Abstract
Background
Borrelia lonestari infects Amblyomma americanum, the tick species that is the most common cause of tick bites in southeast and south-central United States, and this spirochete has been detected in an erythema migrans (EM)–like skin rash in 1 patient. Therefore, B. lonestari is considered to be a leading candidate for the etiologic agent of EM in this region.
Methods
Skin biopsy specimens obtained from patients from the Cape Girardeau area of Missouri who had EM-like lesions were cultured in Barbour-Stoenner-Kelly medium and evaluated by polymerase chain reaction (PCR) targeting multiple genes. Serum specimens were tested by enzyme-linked immunosorbent assay for antibodies against sonicated whole-cell Borrelia burgdorferi. Results were compared with those obtained over the same period for patients from New York State who had EM.
Results
B. lonestari was not detected by PCR in any of 31 skin biopsy specimens collected from 30 Missouri patients. None of 19 cultures of Missouri skin samples that were suitable for evaluation were positive for B. burgdorferi, compared with 89 (63%) of 142 cultures of samples collected from New York State patients (P < .001). None of the 25 evaluable Missouri patients were seropositive for antibodies against B. burgdorferi, compared with 107 (75%) of 143 New York State patients (P < .001).
Conclusions
Neither B. lonestari nor B. burgdorferi is likely to be the cause of EM-like skin lesions in patients from the Cape Girardeau area of Missouri. The etiology of this condition remains unknown.
Lyme disease is the most common tickborne disease in the United States [1]. Although the majority of cases are reported from the mid-Atlantic, northeastern, midwestern, and far western regions of the country, several hundreds of cases annually are reported from the southeast and south-central United States [1, 2]. Many of these cases are associated with an erythema migrans (EM)–like rash [3–6].
This skin lesion has followed the bite of Amblyomma americanum, a tick species found throughout the southeast and south-central United States [4, 7–9], and is alternatively referred to as southern tick–associated rash illness or Masters disease. A. americanum ticks are not infected with Borrelia burgdorferi but may be infected with a borrelial species different from both B. burgdorferi and the other Borrelia genospecies recognized to cause Lyme disease in Eurasia [10–19]. Barbour et al. [11] proposed the name Borrelia lonestari species novum.
Until recently, it remained unclear whether A. americanum could transmit B. lonestari to humans or whether B. lonestari was the cause of (or even associated with) any clinical illness. In 2001, James et al. [18] documented the first such case. This patient developed an EM-like rash following the bite of an A. americanum tick during a trip to Maryland and North Carolina. James et al. [18] demonstrated by PCR amplification techniques that both the tick and the patient were infected with B. lonestari or a closely related bacterium.
An important question is whether B. lonestari is the cause of the many cases reported as Lyme disease in southern and other regions of the United States where A. americanum ticks bite humans. In this article, we report results of a microbiologic evaluation of patients from Missouri with EM-like skin lesions and compare these results with those for patients from New York State with EM who were similarly evaluated during the same period.
Patients and Methods
Patient population
Patients with EM-like lesions provided the specimens used in this study. All but 3 of the patients satisfied the Lyme disease surveillance definition of the Centers for Disease Control and Prevention [20], which specifies a lesion size of ≥ 5 cm; the diameter of the largest EM-like lesion in the 3 patients who were exceptions was 4–4.5 cm (2 of the patients were from New York State, and 1 was from Missouri). The source of patient referral for the Missouri EM-like cases was the office practice of E.M. in Cape Girardeau, Missouri. Control cases consisted of patients from New York State who had EM diagnosed at the Lyme Disease Practice of the West-chester Medical Center (Valhalla, NY). All patients at both sites were evaluated during 2000–2003, all were treated for Lyme disease with conventional antibiotics (e.g., doxycycline or amoxicillin) at the baseline visit, and all of the lesions resolved. The research protocol was approved by the Institutional Review Board of New York Medical College (Valhalla, NY).
Patient research plan
After obtaining written informed consent, a serum specimen (10–20 mL) was obtained, and a biopsy specimen of a primary skin lesion was obtained. Beginning in 2001, additional serum samples were obtained 20–30 days and 3 months after presentation.
Specimen transport
Skin biopsy samples were transported from Missouri to Valhalla by overnight express courier, in accordance with the methods of Berger et al. [21].
Skin biopsy and culture
At both clinical sites, skin biopsy material (2-mm biopsy specimens in New York and 4-mm biopsy specimens in Missouri) was placed into Barbour-Stoenner-Kelly (BSK) media containing 40 μg/mL of rifampin to reduce contamination with skin flora [21]. After receipt in New York, the 4-mm Missouri specimens were sectioned into 2 equal parts, one for PCR and the other for culture. Cultures were performed as described elsewhere [22, 23].
Sample processing and DNA isolation
A total of 312 field-collected A. americanum ticks from Missouri (128 nymphs and 38 adult ticks were from Stoddard County, and 146 nymphs were from Bollinger County) were preserved in 70% ethanol. Nymphal ticks were pooled into groups of 5 and processed for DNA isolation, whereas adult ticks were individually dissected and processed for DNA isolation.
DNA was extracted by means of the IsoQuick DNA extraction kit (Orca Research) from both the skin biopsy specimen and the transport medium (each of which was processed separately) for most of the patient specimens as well as for the tick specimens, as described elsewhere [24, 25]. DNA was re-suspended in 50 μL of water, and 10 μL were used for PCR.
PCR amplification
PCR amplifications were performed in a 50-μL reaction mixture containing 10 mmol/L Tris-HCl (pH 8.3); 1.5 mmol/L MgCl2; 50 mmol/L KCl, 0.1% (w/v) gelatin; 100 μmol/L each of dATP, dGTP, dCTP, and TTP; 1.25 units Taq polymerase; and 20 pmol of each primer. Detection of borrelial DNA in patient specimens and ticks was accomplished by the nested PCR amplification of flaB using primers FlaLL, FlaLS, FlaRL, and FlaRS as described by Barbour et al [11]. PCR of 16S rDNA was performed with broad-range eubacterial primers 8FPL and 1492RPL [26], which yields a product of ∼1.5 kbp. In cases in which no detectable product was obtained, second-round heminested PCR was performed with 8FPL and a reverse primer (519R: 5′-TTACCGCGGCTGCTGGC-3′) targeted at residues 535–518 (numbering corresponds to residues in the 16S RNA sequence of Escherichia coli) in 16S rDNA; this resulted in a fragment of ∼500 bp. Some specimens were also tested by PCR targeted at ospA (forward primer, 5′-CTGCAGCTTGGAATTCAGGCACTTC-3′; reverse primer, 5′-GTTTTGTAATTTCAACTGCTGACCCCTC-3′) and/or recA [27].
A real-time quantitative PCR (qPCR) targeting the B. lonestari–specific glpQ gene [28] was used to detect B. lonestari DNA in many of the patient and tick specimens. For skin biopsy specimens collected from patients in Missouri who had an EM-like lesion, a TaqMan probe–based multiplex qPCR assay for simultaneous amplification of B. lonestari glpQ and human gapdh was employed. PCR was performed with an ABI 7900HT Sequence Detection System in 50-μL reaction mixtures containing 1 × TaqMan universal PCR master mix (which contained deoxyribonucleoside triphosphates [200 μmol/L dATP, 200 μmol/L dCTP, 200 μmol/L dGTP, and 400 μmol/L dUTP] 0.01 U of uracil-N-glycosylase per μL, >2.5 mmol/L MgCl2, and 0.025 U of AmpliTaq Gold per μL, and the reference dye 6-carboxy-X-rhodamine [Applied Biosystems]), 1 μmol/L of each primer, 150 nmol/L of probe, and 5 μL of template DNA. Primers and probes were as follows: for B. lonestari glpQ, the forward primer was 5′-GATCCAGAACTTGATACAACCACAA-3′, the reverse primer was 5′-TGATTTAAGTTCATCTAGTGTGAAGTCAGT-3′, and the probe was 5′-FAM (6-car-boxyfluorescein)-CAACCGAGCTAGGGAAGACGGACGATATTACT-BHQ1 (black hole quencher 1)-3′ (Bacon et al., unpublished data); and for human gapdh, the forward primer was hGAPDH-F (5′-CCTGCCAAATATGATGACATCAAG-3′), the reverse primer was hGAPDH-R (5′-GTGGTCGTTGAGGGCAATG-3′), and the probe was hGAPDH-P (5′-VIC-CTCCTCTGACTTCAACAGCGACACCCA-TAMRA-3′). The amplification program started at 50°C for 2 min and continued at 95°C for 10 min, 40–45 cycles at 95°C for 15 s, and 60°C for 60 s. DNA from ticks that were positive for B. lonestari and water controls were included in each PCR run. The number of gene copies in each PCR reaction was calculated by comparing the threshold cycle number for the sample with those for the standards using the ABI Sequence Detection System Software, version 2.0 (Applied Biosystems). This qPCR has a detection sensitivity of 5 copies of B. lonestari glpQ.
DNA sequencing and phylogenetic analysis
Amplified PCR products were cloned into pT7-Blue3 (Novagen) and transformed into E. coli DH5α, and the inserts of individual ampicillin-resistant clones were sequenced. DNA sequences were determined by a commercial service (Davis Sequencing).
DNA sequences were compared by means of BLAST analysis to those in the bacterial subdivision of GenBank. Sequences of flaB were aligned using ClustalW, and a phylogenetic tree was constructed using the MEGA program, version 2.1.
Serologic testing
Acute- and convalescent-phase serum specimens were tested by use of a polyvalent (IgG/IgM), sonicated whole-cell ELISA (Wampole Laboratories), performed in accordance with the manufacturer's instructions.
Results
PCR of patient samples
Specimens obtained from 30 Missouri patients and from 143 New York State patients for whom EM was clinically diagnosed were evaluated in this study. PCR targeting flaB using genus-wide primers produced a product in 5 (16%) of 31 evaluable skin specimens obtained from patients in Missouri (1 patient underwent biopsy of 2 separate EM-like skin lesions, both of which were thought to be a primary EM on the basis of clinical evidence of distinct bite sites in the lesions). By comparison, 70 (50%) of 139 skin biopsy samples obtained from New York State patients were positive for flaB (P < .001) (table 1). The positive PCR findings for the 5 Missouri specimens, however, were likely to be the result of laboratory contamination with amplicons of B. burgdorferi. The DNA sequences of all 5 of the amplified flaB products were identical to each other and to that of B. burgdorferi B31; they were clearly different from flaB of B. lonestari (GenBank accession number AF273670) (figure 1). These 5 samples were also PCR positive for a second B. burgdorferi gene, recA, but were PCR negative for ospA and 16S rDNA. Additional skin samples from the original EM-like lesions were available for 3 of these 5 patients; all specimens were PCR negative for flaB, ospA, recA, and eubacterial 16S rDNA. Twenty-two skin biopsy specimens obtained in 2001 and 2002 (which included the 5 flaB-positive skin samples) were also tested by multiplex qPCR for B. lonestari glpQ. All 22 specimens tested negative for B. lonestari glpQ.
Table 1. Test results for specimens obtained from patients with erythema migrans (EM) or EM-like lesions.
| Laboratory test | Missouri patients with EM-like lesions (n = 30) |
New York State patients with EM (n = 143) |
Pa |
|---|---|---|---|
| Skin PCR/culture | |||
| Positive flaB PCR | 5/31 (16)b | 70/139 (50) | <.001 |
| Positive eubacterial 16S RNA PCR | 0/20 (0)b | Not done | |
| Positive glpQ PCR | 0/22 (0)b | Not done | |
| Positive Borrelia burgdorferi culture | 0/19 (0) | 89/142 (63) | <.001 |
| Serum serologic analysis | |||
| Acute phase, seropositivec | 0/25 (0) | 81/143 (57) | <.001 |
| Convalescent phase, seropositive (1–3 months) | 0/22 (0) | 99/135 (73) | <.001 |
| Seroconversiond | 0/22 (0) | 25/52 (48) | <.001 |
| Acute or convalescent phase, seropositive | 0/25 (0) | 107/143 (75) | <.001 |
| Overalle | 5/31 (16)b | 130/143 (90.9) | <.001 |
NOTE. Data are no. of specimens with positive test results/no. that were evaluable (%).
By 2-tailed Fisher's exact test.
Two biopsies of separate EM-like lesions were performed for 1 patient.
By ELISA.
Negative-to-positive or equivocal-to-positive results ≤3 months after baseline.
Positive B. burgdorferi culture, positive PCR result, or seropositive.
Figure 1.
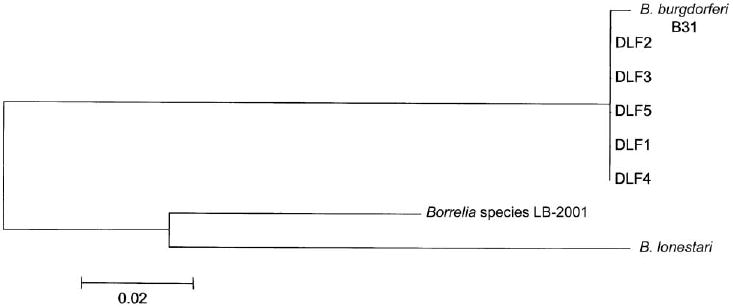
Phylogenetic tree of flaB sequences amplified from skin biopsy specimens obtained from patients in Missouri (the 5 sequences in this study were designated DLF1–DLF5), Borrelia burgdorferi B31 (GenBank accession number AE001126), Borrelia lonestari (GenBank accession number AF273670), and Borrelia species LB-2001 (GenBank accession number AY024344). The bar represents a 2% sequence divergence.
Twenty-six skin biopsy samples obtained from 25 Missouri patients were tested for the presence of any other bacteria using broad-spectrum eubacterial primers targeted at conserved regions of 16S rDNA. No specific PCR products were obtained from any of the 20 evaluable specimens (6 specimens could not be evaluated because the water control tested positive).
Culture and serologic testing of patient samples
B. burgdorferi was not recovered from any of the skin biopsy samples suitable for evaluation of 19 Missouri patients (5 skin samples that were submitted in ethanol and 4 additional skin samples collected from patients being treated with antibiotics were not cultured; 2 additional skin cultures were unevaluable because of contamination with other bacteria), compared with 89 (63%) of 142 evaluable New York State patients (1 skin culture was contaminated) (P < .001) (table 1). The overall rate of seropositivity among acute- or convalescent-phase serum samples was 0 of 25 samples for Missouri patients, compared with 107 (75%) of 143 for New York State patients (P < .001) (table 1).
Evaluation of patient specimens after spontaneous resolution of EM
Specimens were received from 2 additional Missouri patients for whom the EM-like lesion had resolved without antibiotic treatment. Serum specimens plus a skin biopsy sample from the site of the former EM-like lesion were obtained from the 2 patients 25 and 72 days after resolution of the lesion. Both patients were seronegative, borrelial culture negative, and flaB PCR negative.
PCR of tick specimens
A. americanum ticks that were removed from 2 patients in Missouri who developed an EM-like lesion at the tick bite site were examined for the presence of Borrelia species by genus-specific PCR directed at flaB. Both specimens were PCR negative. The ticks were then tested for the presence of any bacteria using eubacterial 16S rDNA primers. One tick yielded a PCR product, which was cloned and subjected to DNA sequencing. DNA database analysis revealed the sequence to be most closely related to an uncharacterized a proteobacterium (GenBank accession number AJ459874), consistent with a contaminating soil bacterium or a tick endosymbiont. The sequence was unrelated to any spirochetal 16S rDNA sequences. None of 312 field-collected A. americanum ticks tested by qPCR assay were positive for B. lonestari DNA (glpQ).
Discussion
The PCR results of this study provide strong evidence that B. lonestari infection is not the explanation for the EM-like illness in the Cape Girardeau area. If the EM-like illness in Missouri has an infectious etiology and if it is caused by a bacterium, our application of broad-spectrum eubacterial rRNA primers was unsuccessful in identifying such a pathogen. Negative results with these primers could be due to low target concentrations or the presence of PCR inhibitors in the DNA extracts from skin biopsy specimens.
The negative PCR findings in this study are unlikely to be the result of inhibitors for several reasons. First, the 22 skin specimens tested yielded a signal for human gapdh in qPCR. Second, these 22 skin biopsy specimens were diluted 1:10 and retested by qPCR; test results for all remained negative. In addition, when several DNA extracts from the Missouri skin biopsy specimens were spiked with 80 gene copies of B. lonestari glpQ, positive results were obtained by qPCR.
Consistent with results of our tests of the clinical specimens, we also could not find evidence of B. lonestari in any of 312 field-collected A. americanum ticks or in 2 A. americanum ticks removed by patients who later developed an EM-like lesion. This observation differs from the findings of Bacon et al. [15], who estimated that the prevalence of B. lonestari infection was 5.6% (95% CI, 2.5%–8.7%) among 185 nymphal and 21 adult A. americanum ticks collected in Butler County, Missouri (which is located ∼60 miles southwest of Cape Girardeau), based on amplification of flaB and/or 16S rDNA gene targets. A difference between the 2 studies is the PCR methodology used for B. lonestari detection; in our study, qPCR for glpQ was used. The discrepancy between the report by Bacon et al. [15] and the findings we describe suggests that B. lonestari infection of A. americanum ticks may be focally distributed within an area of endemicity. However, analogous to our results, Bacon et al. [15] were also unable to detect B. lonestari DNA in 4 skin biopsy samples obtained from patients in Missouri with EM-like lesions.
Subsequent to completion of this study, Varela et al. [29] successfully isolated B. lonestari from an A. americanum tick using an embryonic tick cell line. This represented the first time that B. lonestari was cultured in vitro. Whether this technique could be successfully applied to clinical specimens is unknown, but if it can, it seems unlikely that it would be more sensitive than the PCR tests used in our study, because we used both nested PCR and real-time qPCR directed at 3 different gene targets in B. lonestari. Also, in the only proven human case of B. lonestari infection, the flaB target in a skin biopsy sample was successfully amplified using identical methodology [18].
The microbiologic and serologic results for patients in Missouri with EM differed significantly from those for patients in New York State (table 1). In agreement with previous studies conducted both in Missouri [4] and in southern states other than Missouri [3, 5, 30], our laboratory test results demonstrated that B. burgdorferi is not the cause of the EM-like lesions we studied. The lack of cross-reactivity with a sonicated whole cell B. burgdorferi B31 antigen preparation, as was used in the serologic assay in this study, diminishes the likelihood that any borrelial agent is the cause of EM in Missouri, because even more distantly related spirochetal infections (such as syphilis) lead to the development of cross-reactive antibodies [31].
B. lonestari is not likely to be the cause of EM-like lesions in the Cape Girardeau area. Whether B. lonestari is the etiologic agent of EM-like lesions in other areas of Missouri or in other states with indigenous B. lonestari–infected A. americanum ticks is unclear. Although it is unknown whether this rash illness has an infectious etiology, it is important to emphasize that this study does not indicate the absence of a therapeutic role for antibiotic treatment.
Acknowledgments
We thank Dr. Barbara Johnson and Rendi Bacon (Division of Vector-Borne Infectious Diseases, Centers for Disease Control and Prevention [CDC] Atlanta, GA), for sharing their quantitative PCR protocols and for helpful discussions, and Lisa Giarratano, Katherine Ma, and Donna McKenna, for their assistance.
Financial support. CDC (cooperative agreements U50/CCU219613 [to G.P.W.] and U50/CCU219612 [to J.N.]) and National Institutes of Health (grant AR41511 [to I.S.]).
Footnotes
Potential conflicts of interest. All authors: no conflicts.
References
- 1.Orloski KA, Hayes EB, Campbell GL, Dennis DT. Surveillance for Lyme disease—United States, 1992–1998. MMWR CDC Surveill Summ. 2000;49:1–11. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Lyme disease—United States, 2001–2002. MMWR Morb Mortal Wkly Rep. 2004;53:365–9. [PubMed] [Google Scholar]
- 3.Felz MW, Chandler FW, Jr, Oliver JH, Jr, Rahn DW, Schriefer ME. Solitary erythema migrans in Georgia and South Carolina. Arch Dermatol. 1999;135:1317–26. doi: 10.1001/archderm.135.11.1317. [DOI] [PubMed] [Google Scholar]
- 4.Campbell GL, Paul WS, Schriefer ME, Craven RB, Robbins KE, Dennis DT. Epidemiologic and diagnostic studies of patients with suspected early Lyme disease. J Infect Dis. 1995;172:470–80. doi: 10.1093/infdis/172.2.470. [DOI] [PubMed] [Google Scholar]
- 5.Kirkland KB, Klimko TB, Meriwether RA, et al. Erythema migrans–like rash illness at a camp in North Carolina: a new tick-borne disease. Arch Intern Med. 1997;157:2635–41. [PubMed] [Google Scholar]
- 6.Masters E, Granter S, Duray P, Cordes P. Physician-diagnosed erythema migrans–like rashes following lone star tick bites. Arch Dermatol. 1998;134:955–60. doi: 10.1001/archderm.134.8.955. [DOI] [PubMed] [Google Scholar]
- 7.Felz MW, Durden LA, Oliver JH., Jr Ticks parasitizing humans in Georgia and South Carolina. J Parasitol. 1996;82:505–8. [PubMed] [Google Scholar]
- 8.Goddard J. Ticks and tickborne diseases affecting military personnel. Brooks Air Force Base, TX: US Air Force School of Aerospace Medicine; 1989. [Google Scholar]
- 9.Felz MW, Durden LA. Attachment sites of four tick species (Acari: Ixodidae) parasitizing humans in Georgia and South Carolina. J Med Entomol. 1999;36:361–4. doi: 10.1093/jmedent/36.3.361. [DOI] [PubMed] [Google Scholar]
- 10.Magnarelli LA, Anderson JF, Apperson CS, Fish D, Johnson RC, Chappell WA. Spirochetes in ticks and antibodies to Borrelia burgdorferi in white-tailed deer from Connecticut, New York State, and North Carolina. J Wildl Dis. 1986;22:178–88. doi: 10.7589/0090-3558-22.2.178. [DOI] [PubMed] [Google Scholar]
- 11.Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease–like illness. J Infect Dis. 1996;173:403–9. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- 12.Schulze TL, Bowen GS, Bosler EM, et al. Amblyomma americanum: a potential vector of Lyme disease in New Jersey. Science. 1984;224:601–3. doi: 10.1126/science.6710158. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong PM, Rich SM, Smith RD, Hartl DL, Spielman A, Telford SR., III A new Borrelia infecting lone star ticks. Lancet. 1996;347:67–8. doi: 10.1016/s0140-6736(96)91604-9. [DOI] [PubMed] [Google Scholar]
- 14.Feir D, Santanello CR, Li BW, et al. Evidence supporting the presence of Borrelia burgdorferi in Missouri. Am J Trop Med Hyg. 1994;51:475–82. [PubMed] [Google Scholar]
- 15.Bacon RM, Gilmore RD, Jr, Quintana M, Piesman J, Johnson BJB. DNA evidence of Borrelia lonestari in Amblyomma americanum (Acari:Ixodidae) in Southeast Missouri. J Med Entomol. 2003;40:590–2. doi: 10.1603/0022-2585-40.4.590. [DOI] [PubMed] [Google Scholar]
- 16.Stegall-Faulk T, Clark DC, Wright SM. Detection of Borrelia lonestari in Amblyomma americanum (Acari:Ixodidae) from Tennessee. J Med Entomol. 2003;40:100–2. doi: 10.1603/0022-2585-40.1.100. [DOI] [PubMed] [Google Scholar]
- 17.Rich SM, Armstrong PM, Smith RD, Telford SR., III Lone star tick–infecting borreliae are most closely related to the agent of bovine borreliosis. J Clin Microbiol. 2001;39:494–7. doi: 10.1128/JCM.39.2.494-497.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.James AM, Liveris D, Wormser GP, Schwartz I, Montecalvo MA, Johnson BJB. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J Infect Dis. 2001;183:1810–4. doi: 10.1086/320721. [DOI] [PubMed] [Google Scholar]
- 19.Stromdahl EY, Williamson PC, Kollars TM, Jr, et al. Evidence of Borrelia lonestari DNA in Amblyomma americanum (Acari:Ixodidae) removed from humans. J Clin Microbiol. 2003;41:5557–62. doi: 10.1128/JCM.41.12.5557-5562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Case definitions for infectious conditions under public health surveillance. MMWR Recomm Rep. 1997;46(RR10):1–55. [PubMed] [Google Scholar]
- 21.Berger BW, Johnson RC, Kodner C, Coleman L. Cultivation of Borrelia burgdorferi from erythema migrans lesions and peri-lesional skin. J Clin Microbiol. 1992;30:359–61. doi: 10.1128/jcm.30.2.359-361.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwartz I, Wormser GP, Schwartz JJ, et al. Diagnosis of early Lyme disease by polymerase chain reaction amplification and culture of skin biopsies from erythema migrans lesions. J Clin Microbiol. 1992;30:3082–8. doi: 10.1128/jcm.30.12.3082-3088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowakowski J, Schwartz I, Liveris D, et al. Laboratory diagnostic techniques for patients with early Lyme disease associated with erythema migrans: a comparison of different techniques. Clin Infect Dis. 2001;33:2023–7. doi: 10.1086/324490. [DOI] [PubMed] [Google Scholar]
- 24.Liveris D, Varde S, Iyer R, et al. Genetic diversity of Borrelia burgdorferi in Lyme disease patients as determined by culture versus direct PCR with clinical specimens. J Clin Microbiol. 1999;37:565–9. doi: 10.1128/jcm.37.3.565-569.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz I, Varde S, Nadelman R, Wormser GP, Fish D. Inhibition of efficient PCR amplification of Borrelia burgdorferi DNA in blood-fed ticks. Am J Trop Med Hyg. 1997;56:339–42. doi: 10.4269/ajtmh.1997.56.339. [DOI] [PubMed] [Google Scholar]
- 26.Relman DA. In: Diagnostic molecular microbiology. Persing DH, Smith TF, Tenover FC, White TJ, editors. Washington, DC: American Society for Microbiology; 1993. pp. 489–95. [Google Scholar]
- 27.Liveris D, Wang G, Girao G, et al. Quantity of Borrelia burgdorferi detected in 2-mm skin samples of erythema migrans lesions: correlation with clinical and laboratory findings. J Clin Microbiol. 2002;40:1249–53. doi: 10.1128/JCM.40.4.1249-1253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bacon RM, Pilgard MA, Johnson BJ, Raffel SJ, Schwan TG. Glycero-phosphodiester phosphodiesterase gene (glpQ) of Borrelia lonestari identified as a target for differentiating Borrelia species associated with hard ticks (Acari:Ixodidae) J Clin Microbiol. 2004;42:2326–8. doi: 10.1128/JCM.42.5.2326-2328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varela AS, Luttrell MP, Howerth EW, et al. First culture isolation of Borrelia lonestari, putative agent of southern tick–associated rash illness. J Clin Microbiol. 2004;42:1163–9. doi: 10.1128/JCM.42.3.1163-1169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong PM, Brunet LR, Spielman A, Telford SR., III Risk of Lyme disease: perceptions of residents of a Lone Star tick–infested community. Bull WHO. 2001;79:916–25. [PMC free article] [PubMed] [Google Scholar]
- 31.Magnarelli LA, Anderson JF, Johnson RC. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987;156:183–8. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]


