Abstract
NF-κB transcription factors have been suspected to be involved in cancer development since their discovery because of their kinship with the v-Rel oncogene product. Subsequent work led to identification of oncogenic mutations that result in NF-κB activation in lymphoid malignancies, but most of these mutations affect upstream components of NF-κB signaling pathways, rather than NF-κB family members themselves. NF-κB activation has also been observed in many solid tumors, but so far no oncogenic mutations responsible for NF-κB activation in carcinomas have been identified. In such cancers, NF-κB activation is a result of underlying inflammation or the consequence of formation of an inflammatory microenvironment during malignant progression. Most importantly, through its ability to up-regulate the expression of tumor promoting cytokines, such as IL-6 or TNF-α, and survival genes, such as Bcl-XL, NF-κB provides a critical link between inflammation and cancer.
Inflammation can promote tumor development and progression. Recent work indicates that the NF-κB signaling molecule provides the critical link.
An important chapter in the long saga of NF-κB is the one dealing with its role as a pivotal link between inflammation and cancer. A possible association between NF-κB and cancer has emerged during the early days of RelA/p65 cloning and sequencing, which instantaneously revealed its kinship to c-Rel and its oncogenic derivative v-Rel (Gilmore 2003). However, oncogenic mutations that endow RelA, c-Rel, or other NF-κB proteins with transforming activity were found to be rare and mainly limited to lymphoid malignancies (Gilmore 2003). Yet, not only lymphoid cancers, but most solid tumors as well, exhibit activated NF-κB (Karin et al. 2002). As in most of these cases, no loss-of-function IκB mutations or gain-of-function IKK mutations have been detected. We have suggested that NF-κB activation in cancer may be the result of either exposure to proinflammatory stimuli in the tumor microenvironment or mutational activation of upstream components in IKK–NF-κB signaling pathways (Karin et al. 2002). Further bolstering our belief in the oncogenic potential of “normal” NF-κB activated by stimuli that are extrinsic to the cancer cell were the findings that NF-κB can inhibit apoptosis (Beg and Baltimore 1996; Liu et al. 1996; Van Antwerp et al. 1996; Wang et al. 1996), stimulate cell proliferation (Joyce et al. 2001), as well as promote a migratory and invasive phenotype that is associated with tumor progression (Huang et al. 2001). Concurrently, we became cognizant of a large body of epidemiological and experimental data providing new support for a causal link between inflammation and cancer, an association that was first proposed by Virchow during the 19th century (Balkwill and Mantovani 2001). Considering these findings, together with sightings of activated NF-κB in a large number of cancers, most of which are not associated with genetic alterations in NF-κB, IKK, or upstream components of this signaling system, we proposed that NF-κB may provide a critical mechanistic link between inflammation and cancer (Karin et al. 2002). During the past seven years, this proposal has been subjected to intense scrutiny by a number of labs, in a variety of experimental systems, and although complex and occasionally unpredictable, the role of the NF-κB signaling system in bridging inflammation and cancer is currently well appreciated (Karin 2006). It was also found that some IKK subunits (IKKα) and closely related protein kinases (e.g., IKKε) can play NF-κB independent roles in a variety of cancers (Boehm et al. 2007; Luo et al. 2007). In addition, new work has resulted in the identification of cancer-associated mutations in upstream components of the IKK-NF-κB signaling system that can lead to cell autonomous activation of NF-κB in multiple myeloma (Annunziata et al. 2007; Keats et al. 2007). The goal of this article is to review the experimental evidence for the pathogenic function of NF-κB in cancer and discuss whether and how IKK-NF-κB targeted interventions can be used in cancer prevention and/or therapy.
NF-κB IN LYMPHOID MALIGNANCIES: FROM CELL AUTONOMY TO PARACRINE EFFECTS
As mentioned above, the first hint to a link between NF-κB and cancer had emerged with the cloning of RelA and the realization of its close kinship to the viral oncoprotein v-Rel and its cellular homolog c-Rel (Gilmore 2003). Soon thereafter, the Bcl-3 oncoprotein, a product of a gene activated by chromosomal translocation in B-cell chronic lymphocytic leukemia, was identified as a member of the IκB family (Franzoso et al. 1992; Bours et al. 1993). Later, the NF-κB2 gene was also found to be rearranged in B- and T-cell lymphomas, giving rise to a truncated NF-κB2/p100 protein devoid of the IκB-like activity that is exhibited by native p100 (Neri et al. 1991). These early findings led to an extensive search for mutations affecting the IκB-NF-κB system in other lymphoid malignancies. This effort, however, has netted few new results other than those described previously. For instance, IκBα gene mutations were detected in Hodgkin’s lymphoma (Cabannes et al. 1999), but their contributions to pathogenesis is still not clear. Eventually, this has led to a broader view of the role played by NF-κB in tumorigenesis, according to which, mutations that cause NF-κB activation in malignant cells may occur in genes coding for signaling proteins that feed into the IKK–NF-κB module. Indeed, translocations that lead to Bcl-10 overexpression and activation of IKK–NF-κB signaling were identified in MALT lymphomas (Willis et al. 1999). Another product of a chromosomal translocation in MALT lymphoma is MALT1, a protein with paracaspase homology that interacts with Bcl-10 and Carma-1 to yield IKK activation (Uren et al. 2000). Given its well established antiapoptotic function, especially in B cells (Grossmann et al. 2000; Gugasyan et al. 2000; Pasparakis et al. 2002), activation of NF-κB through MALT1 or Bcl-10 is thought to be one of the hallmarks and a key pathogenic event in MALT lymphoma.
Another B-cell malignancy in which the CARD11:MALT1:Bcl-10 complex plays an important pathogenic role is diffuse large B-cell lymphoma (DLBCL). Staudt and coworkers made extensive use of DNA microarray technology to identify genes that are misregulated in different types of DLBCL and arrived at the conclusion that NF-κB is constitutively active in activated B-cell-like (ABC)-DLBCL, but not in germinal center B-cell-like (GCB)-DLBCL (Davis et al. 2001). Importantly, constitutively active NF-κB is required for the survival of ABC-DLBCL (Davis et al. 2001). An shRNA-based screen for genes, whose expression is required for the survival of DLBCL cells, identified CARD11 as the driver of constitutive NF-κB activity in ABC-DLBCL (Ngo et al. 2006). Furthermore, the CARD11 gene was found to be mutated in about 10% of ABC-DLBCL (Lenz et al. 2008). The mutations all affect residues within the coiled-coil domain of CARD11 and generate a protein that is a constitutive activator of IKK-NF-κB signaling. These results indicate that in a subpopulation of ABC-DLBCL, CARD11 acts as a bona fide oncogene and that its coiled-coil domain serves a negative regulatory function. However, we still do not know what causes the CARD11-dependent activation of NF-κB in the remaining 90% of DLBCL. Nonetheless, it is clear that the CARD11:MALT1:Bcl-10 complex is an important driver of malignant B-cell survival in more than one type of lymphoma.
Another lymphoid malignancy associated with NF-κB activation is multiple myeloma. Although activated NF-κB is a common feature of multiple myeloma, no mutations in NF-κB or IκB encoding genes have been discovered in this disease either. However, extensive genetic analysis of primary tumors and multiple myeloma cell lines have revealed a number of mutations in genes encoding upstream signaling molecules that lead to stabilization and accumulation of NF-κB inducing kinase (NIK), a member of the MAPK kinase kinase (MAP3K) family (Annunziata et al. 2007; Keats et al. 2007). Normally, NIK is a very unstable protein whose activity is kept at a low level because of its rapid turnover (Vallabhapurapu et al. 2008). However, mutations in genes encoding components of an ubiquitin ligase complex responsible for NIK turnover or in the NIK gene itself result in accumulation and self-activation of NIK. These mutations include alterations in either NIK or in TRAF3 that disrupt the interactions between the two proteins (Annunziata et al. 2007; Keats et al. 2007). Normally, the binding of TRAF3 to NIK in nonstimulated cells results in the recruitment to NIK of a protein complex composed of the ubiquitin ligases cIAP1 or cIAP2 and TRAF2 and this complex leads to degradative NIK ubiquitination (Vallabhapurapu et al. 2008). Other multiple myeloma-linked mutations include large deletions affecting the closely linked cIAP1 and cIAP2 loci, resulting in the complete absence of their protein products, thereby preventing degradative polyubiquitination of NIK (Annunziata et al. 2007; Keats et al. 2007). More rare mutations abolish the expression of TRAF2 (Keats et al. 2007). Although related in structure to TRAF3, TRAF2 does not directly interact with NIK and instead serves as an activating ubiquitin ligase for cIAP1 and cIAP2, enhancing their ability to polyubiquitinate NIK (Vallabhapurapu et al. 2008). Based on its known ability to activate IKKα and thereby induce processing of NF-κB2/p100 to NF-κB2/p52, it was expected that the elevated and activated NIK in multiple myeloma exerts its oncogenic activity via IKKα (Senftleben et al. 2001). It was, therefore, much of a surprise that only IKKβ inhibition and not IKKα depletion affected the proliferation and survival of multiple myeloma cells (Annunziata et al. 2007).
NF-κB can also be activated in several other lymphoid malignancies as a result of infection with either DNA or RNA tumor viruses. For instance, Epstein-Barr virus (EBV) activates NF-κB through expression of latent membrane protein 1 (LMP1), a protein that can induce lymphomas when expressed in transgenic mice (Eliopoulos and Young 2001; Thornburg et al. 2006). Curiously, LMP1 can induce NIK-dependent NF-κB2/p100 processing (Luftig et al. 2004), but the specific contribution of NF-κB2/p52 formation to lymphomagenesis is not entirely clear, unless NF-κB2/p100 acts as a general NF-κB inhibitor in nontransformed lymphocytes. Kaposi Sarcoma-associated herpesvirus (KSHV) can induce primary effusion lymphoma (PEL) through expression of vFLIP, a viral version of the cFLIP protein that can lead to IKK activation (Liu et al. 2002). Inhibition of NF-κB induces the apoptotic death of PEL cells (Keller et al. 2000). Human T-cell lymphoma virus (HTLV) leads to NF-κB activation through expression of the Tax oncoprotein, which binds to IKKγ/NEMO and induces IKK activation (Carter et al. 2001). Tax can also activate the alternative, IKKα-dependent, NF-κB pathway (Xiao et al. 2001), but in light of what has been discussed previously, the contribution of alternative NF-κB signaling to lymphomagenesis is not entirely clear.
NF-κB IN COLITIS-ASSOCIATED CANCER: ONCOGENIC COOPERATION BETWEEN NEIGHBORS
Whereas the involvement of NF-κB and its activators in lymphomagenesis was somewhat anticipated, identifying a role for NF-κB in solid malignancies required a conviction in this possibility and the use of specialized mouse models, in which tumor induction depends on inflammation, thus mimicking inflammation-driven cancers in humans. The first such model was a mouse model for colitis-associated cancer (CAC), a type of colon cancer that appears in patients suffering from ulcerative colitis, a chronic inflammatory bowel disease. In this particular model, mice are given azoxymethane (AOM), a procarcinogen that undergoes metabolic activation in intestinal epithelial cells (IEC) and can give use to oncogenic mutations, such as those that lend to activation of β catenin (Greten et al. 2004). Although β-catenin is the most commonly activated oncogene in colon cancer (Morin et al. 1997), AOM alone gives rise to only a small number of large bowel adenomas, which can be strongly augmented through concomitant induction of colonic inflammation that in this model is elicited by repeated administration of the irritant dextrane sulfate sodium (DSS). Using the AOM and DSS model for CAC induction (Okayasu et al. 1996) and conditional disruption of the Ikkβ gene in mice, we found that IKKβ-driven NF-κB activation in IEC is essential for the development of colonic adenomas (Greten et al. 2004). The oncogenic role of NF-κB in IEC appears to be mediated through its antiapoptotic function (Lin and Karin 2003), mainly through induction of Bcl-XL, which prevents the apoptotic elimination of premalignant cells (Greten et al. 2004). In addition to its cell-autonomous function in premalignant IEC, IKKβ-driven NF-κB contributes to CAC development by acting within myeloid cells, most likely within lamina propria macrophages. Activation of NF-κB in these cells was found to stimulate the proliferation of premalignant IEC, through the secretion of growth factors (Greten et al. 2004). No effect of myeloid IKKβ on the survival of IEC was found.
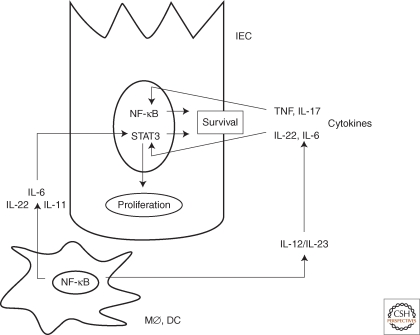
We have searched for NF-κB-dependent factors produced by lamina propria macrophages that stimulate CAC growth. As earlier experiments suggested that IL-6 produced by T cells at late stages of CAC progression enhances adenoma growth (Becker et al. 2004), we first examined the involvement of this cytokine in early tumor promotion. We confirmed that during CAC development, IL-6 is mainly produced by lamina propria macrophages and dendritic cells as initially suspected (Grivennikov et al. 2009). Most importantly, ablation of IL-6 reduced both the multiplicity and size of colonic adenomas in AOM plus DSS-treated mice (Grivennikov et al. 2009). However, unlike the ablation of IKKβ in myeloid cells, which had no effect on the survival of IEC and their premalignant derivatives (Greten et al. 2004), the IL-6 deficiency compromised IEC survival (Grivennikov et al. 2009). Both the proliferative and the survival effects of IL-6 are mediated through activation of the STAT3 transcription factor and the ablation of STAT3 in IEC dramatically compromised IEC survival and greatly reduced CAC growth (Grivennikov et al. 2009). These results suggest that some of the protumorigenic effects of NF-κB activation in myeloid cells could be caused by paracrine signaling to STAT3 in epithelial cells (Fig. 1). In addition, these results suggest that the proproliferative and prosurvival effect of myeloid cell NF-κB on premalignant IEC is predominantly mediated via other cytokines. Likely candidates are IL-22, IL-11, and EGF family members (Bollrath et al. 2009; Pickert et al. 2009). IL-12 family members also play an important role in CAC development and growth, as ablation of the gene encoding the p40 subunit, which is common to both IL-12 and IL-23, greatly diminishes CAC induction and growth (Grivennikov and Karin, unpubl.). However, the effect of these cytokines on IEC appears to be indirect, because IEC do not express IL-12/IL-23 receptors.
Figure 1.
NF-κB-dependent interactions between myeloid (MØ, DC) and intestinal epithelial cells (IEC) drive the development of colitis-associated cancer.
Activation of NF-κB in IEC results in induction of antiapoptotic genes that increase the survival of premalignant cells. In MØ, however, the activation of NF-κB results in production of cytokines, particularly IL-6, IL-11, and IL-22, which drive the proliferation of premalignant IEC. IL-6 and IL-11 exert their proliferative effect via STAT3, which further synergizes with NF-κB to increase the expression of survival genes. NF-κB also drives the production of IL-12/IL-23 cytokines, which amplify the production of prosurvival cytokines.
An alternative mode of action for myeloid cell NF-κB was suggested by Hagemann and coworkers (Hagemann et al. 2008), who found that inhibition of NF-κB activity in tumor-associated macrophages (TAM) through the conditional deletion of IKKβ re-educates these immunosuppressive and protumorigenic cells to acquire a cytotoxic, antitumorigenic phenotype. Importantly, the adoptive transfer of TAMs infected with a dominant–negative IKKβ adenovirus into mice bearing transplanted ovarian carcinomas resulted in inhibition of tumor growth, which was associated with enhanced tumoricidal activity (Hagemann et al. 2008). Thus, according to this work, another tumor promoting function exerted by IKKβ and NF-κB in TAM is the maintenance of a tumor suppressive phenotype characterized by low levels of inducible NO synthase (iNOS) and IL-12 expression, and high levels of IL-10, TNF-α, and arginase-1. Exactly how NF-κB maintains this immunosuppressive phenotype is not clear, but inhibition of NF-κB in TAMs was seen to result in up-regulation of iNOS and IL-12, leading to elevated production of tumoricidal NO and enhanced activation of NK-cell-mediated antitumor immunity, respectively (Hagemann et al. 2008). It remains to be seen whether this outcome of IKKβ inhibition in myeloid cells also contributes to the results obtained in the CAC model that were described previously, as well as those found in the liver cancer model described later, where IKKβ activation in liver myeloid cells also promotes tumor development. So far, however, inhibition of IKKβ-driven NF-κB in lamina propria myeloid cells was not found to result in increased IL-12 production (Greten et al. 2004).
THE COMPLEX ROLE OF NF-κB IN HEPATOCELLULAR CARCINOMA: LOCATION, LOCATION, LOCATION
Another inflammation-linked cancer is hepatocellular carcinoma (HCC), the most common form of liver cancer. HCC most commonly develops in the context of chronic viral hepatitis caused by either HBV or HCV infection. However, as neither virus infects mice, mouse models of HCC are not based on viral hepatitis. Nonetheless, one mouse model in which spontaneous HCC development is dependent on chronic liver inflammation is the Mdr2−/− knockout mouse, which develops hepatosteatosis caused by defective phospholipid and bile acid export (Mauad et al. 1994). Hepatosteatosis in these mice leads to low grade hepatitis, which eventually results in the development of HCC. In this model, Pikarsky and colleagues have examined the role of hepatocyte NF-κB by expressing a nondegradable form of IκBα from a doxycycline-regulated liver-specific promoter (Pikarsky et al. 2004). Inhibition of NF-κB activation in hepatocytes of Mdr2−/− mice retarded and reduced HCC development. Although the initial stimulus leading to NF-κB activation in Mdr2−/− mice has not been fully identified, it appears to be associated with a chronic inflammatory response that is propagated via paracrine TNF-α production, as treatment of these mice with a neutralizing anti-TNF-α antibody inhibits NF-κB activation in hepatocytes and decreases expression of NF-κB-dependent antiapoptotic genes. The major mechanism by which NF-κB was suggested to exert its tumor promoting function in Mdr2−/− mice is the suppression of apoptosis (Pikarsky et al. 2004). However, the published results are also consistent with a role for hepatocyte NF-κB in the maintenance of chronic inflammation in Mdr2−/− mice that is critical for tumor development.
An entirely different scenario applies to the role of NF-κB in HCC development in mice injected with the procarcinogen diethylnitrosamine (DEN). DEN undergoes metabolic activation in zone 3 hepatocytes and if injected into 2-week-old mice, it acts as a “complete” carcinogen that, unlike AOM, does not require assistance from concurrent inflammation. Nonetheless, DEN-induced HCC requires NF-κB activation in myeloid cells, in this case Kupffer cells, the resident liver macrophages (Maeda et al. 2005). As found in CAC, DEN-induced HCC requires the NF-κB-dependent production of IL-6 by Kupffer cells (Naugler et al. 2007) and the activation of STAT3 by IL-6 in hepatocytes (Yu and Karin, unpubl.) (Fig. 2). However, in a striking difference from CAC and HCC in Mdr2−/− mice, development of DEN-induced HCC is strongly enhanced by inhibition of NF-κB activation in hepatocytes through the targeted deletion of IKKβ (Maeda et al. 2005). An even more striking effect on HCC development is seen upon the conditional deletion of hepatocyte IKKγ/NEMO (Luedde et al. 2007). In this case, the “deleted” mice exhibit spontaneous liver damage and sequentially develop hepatosteatosis, hepatitis, liver fibrosis, and HCC even without any injection of a carcinogen. Enhanced chemical hepatocarcinogenesis was also observed in hepatocyte-specific p38α knockout mice (Hui et al. 2007; Sakurai et al. 2008). Mice lacking either IKKβ (IkkβΔhep) or p38α (p38αΔhep) in their hepatocytes exhibit greatly enhanced accumulation of reactive oxygen species (ROS) in zone-3 hepatocytes after DEN exposure (Maeda et al. 2005; Sakurai et al. 2008). As a result of elevated ROS accumulation, which can be prevented by oral administration of antioxidant butylated hydroxyanisol (BHA), both IkkβΔhep and p38αΔhep mice show increased hepatocyte death. However, in the liver, an organ with unusually high regenerative capacity, cell death triggers compensatory proliferation. We proposed that compensatory proliferation acts as a tumor promoter in situations in which liver tumorigenesis is driven by circles of injury and regeneration, rather than low-grade chronic inflammation, and is therefore the major cause of enhanced hepatocarcinogenesis in IkkβΔhep, p38αΔhep, and IkkγΔhep mice (Sakurai et al. 2006). Indeed, in all of these mutant-mouse strains, administration of BHA prevents liver damage and inhibits compensatory proliferation and, where tested, it fully blocks the increase in hepatocellular carcinogenesis (Maeda et al. 2005; Luedde et al. 2007; Sakurai et al. 2008). Reduced hepatocyte death, compensatory proliferation, and hepatocarcinogenesis were also seen upon crossing of IkkβΔhep mice with JNK1-deficient Jnk1−/− mice (Sakurai et al. 2006). Contrary to IKKβ, JNK1 promotes the death of DEN-exposed hepatocytes and at the same time stimulates compensatory proliferation. Furthermore, ablation of hepatocyte IKKβ results in increased JNK activity (Maeda et al. 2005) because of increased ROS accumulation (Kamata et al. 2005). Collectively, these results indicate that the major function of hepatocyte NF-κB in DEN-administered mice or even in unchallenged mice is to maintain hepatocyte survival and liver homeostasis, in part by suppressing cytotoxic ROS accumulation. In the mouse, after 2 weeks of age, most hepatocytes withdraw from the cell cycle and arrest in G0. The same applies to human liver, although in this case cell-cycle withdrawal occurs at a later time point. Carcinogen exposure in a tissue that does not undergo active proliferation, such as the uninjured liver, can not easily give rise to cancer. Therefore, any injury or an alteration that augments hepatocyte death and gives rise to compensatory proliferation will enhance HCC development. However, in the colon, IEC undergo continuous renewal and the absence of NF-κB in such cells does not further enhance cell proliferation, resulting in a net increase in cell death. Under these circumstances, increased elimination of premalignant cells is the dominant outcome of NF-κB inhibition, resulting in reduced tumorigenesis. Thus, by acting in different cells subject to different tissue kinetics, NK-κB can either enhance or suppress tumorigenesis.
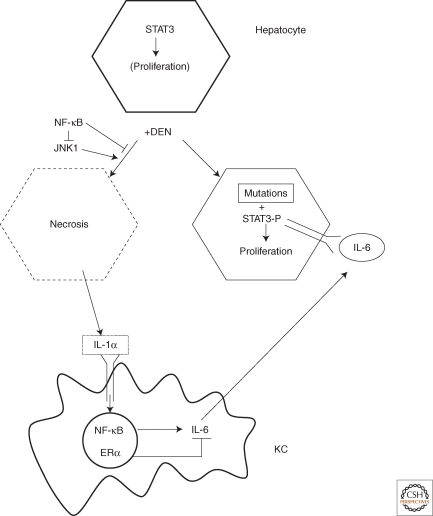
Figure 2.
NF-κB in Kupffer cells (KC) and STAT3 in hepatocytes drive the development of DEN-induced hepatocellular carcinoma (HCC).
Administration of DEN results in induction of oncogenic mutations in some hepatocytes and the necrotic death of others. Necrotic hepatocytes release IL-1α that leads to activation of NF-κB in Kupffer cells. This results in induction of IL-6, which is negatively regulated by estrogen receptor (ER)α. The IL-6 produced by Kupffer cells acts on neighboring hepatocytes to activate STAT3 and induce the expression of proliferation-promoting genes. If these cells harbor oncogenic mutations, their proliferation would eventually give rise to HCC.
Importantly, in all of the models discussed so far, inhibition of NF-κB in myeloid cells reduces tumor development. Furthermore, as found in CAC, the major protumorigenic effect of NF-κB in Kupffer cells is mediated through the induction of IL-6, which is inhibited by activation of estrogen receptor (ER)α (Naugler et al. 2007). We found that DEN administration, especially in IkkβΔhep and p38αΔhep mice, gives rise to NF-κB activation in Kupffer cells in a manner that depends on induction of hepatocyte necrosis (Sakurai et al. 2008). In this case, the primary mediator of NF-κB activation in Kupffer cells is IL-1α, which is released in large amounts by necrotic hepatocytes (Fig. 2). Importantly, mice that are deficient in IL-1 receptor or its adaptor protein MyD88 are quite refractory to DEN-induced hepatocarcinogenesis, demonstrating the importance of the IL-1α-mediated cross talk between dying hepatocytes and Kupffer cells (Sakurai et al. 2008).
Interestingly, the incidence of HCC is three to five times higher in men than in women (Bosch et al. 2004) and the same applies to DEN-induced HCC in mice (Naugler et al. 2007). As mentioned previously, production of IL-6 by Kupffer cells exposed to IL-1α or other NF-κB activators is negatively regulated by ERα. Thus, DEN-treated female mice produce less IL-6 than similarly treated male mice and contain less activated STAT3 in their hepatocytes (Naugler et al. 2007). Ablation of the Il6 gene abolishes the gender difference in HCC induction, whereas ovariectomy enhances IL-6 production and augments HCC induction in female mice (Naugler et al. 2007). It is likely that gender-specific differences in IL-6 expression also affect the incidence of human HCC, as serum IL-6 is higher after menopause (Jilka et al. 1992; Ershler and Keller 2000) and postmenopausal women display higher HCC incidence than premenopausal women (Bosch et al. 2004). Recently, elevated serum IL-6 was found to be associated with rapid progression from chronic viral hepatitis to frank HCC in a large cohort of HBV-positive patients in Hong Kong (Wong et al. 2009).
AN IKK ACTIVATION CASCADE IN PROSTATE CANCER: AN IKKβ-IKKα RELAY
Although HCC and CAC are clearly inflammation-linked cancers, there are many other cancers that rarely arise in the context of underlying inflammation or infection and yet are dependent on inflammatory processes, most of which occur as a consequence of tumor progression. One such cancer is prostate cancer (CaP), which is the most common malignancy in older men. We have used the TRAMP mouse in which CaP development and progression are driven by expression of SV40 T antigen in prostate epithelial cells (Greenberg et al. 1995; Gingrich et al. 1996) to study the role of IKK signaling in prostate tumorigenesis. We first examined whether deletion of IKKβ in prostate epithelial cells has any effect on CaP development and found no effect whatsoever, neither on tumor development and progression, nor on the development of androgen-independent (AI) cancer after castration (Ammirante et al., in prep.). The latter results were surprising, as AI CaP usually exhibits activated NF-κB (Gasparian et al. 2002). These findings led us to examine whether IKKβ in hematopoietic-derived cells has a role in CaP development. Although deletion of IKKβ in the hematopoietic compartment had no effect on development and progression of primary CaP in TRAMP mice, it slowed down the development of AI CaP after castration and inhibited the appearance of metastases (Ammirante et al., in prep.). Similar results were obtained in a different model based on subcutaneous implantation of the androgen-dependent (AD) mouse CaP cell line, Myc-CaP. In this case, the tumors were allowed to grow to a size of 1000 mm3 before castration of the hosts, which subjects the tumor to androgen deprivation, causing near complete regression because of necrotic and apoptotic death of CaP cells, which depend on androgens (testosterone) for survival. However, through mechanisms that are not entirely understood, almost as soon as the original AD CaP disappear, an AI tumor starts growing, as is often the case in prostate cancer patients undergoing androgen ablation therapy. Silencing of endogenous IKKβ in Myc-CaP cells had no effect on their primary tumorigenic growth, regression upon castration, and regrowth as AI CaP. However, deletion of IKKβ in bone-marrow-derived cells (BMDC) of the host substantially slowed down the regrowth of AI CaP in castrated tumor-bearing mice (Ammirante et al., in prep.). A similar delay in the regrowth of AI CaP was seen upon treatment of tumor-bearing hosts with IKKβ inhibitors. As found in both the CAC and HCC models described above, IKKβ ablation in BMDC-inhibited STAT3 activation in CaP cells and a STAT3 inhibitor slowed down the emergence of AI CaP (Ammirante et al., in prep.).
Curiously, the development of AI CaP is associated with the accumulation of activated IKKα in nuclei of CaP cells (Ammirante et al., in prep.). Accumulation of nuclear IKKα was previously found to be linked with and necessary for metastatic progression of CaP in TRAMP mice (Luo et al. 2007). Furthermore, accumulation of nuclear IKKα correlated with progression and clinical grade in human CaP (Luo et al. 2007). Importantly, IKKβ in BMCD is required for activation of nuclear IKKα in CaP cells through the production of IKKα-activating cytokines. The silencing of IKKα in Myc-CaP cells delays the emergence of AI CaP as effectively as the inhibition of IKKβ does.
The mechanism by which nuclear IKKα contributes to the growth of AI CaP remains to be determined, but previous studies on metastatic progression in TRAMP mice revealed that nuclear IKKα enhances metastatic progression by repressing transcription of the metastasis inhibitor maspin (Luo et al. 2007). Although the details of maspin repression by IKKα are not fully known, it is clear that it does not involve activation of either canonical or noncanonical NF-κB signaling (Luo et al. 2007). Repression of maspin requires the kinase activity of IKKα, suggesting that it is exerted through the phosphorylation of another protein, possibly a component of chromatin, which is involved in the regulation of maspin transcription. It remains to be seen whether repression of maspin contributes to the emergence of AI CaP, but maspin was shown to have antiproliferative and proapoptotic activities (Lockett et al. 2006).
Concurrent with the death of AD CaP, androgen withdrawal results in massive inflammatory infiltration of the tumor remnant. Most immune and inflammatory cell types are present within the regressing tumor transiently and only B cells are the ones that remain within the newly emerging AI CaP for at least 3 weeks after castration (Ammirante et al., in prep.). Curiously, we found that about 90% of human prostate tumors, but not normal, hyperplastic, or malignant tissue, contain B cells as well. Most importantly, B cells, but not T cells, were found to be required for the rapid emergence of AI tumors as well as for IKKα and STAT3 activation in CaP cells of such tumors (Ammirante et al., in prep.). Most likely, B cells are required for production of cytokines that lead to IKKα and STAT3 activation within newly emerging AI CaP cells (Fig. 3). The activation of IKKα and STAT3 is likely to be required for the survival and proliferation of these cells.
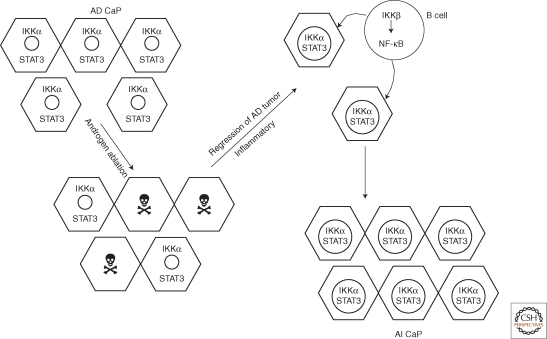
Figure 3.
IKKβ–IKKα cross talk drives the development of androgen-independent (AI) prostate carcinoma (CaP).
In androgen-dependent (AD) CaP, IKKα, and STAT3 are not activated and are located in the cytosol. Androgen ablation results in the death of most AD CaP cells. In response to the release of inflammatory mediators by the dying AD CaP cells, inflammatory and immune cells, including B cells, are recruited into the tumor remnant. In these tumor-infiltrating B cells, IKKβ is activated, resulting in the production of NF-κB-dependent cytokines that lead to activation of STAT3 and nuclear translocation of activated IKKα in remaining CaP cells. STAT3 and IKKα promote the survival of cells that have become independent of androgens, leading to the development of AI CaP. Because of the presence of nuclear IKKα, AI CaP is often metastatic.
In summary, the findings described previously indicate that even a cancer whose development is not associated with an underlying inflammatory condition depends on an NF-κB-regulated inflammatory response. In the case of prostate cancer, tumor-associated inflammation is part of normal progression, but can also be elicited and accelerated as a result of therapy (in this case, androgen ablation)-induced death of the primary tumor. It is possible that in both cases, the localized tumor-associated inflammatory response is triggered by the necrotic death of malignant cells, either as the result of hypoxia during normal progression or as the consequence of therapeutic intervention. In the prostate cancer models described previously, the inflammatory response elicited by androgen deprivation is a major contributor to the emergence of AI CaP. The dependence of this response on IKKβ, B cells, STAT3, and IKKα (Fig. 3) suggests that therapeutic interventions targeting any of these four elements may be used to improve the outcome of androgen ablation therapy and delay the appearance of AI CaP.
CONCLUSIONS AND TRANSLATIONAL IMPLICATIONS
In the three types of epithelial cancers described previously, inactivation of IKKβ in premalignant tumor progenitors or in the neoplastic cell itself can either inhibit tumor development (CAC), enhance tumor development (HCC), or have no discernable effect (CaP). However, in all three cancers, the inactivation or inhibition of IKKβ in cells of the hematopoietic compartment (lamina propria macrophages, Kupffer cells, or B cells) inhibits tumor growth and progression. We assume that the tumor-promoting effect of IKKβ in such cells is mediated via NF-κB and at least in two cases (CAC and HCC), we know that it depends, at least in part, on the production of the NF-κB regulated cytokine IL-6. In all three cases, NF-κB signaling in cells of the hematopoietic compartment results in activation of STAT3, a transcription factor that controls the expression of proliferative and survival genes in premalignant cells and their fully neoplastic derivatives. In one case (CaP), IKKβ signaling in tumor-infiltrating lymphocytes results in the accumulation of activated IKKα in cancer cell nuclei. Thus, it can be generalized that IKKβ-dependent signaling to NF-κB in tumor-associated inflammatory and immune cells results in the production of cytokines that activate signaling pathways that stimulate the proliferation and enhance the survival of malignant carcinoma cells.
These findings suggest that regardless of the direct effect of NF-κB on the survival of neoplastic cells, IKKβ inhibition can be used to slow down tumor growth and enhance susceptibility to cytocidal therapeutics (e.g., genotoxic chemicals, microtubule disruptors, ionizing radiation, and apoptosis-inducing cytokines). In this regard, it is important to realize that the mere disruption of NF-κB or STAT3 signaling does not lead to cell death. Hence, the requirement for classical cytocidal therapy for which IKKβ inhibitors can serve as adjuvants.
It should also be recognized that unless the cytocidal agent being used in conjunction with an IKKβ or another NF-κB inhibitor has a wide safety margin, the systemic inhibition of NF-κB function may result in enhanced toxicity to normal cells and tissues. Two ways to circumvent this problem are: (a) direct the IKKβ or NF-κB inhibitor to the relevant inflammatory or immune cell type; and (b) direct the cytocidal therapy to the neoplastic cell. Another potential complication of IKKβ or NF-κB inhibition is long-lasting immune suppression and unpredictable effects on the inflammatory response. For instance, we have found that instead of reducing sepsis-induced inflammation, systemic administration of a specific IKKβ inhibitor or the ablation of IKKβ in myeloid cells resulted in greatly enhanced inflammation and mortality driven by elevated IL-1β production in mice infected with bacteria or challenged with endotoxin (Greten et al. 2007). Thus, a great deal of caution needs to be exerted during clinical development of IKKβ inhibitors as adjuvants to chemotherapy or radiation. Limiting the duration of treatment with an IKKβ inhibitor to a short period preceding or concurrent with cytocidal therapy may provide a solution to this potential complication.
Given the complexities and unpredictable nature of anti-IKKβ therapy, it is worthwhile considering a more specific approach that targets the IKKβ-dependent cytokine responsible for tumor growth and survival. For instance, in the case of HCC and CAC, it may make sense to target IL-6 by the use of anti-IL-6 receptor antibodies. Although the cytokines that are critical for the growth of AI CaP and its metastatic spread remain to be identified, our initial analysis suggests that they may be RANK ligand (RANKL) or lymphotoxin (LT) (Luo et al. 2007). As both anti-RANKL and anti-LT therapeutics have already been developed for other indications, it is worthwhile to assess their effect on the emergence of AI CaP and its progression into metastatic disease, first in animal models and if positive, in prostate cancer patients receiving androgen ablation therapy.
In summary, much has been learned about the role of inflammation and inflammatory processes in the pathogenesis of cancer by studying the oncogenic functions of NF-κB. It is our hope that the next stage in this long endeavor would see this basic knowledge arriving at the bedside to result in new and improved cancer therapies and preventive agents.
Footnotes
Editors: Louis M. Staudt and Michael Karin
Additional Perspectives on NF-κB available at www.cshperspectives.org
REFERENCES
- Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, et al. 2007. Frequent engagement of the classical and alternative NF-κB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell 12:115–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill F, Mantovani A 2001. Inflammation and cancer: Back to Virchow? Lancet 357:539–545 [DOI] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al. 2004. TGF-β suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 21:491–501 [DOI] [PubMed] [Google Scholar]
- Beg AA, Baltimore D 1996. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274:782–784 [DOI] [PubMed] [Google Scholar]
- Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, Sjostrom SK, Garraway LA, Weremowicz S, Richardson AL, et al. 2007. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell 129:1065–1079 [DOI] [PubMed] [Google Scholar]
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. 2009. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15:91–102 [DOI] [PubMed] [Google Scholar]
- Bosch FX, Ribes J, Diaz M, Cleries R 2004. Primary liver cancer: Worldwide incidence and trends. Gastroenterology 127:S5–S16 [DOI] [PubMed] [Google Scholar]
- Bours V, Franzoso G, Azarenko V, Park S, Kanno T, Brown K, Siebenlist U 1993. The oncoprotein Bcl-3 directly transactivates through κ B motifs via association with DNA-binding p50B homodimers. Cell 72:729–739 [DOI] [PubMed] [Google Scholar]
- Cabannes E, Khan G, Aillet F, Jarrett RF, Hay RT 1999. Mutations in the IkBa gene in Hodgkin’s disease suggest a tumour suppressor role for IκBα. Oncogene 18:3063–3070 [DOI] [PubMed] [Google Scholar]
- Carter RS, Geyer BC, Xie M, Acevedo-Suarez CA, Ballard DW 2001. Persistent activation of NF-κ B by the tax transforming protein involves chronic phosphorylation of IκB kinase subunits IKKβ and IKKγ. J Biol Chem 276:24445–24448 [DOI] [PubMed] [Google Scholar]
- Davis RE, Brown KD, Siebenlist U, Staudt LM 2001. Constitutive nuclear factor κB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med 194:1861–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos AG, Young LS 2001. LMP1 structure and signal transduction. Semin Cancer Biol 11:435–444 [DOI] [PubMed] [Google Scholar]
- Ershler WB, Keller ET 2000. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med 51:245–270 [DOI] [PubMed] [Google Scholar]
- Franzoso G, Bours V, Park S, Tomita-Yamaguchi M, Kelly K, Siebenlist U 1992. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-κB-mediated inhibition. Nature 359:339–342 [DOI] [PubMed] [Google Scholar]
- Gasparian AV, Yao YJ, Kowalczyk D, Lyakh LA, Karseladze A, Slaga TJ, Budunova IV 2002. The role of IKK in constitutive activation of NF-κB transcription factor in prostate carcinoma cells. J Cell Sci 115:141–151 [DOI] [PubMed] [Google Scholar]
- Gilmore TD 2003. The Re1/NF-κ B/I κ B signal transduction pathway and cancer. Cancer Treat Res 115:241–265 [PubMed] [Google Scholar]
- Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, Angelopoulou R, Rosen JM, Greenberg NM 1996. Metastatic prostate cancer in a transgenic mouse. Cancer Res 56:4096–4102 [PubMed] [Google Scholar]
- Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM 1995. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci 92:3439–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, et al. 2007. NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell 130:918–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M 2004. IKKβ links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118:285–296 [DOI] [PubMed] [Google Scholar]
- Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H, Eckmann L, et al. 2009. IL-6 and stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15:103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann M, O’Reilly LA, Gugasyan R, Strasser A, Adams JM, Gerondakis S 2000. The anti-apoptotic activities of Rel and RelA required during B-cell maturation involve the regulation of Bcl-2 expression. EMBO J 19:6351–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugasyan R, Grumont R, Grossmann M, Nakamura Y, Pohl T, Nesic D, Gerondakis S 2000. Rel/NF-κB transcription factors: Key mediators of B-cell activation. Immunol Rev 176:134–140 [DOI] [PubMed] [Google Scholar]
- Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR 2008. “Re-educating” tumor-associated macrophages by targeting NF-κB. J Exp Med 205:1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ 2001. Blockade of NF-κB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 20:4188–4197 [DOI] [PubMed] [Google Scholar]
- Hui L, Bakiri L, Mairhorfer A, Schweifer N, Haslinger C, Kenner L, Komnenovic V, Scheuch H, Beug H, Wagner EF 2007. p38α suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet 39:741–749 [DOI] [PubMed] [Google Scholar]
- Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, Abrams JS, Boyce B, Broxmeyer H, Manolagas SC 1992. Increased osteoclast development after estrogen loss: Mediation by interleukin-6. Science 257:88–91 [DOI] [PubMed] [Google Scholar]
- Joyce D, Albanese C, Steer J, Fu M, Bouzahzah B, Pestell RG 2001. NF-κB and cell-cycle regulation: The cyclin connection. Cytokine Growth Factor Rev 12:73–90 [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M 2005. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120:649–661 [DOI] [PubMed] [Google Scholar]
- Karin M 2006. Nuclear factor-κB in cancer development and progression. Nature 441:431–436 [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW 2002. NF-κB in cancer: From innocent bystander to major culprit. Nat Rev Cancer 2:301–310 [DOI] [PubMed] [Google Scholar]
- Keats JJ, Fonseca R, Chesi M, Schop R, Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, et al. 2007. Promiscuous mutations activate the noncanonical NF-κB pathway in multiple myeloma. Cancer Cell 12:131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SA, Schattner EJ, Cesarman E 2000. Inhibition of NF-κB induces apoptosis of KSHV-infected primary effusion lymphoma cells. Blood 96:2537–2542 [PubMed] [Google Scholar]
- Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, Dave SS, Zhao H, Xu W, Rosenwald A, et al. 2008. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science 319:1676–1679 [DOI] [PubMed] [Google Scholar]
- Lin A, Karin M 2003. NF-κB in cancer: A marked target. Semin Cancer Biol 13:107–114 [DOI] [PubMed] [Google Scholar]
- Liu L, Eby MT, Rathore N, Sinha SK, Kumar A, Chaudhary PM 2002. The human herpes virus 8-encoded viral FLICE inhibitory protein physically associates with and persistently activates the Iκ B kinase complex. J Biol Chem 277:13745–13751 [DOI] [PubMed] [Google Scholar]
- Liu ZG, Hsu H, Goeddel DV, Karin M 1996. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-κB activation prevents cell death. Cell 87:565–576 [DOI] [PubMed] [Google Scholar]
- Lockett J, Yin S, Li X, Meng Y, Sheng S 2006. Tumor suppressive maspin and epithelial homeostasis. J Cell Biochem 97:651–660 [DOI] [PubMed] [Google Scholar]
- Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M 2007. Deletion of NEMO/IKKγ in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 11:119–132 [DOI] [PubMed] [Google Scholar]
- Luftig M, Yasui T, Soni V, Kang MS, Jacobson N, Cahir-McFarland E, Seed B, Kieff E 2004. Epstein-Barr virus latent infection membrane protein 1 TRAF-binding site induces NIK/IKK α-dependent noncanonical NF-κB activation. Proc Natl Acad Sci 101:141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M 2007. Nuclear cytokine-activated IKKα controls prostate cancer metastasis by repressing Maspin. Nature 446:690–694 [DOI] [PubMed] [Google Scholar]
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M 2005. IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121:977–990 [DOI] [PubMed] [Google Scholar]
- Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, van den Bergh Weerman MA, Verkruisen RP, Groen AK, Oude Elferink RP, et al. 1994. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol 145:1237–1245 [PMC free article] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW 1997. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275:1787–1790 [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M 2007. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317:121–124 [DOI] [PubMed] [Google Scholar]
- Neri A, Chang CC, Lombardi L, Salina M, Corradini P, Maiolo AT, Chaganti RS, Dalla-Favera R 1991. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-κ B p50. Cell 67:1075–1087 [DOI] [PubMed] [Google Scholar]
- Ngo VN, Davis RE, Lamy L, Yu X, Zhao H, Lenz G, Lam LT, Dave S, Yang L, Powell J, et al. 2006. A loss-of-function RNA interference screen for molecular targets in cancer. Nature 441:106–110 [DOI] [PubMed] [Google Scholar]
- Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S 1996. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut 39:87–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M, Schmidt-Supprian M, Rajewsky K 2002. IκB kinase signaling is essential for maintenance of mature B cells. J Exp Med 196:743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr H-A, Hirth S, Weigmann B, Wirtz S, et al. 2009. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med 206:1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y 2004. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature 431:461–466 [DOI] [PubMed] [Google Scholar]
- Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M 2008. Hepatocyte necrosis induced by oxidative stress and IL-1 α release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell 14:156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Maeda S, Chang L, Karin M 2006. Loss of hepatic NF-κ B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci 103:10544–10551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, et al. 2001. Activation by IKKα of a second, evolutionary conserved, NF-κ B signaling pathway. Science 293:1495–1499 [DOI] [PubMed] [Google Scholar]
- Thornburg NJ, Kulwichit W, Edwards RH, Shair KH, Bendt KM, Raab-Traub N 2006. LMP1 signaling and activation of NF-κB in LMP1 transgenic mice. Oncogene 25:288–297 [DOI] [PubMed] [Google Scholar]
- Uren AG, O’Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EV, Dixit VM 2000. Identification of paracaspases and metacaspases: Two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol Cell 6:961–967 [DOI] [PubMed] [Google Scholar]
- Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M 2008. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-κB signaling. Nat Immunol 9:1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM 1996. Suppression of TNF-α-induced apoptosis by NF-κB. Science 274:787–789 [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS Jr 1996. TNF- and cancer therapy-induced apoptosis: Potentiation by inhibition of NF-κB. Science 274:784–787 [DOI] [PubMed] [Google Scholar]
- Willis TG, Jadayel DM, Du MQ, Peng H, Perry AR, Abdul-Rauf M, Price H, Karran L, Majekodunmi O, Wlodarska I, et al. 1999. Bcl10 is involved in t(1;14) (p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 96:35–45 [DOI] [PubMed] [Google Scholar]
- Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES, Ng EK, Chan FK, Sung JJ, Chan HL 2009. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer 124:2766–2770 [DOI] [PubMed] [Google Scholar]
- Xiao G, Cvijic ME, Fong A, Harhaj EW, Uhlik MT, Waterfield M, Sun SC 2001. Retroviral oncoprotein Tax induces processing of NF-κB2/p100 in T cells: Evidence for the involvement of IKKα. EMBO J 20:6805–6815 [DOI] [PMC free article] [PubMed] [Google Scholar]