Abstract
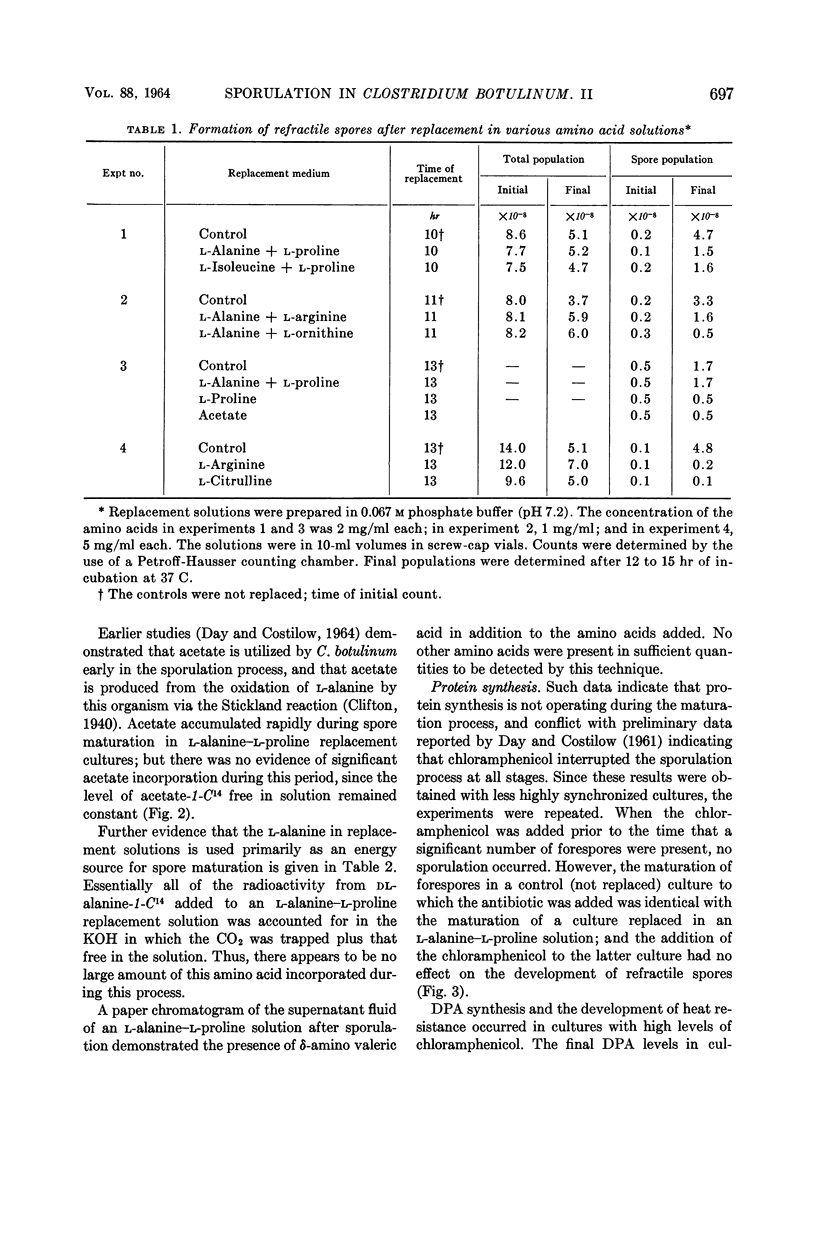
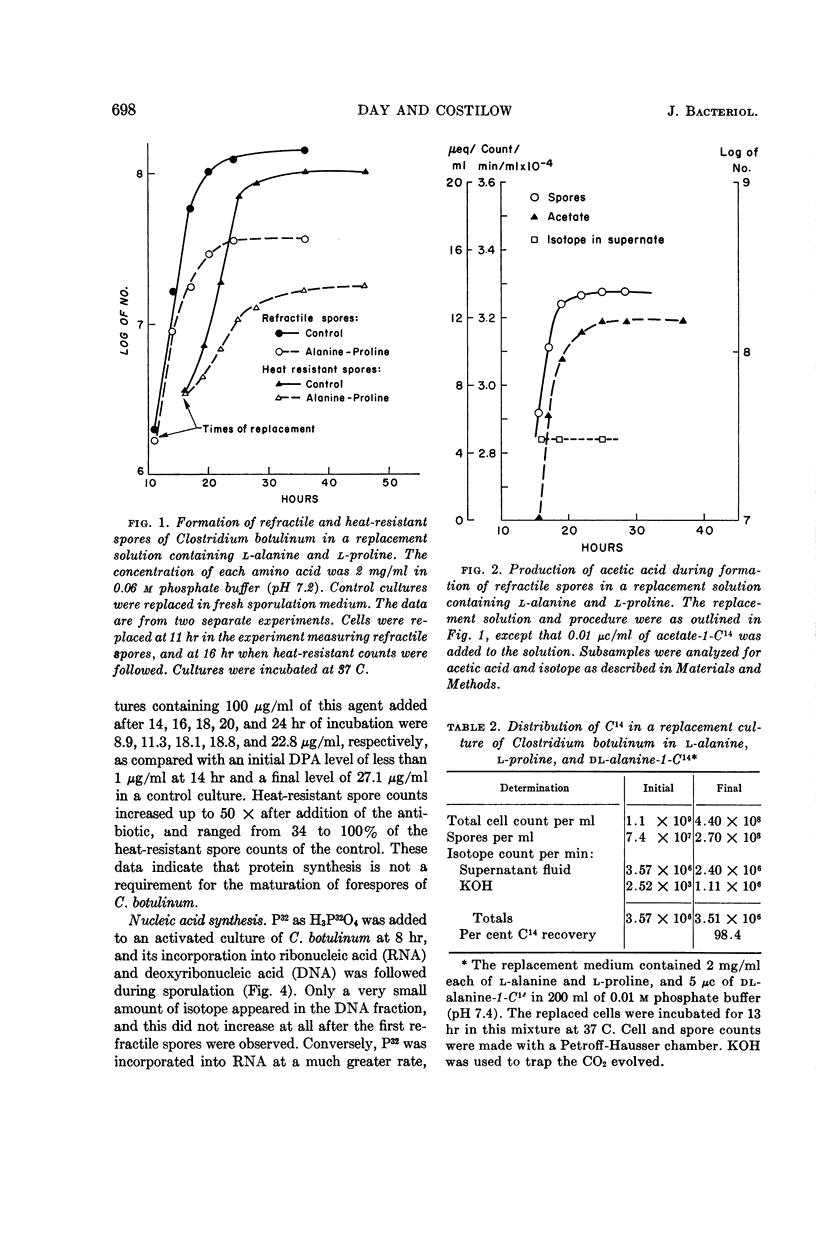
Day, Lawrence E., (Michigan State University, East Lansing) and Ralph N. Costilow. Physiology of the sporulation process in Clostridium botulinum. II. Maturation of forespores. J. Bacteriol. 88:695–701. 1964.—Clostridium botulinum, strain 62-A, did not sporulate endotrophically, but forespores matured to refractile, heat-resistant spores when replaced in solutions containing l-alanine and l-proline, l-isoleucine and l-proline, or l-alanine and l-arginine. Solutions of l-arginine or l-citrulline would not support the maturation process. Acetate, CO2, and δ-amino valeric acid were produced during sporulation in a replacement solution of l-alanine and l-proline, indicating the operation of the Stickland reaction. There was no large uptake of either exogenous l-alanine or acetate during this process. Similarly, there was no apparent protein or nucleic acid synthesis, since high levels of chloramphenicol, 8-azaguanine, or mitomycin C failed to inhibit, and no significant amount of P32 was incorporated into the spore nucleic acids. Dipicolinic acid (DPA) was synthesized during forespore maturation. It is believed that these final steps in sporulation of C. botulinum require only an exogenous source of energy which can be obtained via the Stickland reaction system, and that the synthesis of DPA and other unknown materials relies primarily on endogenous substrates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S. H., HASHIMOTO T., GERHARDT P. Calcium reversal of the heat susceptibility and dipicolinate deficiency of spores formed "endotrophically" in water. Can J Microbiol. 1960 Apr;6:213–224. doi: 10.1139/m60-023. [DOI] [PubMed] [Google Scholar]
- COSTILOW R. N. Fermentative activities of control and radiation-"killed" spores of Clostridium botulinum. J Bacteriol. 1962 Dec;84:1268–1273. doi: 10.1128/jb.84.6.1268-1273.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton C. E. The Utilization of Amino Acids and of Glucose by Clostridium botulinum. J Bacteriol. 1940 May;39(5):485–497. doi: 10.1128/jb.39.5.485-497.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAY L. E., COSTILOW R. N. PHYSIOLOGY OF THE SPORULATION PROCESS IN CLOSTRIDIUM BOTULINUM. I. CORRELATION OF MORPHOLOGICAL CHANGES WITH CATABOLIC ACTIVITIES, SYNTHESIS OF DIPICOLINIC ACID, AND DEVELOPMENT OF HEAT RESISTANCE. J Bacteriol. 1964 Sep;88:690–694. doi: 10.1128/jb.88.3.690-694.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOSTER J. W., PERRY J. J. Intracellular events occurring during endotrophic sporulation in Bacillus mycoides. J Bacteriol. 1954 Mar;67(3):295–302. doi: 10.1128/jb.67.3.295-302.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., BLACK S. H. Permeability of bacterial spores. II. Molecular variables affecting solute permeation. J Bacteriol. 1961 Nov;82:750–760. doi: 10.1128/jb.82.5.750-760.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAWALT P. Use of phosphorus-32 in microassay for nucleic acid synthesis in Escherichia coli. Science. 1959 Aug 14;130(3372):386–387. doi: 10.1126/science.130.3372.386. [DOI] [PubMed] [Google Scholar]
- HARDWICK W. A., FOSTER J. W. On the nature of sporogenesis in some aerobic bacteria. J Gen Physiol. 1952 Jul;35(6):907–927. doi: 10.1085/jgp.35.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODSON P. H., BECK J. V. Origin of deoxyribonucleic acid of the bacterial endospore. J Bacteriol. 1960 May;79:661–665. doi: 10.1128/jb.79.5.661-665.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- NAKATA H. M. EFFECT OF PH ON INTERMEDIATES PRODUCED DURING GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Sep;86:577–581. doi: 10.1128/jb.86.3.577-581.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS W. E., TSUJI K. Sporulation of Clostridium botulinum. II. Effect of arginine and its degradation products on sporulation in a synthetic medium. J Bacteriol. 1962 Jul;84:86–94. doi: 10.1128/jb.84.1.86-94.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY J. J., FOSTER J. W. Non-involvement of lysis during sporulation of Bacillus mycoides in distilled water. J Gen Physiol. 1954 Jan 20;37(3):401–409. doi: 10.1085/jgp.37.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL J. F., HUNTER J. R. Sporulation in distilled water. J Gen Physiol. 1953 May;36(5):601–606. doi: 10.1085/jgp.36.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky R. A., Law J. H. SYNTHESIS AND DEGRADATION OF POLY-beta-HYDROXYBUTYRIC ACID IN CONNECTION WITH SPORULATION OF BACILLUS MEGATERIUM. J Bacteriol. 1961 Jul;82(1):37–42. doi: 10.1128/jb.82.1.37-42.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TINELLI R. Etude de la biochimie de la sporulation chez Bacillus megaterium. II. Modifications biochimiques et échanges gazeux accompagnant la sporulation provoquée par carence de glucose. Ann Inst Pasteur (Paris) 1955 Mar;88(3):364–375. [PubMed] [Google Scholar]
- Woods D. D. Studies in the metabolism of the strict anaerobes (genus Clostridium): Further experiments on the coupled reactions between pairs of amino-acids induced by Cl. sporogenes. Biochem J. 1936 Oct;30(10):1934–1946. doi: 10.1042/bj0301934. [DOI] [PMC free article] [PubMed] [Google Scholar]



