Abstract
Context
Much of pediatric drug use is “off-label” because appropriate pediatric studies have not been conducted and the drugs have not been labeled by the US Food and Drug Administration (FDA) for use in children. In 1997, Congress authorized FDA to grant extensions of marketing rights known as “pediatric exclusivity” if FDA-requested pediatric trials were conducted. As a result, there have been over 100 product labeling changes. The publication status of studies completed for pediatric exclusivity has not been evaluated.
Objective
To quantify the dissemination of results of studies conducted for pediatric exclusivity into the peer-review literature.
Design
Cohort study of all trials conducted for Pediatric Exclusivity, the subsequent labeling changes, and the publication of those studies in peer-reviewed journals. We categorized each study in the exclusivity application as ”successful” or “unsuccessful” based on FDA approval of the indication sought by the sponsor. We categorized any labeling changes resulting from the studies as ”positive” or “negative” for the drug under study. We then evaluated aspects of the studies and product label changes that were associated with subsequent publication in peer-reviewed medical journals.
Main Outcome Measures
Publication of the trial data in peer-reviewed journals.
Results
Between 1998 and 2004, 253 studies were submitted to FDA for pediatric exclusivity: 50% evaluated efficacy, 20% were multi-dose pharmacokinetic, 13% were single-dose pharmacokinetic, and 17% were safety studies. Labeling changes were positive for 127/253 (50%) of studies; only 112/253 (44%) were published. Efficacy studies and those with a favorable labeling change were more likely to be published. Of 100 studies resulting in important labeling changes, only 33 were published.
Conclusions
The pediatric exclusivity program has been successful in encouraging drug studies in children. However, the dissemination of these results in the peer-reviewed literature is limited. The results of these trials and future studies conducted for pediatric exclusivity should be published in peer-reviewed journals. Mechanisms to more widely disperse this information warrant further evaluation.
Keywords: registration, children, research, bias
Introduction
Historically, 75% of drug products have insufficient labeling information for pediatric dosing, safety or efficacy. For a product to be marketed in the United States, the sponsor must submit adequate and well controlled trials to the FDA which demonstrate the efficacy and safety of the product when used as intended. These trials are the basis of the “labeling” or package insert instructions that are provided for each product approved by the Food and Drug Administration (FDA). Children are usually not included in most product submission studies and therefore the data on dosing, efficacy and safety that are available for adults is not usually available for pediatrics. Other data may be available but are often not from the same type of rigorous clinical trials that must be submitted for any product that is seeking marketing to be used by adults.
Inadequate dosing and safety information places children at risk for adverse events and denies them potential therapeutic benefits. Physicians who care for children have therefore been forced to either withhold treatment shown to be effective in older patients, or provide drugs to children in whom the dose, efficacy, and safety have not been studied. This prescription practice is known as “off label use” because there is not adequate pediatric information in the labeling. This “off-label” use may result in benefit, no effect, or harm, depending on how much other information about use of the product in the pediatric population is available. The lack of information has had a negative impact on pediatric therapeutics, including reliance on anecdotal practice patterns, and adaptation of data from adult trials that may not be applicable to children.
Congress passed the Food and Drug Administration Modernization Act (FDAMA) in 1997. One component of this Act is the authorization of a pediatric exclusivity process whereby the study of therapies used in children, and potentially of use in pediatrics, is encouraged via a six month marketing protection extension incentive. The marketing protection extension, “pediatric exclusivity” is in return for performing studies specified by the FDA. The Best Pharmaceuticals for Children Act of 2002 renewed the incentives for the study of marketed drugs, and the pediatric exclusivity program enjoys broad support from pediatricians, professional societies, and other public health advocates(1); however, the program is not without controversy or critics. Critics have asserted that the cost of the program to the public will exceed 14 billion dollars as a result of the sole marketing protection extensions (2) (e.g., extended patent protection) and the resultant delay of generic products into the U.S. market. There are, however, other marketing protections, created by the Hatch-Waxman Act of 1984 and the Orphan Drug Act of 1982. These marketing exclusivities also block any generic of the product from being marketed during the exclusivity period. The marketing exclusivity period varies based upon the type of exclusivity--from 7 years for Orphan drugs to 180 days.
The pediatric exclusivity program has been successful from many perspectives, including labeling, with over 100 labeling changes to date. The subsequent dissemination of results in the peer-reviewed medical literature has not been previously quantified. Because few pediatric studies are performed for products primarily approved and marketed for adults, it is important that the information obtained from such studies are readily available. One standard for dissemination of human experimentation results is peer-reviewed publication in a journal retrievable by Medline search. (3) We evaluated the frequency of such publication of studies completed for pediatric exclusivity. We hypothesized that publication would be incomplete and that positive labeling changes would be associated with increased probability of publication.
Methods
The unit of observation for this article is the clinical trial. The cohort of clinical trials included any informative human study (pharmacokinetic, safety, efficacy, etc.) that we identified through the “Written Request” mechanism of the pediatric exclusivity program. The pediatric exclusivity program was designed to provide economic incentives to companies to conduct pharmacokinetic, safety, and efficacy studies in children. The Written Request, generally issued by FDA prior to initiation of pediatric exclusivity studies, contains required elements of the requested studies, including indication, number of studies, sample sizes, trials design, and age ranges to be studied. The results of the studies comprise much of the final study report that is submitted to the FDA. It is noteworthy that marketing exclusivity is extended regardless of the outcome of the trials requested by the FDA in the Written Request, provided that the companies fairly respond to the terms of the Written Request. From the Written Requests, final study reports, and FDA’s regulatory actions on the submissions, we recorded the basic elements of design and subsequent labeling changes for each product. All studies completed in response to a Written Request and submitted to FDA are evaluated by the Agency. Thus, this cohort captures 100% of the studies evaluated as part of the pediatric exclusivity program within our time frame.
The primary independent variable was based on the labeling change that resulted from the exclusivity submission. Labeling change categories included positive labeling changes, negative labeling changes, submission completed but no labeling change, submission/application withdrawn, or product withdrawn from the market. Examples of positive labeling changes included “safety and effectiveness established”, and “approved for use in children”. Examples of negative labeling changes included “no meaningful clinical activity”, “black box warning”, and “increased mortality reported in the product compared to placebo”. We collapsed the categorical labeling change variable into the primary independent variable. If the labeling change was positive, we categorized the outcome as positive; we otherwise categorized the outcome as negative. The categorization of the labeling changes was adjudicated by the authors affiliated with FDA. Two authors (DKB and JL) first categorized the labeling changes. Label changes were then categorized by three authors (LM, DA, and RR). Differences were then discussed and adjudicated (DA, DKB, DM, JL, LM, and RR). Reconciliation was required for two products.
Drug labeling changes also were classified with respect to public health impact. Labeling changes were classified as “key labeling changes” if the studies resulted in substantive dosing changes, new safety information, or lack of efficacy in phase III testing. Study design was classified as follows: efficacy (randomized trials, either placebo or active comparator), dose-ranging or multi-dose pharmacokinetics, single-dose pharmacokinetics, or safety. Race and ethnicity were not uniformly provided for each study; but most of the trials conducted have submitted the fraction of children enrolled who were white. These data are reported as a marker of racial and ethnic diversity of enrolled children. We recorded the annual sales (http://www.drugtopics.com/drugtopics/) for each product for the most recent available year that the product had sole marketing protection in the US. The number of centers at which each trial was conducted was transformed into a categorical variable—<7 centers, 7–17 centers, 18–34 centers, and > 34 centers.
The primary outcome was publication of the main study results in a peer-reviewed journal. If the results of two or more studies were combined into one publication, (4) then all studies with data reported in the paper were counted as published. If data from a study was combined with data from non-exclusivity trials (e.g., a population pharmacokinetic study), then the study was counted as published. If studies of two products were performed in the same trial and the trial results were published (5) then both studies were counted as published. We only counted as published those articles that could be found by Medline search.
We used four separate search strategies to obtain publication status. One author searched Medline (JL, 6/2006), entered the generic name of the product, and limited the search strategy to “all child (0–18 years)”, “1998–2005”, and “English language”. She read each abstract and compared the abstract with the Written Request or final study report. If the abstract was a potential match, the article was obtained and read. A second author (DKB, 6/2006) entered the product’s generic name, and limited the search to “1998–2005”, “English Language” and ages of trial participants from the Written Request and/or final study report. A third author (PBS) used key words from the study design provided by the Written Request and the generic name. This final strategy allowed for the capture of manuscripts prior to 1998. Each search strategy had high sensitivity; 6 articles were found by only one of the three strategies. When a paper was found to be a match, we recorded the 2004 journal impact factor (http://isiknowledge.com/jcr). Finally, a study coordinator at Duke University requested publications (including those published, in press, or submitted) from studies conducted for Pediatric Exclusivity from each company. This resulted in no additional publications. We did not include publications limited to abstract or publications in trade journals because it was our primary aim to assess ready availability of results to the practicing physician. The analysis was limited to studies for which the data were submitted to FDA’s Pediatric Exclusivity Board by December 1st, 2004 because of the delay between completion of study, manuscript preparation, and publication.
Reported p-values and 95% confidence intervals (CI) are two-tailed. Odds Ratios (OR) and 95% CI were derived from logistic regression modeling (STATA 8.2). We used forward selection logistic regression and the maximum number of variables in the model did not exceed the variables presented in the final model. We limited the number of variables in accordance with previously published regression methods. (6) We repeated the multi-variable analysis using three models: a parsimonious model that included only those variables that were associated (p< 0.05) with publication in forward selection, a model that included variables associated with publication and was adjusted for year of submission, and a model that included variables associated with publication and was adjusted for year of submission and log-sample size (of children enrolled) (7). Variables that were analyzed in forward regression but were not retained included number of centers (analyzed as a continuous and as a categorical variable outlined above), fraction of children enrolled who were categorized as white race, and annual sales of drug in the latest year of patent protection. The Institutional Review Boards and Ethics Committees of the enrolling sites approved the protocols included in this analysis. Dr. Benjamin had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript are independent of any funding organization.
Results
Between January 1st, 1998 and December 31st, 2004, data from 115 therapeutic agents were submitted to the Pediatric Exclusivity Board. The number of studies for each agent ranged from one to seven; 253 studies were submitted (Table 1). The sample size for each study was variable; range n=8 (a single dose pharmacokinetic study) to n=27,065 (an open label safety study); 23% of studies enrolled <30 children, the median enrollment was 103, and two studies enrolled >1,000 children.
Table 1.
Description of the studies conducted for Pediatric Exclusivity
| Type | Allergy Immunology Rheumatology | Anesthesia Pain | Cancer | CNS | Cardio-Renal | Endocrine | GI | ID | Other |
|---|---|---|---|---|---|---|---|---|---|
| Study Design | 40 | 9 | 21 | 66 | 42 | 15 | 14 | 41 | 5 |
| Safety | 18 | 1 | 3 | 8 | 1 | 2 | 9 | 1 | |
| Pharmacokinetic | 6 | 1 | 1 | 8 | 7 | 5 | 3 | 3 | |
| Multiple-dose PK | 3 | 1 | 8 | 11 | 12 | 2 | 4 | 9 | 1 |
| Efficacy | 13 | 6 | 9 | 39 | 22 | 8 | 5 | 20 | 3 |
| Indication | Conjunctivitis, rhinitis (18) | Anesthesia (4) | Solid Tumor (9) | Depression Anxiety OCD (31) | Hypertension (22) | Diabetes (7) | Reflux (14) | HIV (11) | Acne (4) |
| Asthma (10) | Pain (3) | Leukemia Lyphoma (5) | ADHD (11) | Hypercholesterolemia (7) | Obesity (4) | Influenza; URI (9) | McCune-Albright (1) | ||
| JRA (7) | Sedation (2) | Other (7) | Migraine (7) | Arrhythmia (3) | Other (4) | Malaria (6) | |||
| Dermatitis (5) | Other (17) | Other (10) | Other (15) | ||||||
| Age Range | |||||||||
| Neonates | 0 | 4 | 2 | 1 | 6 | 0 | 8 | 10 | 0 |
| Infants | 16 | 4 | 8 | 6 | 16 | 0 | 8 | 21 | 0 |
| Toddlers | 27 | 6 | 20 | 13 | 20 | 2 | 9 | 28 | 1 |
| Children | 26 | 7 | 18 | 54 | 38 | 15 | 9 | 28 | 5 |
| Adolescents | 10 | 6 | 17 | 52 | 35 | 15 | 7 | 12 | 4 |
| Sample Size | |||||||||
| <30 | 5 | 0 | 7 | 15 | 13 | 4 | 7 | 7 | 1 |
| 30–100 | 17 | 1 | 9 | 8 | 12 | 3 | 5 | 9 | 1 |
| 101–200 | 6 | 4 | 4 | 14 | 12 | 4 | 2 | 8 | 2 |
| >200 | 12 | 4 | 1 | 29 | 5 | 4 | 0 | 17 | 1 |
Only 44% (112/253) of the studies were published in peer-reviewed journals. A positive labeling change was observed for 50% of studies (127/253). Efficacy studies and studies that resulted in a favorable labeling change were more likely to be published (Table 2). These associations remained strong in multivariable analysis (Table 3).
Table 2.
Demographics of trials published, and not published, in the peer-reviewed literature
| Result | Total | Not Published | Published | Proportion Published |
|---|---|---|---|---|
| Favorable Results | ||||
| Positive Label Change | 127 | 59 | 68 | 54% |
| Unfavorable Results | 126 | 82 | 44 | 35% |
| Negative Label Change | 75 | 49 | 26 | 35% |
| No Labeling Change | 43 | 28 | 15 | 35% |
| Application Withdrawn | 5 | 2 | 3 | 60% |
| Product Withdrawn from Market | 3 | 3 | 0 | 0% |
| Key Label Change* | 100 | 67 | 33 | 33% |
| Dosing Change | 37 | 19 | 18 | 49% |
| Safety Information | 67 | 38 | 29 | 43% |
| Ineffective | 37 | 23 | 14 | 38% |
| Year Data Submitted | ||||
| 1998 | 8 | 3 | 5 | 63% |
| 1999 | 18 | 11 | 7 | 39% |
| 2000 | 39 | 19 | 20 | 51% |
| 2001 | 51 | 23 | 28 | 55% |
| 2002 | 51 | 29 | 22 | 43% |
| 2003 | 46 | 31 | 15 | 33% |
| 2004 | 40 | 25 | 15 | 38% |
| Type | ||||
| Allergy Immunology Pulmonary | 40 | 21 | 19 | 48% |
| Anesthesia/Pain | 9 | 7 | 2 | 22% |
| Cancer | 21 | 15 | 6 | 29% |
| Central Nervous System | 66 | 37 | 29 | 44% |
| Cardiology/Renal | 42 | 22 | 20 | 48% |
| Endocrine | 15 | 10 | 5 | 33% |
| Gastro-intestinal | 14 | 5 | 9 | 64% |
| Infectious Disease | 41 | 21 | 20 | 49% |
| Other | 5 | 3 | 2 | 40% |
| Study Design | ||||
| Safety | 43 | 32 | 11 | 26% |
| Single Dose Pharmacokinetic | 34 | 19 | 15 | 44% |
| Multiple-dose PK or Dose Ranging | 51 | 28 | 23 | 45% |
| Efficacy | 125 | 62 | 63 | 50% |
Key labeling changes include crucial elements of one or more of dosing, safety, or efficacy that are different from adults; studies could result in more than one key labeling change
Table 3.
Multivariable analysis: the outcome is publication and the unit of observation (n=253) is the clinical trial. Positive labeling changes and efficacy trial design were associated with subsequent peer-reviewed publication
| Variable | OR | 95%CI | p-value |
|---|---|---|---|
| Results | |||
| Unfavorable Result | Referent | ||
| Favorable Result | 2.58 | 1.52, 4.37 | < 0.001 |
| Study Design | |||
| Safety or Outcomes Registry | Referent | ||
| Pharmacokinetic | 2.43 | 0.91, 6.54 | 0.08 |
| Multiple-dose PK or Dose Ranging | 2.62 | 1.06, 6.45 | 0.04 |
| Efficacy | 3.49 | 1.58, 7.74 | 0.002 |
Annual sales of the drug products and number of centers that participated in enrollment were not associated with subsequent publication. These variables were explored as continuous, categorical, and dummy variables. The number of centers is missing from a large number of observations because center reporting was not required prior to 2002. However, at least 73 studies enrolled children in countries outside of the US including Eastern and Western Europe, Russia, Latin America, and sub-Saharan Africa. Neither race nor clinical therapeutic area was associated with subsequent publication.
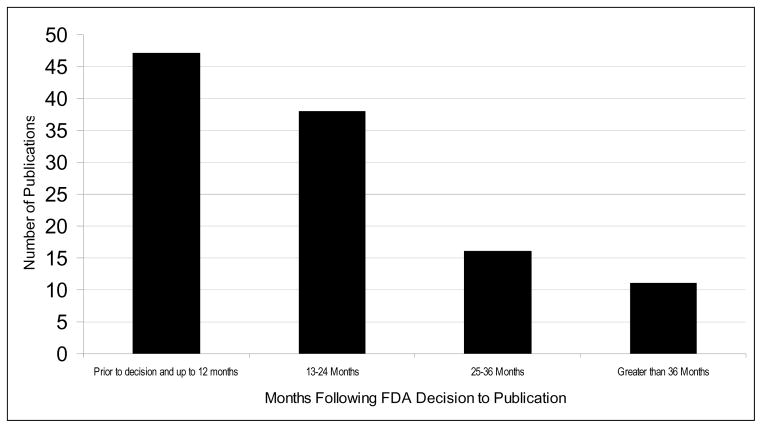
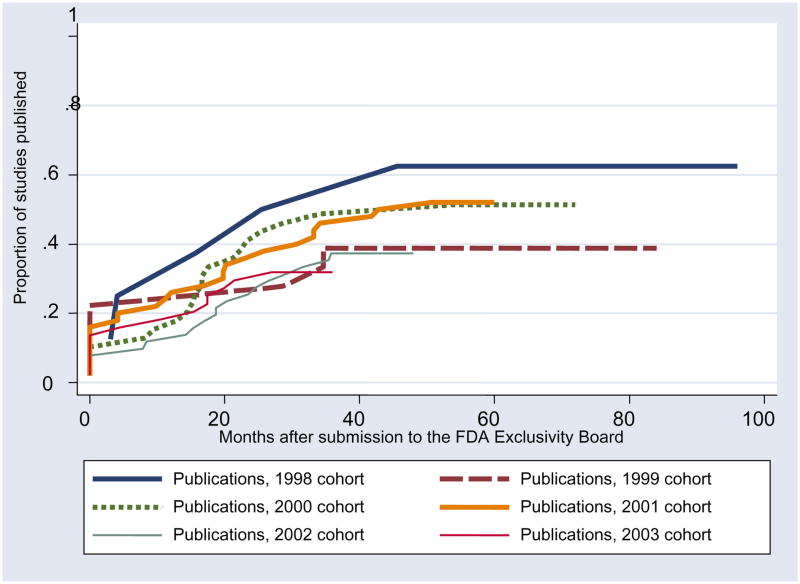
Data for the studies were submitted over the course of 7 years (1998–2004), but year of submission of data was not associated with subsequent publication. We further explored the relationship between the time that the data were submitted to the Exclusivity Board of FDA and subsequent publication (Figures 1 and 2). Only 3 articles were published more than 48 months after the data were submitted. Of note, 202/253 studies (80%) were submitted prior to December 2003: specifically, at least 80% of the studies for this analysis were completed >30 months prior to our evaluation, and all studies were completed (enrollment finished, data analyzed, and data submitted) >18 months prior to our evaluation.
Figure 1.
Time from submission of data to the Pediatric Exclusivity Board of FDA and subsequent peer reviewed publication; the y-axis provides the absolute number of publications, and the x-axis provides the time following submission categorized by year.
Figure 2.
Publication rates by year of submission; each line encompasses all of the submissions for one year of the program. The y-axis provides the proportion of studies published, and the x-axis provides the time following submission to the Pediatric Exclusivity Board of FDA.
Although all studies for several products were published, (4, 8–21) several products with substantial safety concerns (22) were not published as of this writing. The relationship between lack of successful labeling change and subsequent publication was not absolute. Many studies were published that were given a “negative” labeling change or not granted a labeling change. (14, 23–26) The peer-reviewed journals that accepted the publications were high quality. The mean impact factor for journals that accepted publication was 6.
There were 100 clinical trials associated with a key labeling change, but only 33 were published. There were 51 trials that did not result in a labeling change (43 from completed submissions and 8 associated with products that were withdrawn from the market or the application was withdrawn), and only 18 (35%) were published. Thus, 4,520 children were enrolled in 33 clinical trials for 20 products resulting in no labeling change and no dissemination of results.
Discussion
We have found that studies completed for pediatric exclusivity are often not published in the peer-reviewed literature. It is important to note that a product may be granted pediatric exclusivity even if the product fails to show effectiveness or obtain FDA approval for marketing. The rationale behind this approach is that the information collected in a well controlled and designed trial is important even if it fails to demonstrate effectiveness. Because so few pediatric studies are conducted for therapeutics, a well constructed effort to answer a question is seen as deserving of the exclusivity irrespective of the ultimate answer. The primary goal of pediatric exclusivity is to provide an incentive to conduct studies in children that will improve the labeling of products and thereby improve public health. The program has been successful in reinvigorating clinical research involving drugs used in children and improved pediatric labeling (27, 28); but the research has not been consistently disseminated into the peer-reviewed medical literature. Peer-reviewed publication is crucial to public health benefit because such publication often is the primary means of notifying practitioners of information that can update their knowledge of therapeutics and may change their prescribing approach. Our findings are consistent with prior investigations of research and subsequent peer-reviewed publications (29–31).
Clinical research is an activity involving human subjects that contributes to generalizable knowledge.(32) An important first step is the submission of data to FDA for review and subsequent authorization of labeling changes if the data so warrant. However, failure to publish pediatric research in a venue in which the clinical community can study and debate the findings poses an unacceptable public health risk. Selective serotonin reuptake inhibitor agents and propofol are drugs in which this risk was particularly evident. Numerous ethical statements from foundation documents(32,33) and professional organizations(3) have emphasized that publication of both negative and positive results of prospective human studies is an obligation shared by physician-scientists, companies, and the peer-review community.(33)
The physician-investigator obtains the consent from the parent for their child to participate in the experiment. The young child, as a person who cannot provide informed consent, relies on the investigator and sponsor to ensure generalizability of results. This dissemination, in the form of peer-reviewed journal articles, helps to prevent future children from shouldering unnecessary risks. Because this research involves children who cannot speak for themselves, the obligation to publish is especially great. The need for publication is underscored by the funding mechanism for the trials. Although the immediate costs were paid for with private sector funds, these trials were conducted with what amounts to public funding support. The data from these trials resulted in the granting of an additional 6 months of sole market protection in the US for approximately 90% of the products, and thus the public and public institutions pay for the higher-priced products during the 6-month delay in a generic coming to market. By law, pediatric exclusivity must be granted, irrespective of the outcome of the data, if the company conducts the trials requested by FDA and the trials fairly respond to the Written Request. This policy was agreed to because it was understood that “negative” data would often be just as important to practitioners and parents as “positive” data from the clinical trials. Unfortunately, the fraction of studies published for which there was a key change in labeling was <50%; more discouraging is that only 26% of the studies in which the primary endpoint was safety were published. (34)
Strengths and Limitations of the Data
In the context of previously conducted evaluations of research and subsequent publication, this cohort of studies has several unique attributes. Foremost is the development of the cohort. Much of the research that explores human experimentation and subsequent publication captures studies presented at meetings, or works backward from the peer-reviewed literature. This cohort, however, captures 100% of the studies undertaken because of an exceptional component of the pediatric exclusivity program: companies are required to submit all of the studies’ data to FDA if they want to obtain the financial benefit of the extended sole market protection of their product line in the United States. A second unique attribute of these data resides within the purpose of the pediatric exclusivity program. The program provides a financial incentive to companies to obtain robust scientific data that will result in improved labeling of drugs given to children and subsequently improve pediatric health globally. Such is hindered by the lack of publication in the peer-reviewed literature.
These data have several limitations and caution should be applied prior to interpreting the data in the context of any specific products, companies, research groups, or investigators. We searched Medline for publications and did not use multiple search engines, such as EMBASE. These data do not include articles that have been submitted recently or are in press, and the time between completion of the study and subsequent publication of multi-center collaborative research can be lengthy. Nevertheless, the results of at least 32 of the unpublished studies have been known for more than five years. The data are also limited by the search efficacy of the authors. Each strategy resulted in over 5,000 abstracts; several articles published prior to 12/2005 were likely overlooked by all authors; nevertheless, only six articles were found by only one author. Finally, companies were requested to submit all published (accepted, or in press) articles based on studies conducted for pediatric exclusivity: a process that yielded no additional publications. A further limitation is that there is not a ‘control group for these data’; it is possible, that similar pediatric studies with negative results may suffer the same non-publication fate as pediatric exclusivity trials.
Possible Explanations for Lack of Publication
The Pediatric Exclusivity Program does not provide rewards or incentives for publication. Publication, and thus dissemination of knowledge, is a social benefit. It is important to remember that studies with “negative” outcomes can, and often do, contain important information for practitioners and parents regarding the appropriate use of the product.
These data do not point solely at industry sponsorship as the root of the problem for lack of publication. In the current regulatory environment, there are few commercial rewards for publication. Pediatric Exclusivity studies are typically completed late in the drug life cycle; there is little opportunity for the sponsor to use the information for promotional purposes. The economic benefits from pediatric exclusivity typically come from continued marketing protection of sales to adults. These studies are often done for drugs in which use in children is well established (e.g., selective serotonin re-uptake inhibitors), or for drugs in which use in children is very limited (e.g., drugs that improve bone density and reduce fracture risk for osteogenesis imperfecta). Promotion to pediatricians may therefore not be a marketing imperative. Having obtained additional marketing protection, sponsors may simply not see publication as a worthwhile investment of resources. Scientific journals have often been accused of having reluctance to publish ‘negative’ data, but this assertion has been challenged. (35)
Plausible Solutions
It is crucial to public health that currently available pediatric clinical trial data are made public and that future studies are published. Previous investigators have encouraged universal registration of clinical trials. Unfortunately, registration of trials, though increasing, is not adequate despite FDAMA legislation in 1997 that lead to the establishment of ClinicalTrials.gov (36, 37). Universal registration of trials is an important first step in achieving the goal of transparency of human subject experimentation, but is unlikely to ensure complete dissemination of results.
We found that after 36 months following data submission to FDA, publication of pediatric clinical trial data in a peer-reviewed journal is uncommon. A mechanism for data unpublished by 36–48 months following submission to FDA could be peer-reviewed dissemination in a journal supplement. The focus of such results could be communication with the public, public health safety questions, and “lessons learned” in trial design and products within the same class. Peer-reviewed publication of all prospective trials involving children, including those conducted as part of the pediatric exclusivity program, is optimal.
Fully publishing pediatric data submitted for labeling will require a multi-faceted solution that might also include legislative support. The BPCA Congressionally legislated program, has been a substantive regulatory step forward. Congress might consider future legislation in which part of the incentive given for the completion of pediatric trials might be linked to peer-review dissemination of results for those studies submitted early enough in their patent or marketing life cycle to permit such an activity.
The Pediatric Exclusivity Provision has stimulated clinical trials and generated useful prescribing information for children more than any other regulatory or legislative process to date; however, the dissemination of these trial results in the peer-reviewed literature has been limited. Publication of studies conducted for pediatric exclusivity is an important step toward the generalization of the knowledge obtained from the children enrolled in the trials and should be ensured for the benefit of pediatric public health.
Acknowledgments
Drs. Benjamin and Li received support from NICHD 1U10-HD45962-03 and the Food and Drug Administration The Views expressed are those of the authors. No official endorsement by the Food and Drug Administration is provided or should be inferred. No commercial or other conflict of interest exists between the authors and the pharmaceutical companies.
References
- 1.Ward R. America Academy of Pediatrics testimony before the United States Senate committee on health, education, labor, and pensions. [Accessed August 7, 2002];2001 May 8; Available at: http://www.aap.org/advocacy/washing/testimonymay8thBWard.html.
- 2.Public Citizens. Patently offensive: Congress set to extend monopoly patents for Cipro and other drugs. [Accessed November 28, 2005];2001 November 9; Available at: http://www.citizens.org/congress/reform/drug_patents/pediatric/articles.cfrn/ID-6435.
- 3.Principles for Protecting Integrity in the Conduct and Reporting of Clinical Trials: Approved by AAMC Executive Committee, September 15, 2005.
- 4.Szer IS, Simpson K, Stewart JA, Strand V. Leflunomide or Methotrexate for Juvenile Rheumatoid Arthritis. N Engl J Med. 2005;352:1655–66. doi: 10.1056/NEJMoa041810. [DOI] [PubMed] [Google Scholar]
- 5.Rongkavilit C, Thaithumyanon P, Chuenyam T, et al. Pharmacokinetics of Stavudine and Didanosine Coadministered with Nelfinavir in Human Immunodeficiency Virus-Exposed Neonates. Antimicrobial Agents and Chemotherapy. 2001;45(12):3585–90. doi: 10.1128/AAC.45.12.3585-3590.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Haidich AB, Ioannidis JP. Effect of early patient enrollment on the time to completion and publication of randomized controlled trials. A J Epidemiol. 2001;154:873–80. doi: 10.1093/aje/154.9.873. [DOI] [PubMed] [Google Scholar]
- 8.Faucher JF, Binder R, Missinous MA, et al. Efficacy of Atovaquone/Proguanil for Malaria Prophylaxis in Children and Its Effect on the Immunogenicity of Live Oral Typhoid and Cholera Vaccines. Clinical Infectious Diseases. 2002;35:1147–54. doi: 10.1086/342908. [DOI] [PubMed] [Google Scholar]
- 9.Camus D, Djossou F, Schilthuis HJ, et al. Atovaquone-Proguanil in Nonimmune Pediatric Travelers: Results of an International, Randomized, Open-Label Study. Clinical Infectious Diseases. 2004;38:1716–23. doi: 10.1086/421086. [DOI] [PubMed] [Google Scholar]
- 10.Sabchaeron A, Attanath P, Phanuaksook P, et al. Efficacy and Pharmacokinetics of Atovaquone and Proguanil in Children with Multidrug-Resistant Plasmodium Falciparum Malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1998;92:201–6. doi: 10.1016/s0035-9203(98)90749-0. [DOI] [PubMed] [Google Scholar]
- 11.Anabwani G, Canfield CJ, Hutchinson D. Combination Atovaquone and Proguanil Hydrochloride vs. Halofantrine for Treatment of Acute Plasmodium Falciparum Malaria in Children. Pediatr Infect Disease J. 1999;18:456–61. doi: 10.1097/00006454-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Lell B, Luckner D, Ndjave M, Scott T, Kremsner PG. Randomised Placebo-Controlled Study of Atovaquone plus Proguanil for Malaria Prophylaxis in Children. Lancet. 1998;351:709–13. doi: 10.1016/S0140-6736(97)09222-2. [DOI] [PubMed] [Google Scholar]
- 13.Sorof JM, Cargo P, Graepel J, et al. B-Blocker/Thiazide Combination for Treatment of Hypertensive Children: a Randomized Double-blind, Placebo-Controlled Trial. Pediatr Nephrol. 2002;17:345–50. doi: 10.1007/s00467-002-0851-0. [DOI] [PubMed] [Google Scholar]
- 14.Silverman E, Spiegel L, Hawkins D, et al. Long-Term Open-Label Preliminary Study of the Safety and Efficacy of Leflunomide in Patients with Polyarticular-Course Juvenile Rheumatoid Arthritis. Arthritis & Rheumatism. 2005;52:554–62. doi: 10.1002/art.20861. [DOI] [PubMed] [Google Scholar]
- 15.Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the Treatment of Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: A Randomized, Placebo-Controlled, Dose-Response Study. Pediatrics. 2001;108:e83. doi: 10.1542/peds.108.5.e83. [DOI] [PubMed] [Google Scholar]
- 16.Michelson D, Allen AJ, Busner J, et al. Once-Daily Atomoxetine Treatment for Children and Adolescents with Attention Deficit Hyperactivity Disorder: A Randomized, Placebo-Controlled Study. Am J Psychiatry. 2002;159:1896–01. doi: 10.1176/appi.ajp.159.11.1896. [DOI] [PubMed] [Google Scholar]
- 17.Spencer T, Heiligenstein JH, Biederman J, et al. Results From 2 Proof-of-Concept, Placebo-Controlled Studies of Atomoxetine in Children with Attention-Deficit/Hyperactivity Disorder. J Clin Psychiatry. 2002;63:1140–7. doi: 10.4088/jcp.v63n1209. [DOI] [PubMed] [Google Scholar]
- 18.Emslie GJ, Heiligenstein JH, Wagner KD, et al. Fluoxetine for Acute Treatment of Depression in Children and Adolescents: A Placebo-Controlled, Randomized Clinical Trial. J Am Acad Child Adolesc Psychiatry. 2002;41:1205–15. doi: 10.1097/00004583-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Carmody T, Mayes TL. Fluoxetine in Child and Adolescent Depression: Acute and Maintenance Treatment. Depression and Anxiety. 1998;7:32–39. doi: 10.1002/(sici)1520-6394(1998)7:1<32::aid-da4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Geller DA, Hoog SL, Heiligenstein JH, et al. Fluoxetine Treatment for Obsessive-Compulsive Disorder in Children and Adolescents: A Placebo-Controlled Clinical Trial. J Am Acad Child Adolesc Psychiatry. 2001;40:773–9. doi: 10.1097/00004583-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Wilens TE, Cohen L, Biederman J, et al. Fluoxetine Pharmacokinetics in Pediatric Patients. J of Clin Psychopharmacology. 2002;22:568–75. doi: 10.1097/00004714-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Wolf AR, Potter F. Propofol infusion in children: when does an anesthetic tool become an intensive care liability? Pediatric Anesthesia. :20. doi: 10.1111/j.1460-9592.2004.01332.x. [DOI] [PubMed] [Google Scholar]
- 23.Winner P, Rothner AD, Saper J, et al. Randomized, Double-Blind, Placebo-Controlled Study of Sumatriptan Nasal Spray in the Treatment of Acute Migraine in Adolescents. Pediatrics. 2000;106:989–97. doi: 10.1542/peds.106.5.989. [DOI] [PubMed] [Google Scholar]
- 24.Steinherz PG, Seibel NL, Ames MM, et al. Phase I Study of Gemcitabine (difluorodeoxycytidine) in Children with Relapsed or Refractory Leukemia (CCG-0955): A Report From the Children’s Cancer Group. Leukemia & Lymphoma. 2002;43:1945–50. doi: 10.1080/1042819021000015880. [DOI] [PubMed] [Google Scholar]
- 25.Findling RL, Preskorn SH, Marcus RN, et al. Nefazodone Pharmacokinetics in Depressed Children and Adolescents. J Am Acad Adolesc Psychiatry. 2000;39:1008–16. doi: 10.1097/00004583-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Foster KW, Friedlander SF, Panzer H, Ghannoum MA, Elewski BE. A randomized Controlled Trial Assessing the Efficacy of Fluconazole in the Treatment of Pediatric Tinea Capitis. J Am Acad Dermatol. 2005;53:798–08. doi: 10.1016/j.jaad.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Roberts R, Rodriguez W, Murphy D, Crescenzi T. Pediatric Drug labeling improving the safety and efficacy of pediatric therapies. JAMA. 2003;290:905–11. doi: 10.1001/jama.290.7.905. [DOI] [PubMed] [Google Scholar]
- 28.Wilson JT. Pragmatic assessment of medicines available for young children and pregnant or breast-feeding women. In: Morselli PL, Garattini S, Sereni F, editors. Basic and Therapeutic Aspects of Perinatal Pharmacology. New York: Raven Press; 1975. pp. 411–21. [Google Scholar]
- 29.Bhandari M, Busse JW, Jackowski D, et al. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ. 2004;170:477–80. [PMC free article] [PubMed] [Google Scholar]
- 30.Easterbrook PJ, Berlin JA. Publication bias in clinical research. Lancet. 1991;337:867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 31.Ioannidis JPA. Effect of the Statistical Significance of Results on the Time to Completion and Publication of Randomized Efficacy Trials. JAMA. 1998;279:281–86. doi: 10.1001/jama.279.4.281. [DOI] [PubMed] [Google Scholar]
- 32.National Institutes of Health Regulations and Ethical Guidelines. [Accessed November 28, 2005]; Available at: http://ohsr.od.nih.gov/guidelines/belmont.html.
- 33.National Institutes of Health Regulations and Ethical Guidelines. [Accessed November 28, 2005]; Available at: http://www.nihtraining.com/ohsrsite/guidelines/helsinki.html.
- 34.Papanikolaou PN, Ioannidis JP. Availability of large-scale evidence on specific harms from systematic reviews of randomized trials. Am J Med. 2004;117:582–9. doi: 10.1016/j.amjmed.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 35.Olson CM, Rennie D, Cook D, Dickersin K, Flanagin A, Hogan JW, Zhu Q, Reiling J, Pace B. Publication bias in editorial decision making. JAMA. 2002;287:2825–8. doi: 10.1001/jama.287.21.2825. [DOI] [PubMed] [Google Scholar]
- 36.Dickersin K, Drummond R. Registering clinical trials. JAMA. 2003;290:516–23. doi: 10.1001/jama.290.4.516. [DOI] [PubMed] [Google Scholar]
- 37.Zarin DA, Tse T, Ide NC. Trial Registrations at ClinicalTrials.gov between May and October 2005. N Engl J Med. 353(26):2779–87. doi: 10.1056/NEJMsa053234. [DOI] [PMC free article] [PubMed] [Google Scholar]