Abstract
Background
When nicotine-dependent human subjects abstain from cigarette smoking, they exhibit deficits in working memory. An understanding of the neural substrates of such impairments may help to understand how nicotine affects cognition. Our aim, therefore, was to identify abnormalities in the circuitry that mediates working memory in nicotine-dependent subjects after they initiate abstinence from smoking.
Methods
We used blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI) to study eight smokers while they performed a letter version of the N-Back working memory task under satiety (≤1.5 hours abstinence) and abstinence (≥14 hours abstinence) conditions.
Results
Task-related activity in the left dorsal lateral prefrontal cortex (DLPFC) showed a significant interaction between test session (satiety, abstinence) and task load (1-back, 2-back, and 3-back). This interaction reflected the fact that task-related activity in the satiety condition was relatively low during performance of the 1-back task but greater at the more difficult task levels, whereas task-related activity in the abstinence condition was relatively high at the 1-back level and did not increase at the more difficult task levels.
Conclusions
We conclude that neural processing related to working memory in the left DLPFC is less efficient during acute abstinence from smoking than at smoking satiety.
Keywords: Functional magnetic resonance imaging, tobacco, nicotine, withdrawal, brain imaging, prefrontal cortex
Working memory is a limited-capacity system, responsible for the maintenance and manipulation of online information, that contributes to a wide range of cognitive operations (Baddeley and Della Salla 1996; Ragland et al 2002). Acute abstinence from smoking has deleterious effects on working memory in nicotine-dependent subjects, and smoking reverses these effects (e.g., Snyder and Henningfield 1989; Blake and Smith 1997). In an investigation of how working memory load interacts with smoking abstinence, we used a parametric letter version of the N-back task to test a group of smokers (n = 15) that included the subjects studied here with functional magnetic resonance imaging (fMRI). They showed significantly longer response latencies when abstinent (13–16 hours) than when at smoking satiety (Mendrek et al, unpublished data), and mean response latency increased with memory load up to the 3-back level. In the abstinence condition, however, peak latency was observed at the 2-back level and dropped (though not significantly) at the 3-back level. This result, in conjunction with the fact that error rates were especially high at the 3-back level during abstinence (26% during abstinence vs. 18% during satiety), suggested that smokers reach maximum memory load capacity (and put forth maximum effort) at lower levels of task difficulty while in abstinence. Because of the fundamental role of working memory in cognition, impairments in this function can contribute to the symptoms that follow abrupt cessation of cigarette smoking.
Neuroimaging techniques, such as fMRI and positron emission tomography (PET), have been used to assess the neural substrates associated with the cognitive effects of nicotine and abstinence from nicotine in dependent subjects. A general conclusion from studies of smokers and nonsmokers is that nicotine, administered by various routes, can increase task-related activity in the prefrontal, parietal, and occipital cortices, as well as in the caudate nucleus and thalamus, and decrease task-related activity in the anterior cingulate gyrus and cerebellum while subjects perform a variety of cognitive tasks (Ghatan et al 1998; Ernst et al 2001; Lawrence et al 2002; Kumari et al 2003). Two neuroimaging studies have tested the effects of nicotine on working memory in overnight-abstinent smokers (Ernst et al 2001; Jacobsen et al 2004). A reason to test beyond the initial 2 hours of abstinence is that some cognitive deficits emerge at 4 hours of abstinence or later (Hatsukami et al 1989; Snyder et al 1989). Administration of nicotine gum to smokers who were abstinent ≥12 hours improved accuracy on a letter version of the N-back task (2-back) and reduced task-related activity compared with levels after placebo (Ernst et al 2001). Whereas the left prefrontal cortex (Brodmann area [BA] 8, 9), right anterior cingulate cortex (BA 32), and right inferior parietal cortex (BA 40) showed task-related activity after placebo administration, activation was restricted mainly to the inferior frontal gyrus (BA 44) after nicotine gum administration. The findings suggest that abstinence per se increases task-related activity in those areas (i.e., anterior cingulate and parietal areas), whereas nicotine normalizes it (Ernst et al 2001). Another study, which used an auditory N-back task to assess the effects of transdermal nicotine in abstinent (≥15 hours) smokers, presented an opposite view (Jacobsen et al 2004). In the most difficult task condition (dichotic 2-back), nicotine increased task-related activity in the anterior cingulate cortex and thalamus while it impaired performance.
Neuroimaging techniques have also been used to assess brain activity associated with working memory tasks in healthy subjects and patients, such as those with schizophrenia (Schumacher et al 1996; Braver et al 1997; Carlson et al 1998; Cohen et al 1997; D'Esposito et al 1998; LaBar et al 1999; Nystrom et al 2000; Pochon et al 2002; Veltman et al 2003; Kondo et al 2004). Studies using parametric versions of working memory tasks, such as the N-back task, have demonstrated load-sensitive fMRI signal changes in lateral prefrontal, parietal, and medial supplemental motor cortices in healthy subjects and schizophrenic patients (Callicott et al 1999, 2003; Perlstein et al 2001, 2003; Manoach 2003; Jansma et al 2004). In both populations, the relation between working memory load and task-related activity in dorsolateral prefrontal cortex (DLPFC) takes the form of an inverted U-shaped curve. At low task load (1-back), the patients exhibited higher activity in the DLPFC than the healthy subjects, and at high task load (e.g., 3-back), the patients showed decreased activity (from their peak activity) in the DLPFC, while the healthy subjects showed a continued increase or a leveling off of activity in the DLPFC. The patients also showed poorer behavioral performance than the healthy subjects and therefore apparently had less efficient and lower capacity working memory processing in the DLPFC than the healthy subjects (Callicott et al 1999, 2003; Perlstein et al 2001, 2003; Manoach 2003; Jansma et al 2004).
While previous studies have demonstrated the central effects of nicotine, they have not directly addressed the question of how acute abstinence from cigarette smoking affects the neural substrates of working memory in nicotine-dependent subjects using a separate test session in which subjects were not deprived. We now present findings from an fMRI study of smokers performing the N-back task on a day when they had free access to cigarettes (satiety session) and on another day after overnight abstinence from smoking (abstinence session) to delineate the anatomical substrates of abstinence-related deficits in working memory.
Based on findings that patients with schizophrenia performed more poorly and had higher activity than healthy control subjects in the DLPFC at low working memory load, we hypothesized that smokers tested in abstinence would show higher activity in the DLPFC at low working memory load (i.e., 1-back level) and less activity in the DLPFC at higher working memory load (i.e., 2-back, 3-back levels) than they would when tested in satiety. Dopamine in the prefrontal cortex and striatum contributes to working memory processes (Brozoski et al 1979; Tanila et al 1998; Williams and Goldman-Rakic 1998; Ellis and Nathan 2001; Jahanshahi et al 2002), and impaired working memory in schizophrenic patients is related to prefrontal cortical dopaminergic dysfunction (Goldman-Rakic and Selemon 1990; Abi-Dargham et al 2002; Abi-Dargham 2004). Animal studies also found that in rats treated chronically with nicotine, acute abstinence from nicotine disrupts dopaminergic function in the prefrontal cortex (Carboni et al 2000) and striatum (Fung et al 1996) and impairs working memory (Levin et al 1994). Therefore, it is possible that abstinence from smoking in otherwise healthy human subjects may produce working memory deficits through a mechanism involving dopaminergic dysfunction.
Methods and Materials
Experimental Subjects
We recruited potential research participants through flyers and newspaper advertisements. Those that passed an initial telephone screening were invited for a baseline session, in which they provided written informed consent, as approved by the Institutional Review Board at University of California Los Angeles. They later completed questionnaires covering medical and smoking histories, including the Fagerström Test for Nicotine Dependence (Heatherton et al 1991), childhood attention-deficit/hyperactivity disorder (ADHD) (Wender Utah Rating Scale [WURS]) (Ward et al 1993), and depressive symptoms (Beck Depression Inventory [BDI]) (Beck and Beamesderfer 1974). We excluded individuals who were younger than 18 or older than 55 years, reported a debilitating medical condition, or had a score of ≥46 on the WURS. Another exclusion factor was illicit substance abuse, as indicated by self-reports and results on urine drug screens for cocaine, methamphetamine, opiates, cannabinoids, and benzodiazepines at the time of enrollment and at all test sessions. Consumption of ≤10 standard drinks of alcohol per week (one standard drink = 12 ounces of beer, 6 ounces of wine, or a 1.5-ounce shot of 80-proof hard liquor), as indicated by self-report, was allowed. In addition, marijuana use of ≤1 joint per week was allowed, but urine was required to test negative for cannabinoids at each session and the participants were instructed to refrain from using marijuana for the 72 hours prior to each test session. English language fluency, right-handedness (as indicated by a self-report of using the right hand to perform at least six out of seven tasks on the Edinburgh Handedness Questionnaire [Oldfield 1971]), and a self-report of smoking at least 10 cigarettes a day for the 2 years prior to enrollment were inclusion requirements.
Nine smokers met the criteria and participated in this study, but one of them reported to our laboratory only for the satiety session. We therefore report the data from eight participants who participated in fMRI during both satiety and abstinence sessions. The ages of the eight subjects ranged from 20 to 54 years (mean = 35 years), and three of them were women. Individuals smoked 13 to 30 cigarettes per day (mean = 19) and had smoked regularly for 2 to 42 years (mean = 16 years). Their scores on the Fagerström Test for Nicotine Dependence, a 10-point scale (Heatherton et al 1991), ranged from 3 to 7 (mean = 4.1), indicating a moderate degree of dependence. Three subjects completed the satiety session first, and five subjects completed the abstinence session first. Smoking abstinence was confirmed by expired carbon monoxide (CO) values of 2 to 7 parts per million (ppm) in expired air (mean = 3.25 ppm).
Experimental Design
The subjects participated in scanning sessions on each of 2 days. Testing began between 2:00 pm and 4:00 pm, when the normal daily smoking behavior produces a relatively stable plateau of nicotine blood level (Benowitz et al 1983). One session was conducted on a day when the participant was allowed to smoke ad libitum prior to testing. We refer to this session throughout as satiety session. The time between his or her last cigarette and the end of fMRI image acquisition was <1.5 hours. The other session, to which we refer as the abstinence session, was conducted after the participants were required to abstain from smoking for 14 to 16 hours. We selected this duration of abstinence, in part, on the observation that 16 hours of abstinence reliably induces cigarette craving (Jarvik et al 1998). Before each scanning session, smokers were evaluated for cigarette withdrawal using the 25-item Shiffman/Jarvik Withdrawal Scale (Jarvik et al 2000) and for subjective craving using a 10-item Likert Urge To Smoke scale (UTS) (Jarvik et al 2000) and a 7-item Likert craving subscale taken from the Shiffman/Jarvik Withdrawal Scale. We measured expired CO to verify abstinence.
Task Design
A four-step letter version of the N-back task was employed. During fMRI, stimuli were displayed through video goggles (Resonance Technology Corporation, Northridge, California). The test stimuli were individual letters, presented one at a time. Each letter appeared for 400 milliseconds, and the interstimulus interval (ISI) was 1600 milliseconds. Participants were instructed to press the first key of a four-key button-box with the right index finger when they saw a target letter and the second key with their right middle finger when they saw a nontarget letter. At the onset of the 0-back condition, in which the task was primarily vigilance, participants saw the instruction “Find X.” In this case, they were required to press the “target key” when the letter “X” appeared and the “nontarget key” when any other letter appeared. At the onset of the 1-back condition, which required monitoring for change but imposed little memory load, participants saw the instruction “Find 1-back.” In this case, they were required to press the target key when the letter presented was identical to the one immediately preceding it and the nontarget key whenever any other letter appeared. The same procedure was followed for the 2-back and 3-back conditions, which required both effortful attention and substantive working memory load. During the 2-back condition, the target was the letter that was displayed two letters prior; during the 3-back condition, the target letter was the one presented 3 letters before.
The N-back conditions were presented individually in 42-second blocks. In each block, 21 trials were presented that contained 7 (33%) targets and 14 nontargets. There was a 3-second instruction screen before each task block and a 15-second block of rest after each task block. During rest, subjects fixed their eyes on a cross that was displayed at the center of the screen. The task blocks were programmed into four scripts. In each script, each of the four N-back conditions was presented twice in one of four pseudorandom orders, resulting in an 8-minute run of the entire task. Each subject completed two test blocks on each day, with two 8-minute runs in each block. The sequence of presentation of the scripts was counterbalanced.
The behavioral data from the scanner were not acquired due to a program error. To assess participants’ performance, we tested the participants using the same N-back task outside the magnetic resonance imaging (MRI) scanner on another 2 days, one time in the satiety condition and the other time in the abstinence condition.
Scanning Parameters
Functional images were acquired on a 3T MRI scanner (GE, Signa with the echoplanar imaging [EPI] upgrade from Advanced NMR Systems; GE Medical Systems, Milwaukee, Wisconsin), using T2* weighted gradient-recalled EPI with blood oxygen level-dependent (BOLD) contrast (repetition time [TR] = 3000 milliseconds, echo time [TE] = 42 milliseconds, flip angle = 80°, slice thickness = 4 mm with a 1-mm interslice interval, matrix of 64 × 64, in-plane resolution = 3.12 mm2). One hundred sixty images were acquired for each of 16 axial slices through the brain. High-resolution T2-weighted EPI anatomical images of the whole brain (23–25 slices, 4-mm thick) were acquired in each scanning session to help define the locations of the BOLD signals.
Statistical Analysis
Behavioral Data
To assess whether smoking abstinence resulted in working memory impairments, a set of repeated measures analyses of variance (ANOVA) was conducted in which N-back performance was compared between abstinence and satiety sessions. In this analysis, N-back condition (0-back, 1-back, 2-back, and 3-back) was used as a within-subject variable, and separate analyses were conducted for errors and response times (RTs).
Imaging Data
We analyzed the data using a two-stage, random effects procedure. First, we created an average brain of all subjects included in the study, based on the high-resolution coplanar images, using Automatic Image Registration (AIR) software (Woods et al 1998). This average brain was used as a template for co-registration and spatial normalization. We used Statistical Parametric Mapping (SPM2, Wellcome Department of Cognitive Neurology, London, United Kingdom) for motion correction, spatial normalization, smoothing, and statistical analysis. For each subject, the anatomical image was spatially normalized with the study-specific template with 12-parameter affine transformations. The functional scans were aligned to the first functional image and corrected for motion, co-registered with the anatomical image, spatially normalized using the same transformations as the anatomical image, and then smoothed with a 10-mm full width at half maximum (FWHM) Gaussian filter. Subsequently, the functional data were filtered with a 128-second high-pass temporal filter. We constructed model time courses for each N-back task level by convolving a boxcar waveform representing the times of administration of each task level with the canonical hemodynamic response function. We then analyzed the data in comparison with these model time courses using the general linear model.
Our statistical tests involved within-condition comparisons between data obtained at the different N-back levels, as well as between-condition evaluations of the effect of abstinence. The SPM{T} maps for within-condition contrasts (e.g., 1-back minus 0-back, 2-back minus 0-back, 3-back minus 0-back, 3-back minus 1-back, contrast [–2 –1 1 2] for parametric test), or between-condition contrasts (e.g., abstinence [1-back minus 0-back] minus satiety [1-back minus 0-back], satiety [3-back minus 1-back] minus abstinence [3-back minus 1-back]) were created to assess task-related BOLD signal changes in each subject. To study group effects, we carried the contrast images from each subject into a second-level random effects analysis (one-sample t test) to study the effects of task load in both the satiety and the abstinence conditions. A voxel-level threshold of p < .001 (uncorrected) and a cluster level extent threshold of p < .05 (corrected for multiple comparisons) were used to identify significant task-related activity within the whole brain. We performed both whole brain analysis and region of interest (ROI) analysis.
The whole brain analysis (searching for voxel-level differences) compared brain activity at each task level (i.e., 1-back, 2-back, 3-back) and the interaction of activity changes as task load increased from 0-back or 1-back to 3-back between the abstinence and satiety sessions. Only the voxels that exhibited significant effects from this analysis and that were contained within predefined ROIs were further analyzed with ROI analysis. To define ROIs, we combined the imaging data from the two test sessions together to minimize bias in ROI definition toward either test session. We first used a parametric test for significant linear increase in BOLD signal with increasing task load (e.g., –2 –1 1 2). Then, we used the SPM2-compatible ROI analysis tool Marsbar (Brett et al 2002) to define the activity in the SPM{T} map as functional ROIs. The signal changes of voxels within ROIs were extracted with Marsbar and were input to SPSS for further statistical analysis.
The Pearson correlations between the percent signal change of each functional ROI (six ROIs) from the 0-back to the 3-back level and scores on the UTS craving scale and Shiffman/Jarvik Withdrawal Scale in the abstinence sessions were assessed with SPSS. To facilitate comparisons with the literature, the study-specific template and contrast images obtained with SPM were normalized into Talairach space. Then, the Talairach coordinates of the center of each significant cluster were determined by overlaying the corresponding contrast images in Talairach space using mri3dX software (http://www.aston.ac.uk/lhs/staff/sing-hkd/mri3dX/index.shtml).
Results
Subjective Craving and Withdrawal Measures
Comparisons between sessions showed higher self-reporting of craving for cigarettes and negative psychological and physical symptoms of withdrawal in the abstinence session than in the satiety session (Table 1). The expired CO level, which was significantly lower in the abstinence session than in the satiety session, confirmed compliance with the instruction to refrain from smoking.
Table 1.
Withdrawal, Craving, and CO Measures
| Satiety | Abstinence | |
|---|---|---|
| Subscales of Shiffman/Jarvik Withdrawal Scalea | ||
| Craving | 3.70 (.15) | 6.05 (.44)c |
| Psychological Symptoms | 3.16 (.24) | 4.15 (.39)b |
| Physical Symptoms | 1.50 (.21) | 2.56 (.36)b |
| Sedation | 2.88 (.42) | 3.39 (.13) |
| Appetite | 4.00 (.09) | 4.38 (.63) |
| Total Score | 15.2 (.65) | 20.5 (1.10)c |
| Urge to Smoke Scale | 24.1 (2.57) | 62.9 (2.23)c |
| Expired CO (ppm) | 22.5 (4.66) | 3.25 (.60)c |
Values shown are the means (standard errors of the means).
CO, carbon monoxide; ppm, parts per million.
Values listed reflect data obtained on the five subscales (and the total) of the 25-item scale.
p < .05 for difference between satiety and abstinence session by Student t test.
p < .001 for difference between satiety and abstinence session by Student t test.
Behavioral Data
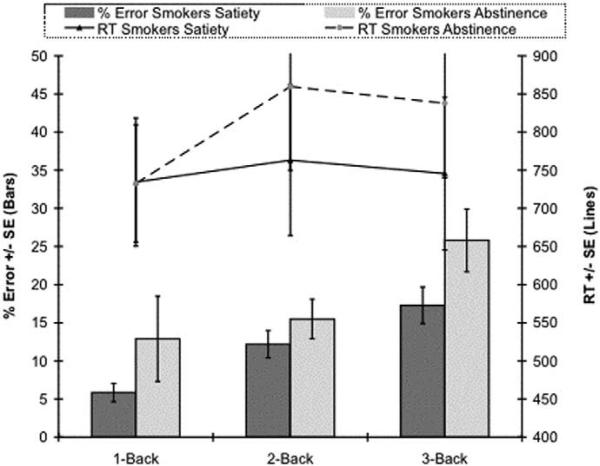
The behavioral data acquired outside of the MRI scanner indicated that the participants tended toward longer overall latencies when tested in abstinence session than they did when tested in satiety [F (1,7) = 5.28, p = .055] (Figure 1). They also showed higher mean error rates when tested during the abstinence session than they did when tested during satiety (Figure 1), although this also was not a statistically significant difference.
Figure 1.
N-back performance of smokers in abstinence and satiety sessions. Lines indicate mean RTs and bars indicate mean percent of errors, both at each N-back level. In the abstinence session, smokers tended to respond more slowly and tended to make more errors compared with the satiety session. RT, response time.
Task-Related Brain Activity
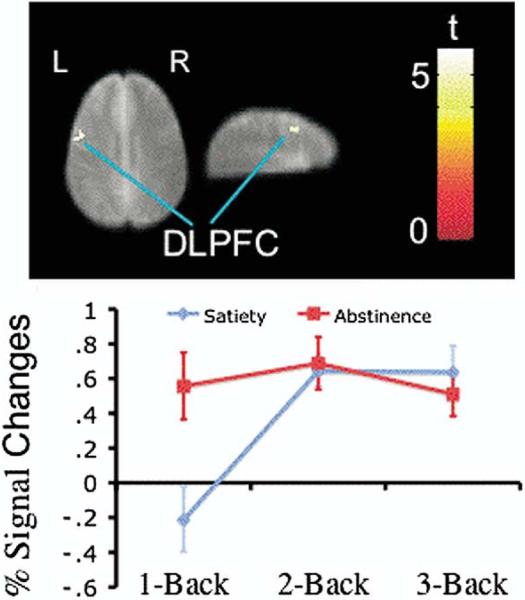
Whole brain (voxel-wise) analysis found no significant difference between the two sessions in task-related activity at 1-back, 2-back, or 3-back levels. Also, no significant interaction was found between sessions and task loads as task loads increased from 0-back to 3-back. When data from the two sessions were combined, a parametric test showed statistically significant task-related activity increases in the left lateral prefrontal cortex (LPFC), dorsal prefrontal cortex, and bilateral medial supplementary motor cortex and parietal cortex (Figure 2, Table 2). The clusters of super-threshold voxels in the five brain regions were defined as ROIs for further analysis. Whole brain, voxel-wise analysis showed a cluster in the left DLPFC (29 voxels, Talairach coordinate: × = –50, y = 7, z = 32) in which there was a significant interaction between satiety and abstinence sessions as task load was increased from 1-back to 3-back. This cluster was within the ROI in the left LPFC defined in analysis of the combined data from two test sessions. Region of interest analysis showed that it survived the statistical correction for multiple comparisons within the ROI (extent corrected p = .012, SPM2 small volume correction). Analysis of signal changes found that this cluster had higher activity at the 1-back level when participants were tested in the abstinence session than in the satiety session (paired t test, p = .027, t = –2.78, df =7). This cluster showed no significant activity changes as task load was increased from 1-back to 2-back and from 2-back to 3-back levels when the participants were tested in the abstinence session (Figure 3). In the satiety condition, it showed significant increases in activity as task load was increased from 1-back to 2-back (paired t test, p = .008, t = –3.69, df = 7) and no further significant changes as task load was increased from 2-back to 3-back (Figure 3). No interactions were found in other brain areas between satiety and abstinence sessions as task load was increased from 1-back to 3-back level. No significant correlations were found between self-reports of craving and withdrawal during the abstinence session and signal change in any ROIs.
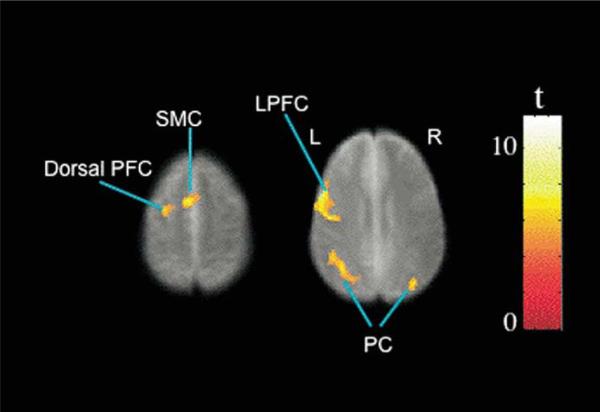
Figure 2.
Brain regions showed activity changes as task load increased from 0-back to 3-back when imaging data from the two test sessions were combined. Colors superimposed on the gray scale image, from the study-specific structural brain template, indicate values of t according to the color bar. Significant effects are shown in the left lateral prefrontal cortex (LPFC) and dorsal frontal cortex (PFC), bilateral supplementary motor cortex (SMC), and parietal cortex (PC). L, left, R, right.
Table 2.
Significant Load Sensitive Activity
| Location | Corrected Cluster p | Cluster Size (No. Contiguous Voxels) | Brodmann Areas | Talairach Coordinates (x,y,z) | |
|---|---|---|---|---|---|
| MFG, IFG | L | <.0005 | 901 | 6, 44, 46 | –46, 7, 25 |
| SFG, MFG | L | <.029 | 127 | 6 | –24, 1, 52 |
| SMC | <.012 | 159 | 6 | –2, 2, 50 | |
| Parietal Cortex | L | <.0005 | 315 | 39, 40 | –27, –68, 31 |
| R | <.034 | 122 | 40 | 46, –60, 30 |
Voxel-level threshold of p < .001, uncorrected; cluster extent p < .05, corrected.
L, left; R, right; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; SFG, superior frontal gyrus; SMC, medial supplementary motor cortex.
Figure 3.
A cluster in left dorsal lateral prefrontal cortex (DLPFC) showed interactions of activity changes between the two test sessions as task load increased from 1-back to 3-back. Colors superimposed on the gray scale image, from the study-specific structural brain template, indicate values of t according to the color bar. The line graph indicates the mean percent signal changes and standard errors as task load increased from 1-back to 3-back exhibited in satiety and abstinence sessions. L, left, R, right.
Discussion
Task-related activity can be categorized as “load-sensitive” or “load-insensitive” (Jansma et al 2000), purportedly reflecting working memory processes (load-sensitive) and supporting processes, such as response selection and perception (load-insensitive), respectively (Jansma et al 2000). Smokers exhibited load-sensitive task-related activity in the left lateral and dorsal prefrontal cortices and bilaterally in the medial supplementary motor cortex and parietal cortex while they performed the N-back working memory task. This finding is generally consistent with prior observations on healthy subjects performing a similar version of the N-back task, except that healthy subjects usually showed activity in bilateral lateral prefrontal cortex (Braver et al 1997; Carlson et al 1998; Callicott et al 1999; Jansma et al 2000). A review of neuroimaging studies of working memory (D'Esposito et al 1998) found that most studies (20/36 studies) reported bilateral activation in lateral prefrontal cortex, and studies using spatial working memory tasks usually found greater activation in the right than in the left hemisphere, while studies using nonspatial working memory tasks usually found greater activation on the left (D'Esposito et al 1998). In our study, smokers showed load-sensitive, task-related activity in the right lateral prefrontal cortex, but this effect did not survive the correction for multiple comparisons. As we used a letter version N-back task, our findings are consistent with previous work with nonspatial working memory tasks, showing a greater effect in the left than the right lateral prefrontal cortex.
The left DLPFC showed an interaction between smoking condition and working memory load. This interaction reflected a higher level of task-related activity at the 1-back task level in the abstinence session than in the satiety session and smaller increases of activity in the abstinence session than in the satiety session when working memory load was increased (1-back to 2-back, and 2-back to 3-back). Moreover, when the subjects were tested in the abstinence session, they tended to exhibit longer latency to respond and less accuracy than they did in the satiety session. Higher activity in the left DLPFC paired with poorer performance, as observed before in schizophrenic patients (Callicott et al 1999, 2003; Perlstein et al 2001, 2003; Jansma et al 2004; for review, see Manoach 2003), suggests that working memory processing in the left DLPFC was less efficient in the abstinence session than in the satiety session. This conclusion is consistent with the findings of fMRI working memory studies from healthy subjects (Rypma et al 2002). Rypma et al (2002) separated participants in a verbal working memory study into higher performers and lower performers. The lower performers exhibited higher task-related activity in the DLPFC than the higher performers at low working memory load, but they showed smaller increases in activation than the higher performers did when working memory load was increased (Rypma et al 2002). In this study, all subjects were right-handed. The language function in right-handed subjects is lateralized to the left hemisphere, while it may be distributed bilaterally or lateralized to the right hemisphere in left-handed subjects (Tzourio et al 1998; Hund-Georgiadis et al 2002). It is possible that left-handed smokers may show interaction between smoking condition and working memory load at different brain regions than the right-handed smokers, e.g., the right DLPFC. This issue can be addressed by testing left-handed smokers with a similar N-back task.
The observed relationship between task-related activity and cognitive load is also consistent with findings in healthy subjects as well as schizophrenic patients. In both populations, the relation between working memory load and activity in DLPFC takes the form of an inverted U-shaped curve (Callicott et al 1999, 2003; Perlstein et al 2001, 2003; Manoach 2003; Jansma et al 2004). Compared with the curve observed in healthy subjects, however, the curve observed in patients is shifted, with peak task-related signal occurring at lower load. At low working memory load (i.e., 1-back), the patients showed higher activity in the DLPFC but poorer performance than healthy subjects (Callicott et al 1999, 2003; Perlstein et al 2003; Jansma et al 2004). This finding was regarded as evidence that the schizophrenic patients had lower efficiency of working memory processes in the DLPFC than healthy subjects (Callicott et al 1999, 2003; Perlstein et al 2001, 2003). Smokers did not show significant decreases in activity in the left DLPFC at the highest task load during either test session, and their performance at the highest load was still above chance. Our data, therefore, do not support the hypothesis that maximal neural recruitment in smokers tested in abstinence would be lower than the maximum during the satiety session. As the participants exhibited significant differences in self-reports of psychological symptoms and sedation, it is possible these conditions affected motivation and that this effect, in turn, impaired performance and task-related activation of the left DLPFC associated with the 2-back and 3-back tasks during the abstinence (compared with the satiety session). Nonetheless, the observation that subjects showed higher task-related activity in the left DLPFC while they performed the 1-back task in abstinence session than when at satiety suggests an impairment of processing efficiency in the left DLPFC.
Some investigators suggest that the abnormal activity associated with working memory in the DLPFC of schizophrenic patients indicates impairment of working memory processes in this region (Callicott et al 1999, 2003; Perlstein et al 2001, 2003), while others suggest that the abnormality in the DLPFC may reflect impairment in other brain regions in the working memory network (Jansma et al 2004). It is possible that the abnormal activity in the DLPFC reflects impairment in other parts of the working memory network. Findings from a structural imaging study, that smokers had smaller gray matter volumes and lower gray matter densities than nonsmokers in the DLPFC and ventrolateral prefrontal cortex bilaterally (Brody et al 2004), suggest that at least part of the problem relates to a deficit in the DLPFC.
Further studies may address the mechanism by which acute abstinence from cigarette smoking impairs working memory. Dopamine in the prefrontal cortex and striatum is critical for working memory processes (Brozoski et al 1979; Williams and Goldman-Rakic 1998; Tanila et al 1998; Ellis and Nathan 2001; Jahanshahi et al 2002), and evidence suggests that dopaminergic dysfunction in these regions contributes to the working memory impairment in schizophrenic patients (Goldman-Rakic and Selemon 1990; Abi-Dargham et al 2002; Abi-Dargham 2004). In rats, removal of nicotine after chronic nicotine treatment produces decreases in dopamine levels in the striatum (Fung et al 1996), increases in the medial prefrontal cortical dopamine (Carboni et al 2000), and impairment of working memory performance (Levin et al 1994). It is reasonable to postulate, therefore, that acute abstinence from cigarette smoking may alter dopaminergic transmission in the striatum and the prefrontal cortex of nicotine-dependent human subjects. Such changes may contribute to the impairment of working memory observed in acutely abstinent chronic cigarette smokers.
One limitation of this study is the fact that task performance was not recorded during the fMRI acquisition. Nonetheless, we did test and record performance of a larger sample of subjects (including those tested here) on the same N-back task in both abstinence and satiety conditions outside the scanning environment and found that subjects responded more slowly and tended to have more errors when tested in abstinence than when tested at satiety (Mendrek et al, unpublished data). The smaller subset of those who participated in the present study provided qualitatively similar results, although they were not statistically significant.
Another consideration is the fact that carbon dioxide (CO2) and nicotine have complex effects on cerebral perfusion (Ghatan et al 1998; Rose et al 2003; Domino et al 2004), which is an essential feature of the BOLD method. Significant differences in task-related activity between the two test sessions, however, were observed only in the left DLPFC, which is critical for working memory processing, and not in other brain areas, indicating that nonspecific effects of CO2 on cerebral perfusion could not easily explain our findings. In addition, nicotine apparently does not alter the coupling between the BOLD signal and activity of the visual cortex in response to photic stimulation (Jacobsen et al 2002).
Other limitations relate to the small sample size, which indicate that replication is warranted, and the fact that the subjects varied widely in age and smoking history; however, the same subjects were tested in the two different conditions, controlling these potentially confounding effects. In addition, light use of marijuana (<1 joint/week) was not exclusionary. Some evidence indicates that the heavy (>5 joints/week) marijuana users have cognitive impairment (Block and Ghoneim et al 1993; Fletcher et al 1996; Bolla et al 2002), may experience withdrawal after initiating abrupt abstinence from marijuana (Wiesbeck et al 1996; Budney et al 2004), and show abnormal brain activation during cognitive testing (Kanayama et al 2004; Eldreth et al 2004; Jacobsen et al 2004). Nonetheless, there is no evidence that light users suffer cognitive impairment (Fletcher et al 1996; Pope et al 2001; Lyketsos et al 1999; Block and Ghoneim et al 1993) or withdrawal signs induced by abstinence from marijuana (Wiesbeck et al 1996).
Consistent with our hypotheses, the findings from this study support the conclusion that in smokers, the left DLPFC has less efficient processing associated with working memory in the abstinence condition than in the satiety condition. Relevant evidence includes high task-related activity at low working memory load and failure to increase activity as task load increases in the abstinence condition..
Acknowledgments
This research was supported by NIH Grants RO1 DA014093.03 (EDL), RO1 DA015059 (ALB), R21 DA 13627 and DA13637 (MSC), MOI RR 00865, NCRR R12169, and RR08655; UC Tobacco-Related Disease Research Program awards 10RT-0091 (EDL) and 11RT-0024 (ALB); VA Merit Review Type I Award (ALB); and Philip Morris USA contract 02066286 (EDL).
We thank Dr. Russell A. Poldrack (Department of Psychology, UCLA) for advice on data analysis. The image data were collected at the Ahmanson Lovelace Brain Mapping Center, which is supported by the Brain Mapping Medical Research Organization, the Brain Mapping Support Foundation, the Pierson-Lovelace Foundation, the Ahmanson Foundation, the Tamkin Foundation, the Jennifer Jones-Simon Foundation, the Capital Group, the Companies Charitable Foundation, the Robson Family, and the Northstar Fund.
References
- Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(suppl 1):S1–S5. doi: 10.1017/S1461145704004110. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Della Salla S. Working memory and executive control. Philos Trans R Soc Lond B Biol Sci. 1996;351:1397–1403. doi: 10.1098/rstb.1996.0123. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A. Assessment of depression: The depression inventory. Psychol Meas Psychopharmacol. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Herning RI, Jacob P III, Jones RT, Osman A-L. Smokers of low-yield cigarettes do not consume less nicotine. N J Med. 1983;309:139–142. doi: 10.1056/NEJM198307213090303. [DOI] [PubMed] [Google Scholar]
- Blake J, Smith A. Effects of smoking and smoking deprivation on the articulatory loop of working memory. Hum Psychopharmacol. 1997;12:259–264. [Google Scholar]
- Block RI, Ghoneim MM. Effects of chronic marijuana use on human cognition. Psychopharmacology. 1993;110:219–228. doi: 10.1007/BF02246977. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey R. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:1140–1141. [abstract] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 1979;205:929–932. doi: 10.1126/science.112679. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Bertolino A, Finn K, Coppola R, Frank JA, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9:20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Carboni E, Bortone L, Giua C, Di Chiara G. Dissociation of physical abstinence signs from changes in extracellular dopamine in the nucleus accumbens and in the prefrontal cortex of nicotine dependent rats. Drug Alcohol Depend. 2000;58:93–102. doi: 10.1016/s0376-8716(99)00064-2. [DOI] [PubMed] [Google Scholar]
- Carlson S, Martinkauppi S, Rama P, Salli E, Korvenoja A, Aronen HJ. Distribution of cortical activation during visuospatial n-back tasks as revealed by functional magnetic resonance imaging. Cereb Cortex. 1998;8:743–752. doi: 10.1093/cercor/8.8.743. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. Functional MRI studies of spatial and nonspatial working memory. Brain Res Cogn Brain Res. 1998;7:1–13. doi: 10.1016/s0926-6410(98)00004-4. [DOI] [PubMed] [Google Scholar]
- Domino EF, Ni L, Xu Y, Koeppe RA, Guthrie S, Zubieta J-K. Regional cerebral blood flow and plasma nicotine after smoking tobacco cigarettes. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:319–327. doi: 10.1016/j.pnpbp.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Ellis KA, Nathan PJ. The pharmacology of human working memory. Int J Neuropsychopharmacol. 2001;4:299–313. doi: 10.1017/S1461145701002541. [DOI] [PubMed] [Google Scholar]
- Ernst M, Matochik JA, Heishman SJ, Van Horn JD, Jons PH, Henningfield JE, et al. Effect of nicotine on brain activation during performance of a working memory task. Proc Natl Acad Sci U S A. 2001;98:4728–4733. doi: 10.1073/pnas.061369098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JM, Page JB, Francis DJ, Copeland K, Naus MJ, Davis CM, et al. Cognitive correlates of long-term cannabis use in Costa Rican men. Arch Gen Psychiatry. 1996;53:1051–1057. doi: 10.1001/archpsyc.1996.01830110089011. [DOI] [PubMed] [Google Scholar]
- Fung YK, Schmid MJ, Anderson TM, Lau YS. Effects of nicotine withdrawal on central dopaminergic systems. Pharmacol Biochem Behav. 1996;53:635–640. doi: 10.1016/0091-3057(95)02063-2. [DOI] [PubMed] [Google Scholar]
- Ghatan PH, Ingvar M, Eriksson L, Stone-Elander S, Serrander M, Ekberg K, et al. Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology (Berl) 1998;136:179–189. doi: 10.1007/s002130050554. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD. New frontiers in basal ganglia research. Trends Neurosci. 1990;13:241–244. doi: 10.1016/0166-2236(90)90103-h. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Fletcher L, Morgan S, Keenan R, Amble P. The effects of varying cigarette deprivation duration on cognitive and performance tasks. J Subst Abuse. 1989;1:407–416. [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fägerström KO. The Fägerström Test for Nicotine Dependence: A revision of the Fägerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hund-Georgiadis M, Lex U, Friederici AD, Cramon DY. Non-invasive regime for language lateralization in right- and left-handers by means of functional MRI and dichotic listeming. Exp Brain Res. 2002;145:166–176. doi: 10.1007/s00221-002-1090-0. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Cyril D'Souza D, Einar Mencl W, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Gore JC, Skudlarski P, Lacadie CM, Jatlow P, Krystal JH. Impact of intravenous nicotine on BOLD signal response to photic stimulation. Magn Reson Imaging. 2002;20:141–145. doi: 10.1016/s0730-725x(02)00494-0. [DOI] [PubMed] [Google Scholar]
- Jacobsen L, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Ann NY Acad Sci. 2004;1021:384–390. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Rowe J, Saleem T, Brown RG, Limousin-Dowsey P, Rothwell JC, et al. Striatal contribution to cognition: Working memory and executive function in Parkinson's disease before and after unilateral posteroventral pallidotomy. J Cogn Neurosci. 2002;14:298–310. doi: 10.1162/089892902317236911. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Coppola R, Kahn RS. Specific versus nonspecific brain activity in a parametric n-back task. Neuroimage. 2000;12:688–697. doi: 10.1006/nimg.2000.0645. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, van der wee NJA, Kahn RS. Working memory capacity in schizophrenia: A parametric fMRI study. Schizophr Res. 2004;68:159–171. doi: 10.1016/S0920-9964(03)00127-0. [DOI] [PubMed] [Google Scholar]
- Jarvik M, Madsen D, Olmstead R, Iwamoto-Schaap P, Elins J, Eisenberger N, et al. Blood nicotine levels and subjective craving for cigarettes. Nicotine Tob Res. 2000;66:553–558. doi: 10.1016/s0091-3057(00)00261-6. [DOI] [PubMed] [Google Scholar]
- Jarvik ME, Schneider NG, Olmstead RE, Iwamoto-Schaap PN, Madsen DC. Sweeteners and cigarette craving: Glucose, aspartame, sorbitol. Am J Health Behav. 1998;22:130–140. [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurelun-Todd DA. Spatial working memory in heavy cannabis users: a functional magnetic resonance imaging study. Psychopharmacology. 2004;176:239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Kondo H, Morishita M, Osaka N, Osaka M, Fukuyama H, Shibasaki H. Functional roles of the cingulo-frontal network in performance on working memory. Neuroimage. 2004;21:2–14. doi: 10.1016/j.neuroimage.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, ffytche DH, Mitterschiffthaler MT, Das D, Zachariah E, et al. Cognitive effects of nicotine in humans: An fMRI study. Neuroimage. 2003;19:1002–1013. doi: 10.1016/s1053-8119(03)00110-1. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: A functional MRI comparision within subjects. Neuroimage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Levin JM, Holman BL, Mendelson JH, Teoh SK, Garada B, Johnson KA, et al. Gender differences in cerebral perfusion in cocaine abuse: Technetium-99m-HMPAO SPECT study of drug-abusing women. J Nucl Med. 1994;35:1902–1909. [PubMed] [Google Scholar]
- Lyketsos CG, Garrett E, Liang KY, Anthony JC. Cannabis use and cognitive decline in persons under 65 years of age. Am J Epidemiol. 1999;149:794–800. doi: 10.1093/oxfordjournals.aje.a009894. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: Reconciling discrepant findings. Schizophr Res. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Nystrom LE, Braver TS, Sabb FW, Delgado MR, Noll DC, Cohen JD. Working memory for letters, shapes, and locations: Functional MRI evidence against stimulus-based regional organization in human prefrontal cortex. Neuroimage. 2000;11:424–446. doi: 10.1006/nimg.2000.0572. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158:1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol Psychiatry. 2003;53:25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, et al. The neural system that bridges reward and cognition in humans: An fMRI study. Proc Natl Acad Sci U S A. 2002;99:5669–5674. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, et al. Working memory for complex figures: An fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16:370–379. [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Mathew RJ, London ED, Hawk TC, et al. PET studies of the influences of nicotine on neural systems in cigarette smokers. Am J Psychiatry. 2003;160:323–333. doi: 10.1176/appi.ajp.160.2.323. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, D'Esposito M. The influence of working-memory demand and subject performance on prefrontal cortical activity. J Cogn Neurosci. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Schumacher EH, Lauber E, Awh E, Jonides J, Smith EE, Koeppe RA. PET evidence for an amodal verbal working memory system. Neuroimage. 1996;3:79–88. doi: 10.1006/nimg.1996.0009. [DOI] [PubMed] [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: Performance decrements assessed on a computerized test battery. Drug Alcohol Depend. 1989;23:259–266. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Snyder FR, Henningfield JE. Effects of nicotine administration following 12 h of tobacco deprivation: Assessment on computerized performance tasks. Psychopharmacology (Berl) 1989;97:17–22. doi: 10.1007/BF00443406. [DOI] [PubMed] [Google Scholar]
- Tanila H, Bjorklund M, Riekkinen P., Jr Cognitive changes in mice following moderate MPTP exposure. Brain Res Bull. 1998;45:577–582. doi: 10.1016/s0361-9230(97)00452-8. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Crivello F, Mellet E, Nkanga-Ngila B, Mazoyer B. Functional anatomy of dominance for speech comprehension in left handers vs right handers. Neuroimage. 1998;8:1–16. doi: 10.1006/nimg.1998.0343. [DOI] [PubMed] [Google Scholar]
- Veltman DJ, Rombouts SARB, Dolan RJ. Maintenance versus manipulation in verbal working memory revisited: An fMRI study. Neuroimage. 2003;18:247–256. doi: 10.1016/s1053-8119(02)00049-6. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: An aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. Am J Psychiatry. 1993;150:885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Wiesbeck GA, Schuckit MA, Kalmijn JA, Tipp JE, Bucholz KK, Smith TL. An evaluation of the history of a marijuana withdrawal syndrome in a large population. Addiction. 1996;91:1469–1478. [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC. Automated image registration: I. General methods and intrasubject, intramodality validation. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]