Abstract
The present study was designed to examine the effect of cigarette smoking and withdrawal on working memory. Participants included 15 smokers and 22 matched non-smokers. For both groups the N-Back Task (of working memory) was administered in two test blocks on each of two days. On one day, smokers were tested after ≥13 h abstinence; on the other day, testing began ≤1 h after smoking. Smokers inhaled one cigarette between the blocks on each test day. Results indicated that performance of smokers after ≥13 h but not ≤1 h abstinence was significantly less accurate than that of non-smokers. A within-subject comparison revealed that in the abstinence session, smokers had significantly longer response latencies (in the 2-back condition) and made more overall errors compared to the satiety session. Smoking between test blocks in the abstinence session did not significantly affect performance although it significantly reduced craving. These findings provide further evidence for a deficit in working memory associated with acute abstinence from smoking, which may contribute to the difficulty of smoking cessation.
Keywords: Cigarette smoking, Working memory, Withdrawal, Nicotine, Attention, Craving
1. Introduction
Working memory is mediated by a system of limited capacity, and it is a component of a wide range of cognitive operations (Baddeley, 1996; Baddeley & Della Salla, 1996). In the simplest case of working memory, information is stored for a few seconds before decaying; in more complex applications, data can be retrieved, manipulated, and associated with other information. Human studies have suggested that chronic smoking, abrupt withdrawal, and acute smoking or nicotine administration can affect performance on tests of working memory, although the results have been inconsistent (Heishman, Henningfield, & Singleton, 2002; Heishman, Snyder, & Henningfield, 1993; Pritchard & Robinson, 1998; Rezvani & Levin, 2001).
Some research reports have suggested associations between smoking history and impairment on tasks purporting to assess cognitive function. The N-Back Task of working memory (Gevins & Cutillo, 1993b), requires the participant to memorize the serial positions of stimuli, presented one at a time, and then, to decide if a given probe was seen 1, 2, or 3 letters prior. Latency for responding to the probe presumably reflects the time needed to mentally scan the items presented. In one study using the N-Back Task, reaction time was shortest in never-smokers, intermediate in ex-smokers, and longest in smokers (Ernst, Heishman, Spurgeon, & London, 2001a). Another study found that smokers, even at smoking satiety, performed more poorly than non-smokers on a recognition memory task in which lists of words were followed by a probe word (Spilich, June, & Renner, 1992). These findings could be interpreted as evidence for a deleterious effect of cigarette smoking. In support of this interpretation, a study of a large cohort found that smokers exhibited greater decline in performance between the ages of 43 and 53 on a word learning task, as compared to non-smokers (Richards, Jarvis, Thompson, & Wadsworth, 2003). Other studies, however, have not demonstrated significant associations between chronic smoking and cognitive decline (Carmelli, Swan, LaRue, & Eslinger, 1997; Schinka et al., 2002).
Deleterious effects of abrupt initiation of abstinence on working memory, and reversal of such effects by smoking or nicotine administration, have also been observed. For example, within 4 h of initiating smoking abstinence, tobacco-dependent research participants showed increased response latency and poorer accuracy on a digit recall task; their performance returned to baseline levels within 24 h of resuming smoking (Snyder, Davis, & Henningfield, 1989). A similar effect was observed in smokers, whose performance in the a serial recall of letters deteriorated after 12 h of deprivation and normalized after smoking a single cigarette (Blake & Smith, 1997). In another study, abstinent smokers and non-smokers performed a variant of the Sternberg Task, in which a set of items was presented for memorization shortly before the presentation of a single probe item (Grobe, Perkins, Goettler-Good, & Wilson, 1998). The latency for responding to the probe presumably reflected the time needed to scan the memory set being held “on-line.” Nicotine administration by nasal spray improved smokers’ performance when distracting stimuli were presented (Grobe et al., 1998). A later study showed that smoking a nicotine-yielding cigarette, but not a de-nicotinized cigarette, shortened reaction times on a Sternberg task (Houlihan, Pritchard, & Robinson, 2001).
Other studies of smoking and/or nicotine manipulations on working memory have yielded results contrasting with those noted above. For example, administration of nicotine gum (4 mg nicotine) did not reduce reaction time or improve accuracy on the N-Back Task in smokers who had abstained for 12 h(Ernst et al., 2001b). Furthermore, in a study in which overnight-abstinent smokers consumed one cigarette whose nicotine content was manipulated, the amount of nicotine delivered was inversely correlated with working memory performance (Williams, 1980). In addition, deficits in spatial working memory were associated with the administration of nicotine nasal spray to abstinent smokers and non-smokers (Park, Knopick, McGurck, & Meltzer, 2000). Summarizing the literature, Pritchard and Robinson (1998) concluded that it “provide[s] no consistent evidence regarding the [acute] effects of smoking/ nicotine on [working memory] capacity” (p. 71).
Complex social and physiological factors contribute to nicotine dependence; and the effects of smoking and of nicotine withdrawal on cognition may contribute to the maintenance and relapse of smoking behavior. The present study therefore aimed to extend knowledge on the interaction of cigarette smoking with working memory performance in humans. We employed a parametric version of the N-Back Task to assess: 1) whether there were differences in working memory between non-smokers and smokers (in abstinence or satiety), 2) whether ≥13 h abstinence, compared to relatively brief abstinence (≤1 h), resulted in working memory impairments among smokers, and 3) whether smoking one cigarette enhanced the working memory performance of smokers who had been abstaining for ≥13 h.
In secondary analyses, we assessed whether self-reported craving was related to working memory performance. In the context of a cognitive test situation, a self-report of craving for a psychoactive entity (e.g., a cigarette) indicates that the research participant is experiencing a cognitive event (Toneatto, 1999), representing intrusive thoughts. Such task-unrelated images or thoughts can capture attention as uncontrolled shifts (Giambra, 1995), and thereby might be expected to interfere with cognitive performance. The effects of withdrawal and smoking history (pack years) also were evaluated.
2. Methods
2.1. Participants
Potential participants were recruited from flyers and newspaper ads. They were screened for eligibility during a telephone interview, which included questions about their current use of medications, prior and current use of illicit drugs, medical and psychiatric conditions, and current and previous cigarette usage. Individuals were excluded if they were younger than 18 or older than 55 years of age, or if they reported smoking marijuana more than once per week, drinking more than 10 alcoholic drinks per week, regularly using recreational drugs other than alcohol or marijuana, were taking medication for mood, sleep, or energy disturbance, or had a history of head trauma. Potential non-smoker participants who reported a lifetime history of smoking more than five cigarettes were excluded. Potential smoker participants were excluded if they reported smoking fewer than 15 or more than 40 cigarettes per day, or had not been smoking regularly for at least two years. As participants from this study potentially could also participate in brain imaging, which might be confounded with handedness, being left-handed was also an exclusion criterion.
Of 1162 individuals who responded to flyers and newspaper ads, 166 both passed the telephone screen and provided informed consent to participate in the study. During the initial baseline visit, additional measures were obtained to assess eligibility. Expired carbon monoxide (CO) was taken as an objective measure of recent smoking (Micro Smokerlyzer II, Bedfont Scientific Instruments), with inclusion criteria of ≥5 parts per million (ppm) for the non-smoker group and ≤10 ppm for the smoker group. The participants also completed questionnaires assessing medical history, smoking history (including the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fägerström, 1991)), childhood Attention Deficit Hyperactivity Disorder (Wender Utah Rating Scale; WURS) (Ward, Wender, & Reimherr, 1993) and depressive symptoms (Beck Depression Inventory; BDI) (Beck & Beamesderfer, 1974). A subject was excluded if he or she reported a debilitating medical condition, or had a score of ≥47 on the WURS. Of the 166 individuals who consented to participate, 78 smokers and 51 non-smokers were excluded for either illicit drug and/or alcohol use, had CO levels outside the specified parameters, or were lost to attrition. The remaining 22 non-smokers and 15 smokers participated in the research reported here.
2.2. Procedures
Subsequent to baseline assessments, both groups were tested in two test blocks on each of two days. On one day, smokers were tested after ≥13 h abstinence (abstinence session); on the other day, testing began ≤1 h after smoking (satiety session). On both days, smokers consumed one cigarette between two test blocks, and measures of cigarette craving and withdrawal were taken before and after each block. Half of the smokers had the abstinence session first and the other half of the group had the abstinence session second. On test days that did not require abstinence, smokers were allowed to smoke ad libitum before arriving in the laboratory.
To diminish the effect of practice, participants were given pre-experimental training on the N-Back Task at the beginning of each of the two test sessions. The training consisted of 2-min blocks of each condition (0-, 1-, 2-, and 3-back), which participants had to perform until attaining a level of at least 60% accuracy without variation of more than 10% on two consecutive blocks of the same condition. Data collection commenced immediately after training. For the smokers, this time was ≤1h after they had smoked their last cigarettes in the satiety session. All testing sessions took place in the afternoon (between 14 : 00 h and 17 : 00 h).
Before each testing session, measurement of expired CO provided verification of compliance with abstinence as required (see above) (Baddeley & Della Salla, 1996; Jarvis, Russell, & Saloojee, 1980). All smokers smoked one cigarette (their usual brands) between the two test blocks on each of the test days. During each block of testing, smokers were evaluated for cigarette withdrawal with the 25-item Shiffman/Jarvik Withdrawal Scale and subjective craving using a 10-item Likert “Urge To Smoke” scale (UTS) (Jarvik et al., 2000) and a 7-item Likert craving subscale from the Shiffman/Jarvik Withdrawal Scale. Average scores on the craving scales were correlated significantly (correlation coefficient = 0.80, p <0.001). We used the UTS scores as the index of craving and the Shiffman/Jarvik Withdrawal Scale as the index of withdrawal to correlate with performance differences in satiety versus abstinence sessions.
2.3. Parametric N-back task
The N-back task used was modified from a prior version (Gevins & Cutillo, 1993a) which only used the 2-back condition with different presentation times. The computerized version we used consisted of randomly presented, 42-s epochs of 0-back, 1-back, 2-back, and 3-back conditions and 18-s periods of rest separating the epochs from one another. Each N-back condition (0-back, 1-back, 2-back, and 3-back) was repeated twice for a total of 8 min of testing, and consisted of a sequence of letters of the alphabet presented in a pseudorandom order, one at a time. Each letter appeared for 400 ms with an inter-stimulus interval (ISI) of 2000 ms. At the onset of the 0-back condition, participants, saw the instruction “Find X” and were required to press one key (“yes” key) when the letter “X” appeared, and a second key (“no” key) when any other letter appeared. At the onset of the 1-back condition, participants saw the instruction “Find 1-back”. They were required to press the “yes” key when the letter presented was identical to the one immediately preceding it, and the “no” key whenever any other letter appeared. The same procedure was followed for the 2- and 3-back conditions, except that during the 2-back condition, the target letter was the letter that was displayed 2 letters prior, and during the 3-back condition the target letter was that presented 3 letters prior. Each epoch contained 21 stimuli: 7 targets and 14 non-targets.
2.4. Analytical plan
Analyses were conducted to address three primary questions: 1) Are there differences in working memory between non-smokers and smokers, either in abstinence or satiety? 2) Does ≥13 h abstinence from smoking produce impairments in working memory compared to ≤1 h abstinence? And 3) Does smoking one cigarette enhance the working memory performance of smokers who had abstained ≥13 h? Each of these questions was addressed by repeated measures Analyses of Variance (ANOVA). To compare the performance between non-smokers and smokers (Question 1), two sets of repeated measures ANOVAs were conducted; one comparing performance of non-smokers to the smokers in abstinence, and one comparing the performance of non-smokers to the smokers in satiety. To assess whether smoking withdrawal resulted in working memory impairments (Question 2), a set of repeated measures ANOVAs were conducted in which smokers’ pre-cigarette N-Back performance was compared between their abstinence and satiety sessions.
The question of whether smoking one cigarette would enhance working memory performance of smokers in the abstinence session (Question 3) required a more complicated approach, given that pre-versus post-cigarette performance was confounded with order of test administration. That is, pre-cigarette testing always preceded post-cigarette testing. To address this confound, repeated measures ANOVAs were conducted that included test block and test day (≥13 h vs. ≥1 h abstinence) as within-subject independent variables. We reasoned that if smokers’ performance improved more across test blocks in the abstinence condition than in the satiety condition (observable as a significant interaction between these variables) then the difference could plausibly (though not definitively) be attributed to the effect of the cigarette rather than repetition.
In all analyses outlined above, N-Back condition (0-, 1-, 2-, and 3-back) was used as a within-subject variable, and separate analyses were conducted for errors and response times to correct responses (RTs). As each trial required a response, the errors consisted of either errors of commission (saying “yes” to a non-target) or errors of omission (saying “no” to a target). In addition, demographic variables that differed across groups were included in analyses that included group comparisons. Finally, in order to explore the relevance of subjective craving, withdrawal symptoms, and lifetime cigarette consumption (pack-years) to any observed significant effects, follow-up repeated measures ANOVAs that included each of these variables (separately) were conducted. We were particularly interested in possible interactions between these variables and both group and experimental condition. The change in UTS craving and scores on the Shiffman/Jarvik Withdrawal Scale across sessions (pre-smoking in the abstinence session minus pre-smoking in the satiety session) were calculated to assess the degree to which change in craving was associated with the abstinence condition.
3. Results
3.1. Participants
Demographic information for the 15 smokers and 22 non-smokers is presented in Table 1. The groups did not differ significantly in age, race, gender or years of education. Smokers did have significantly higher WURS scores (t (33) = 2.14, p = 0.04), suggesting a higher incidence of childhood ADHD in the group. WURS score, therefore, was included in all analyses that included comparisons across groups. The smokers reported a current mean cigarette consumption of 20.4 ± 1.3 (Standard Error) cigarettes per day and an average smoking history of 15.4 ± 2.7 years. Their severity of nicotine dependence, as assessed by Fagerström scores (full range = 0–10), was moderate, 5.06 ± 0.5.
Table 1.
Characteristics of participants
| Non-smokers (n = 22) | Smokers (n = 15) | |
|---|---|---|
| Age (years) | 29 ± 1.7 | 35 ± 2.6 |
| % female | 59.1% | 40% |
| Years of education | 14.5 ± 0.34 | 14 ± 0.48 |
| Race | ||
| Caucasian (non-Hispanic) | 45.5% | 60% |
| Hispanic | 13.6% | 0% |
| African-American | 27.3% | 33.3% |
| Other | 13.6% | 6.7% |
| Beck depression inventory | 3.5 ± 0.81 | 3 ± 1.2 |
| Wender Utah rating scalea | 10.7 ± 1.7 | 22.3 ± 5.8 |
Values represent means ± standard errors of the means.
Group difference significant at p = 0.05.
3.2. N-back performance
3.2.1. Non-smokers versus smokers
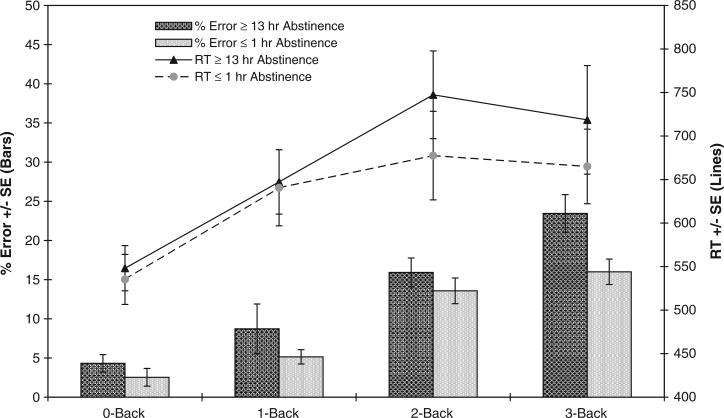
To assess the effect of smoking history on working memory, non-smokers and smokers after ≤1 h and ≥13 h abstinence were compared in separate analyses with WURS score included as a covariate. Data from only the first test block (i.e., performance prior to within-session smoking) of both sessions was compared. Smokers in relative satiety did not significantly differ from non-smokers in either RT (F(1, 32) = 0.34, p = 0.57) or error rate (F(1, 32) = 0.17, p = 0.68). Fig. 1 presents the RT and error rates for non-smokers and smokers abstinent ≥ 13 h. Though the groups did not differ significantly in RT (F(1, 32) = 1.7, p = 2.0), analysis of error rates indicated that smokers performed less accurately than non-smokers during the abstinence session (F(1, 32) = 6.8, p = 0.013).
Fig. 1.
N-Back performance of smokers (in the abstinence session) and non-smokers. Lines indicate mean RT's and bars indicate mean errors, both ± 1 SE, and both at each N-Back level. RT's of smokers in the abstinence session tended to be larger than those of non-smokers in the 2-Back; smokers in this session made more errors overall than non-smokers (p = 0.013).
3.2.2. The effects of abstinence on N-back performance
In order to assess the effects of abstinence on working memory, performance of smokers on test block 1 of the N-Back Task was compared between the abstinence (>13 h abstinence) and satiety (<1 h abstinence) sessions (see Fig. 2). A main effect of session was observed, with smokers performing more slowly in the abstinence session than in the satiety session (F(1, 14) = 5.18, p = 0.039). Correspondingly, smokers made significantly more errors in the abstinence session compared to the satiety session (F(1, 14) = 5.73, p = 0.032). A repeated measures ANOVA indicated that there was a significant interaction for reaction time between condition and session (F(3, 12) = 3.8, p = 0.04). Follow-up t-tests indicated that smokers in the abstinence session had significantly longer response times at the 2-back condition than during the satiety session (t (14) = 3.42, p = 0.004).
Fig. 2.
N-Back performance of smokers in abstinence and satiety sessions. Lines indicate mean RT's and bars indicate mean errors, both ± 1 SE, and both at each N-Back level. In the abstinence session, smokers responded more slowly, especially in the 2-back condition, and tended to make more errors compared with the satiety session ( p's < 0.04).
3.2.3. The effects of one cigarette on performance in the abstinence session
As within-session smoking always took place between the first and second blocks of the N-Back Task, we looked to the interaction between test block and session for possible statistical evidence of an effect that could be attributed to within-session smoking. A repeated measures ANOVA indicated no statistically significant interaction on RT (F(1, 14) = 0.30, p = 0.59) or errors (F(1, 14) = 1.65, p = 0.22).
3.2.4. Subjective craving, withdrawal measures and performance
The UTS craving scores, subscales of the Shiffman/Jarvik Withdrawal Scale and expired CO levels in each test block are presented in Table 2. Smokers reported significantly more negative psychological and physical symptoms in the first test block after >13 h abstinence than in the satiety session. We assessed the relation between the changes in subjective craving of smokers across sessions and the changes in N-Back performance across the same sessions. Since no reliable effect of within-session smoking on performance was observed, we limited this comparison to the first test block of abstinence vs. relative satiety difference scores. Repeated measures ANOVAs were performed to assess possible interactions between UTS and Shiffman/Jarvik change scores and both group and experimental condition. Our results indicated no significant interactions with performance. As a retrospective analysis, we ran a repeated measure ANOVA on performance data from smokers to investigate possible interactions between relative satiety vs. abstinence and smoking history (pack years) and age. After taking age into consideration, there was no significant effect of smoking history on performance by the smokers.
Table 2.
Withdrawal, craving, and CO measures
| Satiety session |
Abstinence session |
|||
|---|---|---|---|---|
| Pre-cigarette | Post cigarette | Pre-cigarette | Post cigarette | |
| Shiffman/Jarvik withdrawal scale | ||||
| Cravinga,b | 4.2 ± 1.5 | 4.3 ± 1.7 | 6.0 ± 0.93 | 4.0 ± 1.3 |
| Psychological symptomsa | 2.6 ± 0.6 | 3.0 ± 0.7 | 3.4 ± 1.2 | 3.1 ± 0.8 |
| Physical symptomsa | 1.7 ± 0.9 | 1.5 ± 0.9 | 2.4 ± 1.3 | 1.8 ± 1.1 |
| Sedationb | 2.2 ± 1.0 | 3.1 ± 1.2 | 2.5 ± 1.1 | 3.2 ± 1.7 |
| Anxiety | 3.9 ± 1.0 | 4.3 ± 0.8 | 4.2 ± 0.8 | 4.1 ± 1.0 |
| Urge to smoke craving scaleb | 4.5 ± 1.5 | 2.0 ± 1.6 | 5.7 ± 1.0 | 1.9 ± 0.83 |
| Carbon monoxide level (ppm)a,b | 18.9 ± 7.7 | 21.9 ± 9.2 | 4.27 ± 2.5 | 10.1 ± 4.0 |
Values indicate the means ± standard errors of the means.
p < 0.001 for difference between pre-cigarette abstinence session and pre-cigarette satiety session.
p < 0.001 for difference between pre-cigarette abstinence session and post-cigarette abstinence session.
3.3. Truncation of data: possible effects
The program for administering the task was written to record responses only after the 400 msec of stimulus presentation, truncating the data collected. The proportion of trials without a response recorded (because the subject responded either during the 400-ms. stimulus presentation or not at all) did not differ significantly across groups (31.16 ± 3.51% for non-smokers and 31.56 ± 6.020% for smokers; (t (35) = 0.06, p = 0.495). Because all analyses were conducted on mean scores across different conditions (N-back levels rather than on individual trials), understanding the effects of the missing data amounts to understanding the relationship between the resulting conditional means and the means that would have been observed without truncation. To examine this question directly, we collected data from 8 participants (4 smokers and 4 non-smokers), of whom 3 participated in the main study and 5 did not. The participants completed the same N-Back Task (administered identically) except that responses during the stimulus presentation were recorded, and the intact data from these 8 participants formed one set. A second set of data was then formed from these data by eliminating all responses made within the first 400 ms (simulating the truncation error in the main study). We then were able to empirically assess the relationship between the conditional means in the absence and presence of the truncation.
The correlation between the data sets was high for both reaction times and error rates (r = 0.97), with the conditional means of the truncated data accounting for nearly 95% of the variance in the conditional means of the intact data on each measure. Curve fitting indicated that the truncated RT data were fit as well to the original data by a linear transformation as by higher order transformations. This comparison is of interest since a linear transformation entails no effect on statistical analyses. A similar analysis suggested that missing data had little effect on mean accuracy scores. Even when accuracy scores for participants were separated by condition, with 0-, 1-, 2- and 3-back included as independent observations, a paired t-test indicated no systematic difference between accuracy rates on the intact data and those on the truncated data (t (31) = 0.47, p = 0.64).
4. Discussion
This study provides evidence for a working memory deficit associated with acute withdrawal in a sample of smokers with a moderate severity of nicotine dependence. Smokers after ≥13 h abstinence, but not in satiety, performed more poorly on the N-Back Task than did non-smokers. More directly, working memory performance by smokers was significantly slower, especially in the 2-back condition, and more prone to errors when the participants had abstained >13 h as compared to when they were in relative satiety. These data support the conclusion that cessation of smoking produces impairments in working memory. Such deficits, in turn, may contribute to the maintenance of smoking behavior.
Although we found a performance deficit in smokers compared to non-smokers, the impairment we saw appeared to be due to abstinence from smoking. An earlier study however, reported that smokers who were abstinent for >12 h had slower responses but demonstrated no difference in errors compared with non-smokers (Ernst et al., 2001a). In contrast, we found that abstinent smokers (>13 h) showed significant deficits in accuracy but not reaction time compared to non-smokers. Also, the abstinent smokers (≥13 h) responded with less speed and less accuracy than when at satiety. The present observations suggest that impaired performance reflects abstinence and is not a trait-like phenomenon.
Although the truncation of the data had minimal impact on the statistical inferences made, there is no guarantee that the effect of the data loss in the main study is the same as that observed in the subsample. However, particularly with respect to the observed effect of withdrawal on reaction times, it is difficult to envision a scenario in which the data truncation would effectively create, or even enhance a true difference across conditions.
Anticipating a deleterious effect of abstinence from smoking on performance of the N-Back Task, we tested for possible improvement from smoking a cigarette. Although nicotine gum previously produced no effect on performance in abstinent smokers (Ernst et al., 2001a), we reasoned that smoking might be effective, as components of the smoking experience, other than nicotine per se, influence behavior. In this regard, nicotine patches and gum do not reduce craving as well as smoking a cigarette (Baddeley & Della Salla, 1996; Schneider & Jarvik, 1984). Furthermore, experienced smokers report that even IV-nicotine, which more closely mimics the pharmacokinetics of smoking than do these dosage forms, produces less relaxation, less satisfaction, and less relief from craving than smoking (Westman, Behm, & Rose, 1996). Still, our results indicated that smoking one cigarette did not improve performance on the N-back task by abstinent smokers.
This lack of effect could reflect an insufficient restoration of nicotine levels by one cigarette. Alternatively, a positive effect may require sustained activation of nicotinic acetylcholine receptors or production of a neuromodulatory product. Notably, a return to baseline performance on a digit recall task was observed within 4–8 h but not 1 h of reinitiating smoking following deprivation for 10 days (Snyder et al., 1989). It is therefore possible that in contrast to the relatively well documented, immediate effect of nicotine to improve sustained and selective attention in abstinent smokers (Pritchard & Robinson, 1998), the normalization of working memory function may require more time. The negative finding of acute smoking on the N-back performance is, nevertheless, in conflict with observations that smoking a single cigarette restored function of the articulatory loop of working memory (Blake & Smith, 1997), and shortened reaction times on a Sternberg task (Houlihan et al., 2001). These contradictory results may reflect differences in the cognitive tests used, and the fact that working memory consists of various subcomponents, such as storage and manipulation of information and rehearsal of performance (Baddeley, 1996). The substrates of these subcomponents have been distinguished from one another neuroanatomically (Awh et al., 1996; Baker, Frith, Frackowiak, & Dolan, 1996; Cohen et al., 1997; McCarthy et al., 1996) and thus may also respond differently to smoking manipulations. It is also possible, particularly given that the effect of the smoking manipulation could only be assessed via an interaction with condition, that the lack of effect was a Type II error.
The present results were mixed with respect to the hypothesized relationship between subjective craving and the effects of smoking manipulations on working memory. Within-session smoking did not produce a statistically significant effect on performance, but it effectively reduced subjective craving and withdrawal symptoms. These observations suggest dissociation between the neural substrates underlying withdrawal-based working memory deficits and the factors underlying subjective craving. Between-session comparisons produced a decrease in craving and withdrawal by individual smokers in abstinence relative to satiety and corresponding decreases in latency, error rate, and CO levels. Although larger sample sizes are required for definitive statements, these data taken together suggest that overlapping but not identical factors contribute to cigarette craving and working memory impairments during withdrawal. To summarize, this study provides evidence for a deficit in working memory associated with acute withdrawal from smoking. Such a deficiency could contribute to the maintenance and relapse of smoking behavior.
Acknowledgments
The following sources of funding contributed to this research:
NIH grants RO1 DA014093.03 (EDL), RO1 DA015059 (ALB), R21 DA 13627 (MSC), and MOIRR 00865; UC Tobacco-Related Disease Research Program 10RT-0091 (EDL) and 11RT-0024 (ALB), Philip Morris USA 01082705 (EDL).
Footnotes
Data was collected at the University of California Los Angeles, Los Angeles, CA, USA.
References
- Awh E, Jonides J, Smith EE, Schumacher EH, Koeppe RA, Katz S. Dissociation of storage and rehearsal in verbal working memory: Evidence from positron emission tomography. Psychological Science. 1996;7:25–31. [Google Scholar]
- Baddeley A. The fractionation of working memory. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:13468–13472. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Della Salla S. Working memory and executive control. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1996;351:1397–1403. doi: 10.1098/rstb.1996.0123. [DOI] [PubMed] [Google Scholar]
- Baker SC, Frith CD, Frackowiak RS, Dolan RJ. Active representation of shape and spatial location in man. Cerebral Cortex. 1996;6:612–619. doi: 10.1093/cercor/6.4.612. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A. Assessment of depression: The depression inventory. Psychological Measurements in Psychopharmacology. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- Blake J, Smith A. Effects of smoking and smoking deprivation on the articulatory loop of working memory. Human Psychopharmacology. 1997;12:259–264. [Google Scholar]
- Carmelli D, Swan GE, LaRue A, Eslinger PJ. Correlates of change in cognitive function in survivors from the Western Collaborative Group Study. Neuroepidemiology. 1997;16:285–295. doi: 10.1159/000109699. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE. Effect of nicotine on brain activation during performance of a working memory task. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4728–4733. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevins A, Cutillo B. Spatiotemporal dynamics of component processes in human working-memory. Electroencephalography and Clinical Neurophysiology. 1993;87:128–143. doi: 10.1016/0013-4694(93)90119-g. [DOI] [PubMed] [Google Scholar]
- Giambra LM. A laboratory method for investigating influences on switching attention to task-unrelated imagery and thought. Consciousness and Cognition. 1995;4:1–21. doi: 10.1006/ccog.1995.1001. [DOI] [PubMed] [Google Scholar]
- Grobe JE, Perkins KA, Goettler-Good J, Wilson A. Importance of environmental distractors in the effects of nicotine on short-term memory. Experimental and Clinical Psychopharmacology. 1998;6:209–216. doi: 10.1037//1064-1297.6.2.209. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fägerström KO. The Fägerström test for nicotine dependence: A revision of the Fägerström tolerance questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Henningfield JE, Singleton EG. Tobacco, nicotine, and human cognition. Nicotine and Tobacco Research. 2002;4:3–4. doi: 10.1080/14622200110101955. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Snyder FR, Henningfield JE. Performance, subjective, and physiological effects of nicotine in non-smokers. Drug Alcohol Dependence. 1993;34:11–18. doi: 10.1016/0376-8716(93)90041-n. [DOI] [PubMed] [Google Scholar]
- Houlihan ME, Pritchard WS, Robinson JH. Effects of smoking/nicotine on performance and event-related potentials during a short-term memory scanning task. Psychopharmacology. 2001;156:388–396. doi: 10.1007/s002130100751. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Madsen DC, Olmstead RE, Iwamoto-Schaap PN, Elins JL, Benowitz NL. Nicotine blood levels and subjective craving for cigarettes. Pharmacology Biochemistry and Behavior. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Jarvis MJ, Russell MA, Saloojee Y. Expired air carbon monoxide: A simple breath test of tobacco smoke intake. British Medical Journal. 1980;281:484–485. doi: 10.1136/bmj.281.6238.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Puce A, Constable RT, Krystal JH, Gore JC, Goldman-Rakic PS. Activation of human prefrontal cortex during spatial and nonspatial working memory tasks measured by functional MRI. Cerebral Cortex. 1996;6:600–611. doi: 10.1093/cercor/6.4.600. [DOI] [PubMed] [Google Scholar]
- Park S, Knopick C, McGurck S, Meltzer HY. Nicotine impairs spatial working memory while leaving spatial attention intact. Neuropsychopharmacology. 2000;22:200–209. doi: 10.1016/S0893-133X(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Pritchard WS, Robinson JH. Effects of nicotine on human performance. In: Snel J, Lorist MM, editors. Nicotine, caffeine and social drinking: Behaviour and brain function. Harwood Academic Publishers; Amsterdam: 1998. pp. 21–81. [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biological Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: Evidence from a prospective birth cohort study. American Journal of Public Health. 2003;93:994–998. doi: 10.2105/ajph.93.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinka JA, Vanderploeg RD, Rogish M, Graves AB, Mortimer JA, Ordoric PI. Effects of the use of alcohol and cigarettes on cognition in elderly adults. Journal of the International Neuropsychological Society. 2002;8:811–818. doi: 10.1017/s135561770286009x. [DOI] [PubMed] [Google Scholar]
- Schneider NG, Jarvik ME. Nicotine vs. placebo gum in the alleviation of withdrawal during smoking cessation. Addictive Behaviors. 1984;9:149–156. doi: 10.1016/0306-4603(84)90052-2. [DOI] [PubMed] [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: Performance decrements assessed on a computerized test battery. Drug and Alcohol Dependence. 1989;23:259–266. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Spilich GJ, June L, Renner J. Cigarette smoking and cognitive performance. British Journal of Addiction. 1992;87:1313–1326. doi: 10.1111/j.1360-0443.1992.tb02740.x. [DOI] [PubMed] [Google Scholar]
- Toneatto T. A metacognitive analysis of craving: Implications for treatment. Journal of Clinical Psychology. 1999;55:527–537. doi: 10.1002/(sici)1097-4679(199905)55:5<527::aid-jclp1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. Wender Utah rating scale: An aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. American Journal of Psychiatry. 1993;150:885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacology Biochemistry and Behavior. 1996;53:309–315. doi: 10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]
- Williams DG. Effects of cigarette smoking on immediate memory and performance in different kinds of smoker. British Journal of Psychology. 1980;71:83–90. doi: 10.1111/j.2044-8295.1980.tb02732.x. [DOI] [PubMed] [Google Scholar]