Abstract
Epithelial cell–cell junctions are formed by apical adherens junctions (AJs), which are composed of cadherin adhesion molecules interacting in a dynamic way with the cortical actin cytoskeleton. Regulation of cell–cell junction stability and dynamics is crucial to maintain tissue integrity and allow tissue remodeling throughout development. Actin filament turnover and organization are tightly controlled together with myosin-II activity to produce mechanical forces that drive the assembly, maintenance, and remodeling of AJs. In this review, we will discuss these three distinct stages in the lifespan of cell–cell junctions, using several developmental contexts, which illustrate how mechanical forces are generated and transmitted at junctions, and how they impact on the integrity and the remodeling of cell–cell junctions.
Assembly, maintenance, and turnover of cell junctions are essential for tissue integrity and remodeling, requiring mechanical forces generated by actin reorganization.
Cell–cell junction formation and remodeling occur repeatedly throughout development. Epithelial cells are linked by apical adherens junctions (AJs) that rely on the cadherin-catenin-actin module. Cadherins, of which epithelial E-cadherin (E-cad) is the most studied, are Ca2+-dependent transmembrane adhesion proteins forming homophilic and heterophilic bonds in trans between adjacent cells. Cadherins and the actin cytoskeleton are mutually interdependent (Jaffe et al. 1990; Matsuzaki et al. 1990; Hirano et al. 1992; Oyama et al. 1994; Angres et al. 1996; Orsulic and Peifer 1996; Adams et al. 1998; Zhang et al. 2005; Pilot et al. 2006). This has long been attributed to direct physical interaction of E-cad with β-catenin (β-cat) and of α-catenin (α-cat) with actin filaments (for reviews, see Gumbiner 2005; Leckband and Prakasam 2006; Pokutta and Weis 2007). Recently, biochemical and protein dynamics analyses have shown that such a link may not exist and that instead, a constant shuttling of α-cat between cadherin/β-cat complexes and actin may be key to explain the dynamic aspect of cell–cell adhesion (Drees et al. 2005; Yamada et al. 2005). Regardless of the exact nature of this link, several studies show that AJs are indeed physically attached to actin and that cadherins transmit cortical forces exerted by junctional acto-myosin networks (Costa et al. 1998; Sako et al. 1998; Pettitt et al. 2003; Dawes-Hoang et al. 2005; Cavey et al. 2008; Martin et al. 2008; Rauzi et al. 2008). In addition, physical association depends in part on α-cat (Cavey et al. 2008) and additional intermediates have been proposed to represent alternative missing links (Abe and Takeichi 2008) (reviewed in Gates and Peifer 2005; Weis and Nelson 2006). Although further work is needed to address the molecular nature of cadherin/actin dynamic interactions, association with actin is crucial all throughout the lifespan of AJs. In this article, we will review our current understanding of the molecular mechanisms at work during three different developmental stages of AJs biology: assembly, stabilization, and remodeling, with special emphasis on the mechanical forces controlling AJs integrity and development.
CELL JUNCTIONS FORMATION
Cell junctions form in two contexts during development. (1) Migrating cells undergo mesenchymal-epithelial transitions (MET) during which they establish membrane contacts with neighbors and initiate assembly of AJs at these sites. Subsequently, cell–cell contacts expand and newly formed AJs serve as landmarks for establishing tissue polarity. (2) In primary embryonic epithelia, cell junctions are formed in a subregion of pre-existing cell contacts, which is defined by upstream polarity cues.
Cell–cell junction formation during MET has been extensively studied in cell cultures: (1) formation of junctions after membrane contacts or after Ca2+ switch (activating cadherin adhesive function by raising the extracellular Ca2+ concentration); (2) adhesion of cells on cadherin-coated substrata. These studies have provided considerable insights into in vivo epitheliogenesis and epithelial sheet sealing processes, which occur during embryogenesis and wound healing. In this section, we first review how cell junctions assemble in cell cultures, focusing on the role of forces generated by actin polymerization and acto-myosin tension. We then examine how these forces are used during epithelial sheet sealing in whole organisms. Finally, we summarize how cell junctions form in a primary embryonic epithelium, i.e., without MET.
Mesenchymal-Epithelial Transitions (MET): Cell Culture Studies
Cell–cell junction formation during MET suggests the following steps:
Membrane protrusions explore the environment to generate initial cell contacts. Cadherin molecules diffusing in the plasma membrane engage in homophilic interactions and form clusters.
Homophilic ligation of cadherins triggers actin cytoskeleton rearrangement by directly controlling the recruitment and activity of several actin regulators.
Actin reorganization drives contact expansion (an increase of the surface of contact between two cells), and is also linked to the stabilization of adhesive interfaces.
Formation of Initial Cell–Cell Contacts
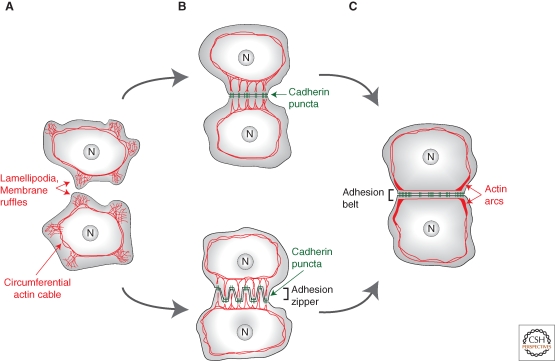
The first step is an opportunistic event resulting from the exploratory behavior of cells extending actin-based protrusions (lamellipodia and membrane ruffles) (Fig. 1A) (reviewed in Vasioukhin and Fuchs 2001). Immunofluoresence and the use of GFP-fusions show that bright cadherin puncta rapidly form where protrusions touch (Fig. 1B). These puncta are thought to represent clusters of homophilic cadherin dimers, a view supported by the fact that they contain cadherin molecules from both contacting cells (Kametani and Takeichi 2007). However, cadherin clustering mechanisms are still not fully understood and remain debated. They may include lateral interactions in the cadherin extracellular domain and intracellular interactions including interactions with actin (reviewed in Leckband and Prakasam 2006; Pokutta and Weis 2007). Upon contact formation, cadherin molecules previously diffusing “freely” in the membrane become immobilized (Adams et al. 1998), presumably by anchoring to actin (Sako et al. 1998; Iino et al. 2001; Lambert et al. 2002). Within a few tens of minutes, cadherin puncta grow, suggesting a continuous addition of new molecules (Yonemura et al. 1995; Adams et al. 1996; Adams et al. 1998).
Figure 1.
Actin reorganization during cell–cell junction formation in cell culture. Actin is shown in red, cadherin in green. (A) Before cell–cell contact, epithelial cells extend protrusions (lamellipodia and membrane ruffles). (B) Cadherin puncta form at the tips of these projections and are connected to the circumferential actin cable via radial actin bundles. (B, bottom) Contacting keratinocytes adopt an intermediate configuration known as the “adhesion zipper” as a consequence of myosin-mediated tension pulling inward on cadherin puncta. (C) As cell–cell contacts expand and mature, actin arcs focus on the edges of the contact. Actin remodeling along the contact results in formation of the adhesion belt.
In the Drosophila embryo, a similar punctate organization of cadherins has been reported (Muller and Wieschaus 1996; Harris and Peifer 2004). Moreover, puncta were recently shown to represent bona fide sites of immobilized E-cad clusters with slower dynamics than outside puncta (Cavey et al. 2008), suggesting that they are equivalent to the spot adherens junctions (SAJs) structures observed at the electron microscopy level (Tepass and Hartenstein 1994; Oda et al. 1998). Therefore the puncta observed in cell cultures and in several epithelia in vivo likely represent the same structures, namely sites of homophilic cadherin dimers enrichment.
Actin Reorganization
Before cell contacts, actin forms concentric ring(s) (the “circumferential actin cable/ring”) and a dense meshwork between the ring and the plasma membrane (Fig. 1A) (Yonemura et al. 1995; Adams et al. 1996; Gloushankova et al. 1997; Adams et al. 1998; Krendel and Bonder 1999; Ehrlich et al. 2002; Vaezi et al. 2002; Ivanov et al. 2005a). As contacts form, cadherin puncta are connected to the actin ring via radial actin bundles (Fig. 1B). Subsequently, actin bundles are replaced by finer ones under the region of contact (the “perijunctional actin belt/adhesion belt”) resembling actin organization in epithelia in vivo (Hirokawa et al. 1983), and thick bundles (“actin arcs”) focus on the contact edges (Fig. 1C). This cytoskeletal reorganization is triggered and controlled by cadherins whose homophilic ligation can directly recruit and activate actin regulators including Rac1, Cdc42 (Nakagawa et al. 2001; Noren et al. 2001; Kovacs et al. 2002a), Abl kinase (Zandy et al. 2007), Arp2/3 (Kovacs et al. 2002b; Verma et al. 2004), Cortactin (Helwani et al. 2004), N-WASP (Ivanov et al. 2005a), Formin1 (Kobielak et al. 2004), and Ena/VASP (Vasioukhin et al. 2000) (for reviews, see Bershadsky 2004; Braga and Yap 2005). Cell–cell contact formation is dependent on actin polymerization (Braga et al. 1997; Adams et al. 1998; Vasioukhin et al. 2000; Ivanov et al. 2005a; Zhang et al. 2005; Yamada and Nelson 2007) and adhesion strength (measured as the force required to detach cell doublets) increases in an F-actin- (Angres et al. 1996), Cdc42-, and Rac1-dependent manner (Chu et al. 2004). Cytoskeletal reorganization likely serves two related purposes examined below: expansion and stabilization of the adhesive interface.
Cell–Cell Contact Expansion
Two forces are coordinated to expand cell contacts: (1) Actin polymerization produces membrane protrusions to generate new sites of contact, and (2) acto-myosin tension focusing on contact edges generates a pulling force to facilitate contact expansion.
(1) Lamellipodia and membrane ruffles initiating cell–cell contacts (Gloushankova et al. 1997; Krendel and Bonder 1999) are generated by branched actin networks (Vaezi et al. 2002; Bershadsky 2004). New sites of contact are then generated in adjacent regions. In keratinocytes, the adhesive interface develops in an “adhesion zipper” structure, because of myosin-mediated tension pulling inward on cadherin puncta (Fig. 1B, bottom). Actin polymerization from the tip of radial actin bundles could provide the force necessary to resolve the two rows of cadherin puncta into a single belt of mature junctions, but the underlying mechanism is not understood (Vasioukhin et al. 2000; Kobielak et al. 2004). In other cell types, lamellipodial activity is initially distributed evenly around the cell periphery but becomes restricted to the region of contact and subsequently propagates to adjacent regions in waves generating new sites of membrane apposition (Ehrlich et al. 2002) (Fig. 2A). Supporting this idea, factors promoting branched actin polymerization are enriched at the leading edge of cells spreading on cadherin-coated substrata (Kovacs et al. 2002a; Helwani et al. 2004) and Rac1 is specifically activated in regions of contact expansion (Yamada and Nelson 2007). Moreover, interfering with actin branching impairs cell spreading or contact expansion (Ehrlich et al. 2002; Kovacs et al. 2002a; Helwani et al. 2004; Verma et al. 2004; Ivanov et al. 2005a; Zandy et al. 2007). Conversely, Rac1 constitutive activation increases cell contact expansion rate and adhesion strength (Ehrlich et al. 2002). Together, these studies show that actin polymerization in lamellipodia is specifically concentrated in regions adjacent to the site of initial contact and provides a pushing force required for membrane apposition. These fluctuating contacts are then ligated and rectified by cadherin homophilic dimers.
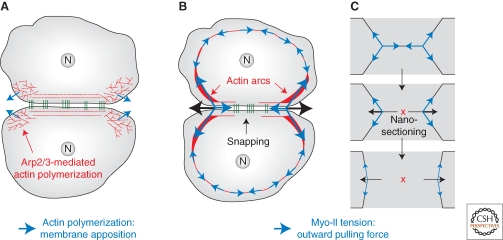
Figure 2.
Forces driving cell–cell contact expansion in cell culture. (A) Polymerization of branched actin networks at the edges of a contact generates a pushing force (blue arrows) necessary to create new sites of membrane apposition, which are then ligated by homophilic cadherin dimers. (B,C) Analogy for the role of myosin-II-mediated tension (blue arrows) during contact expansion in cell culture (B) and after laser nano-sectioning in an epithelium (C). In both cases, the sum of tension forces applied at the contact edges is initially null, but once actin bundles are sectioned by snapping along the cell–cell contact (B) or laser nano-sectioning (C), these forces are not balanced anymore. This produces a net outward pulling force (black arrows), which drives contact expansion.
(2) Myo-II-mediated tension is also important for contact expansion. Myo-II localizes to peripheral actin bundles in epithelial cells (Krendel et al. 1999; Krendel and Bonder 1999; Bertet et al. 2004; Zallen and Wieschaus 2004; Shewan et al. 2005; Zhang et al. 2005; Yamada and Nelson 2007) and is required to bundle actin filaments (Vaezi et al. 2002; Ivanov et al. 2005a; Shewan et al. 2005; Zhang et al. 2005). Cell adhesion induces Myo-II activation (Ivanov et al. 2005a), which can be directly triggered by cadherin homophilic ligation (Shewan et al. 2005). In keratinocytes, Myo-II appears to act negatively on contact expansion by generating the adhesion zipper structure (Vaezi et al. 2002). However, in other cell types Myo-II acts positively on contact expansion (Yamada and Nelson 2007). How can Myo-II activity participate in contact expansion?
As contacts expand, cadherin puncta (Adams et al. 1998) and ConA-coated beads that mark plasma membrane proteins (Gloushankova et al. 1997) move toward the contact edges (i.e., tangentially) at similar speeds, revealing a flow of cortical material toward the edges. The cortical forces responsible for this flow likely result from acto-myosin contractility. Activated Myo-II and activated Rho are enriched on the actin arcs, which contract at the edges of cell contacts (Krendel and Bonder 1999; Yamada and Nelson 2007) (Fig. 2B). In addition, during contact expansion, actin bundles along the contact zone break and retract toward the edges of the contact (i.e., tangentially), in a process termed “actin bundle snapping” (Krendel and Bonder 1999) (Fig. 2B). This suggests a model whereby the local unbalance of cortical acto-myosin forces at contact edges drives expansion. Actin bundles along the contact have to resist the outward pulling forces generated on the actin arcs. However, actin bundle snapping along the contact alleviates such a resistance, resulting in a net outward force pulling on the contact edges that drives contact expansion (Fig. 2B). Snapping along cell contacts could be a consequence of increased tension generated on actin arcs, combined with actin remodeling triggered by cell–cell adhesion. Moreover, retraction of these bundles after snapping could drag cadherin puncta toward the edges (Adams et al. 1998). Such a mechanism would be analogous to laser nano-sectioning experiments performed in live epithelial cells of Drosophila embryos (Cavey et al. 2008; Rauzi et al. 2008). Indeed, nano-sectioning of a tensile acto-myosin cortical network along a cell–cell contact causes the actin network to retract, resulting in a net expansion of the contact (Fig. 2C). Actin retraction induces E-cad puncta redistribution away from the region of sectioning by tethering E-cad puncta to the contractile acto-myosin network (Cavey et al. 2008; Rauzi et al. 2008).
Junction Formation by MET in Embryos
Interfering with AJs components in embryonic tissues results in tissue collapse at varying developmental stages (Larue et al. 1994; Riethmacher et al. 1995; Tepass et al. 1996; Uemura et al. 1996; Torres et al. 1997; Carmeliet et al. 1999; Vasioukhin et al. 2001; De Vries et al. 2004). One interesting phenomenon is when cell–cell contacts are increased during compaction of late 8-cell mouse embryos. Interfering with cell adhesion using anti-E-cad antibodies and Ca2+ depletion both block compaction (Kemler et al. 1977; Hyafil et al. 1980; Pratt et al. 1982; Shirayoshi et al. 1983; Johnson et al. 1986). Compaction may be driven by similar forces as contact expansion in cell culture. Compaction requires actin polymerization (Pratt et al. 1982; Fleming et al. 1986; Clayton et al. 1999) and involves a redistribution of E-cad to the baso-lateral domain where cell contacts expand (Vestweber et al. 1987). Interestingly, numerous membrane protrusions (microvilli) form bridges between cells along regions of contacts (Calarco and Epstein 1973; Fleming et al. 1986), suggesting a role analogous to that of lamellipodia in cell cultures. In later stages, microvilli are excluded from regions of cell contacts (Fleming et al. 1986), as membrane protrusive activity may not be compatible with stabilization of the interface (see below). This may be controlled in part by the ERM (Ezrin-Radixin-Moesin) protein Ezrin, which organizes actin networks to form microvilli and has to be excluded from regions of cell–cell contacts for compaction to be completed (Dard et al. 2001; Dard et al. 2004). A role for acto-myosin tension in driving compaction has not been directly investigated yet, but Rho inhibition (using C3-transferase) disrupts actin and E-cad organization, blocking compaction (Clayton et al. 1999).
Cell–cell contact establishment also occurs during the sealing of epithelial sheets during embryogenesis and wound healing. At the end of embryogenesis, morphogenetic rearrangements leave holes in the dorsal epidermis of Drosophila embryos and in the ventral hypodermis of C. elegans embryos. Similar holes are created when embryonic or adult tissues are wounded. In all cases, migrating epithelial sheets cover and eventually seal these holes (Fig. 3) (reviewed in Jacinto et al. 2001; Martin and Parkhurst 2004). As for the establishment of cell–cell contacts in cell culture, actin protrusions and acto-myosin cables are both involved in epithelial sheet sealing. An acto-myosin cable assembled at the periphery of the hole/wound provides contractile force to progressively close the hole in flies (Harden et al. 1999; Magie et al. 1999; Kiehart et al. 2000; Bloor and Kiehart 2002; Jacinto et al. 2002) during wound healing (Wood et al. 2002)(reviewed in Jacinto et al. 2001; Redd et al. 2004) and possibly in worms as well (Williams-Masson et al. 1997; Raich et al. 1999). Filopodia establish connections over the holes and are required during the final phase to seal the two sheets together. They may exert pulling forces to help sealing (Williams-Masson et al. 1997; Raich et al. 1999; Jacinto et al. 2000; Bloor and Kiehart 2002; Jacinto et al. 2002; Soto et al. 2002; Wood et al. 2002; Gates et al. 2007; Sheffield et al. 2007; Millard and Martin 2008). Electron microscopy studies have revealed interdigitated filopodia during sealing (Redd et al. 2004) harboring AJs puncta at their tips (Raich et al. 1999; Vaezi et al. 2002). This structure appears analogous to the adhesion zipper observed in keratinocytes (Vasioukhin et al. 2000).
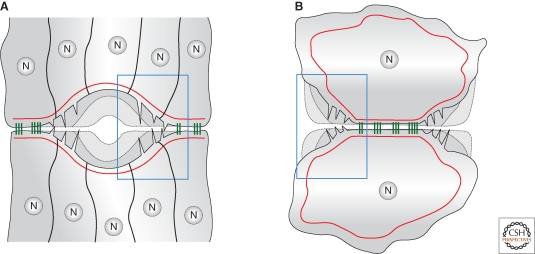
Figure 3.
Parallel between epithelial sheet sealing and contact expansion in cell culture. (A) Epithelial sheet sealing at the end of embryogenesis and during wound healing and (B) cell–cell contact expansion in cell culture. More advanced stages are shown by dashed lines. (A) A contracting acto-myosin cable (red) closes the hole and the final sealing step is facilitated by actin-based protrusions. Note the similarity of structures involved in the two systems (blue rectangles).
Cell–Cell Junctions Formation by Non-MET Processes
In the examples mentioned so far, AJs formation defines a spatial cue for organizing the cytoskeleton and recruiting apical–basal polarity complexes. In turn, these complexes stabilize and maintain AJs, possibly via regulation of the junctional actin cytoskeleton (reviewed in Knust and Bossinger 2002; Nelson 2003; Ebnet 2008). However, in the Drosophila embryonic primary epithelium, AJs formation occurs in a small region of the surface of contact between cells, which is defined by already-present apical–basal polarity cues. The polarity protein Par3/Bazooka (Baz) is the first component recruited to the apical region, in a microtubule- and Dynein-dependent process (Harris and Peifer 2004; Harris and Peifer 2005). Par3/Baz then initiates AJs assembly and recruits further polarity components, which maintain AJs in later stages (Muller and Wieschaus 1996; Bilder et al. 2003; Tanentzapf and Tepass 2003; Harris and Peifer 2004; Hutterer et al. 2004; Harris and Peifer 2005). Par3/Baz oligomers could serve as structural adaptors for AJs integrity (Benton and St Johnston 2003) or could link AJs to actin via the nectin/afadin system (Wei et al. 2005). Polarity complexes are also probably implicated in regulating actin polymerization via the small GTPases. In mammals, Par3 serves as a platform connecting Rho signaling to Rac1 regulation to control front/rear polarity in migrating cells. Rac1 is activated by its GEF Tiam1/2, which is in a complex with Par3/aPKC/Cdc42 (Nishimura et al. 2005). Phosphorylation of Par3 by the RhoA-ROCK pathway separates Rac1 from Tiam1/2, resulting in Rac1 inactivation (Nakayama et al. 2008). In flies, Par3 has also been implicated in organizing the cortical actin cytoskeleton by acting on the recruitment of Moesin, an ERM protein (Pilot et al. 2006) and in the control of phosphoinositides levels (von Stein et al. 2005), which can impact on actin dynamics and organization in many ways (for reviews, see Zheng 2001; Yin and Janmey 2003).
MAINTENANCE OF CELL–CELL JUNCTIONS
Once cell contacts have been established, actin polymerization and acto-myosin tension are required for the stabilization and maturation of adhesive interfaces. Actin network organization and Myo-II-mediated tension control the organization of adhesion molecules at the cell surface and the dynamics of the plasma membrane, which are both crucial for maintaining stable cell interfaces and tissue architecture.
Immobilization of E-cadherin Clusters
The organization of cadherins at cell interfaces depends on the integrity of the actin cytoskeleton (Pilot et al. 2006). In Drosophila embryos, apical–basal polarity cues (Par3/Baz and the phosphoinoside derivative PIP2) recruit the synaptotagmin-like protein Bitesize, which in turn recruits Moesin to the apical region. These factors organize the cortical actin network and ensure its integrity, which is crucial for a homogeneous distribution of adhesion foci at cell interfaces (Pilot et al. 2006). In fact, cortical actin is composed of two intermixed populations of filaments, which control E-cad distribution at two levels (Cavey et al. 2008). (1) Locally, actin filaments with a low turnover control the stability of E-cad molecules within puncta. (2) A peripheral contractile network of acto-myosin controls the mobility (displacement) of these puncta along cell–cell contacts. Myo-II-mediated tension is crucial for effectively tethering E-cad puncta in the plasma membrane and thus for controlling their spatial distribution along cell–cell contacts. This suggests that stabilization of adhesion requires the regulated immobilization of homophilic E-cad clusters by a tensile cortical actin network, independently of cluster stabilization per se (Cavey et al. 2008). These results shed light on previous reports, which implicated Myo-II in the spatial organization of cadherin clusters in various cell types (Gloushankova et al. 1998; Krendel et al. 1999; Vaezi et al. 2002; Conti et al. 2004; Shewan et al. 2005; Zhang et al. 2005).
E-cadherin Clusters Stability
Actin depolymerization studies suggest that actin turnover is reduced in mature cell junctions compared to younger ones (Adams et al. 1998; Braga et al. 1999; Ivanov et al. 2005a). Actin turnover is also specifically lower at cadherin puncta compared to neighboring regions in Drosophila epithelial cells (Wood et al. 2002; Cavey et al. 2008). In mammalian cells, cadherin stability and actin stability may be directly coupled by Eplin, a protein recruited by E-cad/β-cat/α-cat complexes and which is required for stabilizing actin filaments associated with adhesion complexes (Abe and Takeichi 2008). However, recycling of actin filaments associated with adhesion complexes is likely to occur because small GTPases are required to maintain adhesion. Alternatively, GTPase activity may reflect the need to remodel junctions in dynamic epithelia (reviewed in Braga and Yap 2005; Kooistra et al. 2007; Yamazaki et al. 2007).
Role of Junctional Actin Architecture
Adhesion strengthening seems to involve regulation of actin cytoskeleton organization: Branched networks associated with lamellipodial protrusions are replaced by parallel contractile bundles. This transition probably takes place very shortly after the initial clustering of cadherins and appears to be controlled by AJs components. Factors promoting branched actin polymerization (Rac1, Arp2/3, and cortactin) are relatively depleted from “older” regions of cell contacts (Helwani et al. 2004; Verma et al. 2004; Yamada and Nelson 2007). α-cat was proposed to directly control this transition by repressing Arp2/3 activity (Drees et al. 2005). As AJs mature, progressive enrichment of α-cat would result in local inhibition of Arp2/3 (Perez-Moreno and Fuchs 2006; Pokutta and Weis 2007). In addition, factors promoting unbranched F-actin elongation can be recruited to AJs, such as Formin1 and Ena/VASP in keratinocytes (Vasioukhin et al. 2000; Kobielak et al. 2004). Another formin, Diaphanous (Dia), is recruited to cell contacts and required for junction maintenance (Sahai and Marshall 2002; Carramusa et al. 2007; Homem and Peifer 2008). Dia may be directly recruited at junctions by α-cat and p120, which recruit its activator Rho1 (Magie et al. 2002). Bundling factors at nascent AJs may also contribute to the transition of actin organization (for reviews, see Adams 2004; Broderick and Winder 2005; Mege et al. 2006). In this context, direct observation of actin network architecture at AJs is an important, albeit challenging, avenue.
Tension and Rigidity of the Interface
Linear arrays of unbranched actin filaments favor Myo-II tension, which can stabilize cell–cell junctions in two ways.
(1) The protrusive activity of cell membranes is thought to destabilize adhesive interfaces (Gloushankova et al. 1997; Sahai and Marshall 2002; Zhang et al. 2005). Myo-II-mediated tension inhibits the formation of protrusions by its ability to align actin filaments parallel to the cell membrane (Gloushankova et al. 1997). In cell cultures, protrusive activity decreases after cell–cell contact formation (Gloushankova et al. 1997; Ehrlich et al. 2002) and increases upon Myo-II inhibition in keratinocytes (Vaezi et al. 2002; Zhang et al. 2005). Similar results were observed in Drosophila (Bloor and Kiehart 2002; Jacinto et al. 2002). Interfering with Dia function (polymerization of unbranched actin filaments) increases the membrane protrusive activity and creates gaps in cell contacts (Sahai and Marshall 2002). The formation of a continuous belt of adhesion is compromised upon Myo-II inhibition in keratinocytes and MCF-7 cells but whether this is a direct consequence of increased protrusions is unclear (Shewan et al. 2005; Zhang et al. 2005).
(2) Myo-II-mediated tension may also affect the distribution of cadherin molecules at junctions. For instance, applying tension on the cell membrane is sufficient to drive the clustering of cadherins independently of actin. The current model proposes that membrane tension along a cell contact brings the two cell membranes in close proximity, thereby favoring new homophilic cadherin interactions to form and thus trapping these molecules in the region under tension (Delanoe-Ayari et al. 2004).
DYNAMICS AND REMODELING OF CELL–CELL JUNCTIONS
Throughout embryonic development and in adults, morphogenetic processes, which shape tissues and organisms, require constant remodeling of cell junctions. What underlies the dynamics of cell–cell junctions? They first require a constant turnover of AJs components at the cell surface. Second, they result from the regulated balance of two forces at the cell surface: adhesion and cortical tension. Adhesion tends to increase the surface of contacts with neighbors, whereas cortical tension tends to decrease it (for a more in-depth discussion, see Lecuit and Lenne 2007).
Regulation of Adhesion
Cadherin Endocytosis and Recycling
The turnover of AJs components is achieved by endocytosis and recycling of cadherins to the cell surface (reviewed in D'souza-Schorey 2005; Ivanov et al. 2005b; Yap et al. 2007). Indeed, several morphogenetic processes involving junction remodeling are blocked upon inhibition of endocytosis (Jarrett et al. 2002; Classen et al. 2005; Ulrich et al. 2005; Shaye et al. 2008). Several studies point to a role for actin in mediating cadherin endocytosis (Le et al. 2002; Ivanov et al. 2004) (reviewed in Kaksonen et al. 2006). Myosin may facilitate endocytosis in Ca2+-depleted cells by assembling a contractile acto-myosin ring, which could provide mechanical force for vesicle formation at AJs (Ivanov et al. 2004). On the other hand, association with actin has been proposed to protect E-cad from endocytosis based on experiments suggesting that homophilically engaged E-cad is not endocytosed, whereas free E-cad is (Izumi et al. 2004). However, this issue remains controversial because cadherin homophilic dimers—which are presumably associated to actin—can be dissociated by endocytosis (Troyanovsky et al. 2006). Recently, the Par polarity proteins Cdc42, aPKC, and Par6 (but not Par3/Baz) have been implicated in regulating AJs stability in Drosophila by controlling E-cad endocytosis. Cdc42 is thought to couple actin polymerization (via WASp and Arp2/3) with vesicle scission (via Cip4 and Dynamin) to promote E-cad endocytosis and hence turnover (Georgiou et al. 2008; Leibfried et al. 2008; Harris and Tepass 2008).
E-cadherin recycling is equally important for the regulation of AJs dynamics. In Drosophila thoracic epithelial cells, members of the exocyst complex (Sec5, Sec6, and Sec15) directly control the recycling of E-cad to AJs via interactions with β-cat (Langevin et al. 2005). In the Drosophila pupal wing epithelium, irregularly arranged cells remodel their contacts to pack into a highly regular hexagonal array (Classen et al. 2005) (Fig. 4A). This process requires E-cad endocytosis (Rab5), recycling (Rab11), and exocytosis (Sec5). E-cad exocytosis may be under the control of the planar cell polarity pathway, which may bias E-cad recycling to specific cortical locations to promote regular hexagonal packing (Fig. 4A) (Classen et al. 2005).
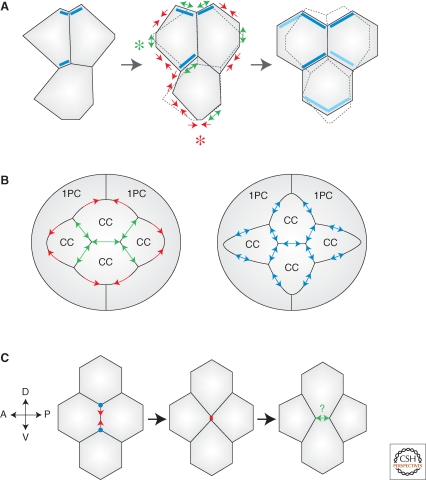
Figure 4.
Remodeling cell–cell junctions during morphogenesis. (A) Hexagonal packing in fly wing epithelial cells. Remodeling involves cell–cell contact shrinking (red arrows), expansion (green arrows), loss of some contacts (red asterisk), and creation of new contacts (green asterisk). The previous shape of cells is indicated by dashed lines in the middle and right panels. Flamingo enrichment (dark blue rectangles) and emergent polarity (light blue rectangles) may spatially bias exocytic delivery of E-cad to promote hexagonal packing. (B) Pattern formation in fly retina. (Left panel) Wild type ommatidial cluster with four cone cells (CC) surrounded by two primary pigment cells (1PC). Strong adhesion (N-cad + E-cad) increases inter-CC contacts (green arrows) at the expense of weaker adhesion (E-cad only) with 1PCs (red arrows). (Right panel) Uniform adhesion in a mutant ommatidium (blue arrows) distorts the cell pattern. (C) Cell intercalation during germ band elongation in fly embryos. The T1 transition involves shrinking of junctions between A/P neighbors (vertical junctions) and creation of new junctions between D/V neighbors (horizontal junctions). Shrinking is triggered by increased acto-myosin tension along A/P junctions (red arrows; vertices are indicated by blue dots). Expansion of the new D/V interface could rely on adhesion (green arrows), as in cell culture systems.
Generation of Tissue Patterns by Differential Adhesion
The concept of differential adhesion states that differences in adhesion strength between different cell types induce cell sorting, that is, the segregation of groups of cells based on their relative affinity for each other (Steinberg 1963). The strength of interaction is hypothesized to be different between homophilic and heterophilic cadherin dimers. However, cell sorting can happen with similar adhesion strengths (Niessen and Gumbiner 2002; Prakasam et al. 2006) and likely depends on additional parameters including the kinetics of the interactions and the actin cytoskeleton (discussed in Leckband and Prakasam 2006; Lecuit and Lenne 2007). Regardless of the exact mechanisms underlying differential adhesion, the idea that it can drive cell sorting has been largely confirmed in vivo (Godt and Tepass 1998; Gonzalez-Reyes and St Johnston 1998). This concept can be extended to the generation of cell patterns, which do not involve the complete separation of different cell types but subtle cell shape changes. One striking example comes from the morphogenesis of the Drosophila ommatidia in the developing retina (Fig. 4B). Ommatidia are composed of four cone cells (CCs) located in the center of a cluster and expressing both N-cad and E-cad, surrounded by two primary pigment cells (1PCs) expressing only E-cad. Genetic analyses have shown that increased adhesion strength in CCs because of N-cad expression maximizes inter-CCs contacts at the expense of CC-1PCs contacts and is responsible for establishing the specific geometry of CCs (Hayashi and Carthew 2004). Another aspect of ommatidial geometry is dictated by differential adhesion, relying on the immunoglobulin (Ig)-domain cell adhesion molecules Hibris and Roughest (Bao and Cagan 2005).
An increase in contact surface is hypothesized to result from increased adhesion strength along the interface. Adhesion strength depends on the surface levels of cadherins (Angres et al. 1996; Duguay et al. 2003; Chu et al. 2004), on the inherent properties (strength, kinetics) of homophilic versus heterophilic interactions (reviewed in Prakasam et al. 2006), as well as on the interaction of cadherins with actin and on actin dynamics (Angres et al. 1996; Imamura et al. 1999; Vasioukhin et al. 2000; Drees et al. 2005; Yamada et al. 2005; Zhang et al. 2005; Cavey et al. 2008). How does an increase in adhesion strength lead to an increase in contact surface? In an ideal system in which no forces resist the deformations induced by changes in contact surface, a zipping-like mechanism could induce contact surface expansion. However, living cells represent a much more complex system in which the deformation of cell shape produces a restoring force—cortical elasticity—which resists to contact expansion. Stronger adhesion alone is thus unlikely to be sufficient to expand the contact surface. As we have seen in the first section, the expansion of cell–cell contacts relies on actin-based forces (protrusions mediated by actin polymerization, and acto-myosin tension), which cooperate to bring cell membranes in close apposition and thereby counteract deformation-induced resistance to contact expansion. Such forces are very likely to be required to increase contact surfaces in the examples mentioned above. One could even imagine that increased adhesion strength only acts via actin remodeling to promote contact surface expansion. The magnitude of adhesion strength (i.e., amount and/or adhesive strength of cadherin dimers) may thus not be directly relevant to contact expansion but may simply reflect the degree of mobilization of actin-based forces. Alternatively, adhesion may regulate the kinetics of contact expansion but not the equilibrium geometry of cell contacts.
Cortical Tension and Junction Dynamics
Recent studies have characterized some important features of junctional mechanics underlying morphogenetic processes (Farhadifar et al. 2007; Kafer et al. 2007; Rauzi et al. 2008). The models were inspired from work done with soap films in which bubbles assemble in geometries almost identical to that of cells in an epithelium and which can be explained as resulting from progressive exploration of local surface energy minima (for details, see Carthew 2005; Lecuit and Lenne 2007). These studies suggest that the extent of cell–cell contacts is dictated by (1) local forces contributed by adhesion and acto-myosin tension, and (2) global forces generated by acto-myosin tension and dependent on the state of cell deformation.
Explaining Steady-State Cell Patterns
This paradigm has been used to explain pattern formation in the fly retina. A simple model that only incorporates the action of adhesive forces cannot recapitulate the patterns observed in wild type ommatidia and mutants in which the expression levels of cadherins are altered. In contrast, these different patterns are faithfully reproduced when including cortical tension as a force resisting cell adhesion (Kafer et al. 2007). Similarly, the steady state geometry of the wing imaginal disc epithelium before hexagonal packing can be modeled in terms of adhesive and elastic tension forces (Farhadifar et al. 2007). Comparing computer simulations to in vivo data yielded estimates of the relative contributions of elastic forces and line tensions at work at cell contacts and suggested that in this epithelium, local acto-myosin contractility predominates over adhesion (Farhadifar et al. 2007).
Tensile Networks Regulating Junction Dynamics
Following similar physical working hypotheses, a recent study probed the spatial distribution of junctional forces underlying the dynamics of tissue elongation (Rauzi et al. 2008). In gastrulating Drosophila embryos, epithelial cells of the ventro-lateral tissue (the germ band) intercalate to promote germ band elongation (GBE). This process involves the regulated disassembly of cell contacts between antero-posterior (A/P) neighbors and the creation of new contacts between dorso-ventral (D/V) neighbors (Bertet et al. 2004) (Fig. 4C). An anisotropy in Myo-II localization, more specifically, Myo-II enrichment along shrinking A/P junctions, is required for intercalation (Bertet et al. 2004), suggesting that polarized Myo-II distribution could generate anisotropic tension that would drive cell intercalation. Computer simulations and comparisons with in vivo data indicated that anisotropic tension could be sufficient to drive GBE. Laser-nanodissection of cortical actin measured the anisotropy at AJs and its dependence on Myo-II (Rauzi et al. 2008).
It is worth noting that tensile forces, which shape cells, need not be generated directly at the cell cortex. This was recently illustrated by the observation that apical cell constriction, which underlies tissue bending and invagination in several developmental contexts (reviewed in Lecuit and Lenne 2007), is driven by contraction of a centrally located acto-myosin network in the plane of AJs (Martin et al. 2008). This contrasts with other models stating that an acto-myosin purse string physically attached to AJs is responsible for constriction. During Drosophila mesoderm invagination, acto-myosin aggregates located in the medial part of cells undergo pulses and pull on the cell cortex to drive constriction as a ratchet (Martin et al. 2008). These recent results add another level of complexity to the description of junctional mechanics controlling cell contacts and cell shape.
Interplay of Adhesion and Cortical Tension
As illustrated throughout this review, actin plays a lead role in the regulation of cell contacts, by sustaining adhesion and cortical tension, whose balance dictates the extent of cell contacts. The cadherin/catenin system provides one among several other mechanical links between the actin skeletons of adjacent cells and thereby integrates intra- and intercellular forces to the scale of the whole tissue during morphogenesis. How such coupling impacts on the amplitude and the spatial range of force transmission at the cell cortex is one of the most important and open fields of investigation now, in which the contributions of different candidate linkers will have to be evaluated in detail. The extent of frictions between cadherins and contacting tensile actin networks is also likely to define the kinetics of cell shape changes. There is evidence that actin filaments are subdivided in functionally distinct populations, which are dedicated to specific purposes (Zhang et al. 2005; Cavey et al. 2008). Future work will have to focus on the mechanisms controlling the functional subdivision of the actin cytoskeleton in subcellular domains. It will be equally important to understand how the balance of forces is fine-tuned to reach equilibrium and obtain stable cell patterns in a tissue. Many experimental and pathological conditions show that misregulating the central players of adhesion and cortical tension leads to disequilibrium and has dramatic consequences on tissue integrity. For instance, excess tension can rupture cell–cell contacts (Sahai and Marshall 2002; Diogon et al. 2007), whereas the loss of cell–cell adhesion triggers epithelia–mesenchymal transitions (EMT) and is associated with cancer (reviewed in Thiery and Sleeman 2006; Baum et al. 2008).
Vertices as Central Regulatory Units of Epithelial Remodeling
Vertices are geometrical points in a tissue where three or more cells meet and can define both cell shape and cell dynamics. The number of neighbors a cell has simply reflects the number of vertices it is part of and the length of cell–cell contacts reflects the inter-vertices distances. Thus, epithelial remodeling can be explained as the displacement of vertices and most importantly the subsequent exchange of neighbors at vertices. Understanding how these two processes are regulated is thus key to understanding epithelial remodeling.
A drastic change in inter-vertices distance occurs during GBE in Drosophila embryos, when A/P junctions shrink during T1 transitions (Fig. 4C) (Bertet et al. 2004; Rauzi et al. 2008). Theoretically, anchoring of acto-myosin contractile bundles to the cell membrane specifically at vertices could be sufficient to remodel cell contacts. However, acto-myosin bundles are anchored to AJs puncta all along cell–cell junctions and not simply at vertices (Cavey et al. 2008). Moreover, vertices impose structural limits on the movement of stable E-cad clusters that cannot “cross” a vertex. Consistent with this, nano-ablation experiments indicate that vertices represent physical barriers to the lateral displacement of retracting actin bundles and E-cad clusters (Cavey et al. 2008; Rauzi et al. 2008), supporting the idea that vertices represent special sites of attachment of acto-myosin bundles to the cortex.
The regulation of AJs stability specifically at vertices also requires further understanding. Anchoring of acto-myosin bundles to the cortex depends on AJs (Dawes-Hoang et al. 2005; Martin et al. 2008) suggesting that the stability of AJs complexes, namely homophilic E-cad clusters, at vertices is essential to drive junction shrinkage. However, T1 transitions, the process whereby four cells meeting at a vertex after junction shrinkage produce two new three-way vertices (Fig. 4C), requires local remodeling of homophilic E-cad clusters. How E-cad stability is regulated during this step is currently unknown. There may be specific mechanisms targeted to vertices to regulate AJs integrity, which remain to be characterized.
ACKNOWLEDGMENTS
M.C. was supported by a fellowship from Association pour la Recherche sur le Cancer (ARC) and the Fondation Schlumberger pour l'Education et la Recherche (FSER). T.L. is supported by the CNRS, the ANR, the Fondation Recherche Médicale (équipe FRM), a subvention fixe from ARC, and a program grant from the HFSP.
Footnotes
Editors: W. James Nelson and Elaine Fuchs
Additional Perspectives on Cell Junctions available at www.cshperspectives.org
REFERENCES
- Abe K, Takeichi M 2008. EPLIN mediates linkage of the cadherin catenin complex to F-actin and stabilizes the circumferential actin belt. Proc Natl Acad Sci 105:13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JC 2004. Roles of fascin in cell adhesion and motility. Current Opinion in Cell Biol 16:590–596 [DOI] [PubMed] [Google Scholar]
- Adams CL, Nelson WJ, Smith SJ 1996. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell-cell adhesion. J Cell Biol 135:1899–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CL, Chen YT, Smith SJ, Nelson WJ 1998. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol 142:1105–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angres B, Barth A, Nelson WJ 1996. Mechanism for transition from initial to stable cell-cell adhesion: Kinetic analysis of E-cadherin-mediated adhesion using a quantitative adhesion assay. J Cell Biol 134:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Cagan R 2005. Preferential adhesion mediated by Hibris and Roughest regulates morphogenesis and patterning in the Drosophila eye. Develop Cell 8:925–935 [DOI] [PubMed] [Google Scholar]
- Baum B, Settleman J, Quinlan MP 2008. Transitions between epithelial and mesenchymal states in development and disease. Sem Cell Develop Biol 19:294–308 [DOI] [PubMed] [Google Scholar]
- Benton R, St Johnston D 2003. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115:691–704 [DOI] [PubMed] [Google Scholar]
- Bershadsky A 2004. Magic touch: How does cell-cell adhesion trigger actin assembly? Trends Cell Biol 14:589–593 [DOI] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T 2004. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429:667–671 [DOI] [PubMed] [Google Scholar]
- Bilder D, Schober M, Perrimon N 2003. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol 5:53–58 [DOI] [PubMed] [Google Scholar]
- Bloor JW, Kiehart DP 2002. Drosophila RhoA regulates the cytoskeleton and cell-cell adhesion in the developing epidermis. Development 129:3173–3183 [DOI] [PubMed] [Google Scholar]
- Braga VM, Yap AS 2005. The challenges of abundance: Epithelial junctions and small GTPase signalling. Current Opinion Cell Biol 17:466–474 [DOI] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA 1997. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol 137:1421–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Del Maschio A, Machesky L, Dejana E 1999. Regulation of cadherin function by Rho and Rac: Modulation by junction maturation and cellular context. Mol Biol Cell 10:9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick MJ, Winder SJ 2005. Spectrin, α-actinin, and dystrophin. Adv Protein Chem 70:203–246 [DOI] [PubMed] [Google Scholar]
- Calarco PG, Epstein CJ 1973. Cell surface changes during preimplantation development in the mouse. Develop Biol 32:208–213 [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M 1999. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98:147–157 [DOI] [PubMed] [Google Scholar]
- Carramusa L, Ballestrem C, Zilberman Y, Bershadsky AD 2007. Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J Cell Sci 120:3870–3882 [DOI] [PubMed] [Google Scholar]
- Carthew RW 2005. Adhesion proteins and the control of cell shape. Current Opinion Gen Develop 15:358–363 [DOI] [PubMed] [Google Scholar]
- Cavey M, Rauzi M, Lenne PF, Lecuit T 2008. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453:751–756 [DOI] [PubMed] [Google Scholar]
- Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dufour S 2004. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol 167:1183–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classen AK, Anderson KI, Marois E, Eaton S 2005. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Develop Cell 9:805–817 [DOI] [PubMed] [Google Scholar]
- Clayton L, Hall A, Johnson MH 1999. A role for Rho-like GTPases in the polarisation of mouse eight-cell blastomeres. Develop Cell 205:322–331 [DOI] [PubMed] [Google Scholar]
- Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS 2004. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem 279:41263–41266 [DOI] [PubMed] [Google Scholar]
- Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR 1998. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J Cell Biol 141:297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'souza-Schorey C 2005. Disassembling adherens junctions: Breaking up is hard to do. Trends Cell Biol 15:19–26 [DOI] [PubMed] [Google Scholar]
- Dard N, Louvet S, Santa-Maria A, Aghion J, Martin M, Mangeat P, Maro B 2001. In vivo functional analysis of ezrin during mouse blastocyst formation. Develop Biol 233:161–173 [DOI] [PubMed] [Google Scholar]
- Dard N, Louvet-Vallee S, Santa-Maria A, Maro B 2004. Phosphorylation of ezrin on threonine T567 plays a crucial role during compaction in the mouse early embryo. Develop Biol 271:87–97 [DOI] [PubMed] [Google Scholar]
- Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF 2005. folded gastrulation, cell shape change and the control of myosin localization. Development 132:4165–4178 [DOI] [PubMed] [Google Scholar]
- De Vries WN, Evsikov AV, Haac BE, Fancher KS, Holbrook AE, Kemler R, Solter D, Knowles BB 2004. Maternal β-catenin and E-cadherin in mouse development. Development 131:4435–4445 [DOI] [PubMed] [Google Scholar]
- Delanoe-Ayari H, Al Kurdi R, Vallade M, Gulino-Debrac D, Riveline D 2004. Membrane and acto-myosin tension promote clustering of adhesion proteins. Proc Natl Acad Sci 101:2229–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogon M, Wissler F, Quintin S, Nagamatsu Y, Sookhareea S, Landmann F, Hutter H, Vitale N, Labouesse M 2007. The RhoGAP RGA-2 and LET-502/ROCK achieve a balance of actomyosin-dependent forces in C. elegans epidermis to control morphogenesis. Development 134:2469–2479 [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI 2005. α-catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly. Cell 123:903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguay D, Foty RA, Steinberg MS 2003. Cadherin-mediated cell adhesion and tissue segregation: Qualitative and quantitative determinants. Develop Biol 253:309–323 [DOI] [PubMed] [Google Scholar]
- Ebnet K 2008. Organization of multiprotein complexes at cell-cell junctions. Histochem Cell Biol 130:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich JS, Hansen MD, Nelson WJ 2002. Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Develop Cell 3:259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadifar R, Roper JC, Aigouy B, Eaton S, Julicher F 2007. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr Biol 17:2095–2104 [DOI] [PubMed] [Google Scholar]
- Fleming TP, Pickering SJ, Qasim F, Maro B 1986. The generation of cell surface polarity in mouse 8-cell blastomeres: The role of cortical microfilaments analysed using cytochalasin D. J Embryol Exp Morph 95:169–191 [PubMed] [Google Scholar]
- Gates J, Peifer M 2005. Can 1000 reviews be wrong? Actin, α-Catenin, and adherens junctions. Cell 123:769–772 [DOI] [PubMed] [Google Scholar]
- Gates J, Mahaffey JP, Rogers SL, Emerson M, Rogers EM, Sottile SL, Van Vactor D, Gertler FB, Peifer M 2007. Enabled plays key roles in embryonic epithelial morphogenesis in Drosophila. Development 134:2027–2039 [DOI] [PubMed] [Google Scholar]
- Georgiou M, Marinari E, Burden J, Baum B 2008. Cdc42, Par6, and aPKC Regulate Arp2/3-Mediated Endocytosis to Control Local Adherens Junction Stability. Curr Biol 18:1631–1638 [DOI] [PubMed] [Google Scholar]
- Gloushankova NA, Alieva NA, Krendel MF, Bonder EM, Feder HH, Vasiliev JM, Gelfand IM 1997. Cell-cell contact changes the dynamics of lamellar activity in nontransformed epitheliocytes but not in their ras-transformed descendants. Proc Natl Acad Sci 94:879–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloushankova NA, Krendel MF, Alieva NO, Bonder EM, Feder HH, Vasiliev JM, Gelfand IM 1998. Dynamics of contacts between lamellae of fibroblasts: Essential role of the actin cytoskeleton. Proc Natl Acad Sci 95:4362–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt D, Tepass U 1998. Drosophila oocyte localization is mediated by differential cadherin-based adhesion. Nature 395:387–391 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Reyes A, St Johnston D 1998. The Drosophila AP axis is polarised by the cadherin-mediated positioning of the oocyte. Development 125:3635–3644 [DOI] [PubMed] [Google Scholar]
- Gumbiner BM 2005. Regulation of cadherin-mediated adhesion in morphogenesis. Nature Rev 6:622–634 [DOI] [PubMed] [Google Scholar]
- Harden N, Ricos M, Ong YM, Chia W, Lim L 1999. Participation of small GTPases in dorsal closure of the Drosophila embryo: Distinct roles for Rho subfamily proteins in epithelial morphogenesis. J Cell Sci 112:273–284 [DOI] [PubMed] [Google Scholar]
- Harris TJ, Peifer M 2004. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J Cell Biol 167:135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Peifer M 2005. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol 170:813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KP, Tepass U 2008. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol 183:1129–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Carthew R 2004. Surface mechanics mediate pattern formation in the developing retina. Nature 431:647–652 [DOI] [PubMed] [Google Scholar]
- Helwani FM, Kovacs EM, Paterson AD, Verma S, Ali RG, Fanning AS, Weed SA, Yap AS 2004. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J Cell Biol 164:899–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M 1992. Identification of a neural α-catenin as a key regulator of cadherin function and multicellular organization. Cell 70:293–301 [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Keller TC 3rd, Chasan R, Mooseker MS 1983. Mechanism of brush border contractility studied by the quick-freeze, deep-etch method. J Cell Biol 96:1325–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homem CC, Peifer M 2008. Diaphanous regulates myosin and adherens junctions to control cell contractility and protrusive behavior during morphogenesis. Development 135:1005–1018 [DOI] [PubMed] [Google Scholar]
- Hutterer A, Betschinger J, Petronczki M, Knoblich JA 2004. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Develop Cell 6:845–854 [DOI] [PubMed] [Google Scholar]
- Hyafil F, Morello D, Babinet C, Jacob F 1980. A cell surface glycoprotein involved in the compaction of embryonal carcinoma cells and cleavage stage embryos. Cell 21:927–934 [DOI] [PubMed] [Google Scholar]
- Iino R, Koyama I, Kusumi A 2001. Single molecule imaging of green fluorescent proteins in living cells: E-cadherin forms oligomers on the free cell surface. Biophys J 80:2667–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y, Itoh M, Maeno Y, Tsukita S, Nagafuchi A 1999. Functional domains of α-catenin required for the strong state of cadherin-based cell adhesion. J Cell Biol 144:1311–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, McCall IC, Parkos CA, Nusrat A 2004. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell 15:2639–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA 2005a. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell 16:2636–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov AI, Nusrat A, Parkos CA 2005b. Endocytosis of the apical junctional complex: Mechanisms and possible roles in regulation of epithelial barriers. Bioessays 27:356–365 [DOI] [PubMed] [Google Scholar]
- Izumi G, Sakisaka T, Baba T, Tanaka S, Morimoto K, Takai Y 2004. Endocytosis of E-cadherin regulated by Rac and Cdc42 small G proteins through IQGAP1 and actin filaments. J Cell Biol 166:237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacinto A, Wood W, Balayo T, Turmaine M, Martinez-Arias A, Martin P 2000. Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr Biol 10:1420–1426 [DOI] [PubMed] [Google Scholar]
- Jacinto A, Martinez-Arias A, Martin P 2001. Mechanisms of epithelial fusion and repair. Nat Cell Biol 3:E117–123 [DOI] [PubMed] [Google Scholar]
- Jacinto A, Wood W, Woolner S, Hiley C, Turner L, Wilson C, Martinez-Arias A, Martin P 2002. Dynamic Analysis of Actin Cable Function during Drosophila Dorsal Closure. Curr Biol 12:1245. [DOI] [PubMed] [Google Scholar]
- Jaffe SH, Friedlander DR, Matsuzaki F, Crossin KL, Cunningham BA, Edelman GM 1990. Differential effects of the cytoplasmic domains of cell adhesion molecules on cell aggregation and sorting-out. Proc Natl Acad Sci 87:3589–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett O, Stow JL, Yap AS, Key B 2002. Dynamin-dependent endocytosis is necessary for convergent-extension movements in Xenopus animal cap explants. Int J Develop Biol 46:467–473 [PubMed] [Google Scholar]
- Johnson MH, Maro B, Takeichi M 1986. The role of cell adhesion in the synchronization and orientation of polarization in 8-cell mouse blastomeres. J Embryol Exp Morph 93:239–255 [PubMed] [Google Scholar]
- Kafer J, Hayashi T, Maree AF, Carthew RW, Graner F 2007. Cell adhesion and cortex contractility determine cell patterning in the Drosophila retina. Proc Natl Acad Sci 104:18549–18554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG 2006. Harnessing actin dynamics for clathrin-mediated endocytosis. Nature Rev 7:404–414 [DOI] [PubMed] [Google Scholar]
- Kametani Y, Takeichi M 2007. Basal-to-apical cadherin flow at cell junctions. Nat Cell Biol 9:92–98 [DOI] [PubMed] [Google Scholar]
- Kemler R, Babinet C, Eisen H, Jacob F 1977. Surface antigen in early differentiation. Proc Natl Acad Sci 74:4449–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA 2000. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol 149:471–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knust E, Bossinger O 2002. Composition and formation of intercellular junctions in epithelial cells. Science 298:1955–1959 [DOI] [PubMed] [Google Scholar]
- Kobielak A, Pasolli HA, Fuchs E 2004. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol 6:21–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra MR, Dube N, Bos JL 2007. Rap1: A key regulator in cell-cell junction formation. J Cell Sci 120:17–22 [DOI] [PubMed] [Google Scholar]
- Kovacs EM, Ali RG, McCormack AJ, Yap AS 2002a. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J Biol Chem 277:6708–6718 [DOI] [PubMed] [Google Scholar]
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS 2002b. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol 12:379–382 [DOI] [PubMed] [Google Scholar]
- Krendel MF, Bonder EM 1999. Analysis of actin filament bundle dynamics during contact formation in live epithelial cells. Cell Mot Cytoskel 43:296–309 [DOI] [PubMed] [Google Scholar]
- Krendel M, Gloushankova NA, Bonder EM, Feder HH, Vasiliev JM, Gelfand IM 1999. Myosin-dependent contractile activity of the actin cytoskeleton modulates the spatial organization of cell-cell contacts in cultured epitheliocytes. Proc Natl Acad Sci 96:9666–9670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert M, Choquet D, Mege RM 2002. Dynamics of ligand-induced, Rac1-dependent anchoring of cadherins to the actin cytoskeleton. J Cell Biol 157:469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin J, Morgan MJ, Sibarita JB, Aresta S, Murthy M, Schwarz T, Camonis J, Bellaiche Y 2005. Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Develop Cell 9:355–376 [DOI] [PubMed] [Google Scholar]
- Larue L, Ohsugi M, Hirchenhain J, Kemler R 1994. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci 91:8263–8267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TL, Joseph SR, Yap AS, Stow JL 2002. Protein kinase C regulates endocytosis and recycling of E-cadherin. Am J Physiol 283:C489–499 [DOI] [PubMed] [Google Scholar]
- Leckband D, Prakasam A 2006. Mechanism and dynamics of cadherin adhesion. Ann Rev Biomed Eng 8:259–287 [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF 2007. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat Rev 8:633–644 [DOI] [PubMed] [Google Scholar]
- Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y 2008. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol 18:1639–1648 [DOI] [PubMed] [Google Scholar]
- Magie CR, Meyer MR, Gorsuch MS, Parkhurst SM 1999. Mutations in the Rho1 small GTPase disrupt morphogenesis and segmentation during early Drosophila development. Development 126:5353–5364 [DOI] [PubMed] [Google Scholar]
- Magie CR, Pinto-Santini D, Parkhurst SM 2002. Rho1 interacts with p120ctn and α-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development 129:3771–3782 [DOI] [PubMed] [Google Scholar]
- Martin P, Parkhurst SM 2004. Parallels between tissue repair and embryo morphogenesis. Development 131:3021–3034 [DOI] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF 2008. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457:495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki F, Mege RM, Jaffe SH, Friedlander DR, Gallin WJ, Goldberg JI, Cunningham BA, Edelman GM 1990. cDNAs of cell adhesion molecules of different specificity induce changes in cell shape and border formation in cultured S180 cells. J Cell Bio 110:1239–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mege RM, Gavard J, Lambert M 2006. Regulation of cell-cell junctions by the cytoskeleton. Current Opinion Cell Biol 18:541–548 [DOI] [PubMed] [Google Scholar]
- Millard TH, Martin P 2008. Dynamic analysis of filopodial interactions during the zippering phase of Drosophila dorsal closure. Development 135:621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HA, Wieschaus E 1996. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J Cell Biol 134:149–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K 2001. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci 114:1829–1838 [DOI] [PubMed] [Google Scholar]
- Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, Amano M, Kaibuchi K 2008. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Develop Cell 14:205–215 [DOI] [PubMed] [Google Scholar]
- Nelson WJ 2003. Adaptation of core mechanisms to generate cell polarity. Nature 422:766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen CM, Gumbiner BM 2002. Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J Cell Biol 156:389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K 2005. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol 7:270–277 [DOI] [PubMed] [Google Scholar]
- Noren NK, Niessen CM, Gumbiner BM, Burridge K 2001. Cadherin engagement regulates Rho family GTPases. J Biol Chem 276:33305–33308 [DOI] [PubMed] [Google Scholar]
- Oda H, Tsukita S, Takeichi M 1998. Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Develop Biol 203:435–450 [DOI] [PubMed] [Google Scholar]
- Orsulic S, Peifer M 1996. An in vivo structure-function study of armadillo, the β-catenin homologue, reveals both separate and overlapping regions of the protein required for cell adhesion and for wingless signaling. J Cell Biol 134:1283–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama T, Kanai Y, Ochiai A, Akimoto S, Oda T, Yanagihara K, Nagafuchi A, Tsukita S, Shibamoto S, Ito F 1994. A truncated β-catenin disrupts the interaction between E-cadherin and α-catenin: A cause of loss of intercellular adhesiveness in human cancer cell lines. Cancer Res 54:6282–6287 [PubMed] [Google Scholar]
- Perez-Moreno M, Fuchs E 2006. Catenins: Keeping cells from getting their signals crossed. Develop Cell 11:601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt J, Cox EA, Broadbent ID, Flett A, Hardin J 2003. The Caenorhabditis elegans p120 catenin homologue, JAC-1, modulates cadherin-catenin function during epidermal morphogenesis. J Cell Biol 162:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot F, Philippe J, Lemmers C, Lecuit T 2006. Spatial control of actin organization at adherens junctions by a synaptotagmin like protein Btsz. Nature 442:580–584 [DOI] [PubMed] [Google Scholar]
- Pokutta S, Weis WI 2007. Structure and mechanism of cadherins and catenins in cell-cell contacts. Ann Rev Cell Develop Biol 23:237–261 [DOI] [PubMed] [Google Scholar]
- Prakasam AK, Maruthamuthu V, Leckband DE 2006. Similarities between heterophilic and homophilic cadherin adhesion. Proc Natl Acad Sci 103:15434–15439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt HP, Ziomek CA, Reeve WJ, Johnson MH 1982. Compaction of the mouse embryo: An analysis of its components. J Embryol Exp Morph 70:113–132 [PubMed] [Google Scholar]
- Raich WB, Agbunag C, Hardin J 1999. Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr Biol 9:1139–1146 [DOI] [PubMed] [Google Scholar]
- Rauzi M, Verant P, Lecuit T, Lenne PF 2008. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol 10:1401–1410 [DOI] [PubMed] [Google Scholar]
- Redd MJ, Cooper L, Wood W, Stramer B, Martin P 2004. Wound healing and inflammation: Embryos reveal the way to perfect repair. Phil Trans Roy Soc London 359:777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethmacher D, Brinkmann V, Birchmeier C 1995. A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc Natl Acad Sci 92:855–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ 2002. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol 4:408–415 [DOI] [PubMed] [Google Scholar]
- Sako Y, Nagafuchi A, Tsukita S, Takeichi M, Kusumi A 1998. Cytoplasmic regulation of the movement of E-cadherin on the free cell surface as studied by optical tweezers and single particle tracking: Corralling and tethering by the membrane skeleton. J Cell Biol 140:1227–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaye DD, Casanova J, Llimargas M 2008. Modulation of intracellular trafficking regulates cell intercalation in the Drosophila trachea. Nat Cell Biol 10:964–970 [DOI] [PubMed] [Google Scholar]
- Sheffield M, Loveless T, Hardin J, Pettitt J 2007. C. elegans Enabled exhibits novel interactions with N-WASP, Abl, and cell-cell junctions. Curr Biol 17:1791–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS 2005. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell 16:4531–4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayoshi Y, Okada TS, Takeichi M 1983. The calcium-dependent cell-cell adhesion system regulates inner cell mass formation and cell surface polarization in early mouse development. Cell 35:631–638 [DOI] [PubMed] [Google Scholar]
- Soto MC, Qadota H, Kasuya K, Inoue M, Tsuboi D, Mello CC, Kaibuchi K 2002. The GEX-2 and GEX-3 proteins are required for tissue morphogenesis and cell migrations in C. elegans. Genes Develop 16:620–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MS 1963. Reconstruction of tissues by dissociated cells. Some morphogenetic tissue movements and the sorting out of embryonic cells may have a common explanation. Science 141:401–408 [DOI] [PubMed] [Google Scholar]
- Tanentzapf G, Tepass U 2003. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat Cell Biol 5:46–52 [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V 1994. The development of cellular junctions in the Drosophila embryo. Develop Biol 161:563–596 [DOI] [PubMed] [Google Scholar]
- Tepass U, Gruszynski-DeFeo E, Haag TA, Omatyar L, Torok T, Hartenstein V 1996. shotgun encodes Drosophila E-cadherin and is preferentially required during cell rearrangement in the neurectoderm and other morphogenetically active epithelia. Genes Develop 10:672–685 [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP 2006. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev 7:131–142 [DOI] [PubMed] [Google Scholar]
- Torres M, Stoykova A, Huber O, Chowdhury K, Bonaldo P, Mansouri A, Butz S, Kemler R, Gruss P 1997. An α-E-catenin gene trap mutation defines its function in preimplantation development. Proc Natl Acad Sci 94:901–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyanovsky RB, Sokolov EP, Troyanovsky SM 2006. Endocytosis of Cadherin from Intracellular Junctions Is the Driving Force for Cadherin Adhesive Dimer Disassembly. Mol Biol Cell 17:3484–3493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Oda H, Kraut R, Hayashi S, Kotaoka Y, Takeichi M 1996. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Develop 10:659–671 [DOI] [PubMed] [Google Scholar]
- Ulrich F, Krieg M, Schotz EM, Link V, Castanon I, Schnabel V, Taubenberger A, Mueller D, Puech PH, Heisenberg CP 2005. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Develop Cell 9:555–564 [DOI] [PubMed] [Google Scholar]
- Vaezi A, Bauer C, Vasioukhin V, Fuchs E 2002. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Develop Cell 3:367–381 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Fuchs E 2001. Actin dynamics and cell-cell adhesion in epithelia. Current Opinion Cell Biol 13:76–84 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E 2000. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100:209–219 [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E 2001. Hyperproliferation and defects in epithelial polarity upon conditional ablation of α-catenin in skin. Cell 104:605–617 [DOI] [PubMed] [Google Scholar]
- Verma S, Shewan AM, Scott JA, Helwani FM, den Elzen NR, Miki H, Takenawa T, Yap AS 2004. Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J Biol Chem 279:34062–34070 [DOI] [PubMed] [Google Scholar]
- Vestweber D, Gossler A, Boller K, Kemler R 1987. Expression and distribution of cell adhesion molecule uvomorulin in mouse preimplantation embryos. Develop Biol 124:451–456 [DOI] [PubMed] [Google Scholar]
- von Stein W, Ramrath A, Grimm A, Muller-Borg M, Wodarz A 2005. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Development 132:1675–1686 [DOI] [PubMed] [Google Scholar]
- Wei SY, Escudero LM, Yu F, Chang LH, Chen LY, Ho YH, Lin CM, Chou CS, Chia W, Modolell J 2005. Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Develop Cell 8:493–504 [DOI] [PubMed] [Google Scholar]
- Weis WI, Nelson WJ 2006. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem 281:35593–35597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Masson EM, Malik AN, Hardin J 1997. An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development 124:2889–2901 [DOI] [PubMed] [Google Scholar]
- Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P 2002. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol 4:907–912 [DOI] [PubMed] [Google Scholar]
- Yamada S, Nelson WJ 2007. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol 178:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ 2005. Deconstructing the cadherin-catenin-actin complex. Cell 123:889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D, Oikawa T, Takenawa T 2007. Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell-cell adhesion. J Cell Sci 120:86–100 [DOI] [PubMed] [Google Scholar]
- Yap AS, Crampton MS, Hardin J 2007. Making and breaking contacts: The cellular biology of cadherin regulation. Current Opinion Cell Biol 19:508–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HL, Janmey PA 2003. Phosphoinositide regulation of the actin cytoskeleton. Ann Rev Physiol 65:761–789 [DOI] [PubMed] [Google Scholar]
- Yonemura S, Itoh M, Nagafuchi A, Tsukita S 1995. Cell-to-cell adherens junction formation and actin filament organization: Similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci 108:127–142 [DOI] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E 2004. Patterned gene expression directs bipolar planar polarity in Drosophila. Develop Cell 6:343–355 [DOI] [PubMed] [Google Scholar]
- Zandy NL, Playford M, Pendergast AM 2007. Abl tyrosine kinases regulate cell-cell adhesion through Rho GTPases. Proc Natl Acad Sci 104:17686–17691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Betson M, Erasmus J, Zeikos K, Bailly M, Cramer LP, Braga VM 2005. Actin at cell-cell junctions is composed of two dynamic and functional populations. J Cell Sci 118:5549–5562 [DOI] [PubMed] [Google Scholar]
- Zheng Y 2001. Dbl family guanine nucleotide exchange factors. Trends Biochem Sci 26:724–732 [DOI] [PubMed] [Google Scholar]