Abstract
In untreated smokers, exposure to cigarette-related cues increases both the intensity of cigarette craving and relative glucose metabolism of the perigenual/ventral anterior cingulate cortex (ACC). Given that treatment with bupropion HCl reduces overall cigarette craving levels in nicotine dependent subjects, we performed a preliminary study of smokers to determine if bupropion HCl treatment attenuates cue-induced cigarette craving and associated brain metabolic activation. Thirty-seven, otherwise healthy smokers (20 untreated and 17 who had received open-label treatment with bupropion HCl) underwent two 18F-fluorodeoxyglucose positron emission tomography scanning sessions in randomized order—one when presented with neutral cues and the other when presented with cigarette-related cues. Bupropion-treated smokers had smaller cigarette cue-induced increases in craving scores on the Urge to Smoke (UTS) Scale and less activation of perigenual/ventral ACC metabolism from the neutral to the cigarette cue scan than untreated smokers. Thus, in addition to its known effects on spontaneous cigarette craving and withdrawal symptoms, bupropion HCl diminishes cue-induced cigarette craving and appears to attenuate cigarette cue-induced ACC activation. These results are consistent with the known effects of bupropion HCl, including its enhancement of catecholaminergic neurotransmission.
Keywords: Positron emission tomography, Nicotine dependence, Brain metabolism, Anterior cingulate cortex, Orbitofrontal cortex, Bupropion hydrochloride
1. Introduction
In cigarette smokers attempting abstinence, craving is associated with relapse into usage (Niaura et al., 1989b; Swan et al., 1996; Killen and Fortmann, 1997; Catley et al., 2000). Craving for cigarettes occurs naturally within the first several hours of abstinence in nicotine dependent smokers (Schuh and Stitzer, 1995; Jarvik et al., 2000), and can also be elicited reliably in the laboratory through exposure to cigarette-related cues (Sayette and Hufford, 1994; Droungas et al., 1995; Mucha et al., 1998; Morgan et al., 1999; Mucha et al., 1999; Conklin et al., 2000; Taylor et al., 2000; Brody et al., 2002). Using positron emission tomography (PET), our group recently reported that exposure to cigarette-related cues results in regional brain metabolic activation of the perigenual/ventral anterior cingulate cortex (ACC) in untreated smokers (Brody et al., 2002). We also found positive correlations between intensity of cigarette craving and relative glucose metabolism in the orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), and anterior insula. These findings are consistent with brain imaging studies of exposure to cues for dependent drugs other than nicotine, in which ACC activation and similar correlations between craving and brain activity have been reported (Grant et al., 1996; Maas et al., 1998; Childress et al., 1999; Volkow et al., 1999; Garavan et al., 2000; Sell et al., 2000; Daglish et al., 2001; Kilts et al., 2001; Wexler et al., 2001; Bonson et al., 2002). While the brain structures that are associated with craving in untreated substance-dependent subjects have been described, no one has yet reported the effects of standardized treatment on regional brain activation associated with exposure to drug-related cues. A better understanding of the neural substrates of medication effects on cue-induced cigarette craving may, in the future, lead to improved treatments for nicotine dependence.
Bupropion HCl is an effective, first-line medication for nicotine dependence (Hurt et al., 1997; Jorenby et al., 1999; Fiore et al., 2000). In addition to its overall efficacy for promoting smoking abstinence, treatment with bupropion HCl results in decreased overall levels of craving (Durcan et al., 2002) and other symptoms of nicotine withdrawal, such as depression and irritability (Shiffman et al., 2000). Despite the widespread use of bupropion HCl for nicotine dependence, no study to date has reported the effects of this medication on brain function in subjects with nicotine dependence.
In this preliminary study, we compared bupropion-treated with untreated smokers in both clinical and brain metabolic responses to cigarette-related (compared with neutral) cues. We also investigated associations between cigarette craving and regional brain metabolism in bupropion-treated subjects to determine if brain regions that mediate craving in untreated smokers also mediate craving in treated smokers. We hypothesized that (1) smokers treated with bupropion HCl would have less overall and cue-induced craving than untreated smokers; (2) bupropion-treated smokers would have less cue-induced metabolic activation of the perigenual/ventral ACC [only the ACC was studied here because it is the only region consistently found to activate in prior drug craving studies and in our previous study of brain mediation of cigarette craving (Brody et al., 2002)]; and (3) positive correlations between intensity of craving and regional metabolism in the OFC, DLPFC, and anterior insula (found in untreated smokers) would remain intact in bupropion-treated smokers.
2. Materials and methods
2.1. Subjects
Thirty-seven otherwise healthy smokers (≥ 20 cigarettes/d) were recruited through local newspaper advertisements. Twenty subjects had no treatment for smoking cessation prior to PET scanning and results from that group are reported elsewhere (Brody et al., 2002), while 17 subjects underwent short-term standardized treatment with bupropion HCl (as described below) prior to PET scanning. Subjects were adults (21–65 years of age) and were screened initially during a telephone interview in which medical, psychiatric, and substance abuse histories were obtained. All subjects passing this initial interview were then assessed in person using screening questions from the Structured Clinical Interview for DSM-IV (First et al., 1995). Participating subjects met DSM-IV criteria for nicotine dependence, and were excluded for any history of or current Axis I psychiatric diagnosis including mood, anxiety, or psychotic disorders, or history of substance abuse or dependence other than nicotine dependence. Subjects were also excluded if they were currently taking medication or had any history of or current medical condition that might affect the central nervous system at the time of scanning (e.g. current treatment with a beta blocker or analgesic medication, or history of head trauma or epilepsy). Pregnancy was an exclusion criterion due to the potential risk of radiation exposure to a fetus. Subjects who occasionally used alcohol, caffeine, or other drugs, but did not meet criteria for dependence were allowed to participate in the study, but were instructed to abstain from these substances for 24 h prior to scanning (72 h for marijuana; verified by urine toxicology screen, if subjects reported a history of drug use). Subjects who drank more than the equivalent of two cups of coffee per day (200–300 mg caffeine/d) were excluded, as were subjects who experienced caffeine withdrawal symptoms (such as irritability, flushing, or headache) associated with cessation of caffeine intake. There was partial temporal overlap between scanning of the two study groups, with the treated group being scanned during and after the untreated group. One additional subject completed bupropion treatment, but a major depressive episode emerged when he quit smoking (a known possibility during smoking cessation attempts) (Glassman, 1993; Tsoh et al., 2000). Therefore, this subject’s data were not used in the present study.
2.2. General experimental design
Both untreated and treated smokers underwent two 18F-fluorodeoxyglucose (FDG) PET scanning sessions roughly 7–10 days apart, in randomized order, as described in our previous report on the untreated group alone (Brody et al., 2002). In one PET session, the subject was presented with neutral cues; and in the other, the subject was presented with cigarette-related cues. Subjects also underwent a magnetic resonance imaging (MRI) scan in the interval between PET scans (as described below).
All subjects were interviewed initially to determine smoking history, including current usage, periods of abstinence, and usage levels during each 5-year period of their lives. The study was described to subjects at the initial visit, and written informed consent was obtained using a form approved by the Veterans Affairs Greater Los Angeles Healthcare System Institutional Review Board. Subjects in the treatment group were provided medication free of charge, and all subjects were compensated $20 per hour for brain scanning time.
2.3. Standardized bupropion HCl treatment
For treated subjects, an exhaled carbon monoxide (CO) level (Bedfont EC-50 Microsmokerlyzer II) was obtained at the initial visit as a rough measure of recent cigarette use. These subjects were then started on a standardized course of bupropion HCl Sustained Release (SR) formula. They received a supply of this medication for 1 week, and were instructed to start on one pill per day (150 mg), with the dosage increased to one pill twice daily after 3 days of treatment. Subjects were instructed to have a target quit date of 8 days after initiation of treatment. They were also advised of potential side effects of bupropion, including headache, anxiety, insomnia, dry mouth, and nausea (Jorenby et al., 1999; Settle et al., 1999). Participants were instructed not to seek complementary interventions (e.g. counseling or medication) outside of the study.
Subjects met weekly with the P.I. for medication management visits (15 min), which consisted of distribution of medication to the subject, titration of dosage, discussion of medication compliance, review of side effects, and monitoring of cigarette usage. To isolate the effects of bupropion HCl, no counseling for cessation of tobacco use took place during these visits. Treatment was provided for a minimum of 4 weeks prior to PET and MRI scanning (mean 5.6 [± 1.4] weeks).
2.4. Rating scales, craving induction, and PET image acquisition
PET sessions commenced between 13:00 and 16:00 h, and subjects were instructed to smoke as per their current habit until the time of each scanning session. The Hamilton Depression (Hamilton, 1967) and Anxiety (Hamilton, 1969) Rating scales (HAM-D and HAM-A) were then administered to all subjects, along with the Shiffman–Jarvik Scale (Shiffman and Jarvik, 1976), to measure cigarette withdrawal symptoms. Immediately prior to injection of FDG, exhaled CO levels were measured, to approximately quantify recent cigarette use. This rating scale period (including CO monitoring) lasted 30 min so that cue exposure began when smokers would be expected to have mild craving that could either be stimulated by cigarette-related cues or unaffected by neutral cues, and that would not to be confounded by the acute effects of nicotine intake.
Subjects then received an injection of 10 mCi FDG over a 30-s period into the right antecubital vein, while seated 100 cm in front of a video monitor in a room adjacent to the PET camera. During the subsequent 30-min uptake period of FDG, subjects either watched the cigarette cue video and held one of their own cigarettes in the dominant hand, or watched the neutral (nature video) and held a neutral object (pen) in the dominant hand. The videos for this study consisted primarily of material that elicited differing intensities of cigarette craving in smokers in earlier work (Droungas et al., 1995). The cigarette-cue video depicts a man and woman smoking in a variety of situations (e.g. during breakfast, while waiting for a bus, sitting at a desk) on a day when the man is going on a job interview. The neutral video presented educational material about birds. Because these videos did not last for the full 30-min uptake period, additional videotaped material from our laboratory was added to the original videos. For the cigarette-cue condition, the remaining video depicted writing a letter while smoking a cigarette with the camera positioned to provide the first person point of view. In this portion of the video, a cigarette is lit on camera and a lighter and ashtray are seen. For the neutral video, additional educational material about nature was shown.
During the FDG-uptake period, the Urge to Smoke (UTS) Scale (Jarvik et al., 2000) was administered every 10 min. This scale consists of 10 craving-related items with analog ratings (0 = definitely not to 6 = definitely) and takes approximately 1 min to complete, with increased urges indicated by higher values (Sweeney et al., 1996).
After the 30-min uptake period, emission scanning commenced. A 1-min transmission scan was obtained for positioning, using a 68Ge external source, followed by a 40-min emission scan. PET images were acquired with the ECAT 953 tomograph (CTI-Siemens, Knoxville, TN) with 31 slices in the two-dimensional mode, in a dimly lit, quiet room. The average transaxial resolution was 5–6 mm FWHM, plane spacing 3.125 mm, and axial FOV 10.8 cm. Reconstruction was performed using filtered back-projection with an automatically computed attenuation correction. Immediately after each PET session and at a 1-week follow-up visit, subjects were interviewed to determine whether cue exposure resulted in a worsening of nicotine dependence.
2.5. MRI scanning, PET-to-MRI realignment, and region of interest drawing
The MRI scan had the following specifications: three-dimensional Fourier-transform spoiled-gradient-recalled acquisition with TR = 30 ms, TE = 7 ms, 30° angle, 2 acquisitions, 256×192 view matrix. The acquired volume was reconstructed as roughly 90 contiguous, 1.5-mm thick transaxial slices.
MRI and PET scans were retrieved onto a SUN Ultra one Workstation (SUN Microsystems, Mountain View, CA), and were coregistered for more precise localization of hand-drawn regions of interest. PET-to-MRI coregistration was performed using MEDx 3.3 (Sensor Systems Inc, Sterling, VA), a UNIX-based medical image visualization and analysis program. Within MEDx, the Automated Image Registration method was used for PET-to-MRI coregistration (Woods et al., 1993).
Four regions of interest (ROIs) were drawn bilaterally on MRI and transferred onto coregistered PET scans (Fig. 1). Based on a priori hypotheses derived from the literature cited above, these regions were the ventral ACC, OFC, DLPFC, and anterior insula. The ventral half of the ACC was drawn in approximately 11 planes. The OFC, consisting of the medial and lateral orbital gyri, was drawn in approximately 14 planes. The DLPFC consisted of the dorsal portion of the middle frontal gyrus, and was drawn in approximately 11 planes. The anterior insula region was drawn as the anterior half of insular cortex in approximately 17 planes. Whole brain was also drawn so that ratios of ROI/global metabolism could be calculated for statistical analysis.
Fig. 1.
Regions of interest drawn on a study subject’s magnetic resonance image for transfer onto co-registered PET scans. Images are presented from dorsal (top) to ventral (bottom). DLPFC = dorsolateral prefrontal cortex; ACC = anterior cingulate cortex; Ant = anterior; OFC = orbitofrontal cortex.
2.6. Statistical analyses
Mean (± standard deviation) scores for all clinical rating scales were determined for the neutral-and cigarette-cue scans within the untreated and bupropion-treated groups. To determine similarities and differences between the untreated and bupropion-treated groups, Student’s t-tests were performed for clinical ratings at the time of scanning (mean cigarettes/d, exhaled CO levels, HAM-D, HAM-A, Shiffman–Jarvik Scale, UTS scale, and difference between the neutral and cigarette-cue scan in UTS scale).
For analyzing PET data, separate statistical analyses were performed using the computer software program Statistical Parametric Mapping (SPM99) and hand drawn ROIs. Both methods were used to examine differences between groups in regional brain activation from the neutral- to the cigarette-cue conditions (group-by-condition interaction), and to determine associations between subjective craving and regional brain metabolism for the treated group (associations between craving and regional metabolism were reported previously for the untreated group (Brody et al., 2002)). Results from both methods were used and compared, given the limitations of each (Steinmetz and Seitz, 1991; Friston et al., 1994; Nadeau and Crosson, 1995; Rajkowska and Goldman-Rakic, 1995). Thus, four primary analyses were performed – SPM and ROI for group-by-condition interactions, and SPM and ROI for associations between craving and normalized metabolism in the treated group.
For the SPM analysis of group-by-condition interaction, the Z-statistic was determined to identify regions that had significantly different between-group activations in response to cigarette-related (compared with neutral) cues. Within-group analyses were also performed with SPM to assess effect of condition, in order to determine which group changes accounted for the interactions in the preceding analysis. A significance threshold of P<0.005 (Z>2.72), uncorrected, was used for this analysis, along with a minimum extent threshold of 25 voxels. This threshold is similar or identical to those used in other published studies using PET and SPM to examine drug craving (Sell et al., 2000; Kilts et al., 2001; Bonson et al., 2002; Brody et al., 2002). Locations of significant voxels were determined by mapping coordinates onto group mean images of study PET and MRI scans, the PET template accompanying the SPM program, and a standard atlas (Talairach and Tournoux, 1988). The mapping of coordinates onto the mean PET scan of actual study subjects is reported here if discrepancies were found between these localization methods. Results are presented using the voxel of peak significance.
For the ROI analysis of group-by-condition interaction, Student’s t-tests were performed for the a priori regions (left and right ventral ACC), with change in normalized metabolic rate (ROI/global) as the variable of interest. Identical statistical tests were performed using the ROIs drawn for the correlational analysis (below) as control regions. The statistical criterion for ROI tests of the two a priori regions and six control regions (left and right OFC, DLPFC, and anterior insula) was P<0.05 (two-tailed), a threshold similar or identical to other hypothesis-driven PET analyses in this line of research (Grant et al., 1996; Childress et al., 1999; Volkow et al., 1999).
Based on significant results from the group-by-condition analyses, a secondary analysis was performed using SPM and ROIs and similar statistical methodology. As an approximate measure of resting differences in ACC metabolism between untreated and bupropion-treated smokers, a comparison of normalized ACC metabolism during the neutral cue scans was performed between untreated and bupropion-treated smokers (effect of group).
To determine associations between craving and regional metabolism in the bupropion-treated group, we examined the within-subject dependence of relative regional metabolism on craving (UTS score), using SPM99 and ROIs in a similar manner to our previous report on untreated smokers (Brody et al., 2002). For the SPM analysis, an ANCOVA was performed with UTS score as the covariate. Using the SPM option of multi-subject covariate only, this design determined the main effect of covariate within the bupropion-treated group, with voxels considered significant at P<0.005, uncorrected for multiple comparisons. For the ROI analysis, this relationship was determined with Pearson correlation coefficients between change in UTS score and change in normalized ROI value from the neutral to the cigarette-cue scans for the three hypothesized ROIs (OFC, DLPFC and anterior insula) bilaterally. The significance threshold for ROI calculations was P<0.05 (two-tailed).
3. Results
3.1. Sample characteristics and clinical results
Prior to treatment, the bupropion-treated and untreated groups were similar in age, number of cigarettes smoked per day, number of years smoking, and exhaled CO levels (Student’s t test, all P>0.05) (Table 1). The groups were also similar in sex, handedness, and interval between scans. HAM-D and HAM-A scores were low overall, and were similar between groups (see Table 1, Student’s t-tests, all P>0.05).
Table 1.
Clinical variables of study groups (Mean±S.D.)
| Variable | Untreated group |
Untreated group |
Bupropion group |
Bupropion group |
Bupropion group |
|---|---|---|---|---|---|
| Neutral scan (n=20) | Cig cue scan | Pre-treatment (n=17) | Neutral scan | Cig cue scan | |
| Age | 42.5 (±10.6) | – | 41.3 (±13.3) | – | – |
| % Female | 30 | – | 24 | – | – |
| % Left-handed | 10 | – | 6 | – | – |
| Scan interval (days) | 10.9 (±9.2) | – | 8.1 (±4.5) | – | – |
| Cigarettes/day | 32.6 (±12.4) | – | 27.1 (±8.7) | 3.9 (±5.0)c | 3.4 (±4.6)c |
| Years smoking | 25.4 (±8.9) | – | 22.0 (±12.8) | – | – |
| Urge to smoke | 2.9 (±1.6) | 4.1 (±1.6)a | – | 1.1 (±1.4)c | 1.4 (±1.4)c |
| Exhaled CO (ppm) | 24.1 (±9.1) | 23.1 (±8.9) | 23.0 (±7.9) | 8.3 (±6.6)c | 8.7 (±6.6)c |
| HAM-D | 2.0 (±2.6) | 2.2 (±2.7) | – | 2.5 (±2.8) | 1.9 (±2.4) |
| HAM-A | 2.2 (±2.0) | 2.6 (±2.2) | – | 3.6 (±2.9) | 3.0 (±3.4) |
| S-J (mean per item) | 3.4 (±0.7) | 3.7 (±1.0) | – | 3.0 (±0.9)b | 2.8 (±0.7)b |
CO=carbon monoxide; ppm=parts per million; HAM-D=Hamilton Depression Rating Scale (17-item) (range 0–52); HAM-A=Hamilton Anxiety Rating Scale (range 0–56); S-J=Shiffman–Jarvik Rating Scale mean (±S.D.) per item score.
Student’s t-test comparing differences in Urge to Smoke Scale scores between groups from the neutral to the cigarette cue scan, P=0.05.
Student’s t-test between groups for corresponding scan, P<0.05.
Student’s t-test between groups for corresponding scan, P<0.0005.
Following treatment, bupropion-treated smokers had lower Urge to Smoke (UTS) Scale scores during both the neutral and cigarette-cue sessions (both Student’s t-tests, P<0.0005) and less of a difference in UTS from the neutral to the cigarette-cue conditions than untreated smokers (Student’s t-test, two-tailed, P=0.05) (Table 1), indicating that bupropion-treated smokers had reduced overall craving levels and diminished cigarette cue-induced craving compared with untreated smokers. The bupropion-treated group reported smoking fewer cigarettes per day, had lower exhaled CO levels, and had lower scores on the Shiffman–Jarvik Scale than untreated smokers during both conditions (Student’s t-tests, P<0.0005 for cigarettes/day and CO levels, and P<0.05 for the Shiffman–Jarvik Scale), indicating that bupropion-treated smokers had reduced cigarette use and withdrawal symptoms. Five bupropion-treated subjects met full criteria for having quit smoking (7-day self-report of abstinence and an exhaled CO of ≤ 8 ppm) (Jarvik et al., 1997), while the majority of the remainder of the group had diminished usage. No subjects reported a worsening of their nicotine dependence related to cigarette cue exposure.
3.2. PET results: brain metabolic changes associated with cue presentation
In the SPM analysis of group-by-condition interaction, untreated and bupropion-treated smokers had significantly different metabolic activation from the neutral to the cigarette-cue scans in the perigenual ACC (Z=3.25, x y z coordinates= −2 40 20, P=0.001; 25 voxels) (roughly corresponding to Brodmann areas [BA] 24/32) (Fig. 2). The untreated group alone had an area of ACC activation (183 voxels), including a voxel of peak significance (Z=3.86, x y z = −2 46 18), with a similar location to the difference between groups found here. These results indicate that untreated smokers had significantly more ACC activation than bupropion-treated smokers in response to cigarette-related (compared with neutral) cues. Unexpectedly, the left lateral posterior temporal lobe had a greater metabolic activation in the treated than the untreated group (Z=3.50, x y z= −54 −34 −24, P=0.001, 43 voxels for the interaction). No other significant differences in metabolic activation or deactivation between study groups were found.
Fig. 2.
Statistical parametric mapping results for the untreated smoker group alone (n=20) showing significant activation (P<0.005) of the perigenual anterior cingulate cortex (ACC) from the neutral to the cigarette-cue conditions (Panel 1). Panel 2 shows a small area of activation in the bupropion-treated group (n=17) alone. Panel 3 shows the ACC region of activation that was greater for untreated than bupropion-treated smokers.
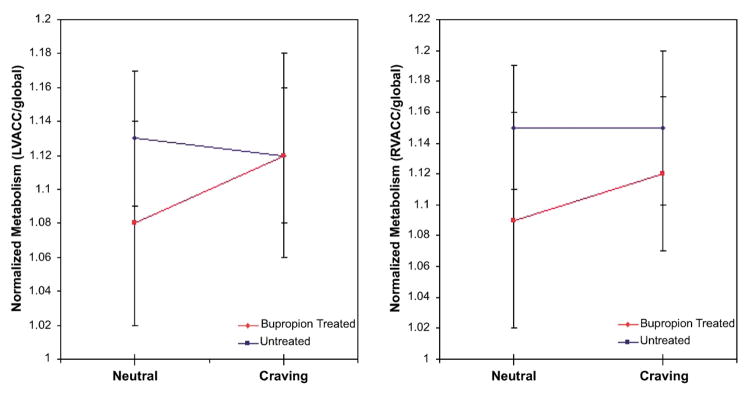
In the ROI analysis examining the group-by-condition interaction, effects for both the left and right ventral ACC were significant (P<0.05 for both). These effects were due to ACC activation in the untreated group (left 0.034 [±0.051] and right 0.030 [±0.050]), without significant activations or deactivations in the bupropion-treated group (left −0.007 [±0.065] and right −0.001 [±0.043]) (Fig. 3). No control regions had significant group-by-condition interactions (all P’s> 0.16).
Fig. 3.
Region of interest analysis showing activation from the neutral to the cigarette-cue state of normalized left (panel 1) and right (panel 2) ventral anterior cingulate cortex (ACC) metabolism for untreated (red), but not bupropion-treated (blue), smokers. A higher mean neutral state ACC metabolic rate is seen in the bupropion-treated than the untreated group.
In the secondary analysis comparing neutral state normalized metabolism between untreated and bupropion-treated smokers, SPM revealed that bupropion-treated smokers had higher normalized metabolism in the right ventral ACC (Z=3.08, x y z=12 52 16, P=0.002) than untreated smokers. In the ROI analysis, bupropion-treated smokers had higher normalized metabolism than untreated smokers in both the left (1.13 [±0.04] vs. 1.08 [±0.06], P=0.01) and right (1.15 [±0.04] vs. 1.09 [±0.07], P=0.001) ventral ACC (Fig. 3). These results indicate that bupropion-treated smokers had higher ventral ACC metabolism in the neutral state than untreated smokers.
3.3. PET results: associations between craving and regional brain metabolism in the bupropion-treated group
In the SPM analysis of within-subject dependence of relative regional metabolism on craving in the bupropion-treated group, positive associations were found in the posterior left (Z=3.30, x y z= −40 32 −8, P=0.002) and right (Z=3.28, x y z=46 32 −10, P=0.001) OFC (Fig. 4). Unexpected positive associations were also found in the left (Z=4.37, x y z=−52 −16 −24, P<0.0005) and right (Z=4.25, x y z=60 −30 −10, P<0.0005) lateral temporal lobe, and right ACC (Z= 4.27, x y z=2 48 0, P<0.0005). An unexpected negative association was found in the right post-central gyrus (Z=6.35, x y z=42 −22 50, P<0.0005). In the ROI analysis, a positive correlation was found between change in UTS and change in normalized metabolism in the right OFC (Pearson r=0.50, P=0.04). No other significant correlations between UTS and ROI values were found.
Fig. 4.
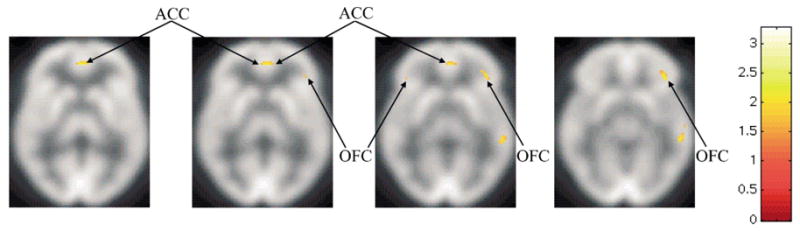
Statistical parametric mapping analysis showing regions with significant associations (P<0.005) between subjective craving and regional metabolism for the bupropion-treated smoker group alone (n=17). These regions included the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC).
4. Discussion
In this study, smokers treated with bupropion HCl had lower overall cigarette craving and less cue-induced cigarette craving than untreated smokers. This diminished cue-induced cigarette craving was accompanied by reduced activation of perigenual/ventral ACC metabolism, possibly related to increased neutral state metabolism in this region. In bupropion-treated smokers, intensity of craving was associated with normalized metabolism in the OFC bilaterally [similar to untreated smokers (Brody et al., 2002)], indicating that this region may mediate urge to smoke regardless of treatment status.
The clinical findings here extend previous research on the effects of bupropion HCl treatment for nicotine dependence. While decreased overall levels of cigarette craving and withdrawal have been reported in prior studies of bupropion-treated smokers (Shiffman et al., 2000; Durcan et al., 2002), the finding of diminished cigarette cue reactivity has not yet been reported and may provide an additional explanation for the efficacy of bupropion HCl, since responsiveness to cigarette-related cues contributes to relapse in smokers attempting to quit (Niaura et al., 1989a).
Attenuation of cigarette cue-induced ACC activation is consistent with reports of the mechanism of action of bupropion HCl. Among its many reported properties, bupropion inhibits pre-synaptic reuptake of norepinephrine (NE) (Cooper et al., 1994; Horst and Preskorn, 1998) and dopamine (DA) (Horst and Preskorn, 1998; Nomikos et al., 1989; Meyer et al., 2002), and increases NE release (Dong and Blier, 2001). The effect of bupropion is greater on NE reuptake and inhibition of NE cell firing than on these mechanisms within the DA system (Cooper et al., 1994; Dong and Blier, 2001; Meyer et al., 2002). Many studies demonstrate that NE cells originating in the locus ceruleus and DA cells originating in the ventral tegmental area innervate the ventral ACC directly, in an overlapping distribution (Levitt et al., 1984; Takada and Hattori, 1987; Mitchell et al., 1994; Sesack et al., 1998; Williams and Goldman-Rakic, 1998; Ciliax et al., 1999; Mundorf et al., 2001) (though the NE system is widespread throughout the entire cortex, while the DA system projects primarily to the nucleus accumbens and forebrain) (Levitt et al., 1984). Treatment with medications that block reuptake of NE and DA have been shown to increase extracellular concentrations of these catecholamines (Page and Lucki, 2002) and to prevent stress-induced surges in NE and DA release (Dazzi et al., 2001, 2002a,b). These reports mirror the results of the current study in which elevated baseline ACC activity was found in bupropion-treated smokers (possibly due to enhanced extracellular catecholamine concentration leading to increased metabolism), accompanied by attenuated ACC activation in response to cigarette cues (possibly due to diminished surges in catecholamine release), although alteration of ACC reactivity through indirect cortical or thalamic mechanisms are also possible explanations for study results.
The unexpected finding here of greater activation (from the neutral to the cigarette-cue scans) in treated than untreated smokers in the left posterior lateral temporal lobe may reflect functions of this region that are more responsive to videotaped cigarette cues in the treated group. In other functional imaging studies, activation of this region has been associated with recognizing the meaning of an object (Whatmough et al., 2002), recognizing images of the human body (Downing et al., 2001), listening to complex sentences (Michael et al., 2001), and visual recognition of speech (Bernstein et al., 2002). The present study indicates that treated smokers may have been more able to attend to these aspects of the cigarette cue video (which had several scenes of subjects conversing while smoking) than untreated smokers (who may have been focused on urge to smoke during the cigarette cue video).
The finding here of a positive correlation between craving and OFC metabolism adds to the many reports of this association in untreated substance-dependent subjects (Volkow et al., 1991, 1999; Sell et al., 2000; Daglish et al., 2001; Bonson et al., 2002; Brody et al., 2002). The link between drug craving and OFC activity is the most common association found in this type of functional imaging work, and has led to the hypothesis that the OFC mediates drive and compulsive behavior associated with drug dependence (Volkow and Fowler, 2000). This region is also thought to mediate decision making that leads to reward (London et al., 2000; O’Doherty et al., 2001a; Ernst et al., 2002) and to act as a secondary processing center for gustatory (Zald et al., 1998; O’Doherty et al., 2001b) and olfactory (Zatorre et al., 1992; Levy et al., 1997; Zald and Pardo, 1997) stimuli. These putative functions of the OFC may help explain the consistent positive association between metabolism in this region and craving.
There are three central limitations of this study. The primary one is the absence of double-blind, placebo-controlled treatment, which would control for aspects of treatment other than the pharmacological effect of bupropion (e.g. patient motivation and expectations, visits with a physician, and repeated administration of rating scales). Another limitation is the inability to distinguish between effects of diminished cigarette usage vs. bupropion HCl treatment, so that study results may be due to administration of bupropion HCl, diminished cigarette usage (and resultant decreases in carbon monoxide levels), or both. To separate these effects, sample sizes will be needed that are large enough to have adequate power to distinguish quitters from non-quitters. In addition, the absence of PET and cigarette-cue response data on the bupropion-treated group prior to treatment precluded a direct assessment of pre-treatment between-group differences in ACC metabolism or ACC reactivity. However, prior to treatment, the bupropion-treated group was similar to the untreated group in all demographic and rating scale measurements, and cigarette-related cues have consistently been shown to heighten cigarette craving in untreated smokers (Sayette and Hufford, 1994; Droungas et al., 1995; Mucha et al., 1998, 1999; Morgan et al., 1999; Conklin et al., 2000; Taylor et al., 2000). Other limitations of the PET scanning and cue exposure procedures are described in our earlier report (Brody et al., 2002).
Despite these limitations, this study also had several strengths that enhance confidence in the findings. First, study samples were free of psychiatric, neurological, substance abuse, and other medical comorbidities (other than nicotine dependence) that could affect PET scanning results. Second, the sample size for a study of this type was relatively large, which enhanced power to detect group differences. And third, substantial overlap in the results was found between two methods of data analysis, which enhances the validity of the findings. To summarize, this study provides preliminary evidence that bupropion HCl attenuates cue-induced cigarette craving and associated ACC activation; additional studies, including both placebo control and repeated imaging, will be needed to confirm and expand upon these results.
Acknowledgments
The research reported was supported by a Veterans Affairs Type I Merit Review Award (A.L.B.), the Tobacco-Related Disease Research Program (A.L.B. [7KT-0098 and 11RT-0024] and E.D.L. [10RT-0091]), the National Institute on Drug Abuse (A.L.B. [R01 DA15059] and E.D.L. [RO1 DA14093]), and the National Institute of Mental Health (S.S. [K23 MH001694]). The authors thank Nayda Quinones and Michael Clark for technical support in performing PET and MRI scans, respectively. An earlier version of this report was presented in part at the American College of Neuropsychopharmacology Annual Meeting, December 9, 2002, San Juan, PR and the Society for Research on Nicotine and Tobacco Annual Meeting, February 21, 2003.
References
- Bernstein LE, Auer ETJ, Moore JK, Ponton CW, Don M, Sigh M. Visual speech perception without primary auditory cortex activation. Neuroreport. 2002;13:311–315. doi: 10.1097/00001756-200203040-00013. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Bota RG, Ho ML, Lee GS, Saxena S, Baxter LR, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Archives of General Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Catley D, O’Connell KA, Shiffman S. Absentminded lapses during smoking cessation. Psychology of Addictive Behaviors. 2000;14:73–76. doi: 10.1037//0893-164x.14.1.73. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. American Journal of Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliax BJ, Drash GW, Staley JK, Haber S, Mobley CJ, Miller GW, Mufson EJ, Mash DC, Levey AI. Immunocytochemical localization of the dopamine transporter in human brain. Journal of Comparative Neurology. 1999;409:38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST, Vrana SR. The impact of imagining completed vs. interrupted smoking on cigarette craving. Experimental and Clinical Psychopharmacology. 2000;8:68–74. doi: 10.1037//1064-1297.8.1.68. [DOI] [PubMed] [Google Scholar]
- Cooper BR, Wang CM, Cox RF, Norton R, Shea V, Ferris RM. Evidence that the acute behavioral and electrophysiological effects of bupropion (Wellbutrin) are mediated by a noradrenergic mechanism. Neuropsychopharmacology. 1994;11:133–141. doi: 10.1038/npp.1994.43. [DOI] [PubMed] [Google Scholar]
- Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, Brewer C, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. American Journal of Psychiatry. 2001;158:1680–1686. doi: 10.1176/appi.ajp.158.10.1680. [DOI] [PubMed] [Google Scholar]
- Dazzi L, Serra M, Spiga F, Pisu MG, Jentsch JD, Biggio G. Prevention of the stress-induced increase in frontal cortical dopamine efflux of freely moving rats by long-term treatment with antidepressant drugs. European Neuropsychopharmacology. 2001;11:343–349. doi: 10.1016/s0924-977x(01)00105-5. [DOI] [PubMed] [Google Scholar]
- Dazzi L, Ladu S, Spiga F, Vacca G, Rivano A, Pira L, Biggio G. Chronic treatment with imipramine or mirtazapine antagonizes stress- and FG7142-induced increase in cortical norepinephrine output in freely moving rats. Synapse. 2002a;43:70–77. doi: 10.1002/syn.10024. [DOI] [PubMed] [Google Scholar]
- Dazzi L, Vignone V, Seu E, Ladu S, Vacca G, Biggio G. Inhibition by venlafaxine of the increase in norepinephrine output in rat prefrontal cortex elicited by acute stress or by the anxiogenic drug FG 7142. Journal of Psychopharmacology. 2002b;16:125–131. doi: 10.1177/026988110201600202. [DOI] [PubMed] [Google Scholar]
- Dong J, Blier P. Modification of norepinephrine and serotonin, but not dopamine, neuron firing by sustained bupropion treatment. Psychopharmacology. 2001;155:52–57. doi: 10.1007/s002130000665. [DOI] [PubMed] [Google Scholar]
- Downing PE, Jiang Y, Shuman M, Kanwisher N. A cortical area selective for visual processing of the human body. Science. 2001;293:2405–2407. doi: 10.1126/science.1063414. [DOI] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addictive Behaviors. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Durcan MJ, Deener G, White J, Johnston JA, Gonzales D, Niaura R, Rigotti N, Sachs DP. The effect of bupropion sustained-release on cigarette craving after smoking cessation. Clinical Therapeutics. 2002;24:540–551. doi: 10.1016/s0149-2918(02)85130-x. [DOI] [PubMed] [Google Scholar]
- Ernst M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, London ED. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, Heyman RB, Jaen CR, Kottke TE, Lando HA, Mecklenburg RE, Mullen PD, Nett LM, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME. Clinical Practice Guideline. U.S. Department of Health and Human Services. Public Health Service; Rockville, MD: 2000. Treating Tobacco Use and Dependence. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders–Patient Edition (SCID-I/P, version 2.0) 1995 [Google Scholar]
- Friston KJ, Worsley K, Frackowiak RSJ, Mazziotta J, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1994;1:214–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Glassman AH. Cigarette smoking: implications for psychiatric illness. American Journal of Psychiatry. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi CS, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences of the USA. 1996;93:12 040–12 045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. Diagnosis and rating of anxiety. British Journal of Psychiatary. 1969;3:76–79. [Google Scholar]
- Horst WD, Preskorn SH. Mechanisms of action and clinical characteristics of three atypical antidepressants: venlafaxine, nefazodone, bupropion. Journal of Affective Disorders. 1998;51:237–254. doi: 10.1016/s0165-0327(98)00222-5. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, Glover ED, Offord KP, Johnston JA, Dale LC, Khayrallah MA, Schroeder DR, Glover PN, Sullivan CR, Croghan IT, Sullivan PM. A comparison of sustained-release bupropion and placebo for smoking cessation. New England Journal of Medicine. 1997;337:1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen D, Shoptaw S, Frosch D. In: Harris LS, editor. Ambient and outdoor carbon monoxide levels among non-smokers in Los Angeles; Problems of Drug Dependence 1996: Proceedings of the 58th Annual Scientific Meeting, the College on Problems of Drug Dependence: NIDA Research Monograph; 1997. pp. 174–210. [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacology Biochemistry and Behavior. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, Nides MA, Rennard SI, Johnston JA, Hughes AR, Smith SS, Muramoto ML, Daughton DM, Doan K, Fiore MC, Baker TB. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. New England Journal of Medicine. 1999;340:685–691. doi: 10.1056/NEJM199903043400903. [DOI] [PubMed] [Google Scholar]
- Killen JD, Fortmann SP. Craving is associated with smoking relapse: findings from three prospective studies. Experimental and Clinical Psychopharmacology. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KPG. Neural activity related to drug craving in cocaine addiction. Archives of General Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Levitt P, Rakic P, Goldman-Rakic P. Region-specific distribution of catecholamine afferents in primate cerebral cortex — a fluorescence histochemical analysis. Journal of Comparative Neurology. 1984;227:23–36. doi: 10.1002/cne.902270105. [DOI] [PubMed] [Google Scholar]
- Levy LM, Henkin RI, Hutter A, Lin CS, Martins D, Schellinger D. Functional MRI of human olfaction. Journal of Computer Assisted Tomography. 1997;21:849–856. doi: 10.1097/00004728-199711000-00002. [DOI] [PubMed] [Google Scholar]
- London ED, Ernst M, Grant S, Bonson K, Weinstein A. Orbitofrontal cortex and human drug abuse: functional imaging. Cerebral Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers V, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. American Journal of Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S. Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology. 2002;163:102–105. doi: 10.1007/s00213-002-1166-3. [DOI] [PubMed] [Google Scholar]
- Michael EB, Keller TA, Carpenter PA, Just MA. FMRI investigation of sentence comprehension by eye and by ear: modality fingerprints on cognitive processes. Human Brain Mapping. 2001;13:239–252. doi: 10.1002/hbm.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K, Oke AF, Adams RN. In-vivo dynamics of norepinephrine release reuptake in multiple terminal field regions of rat brain. Journal of Neurochemistry. 1994;63:917–926. doi: 10.1046/j.1471-4159.1994.63030917.x. [DOI] [PubMed] [Google Scholar]
- Morgan MJ, Davies GM, Willner P. The questionnaire of smoking urges is sensitive to abstinence and exposure to smoking-related cues. Behavioural Pharmacology. 1999;10:619–626. doi: 10.1097/00008877-199911000-00008. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Geier A, Pauli P. Modulation of craving by cues having differential overlap with pharmacological effect: evidence for cue approach in smokers and social drinkers. Psychopharmacology. 1999;147:306–313. doi: 10.1007/s002130051172. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Pauli P, Angrilli A. Conditioned responses elicited by cues for smoking. Canadian Journal of Physiology and Pharmacology. 1998;76:259–268. [PubMed] [Google Scholar]
- Mundorf ML, Joseph JD, Austin CM, Caron MG, Wightman RM. Catecholamine release and uptake in the mouse prefrontal cortex. Journal of Neurochemistry. 2001;79:130–142. doi: 10.1046/j.1471-4159.2001.00554.x. [DOI] [PubMed] [Google Scholar]
- Nadeau SE, Crosson B. A guide to functional imaging of cognitive processes. Neuropsychiatry, Neuropsychology and Behavioral Neurology. 1995;8:143–162. [PubMed] [Google Scholar]
- Niaura R, Abrams D, Demuth B, Pinto R, Monti P. Responses to smoking-related stimuli and early relapse to smoking. Addictive Behaviors. 1989a;14:419–428. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Monti PM, Pedraza M. Reactivity to high risk situations and smoking cessation outcome. Journal of Substance Abuse. 1989b;1:393–405. [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Acute effects of bupropion on extracellular dopamine concentrations in rat striatum and nucleus accumbens studied by in vivo microdialysis. Neuropsychopharmacology. 1989;2:273–279. doi: 10.1016/0893-133x(89)90031-6. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neuroscience. 2001a;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. Journal of Neurophysiology. 2001b;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- Page ME, Lucki I. Effects of acute and chronic reboxetine treatment on stress-induced monoamine efflux in the rat frontal cortex. Neuropsychopharmacology. 2002;27:237–247. doi: 10.1016/S0893-133X(02)00301-9. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach coordinate system. Cerebral Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Hufford MR. Effects of cue exposure and deprivation on cognitive resources in smokers. Journal of Abnormal Psychology. 1994;103:812–818. doi: 10.1037//0021-843x.103.4.812. [DOI] [PubMed] [Google Scholar]
- Schuh KJ, Stitzer ML. Desire to smoke during spaced smoking intervals. Psychopharmacology. 1995;120:289–295. doi: 10.1007/BF02311176. [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug and Alcohol Dependence. 2000;60:207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. Journal of Neuroscience. 1998;18:2697–2708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settle EC, Stahl SM, Batey SR, Johnston JA, Ascher JA. Safety profile of sustained-release bupropion in depression: results of three clinical trials. Clinical Therapeutics. 1999;21:454–463. doi: 10.1016/s0149-2918(00)88301-0. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Johnston JA, Khayrallah M, Elash CA, Gwaltney CJ, Paty JA, Gnys M, Evoniuk G, DeVeaugh-Geiss J. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology. 2000;148:33–40. doi: 10.1007/s002130050022. [DOI] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME. Smoking withdrawal symptoms in 2 weeks of abstinence. Psychopharmacology. 1976;50:35–39. doi: 10.1007/BF00634151. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Seitz RJ. Functional anatomy of language processing: neuroimaging and the problem of individual variability. Neuropsychologia. 1991;29:1149–1161. doi: 10.1016/0028-3932(91)90030-c. [DOI] [PubMed] [Google Scholar]
- Swan GE, Ward MM, Jack LM. Abstinence effects as predictors of relapse in smokers. Addictive Behaviors. 1996;21:481–490. doi: 10.1016/0306-4603(95)00070-4. [DOI] [PubMed] [Google Scholar]
- Sweeney CT, Pillitteri JL, Kozlowski LT. Measuring drug urges by questionnaire. Addictive Behaviors. 1996;21:199–204. doi: 10.1016/0306-4603(95)00044-5. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; New York, NY: 1988. [Google Scholar]
- Takada M, Hattori T. Organization of ventral tegmental area cells projecting to the occipital cortex and forebrain in the rat. Brain Research. 1987;418:27–33. doi: 10.1016/0006-8993(87)90958-9. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Harris NA, Singleton EG, Moolchan ET, Heishman SJ. Tobacco craving: intensity-related effects of imagery scripts in drug abusers. Experimental and Clinical Psychopharmacology. 2000;8:75–87. doi: 10.1037//1064-1297.8.1.75. [DOI] [PubMed] [Google Scholar]
- Tsoh JY, Humfleet GL, Munoz RF, Reus VI, Hartz DT, Hall SM. Development of major depression after treatment for smoking cessation. American Journal of Psychiatry. 2000;157:368–374. doi: 10.1176/appi.ajp.157.3.368. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereberal Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. American Journal of Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Hitzemann R, Angrist B, Gatley SJ, Logan J, Ding YS, Pappas N. Association of methylphenidate-induced craving with changes in right striato-orbitofrontal metabolism in cocaine abusers: implications in addiction. American Journal of Psychiatry. 1999;156:19–26. doi: 10.1176/ajp.156.1.19. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. American Journal of Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Whatmough C, Chertkow H, Murtha S, Hanratty K. Dissociable brain regions process object meaning and object structure during picture naming. Neuropsychologia. 2002;40:174–186. doi: 10.1016/s0028-3932(01)00083-5. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereberal Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- Zald DH, Lee JT, Fluegel KW, Pardo JV. Aversive gustatory stimulation activates limbic circuits in humans. Brain. 1998;121:1143–1154. doi: 10.1093/brain/121.6.1143. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proceedings of the National Academy of Sciences of the Unites States of America. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Jonesgotman M, Evans AC, Meyer E. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339–340. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]