Summary
Despite advances in promoting axonal regeneration after acute spinal cord injury (SCI), elicitation of bridging axon regeneration after chronic SCI remains a formidable challenge. We report that combinatorial therapies administered 6 weeks, and as long as 15 months, after SCI promote axonal regeneration into and beyond a mid-cervical lesion site. Provision of peripheral nerve conditioning lesions, grafts of marrow stromal cells and establishment of NT-3 gradients, supports bridging regeneration. Controls receiving partial components of the full combination fail to exhibit bridging. Notably, intraneuronal molecular mechanisms recruited by delayed therapies mirror those of acute injury, including activation of transcriptional activators and regeneration-associated genes. Collectively, these findings provide evidence that regeneration is achievable at unprecedented post-injury time points.
INTRODUCTION
Injured axons fail to spontaneously regenerate in either acute or chronic stages of spinal cord injury (SCI), resulting in persistent functional loss. Knowledge gained over the last several years indicates that the failure of axonal regeneration after CNS injury is attributable at least in part to: 1) an absence of permissive substrates to support axonal attachment and extension through lesion sites (Bunge, 2001), 2) a lack of neurotrophic stimulation (Jones et al., 2001), 3) myelin-based (Filbin, 2003) and extracellular matrix inhibitors in the injured region (Fawcett, 2006), 4) partial deficiency in the intrinsic growth capacity of adult neurons (Neumann et al., 2002; Qiu et al., 2002), and 5) extensive secondary damage resulting from inflammatory mechanisms (Jones et al., 2005). Chronic SCI presents additional obstacles: 1) glial scars and inhibitory extracellular matrices become well-established around the lesion site (Busch and Silver, 2007), a mechanism that is thought to increase the refractory nature of the chronically injured spinal cord to regeneration; 2) chronically injured neurons downregulate regeneration-associated genes and become atrophic (Kwon et al., 2002); 3) injured axons exhibit varying degrees of retraction and retrograde degeneration (Pallini et al., 1988), and 4) progressive anterograde degeneration in white matter beyond lesion sites potentially generates a particularly refractory milieu for axonal extension (Houle and Tessler, 2003). Collectively, these mechanisms constitute formidable barriers to regeneration in the chronically injured state.
Recent studies indicate that experimental strategies targeting multiple mechanisms that limit adult CNS plasticity and regeneration may be capable of eliciting axonal regeneration into and beyond sites of CNS injury, when administered immediately after injury (Lu et al., 2004; Pearse et al., 2004; Houle et al., 2006). These mechanisms include trophic factor availability (Lu et al., 2004), the provision of permissive cellular or extracellular matrices within injury sites ( Lu et al., 2004; Pearse et al., 2004; Houle et al., 2006), and augmentation of the intrinsic growth state of the neuron by pre-conditioning lesions (pre-CL) or cAMP administration (Lu et al., 2004; Pearse et al., 2004). We hypothesized that combinatorial therapies administered at prolonged time points after SCI: 1) would exhibit persistent effects in eliciting the expression of regeneration-associated genes, 2) thereby enhance the intrinsic growth capacity of sensory neurons, and 3) when combined with delayed application of neurotrophic factors beyond the lesion site, would be sufficient to elicit bridging axonal regeneration. We now report successful bridging regeneration of adult CNS axons when treatment is initiated from 6 weeks to as long as 15 months after the original injury, generated by modification of both cell-extrinsic and cell-intrinsic growth mechanisms.
RESULTS
Combinatorial Treatments Promote Bridging Axonal Regeneration When Administered Six Weeks After SCI
We initially examined whether combinatorial therapies would promote bridging sensory axonal regeneration when treatment was delayed 6 weeks after SCI. A total of 51 adult F344 rats underwent C3 spinal cord dorsal column wire knife lesions (Lu et al., 2004; Taylor et al., 2006). Six weeks later, combinatorial therapies were applied consisting of: 1) a peripheral CL (sciatic nerve crush bilaterally) to upregulate neuron-intrinsic regeneration-associated gene expression (Neumann and Woolf, 1999); 2) placement of a syngenic bone marrow stromal cell (MSC) graft mixed with 1µg NT-3 protein in the established C3 cystic lesion cavity, 1 week after CL (7 weeks after the initial SCI) (Lu et al., 2004);and 3) provision of a neurotrophic factor gradient by injection of lentivirus expressing neurotrophin-3 (NT-3) into dorsal column white matter (Taylor et al., 2006), 2.5mm rostral to the lesion site, 1 week after CL (7 weeks after the initial SCI, n=16 animals; Fig. 1A). In addition, 4 control groups were examined: a) 9 subjects received C3 lesions, CL 6 weeks later, and a cell graft in the lesion site an additional week later; neither NT-3 protein nor vector were administered; b) 9 subjects received C3 lesions, a CL 6 weeks later, then a cell graft mixed with 1µg NT-3 protein in the lesion site and lentivirus expressing the reporter gene Green Fluorescent Protein (GFP) into the dorsal white matter 2.5mm beyond the lesion an additional week later; NT-3 beyond the lesion was not provided; c) 8 subjects underwent C3 lesions, no CL, and delivery of 1µg NT-3 protein into a cell graft in the lesion site plus Lenti-NT-3 vector 7 weeks after the initial injury; and d) 9 subjects underwent C3 lesions and a cell graft 7 weeks after the initial injury. Of note, chronic scars surrounding the cystic lesion cavity were not resected in any case, to avoid damage to the surrounding spinal cord (Tuszynski et al., 2003); thus, bridging axonal regeneration in this paradigm would require growth into and beyond the peri-lesion scar. An additional 6 weeks later, or 13 weeks after the original C3 lesion, dorsal column sensory axons were traced transganglionically by injections of cholera toxin B (CTB) subunit into the sciatic nerve, and animals were sacrificed 3 days later.
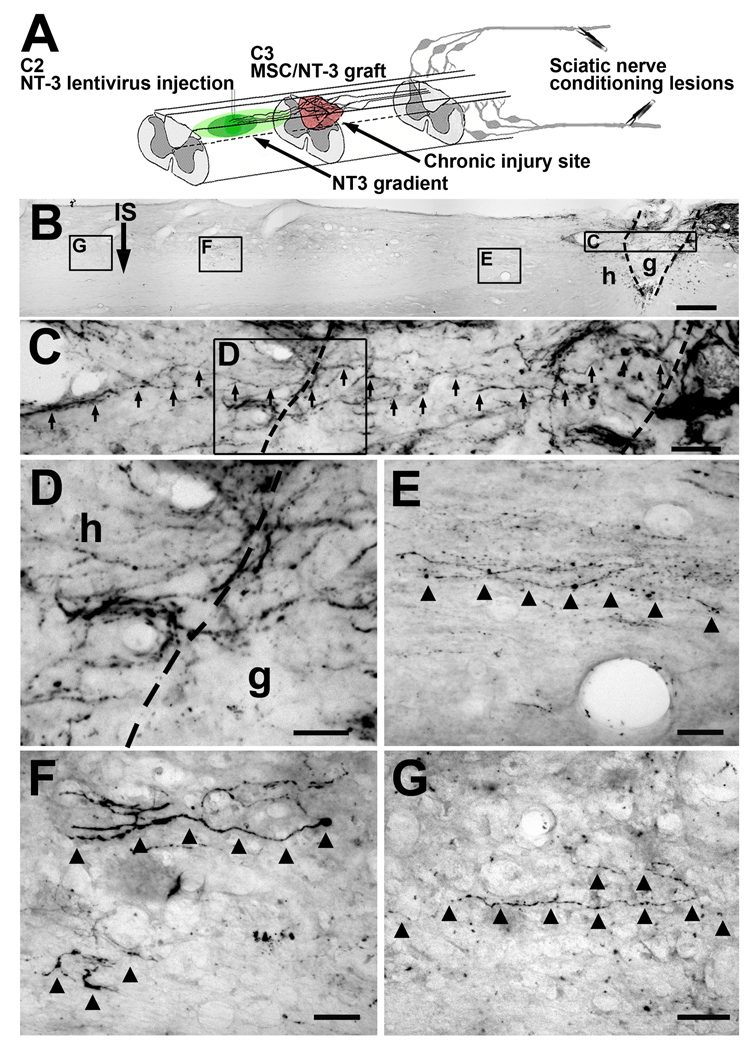
Figure 1.
Sensory axons regenerate beyond the lesion site when treatments influencing both neuron-intrinsic and environmental mechanisms are applied 6 weeks after the original SCI. (A) Injury model and delayed combination therapy. Dorsal column sensory axons were completely transected at C3. Six weeks or 15 months later, L4-6 DRG neurons were conditioned by sciatic nerve crush. One week later, MSCs were injected into the lesion site, and NT-3-expressing lentivirus was injected 2.5mm rostral to the lesion site. Animals survived an additional 6 weeks. (B) Low magnification sagittal overview from subject that received delayed combination therapy. CTB-labeled dorsal column sensory axons are evident approaching lesion/graft site on upper right side of panel. Rostral left, caudal right. g, graft; h, host; IS, lenti-NT-3 injection site 2.5mm rostral to lesion border. Scale bar 200µm. Areas of higher magnification are indicated in boxed regions. (C) Higher magnification of graft: axons indicated by arrows course through graft in irregular trajectories; several axons cross rostral interface with host (dashed line in box C) to enter host spinal cord beyond the lesion. (D) High magnification of panel C at interface of graft border with host beyond the lesion, demonstrating sensory axons crossing interface. Notably, axons cross at several dorsoventral levels of host/graft interface, not simply at most dorsal or ventral aspects of grafts where spared axons might be mistaken for regenerating axons. (E) Several varicose axons continue to extend 500µm beyond lesion site in host white matter. (F) 2mm and (G) 2.5 mm beyond the lesion, bridging axons remain visible in host white matter. Lesion completeness was confirmed by sectioning medulla, showing absence of CTB labeling (Suppl. Fig. 1). Scale bar C = 40µm; D–G = 20µm.
Notably, axonal bridging beyond the lesion occurred only in animals that received combinatorial treatment with CLs, MSC grafts in the lesion cavity, and lenti-NT-3 injections (Fig. 1, 2). 10-of-16 animals in the full treatment group exhibited axonal bridging beyond the lesion site; in the 6 remaining animals, lenti-NT-3 failed to diffuse from the rostral injection site to the host/graft interface (Suppl. Fig. 1), and no axonal bridging was observed in these subjects. In subjects with complete vector diffusion from the injection site to the graft in the lesion cavity, axons extended in several instances the full 2.5mm distance beyond the lesion to the rostral site of lenti-NT-3 injection. Further, growth occurred through host white matter undergoing Wallerian degeneration. The morphology of extending axons was not typical of intact axons: circuitous trajectories and frequent swellings of axons were evident (Fig. 1, 2). Lesions were complete, confirmed by absence of CTB label in the nucleus gracilis of all subjects (Suppl. Fig. 1). Thus, regeneration of axons into and beyond the lesion site in combination-treated animals was not an artifact of axon sparing.
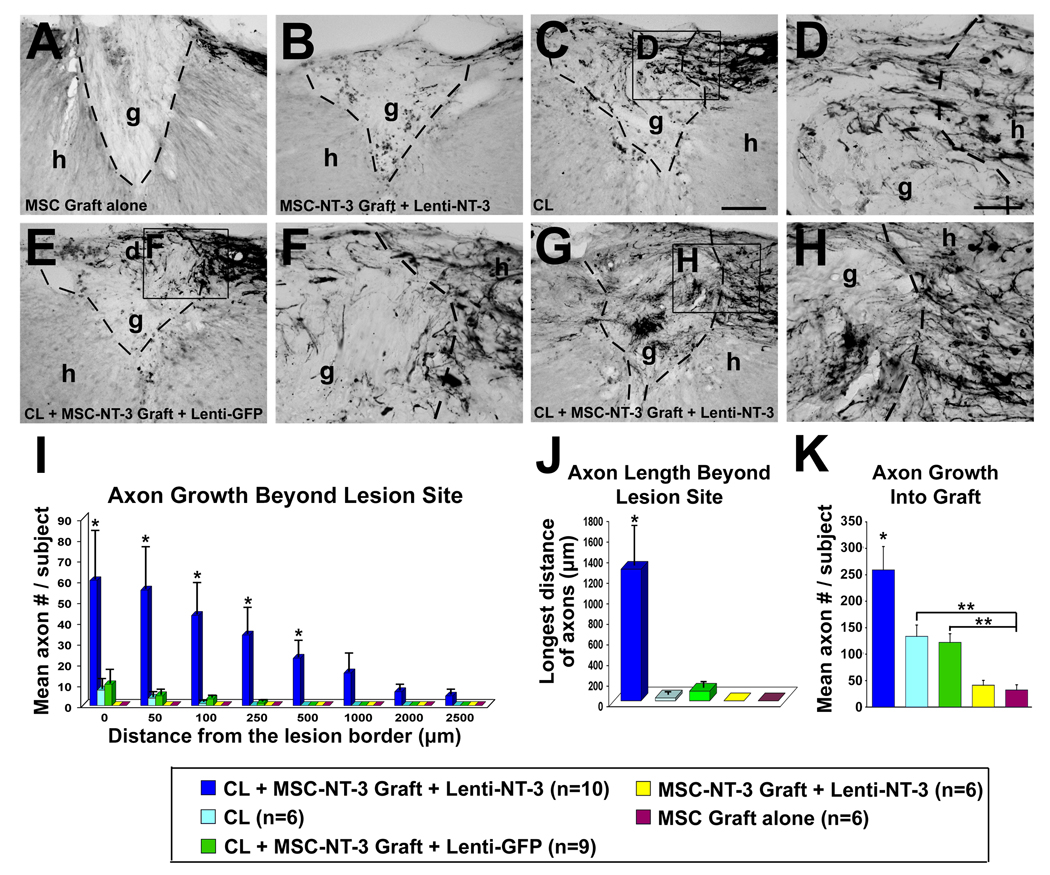
Figure 2.
Penetration of lesion site and quantification of regeneration. Sagittal sections of C3 lesion/graft site, 3 months after original injury. Rostral left, caudal right. (A) Subjects treated only with MSC grafts demonstrate rare penetration of CTB-labeled sensory axons into graft, and no bridging. g, graft; h, host. Lines indicate host/graft interface. (B) Injection of lenti-NT-3 vector beyond lesion site, and NT-3 protein into MSC graft in lesion site, results in slightly enhanced axon penetration of graft but no axonal bridging beyond graft. (C, D) CLs significantly enhance axon penetration into graft, but axons rarely extend beyond the lesion, and never for distances greater than 100μm. D is higher magnification of panel C, showing axonal entry into graft from caudal host/graft interface. (E, F) Treatment with CL and NT-3 protein within MSC graft, and Lenti-GFP injection beyond, fails to support axonal bridging beyond that observed with CL alone. F is higher magnification of panel E, showing axonal entry into graft from caudal host/graft interface. (G, H) As noted in Figure 1, combinatorial treatment with CL and NT-3 gradients beyond the lesion support axon entry into graft and significant axonal bridging beyond lesion. H is higher magnification of panel G, showing axonal entry into graft. (I) Quantification of regeneration beyond lesion/graft site. Animals that received CLs plus NT-3 gradients exhibit significantly more sensory axons regenerating up to 500μm beyond the lesion (Kruskall Wallis test, χ2 p<0.03; Dunn post-hoc tests with Bonferroni correction p<0.005). (J) Similarly, quantification of longest bridging axon per subject demonstrates significant effects of combinatorial treatments (Kruskall Wallis test, χ2 p<0.01; Dunn post-hoc with Bonferroni correction p<0.005). (K) Quantification of axon number within graft demonstrates greatest growth into grafts in subjects receiving CLs and NT-3 (ANOVA p<0.0001; posthoc Fisher’s *p<0.005 compared to all groups). Subjects that received CLs with or without single injections of NT-3 protein in the graft also showed greater axon penetration than subjects with grafts only (posthoc Fisher’s *p<0.05 in both cases). Scale bar A, B, C, E, G = 100 µm; D, F, H= 50 µm.
Axon quantification beyond the lesion revealed significantly greater regeneration in combination-treated subjects compared to all other groups (χ2 p<0.03; Dunn posthoc with Bonferroni correction, p<0.005; Fig. 2I). Similarly, the maximum distance of axonal regeneration was significantly greater in animals receiving combination treatment (χ2 p<0.01; Dunn posthoc with Bonferroni correction, p<0.005;Fig. 2J). Among controls, axon growth beyond the lesion was detectable only among subjects that received MSC grafts plus CLs, and only for very short distances of 100µm or less (Fig. 2I). Unlike findings in models of acute SCI (Taylor et al., 2006), treatment with MSC grafts plus lenti-NT-3, in the absence of a CL, failed to elicit detectable axon extension for even short distances beyond the lesion (Fig. 2I). On the other hand, CLs with or without NT3 protein in the graft significantly increased penetration of sensory axons into MSC grafts in the lesion cavity, relative to non-CL controls (ANOVA p<0.0001; post-hoc Fisher’s p<0.05; Fig. 2K).
Combinatorial Treatments Promote Bridging Axonal Regeneration When Administered More Than One Year After SCI
Next, we determined whether the same combinatorial therapies would promote bridging axonal regeneration when administered 15 months after SCI. 27 adult Fischer 344 rats underwent C3 dorsal column lesions as described above. 15 months later, subjects underwent bilateral CLs of the sciatic nerve. One week later, the chronic lesion site was re-exposed, and syngenic MSCs were grafted into the chronic lesion cavity and lenti--NT-3 was injected 2.5mm rostral to the lesion site (n=11 animals). In addition to this “full treatment” group, the following controls were examined. a) 4 subjects received MSC grafts, no CL, and injection of lenti-GFP 2.5mm rostral to the lesion (i.e., no NT-3 gradient); b) 6 subjects received MSC grafts, no CL, and injection of lenti-NT-3 2.5mm rostral to the lesion; and c) 6 subjects received MSC grafts, CLs, and lenti-GFP 2.5mm rostral to the lesion.
Of note, regeneration beyond the lesion was observed in full combination treatment animals when treatment was initiated 15 months after the original lesion (Fig. 3). 5-of-11 animals in the full treatment group exhibited axonal bridging beyond the lesion site; in 2 out of the 6 remaining animals, lenti-NT-3 vector failed to diffuse from the rostral injection site to the host/graft interface (Suppl. Fig. 1); in 1 out of the 6 remaining animals, the graft did not survive. CTB immunolabeling demonstrated dorsal column sensory axons first penetrating, and then emerging from, the graft/host interface and extending into rostral host white matter. Axons exhibited varicose morphology with frequent swellings, and circuitous trajectories, unlike spared axons (Fig. 3, 4). Further indicating that these were not spared axons, medullary sectioning confirmed absence of CTB labeling (Suppl. Fig. 1). Triple labeling for CTB, GFP, and GFAP demonstrated that regenerating axons passed through the GFAP immunoreactive border within the lesion site, and beyond into host white matter. Notably, axons regenerating in host white matter were invariably associated with regions of lenti-NT-3 expression, indicated by co-association of axons with the reporter gene GFP (Fig. 4A–F). As noted above, the peri-lesion scar was not resected following the original lesion to avoid further damage to the spinal cord. Control groups lacking the combination treatment did not exhibit axonal bridging (Fig. 4G–I).
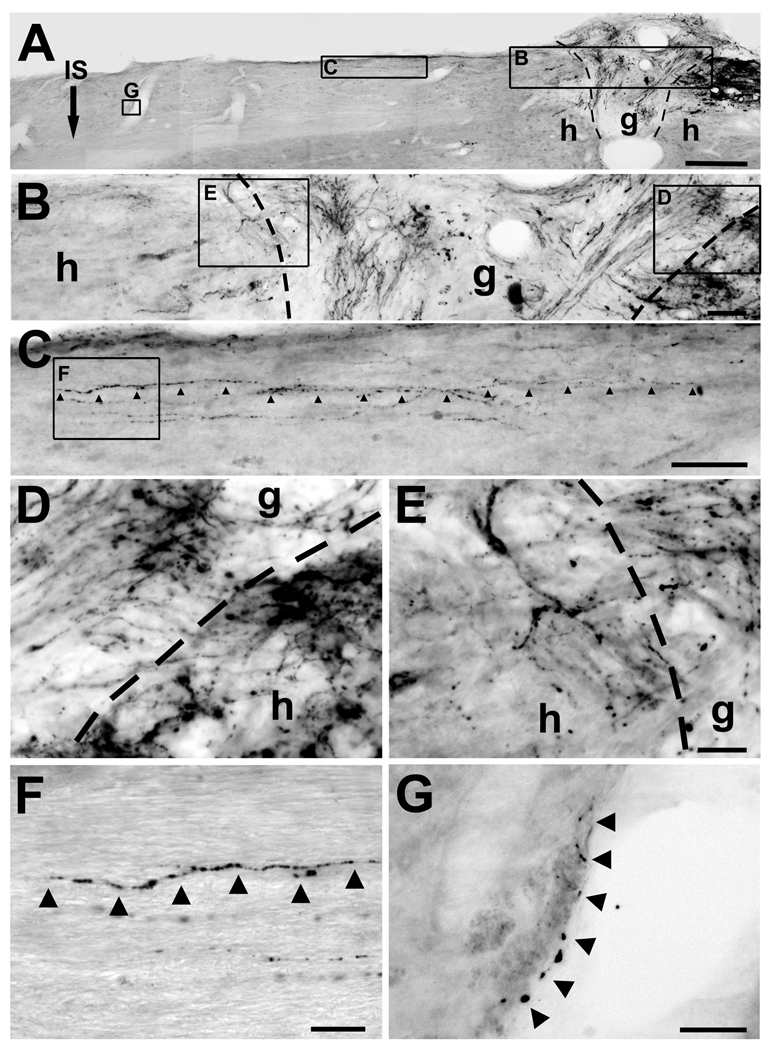
Figure 3.
15 months delayed combinatorial treatment with CLs, NT-3 gradients, and MSC graft in lesion site promotes sensory axonal bridging in chronic SCI. (A) Low magnification sagittal overview. CTB-labeled sensory axons are evident approaching lesion/graft site on upper right side of panel. Rostral left, caudal right. g, graft; h, host; IS, lenti-NT-3 injection site, 2.5 mm rostral to lesion; dashed lines, graft/lesion border. Scale bar 300µm. (B) Higher magnification of graft/lesion region. CTB-labeled sensory axons enter graft (box D) and extend beyond graft into host spinal cord rostral to lesion (box E). (D, E) Higher magnification of panel B, including caudal (D) and rostral (E) interface of graft with host. Sensory axons cross host/graft interface at several dorsoventral levels. (C) High magnification of panel A: arrowheads indicate a varicose axon extending 1200µm beyond lesion site in host white matter. (F) High magnification of panel C. Arrowheads indicate a regenerating, varicose axon. (G) High magnification of panel A demonstrates a regenerating axon 2200µm beyond lesion site in host white matter, extending in close association with blood vessel wall. All lesions were complete, indicated by sectioning of medulla (Suppl. Fig. 1). Scale bar B, C = 50µm; D, E = 20µm, F = 20µm, G = 25µm.
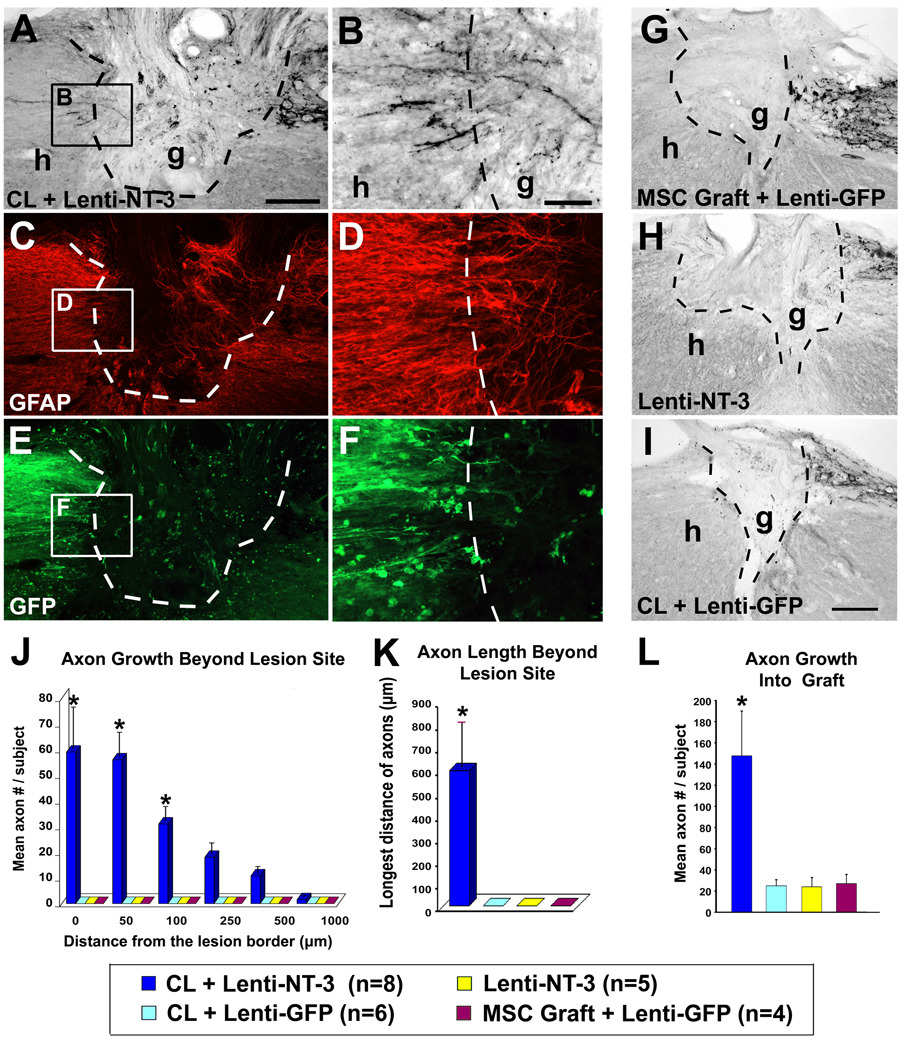
Figure 4.
Only subjects undergoing stimulation of both the chronically injured neuron and the lesioned environment exhibit significant axonal bridging, when treatment is initiated 15 months after SCI. (A–F) Sagittal sections of C3 lesion/graft site, 16.5 months after the original injury, triple labeled for CTB (A, B), GFAP (C, D) and GFP (E, F); rostral left, caudal right. In subjects that received CLs, cell grafts and NT-3 vector injections beyond the lesion site 15 months after the original injury, (A, B) CTB-labeled sensory axons extend into and then (B) emerge from the lesion site. (C, D) GFAP labeling outlines the lesion border; regenerating axons extend into and beyond the GFAP-reactive lesion boundary. (E, F) GFP labeling indicates extension of the lentiviral-NT-3 trophic gradient from the site of vector injection to the immediate host/graft interface: axons bridged beyond the lesion site only when lentiviral-NT-3 vector spread to the lesion/graft boundary (see Suppl. Fig. 1). CTB-labeled sensory axons penetrated grafts, but did not bridge beyond the lesion site, in subjects treated with (G) cell grafts alone, (H) lentiviral-NT-3 gradients + cell grafts but no CL, or (I) CLs + cell grafts, but no NT-3 gradient. (J) Quantification of axon growth beyond lesion/graft site. Only animals that received full treatment exhibited sensory axonal regeneration beyond the lesion site. (Kruskall Wallis test, χ2 p<0.003; Dunn post-hoc with Bonferroni correction p<0.008). (K) Longest bridging axon beyond the graft, per subject. (Kruskall Wallis test, χ2 p<0.003; Dunn post-hoc with Bonferroni correction p<0.008) (L) Quantification of axon number within graft demonstrates significantly greater axonal penetration in subjects receiving full treatment (ANOVA p<0.01; post-hoc Fisher’s *p<0.01 comparing combination treatment to all other groups). Scale bar A, C, E = 200µm; B, D, F = 50µm, G, H, I = 200µm.
Quantification of axon number beyond the lesion revealed significantly greater regeneration in combination-treated subjects compared to all other groups (χ2 p<0.003; Dunn posthoc with Bonferroni correction, p<0.008; Fig. 4J). Similarly, the maximum distance of axonal regeneration was significantly greater in animals receiving combinatorial treatment (χ2 p<0.003; post-hoc p<0.008; Fig. 4K). Groups treated with CLs alone, or lenti-NT-3 injections alone, exhibited few sensory axons regenerating into grafts and no regeneration beyond the lesion (Fig. 4J–L). Thus, axons emerged from the graft and regenerated beyond only when treatments modified both the lesioned environment and the intrinsic state of neuronal activation.
Delayed Conditioning Lesions Increase Neuron-Intrinsic Growth Programs
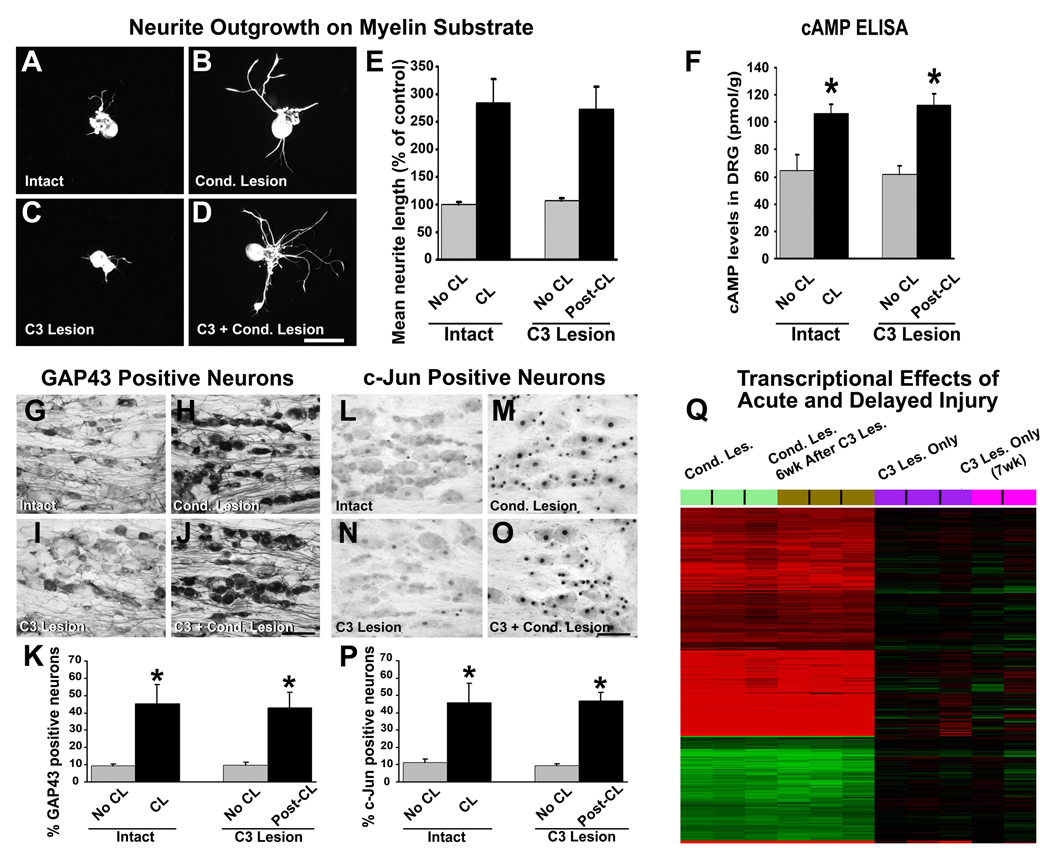
To investigate intrinsic neuronal mechanisms related to enhanced axonal regeneration at prolonged times after SCI, we examined the expression of neuronal growth programs. This is particularly important as a previous study reported that CLs must be applied before SCI to promote regeneration (Neumann et al., 1999). We performed both in vitro assays and gene array analyses. For in vitro studies, adult rats underwent C3 dorsal column lesions, and 6 weeks later sciatic nerves were crushed. One week later, sensory neurons of lumbar dorsal root ganglia (DRG) L4-6 were cultured on myelin substrates for 48 hr. Neurite outgrowth was compared to sensory neurons obtained from: 1) intact subjects, 2) animals that underwent C3 lesions 6 weeks earlier, without subsequent CLs, and 3) animals that underwent CLs only, 1 week prior to explant. As previously described, sensory neurons from animals that underwent CL only (no central lesion) exhibited longer neurites compared to neurons from intact rats (p<0.0001 ANOVA; p<0.0001 post-hoc Fisher’s; Fig. 5E) (Smith and Skene, 1997). Sensory neurons from animals that underwent CLs 6 weeks after a C3 lesion also exhibited enhanced neurite outgrowth compared to neurons from intact rats (p<0.0001 post-hoc Fisher’s; Fig. 5E) that was equal in degree to animals that underwent CLs alone (Fig. 5E). In contrast, central lesions alone, without a CL, did not enhance outgrowth (p=0.54 post-hoc Fisher’s). Thus, growth of injured sensory neurons can be enhanced by CLs applied even 6 weeks after central injury.
Figure 5.
Delayed CLs increase neuron-intrinsic growth programs. (A–D) Lumbar DRG neurons were cultured on myelin for 48 hours and labeled with NF200. Neurons from (A) naïve controls extend shorter neurites than (B) CLs 1 week prior to isolation (pre-CL). (C) Dorsal column lesions 6 weeks prior to isolation have no influence on neurite extension, whereas (D) CLs applied 6 weeks following C3 lesions (post-CL) significantly enhance neurite extension. Scale bar A–D = 80 µm. (E) Quantification of neurite outgrowth as mean percentage of control (A) indicates that both pre-CL and post-CL significantly enhance neurite outgrowth on myelin compared to intact animals and animals that received only C3 lesions (ANOVA p<0.0001; post-hoc Fisher’s * p<0.0001). (F) Measurement of cAMP levels by ELISA 1 week after either pre- or post-CL demonstrated significant elevations over basal levels (ANOVA p<0.003; post-hoc Fisher’s * p<0.001); C3 lesions alone have no effect. (G–K) Immunolabeling for GAP43 in lumbar DRG neurons shows weak immunoreactivity in (G) DRGs from naïve control animals. (H) Pre-CLs increase intensity and number of labeled neurons. (I) C3 lesions alone placed 6 weeks earlier do not increase GAP43 immunolabeling, whereas (J) post-CLs increase GAP43 labeling to same degree as pre-CL. (K) Quantification of percentage of GAP43-labeled neurons reveals significant increase after pre- and post-CL (ANOVA p<0.01; post-hoc Fisher’s * p<0.01). (L–P) Immunolabeling for c-Jun in lumbar DRG neurons also shows few labeled nuclei in (L) naïve control animals. (M) Pre-CL increases the number and intensity of neurons labeled for c-Jun. (N) C3 lesions alone, 6 weeks earlier, do not increase c-Jun, whereas (O) post-CL 6 weeks after C3 lesions increases c-Jun labeling to same degree as pre-CL. (P) Quantification shows significant increase in percentage of c-Jun-labeled neurons after pre- and post-CLs (ANOVA p<0.003; post-hoc Fisher’s *p<0.005). Scale bar G–J, L–O = 80 µm. (Q) Heatmap illustrating hierarchical clustering of gene expression from rat DRG extracted 1 week after pre- or post-CLs, or after C3 lesion alone. Comparisons in each case are made to intact DRGs. Red indicates increased, and green indicates decreased, gene expression. Groups of samples are color-coded. (Light green) Pre-CL upregulates more than 3,000 probes compared to intact DRG. (Brown) Post-CL persistently regulates, with similar intensity, a nearly identical set of probes compared to a pre-CL. In contrast to gene changes observed after CL, a C3 central lesion results in perturbation of remarkably fewer probe sets, whether gene changes are examined acutely after C3 lesion (purple) or seven weeks after C3 injury (pink).
Next, we examined expression of the regeneration-associated genes GAP43 and c-Jun in DRG neurons (Raivich et al., 2004). A 4-fold increase in the number of neurons labeled for GAP43 and c-Jun protein was observed when CLs were applied 6 weeks after C3 injury compared to intact DRGs (p<0.01 ANOVA; p<0.01 post-hoc Fisher’s), equal to increases observed when CLs were applied without a central lesion (“pre-CL”) (Fig. 5G–P). C3 lesions alone, without CL, did not increase GAP-43 or c-Jun. cAMP levels in DRGs, measured 1 week after placement of CLs, were also elevated equally when CLs were placed 6 weeks after a central lesion or as “pre-CLs” (Fig. 5F).
Next, we performed gene array analysis using Affymetrix whole-genome chips in 4 groups of adult rats: Group 1 DRGs were harvested 1week after CL (n=9 animals, pooling mRNA from 3 subjects, providing 3 arrays per group); Group 2 DRGs were harvested 7 weeks after C3 spinal cord lesion and 1 week after CL (i.e., CLs were placed 6 weeks after SCI; n=9 animals, 3 arrays); Group 3 DRGs were harvested 1 week after C3 lesion (no CL; n=9, 3 arrays); and Group 4 DRGs were harvested 7 weeks after C3 lesion (no CL; n=9, degradation of pooled mRNA in one group resulted in 2 arrays from 6 animals in this group). mRNA was also obtained from DRGs of 12 intact animals (4 arrays). Using a significance criterion of 5% false discovery rate (FDR) and a log ratio greater than 0.2 (15%), we found 5,609 differentially expressed probes in subjects that underwent CLs (Group 1) compared to intact animals: of these, 2,936 probes were upregulated and 2,673 were downregulated (Fig. 5Q). In marked contrast, no probes changed at this level of significance in samples from animals with C3 lesions only (no CL, Groups 3 and 4). Thus, peripheral but not central lesions led to extensive and long-lasting changes in gene expression. Notably, CLs performed 6 weeks after C3 lesions also resulted in extensive changes in gene expression (6,092 differentially expressed probes), and these changes overlapped to an exceptionally high degree with animals that underwent pre-CLs. Indeed, 4,244 probes, corresponding to 76% of all probes differentially expressed in animals that received pre-CLs, were also differentially expressed in animals that underwent “post-CLs”. Probes for GAP-43 and c-Jun were elevated in subjects with pre- and post-CLs (+85−92% and +227−201% elevations, respectively, relative to controls), providing independent confirmation of probe data. Functional annotation identified, among others, modulation of transcriptional networks centered around GAP-43 and c-Jun in both groups with CLs, whereas a C3 lesion had little effect on these pathways (Suppl. Fig. 2).
DISCUSSION
These findings indicate that bridging axonal regeneration in the adult CNS can be induced when experimental treatments are administered at unprecedented delays of more than a year after SCI. Regeneration after established injury requires modification of both the intrinsic growth state of the neuron, achieved by CLs, and modification of the non-permissive injury environment, accomplished by cell grafting and placement of growth factor gradients beyond the lesion site. Manipulation of the intrinsic neuronal growth state alone, or the environment alone, are insufficient to support axonal bridging beyond the lesion. Delayed conditioning of the injured neuron elicits modulation of broads set of genes remarkably similar in profile to CLs placed before central injury, suggesting extensive recruitment of intrinsic molecular mechanisms that contribute to axonal regeneration. Thus, neuron-intrinsic mechanisms and the injured environment can both be modified at extended delays after injury to successfully elicit axonal bridging into, and beyond, sites of SCI.
Few studies have examined axonal regeneration when therapies are administered at delays after injury, and most of these have examined relatively brief delays of 1–8 weeks (Houle and Tessler, 2003). Further, most delay treatment paradigms resect the “chronic scar” (Coumans et al., 2001; Jin et al., 2000), risking spinal cord re-injury (Tuszynski et al., 2003). Of these studies, only one to our knowledge has reported axonal growth beyond the lesion, using a combination of a fetal graft and growth factors applied to a re-lesioned spinal cord 4 weeks after the original injury (Coumans et al., 2001). The latter study reported regeneration through both gray matter and inhibitory white matter below the lesion resulting purely from cell-extrinsic experimental manipulations within the lesion site; however, examples of specific axons leaving the graft site were not provided. In contrast, axonal bridging in the present study is observed when experimental therapies are provided at exceptionally prolonged delays after the original injury, and only when both the intrinsic neuronal growth state and the surrounding, inhibitory environment are modified, consistent with findings in acute injury models (Lu et al., 2004). Re-lesioning the spinal cord was not necessary to achieve axonal growth either into or beyond the chronic lesion site in this study, enhancing clinical relevance: re-lesioning the spinal cord risks further deterioration of function, particularly in cervically injured subjects that are critically dependent of neural systems spared immediately above the lesion site to support residual function.
We examined a long-projecting axonal system, the dorsal column sensory tract, as a model system because the projection is well defined anatomically, it normally fails to regenerate after central injury, and lesion completeness is readily verifiable by examination of the gracilar target of this projection (Lu et al., 2004; Taylor et al., 2006). Five observations suggest that axons observed beyond the lesion actually regenerated, and were not simply spared. First, the morphology of axons extending beyond the lesion site was circuitous and their course was nonlinear, unlike intact axons (Fig. 1, 3). Second, CTB-labeled axons emerged across the lesion-host interface at different dorsoventral levels of the graft, not simply the most ventral or dorsal aspects of the graft/lesion site where spared axons might be located (Fig. 1–Fig 4). Third, CTB-labeled axons were not observed ventral to the lesion site or in lateral, unlesioned portions of the spinal cord. Fourth, the lesion site was devoid of GFAP immunoreactivity, which would otherwise be present in strands of spared spinal cord parenchyma (Fig. 4). Fifth, sectioning of the medulla through the entire extent of the nucleus gracilis revealed an absence of CTB-labeled axons in all lesioned subjects, indicating that lesions were complete, whereas dense labeling was readily detected in unlesioned subjects (Suppl. Fig. 1). The latter observation also indicates that axons did not regenerate over extended distances to re-enter the denervated nucleus.
Having established that bridging axonal regeneration is feasible in the sensory model at remarkably extended delays after injury, findings can be adapted to descending motor projections of the spinal cord. While pools of axons recruitable for regeneration persist at prolonged time points post-injury, the number of sensory axons regenerating beyond the lesion site is lower when therapy is applied 15-months post-injury compared to 6 weeks post-injury, and the distance that axons regenerate beyond the lesion is also reduced after prolonged treatment times (Fig. 2, 4). For these reasons, attempts to achieve chronic functional recovery should likely target cervical level lesions, because potential neuronal targets of regenerating axons can be contacted immediately below the lesion site. If reinnervation of denervated motor neuron pools even one level below a lesion were achieved, the impact on quality of life in spinal cord-injured subjects could be substantial, potentially providing movement of a wrist or hand that could support greater independence. Incremental improvements in function are a more tractable goal for human translation, and may be achievable with rational, safe therapies that target neuron-intrinsic and environmental mechanisms.
Experimental Procedures (see also Supplemental Information)
Surgery and Tissue Processing
Female Fisher 344 rats (n=182) weighing 160–200g were experimental subjects. C3 dorsal columns were completely transected bilaterally using a tungsten wire knife (Lu et al., 2004). Peripheral CLs were made by firm compression of the exposed nerve at mid-thigh level using jeweler’s forceps for 15sec._ MSCs were isolated as described previously (Azizi et al., 1998). The chronic lesion site was re-exposed and 2µl (75,000 cells/µl) of passage 4 MSCs, mixed with fibrin glue, were grafted into the lesion cavity. Lenti-NT-3 vector and Lenti-GFP vector were generated as previously described (Taylor et al., 2006) and 2.5µl were injected 2.5mm rostral to the lesion site in dorsal column white matter (titer 100 µg/ml p24, ~1×108 Infectious Units/ml). Dorsal-column sensory axons were labeled transganglionically by CTB injection into the sciatic nerve proximal to the CL site (2µl 1% solution) 3 days before perfusion. Animals were perfused with 4%PFA and subjected to CTB, GFP and GFAP immunocytochemistry on 30µm-thick spinal cord sections.
Axonal Quantification
Axon number was quantified in 1-in-6 sections within and beyond the lesion site. Lesion margins were determined using GFAP immunoreactivity (Taylor et al., 2006), and axons crossing a vertical line placed in sagittal sections within the graft midpoint, and 0, 50, 100, 250, 500, 1000, 2000 and 2500 µm rostral to the rostral lesion border, were counted. Total axon number/subject was calculated by multiplying the counted number by 6. The longest distance of regenerating axons from the rostral lesion border was also measured. Observers were blinded to group identity.
DRG Isolation, Culture and Labeling
Dissected adult L4-6 DRGs were digested for 1h in 0.25% collagenase type XI, triturated, and resuspended on 35mm cell culture dishes coated with myelin (18 µg/ml/ per well). Cells were fixed 48h later with 4%PFA and labeled for NF200. Longest neurite length/cell was measured from a minimum of 150 cells/animal (n=4 DRGs/group). 35μm sections of L4-6 adult DRG were immunolabeled for GAP43 and c-Jun and counterstained with Hoechst 33342. %GAP43- or c-Jun-labeled neurons was quantified as described, n=3 DRGs/group (Qiu et al., 2005).
cAMP ELISA
L4-6 DRGs were dissected, homogenized and subjected to cAMP ELISA (Assay designs), n=7 DRGs/group.
Array Methods
L4-6 DRGs were dissected, frozen at −80°C, and RNA extracted. RNA samples were reverse transcribed and labeled per manufacturer’s instructions, and hybridized to Affymetrix high-density oligonucleotide GeneChip Rat Genome 230 2.0 Arrays (Affymetrix).
Statistical Analysis
All experiments and analyses were conducted under blinded conditions. Quantification of axon number beyond lesion sites and axonal length was assessed by Kruskall Wallis followed by Dunn post-hoc and Bonferroni adjustment. In all other quantifications, multiple group comparisons were made by ANOVA, with post-hoc Fisher’s. A significance criterion of p<0.05 was used. Data presented as mean ± SEM.
Supplementary Material
ACKNOWLEDGMENTS
We thank Maya Culbertson, Lori Graham and Fuying Gao for experimental assistance. Funded by NIH (R01 NS09881and NS054883), the Veterans Administration, International Spinal Research Trust, the Bernard and Anne Spitzer Charitable Trust and the Dr. Miriam and Sheldon G Adelson Medical Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB. Bridging areas of injury in the spinal cord. Neuroscientist. 2001;7:325–339. doi: 10.1177/107385840100700409. [DOI] [PubMed] [Google Scholar]
- Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21:9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW. Overcoming inhibition in the damaged spinal cord. J Neurotrauma. 2006;23:371–383. doi: 10.1089/neu.2006.23.371. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tessler A. Repair of chronic spinal cord injury. Exp Neurol. 2003;182:247–260. doi: 10.1016/s0014-4886(03)00029-3. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system "bridge" and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Tessler A, Fischer I, Jin Y, Tessler A, Fischer I, Houle JD. Fibroblasts genetically modified to produce BDNF support regrowth of chronically injured serotonergic axons. Neurorehabil Neural Repair. 2000;14:311–317. doi: 10.1177/154596830001400407. [DOI] [PubMed] [Google Scholar]
- Jones LL, Oudega M, Bunge MB, Tuszynski MH. Neurotrophic factors, cellular bridges and gene therapy for spinal cord injury. J Physiol. 2001;533:83–89. doi: 10.1111/j.1469-7793.2001.0083b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TB, McDaniel EE, Popovich PG. Inflammatory-mediated injury and repair in the traumatically injured spinal cord. Curr Pharm Des. 2005;11:1223–1236. doi: 10.2174/1381612053507468. [DOI] [PubMed] [Google Scholar]
- Kwon BK, Liu J, Messerer C, Kobayashi NR, McGraw J, Oschipok L, Tetzlaff W. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci U S A. 2002;99:3246–3251. doi: 10.1073/pnas.052308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Pallini R, Fernandez E, Sbriccoli A. Retrograde degeneration of corticospinal axons following transection of the spinal cord in rats. A quantitative study with anterogradely transported horseradish peroxidase. J Neurosurg. 1988;68:124–128. doi: 10.3171/jns.1988.68.1.0124. [DOI] [PubMed] [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17:646–658. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Grill R, Jones LL, Brant A, Blesch A, Low K, Lacroix S, Lu P. NT-3 gene delivery elicits growth of chronically injured corticospinal axons and modestly improves functional deficits after chronic scar resection. Exp Neurol. 2003;181:47–56. doi: 10.1016/s0014-4886(02)00055-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.