Abstract
A sample of 335 five-year-old children participating in an ongoing longitudinal study was the focus of a study on the effects of emotional and behavioral challenge on cardiac activity in children with different patterns of early childhood behavior problems. The children were placed in one of three behavior problem groups (low behavior problems, risk for externalizing problems, risk for mixed externalizing/internalizing problems) based on their scores on the Child Behavior Checklist for 4-18 year-olds (Achenbach, 1991), completed by their mothers. To assess cardiac vagal regulation, resting measures of respiratory sinus arrhythmia (RSA) and RSA change (vagal withdrawal) to five emotionally and behaviorally challenging tasks were derived. In addition, Heart period (HP) and Heart period change (HR acceleration) was examined. Results indicated that the behavior problem groups did not differ in terms of resting measures of either RSA or HP. Analyses of the challenge tasks indicated that the children at risk for mixed problems displayed greater cardiac vagal withdrawal across the five tasks than did the other two groups of children. There was a trend for the children at risk for externalizing problems to display less vagal withdrawal than the control group. In addition, the children at risk for mixed problems displayed greater heart rate acceleration to the tasks than did the other two groups of children. Follow-up analyses indicated that the greater cardiac acceleration observed in the mixed group was largely a function of greater vagal withdrawal. These findings are discussed in terms of the emotion regulatory function of cardiac vagal regulation, and its implications for patterns of risk for behavior problems in young children.
Keywords: Cardiac vagal control, Cardiac vagal tone, Behaviour problems, Vagal regulation, Externalizing
Recent work in both developmental and clinical psychology has identified the construct of emotion regulation as one that is critical to understanding adaptive functioning (Baumeister & Vohs, 2004; Calkins & Dedmon, 2000; Calkins & Fox, 2002; Keenan, 2000; Shaw, Keenan, Vondra, Delliquadri, & Giovannelli, 1997). The ability to exercise self-control over the expression of emotion, particularly negative emotions, and emotion-related behavior, develops over the first years of life and has particular importance for the development of appropriate and adaptive social behavior during the preschool and school years (Calkins, 1994; in press; Kopp, 1982; Eisenberg, Murphy, Maszk, Smith, & Karbon, 1995; Eisenberg et al., 1996; Thompson, 1994). Moreover, the lack of adequate development of control over emotion, including over-control of emotion, and behavior may be a precursor to the development of psychopathology, particularly early problems characterized by aggression, hyperactivity, and anxiety (Keenan & Shaw, 2003; Nigg & Huang-Pollock, 2003).
Research in the area of early emotion regulation has focused on both intrinsic (biological) and extrinsic (familial) contributors to both normative development and individual differences in emotion regulation (Fox & Calkins, 2003). Biological substrates of emotion regulation have been the focus of both theoretical (Calkins, 1994; Fox, 1994) and empirical work (Blair, 2003; Calkins & Dedmon, 2000; Calkins & Keene, 2004). Theories of emotion regulation that focus on underlying biological components of regulation assume that maturation of different biological support systems lays the foundation for increasingly sophisticated emotional and behavioral regulation that is observed across childhood. Fox (1989; 1994), for example, has noted that the frontal lobes of the brain are differentially specialized for approach versus avoidance and that these tendencies influence the behaviors that children engage in when emotionally and behaviorally aroused. He further notes that maturation of the frontal cortex provides a mechanism for the more sophisticated and planful regulatory behaviors of older children versus infants. Porges’ Polyvagal theory (Porges, Doussard-Roosevelt & Maita, 1994; Porges, 1996, 2001, 2003) also describes an important role for biological maturation, specifically maturation of the parasympathetic nervous system that plays a key role in regulation of state, motor activity, and emotion. Moreover, Porges notes that individual differences in nervous system functioning might mediate the expression and regulation of emotion and, by extension, be important elements of the system tat supports appropriate social engagement (Porges et al., 1994; Porges, 2001; 2003). Porges and others have found that parasympathetic nervous system functioning, as reflected in heart rate variability influenced by the vagal system, is related to the control of attention, emotion, and behavior (Calkins, 1997; Calkins & Dedmon, 2000; DeGangi, DiPietro, Greenspan, & Porges, 1991; Huffman et al., 1998; Porges, Doussard-Roosevelt, Portales, & Greenspan, 1996).
Although there are multiple ways to measure this variability, Porges (1985, 1991, 1996) and colleagues developed a method that measures the amplitude and period of the oscillations associated with inhalation and exhalation. This measure refers to the variability in heart rate that occurs at the frequency of breathing (respiratory sinus arrhythmia, RSA) and is thought to reflect the parasympathetic influence on heart rate variability via the vagus nerve. Porges has termed this measure of heart rate variability vagal tone (Vna; Porges & Byrne, 1992; Porges, 1996). Although there are other components of HR variability, the RSA measure has been identified as suitable for the study of physiological links to multiple dimensions of behavioral functioning in young children (Huffman et al., 1998; Richards, 1985, 1987). For example, high resting RSA is one index of autonomic functioning that has been associated with appropriate emotional reactivity (Stifter & Fox, 1990) and good attentional ability (Richards, 1985, 1987; Suess, Porges, & Plude, 1994). Several studies have linked high RSA in newborns with good developmental outcomes, suggesting that it may be an important physiological component of appropriate engagement with the environment (Hofheimer, Wood, Porges, Pearson, & Lawson, 1995; Richards & Cameron, 1989).
Porges’ theory further suggests that one particular measure of cardiac activity that may be more directly related to the kinds of regulatory behaviors children begin to display in toddlerhood and early childhood is vagal regulation of the heart as indexed by a decrease in RSA (vagal withdrawal) during situations where coping or emotional and behavioral regulation is required (Porges, 2001; 2003). Vagal regulation in the form of decreases in RSA during demanding tasks may reflect physiological processes that allow the child to shift focus from internal homeostatic demands to demands that require internal processing or the generation of coping strategies to control affective or behavioral arousal. Thus, vagal withdrawal is thought to be a physiological strategy that permits sustained attention and behaviors indicative of active coping that are mediated by the parasympathetic nervous system (Porges, 1991; 1996; Wilson & Gottman, 1996), and that results in greater cardiac output in the form of HR acceleration.
Considerable research indicates that greater vagal withdrawal during challenging situations is related to better state regulation, greater self-soothing and more attentional control in infancy (DeGangi, et al., 1991; Huffman et al., 1998), fewer behavior problems and more appropriate emotion regulation in preschool (Calkins, 1997; Calkins & Dedmon, 2000; Calkins & Keane, 2004; Porges et al., 1996), and sustained attention in school-age children (Suess et al., 1994). Moreover, recent research comparing the magnitude of RSA response to different types of challenges indicates that children display significantly greater decreases in RSA when provided with parental support during a task than when confronted with a challenge independent of support (Calkins & Keane, 2004), and that the magnitude of this response is an individual difference that is moderately stable across early development and that predicts a range of indicators of adaptive functioning (Calkins & Keane, 2004; El-Sheikh, 2005). The extension of these research findings is that while vagal withdrawal may be related to complex responses involving the regulation of attention and behavior, a deficiency in this ability may be related to early adjustment problems, particularly problems characterized by a lack of behavioral and emotional regulation (Calkins & Dedmon, 2000; Porges, 1996; Wilson & Gottman, 1996).
Lack of behavioral and emotional control is considered a core deficit for children with externalizing-type behavior problems (Gilliom & Shaw; 2004; Keenan & Shaw, 2003). Children with externalizing problems display patterns of aggressive, destructive, undercontrolled behavior that remains stable from preschool to middle childhood (Gilliom & Shaw, 2004) and that often results in more severe conduct problems in adolescence and young adulthood (Olweus, 1979). Given that such problems are believed to have both biological and socialization origins (Moffitt, 1993), one question that may be asked is whether these children display a pattern of physiological dysregulation that impairs their ability to generate and engage appropriate regulatory strategies in situations that are emotionally or behaviorally challenging. A small number of studies have addressed this question. Pine and colleagues (Pine et al., 1998) reported that 11-year-old boys with externalizing symptoms had lower heart period variability. Mezzacappa and colleagues (Mezzacappa et al., 1997) report similar findings among adolescent males. Both researchers conclude that such relations may occur because of parasympathetic links to regulatory abilities involving attentional and behavioral control.
These early seminal studies of RSA and externalizing problems are limited in their focus on adolescent male samples. More recent work has addressed the question of whether such findings may be observed in sample of younger girls and boys. In one study, children at high risk for the development of aggressive behavior problems were identified at age 2 and assessed in a number of challenging tasks (Calkins & Dedmon, 2000). These children displayed significantly lower RSA suppression (less vagal withdrawal) across these tasks than did children at low risk for behavior problems. In a follow-up of these same children, continued behavioral difficulties, including social problems and difficulties with emotion regulation, were characteristic of the children who displayed, across the preschool period, a stable pattern of physiological dysregulation, in the form of lower vagal withdrawal to challenge (Calkins & Keane, 2004). Interestingly, children who displayed a pattern of lower withdrawal at age 2, but who displayed a decrease in RSA (indicating vagal withdrawal) at age 4 had continued behavioral difficulties, suggesting that the early pattern of cardiac vagal regulation may have constrained the acquisition of regulatory skill that affected behavior later in the preschool period.
These limited findings suggest that there may be a physiological profile of poorer vagal regulation of HR activity that may be characteristic of children at risk for early externalizing problems. However, one challenge to the study of physiological regulation among children with behavior problems characterized by aggression is that these problems often present with comorbid internalizing symptoms (anxiety, withdrawal) (Achenbach, Howell, Quay & Connors, 1991; Gilliom & Shaw, 2004). These comorbid problems are often ignored, either because they are thought to be a consequence of single-reporter bias, or because the sample sizes in most studies of children’s behavior problems are too small to allow for separate consideration of pure versus comorbid problems (Calkins & Dedmon, 2000). However, in a recent large-scale study of early externalizing behavior problems, researchers identified differential behavioral and environmental correlates and predictors of pure versus mixed patterns of externalizing behavior problems (Keilly, Lofthouse, Bates, Dodge, & Pettit, 2003). Clearly, it is important to examine whether these different behavioral patterns may be distinguished by cardiac vagal regulation in the form of RSA decreases to emotional and behavioral challenges. One hypothesis is that the comorbid anxiety symptoms, which may associated with overcontrol of emotion, may indicate less severe behavior problems (Lillienfield, 2003) and may be reflected in greater cardiac vagal regulation compared to children with pure externalizing problems. However, excessive vagal withdrawal may also be a marker of emotional lability and a flight/fight response on the part of the child with anxiety symptoms (Beauchaine, 2001). Another possibility is that comorbid problems may be considered more severe than pure internalizing or externalizing problems (Hinshaw, Lahey, & Hart, 1993), and may result in significantly poorer cardiac vagal regulation compared to children with pure externalizing problems.
Although there is a clear rationale for examining cardiac vagal regulation as an indicator of emotional and behavioral regulation, and for hypothesizing that children at risk for behavior problems characterized by a lack of emotional and behavioral regulation would display poorer cardiac vagal regulation, other cardiac measures may also be related to behavior problems in young children. In the adult personality and psychopathology literature, a number of studies suggest links between aggressive and antisocial behavior and autonomic functioning using such indicators as heart rate and skin conductance (see Lahey, Hart, Pliska, Applegate, & McBurnett, 1993; and Raine, 1996 for reviews). One conclusion to be drawn from this literature is that underarousal of the autonomic nervous system as reflected in low heart rate (HR) in particular may be a core characteristic of aggressive behavior in adolescents and adults (Lahey et al., 1993; Raine, Venables & Williams, 1990; Raine, 1996).
Raine and colleagues have also linked low resting heart rate to behavior problems in late childhood (Raine & Jones, 1987; Raine, Venables & Mednick, 1997) and have found that low resting HR in adolescence is predictive of criminal behavior in adulthood. Raine has suggested that low autonomic nervous system (ANS) arousal may be a genetic marker for aggressive behavior (Raine et al., 1990). However, this finding has not been widely replicated in the developmental literature, though the studies examining relations between resting measures of heart rate and aggression in children are few and the behavior of the children tested is typically not as extreme as in studies of adolescent or adult aggression. For example, Eisenberg and colleagues (Eisenberg et al., 1996) examined relations between resting HR in normal school age children and parent report of behavior problems and found modest relations between low resting HR and the incidence of problem behavior. Zahn-Waxler and colleagues found no association between problem behavior in preschoolers and resting heart rate (Zahn-Waxler, Cole, Welsh & Fox, 1995) or between early childhood measures of resting HR and behavior problems at age 7. Using a sample at subclinical risk for behavior problems, Calkins (Calkins & Dedmon, 2000) found no relation between resting HR and toddler behavior problems or preschool social and behavioral functioning (Calkins & Keane, 2004). Clearly more data on the relation between childhood behavior problems, and risk for behavior problems, and resting HR are needed.
HR reactivity has also been examined as a correlate of aggressive behavior in children. Eisenberg (Eisenberg et al., 1996) found an association between boys’ problem behavior and changes in HR during distressing film stimuli, but argued that this relation was due to the lower resting HR measure observed in boys as opposed to any independent change in HR. Wilson and Gottman (1996) hypothesized that excessive heart rate reactivity may interfere with the effective allocation of attentional abilities to meet task demands. Given that resting measures of cardiac activity and response measures have been shown to be related in children (Calkins, 1997; Suess et al., 1994), and that these measures are influenced both sympathetically and parasympathetically, it would be important to examine these effects independently in a sample of children with behavior problems.
The aims of the present study were to examine multiple measures of cardiac functioning in a large sample of male and female children, to try to address several gaps in the current literature. First, the study was designed to look at resting measures of cardiac activity and changes in cardiac activity, across several emotional and behaviorally challenging tasks. As a way of validating prior psychophysiological studies of older children and males, and to test Porges’ Polyvagal theory that parasympathetic functioning, in particular, is related to more adaptive regulatory behavior in young children. A large community sample, overselected for externalizing problems, was chosen as the appropriate group in which to test the theory as this group would have sufficient numbers of boys and girls displaying both mixed and pure patterns of externalizing problems indicative of different patterns of emotion regulation and adaptation. Children were classified as at risk for externalizing problems, at risk for mixed problems or low in problem behavior using borderline clinical cut-offs on a widely-used measure of childhood behavior problems. Such an approach is less stringent than using clinical cut-offs or clinical-referred children, but may be appropriate in longitudinal studies of young children given that considerable discontinuity in behavioral adaptation is observed across early development (NICHD SECC, 2005). Examining children at risk for problems provides a window on interactions between children and their environments as factors in behavioral change across time.
Several questions were addressed in the present study. The first question was whether there would be differences in resting measures of RSA and HP among groups of children displaying different patterns of behavior problems. It was hypothesized that there would be differences in the RSA measure as this measure has been found in numerous studies to index emotional reactivity (c.f. Calkins, 1997). No difference was predicted among the groups in terms of resting HP. Although some researchers speculate that low resting HR (high resting HP) is an index of low arousal that is characteristic of children with conduct problems, little evidence of such a phenomenon exists in community samples of young children or in children at lower risk for behavior problems. Moreover, in this sample, conduct problems were likely to be low occurring. Sex was also examined as part of this question as sex differences have been observed in both levels of behavior problems and in measures of resting HR (Eisenberg et al., 1996). The second question addressed in the study was whether there would be behavior problem group differences in RSA change across tasks. It was hypothesized that the children with behavior problems would display lower vagal withdrawal (smaller decreases in RSA) than the children in the low problem group, as children with behavior problems display significant difficulties in the regulation of affect and behavior (Calkins & Dedmon, 2000). It was unclear whether the mixed children would show the smallest decrease in RSA since they are considered to have a higher level of problem behavior, or whether the anxiety symptoms these children display would result in excessive vagal regulation, which might manifest in a greater degree of RSA suppression (Beauchaine, 2001). The third question addressed in this study was whether there would be behavior problem group differences in HP change across challenge tasks. It was hypothesized that the behavior problem groups would not differ on measures of HP change. Again, although there has been some data to suggest that behavior problems characterized by conduct problems versus anxiety might lead to differences in HP response to challenge, these changes may in fact be a function of differences in the ability to suppress RSA, as opposed to other non-parasympathetic influences on HR (such as sympathetic, homeostatic, or hormonal).
Method
Participants
Participants for this study were recruited as part of an ongoing longitudinal study that began when children were 2-years-old. At the original 2-year assessment, 447 families participated (215 males). Because the original study was designed to the trajectories of early externalizing behavior problems, the original sample was overselected for externalizing problems, with 37% of the sample displaying elevated externalizing symptoms at age 2. Further details about the original sample recruitment strategy may be found in Calkins & Keane, 2004; Smith, Calkins, Keane, Anastopoulos & Shelton (2004) and Calkins and Dedmon (2000). Assessments at age two and each follow-up age (4 and 5 years) consisted of multiple assessments in the home, school, and laboratory. Parents were compensated for their participation in each assessment.
At the five-year assessment that is the focus of the current study, 335 children (156 males, 179 females, mean age = 66 months, SD = 3.2 months) participated in the laboratory portion of the study that included the collection of cardiac data. Attrition from age 2 to age 5 was due to refusal (approximately 6%, as many of the original participants were not told that there would be follow-up as continued funding was unknown at the time), movement from the area (7%) or movement within the area to an unknown address (11%). Of the 335 families participating in this follow-up, 67% were Caucasian, and the remaining families were African American (30%) or mixed race (3%). Sixty four percent of the children were from intact families. Fifty-six percent of the parents of the children had college degrees, 30% had attended some college, and 12% had a high school education. Socioeconomic status of the participants ranged from lower to upper-middle class, with mean Hollingshead score = 43, SD = 10.5. There were no differences between children who completed the 5-year assessment and those who did not on any demographic or behavior problem measure collected at age 2.
Procedures
Overview
Mothers accompanied their children to the laboratory for this assessment. In the laboratory, a number of individual child tasks and mother-child tasks were conducted. Following informed consent, each child had his or her weight and height measured. Then, the child and the mother were observed in a series of individual and dyadic tasks. Several of these tasks were conducted while collecting psychophysiological data from the children. The six tasks that were conducted with the child observed independently of the mother are the focus of the present report. Parents also completed questionnaires concerning their child’s adjustment at preschool. Psychophysiological data were collected during the first half of the session. Sessions typically lasted between 90 and 120 minutes (including informed consent and completion of questionnaires by the mother).
Laboratory assessment
Parents who had participated in prior assessments when their children were four years old were contacted to schedule a laboratory assessment. Mothers were asked to accompany their children to the laboratory where the children were assessed in a laboratory playroom during several procedures. First, the experimenter placed three disposable pediatric electrodes in an inverted triangle pattern on the child’s chest while the child was seated at a table next to the mother. The electrodes were connected to a preamplifier, the output of which was transmitted to a vagal tone monitor (VTM-I, Delta Biometrics, Inc, Bethesda, MD) for R-wave detection. The vagal tone monitor displayed ongoing heart rate (HR) and computed and displayed RSA (vagal tone) every 30 seconds. A data file containing the interbeat intervals (IBIs) for the entire period of collection was transferred to a laptop computer for later artifact editing (resulting from child movement) and analysis.
While connected to the HR collection equipment, the child was observed during a multiepisode sequence that was derived from the Laboratory Temperament Assessment Battery (LAB-TAB; Goldsmith & Rothbart, 1993) and prior work (Calkins, 1997; Calkins & Johnson, 1998; Calkins & Keane, in press; Kochanska, Murray & Coy, 1997). The baseline episode consisted of a 5-minute segment of the videotape “Spot,” a short story about a puppy that explores its neighborhood. While this episode was not a true baseline given that the child’s attention was engaged in an external stimulus, it was sufficient to keep the child sitting quietly and showing little affect. Given the ages of the participants, such stimulus was necessary in order to keep the child seated at the table and to limit movement artifact in the HR data. Following the baseline episode, the child was observed in several situations designed to elicit physiological stress and coping. The onset and end of each challenge episode was marked on the computer file of the IBI data through the use of an electronic signal controlled by the experimenter. In the first episode, an effortful control task was designed to assess the child’s ability to slow down gross and fine motor activity. The child was asked to draw some shapes (circles and stars) between boundary lines at varying speeds (regular, slow, and fast). This episode lasted 6 minutes. The next episode was a second effortful control task similar to a Stroop task. The child was presented with large pictures representing large shapes (animals, geometric figures). Within the larger pictures, smaller shapes were depicted. In half of the trials the small shapes were consistent with the large shape (e.g., a large cat was made up of identical smaller cats), and in the other half the shapes were inconsistent (e.g. large circle made up of small squares). The child was asked to recognize only the smaller shapes in the pictures presented and were instructed to answer as fast as they could. This episode lasted for 4 minutes. The next episode was an attentional persistence task, during which the child was asked to sort a large number of beads by color and place them in a container. This episode lasted 3 minutes. The positive episode that followed consisted of a 4-minute segment in which the child was surprised with a pop up snake and then was instructed to surprise his/her mother. Lastly, a frustration episode consisted of a 4-minute segment in which a second experimenter presented the experimenter and child with candy to be evenly divided. The experimenter proceeded to share the candy unevenly, eating some of the child’s candy, and slowly taking all the candy away from the child while preventing the child from eating any of it.
Each challenge episode was separated from the subsequent episode by a very brief (2-3 min) period during which the child was free to interact with the mother while the experimenter gathered materials for the next episode. This period was necessary because the children’s tolerance for the HR collection (and in particular, remaining seated for collection) was often low. In addition, this break was not considered to be an additional resting measure of cardiac activity with which to contrast the subsequent challenge episode given that the child was almost always engaged with the mother or moving around (or both). Moreover, there was some concern that there would be carry-over effects from the episode to the break that would call into question the validity of using the break to derive resting measures. For these reasons, only the initial baseline measure was considered for analyses involving contrasts with the challenge episodes.
Questionnaires
Mothers were given a packet of questionnaires to complete at this assessment. Of interest for this report was The Child Behavior Checklist for 4-18 year olds (CBCL; Achenbach, 1991). This measure has been widely used by researchers studying early social adjustment and has adequate reliability and validity.
Measures
Cardiac Measures
Two types of physiological measures were derived from the laboratory assessments. These were: cardiac vagal regulation measures (RSA and RSA change), and heart period measures (HP and HP change). To generate measures of cardiac activity from which to derive measures of RSA and RSA in response to challenge, the IBI files were edited and analyzed using MXEDIT software (Delta Biometrics, Bethesda, MD). Editing the files consisted of scanning the data for outlier points relative to adjacent data, and replacing those points by dividing them or summing them so that they would be consistent with the surrounding data. Data files that required editing of more than 5% of the data were not included in the analyses.
Analysis of the IBI data consisted of applying the Porges (1985) method of calculating RSA. This method applies an algorithm to the sequential heart period (HP) data. The algorithm uses a moving 21-point polynomial to detrend periodicities in HP slower than RSA. Then, a band-pass filter extracts the variance of HP within the frequency band of spontaneous respiration in young children, .24-1.04 Hz. Although lower frequency bands may be studied, research with young children has consistently examined this band and identified associations to child functioning (Porges et al., 1996; Stifter & Fox, 1990; Huffman et al., 1998). The estimate of RSA is derived by calculating the natural log of this variance and is reported in units of ln(msec)2. Heart period and RSA were calculated every 30 sec for the five min baseline period and for each of the challenge episodes. These epoch durations are typical for studies of short duration tasks (Huffman et al., 1998; Doussard-Roosevelt, Montgomery, & Porges, (2003). The mean RSA of the epochs within each episode was used in subsequent analyses. If the standard deviation across the epochs was greater than 1.00 for RSA (indicating a high degree of variability over the course of the episode and calling into question the validity of the mean RSA value), that episode was excluded from subsequent analyses. Descriptive statistics for RSA and HP for the baseline and challenge episodes are reported in Table 1.
Table 1.
Descriptive Statistics for RSA, RSA Change, HP, HP Change
| M | SD | Min | Max | n | |
|---|---|---|---|---|---|
| Age 5 RSA | |||||
| Baseline RSA | 6.08 | 1.15 | 3.28 | 9.42 | 296 |
| Effortful control 1 RSA | 6.09 | 1.13 | 3.09 | 9.09 | 293 |
| Effortful control 2 RSA | 5.87 | 1.18 | 3.01 | 8.80 | 291 |
| Attention RSA | 5.57 | 1.13 | 2.72 | 8.85 | 288 |
| Positive RSA | 6.02 | 1.01 | 2.99 | 8.44 | 280 |
| Frustration RSA | 5.81 | 1.11 | 2.68 | 9.41 | 287 |
| Age 5 RSA Change | |||||
| Effortful control 1 | -.02 | .55 | -2.88 | 2.05 | 293 |
| Effortful control 2 | .20a | .77 | -3.47 | 3.34 | 290 |
| Attention | .49 | .78 | -3.02 | 3.24 | 287 |
| Positive | .06 | .75 | -2.44 | 2.27 | 279 |
| Frustration | .25 | .72 | -2.66 | 2.50 | 286 |
| Age 5 HP | |||||
| Baseline HP | 642.82 | 70.88 | 501.90 | 913.56 | 296 |
| Effortful control 1 HP | 626.62 | 66.94 | 487.45 | 893.86 | 293 |
| Effortful attention 2 HP | 621.84 | 64.46 | 489.89 | 862.02 | 291 |
| Attention HP | 595.75 | 59.75 | 473.35 | 798.23 | 288 |
| Positive HP | 603.56 | 59.59 | 453.32 | 833.66 | 280 |
| Frustration HP | 614.50 | 62.75 | 491.67 | 847.91 | 287 |
| Age 5 HP Change | |||||
| Effortful control 1 | 15.95 | 25.00 | -53.60 | 133.82 | 293 |
| Effortful control 2 | 20.04 | 35.07 | -75.39 | 176.32 | 287 |
| Attention | 46.05 | 35.93 | -54.30 | 195.11 | 287 |
| Positive | 38.34 | 41.43 | -60.10 | 220.88 | 279 |
| Frustration | 27.25 | 37.44 | -74.80 | 223.44 | 286 |
RSA, respiratory sinus arrhythmia; HP, heart period
Positive change scores indicate greater decrease from baseline to task
As the table indicates, the data files of some children were not included in some of the analyses. Several situations led to missing data across all 6 tasks. A few children would not allow the experimenter to apply the HR electrodes (7% of missing). In addition, the HR data collection equipment failed on several occasions (72% of missing). A third explanation for missing data was that the child pulled on, or touched, the HR leads from the beginning of the collection procedure (21%). Individual trials may have also been compromised due to excessive movement artifact affecting greater than 5% of the data in the HR file. A comparison of children with complete missing HR data from those without missing data revealed that these children did not differ in terms of behavior problem group status.
Change scores were computed for each challenge episode by subtracting the challenge episode RSA from the baseline RSA, and by subtracting the challenge HP from the baseline HP. These change scores appear in Table 1. High or positive change scores for RSA indicated that the child displayed a decrease in RSA (vagal withdrawal) from baseline to task. High or positive scores for HP indicated that the child displayed a decrease in HP (or HR acceleration) from baseline to task.
Child behavior problems
The CBCL completed by the parent was used to generate groups of children with different behavior problem profiles that might map onto different patterns of emotion regulation. Two broadband scales, representing externalizing behavior problems (attention, aggression, destructive behavior problems) and internalizing behavior problems (withdrawal, anxiety, and depression) were the focus of the study. Children were selected for the “Low problem” group if their t-scores on the two scales were below 60 (n=249, 111 males), which is considered the borderline clinical cut-off (Achenbach, 1991); children who score at or above this cut-off are ten times more likely to be referred for clinical services than children who score below the cut-off (Achenbach, 1991). Children were selected for the “Risk for Externalizing Problems” group if their externalizing t-score was at or above 60 and their internalizing t-score was below 60 (n = 47, 21 males, 14 above the clinical cut-off of 70). Children were selected for the “Risk for Mixed Problems” group if their t-scores on both the externalizing and internalizing scales were at or above 60 (n = 29, 13 males, 4 above the clinical cut-off of 70 on both scales). Because externalizing behavior problems were the focus of the study and children were not originally included in the sample at age two if their t-scores on the internalizing scale was high, this “Risk for Internalizing Problems” group (n = 13, 10 males, 2 above the clinical cut-off of 70) was not considered representative of children at age 5 with internalizing problems and was not included in the analyses. In addition, 7 mothers failed to complete the CBCL and these children were not included in the analyses.
Descriptive statistics for the CBCL subscales and the broadband measures of child internalizing and externalizing behavior problems appear in Table 2.
Table 2.
CBCL Descriptive Statistics for Problem Groups
| Low problem |
Externalizing |
Mixed |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | N | M | SD | N | M | SD | N | |
| Internalizing T Score | 43.51 | 6.80 | 240 | 50.19 | 6.11 | 47 | 65.69 | 4.94 | 28 |
| Externalizing T Score | 47.22 | 7.32 | 240 | 64.36 | 4.40 | 47 | 68.07 | 5.87 | 28 |
| Withdrawn T Score | 51.14 | 2.86 | 240 | 52.23 | 3.53 | 47 | 60.69 | 7.02 | 28 |
| Somatic Complaints T Score | 51.94 | 3.53 | 240 | 53.79 | 5.01 | 47 | 60.97 | 9.10 | 28 |
| Anxious/Depressed T Score | 50.78 | 2.10 | 240 | 53.32 | 3.71 | 47 | 65.10 | 6.71 | 28 |
| Social Problems T Score | 51.52 | 3.56 | 240 | 55.51 | 6.06 | 47 | 60.97 | 8.92 | 28 |
| Thought Problems T Score | 52.24 | 4.62 | 240 | 55.91 | 6.89 | 47 | 61.24 | 9.17 | 28 |
| Attention Problems T Score | 52.41 | 4.50 | 240 | 58.60 | 6.74 | 47 | 65.79 | 9.05 | 28 |
| Delinquent Behavior T Score | 52.36 | 3.90 | 240 | 60.11 | 5.96 | 47 | 62.17 | 6.50 | 28 |
| Aggressive Behavior T Score | 51.83 | 2.91 | 240 | 65.17 | 6.29 | 47 | 69.93 | 8.42 | 28 |
CBCL, Child Behavior Checklist (Achenbach, 1991).
These data were analyzed using an ANOVA comparing the three groups on the two broadband subscales. The analysis indicated significant group differences on both scales, F (2,314) = 146.72, p < .001 for internalizing and F (2,314) = 208.90, p < .001 for externalizing. Follow-up post-hoc tests comparing the three groups indicated that the children in the low problem group were rated by their mothers as lower in externalizing symptoms than both the risk for externalizing group and the risk for mixed problems group (Tukey’s HSD, p < .001 for both contrasts), but that the two behavior problem groups did not differ in terms of externalizing score. The three groups differed from each other in terms of internalizing score, with the low problem group receiving the lowest internalizing score and the mixed group receiving the highest (Tukey’s HSD, p < .001 for each contrast). Additional analyses indicated that the three groups differed from each other on every subscale (p< .001 for all contrasts, Tukey’s HSD) except the withdrawn subscale, in which the mixed problem group was significantly higher (p< .001 for all contrasts, Tukey’s HSD) than both the low problem group and the externalizing group, who did not differ from each other.
Results
Preliminary Analyses
Preliminary analyses examined whether there were any relations between sex, race, age, height, weight, or SES and the physiological measures and behavior problem groups. The behavior problem groups did not differ on any of these measures. The physiological measures of baseline RSA and HP and task RSA and HP were unrelated to all the measures except sex. Across all 5 tasks, boys displayed higher HP (lower HR) than did girls, F(1,259) = 4.09, p < .04. Subsequent analyses of HP and HP change incorporated both sex and behavior problem group.
Behavior problem group differences in resting RSA and HP
To examine whether the behavior problem groups differed in terms of resting RSA and resting HP, two ANOVA’s were conducted with RSA and HP as the dependent measures and sex and behavior problem group as the independent variables. Group means for this analysis are presented in table 3.
Table 3.
Descriptive Statistics for Baseline RSA & HP for Problem Groups by Gender
| Low problem |
Externalizing |
Mixed |
||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| 5 yr Baseline RSA | 6.05 | 6.03 | 6.18 | 6.13 | 6.68 | 6.16 |
| 5 yr Baseline HP | 647.61 | 636.50 | 656.25 | 639.50 | 687.40 | 624.44 |
RSA, respiratory sinus arrhythmia; HP, heart period
The first ANOVA analysis indicated that there was no difference between girls and boys or among the behavior problem groups in terms of resting RSA. Nor was there an interaction of sex and behavior problem group. The second analysis indicated that there was an effect of sex on HP, F (1,280) = 5.73, p < .02. Boys displayed significantly higher resting HP, lower HR, than did girls. No effect of behavior problem group and no interaction of sex and behavior problem group were found.
Behavior problem group differences in challenge RSA
To examine whether the behavior problem groups differed in terms of RSA change to the five challenge tasks, a repeated measures ANOVA was conducted with the three behavior problem groups. Sex was not examined as no sex differences were found in any of the measures of RSA. In addition, although initial levels of RSA are predictive of change scores of RSA, these initial levels did not differ across behavior problem groups, and were not covaried in this analysis. Group means for this analysis are presented in Figure 1.
Figure 1. RSA Change Scores for Problem Groups.
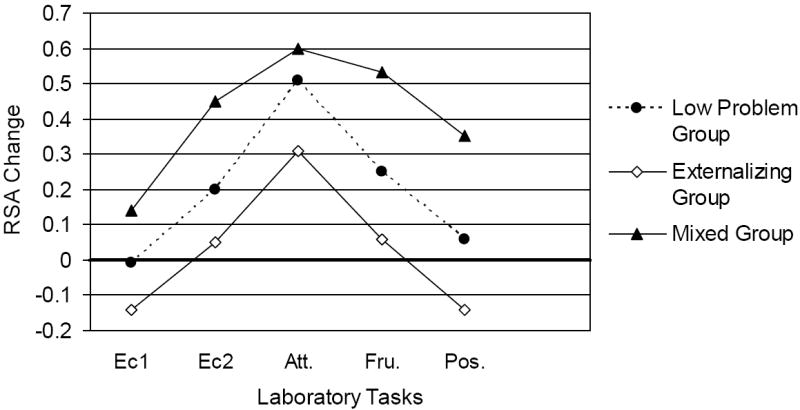
RSA = Respiratory sinus arrhythmia, Positive change scores indicate greater decrease from baseline to task or vagal withdrawal.
Ec1 = effortful control task #1
Ec2 = effortful control task #2
Att. = attention task
Fru.= frustration task
Pos. = positive task
This analysis revealed a main effect of behavior problem group on the measures of RSA change, F (2,260) = 3.91, p < .02, partial eta-squared = .03. No interaction of task and behavior problem group was found. Across all five challenge tasks, the behavior problem groups differed in the measures of RSA change. Follow-up contrast tests, repeated measures ANOVA comparing each of the two groups revealed that the mixed group differed from the externalizing group, F (1,58) = 5.44, p < .02. Children in the mixed group displayed significantly greater RSA decreases (greater vagal withdrawal) across tasks than did children in the externalizing only group. The analysis comparing the mixed group and the low problem group indicated a trend toward a significant difference, F (1, 226) = 3.13, p = .08. Similarly, a trend toward a significant difference emerged between the externalizing group and the low problem group F (1, 232) = 2.69, p = .10. Children in the mixed problem group displayed the greatest decrease in RSA (greater vagal withdrawal) and children in the externalizing only group displayed the smallest decrease, with the low problem children in the middle of these two groups.
Behavior problem group differences in HP change
To examine whether the behavior problem groups differed in terms of HP change to the five challenge tasks, a repeated measures ANOVA was conducted with the three behavior problem groups. Sex was examined because sex differences were found across all of the HP measures. However, because there was a sex difference in terms of resting HP, which is used to compute the HP change score, resting HP was used as a covariate in this analysis. Descriptive statistics for this analysis are presented in Table 4.
Table 4.
Heart Period Change Scores for Problem Groups by Gender
| Low problem |
Externalizing |
Mixed |
||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Effortful Control 1 | 16.61a | 16.47 | 12.97 | 11.64 | 24.27 | 19.82 |
| Effortful Control 2 | 21.30 | 18.84 | 13.80 | 9.42 | 43.20 | 33.57 |
| Attention | 44.21 | 49.64 | 38.10 | 33.06 | 53.34 | 47.47 |
| Positive | 39.15 | 37.02 | 31.94 | 30.15 | 60.74 | 45.31 |
| Frustration | 28.76 | 25.78 | 25.91 | 18.00 | 49.45 | 30.96 |
Positive change scores indicate greater decrease from baseline to task, Heart period change scores adjusted for baseline heart period
This analysis revealed that after controlling for the effects of resting HP, which was a significant predictor of HP change, p < .001, there was a main effect of behavior problem group on the measures of HP change, F (1,260) = 4.41, p < .01, partial eta-squared = .04, no effect of sex, and no interaction of sex and behavior problem group. There were no interactions involving tasks. Across all five challenge tasks, the behavior problem groups differed in the measures of HP change. Follow-up contrast tests, repeated measures ANOVA, comparing each pair of the problem groups revealed that the mixed group differed from the externalizing group, F (1,58) = 5.18 p < .03. Children in the mixed group displayed significantly greater HP change (HR acceleration) across tasks than did children in the externalizing only group. The analysis comparing the mixed group and the low problem group also indicated a significant difference in terms of HP change, F (1, 226) = 4.98, p = .03. No significant difference in HP change was found between the externalizing group and the low problem group. Children in the mixed problem group displayed the highest level of HP change across the five challenge tasks.
To examine whether the HP change difference that was observed among the three problem groups was a function of the group difference in RSA withdrawal that was observed, individual ANOVA’s were conducted comparing the three groups on HP change in each of the 5 tasks, controlling for RSA change scores for each of the five tasks. In each case, the covariate RSA change variable accounted for a significant portion of the variance in HP change, p < .001 for each analysis. However, after controlling for the effects of RSA suppression, the behavior problem groups no longer differed in terms of HP change.
Discussion
One broad goal of this study was to understand the patterns of cardiac activity and regulation that may be observed in young children at risk for different patterns of externalizing behavior problems. Although these children are thought to have significant deficits in the regulation of behavior and emotion (Keenan, 2003), prior work on the physiological indicators of such problems has been limited in its focus on older boys with little research conducted on younger samples of boys and girls. Moreover, prior work has not sought to disentangle physiological profiles of children with only externalizing problems from those of children experiencing both externalizing and internalizing symptoms. Our focus on the early childhood period, and on children at borderline risk for behavior problems, was motivated by the observation in other research that considerable development occurs in the domain of emotion regulation during this period, that behavior problems are becoming relatively stable by this time, and by the fact that relatively few studies of physiological functioning have been conducted with children of this age.
The first issue examined in this study was whether there would be differences in resting measures of RSA and HP among groups of children at risk for different patterns of behavior problems. It was hypothesized that there would be differences in the resting RSA measure as such differences have been found in numerous studies of emotional reactivity (c.f. Calkins, 1997), with children displaying greater negative emotion also displaying higher resting RSA (Calkins et al., 2002). However, this hypothesis was not confirmed. One explanation for the null finding is that children with different patterns of behavior problems may experience and express different patterns of negative emotion. Fear, frustration, and sadness are all negative emotions. However, these three emotions have not been examined separately in studies of RSA. It may be the case that different negative emotions are each differentially influenced by parasympathetic functioning. Given that externalizing and comorbid externalizing and internalizing behavior problems may be characterized by different patterns of these negative emotions, it may be difficult to differentiate among them with a single physiological measure.
The analysis of resting HP (the inverse of HR) also indicated that the behavior problem groups did not differ from one another or from the control group. The findings of the present study replicate a number of null findings (Calkins & Dedmon, 2000; Calkins & Keane, 2004; Zahn-Waxler et al., 1996; Van Hulle, Corley, Zahn-Waxler, Kagan, & Hewitt, 2000) that suggest that early aggression is likely determined by socialization and biological factors that are quite complex. Moreover, as the current body of literature clearly demonstrates, HR is multiply determined by sympathetic, parasympathetic, homeostatic and hormonal processes. Finally, children at risk early in development, or those coping with mild regulatory challenges, may not develop serious conduct problems (i.e., the kind that have been associated with lower resting HR) later in development. Although behavior problems can be highly stable throughout childhood and adolescence, increases in language development, cognitive abilities, and self-regulation during toddlerhood should allow children to learn to control early normative noncompliant, aggressive, and impulsive tendencies, leading to a decline in serious and debilitating problem behavior (Campbell, 2002).
A second question addressed in this study was whether children with different patterns of behavior problems would display different patterns of RSA response to environmental challenges. Such a question follows directly from Porges’ Polyvagal theory (2001, 2003), which notes that vagal regulation of cardiac activity is likely to be implicated in a variety of conditions characterized by deficits in emotional and social functioning. Moreover, numerous studies document that RSA does change under conditions in which the individual must generate a response to emotional, cognitive, and behavioral challenges (e.g. Calkins & Dedmon, 2000; El-Sheikh, 2005). And, young children who display symptoms of early behaviors problems are presumed to have difficulty regulating their emotions and their behaviors (Keenan, 2003). Presumably, such behavioral difficulties would be reflected in the fundamental ability to regulate cardiac activity under conditions of challenge. Five tasks were chosen for examination because they have been demonstrated in prior research to elicit a significant parasympathetic response and because they present clear emotional and behavioral challenges to the children (Calkins, 1997; Calkins & Dedmon, 2000). Because no study to date had examined whether the RSA response would vary as a function of early behavior problem subtypes, multiple hypotheses were proposed. Given the comorbid anxiety, withdrawal, and depression symptoms of the mixed group, one hypothesis was children with both externalizing and internalizing symptoms would exhibit greater vagal withdrawal, indicative of either better physiological regulation, or perhaps excessive physiological regulation. Alternatively, it was hypothesized that these children would display a pattern of less vagal withdrawal, indicative of poorer regulation, because of the pattern of multiple behavior problem symptoms with which they present. The data supported the first hypothesis. Children at risk for a mixed profile of externalizing and internalizing behavior problems displayed the greatest cardiac vagal regulation whereas children with a pure externalizing profile displayed the least regulation. This pattern of findings is suggestive of support for Beauchaine’s (2001) hypothesis that there may be an optimal or moderate level of vagal regulation (in this case displayed by the low problem group) that is associated with both preparedness to respond to, and constructive engagement with, environmental challenge. And, moreover, that excessive vagal withdrawal in response to challenge (in this case displayed by the mixed problem group) reflects greater emotional lability, which is a likely characteristic of the children with a mixed behavior problem profile.
From a developmental point of view, it would be important for future research to identify which pattern of symptoms, internalizing or externalizing, if either, emerge first. Perhaps a child’s initial internalizing symptoms impact his or her ability to effectively communicate with parents and/or peers, which may lead a child to feel more frustrated during social interactions and eventually react in a more impulsive, blunt, or aggressive manner. On the other hand, a child’s initial externalizing symptoms may also impact his or her ability to effectively communicate with parents and/or peers which may lead that child to being rejected at school and potentially experiencing internalizing symptoms. The cross-sectional nature of this data set precludes answering these questions, although, we know that because this sample was overselected for externalizing problems, in all likelihood, for many of the children, these symptoms emerged first.
A third issue addressed in this study concerned the HP change in response to challenge. The data indicated that sex differences in HP response were largely a function of sex difference in resting HP. In addition to this sex difference, there was a behavior problem group difference, with the mixed group displaying significantly greater HP change (decrease, indicative of greater HR acceleration) than the other two groups of children. Such a difference might indicate greater sympathetic activation which is thought to be characteristic of children with anxiety. However, because RSA suppression is thought to facilitate HR increases under conditions of challenge, it was important to control for these parasympathetic influences prior to concluding that HR differences might reflect sympathetic influences. In individual ANOVA’s examining HP change in each of the five tasks, controlling for the parasympathetic influences measured by RSA suppression, there were no differences observed among the three behavior problem groups. Thus, the group differences in HP change (HR acceleration) appeared to have been largely a function of parasympathetic influence, in the form of vagal withdrawal, on HR.
The data from this study suggest that children at risk for different patterns of behavior problems, patterns that may reflect lack of control of emotion and aggression versus aggression with anxious symptomology, also display a distinct pattern of parasympathetic nervous system functioning that has been linked in past research to the regulation of attention, affect, and behavior (Calkins, 1997; Calkins & Dedmon, 2000; Calkins & Keane, 2004). Differentiating among subtypes at the behavioral as well as physiological level is critical, as such differentiation may suggest sources of these behavioral differences as well as factors that influence the outcomes of these behavioral patterns. Future research must determine whether the parasympathetic processes precede the behavioral pattern or are a consequence of it. Moreover future research should also examine whether the greater cardiac vagal regulation of the anxious/aggressive group might be indicative of a more severe form of behavioral and physiological dysfunction.
This study has several limitations that must be acknowledged. First, the data were collected in one brief laboratory session. Questions about whether such a brief observation period allow for adequate characterization of children’s responding are unanswered. Second, the effects sizes for the behavior problem group differences were quite modest. Third, the sample itself was not comparable to many other samples of children with more serous levels of conduct problems. Finally, no effort was made in this report to examine possible moderating effects on early cardiac physiological regulation, such as parenting behavior. Such an analysis would likely shed more light on the multiple developmental processes implicated in the emergence of child regulation skills across the preschool period. Nevertheless, the data from this study demonstrate that there are physiological differences among children with different patterns of behavior problems, and provide support for Polyvagal theory (Porges, 2001; 2003). The extent to which these differing patterns influence subsequent function, and may be influenced by numerous environmental factors, are important questions for future research.
Acknowledgments
This research was supported by National Institute of Mental Health awards (MH 55625 and MH 55584) to the first author and an NIMH award (MH 58144) to the first and third authors. The authors would like to thank Kathryn Degnan, Rachel Nas, Cynthia Smith, Caitlin Stone, and Michelle Wilkinson for their help in subject recruitment and data collection and coding. The authors also thank the families who generously gave their time to participate in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
List of References
- Achenbach TM. Integrative guide for the 1991 CBCL/4-18, YSR & TRF profiles. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Achenbach TM, Howell CT, Quay HC, Connors CK. National survey of problems and competencies among four-to-sixteen-year-olds: Parents’ reports for normative and clinical samples. Monographs of the Society for Research in Child Development. 1991;56(Serial No 225 Whole No 3) [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD. Handbook of self-regulation: Research, theory, and application. New York: The Guilford Press; 2004. [Google Scholar]
- Blair C. Behavioral inhibition and behavioral activation in young children: Relations with self-regulation and adaptation to preschool children attending Head Start. Developmental Psychobiology. 2003;42:301–311. doi: 10.1002/dev.10103. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Origins and outcomes of individual differences in emotional regulation. In: Fox NA, editor. Emotion regulation: Behavioral and biological considerations, Monographs of the Society for Research in Child Development. Nos 23 Serial No 240. 1994. pp. 53–72. [PubMed] [Google Scholar]
- Calkins SD. Cardiac vagal tone indices of temperamental reactivity and behavioral regulation in young children. Developmental Psychobiology. 1997;31:125–135. doi: 10.1002/(sici)1098-2302(199709)31:2<125::aid-dev5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Calkins SD. Regulatory competence and early disruptive behavior problems: The role of physiological regulation. In: Olson S, Sameroff A, editors. Regulatory Processes in the Development of Behavior Problems: Biological, Behavioral, and Social-Ecological Interactions. Cambridge University Press; in press. [Google Scholar]
- Calkins SD, Dedmon SE. Physiological and behavioral regulation in two-year-old children with aggressive/destructive behavior problems. Journal of Abnormal Child Psychology. 2000;28:103–118. doi: 10.1023/a:1005112912906. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Dedmon S, Gill K, Lomax L, Johnson L. Frustration in infancy: Implications for emotion regulation, physiological processes, and temperament. Infancy. 2002;3:175–198. doi: 10.1207/S15327078IN0302_4. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Fox NA. Self-regulatory processes in early personality development: A multilevel approach to the study of childhood social withdrawal and aggression. Development and Psychopathology. 2002;14:477–498. doi: 10.1017/s095457940200305x. [DOI] [PubMed] [Google Scholar]
- Calkins SD, Johnson M. Toddler regulation of distress to frustrating events: Temperamental and maternal correlates. Infant Behavior and Development. 1998;21:379–395. [Google Scholar]
- Calkins SD, Keane SP. Cardiac vagal regulation across the preschool period: Stability, continuity, and implications for childhood adjustment. Developmental Psychobiology. 2004;45:101–112. doi: 10.1002/dev.20020. [DOI] [PubMed] [Google Scholar]
- Campbell SB. Behavior problems in preschool children: Clinical and developmental issues. 2. New York: Guilford Press; 2002. [Google Scholar]
- DeGangi G, DiPietro J, Greenspan S, Porges SW. Psychophysiological characteristics of the regulatory disordered infant. Infant Behavior and Development. 1991;14:37–50. [Google Scholar]
- Doussard-Roosevelt, Montgomery L, Porges S. Short-term stability of physiological measures in kindergarten children: Respiratory sinus arrhythmia, heart period, and cortisol. Developmental Psychobiology. 2003;43:230–242. doi: 10.1002/dev.10136. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes R, Guthrie I, Murphy B, Maszk P, Holmgren R, Suh K. The relations of regulation and emotionality to problem behavior in elementary school. Development and Psychopathology. 1996;8:141–162. [Google Scholar]
- Eisenberg N, Fabes R, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children’s social functioning: A longitudinal study. Child Development. 1995;66:1360–1384. [PubMed] [Google Scholar]
- El-Sheik M. Stability of respiratory sinus arrhythmia in children and young adolescents. Developmental psychobiology. 2005;46:307–317. doi: 10.1002/dev.20036. [DOI] [PubMed] [Google Scholar]
- Fox NA. Psychophysiological correlates of emotional reactivity during the first year of life. Developmental Psychology. 1989;25:364–372. [Google Scholar]
- Fox NA. Dynamic cerebral processes underlying emotion regulation. In: Fox NA, editor. Emotion regulation: Behavioral and biological considerations, Monographs of the Society for Research in Child Development. Nos 23 Serial No 240. 1994. pp. 152–166. [PubMed] [Google Scholar]
- Fox N, Calkins SD. The development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion. 2003;23:7–26. [Google Scholar]
- Gilliom M, Shaw DS. Codevelopment of externalizing and internalizing problems in early childhood. Development and Psychopathology. 2004;16:313–333. doi: 10.1017/s0954579404044530. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. The laboratory temperament assessment battery (LAB-TAB) University of Wisconsin; 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw SP, Lahey BB, Hart E. Issues of taxonomy and comorbidity in the development of conduct disorder. Development and Psychopathology. 1993;5:31–49. [Google Scholar]
- Hoffheimer JA, Wood BR, Porges SW, Pearson E, Lawson E. Respiratory sinus arrhthymia and social interaction patterns in preterm newborns. Infant Behavior and Development. 1995;18:233–245. [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University; 1975. [Google Scholar]
- Huffman LC, Bryan Y, del Carmen R, Pederson F, Doussard-Roosevelt J, Porges S. Infant temperament and cardiac vagal tone: Assessments at twelve weeks of age. Child Development. 1998;69:624–635. [PubMed] [Google Scholar]
- Keenan K. Emotion dysregulation as a risk factor for child psychopathology. Clinical Psychology: Science and Practice. 2000;7:418–434. [Google Scholar]
- Keenan K, Shaw DS. Exploring the Etiology of Antisocial Behavior in the First Years of Life. In: Lahey BB, Moffitt TE, Caspi A, editors. Causes of Conduct Disorder and Juvenile Delinquency. New York: The Guilford Press; 2003. pp. 153–181. [Google Scholar]
- Keiley MK, Lofthouse N, Bates JE, Dodge KA, Pettit GS. Differential risks of covarying and pure components in mother and teacher reports of externalizing and internalizing behavior across ages 5 to 14. Journal of Abnormal Child Psychology. 2003;31:267–283. doi: 10.1023/a:1023277413027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Murray K, Coy KC. Inhibitory control as a contributor to conscience in childhood: From toddler to early school age. Child Development. 1997;68:264–277. [PubMed] [Google Scholar]
- Kopp C. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18:199–214. [Google Scholar]
- Lahey B, Hart E, Pliska S, Applegate B, McBurnett R. Neurophysiological correlates of conduct disorder: A rationale and review of research. Journal of Clinical Child Psychology. 1993;22:141–153. [Google Scholar]
- Lilienfeld SO. Comorbidity between and within childhood externalizing and internalizing disorders: Reflections and directions. Journal of Abnormal Child Psychology. 2003;31:285–291. doi: 10.1023/a:1023229529866. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E, Tremblay R, Kindlon D, Saul J, Arseneault L, Seguin J, Pihl R, Earls F. Anxiety, antisocial behavior, and heart rate regulation in adolescent males. Journal of Child Psychology and Psychiatry. 1997;38:457–469. doi: 10.1111/j.1469-7610.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100(4):674–701. [PubMed] [Google Scholar]
- NICHD SECC. Trajectories of physical aggression from toddlerhood to middle childhood. Monographs of the Society for Research in Child Development. 2004;69:278. doi: 10.1111/j.0037-976x.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Huang-Pollock CL. An early-onset model of the role of executive functions and intelligence in conduct disorder/delinquency. In: Lahey BB, Moffit TE, Caspi A, editors. Causes of Conduct Disorder and Juvenile Delinquency. New York: Guilford Press; 2003. pp. 227–253. [Google Scholar]
- Olweus D. Stability of aggressive reactive patterns in males: A Review. Psychological Bulletin. 1979;86:852–875. [PubMed] [Google Scholar]
- Pine D, Wasserman G, Miller L, Coplan J, Bagiella E, Kovelenku P, Myers M, Sloan R. Heart period variability and psychopathology in urban boys at risk for delinquency. Psychophysiology. 1998;35:521–529. doi: 10.1017/s0048577298970846. [DOI] [PubMed] [Google Scholar]
- Porges SW. Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. 1985 United States patent no 4520944. [Google Scholar]
- Porges SW. Vagal tone: An autonomic mediator of affect. In: Garber J, Dodge KA, editors. The development of emotional regulation and dysregulation. Cambridge: Cambridge University Press; 1991. pp. 111–128. [Google Scholar]
- Porges SW. Physiological regulation in high-risk infants: A model for assessment and potential intervention. Development and Psychopathology. 1996;8:43–58. [Google Scholar]
- Porges SW. The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The Polyvagal Theory: Phylogenetic contributions to social behavior. Physiology and Behavior. 2003:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Porges SW, Byrne EA. Research methods for measurement of heart rate and respiration. Biological Psychology. 1992;34:93–130. doi: 10.1016/0301-0511(92)90012-j. [DOI] [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt J, Maita AK. Vagal tone and the physiological regulation of emotion. Monographs of the Society for Research in Child Development. 1994;59(Nos 23 Serial No 240):167–186. [PubMed] [Google Scholar]
- Porges SW, Doussard-Roosevelt J, Portales L, Greenspan SI. Infant regulation of the Vagal “Brake” predicts child behavior problems: A psychobiological model of social behavior. Developmental Psychobiology. 1996;29:697–712. doi: 10.1002/(SICI)1098-2302(199612)29:8<697::AID-DEV5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Raine A. Autonomic nervous system activity and violence. In: Stoff DM, Cairns RB, editors. Aggression and Violence. Hillsdale, NJ: Erlbaum; 1996. pp. 145–168. [Google Scholar]
- Raine A, Jones F. Attention, autonomic arousal, and personality in behaviorally disordered children. Journal of Abnormal Child Psychology. 1987;15:583–599. doi: 10.1007/BF00917243. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables P, Mednick S. Low resting heart rate at age three years predisposes to aggression at age 11 years: Evidence from the Mauritius Child Health Project. Journal of the Academy of Child and Adolescent Psychiatry. 1997;36:1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- Raine A, Venable P, Williams M. Relationship between CNS and ANS measures of arousal at age 15 and criminality at age 24. Archives of General Psychiatry. 1990;47:1003–1007. doi: 10.1001/archpsyc.1990.01810230019003. [DOI] [PubMed] [Google Scholar]
- Richards JE. Respiratory sinus arrhythmia predicts heart rate and visual responses during visual attention in 14- and 20-week-old infants. Psychophysiology. 1985;22:101–109. doi: 10.1111/j.1469-8986.1985.tb01567.x. [DOI] [PubMed] [Google Scholar]
- Richards JE. Infant visual sustained attention and respiratory sinus arrhythmia. Child Development. 1987;58:488–496. [PubMed] [Google Scholar]
- Richards JE, Cameron D. Infant heart rate variability and behavioral developmental status. Infant Behavior and Development. 1989;12:45–58. [Google Scholar]
- Rubin KH, Burgess KB, Dwyer KM, Hastings PD. Predicting preschoolers’ externalizing behaviors from toddler temperament, conflict, and maternal negativity. Developmental Psychology. 2003;39:164–176. [PubMed] [Google Scholar]
- Shaw DS, Keenan K, Vondra J, Delliquadri E, Giovanelli J. Antecedents of preschool children’s internalizing problems: A longitudinal study of low-income families. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:1760–1767. [PubMed] [Google Scholar]
- Smith C, Calkins S, Keane SP, Anastopoulos A, Shelton T. Predicting stability and change in toddler behavior problems: Contributions of maternal behavior and child sex. Developmental Psychology. 2004;40:29–42. doi: 10.1037/0012-1649.40.1.29. [DOI] [PubMed] [Google Scholar]
- Stifter CA, Fox NA. Infant reactivity: Physiological correlates of newborn and 5-month temperament. Developmental Psychology. 1990;26:582–588. [Google Scholar]
- Suess PE, Porges SW, Plude DJ. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology. 1994;31:17–22. doi: 10.1111/j.1469-8986.1994.tb01020.x. [DOI] [PubMed] [Google Scholar]
- Thompson RA. Emotion regulation: A theme in search of a definition. In: Fox NA, editor. Emotion regulation: Behavioral and biological considerations, Monographs of the Society for Research in Child Development. Nos 23 Serial No 240. 1994. pp. 25–52. [PubMed] [Google Scholar]
- Van Hulle CA, Corley R, Zahn-Waxler C, Kagan J, Hewitt JK. Early childhood heart rate does not predict externalizing behavior problems at age 7 years. Journal of the American Academy of Child and Adolescent Psychiatry. 2000;39:1238–1244. doi: 10.1097/00004583-200010000-00010. [DOI] [PubMed] [Google Scholar]
- Wilson B, Gottman J. Attention--the shuttle between emotion and cognition: Risk, Resiliency, and physiological bases. In: Hetherington E, Blechman E, editors. Stress, coping and resiliency in children and families. Mahwah, N.J.: Lawrence Erlbaum Associates; 1996. [Google Scholar]
- Zahn-Waxler C, Cole P, Welsh J, Fox N. Psychophysiological correlates of empathy and prosocial behaviors in preschool children with behavior problems. Development and Psychopathology. 1995;7:27–48. [Google Scholar]


