular junction assembly. J Cell Biol 134:985–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrmann CM, Mertens C, Schmidt A, Langbein L, Kuhn C, Franke WW 2000. Molecular diversity of plaques of epithelial adhering junctions. Ann NY Acad Sci 915:144–150 [DOI] [PubMed] [Google Scholar]
- Brabletz T 2004. In vivo functions of catenins. Cell adhesion (eds. Behrens J., Nelson W.J.), Handbook Exp. Pharmacol., Vol. 165, pp. 105–135 Springer, Berlin: [DOI] [PubMed] [Google Scholar]
- Brandner JM, Kief S, Grund C, Rendl M, Houdek P, Kuhn C, Tschachler E, Franke WW, Moll I 2002. Organization and formation of the tight junction-system in human epidermis and cultured keratinocytes. Eur J Cell Biol 81:253–263 [DOI] [PubMed] [Google Scholar]
- Bretscher A 1986. Purification of the intestinal microvillus cytoskeletal proteins villin, fimbrin, and ezrin. Meth Enzymol 134:24–37 [DOI] [PubMed] [Google Scholar]
- Brill S, Li S, Lyman CW, Church DM, Wasmuth JJ, Weissbach L, Bernards A, Snijders AJ 1996. The Ras GTPase-activating-protein-related human protein IQGAP2 harbors a potential actin binding domain and interacts with calmodulin and Rho family GTPases. Mol Cell Biol 16:4869–4878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R, White TW, Paul DL 1996. Connections with connexins: The molecular basis of direct intercellular signalling. Eur J Biochem 238:1–27 [DOI] [PubMed] [Google Scholar]
- Bussemakers MJG, van Bokhoven A, Mees SGM, Kemler R, Schalken JA 1993. Molecular cloning and characterization of the human E-cadherin cDNA. Mol Biol Rep 17:123–128 [DOI] [PubMed] [Google Scholar]
- Buxton RS, Magee AI 1992. Structure and interactions of desmosomal and other cadherins. Semin Cell Biol 3:157–167 [DOI] [PubMed] [Google Scholar]
- Buxton RS, Cowin P, Franke WW, Garrod DR, Green KJ, King IA, Koch PJ, Magee AI, Rees DA, Stanley JR, et al. 1993. Nomenclature of the desmosomal cadherins. J Cell Biol 121:481–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Peifer M 2009. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol 1:a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell RD, Campbell JH 1971. Origin and continuity of desmosomes. Origin and continuity of cell organelles (eds. Reinert J., Ursprung H.), pp. 261–298 Springer Verlag, Berlin [Google Scholar]
- Caspar D, Goodenough D, Makowski L, Phillips W 1977. Gap junction structures. I. Correlated electron microscopy and x-ray diffraction. J Cell Biol 74:605–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M, Anderson J (eds.) 2001. Tight Junctions 2. edn CRC Press, Boca Raton, FL [Google Scholar]
- Chen X, Bonné S, Hatzfeld M, van Roy F, Green KJ 2002. Protein binding and functional characterization of plakophilin 2. J Biol Chem 277:10512–10522 [DOI] [PubMed] [Google Scholar]
- Chitaev NA, Troyanovsky SM 1997. Direct Ca2+-dependent heterophilic interaction between desmosomal cadherins, desmoglein and desmocollin, contributes to cell–cell adhesion. J Cell Biol 138:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitaev NA, Leube RE, Troyanovsky RB, Eshkind LG, Franke WW, Troyanovsky SM 1996. The binding of plakoglobin to desmosomal cadherins: Patterns of binding sites and topogenic potential. J Cell Biol 133:359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choate KA, Lu Y, Lifton RP 2001. Claudins mediate specific paracellular fluxes in vivo: Paracellin-1 is required for paracellular Mg2+ flux. Tight junctions (eds. Cereijido M., Anderson J.), pp. 483–492 CRC Press, Boca Raton, FL [Google Scholar]
- Choi H-J, Weis WI 2004. Structural aspects of adherens junctions and desmosomes. In Cell adhesion (eds. Behrens J., Nelson W.J.), Handbook Exp. Pharmacol., Vol. 165, pp. 23–52 Springer, Berlin: [DOI] [PubMed] [Google Scholar]
- Citi S 2001. The cytoplasmic plaque proteins of the tight junction. Tight junctions (eds. Cereijido M., Anderson J.), pp. 231–264 CRC Press, Boca Raton, FL [Google Scholar]
- Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J 1988. Cingulin, a new peripheral component of tight junctions. Nature 333:272–276 [DOI] [PubMed] [Google Scholar]
- Citi S, Sabanay H, Kendrick-Jones J, Geiger B 1989. Cingulin: Characterization and localization. J Cell Sci 93:107–122 [DOI] [PubMed] [Google Scholar]
- Citi S, Amorosi A, Franconi F, Giotti A, Zampi G 1991. Cingulin, a specific protein component of tight junctions, is expressed in normal and neoplastic human epithelial tissues. Am J Pathol 138:781–789 [PMC free article] [PubMed] [Google Scholar]
- Claude P 1978. Morphological factors influencing transepithelial permeability: A model for the resistance of the zonula occludens. J Membr Biol 10:219–232 [DOI] [PubMed] [Google Scholar]
- Claude P, Goodenough DA 1973. Fracture faces of zonulae occludentes from “tight” and “leaky” epithelia. J Cell Biol 58:390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Gorbsky GJ, Steinberg MS 1983. Immunochemical characterization of related families of glycoproteins in desmosomes. J Biol Chem 258:2621–2627 [PubMed] [Google Scholar]
- Colaco CALS, Evans WH 1981. A biochemical dissection of the cardiac intercalated disk: Isolation of subcellular fractions containing fascia adherentes and gap junctions. J Cell Sci 52:313–325 [DOI] [PubMed] [Google Scholar]
- Collins JE, Legan PK, Kenny TP, MacGarvie J, Holton JL, Garrod DR 1991. Cloning and sequence analysis of desmosomal glycoproteins 2 and 3 (desmocollins): Cadherin-like desmosomal adhesion molecules with heterogeneous cytoplasmic domains. J Cell Biol 113:381–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M, D’Atri F, Hammar E, Parry DA, Kendrick-Jones J, Shore D, Citi S 1999. Cingulin contains globular and coiled-coil doamins and interacts with ZO-1, ZO-2, ZO-3, and myosin. J Cell Biol 147:1569–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P, Garrod DR 1983. Antibodies to epithelial desmosomes show wide tissue and species cross-reactivity. Nature 302:148–150 [DOI] [PubMed] [Google Scholar]
- Cowin P, Kapprell H-P, Franke WW 1985b. The complement of desmosomal plaque proteins in different cell types. J Cell Biol 101:1442–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P, Mattey DM, Garrod DR 1984a. Distribution of desmosomal components in the tissues of vertebrates, studied by fluorescent antibody staining. J Cell Sci 66:119–132 [DOI] [PubMed] [Google Scholar]
- Cowin P, Mattey DM, Garrod DR 1984b. Identification of desmosomal surface components (desmocollins) and inhibition of desmosome formation by specific Fab. J Cell Sci 70:41–60 [DOI] [PubMed] [Google Scholar]
- Cowin P, Franke WW, Grund C, Kapprell HP, Kartenbeck J 1985a. The desmosome-intermediate filament complex. In The cell in contact (eds. Edelman G.M., Thiery J.-P.), pp. 427–460 John Wiley and Sons, New York [Google Scholar]
- Cowin P, Kapprell H-P, Franke WW, Tamkun J, Hynes RO 1986. Plakoglobin: A protein common to different kinds of intercellular adhering junctions. Cell 46:1063–1073 [DOI] [PubMed] [Google Scholar]
- Cunningham BA 1985. Structures of cell adhesion molecules. In The cell in contact (eds. Edelman G.M., Thiery J.-P.), pp. 197–217 John Wiley and Sons, New York [Google Scholar]
- Cunningham BA, Leutzinger Y, Gallin WJ, Forkin BC, Edelman GM 1984. Linear organization of the liver cell adhesion molecule L-CAM. Proc Natl Acad Sci 81:5787–5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky CH, Richa J, Solter D, Knudsen K, Buck CA 1983. Identification and purification of a cell surface glycoprotein mediating intercellular adhesion in embryonic and adult tissue. Cell 34:455–466 [DOI] [PubMed] [Google Scholar]
- Damsky CH, Richa J, Wheelock M, Damjanov I, Buck CA 1985. Characterization of cell-CAM 120/80 and the role of surface membrane adhesion glycoproteins in early events in mouse embryo morphogenesis. The cell in contact (eds. Edelman G.M., Thiery J.-P.), pp. 233–255 John Wiley and Sons, New York [Google Scholar]
- Dbouk HA, Mroue RM, El-Sabban MW, Talhouk RS 2009. Connexins: A myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal 7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi M, Iizuka T, Hata Y, Nishimury W, Hirao K, Yao I, Kawabe H, Takai Y 2000. PAPIN. A novel multiple PSD95/Dlg-A/ZO-1 protein interacting with neural plakophilin-related armadillo repeat protein/δ-catenin and p0071. J Biol Chem 275:29875–29880 [DOI] [PubMed] [Google Scholar]
- Dejana E 1996. Endothelial adherens junctions: Implications in the control of vascular permeability and angiogenesis. J Clin Invest 98:1949–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Lampugnani MG, Martinez-Estrada O, Bazzoni G 2000. The molecular organization of endothelial junctions and their functional role in vascular morphogenesis and permeability. Int J Dev Biol 44:743–748 [PubMed] [Google Scholar]
- Delva E, Tucker DK, Kowalczyk AP 2009. The desmosome. Cold Spring Harb Perspect Biol 1:a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demlehner MP, Schäfer S, Grund C, Franke WW 1995. Continual assembly of half-desmosomal structures in the absence of cell contacts and their frustrated endocytosis: A coordinated Sisyphus cycle. J Cell Biol 131:745–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk H, Lackinger E, Cowin P, Franke WW 1985a. Maintenance of desmosomes in mouse hepatocytes after drug-induced rearrangement of cytokeratin filament material. Exp Cell Res 161:161–171 [DOI] [PubMed] [Google Scholar]
- Denk H, Weybora W, Ratschek M, Sohar R, Franke WW 1985b. Distribution of vimentin, cytokeratins, and desmosomal-plaque proteins in human nephroblastomas revealed by specific antibodies: Co-existence of cell groups of different degrees of epithelial differentiation. Differentiation 29:88–97 [DOI] [PubMed] [Google Scholar]
- Dermietzel R, Leibstein A, Frixen U, Janssen-Timmen U, Traub O, Willecke K 1984. Gap junctions in several tissues share antigenic determinants with liver gap junctions. EMBO J 3:2261–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey MM, Barr L 1964. A study of the structure and distribution of the nexus. J Cell Biol 23:553–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JM 1977. The epithelial junction: Bridge, gate, and fence. Physiologist 20:10–18 [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI 2005. α-Catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly. Cell 123:903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drochmans P, Freudenstein C, Wanson J-C, Laurent L, Keenan TW, Stadler J, Leloup R, Franke WW 1978. Structure and biochemical composition of desmosomes and tonofilaments isolated from calf muzzle epidermis. J Cell Biol 79:427–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duden R, Franke WW 1988. Organization of desmosomal plaque proteins in cells growing at low calcium concentrations. J Cell Biol 107:1049–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM 1985. Specific cell adhesion in histogenesis and morphogenesis. In The cell in contact (eds. Edelman G.M., Thiery J.-P.), pp. 139–168 John Wiley and Sons, New York [Google Scholar]
- Edelman GM, Gallin WJ, Delouvée A, Cunningham BA, Thiery J-P 1983. Early epochal maps of two different cell adhesion molecules. Proc Natl Acad Sci 80:4384–4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS 2001. Organization and regulation of the tight junction by the actin-myosin cytoskeleton. In Tight junctions (eds. Cereijido M., Anderson J.), pp. 265–284 CRC Press, Boca Raton, FL [Google Scholar]
- Fanning AS, Ma TY, Anderson JM 2002. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J 16:1835–1837 [DOI] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM 1998. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273:29745–29753 [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE 1963. Junctional complexes in various epithelia. J Cell Biol 17:375–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett DW, McNutt S 1969. The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. J Cell Biol 42:1–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreri DM, Vincent PA 2008. Signaling to and through the endothelial adherens junction. In Cell Junctions (eds. La Flamme S.E., Kowalczyk A.P.), pp. 169–195 Wiley-VCH, Weinheim [Google Scholar]
- Finbow ME 1997. Vertebrate and invertebrate gap junctions: A common molecular basis? Cell Biol Int 21:329–331 [DOI] [PubMed] [Google Scholar]
- Finbow ME, Pitts JD 1993. Is the gap junction channel—the connexon—made of connexin or ductin? J Cell Sci 106:463–472 [DOI] [PubMed] [Google Scholar]
- Finbow ME, Buultjens TEJ, John S, Kam E, Meagher L, Pitts JD 1985. Molecular structure of the gap junctional channel. In Junctional complexes of epithelial cells, Ciba Found. Symp 125, pp. 92–107 J. Wiley and Sons, Chichester: [DOI] [PubMed] [Google Scholar]
- Förster C 2008. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol 130:55–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouquet B, Zimbelmann R, Franke WW 1992. Identification of plakoglobin in oocytes and early embryos of Xenopus laevis: Maternal expression of a gene encoding a junctional plaque protein. Differentiation 51:187–194 [DOI] [PubMed] [Google Scholar]
- Franke WW, Kapprell H-P, Cowin P 1987b. Immunolocalization of plakoglobin in endothelial junctions: Identification as a special type of Zonulae adhaerentes. Biol Cell 59:205–218 [DOI] [PubMed] [Google Scholar]
- Franke WW, Borrmann CM, Grund C, Pieperhoff S 2006. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur J Cell Biol 85:69–82 [DOI] [PubMed] [Google Scholar]
- Franke WW, Cowin P, Schmelz M, Kapprell H-P 1987a. The desmosomal plaque and the cytoskeleton. Junctional complexes of epithelial cells, Ciba Found. Symp.125, pp. 26–44 J. Wiley and Sons, Chichester: [DOI] [PubMed] [Google Scholar]
- Franke WW, Goldschmidt MD, Zimbelmann R, Mueller HM, Schiller DL, Cowin P 1989. Molecular cloning and amino acid sequence of human plakoglobin, the common junctional plaque protein. Proc Natl Acad Sci 86:4027–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Grund C, Kuhn C, Jackson BW, Illmensee K 1982a. Formation of cytoskeletal elements during mouse embryogenesis. III. Primary mesenchymal cells and the first appearance of vimentin filaments. Differentiation 23:43–59 [DOI] [PubMed] [Google Scholar]
- Franke WW, Moll R, Müller H, Schmid E, Kuhn C, Krepler R, Artlieb U, Denk H 1983a. Immunocytochemical identification of epithelium-derived human tumors with antibodies to desmosomal plaque proteins. Proc Natl Acad Sci 80:543–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Mueller H, Mittnacht S, Kapprell H-P, Jorcano JL 1983b. Significance of two desmosome plaque-associated polypeptides of molecular weights 75,000 and 83,000. EMBO J 2:2211–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Rickelt S, Barth M, Pieperhoff S 2009. The junctions that don’t fit the scheme: Special symmetrical junctions of their own kind. Cell Tissue Res, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Schmid E, Grund C, Müller H, Engelbrecht I, Moll R, Stadler J, Jarasch E-D 1981. Antibodies to high molecular weight polypeptides of desmosomes: Specific localization of a class of junctional proteins in cells and tissues. Differentiation 20:217–241 [DOI] [PubMed] [Google Scholar]
- Franke WW, Schmid E, Schiller DL, Winter S, Jarasch E-D, Moll R, Denk H, Jackson BW, Illmensee K 1982b. Differentiation-related patterns of expression of proteins of intermediate-size filaments in tissues and cultured cells. Cold Spring Harb Symp Quant Biol 46:431–453 [DOI] [PubMed] [Google Scholar]
- Franke WW, Troyanovsky SM, Koch PJ, Troyanovsky R, Fouquet B, Leube RE 1992. Desmosomal proteins— mediators of intercellular coupling and intermediate filament anchorage. Cold Spring Harbor Symp Quant Biol 57:643–652 [DOI] [PubMed] [Google Scholar]
- Friend DS, Gilula NB 1972. Variations in tight and gap-junctions in mammalian tissues. J Cell Biol 53:758–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frixen U, Behrens J, Sachs M 1991. E-cadherin-mediated cell–cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol 113:173–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromaget C, Al Aoumari A, Dupont E, Briand JP, Gros D 1990. Changes in the expression of Cx43, a cardiac gap-junctional protein, during mouse heart development. J Mol Cell Cardiol 22:1245–1258 [DOI] [PubMed] [Google Scholar]
- Fromaget C, El Aoumari A, Gros D 1992. Distribution pattern of connexin 43, a gap junctional protein, during the differentiation of mouse heart myocytes. Differentiation 51:9–20 [DOI] [PubMed] [Google Scholar]
- Fromaget C, El Aoumari A, Jarry T, Briand J-P, Maurice M, Feldmann G, Maro B, Gros D 1993. Expression of Cx43 in rat and mouse liver. In Gap junctions (eds. Hall J.E., Zampighi G.A., Davis R.M.), Progr Cell Res Vol. 3, pp. 25–31 Elsevier, Amsterdam [Google Scholar]
- Funayama N, Nagafuchi A, Sato N, Tsukita S, Tsukita S 1991. Radixin is a novel member of the band 4.1 family. J Cell Biol 115:1039–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Tsukita Sh 1999. Manner of interaction of heterogenous claudin species within and between tight junction strands. J Cell Biol 147:891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Fujimoto K, Sato N, Hirase T, Tsukita Sa, Tsukita Sh 1996. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J Cell Sci 109:429–435 [DOI] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita Sh 1998a. Claudin-1 and -2: Novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141:1539–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita Sh 2002. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J Cell Biol 156:1099–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita Sa, Tsukita Sh 1993. Occludin: A novel integral membrane protein localizing at tight junctions. J Cell Biol 123:1777–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita Sa, Tsukita Sh 1994. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol 127:1617–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Fujimoto K, Tsukita Sh 1998b. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol 143:391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin WJ, Edelman GM, Cunningham BA 1983. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci 80:1038–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin WJ, Sorkin BC, Edelman GM, Cunningham BA 1987. Sequence analysis of a cDNA clone encoding the liver cell adhesion molecule, L-CAM. Proc Natl Acad Sci 84:2808–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicano GI, Bauer C, Fuchs E 2001. Rescuing desmoplakin function in extra-embryonic extoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasuclature. Development 128:929–941 [DOI] [PubMed] [Google Scholar]
- Gallicano GI, Kouklis P, Bauer C, Yin M, Vasioukhin V, Degenstein L, Fuchs E 1998. Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J Cell Biol 143:2009–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary R, Bretscher B 1993. Heterotypic and homotypic associations between ezrin and moesin, two putative membrane-cytoskeletal linking proteins. Proc Natl Acad Sci 90:10846–10850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D, Chidgey M, North A 1996. Desmosomes: Differentiation, development, dynamics and disease. Curr Opin Cell Biol 8:670–678 [DOI] [PubMed] [Google Scholar]
- Garrod DR, Merritt AJ, Nie Z 2002. Desmosomal cadherins. Curr Opin Cell Biol 14:537–545 [DOI] [PubMed] [Google Scholar]
- Geiger B, Schmid E, Franke WW 1983. Spatial distribution of proteins specific for desmosomes and adhaerens junctions in epithelial cells demonstrated by double immunofluorescence microscopy. Differentiation 23:189–205 [DOI] [PubMed] [Google Scholar]
- Geiger B, Volk T, Volberg T 1985b. Molecular heterogeneity of adherens junctions. J Cell Biol 101:1523–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Avnur Z, Volberg T, Volk T 1985a. Molecular domains of adherens junctions. In The cell in contact (eds. Edelman G.M., Thiery J.-P.), pp. 461–489 John Wiley and Sons, New York [Google Scholar]
- Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA et al. 2004. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet 36:1162–1164 [DOI] [PubMed] [Google Scholar]
- Giagtzoglou N, Ly CV, Bellen HJ 2009. Cell adhesion, the backbone of the synapse: “Vertebrate” and “invertebrate” perspectives. Cold Spring Harb Perspect Biol 1:a003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giepmans BN, Molenaar WH 1998. The gap junction protein connexin 43 interacts with the second PDZ domain of the zona occludes-1 protein. Curr Biol 8:931–934 [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Verlaan I, Moolenaar WH 2001. Connexin-43 interactions with ZO-1 and α- and β-tubulin. Cell Commun Adhes 8:213–217 [DOI] [PubMed] [Google Scholar]
- Gilula NB 1985. Gap junctional contact between cells. In The cell in contact (eds. Edelman G.M., Thiery J.-P.), pp. 395–409 John Wiley and Sons, New York [Google Scholar]
- Gilula NB 1987. Topology of gap junction protein and channel function. In Junctional complexes of epithelial cells, Ciba Found. Symp.125, pp. 128–139 J. Wiley and Sons, Chichester: [DOI] [PubMed] [Google Scholar]
- Gilula NB 1990. Gap junction protein and its organization into the cell–cell channel. In Morphoregulatory molecules (eds. Edelman G.M., Cunningham B.A., Thiery J.P.), pp. 371–382 John Wiley and Sons, New York [Google Scholar]
- Giudice GJ, Cohen SM, Patel N, Steinberg MS 1984. Immunological comparison of desmosomal components from several bovine tissues. J Cell Biochem 26:35–45 [DOI] [PubMed] [Google Scholar]
- Godsel LM, Getsios S, Huen AC, Green KJ 2004. The molecular composition and function of desmosomes. In Cell adhesion (eds. Behrens J., Nelson W.J.), Handbook Exp. Pharmacol., Vol. 165, pp. 137–193 Springer, Berlin: [DOI] [PubMed] [Google Scholar]
- Golenhofen N, Drenckhahn D 2000. The catenin, p120ctn, is a common membrane-associated protein in various epithelial cells and tissues. Histochem Cell Biol 114:147–155 [DOI] [PubMed] [Google Scholar]
- González-Mariscal L, Betanzos S, Nava P, Jaramillo BE 2003. Tight junction proteins. Progr Biophys Mol Biol 81:1–44 [DOI] [PubMed] [Google Scholar]
- Goodenough DA 1974. Bulk isolation of mouse hepatocyte gap junctions. Characterizations of the principal protein, connexin. J Cell Biol 61:557–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA 1990. Gap junctions and intercellular communication. In Morphoregulatory molecules (eds. Edelman G.M., Cunningham B.A., Thiery J.P.), pp. 357–369 John Wiley and Sons, New York [Google Scholar]
- Goodenough DA, Revel JP 1970. A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol 45:272–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL 2009. Gap Junctions. Cold Spring Harb Perspect Biol 1:a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA, Revel JP 1971. The permeability of isolated and in situ mouse hepatic gap junctions studied with enzymatic tracers. J Cell Biol 50:81–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL 1996. Connexins, Connexons, and intercellular communication. Annu Rev Biochem 65:475–502 [DOI] [PubMed] [Google Scholar]
- Goodwin L, Hill JE, Raynor K, Raszi L, Manabe M, Cowin P 1990. Desmoglein shows extensive homology to the cadherin family of cell adhesion molecules. Biochem Biophys Res Commun 173:1224–1230 [DOI] [PubMed] [Google Scholar]
- Goossens S, Janssens B, Bonne S, De Rycke R, Braet F, van Hengel J, van Roy F 2007. A unique and specific interaction between αT-catenin and plakophilin-2 in the area composita, the mixed-type junctional structure of cardiac intercalated discs. J Cell Sci 120:2126–2136 [DOI] [PubMed] [Google Scholar]
- Gorbsky G, Steinberg MS 1981. Isolation of the intercellular glycoproteins of desmosomes. J Cell Biol 90:243–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky G, Cohen SM, Shida H, Giudice GJ, Steinberg MS 1985. Isolation of the non-glycosylated proteins of desmosomes and immunolocalization of a third plaque protein: Desmoplakin III. Proc Natl Acad Sci 82:810–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi CJ, Niessen CM 2008. Tight junctions in simple and stratified epithelium. In Cell Junctions (eds. La Flamme S.E., Kowalczyk A.P.), pp. 217–233 Wiley-VCH, Weinheim [Google Scholar]
- Gottardi CJ, Arpin M, Fanning AS, Louvard D 1996. The junction-associated protein, zonula occludens-1, localizes to the nucleus before the maturation and during the remodeling of cell–cell contacts. Proc Natl Acad Sci 93:10779–10784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould KL, Bretscher A, Esch FS, Hunter T 1989. cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1. EMBO J 8:4133–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw J, Löster J, Doewarto D, Fuchs H, Meyer B, Reis A, Wolf E, Balling R, Hrabé de Angelis M 2001. Characterization of a mutation in the lens-specific MP70 encoding gene of the mouse leading to a dominant cataract. Exp Eye Res 73:867–876 [DOI] [PubMed] [Google Scholar]
- Green KJ, Gaudry CA 2000. Are desmosomes more than tethers for intermediate filaments? Nat Rev Mol Cell Biol 1:208–216 [DOI] [PubMed] [Google Scholar]
- Green KJ, Goldman RD, Chisholm RL 1988. Isolation of cDNAs encoding desmosomal plaque proteins: Evidence that bovine desmoplakins I and II are derived from two mRNAs and a single gene. Proc Natl Acad Sci 85:2613–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Parry DAD, Steinert PM, Virata MLA, Wagner RM, Angst BD, Nilles LA 1990. Structure of the human desmoplakins. Implications for function in the desmosomal plaque. J Biol Chem 265:2603–2612 [PubMed] [Google Scholar]
- Green KJ, Getsios S, Troyanovsky S, Godsel LM 2009. Intercellular junction assembly, dynamics and homeostasis. Cold Spring Harb Perspect Biol 2:a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann KS, Grund C, Huelsken J, Behrend M, Erdmann B, Franke WW, Birchmeier W 2004. Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J Cell Biol 167:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM 1993. Breaking through the tight junction barrier. J Cell Biol 123:1631–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B 1996. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 84:345–357 [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Lowenkopf T, Apatira D 1991. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci 88:3460–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij UC, Hunziker W, Fromm M, Müller D 2009. Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci 122:1507–1517 [DOI] [PubMed] [Google Scholar]
- Halbleib JM, Nelson WJ 2006. Cadherins in development: Cell adhesion, sorting, and tissue morphogenesis. Genes Dev 20:3199–3214 [DOI] [PubMed] [Google Scholar]
- Hart MJ, Callow MG, Souza B, Polakis P 1996. IQGAP1, a calmodulin-binding protein with a rasGAP-related domain, is a potential effector for cdc42Hs. EMBO J 15:2997–3005 [PMC free article] [PubMed] [Google Scholar]
- Haskins J, Lijie G, Wittchen ES, Hibbard J, Stevenson BR 1998. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol 141:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K, Takeichi M 1986. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature 320:447–449 [DOI] [PubMed] [Google Scholar]
- Hatta K, Nose A, Nagafuchi A, Takeichi M 1988. Cloning and expression of cDNA encoding a neural calcium-dependent cell adhesion molecule: Its identity in the cadherin gene family. J Cell Biol 106:873–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K, Takagi S, Fujisawa H, Takeichi M 1987. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryo. Dev Biol 120:215–227 [DOI] [PubMed] [Google Scholar]
- Hatzfeld M 1999. The armadillo family of structural proteins. Int Rev Cytol 186:179–224 [DOI] [PubMed] [Google Scholar]
- Hatzfeld M 2005. The p120 family of cell adhesion molecules. Eur J Cell Biol 84:205–214 [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Nachtsheim C 1996. Cloning and characterization of a new armadillo family member, p0071, associated with the junctional plaque: Evidence for a subfamily of closely related proteins. J Cell Sci 109:2767–2778 [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Kristjansson GI, Plessmann U, Weber K 1994. Band 6 protein, a major constituent of desmosomes from stratified epitehlia, is a novel member of the armadillo multigene family. J Cell Sci 107:2259–2270 [DOI] [PubMed] [Google Scholar]
- Hecht G 2001. Microbial pathogens that affect tight junctions. In Tight junctions (eds. Cereijido M., Anderson J.), pp. 493–515 CRC Press, Boca Raton, FL [Google Scholar]
- Henderson D, Eibl H, Weber K 1979. Structure and biochemistry of mouse hepatic gap junctions. J Mol Biol 132:193–218 [DOI] [PubMed] [Google Scholar]
- Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R 1991. The uvomorulin-anchorage protein α catenin is a vinculin homologue. Proc Natl Acad Sci 88:9156–9160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg EL 1984. A detergent-independent procedure for the isolation of gap junctions from rat liver. J Biol Chem 259:9936–9943 [PubMed] [Google Scholar]
- Hertzberg EL, Gilula NB 1979. Isolation and characterization of gap junctions from rat liver. J Biol Chem 254:2138–2147 [PubMed] [Google Scholar]
- Hertzberg EL, Skibbens RV 1984. A protein homologous to the 27,000 dalton liver gap junction protein is present in a wide variety of species and tissues. Cell 39:61–69 [DOI] [PubMed] [Google Scholar]
- Hertzberg AL, Spray DC, Bennett MVL 1985. Reduction of gap junctional conductance by microinjection of antibodies against the 27-kDa liver gap junction polypeptide. Proc Natl Acad Sci 82:2412–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg EL, Anderson DJ, Friedlander M, Gilula NB 1982. Comparative analysis of the major polypeptides from liver gap junctions and lens fiber junctions. J Cell Biol 92:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuberger J, Birchmeier W 2009. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol 2:002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heynkes R, Kozjek G, Traub O, Willecke K 1986. Identification of a rat liver cDNA and mRNA coding for the 28 kDa gap junction. FEBS Lett 205:56–60 [DOI] [PubMed] [Google Scholar]
- Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister J-J 2004. Myofibroblast development is characterized by specific cell–cell adherens junctions. Mol Biol Cell 15:4310–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita Sh, Tsukita Sa 1996. Regulation mechanism of ERM (Ezrin/Radixin/Moesin) protein/plasma membrane association: Possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol 135:37–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C, Zhou J, Medina M, Goto T, Jacobson M, Bhide PG, Kosik KS 2000. δ-Catenin is a nervous system-specific adherens junction protein which undergoes dynamic relocalization during development. J Comp Neurol 420:261–276 [PubMed] [Google Scholar]
- Hobmayer E, Hatta M, Fischer R, Fujisawa T, Holstein TW, Sugiyama T 1996. Identification of a Hydra homologue of the β-catenin/plakoglobin/armadillo gene family. Gene 172:155–159 [DOI] [PubMed] [Google Scholar]
- Hobmayer B, Rentzsch F, Kuhn K, Happel CM, Cramer von Laue C, Snyder P, Rothbächer U, Holstein TW 2000. WNT signalling molecules act in axis formation in the diploblastic metazoan Hydra. Nature 407:186–189 [DOI] [PubMed] [Google Scholar]
- Hofmann I, Kuhn C, Franke WW 2008. Protein p0071, a major plaque protein of non-desmosomal adhering junctions, is a selective cell-type marker. Cell Tissue Res 334:381–399 [DOI] [PubMed] [Google Scholar]
- Hofmann I, Mertens C, Brettel M, Nimmrich V, Schnölzer M, Herrmann H 2000. Interaction of plakophilins with desmoplakin and intermediate filament proteins: An in vitro analysis. J Cell Sci 113:2471–2483 [DOI] [PubMed] [Google Scholar]
- Hofmann I, Schlechter T, Kuhn C, Hergt M, Franke WW 2009. Protein p0071 – an armadillo plaque protein that characterizes a specific subtype of adherens junctions. J Cell Sci 122:21–24 [DOI] [PubMed] [Google Scholar]
- Holtfreter J 1939. Gewebeaffinität, ein Mittel der embryonalen Formbildung. Arch Exp Zellforsch 23:169–209 [Google Scholar]
- Holthöfer B, Windoffer R, Troyanovsky S, Leube RE 2007. Structure and function of desmosomes. Int Rev Cytol 264:65–163 [DOI] [PubMed] [Google Scholar]
- Holton JL, Kenny TP, Legan PK, Collins JE, Keen JN, Sharma R, Garrod DR 1990. Desmosomal glycoproteins 2 and 3 (desmocollins) show N-terminal similarity ot calcium-dependent cell–cell adhesion molecules. J Cell Sci 97:239–246 [DOI] [PubMed] [Google Scholar]
- Horwarth AG, Hughes MR, Stevenson BR 1992. Detection of the tight junction-associated protein ZO-1 in astrocytes and other nonepithelial cell types. Am J Physiol 262:C461–C469 [DOI] [PubMed] [Google Scholar]
- Hsu S-N, Yonekura S, Ting C-Y, Robertson HM, Iwai Y, Uemura T, Lee C-H, Chiba A 2009. Conserved alternative splicing and expression patterns of arthropod N-cadherin. PLoS Genet 5:e1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AH, Nelson WJ, Weis WI 1997. Three-dimensional structure of the armadillo repeat region of β-catenin. Cell 90:871–882 [DOI] [PubMed] [Google Scholar]
- Hull BE, Staehelin LA 1976. Functional significance of the variations in the geometrical organization of tight junction networks. J Cell Biol 68:688–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hülsken J, Birchmeier W, Behrens J 1994. E-cadherin and APC compete for the interaction with β-catenin and the cytoskeleton. J Cell Biol 127:2061–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyafil F, Morello D, Babinet C, Jacob F 1980. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell 21:927–934 [DOI] [PubMed] [Google Scholar]
- Hyafil F, Babinet C, Jacob F 1981. Cell–cell interactions in early embryogenesis: A molecular approach to the role of calcium. Cell 26:447–454 [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita Sa, Tsukita Sh 2005. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 171:939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof BA, Vollmers HP, Goodman SL, Birchmeier W 1983. Cell–cell interaction and polarity of epithelial cells: Specific perturbation using a monoclonal antibody. Cell 36:667–675 [DOI] [PubMed] [Google Scholar]
- Itoh M, Morita K, Tsukita Sh 1999. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and α catenin. J Biol Chem 274:5981–5986 [DOI] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S 1993. The 200-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol 131:391–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Yonemura S, Nagafuchi A, Tsukita S, Tsukita S 1991. A 220-kD undercoat-constitutive protein: Its specific localization at cadherin-based cell–cell adhesion sites. J Cell Biol 115:1449–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie K, Shimizu K, Sakisaka T, Ikeda W, Takai Y 2004. Roles of nectins in cell adhesion, signaling and polarization. In Cell adhesion (eds. Behrens J., Nelson W.J.), Handbook Exp. Pharmacol., Vol. 165, pp. 343–372 Springer, Berlin: [DOI] [PubMed] [Google Scholar]
- Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA 2004. VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 286:L1143–L1153 [DOI] [PubMed] [Google Scholar]
- Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y 2004. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J Cell Biol 166:237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Timmen U, Dermietzel R, Frixen U, Leibstein A, Traub O, Willecke K 1983. Immunocytochemical localization of the gap junction 26 K protein in mouse liver plasma membranes. EMBO J 2:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens B, Goossens S, Staes K, Gilbert B, van Hengel J, Colpaert C, Bruyneel E, Mareel M, van Roy F 2001. αT-catenin: A novel tissue-specific β-catenin-binding protein mediating strong cell–cell adhesion. J Cell Sci 114:3177–3188 [DOI] [PubMed] [Google Scholar]
- Jin C, Martyn KD, Kurata WE, Warn-Cramer BJ, Lau AF 2004. Connexin43 PDZ2 binding domain mutants create functional gap junctions and exhibit altered phosphorylation. Cell Commun Adhes 11:67–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Oda T, Shimoyama Y, Ochiai A, Oyama T, Yoshiura K, Akimoto S, Yamada T, Hirohashi S 1994. Alterations of the cadherin-catenin cell adhesion system in cancers. In Molecular and cellular basis for cell to cell interaction: Its significance in cancer (eds. Hirohashi S., Moses H.L., Ruoslahti E., Sugimura T., Takeichi M., Terada M.; Proc. 24th Int. Symp. Princess Takamatsu Cancer Res. Fund), pp. 51–62 Princeton Scientific Publishing, Princeton, NJ: [PubMed] [Google Scholar]
- Kanter HL, Saffitz JE, Beyer EC 1991. Canine cardiac myocytes express multiple gap-junction proteins. Clin Res 39: p193A. [DOI] [PubMed] [Google Scholar]
- Kapprell H-P, Cowin P, Franke WW 1987. Biochemical characterization of the soluble form of the junctional plaque protein, plakoglobin, from different cell types. Eur J Biochem 166:505–517 [DOI] [PubMed] [Google Scholar]
- Kapprell H-P, Owaribe K, Franke WW 1988. Identification of a basic protein of Mr 75,000 as an accessory desmosomal plaque protein in stratified and complex epithelia. J Cell Biol 106:1679–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J, Franke WW, Moser JG, Stoffels U 1983. Specific attachment of desmin filaments to desmosomal plaques in cardiac myocytes. EMBO J 2:735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J, Schmid E, Franke WW, Geiger B 1982. Different modes of internalization of proteins associated with adhaerens junctions and desmosomes: Experimental separation of lateral contacts induces endocytosis of desmosomal plaque material. EMBO J 1:725–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J, Schwechheimer K, Moll R, Franke WW 1984. Attachment of vimentin filaments to desmosomal plaques in human meningiomal cells and arachnoidal tissue. J Cell Biol 98:1072–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J, Schmelz M, Franke WW, Geiger B 1991. Endocytosis of junctional cadherins in bovine kidney epithelial (MDBK) cells cultured in low Ca2+ ion medium. J Cell Biol 113:881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann U, Zuppinger C, Waibler Z, Rudiger M, Urbich C, Martin B, Jockusch BM, Eppenberger H, Starzinski-Powitz A 2000. The armadillo repeat region targets ARVCF to cadherin-based cellular junctions. J Cell Sci 113:4121–4135 [DOI] [PubMed] [Google Scholar]
- Kausalya PJ, Phua DCY, Hunziker W 2004. Association of ARVCF with zonula occludens (ZO)-1 and ZO-2: Binding to PDZ-domain proteins and cell–cell adhesion regulate plasma membrane and nuclear localization of ARVCF. Mol Biol Cell 15:5503–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausalya PJ, Reichert M, Hunziker W 2001. Connexin45 directly binds to ZO-1 and localizes to the tight junction region in epithelial MDCK cells. FEBS Lett 505:92–96 [DOI] [PubMed] [Google Scholar]
- Kemler R, Babinet C, Eisen H, Jacob F 1977. Surface antigen in early differentiation. Proc Natl Acad Sci 74:4449–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King N, Hittinger CT, Carroll SB 2003. Evolution of key cell signaling and adhesion protein families predates animal origins. Science 301:361–363 [DOI] [PubMed] [Google Scholar]
- King IA, O’Brien TJ, Buxton RS 1996. Expression of the “skin-type” desmosomal cadherin DSC1 is closely linked to the keratinization of epithelial tissues during mouse development. J Invest Dermatol 107:531–538 [DOI] [PubMed] [Google Scholar]
- King IA, Angst BD, Hunt DM, Kruger M, Arnemann J, Buxton RS 1997. Hierarchical expression of desmosomal cadherins during stratified epithelial morphogenesis in the mouse. Differentiation 62:83–96 [DOI] [PubMed] [Google Scholar]
- King IA, Arnemann J, Spurr NK, Buxton RS 1993a. Cloning of the cDNA (DSC1) coding for human type 1 desmocollin and its assignment to chromosome 18. Genomics 18:185–194 [DOI] [PubMed] [Google Scholar]
- King IA, Sullivan KH, Bennett Jr, Buxton RS 1995. The desmocollins of human foreskin epidermis: Identification and chromosomal assignment of a third gene and expression patterns of the three isoforms. J Invest Dermatol 105:314–321 [DOI] [PubMed] [Google Scholar]
- King IA, Tabiowo A, Purkis P, Leigh I, Magee AI 1993b. Expression of distinct desmocollin isoforms in human epidermis. J Invest Dermatol 100:373–379 [DOI] [PubMed] [Google Scholar]
- Kljuic A, Bazzi H, Sundberg JP, Martinze-Mir A, S’shaughnessy R, Mahoney MG, Levy M, Montagutelli X, Ahmad W, Aita VM, et al. 2003. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: Evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell 113:249–260 [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Wheelock MJ 1992. Plakoglobin, or an 83-kD homologue distinct from β-catenin, interacts with E-cadherin and N-cadherin. J Cell Biol 118:671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch PJ, Franke WW 1994. Desmosomal cadherins: Another growing multigene family of adhesion molecules. Curr Opin Cell Biol 6:682–687 [DOI] [PubMed] [Google Scholar]
- Koch PJ, Goldschmidt MD, Walsh MJ, Zimbelmann R, Franke WW 1991a. Complete amino acid sequence of the epidermal desmoglein precursor polypeptide and identification of a second type of desmoglein gene. Eur J Cell Biol 55:200–208 [PubMed] [Google Scholar]
- Koch PJ, Goldschmidt MD, Walsh MJ, Zimbelmann R, Schmelz M, Franke WW 1991b. Amino acid sequence of bovine muzzle epithelial desmocollin derived from cloned cDNA: A novel subtype of desmosomal cadherins. Differentiation 47:29–36 [DOI] [PubMed] [Google Scholar]
- Koch PJ, Goldschmidt MD, Zimbelmann R, Troyanovsky R, Franke WW 1992. Complexity and expression patterns of the desmosomal cadherins. Proc Natl Acad Sci 89:353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch PJ, Walsh MJ, Schmelz M, Goldschmidt MD, Zimbelmann R, Franke WW 1990. Identification of desmoglein, a constitutive desmosomal glycoprotein, as a member of the cadherin family of cell adhesion molecules. Eur J Cell Biol 53:1–12 [PubMed] [Google Scholar]
- Koeser J, Troyanovsky SM, Grund C, Franke WW 2003. De novo formation of desmosomes in cultured cells upon transfection of genes encoding specific desmosomal components. Exp Cell Res 285:114–130 [DOI] [PubMed] [Google Scholar]
- Korman NJ, Eyre RW, Klaus-Kovtun V, Stanley JR 1989. Demonstration of an adhering-junction molecule (plakoglobin) in the autoantigens of Pemphigus foliaceus and Pemphigus vulgaris. N Engl J Med 321:631–635 [DOI] [PubMed] [Google Scholar]
- Kosik KS, Donahue CP, Israely I, Liu X, Ochiishi T 2005. δ-Catenin at the synaptic-adherens junction. Trends Cell Biol 15:172–178 [DOI] [PubMed] [Google Scholar]
- Koslov ER, Maupin P, Pradhan D, Morrow JS, Rimm DL 1997. α-Catenin can form asymmetric homodimeric complexes and/or heterodimeric complexes with β-catenin. J Biol Chem 272:27301–27306 [DOI] [PubMed] [Google Scholar]
- Kottke MD, Delva E, Kowalczyk AP 2006. The desmosome: Cell science lessons from human diseases. J Cell Sci 119:797–806 [DOI] [PubMed] [Google Scholar]
- Koval M 2008. Gap junctions: Connexin functions and roles in human disease. In Cell Junctions (eds. La Flamme S.E., Kowalczyk A.P.), pp. 197–216 Wiley-VCH, Weinheim [Google Scholar]
- Kowalczyk AP, Bornslaeger EA, Borgwardt JE, Palka HL, Dhaliwal AS, Corcoran CM, Denning MF, Green KJ 1997. The amino-terminal domain of desmoplakin binds to plakoglobin and clusters desmosomal cadherin-plakoglobin complexes. J Cell Biol 139:773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk AP, Navarro P, Dejana E, Bornslaeger EA, Green KJ, Kopp DS, Borgwardt JE 1998. VE-cadherin and desmoplakin are assembled into dermal microvascular endothelial intercellular junctions: A pivotal role for plakoglobin in the recruitment of desmoplakin to the intercellular junctions. J Cell Sci 111:3045–3057 [DOI] [PubMed] [Google Scholar]
- Kowalczyk AP, Palka HL, Luu HH, Nilles LA, Anderson JE, Wheelock MJ, Green KJ 1994. Posttranslational regulation of plakoglobin expression. Influence of the desmosomal cadherins on plakoglobin metabolic stability. J Biol Chem 269:31214–31223 [PubMed] [Google Scholar]
- Kreft B, Berndorff D, Böttinger A, Finnemann S, Wedlich D, Hortsch M, Tauber R, Geßner R 1997. LI-Cadherin-mediated cell–cell adhesion does not require cytoplasmic interactions. J Cell Biol 136:1109–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar NM, Gilula NB 1986. Cloning and characterization of human and rat liver cDNAs coding for a gap junction protein. J Cell Biol 103:767–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Gilula NB 1996. The gap junction communication channel. Cell 84:381–388 [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Kobayashi K, Nakafuku M, Nomura N, Iwamatsu A, Kaibuchi K 1996. Identification of IQGAP as a putative target for the small GTPases, Cdc42 and Rac1. J Biol Chem 271:23363–23367 [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, et al. 1998. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell–cell adhesion. Science 281:832–835 [DOI] [PubMed] [Google Scholar]
- Laing JG, Manley-Markowski RN, Koval M, Civitelli R, Steinberg TH 2001a. Connexin45 interacts with zonula occludens-1 and connexin43 in osteoblastic cells. J Biol Chem 276:23051–23055 [DOI] [PubMed] [Google Scholar]
- Laing JG, Manley-Markowski RN, Koval M, Civitelli R, Steinberg TH 2001b. Connexin45 interacts with zonula occludens-I in osteoblastic cells. Cell Commun Adhes 8:209–212 [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E 1995. The molecular organization of endothelial cell to cell junctions: Differential association of plakoglobin, β-catenin, and α-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 129:203–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbein L, Grund C, Kuhn C, Praetzel S, Kartenbeck J, Brandner JM, Moll I, Franke WW 2002. Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Eur J Cell Biol 81:419–435 [DOI] [PubMed] [Google Scholar]
- Langbein L, Pape U-F, Grund C, Kuhn C, Praetzel S, Moll I, Moll R, Franke WW 2003. Tight junction-related structures in the absence of a lumen: Occludin, claudins and tight junction plaque proteins in densely packed cell formations of stratified epithelia and squamous cell carcinomas. Eur J Cell Biol 82:385–400 [DOI] [PubMed] [Google Scholar]
- Legan PK, Yue KKM, Chidgey MAJ, Holton JL, Wilkinson RW, Garrod DR 1994. The bovine desmocollin family: A new gene and expression patterns reflecting epithelial cell proliferation and differentiation. J Cell Biol 126:507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen S, Ryan JJ, Kudlicka K, Iino N, Zhou H, Farquhar MG 2005. Cell junction-associated proteins IQGAP1, MAGI-2, CASK, spectrins, and α-actinin are components of the nephrin multiprotein complex. Proc Natl Acad Sci 102:9814–9819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leloup R, Laurent L, Ronveaux M-F, Drochmans P, Wanson J-C 1979. Desmosomes and desmogenesis in the epidermis of calf muzzle. Biol Cell 34, 137–152 [Google Scholar]
- Lewis-Tuffin JJ, Anastasiadis PZ 2008. Armadillo repeat proteins at epithelial adherens junctions. In Cell Junctions (eds. La Flamme S.E., Kowalczyk A.P.), pp. 153–167 Wiley-VCH, Weinheim [Google Scholar]
- Loewenstein WR 1981. Junctional intercellular communication: The cell-to-cell membrane channel. Physiol Rev 61:829–913 [DOI] [PubMed] [Google Scholar]
- Loh YH, Christoffels A, Brenner S, Hunziker W, Venkatesh B 2004. Extensive expansion of the claudin gene family in the teleost fish, Fugu rubripes. Genome Res 14:1248–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Paredes M, Medina M, Zhou J, Cavallo R, Peifer M, Orecchio L, Kosik KS 1999. δ-Catenin, and adhesive junction-associated protein which promotes cell scattering. J Cell Biol 144:519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui WY, Mruk DD, Cheng CY 2005. Interactions among IQGAP1, Cdc42, and the cadherin/catenin protein complex regulate Sertoli-germ cell adherens junction dynamics in the testis. J Cell Phsiol 202:49–66 [DOI] [PubMed] [Google Scholar]
- Madara JL, Dharmsathaphorn K 1985. Occluding junction structure-function relationships in a cultured epithelial monolayer. J Cell Biol 101:2124–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, Matsuda Y, Tsukita S, Takai Y 1997. Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol 139:517–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcozzi C, Burdett IDJ, Buxton RS, Magee AI 1998. Coexpression of both types of desmosomal cadherin and plakoglobin confers strong intercellular adhesion. J Cell Sci 111:495–509 [DOI] [PubMed] [Google Scholar]
- Mareel MM, Behrens J, Birchmeier W, De Bruyne GK, Vleminckx K, Hoogewijs A, Fiers WC, Van Roy FM 1991. Downregulation of E-cadherin expression in Madin Darby canine kidney (MDCK) cells inside nude mice tumors. Int J Cancer 47:922–928 [DOI] [PubMed] [Google Scholar]
- Mareel M, Vleminckx K, Vermeulen S, Yan G, Bracke M, van Roy F 1994. Downregulation in vivo of the invasion-suppressor molecule E-cadherin in experimental and clinical cancer. In Molecular and cellular basis for cell to cell interaction: Its significance in cancer (eds. Hirohashi S., Moses HL, Ruoslahti E., Sugimura T., Takeichi M., Terada M.; Proc. 24th Int. Symp. Princess Takamatsu Cancer Res. Fund), pp. 63–80 Princeton Scientific Publishing, Princeton, NJ: [PubMed] [Google Scholar]
- Mariner DJ, Sirotkin H, Daniel JM, Lindman BR, Mernaugh RL, Patten AK, Thoreson MA, Reynolds AB 1999. Production and characterization of monoclonal antibodies to ARVCF. Hybridoma 18:343–349 [DOI] [PubMed] [Google Scholar]
- Mariner DJ, Wang J, Reynolds AB 2000. ARVCF localizes to the nucleus and adherens junction and is mutually exclusive with p120(ctn) in E-cadherin complexes. J Cell Sci 113:1481–1490 [DOI] [PubMed] [Google Scholar]
- Matsunaga M, Hatta K, Takeichi M 1988. Role of N-cadherin cell adhesion molecules in the histogenesis of neural retina. Neuron 1:289–295 [DOI] [PubMed] [Google Scholar]
- Matter K, Balda M 1999. Occludin and the function of tight junctions. Int Rev Cytol 186:117–146 [DOI] [PubMed] [Google Scholar]
- McCallum SJ, Su WJ, Cerione RA 1996. Identification of a putative effector for Cdc42Hs with high sequence similarity to the RasGAP-related protein IQGAP1 aqnd Cdc42Hs binding partner with similarity to IQGAP2. J Biol Chem 271:21732–21737 [DOI] [PubMed] [Google Scholar]
- McCrea PD, Gumbiner BM 1991. Purification of a 92-kDa cytoplasmic protein tightly associated with the cell–cell adhesion molecule E-cadherin (uvomorulin). Characterization and extractability of the protein complex from the cell cytostructure. J Biol Chem 266:4514–4520 [PubMed] [Google Scholar]
- McCrea PD, Turck CW, Gumbiner B 1991. A homolog of the armadillo protein in Drosophila (plakoglobin) associated with E-cadherin. Science 254:1359–1361 [DOI] [PubMed] [Google Scholar]
- McNutt NS, Weinstein RS 1970. The ultrastructure of the nexus: A correlated thin-section and freeze-cleave study. J Cell Biol 47:666–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanic S, Raynor K, Hill JE, Cowin P 1991. Desmocollins form a distinct subset of the cadherin family of cell adhesion molecules. Proc Natl Acad Sci 88:4476–4480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Takeichi M 2009. Adherens junction: Molecular architecture and regulation. Cold Spring Harb Perspect Biol 1:002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens C, Kuhn C, Franke WW 1996. Plakophilins 2a and 2b: Constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol 135:1009–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens C, Kuhn C, Moll R, Schwetlick I, Franke WW 1999. Desmosomal plakophilin 2 as a differentiation marker in normal and malignant tissues. Differentiation 64:277–290 [DOI] [PubMed] [Google Scholar]
- Mertens C, Hofmann I, Wang Z, Teichmann M, Sepehri Chong S, Schnölzer M, Franke WW 2001. Nuclear particles containing RNA polymerase III complexes associated with the junctional plaque protein plakophilin 2. Proc Natl Acad Sci 98:7795–7800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meşe G, Richard G, White TW 2007. Gap junctions: Basic structure and function. J Invest Dermatol 127:2516–2524 [DOI] [PubMed] [Google Scholar]
- Mitic LL, Anderson JM 1998. Molecular architecture of tight junctions. Annu Rev Physiol 60:121–142 [DOI] [PubMed] [Google Scholar]
- Moll R, Cowin P, Kapprell H-P, Franke WW 1986. Biology of disease. Desmosomal proteins: New markers for identification and classification of tumors. Lab Invest 54:4–25 [PubMed] [Google Scholar]
- Montesano R, Friend DS, Perrelet A, Orci L 1975. In vivo assembly of tight junctions in fetal rat liver. J Cell Biol 67:310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Itoh M, Saitou M, Ando-Akatsuka Y, Furuse M, Yoneda K, Imamura S, Tsukita Sh 1998. Subcellular distribution of tight junction-associated proteins (occludin, ZO-1 and ZO-2) in rodent skin changes. J Invest Dermatol 110:862–866 [DOI] [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita Sh 1999a. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci 96:511–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita Sh 1999b. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J Cell Biol 145:579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita Sh 1999c. Endothelial claudin: Claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 147:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Furuse M, Yoshida Y, Itoh M, Sasaki H, Tsukita Sh, Miyachi Y 2002. Molecular architecture of tight junctions of periderm differs from that of the maculae occludentes of epidermis. J Invest Dermatol 118:1073–1079 [DOI] [PubMed] [Google Scholar]
- Moscona AA 1962. Analysis of cell recombinations in experimental synthesis of tissues in vitro. J Cell Comp Physiol 60(Suppl. 1): 65–80 [Google Scholar]
- Mueller H, Franke WW 1983. Biochemical and immunological characterization of desmoplakins I and II, the major polypeptides of the desmosomal plaque. J Mol Biol 163:647–671 [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Tsukita S 1994. The loss of the expression of α catenin, the 102 kD cadherin associated protein, in central nervous tissues during development. Dev Growth Differ 36:59–71 [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M, Tsukita S 1991. The 102 kD cadherin-associated protein: Similarity to vinculin and posttranscriptional regulation of expression. Cell 65:849–857 [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Ishihara S, Tsukita S 1994. The roles of catenins in the cadherin-mediated cell adhesion: Functional analysis of E-cadherin-α catenin fusion molecules. J Cell Biol 127:235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näthke IS, Hinck L, Swedlow JR, Papkoff J, Nelson WJ 1994. Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol 125:1341–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols SA, Dirks W, Pearse JS, King N 2006. Early evolution of animal cell signaling and adhesion genes. Proc Natl Acad Sci 103:12451–12456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson BJ, Gros DB, Kent SBH, Hood LE, Revel J-P 1985. The Mr 28,000 gap junction proteins from rat heart and liver are different but related. J Biol Chem 260:6514–6517 [PubMed] [Google Scholar]
- Nielsen PA, Baruch A, Giepmans BN, Kumar NM 2001. Characterization of the assocation of connexins and ZO-1 in the lens. Cell Commun Aches 8:219–223 [DOI] [PubMed] [Google Scholar]
- Nielsen PA, Baruch A, Shestopalov VI, Giepmans BN, Dunia I, Benedetti EL, Kumar NM 2003. Lens connexins α3Cx46 and α8Cx50 interact with zonula occludens protein-1 (ZO-1). Mol Biol Cell 14:2470–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen PA, Geahm DL, Giepmans BN, Baruch A, Hall JE, Kuman NM 2002. Molecular cloning, functional expression, and tissue distribution of a novel human gap junction-forming protein, connexin-31.9. Interaction with zona occludens protein-1. J Biol Chem 277:38272–38283 [DOI] [PubMed] [Google Scholar]
- Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR, Wheelock MJ 1997. Characterization of the interactions of α-catenin with α-actinin and β-catenin/plakoglobin. J Cell Sci 110:1013–1022 [DOI] [PubMed] [Google Scholar]
- Nilles LA, Parry DA, Powers EE, Angst BD, Wagner RM, Green KJ 1991. Structural analysis and expression of human desmoglein: A cadherin-like component of the desmosome. J Cell Sci 99:809–821 [DOI] [PubMed] [Google Scholar]
- Nollet F, Kools P, van Roy F 2000. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol 299:551–572 [DOI] [PubMed] [Google Scholar]
- Noritake J, Fukata M, Sato K, Nakagawa M, Watanabe T, Izumi N, Wang S, Fukata Y, Kaibuchi K 2004. Positive role of IQGAP1, an effector of Rac1, in actin-meshwork formation at sites of cell–cell contact. Mol Biol Cell 15:1065–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North AJ, Bardsley WG, Hyam J, Bornslaeger EA, Cordingley HC, Trinnaman B, Hatzfeld M, Green KJ, Magee AI, Garrod DR 1999. Moleclar map of the desmosomal plaque. J Cell Sci 112:4325–4336 [DOI] [PubMed] [Google Scholar]
- Nose A, Takeichi M 1986. A novel cadherin cell adhesion molecule: Its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol 103:2649–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M 1987. Isolation of placental cadherin cDNA: Identification of a novel gene family of cell–cell adhesion molecules. EMBO J 6:3655–3661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuber UA, Schäfer S, Schmidt A, Koch PJ, Franke WW 1995. The widespread human desmocollin Dsc2 and tissue-specific patterns of synthesis of various desmocollin subtypes. Eur J Cell Biol 66:69–74 [PubMed] [Google Scholar]
- Nuber UA, Schäfer S, Stehr S, Rackwitz H-R, Franke WW 1996. Patterns of desmocollin synthesis in human epithelia: Immunolocalization of desmocollins 1 and 3 in special epithelia and in cultured cells. Eur J Cell Biol 71:1–13 [PubMed] [Google Scholar]
- Ohkubo T, Ozawa M 1999. p120ctn binds to the membrane-proximal region of the E-cadherin cytoplasmic domain and is involved in modulation of adhesion activity. J Biol Chem 274:21409–21415 [DOI] [PubMed] [Google Scholar]
- Okada TS 1996. The path leading to the discovery of cadherin: In retrospect. Develop Growth Differ 38:583–596 [DOI] [PubMed] [Google Scholar]
- Ozawa M, Kemler R 1992. Molecular organization of the uvomorulin-catenin complex. J Cell Biol 116:989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R 1989. The cytoplasmic domain of the cell adhesion molecules uvomorulin associates with three independent proteins structurally related in different species. EMBO J 8:1711–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Ringwald M, Kemler R 1990b. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci 87:4246–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Hoschützky M, Herrenknecht K, Kemler R 1990a. A possible new adhesive site in the cell-adhesion molecule uvomorulin. Mech Dev 33:49–56 [DOI] [PubMed] [Google Scholar]
- Paffenholz R, Franke WW 1997. Identification and localization of a neurally expressed member of the plakoglobin/armadillo multigene family. Differentiation 61:293–304 [DOI] [PubMed] [Google Scholar]
- Paffenholz R, Kuhn C, Grund C, Stehr S, Franke WW 1999. The arm-repeat protein NPRAP (neurojungin) is a constituent of the plaques of the outer limiting zone in the retina, defining a novel type of adhering junction. Exp Cell Res 250:452–464 [DOI] [PubMed] [Google Scholar]
- Palka HL, Green KJ 1997. Roles of plakoglobin end domains in desmosome assembly. J Cell Sci 110:2359–2371 [DOI] [PubMed] [Google Scholar]
- Park DJ, Wallick CJ, Martyn KD, Lau AF, Jin C, Warn-Cramer BJ 2007. Akt phosphorylates connexin43 on Ser373, a “mode-1” binding site for 14-3–3. Cell Commun Adhes 14:211–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AE, Wheeler GN, Arnemann J, Pidsley SC, Ataliotis P, Thomas CL, Rees DA, Magee AI, Buxton RS 1991. Desmosomal glycoproteins II and III. Cadherin-like junctional molecules generated by alternative splicing. J Biol Chem 266:10438–10445 [PubMed] [Google Scholar]
- Parrish EP, Garrod DR, Mattey DL, Hand L, Steart P, Weller RO 1986. Mouse antisera specific for desmosomal adhesion molecules of suprabasal skin cells, meninges, and meningioma. Proc Natl Acad Sci 83:2657–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish EP, Marston JE, Mattey DL, Measures HR, Venning R, Garrod DR 1990. Size heterogeneity, phosphorylation and transmembrane organisation of desmosomal glycoproteins 2 and 3 (desmocollins) in MDCK cells. J Cell Sci 96:239–248 [DOI] [PubMed] [Google Scholar]
- Parrish EP, Steart PV, Garrod DR, Weller RO 1987. Antidesmosomal monoclonal antibody in the diagnosis of intracranial tumours. J Pathol 153:265–273 [DOI] [PubMed] [Google Scholar]
- Pasdar M, Nelson WJ 1988a. Kinetics of desmosome assembly in Madin-Darby canine kidney epithelial cells: Temporal and spatial regulation of desmoplakin organization and stabilization upon cell–cell contact. I. Biochemical analysis. J Cell Biol 106:677–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasdar M, Nelson WJ 1988b. Kinetics of desmosome assembly in Madin-Darby canine kidney epithelial cells: Temporal and spatial regulation of desmoplakin organization and stabilization upon cell–cell contact. II. Morphological analysis. J Cell Biol 109:163–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul DL 1985. Antibody against liver gap junction 27-kD protein is tissue-specific and cross-reacts with a 54-kD protein. In Gap junctions (eds. Bennett M.V.L., Spray D.C.), pp. 107–122 Cold Spring Habor Laboratory, Cold Spring Harbor, New York [Google Scholar]
- Paul D 1986. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol 103:123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson AF, Mooney E, Fang X, Ji H, McCrea PD 2000. Xarvcf, Xenopus member of the p120 catenin subfamily associating with cadherin juxtamembrane region. J Biol Chem 275:30124–30131 [DOI] [PubMed] [Google Scholar]
- Peifer M, Wieschaus E 1990. The segment polarity gene armadillo encodes a functionally modular protein that is the Drosophila homolog of human plakoglobin. Cell 63:1167–1176 [DOI] [PubMed] [Google Scholar]
- Peifer M, Berg S, Reynolds AB 1994. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell 76:789–791 [DOI] [PubMed] [Google Scholar]
- Peifer M, McCrea PD, Green KJ, Wieschaus E, Gumbiner BM 1992. The vertebrate adhesive junction proteins β-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J Cell Biol 118:681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peracchia C (ed.) 2000. Gap Junctions. Molecular Basis of Cell Communication and Health and Disease Curr. Top. in Membranes, Vol. 49 Academic Press, San Diego [Google Scholar]
- Perez TD, Nelson WJ 2004. Cadherin adhesion: mechanisms and molecular interactions. In Cell adhesion (eds. Behrens J., Nelson W.J.), Handbook Exp. Pharmacol., Vol. 165, pp. 3–21 Springer, Berlin: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrieras N, Hyafil F, Louvard D, Ploegh H, Jacob F 1983. Uvomorulin: A nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci 80:6274–6277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochnow N, Dermietzel R 2008. Connexons and cell adhesion: A romantic phase. Histochem Cell Biol 130:71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pummi K, Malminen M, Aho H, Karvonen S-L, Peltonen J, Peltonen S 2001. Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes. J Invest Dermatol 117:1050–1058 [DOI] [PubMed] [Google Scholar]
- Ranscht B, Dours-Zimmermann MT 1991. T-cadherin, a novel cadherin cell adhesion molecule in the nervous system lacks the conserved cytoplasmic region. Neuron 7:391–402 [DOI] [PubMed] [Google Scholar]
- Revel JP, Yancey SB, Nicholson B 1985. Biology of gap junction molecules and development. In The cell in contact (eds. Edelman G.M., Thiery J.-P.), pp. 411–425 John Wiley and Sons, New York [Google Scholar]
- Revel JP, Yancey SB, Nicholson B, Hoh J 1987. Sequence diversity of gap junction proteins. In Junctional complexes of epithelial cells, Ciba Found. Symp 165, pp. 108–127 J. Wiley and Sons, Chichester: [DOI] [PubMed] [Google Scholar]
- Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zang Z 1994. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol 14:8333–8342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AB, Daniel JM, Mo Y-Y, Wu J, Zhang Z 1996. The novel catenin p120cas binds classical cadherins and induces an unusual morphological phenotype in NIH3T3 fibroblasts. Exp Cell Res 225:328–337 [DOI] [PubMed] [Google Scholar]
- Reynolds AB, Herbert L, Cleveland JL, Berg ST, Gaut JR 1992. p120, a novel substrate of protein tyrosine kinase receptors and p60v-src, is related to cadherin-binding factors β-catenin, plakoglobin and armadillo. Oncogene 7:2439–2445 [PubMed] [Google Scholar]
- Rickelt S, Franke WW, Doerflinger Y, Goerdt S, Brandner JM, Peitsch WK 2008. Subtypes of melanocytes and melanoma cells distinguished by their intercellular contacts: Heterotypic adherens junctions, adhesive associations, and dispersed desmoglein 2 glycoproteins. Cell Tissue Res 334:401–422 [DOI] [PubMed] [Google Scholar]
- Rickelt S, Winter-Simanowski S, Noffz E, Kuhn C, Franke WW 2009. Upregulation of plakophilin-2 and its acquisition to adherens junctions identifies a novel molecular ensemble of cell–cell-attachment characteristic for transformed mesenchymal cells. Int J Cancer, in press; published online May 4. 2009; DOI: 10.1002/ijc.24552 [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS 1995. α1(E)-catenin is an actin-binding and -bundling protein mediating the attachement of F-actin to the membrane adhesion complex. Proc Natl Acad Sci 92:8813–8817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringwald M, Schuh R, Vestweber D, Eistetter H, Lottspeich F, Engel J, Dölz R, Jähnig F, Epplen J, Mayer S et al. , 1987. The structure of cell adhesion molecule uvomorulin. Insights into the molecular mechanism of Ca2+-dependent cell adhesion. EMBO J 6:3647–3653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C, Watt FM 1997. The plakin family: Versatile organizers of cytoskeletal architecture. Curr Opin Gen Dev 7:392–397 [DOI] [PubMed] [Google Scholar]
- Ruiz P, Brinkmann V, Ledermann B, Behrend M, Grund C, Thalhammer C, Vogel F, Birchmeier C, Günthert U, Franke WW, Birchmeier W 1996. Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J Cell Biol 135:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahakibara A, Furuse M, Saitou M, Ando-Akatsuka Y, Tsukita Sh 1997. Possible involvement of phosphorylation of occludin in tight junction formation. J Cell Biol 137:1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Akatsuka-Ando Y, Itoh M, Furuse M, Inazawa J, Fujimoto K, Tsukita Sh 1997. Mammalian occludin in epithelial cells: Its expression and subcellular distribution. Eur J Cell Biol 73:222–231 [PubMed] [Google Scholar]
- Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita Sh 1998. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol 141:397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke J, Fromm M, Takano H, Noda T, Tsukita Sh 2000. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol Biol Cell 11:4131–4142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S 1992. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci 103:131–143 [DOI] [PubMed] [Google Scholar]
- Sato N, Yonemura S, Obinata T, Tsukita S, Tsukita S 1991. Radixin, a barbed end-capping actin-modulating protein, is concentrated at the cleavage furrow during cytokinesis. J Cell Biol 113:321–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y 2000. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell–cell adhesion activities. J Biol Chem 275:10291–10299 [DOI] [PubMed] [Google Scholar]
- Schäfer S, Koch PJ, Franke WW 1994. Identification of the ubiquitous human desmoglein, Dsg2, and the expression catalogue of the desmoglein subfamily of desmosomal cadherins. Exp Cell Res 211:391–399 [DOI] [PubMed] [Google Scholar]
- Schäfer S, Stumpp S, Franke WW 1996. Immunological identification and characterization of the desmosomal cadherin Dsg2 in coupled and uncoupled epithelial cells and in human tissues. Differentiation 60:99–108 [DOI] [PubMed] [Google Scholar]
- Schäfer S, Troyanovsky SM, Heid HW, Eshkind LG, Koch PJ, Franke WW 1993. Cytoskeletal architecture and epithelial differentiation: Molecular determinants of cell interaction and cytoskeletal filament anchorage. C R Acad Sci III Sci Vie 316:1316–1323 [PubMed] [Google Scholar]
- Schlüter H, Moll I, Wolburg H, Franke WW 2007. The different structures containing tight junction proteins in epidermal and other stratified epithelial cells, including squamous cell metaplasia. Eur J Cell Biol 86:645–655 [DOI] [PubMed] [Google Scholar]
- Schlüter H, Wepf R, Moll I, Franke WW 2004. Sealing the live part of the skin: The integrated meshwork of desmosomes, tight junctions and curvilinear ridge structures in the cells of the uppermost granular layer of the human epidermis. Eur J Cell Biol 83:655–665 [DOI] [PubMed] [Google Scholar]
- Schmelz M, Duden R, Cowin P, Franke WW 1986a. A constitutive transmembrane glycoprotein of Mr 165,000 (desmoglein) in epidermal and non-epidermal desmosomes: I. Biochemical identification of the polypeptide. Eur J Cell Biol 42:177–183 [PubMed] [Google Scholar]
- Schmelz M, Duden R, Cowin P, Franke WW 1986b. A constitutive transmembrane glycoprotein of Mr 165,000 (desmoglein) in epidermal and non-epidermal desmosomes: II. Immunolocalization and microinjection studies. Eur J Cell Biol 42:184–199 [PubMed] [Google Scholar]
- Schmidt A, Koch PJ 2008. Desmosomes in development and disease. In Cell Junctions (eds. La Flamme S.E., Kowalczyk A.P.), pp. 235–249 Wiley-VCH, Weinheim [Google Scholar]
- Schmidt A, Heid HW, Schäfer S, Nuber UA, Zimbelmann R, Franke WW 1994. Desmosomes and cytoskeletal architecture in epithelial differentiation: Cell type-specific plaque components and intermediate filament anchorage. Eur J Cell Biol 65:229–245 [PubMed] [Google Scholar]
- Schmidt A, Langbein L, Prätzel S, Rode M, Rackwitz H-R, Franke WW 1999. Plakophilin 3 – a novel cell-type-specific desmosomal plaque protein. Differentiation 64:291–306 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Langbein L, Rode M, Prätzel S, Zimbelmann R, Franke WW 1997. Plakophilins 1a and 1b: Widespread nuclear proteins recruited in specific epithelial cells as desmosomal plaque components. Cell Tissue Res 290:481–499 [DOI] [PubMed] [Google Scholar]
- Schmitt CJ, Franke WW, Goerdt S, Falkowska-Hansen B, Rickelt S, Peitsch WK 2007. Homo- and heterotypic cell contacts in malignant melanoma cells and desmoglein 2 as a novel solitary surface glyocprotein. J Invest Dermatol 127, 2191–2206 [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD 1992. Structure, function and regulation of cellular tight junctions. Am J Physiol 262:L647–L661 [DOI] [PubMed] [Google Scholar]
- Schuh R, Vestweber D, Riede I, Ringwald M, Rosenberg UB, Jäckle H, Kemler R 1986. Molecular cloning of the mouse cell adhesion molecule uvomorulin: cDNA contains a B1-related sequence. Proc Natl Acad Sci 83:1364–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwechheimer K, Kartenbeck J, Moll R, Franke WW 1984. Vimentin filament-desmosome cytoskeleton of diverse types of human meningiomas. A distinctive diagnostic feature. Lab Invest 51:584–591 [PubMed] [Google Scholar]
- Setzer SV, Calkins CC, Garner J, Summers S, Green KJ, Kowalczyk AP 2004. Comparative analysis of armadillo family proteins in the regulation of A431 epithelial cell junction assembly, adhesion and migration. J Invest Dermatol 123:426–433 [DOI] [PubMed] [Google Scholar]
- Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Miyazawa K, Kitamura N, Johnson KR, Wheelock MJ, Matsuyoshi N, Takeichi M, Ito F 1995. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. J Cell Biol 128:949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP 1990. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285:103–106 [DOI] [PubMed] [Google Scholar]
- Simons K 1990. The epithelial tight junction: Occluding barrier and fence. Morphoregulatory molecules (eds. Edelman G.M., Cunningham B.A., Thiery J.P.), pp. 341–356 John Wiley and Sons, New York [Google Scholar]
- Singh D, Solan JL, Taffet SM, Javier R, Lampe PD 2005. Connexin43 interacts with zona occludens-I and -2 proteins in a cell cycle stage-specific manner. J Biol Chem 280:30416–30421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotkin H, O’Donnell H, DasGupta R, Halford S, St. Jore B, Puech A, Parimoo S, Morrow S, Skoultchi A, Weissman SM, et al. 1997. Identification of a new human catenin gene family member (ARVCF) from the region deleted in velo-cardiofacial syndrome. Genomics 41:75–83 [DOI] [PubMed] [Google Scholar]
- Skerrow CJ 1986. Desmosomal proteins. In Biology of the integument 2 Vertebrates (eds. Bereiter-Hahn J., Matoltsy A.G., Richard K.S.), pp. 762–787 Springer-Verlag, Berlin [Google Scholar]
- Skerrow CJ, Matoltsy AG 1974a. Isolation of epidermal desmosomes. J Cell Biol 63:515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skerrow CJ, Matoltsy AG 1974b. Chemical characterization of epidermal desmosomes. J Cell Biol 63:524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EA, Fuchs E 1998. Defining the interactions between intermediate filaments and desmosomes. J Cell Biol 141:1229–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda N, Furuse M, Sasaki H, Yonemura S, Katahira J, Horiguchi A, Tsukita Sh 1999. Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier. J Cell Biol 147:195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky G 2000. Gap junction structure: New structures and new insights. In Gap Junctions. Molecular Basis of Cell Communication and Health and Disease (ed. Peracchia C.), Curr. Top. Membr., Vol. 49, pp. 1–22 Academic Press, San Diego [Google Scholar]
- Staehelin LA 1974. Structure and function of intercellular junctions. Int Rev Cytol 39:191–283 [DOI] [PubMed] [Google Scholar]
- Stappenbeck TS, Bornslaeger EA, Corcoran CM, Luu HH, Virata ML, Green KJ 1993. Functional analysis of desmoplakin domains: Specification of the interaction with keratin versus vimentin intermediate filament networks. J Cell Biol 123:691–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappert J, Kemler R 1994. A short core region of E-cadherin is essential for catenin binding and is highly phosphorylated. Cell Adhes Commun 2:319–327 [DOI] [PubMed] [Google Scholar]
- Stauffer KA, Kumar NM, Gilula NB, Unwin N 1993. Biochemistry of gap-junction channels. In Gap junctions (eds. Hall J.E., Zampighi G.A., Davis R.M.), Progr. Cell Res. Vol. 3, pp. 57–59 Elsevier, Amsterdam [Google Scholar]
- Steinberg M 1958. On the chemical bonds between animal cells. A mechanism for type-specific association. Am Nat 92:65–81 [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker JD, Goodenough DA 1986. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103:755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes DL 2007. Desmosomes from a structural perspective. Curr Opin Cell Biol 19:565–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumane K, Berx G, Van Roy F 2004. Cadherins in cancer. In Cell adhesion (eds. Behrens J., Nelson W.J.), Handbook Exp. Pharmacol., Vol. 165, pp. 69–103 Springer, Berlin: [DOI] [PubMed] [Google Scholar]
- Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ideda W, Yamamoto Y, Nagafuchi A, Tsukita S, Takai Y 2000. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol 150:1161–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh Y, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, et al. 1999. Nectin/PRR: An immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J Cell Biol 145:539–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Nakanishi H 2003. Nectin and afadin: Novel organizers of intercellular junctions. J Cell Sci 116:17–27 [DOI] [PubMed] [Google Scholar]
- Takai Y, Irie K, Shimizu K, Sakisaka T, Ikeda W 2003. Nectins and nectin-like molecules: Roles in cell adhesion, migration, and polarization. Cancer Sci 94:655–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Ikeda W, Ogita H, Rikitake Y 2008a. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu Rev Cell Dev Biol 24:309–342 [DOI] [PubMed] [Google Scholar]
- Takai Y, Miyoshi J, Ideda W, Ogita H 2008b. Nectins and nectin-like molecules: Roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol 9:603–615 [DOI] [PubMed] [Google Scholar]
- Takeda H, Shimoyama Y, Nagafuchi A, Hirohashi S 1999. E-cadherin functions as a cis-dimer at the cell–cell adhesive interface in vivo. Nat Struct Biol 6:310–312 [DOI] [PubMed] [Google Scholar]
- Takeichi M 1977. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol 75:464–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M 1988. The cadherins: Cell–cell adhesion molecules controlling animal morphogenesis. Development 102:639–655 [DOI] [PubMed] [Google Scholar]
- Takeichi M 1990. Cadherins: A molecular family important in selective cell–cell adhesion. Annu Rev Biochem 59:237–252 [DOI] [PubMed] [Google Scholar]
- Takeichi M, Yoshida-Noro C, Shirayoshi Y, Hatta K 1985. Calcium-dependent cell–cell adhesion system: Its molecular nature, cell type specificity, and morphogenetic role. In The cell in contact (eds. Edelman G.M., Thiery J.-P.), pp. 219–232 John Wiley and Sons, New York [Google Scholar]
- Talhouk RS, Mroue RM, Mokalled M, Abi-Mosleh L, Nehme R, Imsail A, Khalil A, Zaatari M, El-Sabban ME 2008. Heterocellular interaction enhances recruitment of α and β-catenins and ZO-2 into functional gap junction complexes and gap junction-dependent differentiation of mammary epithelial cells. Exp Cell Res 314:3275–3291 [DOI] [PubMed] [Google Scholar]
- Tanihara H, Sano K, Heimark RL, John T St, Suzuki S 1994. Cloning of five human cadherins clarifies characteristic features of cadherin extracellular domain and provides further evidence for two structurally different types of cadherin. Cell Adhes Commun 2:15–26 [DOI] [PubMed] [Google Scholar]
- Theis DG, Koch PJ, Franke WW 1993. Differential synthesis of type 1 and type 2 desmocollin mRNAs in human stratified epithelia. Int J Dev Biol 37:101–110 [PubMed] [Google Scholar]
- Thiery J-P, Duband J-L, Delouvée A 1985. The role of cell adhesion in morphogenetic movements during early embryogenesis. In The cell in contact (eds. Edelman G.M., Thiery J.-P.), pp. 169–196 John Wiley and Sons, New York [Google Scholar]
- Thiery JP, Delouvée A, Gallin W, Cunningham BA, Edelman GM 1984. Ontogenetic expression of cell adhesion molecules: L-CAM is found in epithelia derived from the three primary germ layers. Dev Biol 102:61–78 [DOI] [PubMed] [Google Scholar]
- Thomson RB, Igarashi P, Biemesderfer D, Kim R, Abu-Alfa A, Soleimani M, Aronson PS 1995. Isolation and cDNA cloning of Ksp-cadherin, a novel kidney-specific member of the cadherin multigene family. J Biol Chem 270:17594–17601 [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Akamatsu Y, Zhang H, Kuzuya T, Tada M, Hori M 2001. c-SRC regulates the interaction between connexin-43 and ZO-1 in cardiac myocytes. J Biol Chem 276:1780–1788 [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M 1998. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 273:12725–12731 [DOI] [PubMed] [Google Scholar]
- Traub O, Look J, Dermietzel R, Brummer F, Hülser D, Willecke K 1989. Comparative characterization of the 21-kDa and 26-kDa gap-junction proteins in murine liver and cultured hepatocytes. J Cell Biol 108:1039–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky SM 2005. Cadherin dimers in cell–cell adhesion. Eur J Cell Biol 84:225–233 [DOI] [PubMed] [Google Scholar]
- Troyanovsky RB, Chitaev NA, Troyanovsky SM 1996. Cadherin binding sites of plakoglobin: Localization, specificity and role in targeting to adhering junctions. J Cell Sci 109:3069–3078 [DOI] [PubMed] [Google Scholar]
- Troyanovsky SM, Eshkind LG, Troyanovsky RB, Leube RE, Franke WW 1993. Contributions of cytoplasmic domains of desmosomal cadherins to desmosome assembly and intermediate filament anchorage. Cell 72:561–574 [DOI] [PubMed] [Google Scholar]
- Troyanovsky SM, Troyanovsky RB, Eshkind LG, Krutovskikh VA, Leube RE, Franke WW 1994a. Identification of the plakoglobin-binding domain in desmoglein and its role in plaque assembly and intermediate filament anchorage. J Cell Biol 127:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky SM, Troyanovsky RB, Eshkind LG, Leube RE, Franke WW 1994b. Identification of amino acid sequence motifs in desmocollin, a desmosomal glycoprotein, that are required for plakoglobin binding and plaque formation. Proc Natl Acad Sci 91:10790–10794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita Sh, Furuse M 1998. Overcoming barries in the study of tight junction functions: From occludin to claudin. Genes Cells 3:569–573 [DOI] [PubMed] [Google Scholar]
- Tsukita Sh, Furuse M 2000. Pores in the wall: Claudins constitute tight junction strands containing aqueous pores. J Cell Biol 149:13–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita Sh, Furuse M 2002. Claudin-based barrier in simple and stratified cellular sheets. Curr Opin Cell Biol 14:531–536 [DOI] [PubMed] [Google Scholar]
- Tsukita Sh, Tsukita Sa 1989. Isolation of cell-to-cell adherens junctions from rat liver. J Cell Biol 108:31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita Sh, Furuse M, Itoh M 2001. Multi-functional strands in tight junctions. Nat Rev Mol Cell Biol 2:285–293 [DOI] [PubMed] [Google Scholar]
- Tsukita Sa, Hieda Y, Tsukita Sh 1989. A new 82-kD barbed end-capping protein (radixin) localized in the cell-to-cell adherens junction: Purification and characterization. J Cell Biol 108:2369–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita Sa, Yonemura S, Tsukita Sh 1997a. ERM (ezrin/radixin/moesin) family: From cytoskeleton to signal transduction. Curr Opin Cell Biol 9:70–75 [DOI] [PubMed] [Google Scholar]
- Tsukita Sa, Yonemura S, Tsukita Sh 1997b. ERM proteins: Head-to-tail regulation of actin-plasma membrane interaction. Trends Biochem Sci 22:53–58 [DOI] [PubMed] [Google Scholar]
- Tsukita Sa, Oishi K, Sato N, Sagara J, Kawai A, Tsukita Sh 1994. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeleton. J Cell Biol 126:391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Honjo Y, Kohnson KR, Wheelock MJ, Takeichi M 1996. The catenin/cadherin adhesion system is localized in synaptic junctions bordering trnsmitter release zones. J Cell Biol 135:767–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unwin PNT 1987. Gap junction structure and the control of cell-to-cell communication. In Junctional complexes of epithelial cells, Ciba Found. Symp 125, pp. 78–91 J. Wiley and Sons, Chichester: [DOI] [PubMed] [Google Scholar]
- Unwin PNT, Zampighi G 1980. Structure of the junctions between communicating cells. Nature 283:545–549 [DOI] [PubMed] [Google Scholar]
- Van Itallie CM, Anderson JM 2006. Claudins and epithelial paracellular transport. Annu Rev Physiol 68:403–429 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E 2000. Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell 100:209–219 [DOI] [PubMed] [Google Scholar]
- Vestweber D, Kemler R 1984. Rabbit antiserum against a purified surface glycoprotein decompacts mouse preimplantation embryos and reacts with specific adult tissues. Exp Cell Res 152:169–178 [DOI] [PubMed] [Google Scholar]
- Vestweber D, Kemler R 1985. Identification of a putative cell adhesion domain of uvomorulin. EMBO J 4:3393–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilela MJ, Parrish EP, Wright DH, Garrod DR 1987. Monoclonal antibody to desmosomal glycoprotein 1 – A new epithelial marker for diagnostic pathology. J Pathol 153:365–375 [DOI] [PubMed] [Google Scholar]
- Virata MLA, Wagner RM, Parry DAD, Green KJ 1992. Molecular structgure of the human desmoplakin I and II amino terminus. Prod Natl Acad Sci 89:544–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vleminckx K, Vakaet L Jr, Mareel M, Fiers W, Van Roy F 1991. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66:107–119 [DOI] [PubMed] [Google Scholar]
- Volk T, Geiger B 1984. A 135-kD membrane protein of intercellular adherens junctions. EMBO J 3:2249–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl JK, Sacco PA, McGranahan-Sadler TM, Sauppe LM, Wheelock MJ, Johnson KR 1996. Plakoglobin domains that define its association with the desmosomal cadherins and the classical cadherins: Identification of unique shared domains. J Cell Sci 109:1143–1154 [DOI] [PubMed] [Google Scholar]
- Waibler Z, Schäfer A, Starzinski-Powitz A 2001. mARVCF cellular localisation and binding to cadherins is influenced by the cellular context but not by alternative splicing. J Cell Sci 114:3873–3884 [DOI] [PubMed] [Google Scholar]
- Walter B, Schlechter T, Hergt M, Berger I, Hofmann I 2008. Differential expression pattern of protein ARVCF in nephron segments of human and mouse kidney. Histochem Cell Biol 130:943–956 [DOI] [PubMed] [Google Scholar]
- Warner AE, Guthrie SC, Gilula NB 1984. Antibodies to gap junctional protein selectively disrupt junctional communication in the early amphibian embryo. Nature 311:127–131 [DOI] [PubMed] [Google Scholar]
- Waschke J 2008. The desmosome and pemphigus. Histochem Cell Biol 130:130–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson ED, Takeichi M 1998. α-catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol 142:847–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Mattey DL, Garrod DR 1984. Calcium-induced reorganization of desmosomal components in cultured human keratinocytes. J Cell Biol 99:2211–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EE, Kroemker M, Rüdiger A-H, Jockusch BM, Rüdiger M 1998. Vinculin is part of the cadherin-catenin junctional complex: Complex formation between α-catenin and vinculin. J Cell Biol 141:755–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler GN, Buxton RS, Parker AE, Arnemann J, Rees DA, King IA, Magee AI 1991a. Desmosomal glycoproteins I, II and III: Novel members of the cadherin superfamily. Biochem Soc Trans 19:1060–1064 [DOI] [PubMed] [Google Scholar]
- Wheeler GN, Parker AE, Thomas CL, Ataliotis P, Poynter D, Arnemann J, Rutman AJ, Pidsley SC, Watt FM, Rees DA, et al. 1991b. Desmosomal glycoprotein DGI, component of intercellular desmosome junctions, is related to the cadherin family of cell adhesion molecules. Proc Natl Acad Sci 88:4796–4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Johnson KR 2003. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol 19:207–235 [DOI] [PubMed] [Google Scholar]
- Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR 2008. Cadherin switching. J Cell Sci 121:727–735 [DOI] [PubMed] [Google Scholar]
- Willecke K, Dermietzel R, Drüge PM, Frixen U, Janssen-Timmen U, Schäfer R, Traub O 1982. Biochemical and genetic investigations on gap junctions from mammalian cells. Biophys Struct Mech 9:103–107 [DOI] [PubMed] [Google Scholar]
- Willecke K, Hennemann H, Dahl E, Jungbluth S 1993. The mouse connexin family. In Gap junctions (eds. Hall J.E., Zampighi G.A., Davis R.M.), Progr. Cell Res. Vol. 3, pp. 33–37 Elsevier, Amsterdam [Google Scholar]
- Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM 1993. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci 90:7834–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HV 1907. On some phenomena of coalescence and regeneration in sponges. J Exp Zool 5:245–258 [Google Scholar]
- Witcher LL, Collins R, Puttagunta S, Mechanic SE, Munson M, Gumbiner B, Cowin P 1996. Desmosomal cadherin binding domains of plakoglobin. J Biol Chem 271:10904–10909 [DOI] [PubMed] [Google Scholar]
- Wittchen ES, Haskins J, Stevenson BR 1999. Protein interaction at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem 274:35179–35185 [DOI] [PubMed] [Google Scholar]
- Yeager M 1993. Structure and design of cardiac gap-junction membranes channels. In Gap junctions (eds. Hall J.E., Zampighi G.A., Davis R.M.), Progr. Cell Res. Vol. 3, pp. 47–55 Elsevier, Amsterdam [Google Scholar]
- Yonemura S, Itoh M, Nagafuchi A, Tsukita S 1995. Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci 108:127–142 [DOI] [PubMed] [Google Scholar]
- Yoshida-Noro C, Takeichi M 1982. Teratocarcinoma cell adhesion: Identification of cell surface protein involved in calcium-dependent cell aggregation. Cell 28:217–224 [DOI] [PubMed] [Google Scholar]
- Yoshida-Noro C, Suzuki N, Takeichi M 1984. Molecular nature of the calcium-dependent cell–cell adhesion sstem in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol 101:19–27 [DOI] [PubMed] [Google Scholar]
- Zhang M-T, Nicholson B 1989. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol 109:3391–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Liyanage U, Medina M, Ho C, Simmons AD, Lovett M, Kosiks KS 1997. Presenilin 1 interaction in the brain with a novel member of the armadillo family. NeuroReport 8:1489–1494 [DOI] [PubMed] [Google Scholar]
- Zhou X, Stuart A, Dettin LE, Rodriguez G, Hoel B, Gallicano GI 2004. Desmoplakin is required for microvascular tube formation in culture. J Cell Sci 117:3129–3140 [DOI] [PubMed] [Google Scholar]
- Zhurinsky J, Shtutman M, Ben-Ze’ev A 2000. Plakoglobin and β-catenin: Protein interactions, regulation and biological roles. J Cell Sci 113:3127–3139 [DOI] [PubMed] [Google Scholar]