Abstract
Bronchiolar stem cells have been functionally defined in vivo on the basis of their resistance to chemical (naphthalene) injury, their infrequent proliferation relative to other progenitor cell types, and their coexpression of the airway and alveolar secretory cell markers Clara cell secretory protein and pro-surfactant protein C, respectively. Cell surface markers that have previously been used for their prospective isolation included Sca-1 and CD34. Using transgenic animal models associated with stem cell expansion, ablation, and lineage tracing, we demonstrate that CD34pos cells do not belong to the airway epithelial lineage and that cell surface Sca-1 immunoreactivity does not distinguish between bronchiolar stem and facultative transit-amplifying (Clara) cell populations. Furthermore, we show that high autofluorescence (AFhigh) is a distinguishing characteristic of Clara cells allowing for the fractionation of AFlow bronchiolar stem cells. On the basis of these data we show that the defining phenotype of the bronchiolar stem cell is CD45neg CD31neg CD34neg Sca-llow AFlow. This refinement in the definition of bronchiolar stem cells provides a critical tool by which to assess functional and molecular distinctions between bronchiolar stem cells and the more abundant pool of facultative transit-amplifying (Clara) cells.
Keywords: Bronchiolar stem cell, Bronchioalveolar stem cell, Clara, Progenitor, Fractionation, Epithelium
Introduction
The lung epithelium turns over slowly in the normal condition but has significant regenerative capacity that can be activated following injury [1, 2]. This regenerative capacity is critical for homeostasis because of continual exposure of the lung to injurious stimuli by either systemic or inhalation routes. Chronic injury leads to the establishment of disease states such as those seen with idiopathic pulmonary fibrosis, asthma, chronic obstructive pulmonary disease, and lung cancer [3]. Bronchiolar airways harbor two progenitor cell types that contribute to epithelial maintenance and repair within bronchioles: an abundant facultative transit-amplifying (TA) cell commonly referred to as the Clara cell, and rare bronchiolar stem cells [4, 5]. We define a facultative TA cell as one that in the steady state fulfills differentiated functions, but has the potential to transiently re-enter the cell cycle and generate differentiated progeny in a manner that is analogous to TA cells in the intestine. In contrast, ciliated cells represent a postmitotic cell type that in the adult bronchiole are derived from Clara cells [2, 6]. In previous work by us and others, bronchiolar stem cells were characterized as naphthalene-resistant, label-retaining, Clara cell secretory protein (CCSP)-expressing cells that are spatially localized to either bronchioalveolar duct junctions or branch point-associated neuroepithelial bodies [7–10]. On the basis of these criteria, bronchiolar stem cells have been termed either variant Clara cells (Clara(v)) according to their unique functional properties but similarity with Clara cells in their expression of CCSP [7, 8], or bronchioalveolar stem cells because of their coexpression of the airway marker CCSP and alveolar marker pro-surfactant protein C (Pro-SPC), their Sca-1pos CD34pos cell surface phenotype, and their ability to express marker genes for both airway and alveolar epithelium in vitro [10].
In the present study, we used transgenic animal models allowing ablation [7, 11], lineage tagging [12, 13], and expansion [12] of the stem cell population to verify the cell surface phenotype of bronchiolar stem cells. We found that Sca-1 does not distinguish bronchiolar stem cells from the more abundant pool of facultative TA (Clara) cells and that neither population expresses cell surface CD34. We determine that bronchiolar stem and facultative TA (Clara) cells share the common property of low cell surface expression of Sca-1 that distinguished them from Sca-1high lung cells and can be distinguished from each other on the basis of their autofluorescence (AF) characteristics. Accordingly, bronchiolar stem cells can be defined on the basis of their CD45neg CD31neg CD34neg Sca-11ow AFlow phenotype.
Materials and Methods
Animal Husbandry
Mice were bred and maintained in Association for Assesment and Accreditation of Laboratory Animal Care-approved facilities at University of Pittsburgh and Duke University. Animals used for experiments were maintained in pathogen-free conditions with unrestricted access to food and water on a 12-hour light/dark cycle. All experiments were performed according to Institutional Animal Care and Use Committee-approved protocols on mice between 2 and 6 months of age.
Genotyping
Genotyping was performed by polymerase chain reaction (PCR) amplification of genomic DNA isolated from the mouse tail by previously published methods. Primer pairs and PCR conditions have been previously described [12, 14].
Ganciclovir Treatment of CCSP-HSVtk Animals
Ganciclovir (GCV) was administered as detailed previously using a miniosmotic pump (Alzet Osmotic Pumps, Cupertino, CA, http://www.alzet.com) loaded with 50 mg/ml solution (wt/vol) in phosphate-buffered saline (PBS) for delivery at a rate of 8 μl/hour over a 24-hour period [7].
Cell isolation
Airway epithelial cells were isolated on the basis of a previously published method [12, 15]. Mice were anesthetized and exsanguinated, the thoracic cavity was opened, the lungs were exposed, and the trachea was cannulated. Lungs were perfused with 10 ml of PBS and lavaged four times with 1 ml of PBS and 0.2 μM EGTA. One milliliter of 4 U/ml Elastase solution (Worthington Biochemical, Lakewood, NJ, http://www.worthington-biochem.com) was instilled in the airways and incubated for 5 minutes at 37°C followed by three 0.5-ml instillations for 5 minutes each. Lung lobes were removed following Elastase digestion, minced, and incubated with DNase I solution for 10 minutes at 37°C Cells were passed through a 100 μm cell strainer, red blood cells (RBCs) were lysed using RBC lysing buffer (eBioscience Inc., San Diego, http://www.ebioscience.com), and live cells were counted on the basis of trypan blue exclusion.
Flow Cytometry
Isolated cells were resuspended at 1 × 106 cells in 100 μl of Hanks' balanced saline solution, 10 mM HEPES, 2% fetal bovine serum (staining solution) and incubated with the indicated antibodies for 30 minutes at 4°C in the dark. Antibodies used were phycoerythrin (PE)-Cy7 anti-CD31 (1:50; eBioscience), PE-Cy7 anti-CD45 (1:100; BD Pharmingen, San Diego, http://www.bdbiosciences.com/index_us.shtml), Alexa Fluor 647 anti-CD34 (13:200; eBioscience), and PE anti-Sca-1 (1:50; BD Pharmingen). Cells were washed with 1 ml of staining solution, resuspended in 300 μl of staining solution, and analyzed using either a FACS-Vantage (BD Biosciences, San Diego, http://www.bdbiosciences.com), a FACSCanto (BD Biosciences), or a FACSCanto II (BD Biosciences). Propidium iodide (PI) or 7-aminoactinomycin D (7-AAD) was used for dead cell discrimination. For intracellular staining, cells were fixed in 1% paraformaldehide solution on ice for one hour, permeabilized using a 0.2% (volume/volume)Tween 20 (Sigma)solution for 20 minutes at 37°C, and incubated with an Alexa Fluor 488 directly conjucated rabbit-anti CCSP (in house, 1:10,000). The antibody was directly conjugated using the Zenon® Alexa Fluor® 488 Rabbit IgG Labeling Kit (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) following manufacturer's instructions. Sorting experiments were performed using a FACSVantage (BD Biosciences) or a FACSAria (BD Biosciences) sorter. Data were analyzed using FlowJo 7.2.4 software (Tree Star, Ashland, OR, http://www.treestar.com).
Immunofluorescence
Cells were cytospun onto glass slides and fixed in 10% neutral buffered formalin for 30 minutes. Cells were permeabilized using 0.5% Triton X-100 (vol/vol) in PBS for 30 minutes, blocked in 5% bovine serum albumin (BSA) (wt/vol) in PBS for 30 minutes, and incubated with the primary antibody for 1 hour at room temperature: polyclonal rabbit anti-CCSP (in-house, 1:10,000), polyclonal goat anti-CCSP (in house, 1:10,000), and rabbit anti-Pro-SPC (1:2,500, kind gift from Y.P. Di from the University of Pittsburgh). A fluorescently labeled secondary antibody was added for 1 hour, and slides were mounted using Fluoromount G (SouthernBiotech, Birmingham, AL, http://www.southernbiotech.com) with 4,6-diamidino-2-phenylindole (DAPI; 2 μg/ml) as a nuclear counterstain. For immunofluorescent staining of lung tissue, 5-μm cryosections were dehydrated in 100% ethanol and rehydrated in decreasing concentrations of ethanol in PBS. When necessary, antigen retrieval was performed using incubation in 0.05% trypsin/EDTA tissue culture grade (Cellgro; Mediatech, Inc., Manassas, VA, http://www.cellgro.com) for 10 minutes at 37°C. Slides were blocked in 5% BSA (wt/vol) in PBS, and the primary antibody was added for 3 hours at room temperature. Slides were incubated for 1 hour at room temperature with a fluorescent secondary antibody and mounted in DAPI containing Fluoromount-G (2 μg/ml). Fluorescent images were acquired using an Olympus Provis AX70 microscope (Olympus, Tokyo, http://www.olympus-global.com) equipped with a Spot RT digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI, http://www.diaginc.com/camRT.shtml). The same acquisition and analysis parameters were used for all samples in each experiment. Images were processed and analyzed using Adobe Photoshop (Adobe Systems Inc., San Jose, CA, http://www.adobe.com).
RNA Isolation and Real-Time PCR
Total RNA was isolated from the left lung lobe as previously described [12]. RNA was reverse-transcribed using Superscript First Strand Synthesis Kit (Invitrogen), and the relative mRNA abundance of genes of interest was assessed using TaqMan FAM-labeled probes (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com) and the AB TaqMan 7000 Real-Time System (Applied Biosystems).
Results
Sca-1 and CD34 Expression in Single-Cell Preparations of Lung Cells
It has previously been proposed that the bronchiolar stem cells can be distinguished from other lung epithelial cell types by virtue of their cell surface expression of Sca-1 and CD34. In their analysis, Kim et al. prepared lung epithelial cells using methods that enrich for alveolar epithelial cells [10]. We sought to determine whether cell surface expression of Sca-1 and CD34 allowed the prospective fractionation of bronchiolar stem cells from the abundant pool of facultative TA (Clara) cells. To address this question we interrogated expression of Sca-1 and CD34 on the surface of lung epithelial cells isolated using established methods that enrich populations of airway epithelial cells [12, 15]. Single-cell preparations were obtained by Elastase digestion of normal mouse lung and further enriched on the basis of negative selection for cell surface CD45 and CD31 and positive selection for viable cells based on exclusion of PI or 7-AAD (supporting information Fig. 1). The profile of the CD45neg CD31neg live population prior to staining for Sca-1 and CD34 (Fig. 1A, upper left panel) revealed two distinct populations based on their autofluorescence in the Alexa Fluor 647 (AF647) and PE channels. Staining of this population for Sca-1 (Fig. 1A, upper right panel) revealed two distinct Sca-1-positive populations: a less abundant Sca-1high (R4 gate = 5.33% of CD45neg CD31neg live population) and a more abundant Sca-1low population (R3 gate = 18.3% of CD45neg CD31neg live population). The Sca-1high population was composed exclusively of AFlow cells, whereas the Sca-1 low population was composed of both AFhigh and AFlow cells. Staining the CD45neg CD31neg PIneg population for CD34 (Fig. 1A, lower left panel) revealed a CD34pos fraction (R1 gate = 12.11%) derived exclusively from the AFlow population. Staining for the combination of all markers and analysis of the CD45neg CD31neg live population revealed that Sca-1pos CD34pos cells (R2 gate) accounted for 4.62% of the population (Fig. 1A, lower right panel). We next determined the spatial localization of Sca-1-positive cells by immunofluorescent staining of lung tissue sections (Fig. 1C). Sca-1 immunoreactivity was evident in cells outside the airway epithelium, with apparently high expression levels detected within endothelial cells lining blood vessels. Consistent with this pattern of immunolocalization was the observation that more than 95% of the CD31 (PECAM)-positive cells from lung cell preparations were Sca-1-positive (data not shown; Kotton et al. [16]). Within the airway epithelium, Sca-1 immunoreactivity was detected only on the basolateral membrane of epithelial cells residing within proximal intrapulmonary airways. Epithelial cells of the distal conducting airway showed no evidence of Sca-1 immunoreactivity above baseline levels in this analysis. Taken together, these data demonstrate that Sca-1 is a broadly expressed marker in the lung and that levels of Sca-1 immunoreactivity vary significantly between cell types. The high proportion of CD45neg CD31neg Sca-1pos CD34pos cells in isolated cells raised the question of whether Sca-1 or CD34 are appropriate and/or specific cell surface markers for rare bronchiolar stem cells.
Figure 1.

Sca-1 and CD34 expression in the lung. (A): Flow cytometry analysis of Sca-1 and CD34 expression on the cell surface of CD45neg CD31neg live single-cell preparations from mouse lung (parameters used for initial selection of cells to be interrogated are given in supporting information Fig. 1). Panels represent unstained control (upper left), Sca-1-stained sample (upper right), CD34-stained sample (lower left), and Sca-1- and CD34-stained sample (lower right). Gates identify populations as follows: R1, CD34-positive population; R2, Sca-1 and CD34 dual-positive population; R3, Sca-1low population; R4, Sca-1high population. Unstained sample: R1 = 0.27%, R2 = 0.00, R3+R4 = 0.17%; Sca-1-stained sample: R1 = 0.14%, R2 = 0.01%, R3 = 18.3, R4 = 5.33%; CD34-stained sample: R1 = 12.11%, R2 = 0.00%, R3 = 0.38%, R4 = 0.00%; Sca-1 CD34 dual-stained sample: R1 = 2.75%, R2 = 6.07%, R3 = 11.94%, R4 = 0.30%. (B): Quantitative representation of data in (A). Data represent averages with SEM for seven samples from three separate experiments. Data represent percentage of live CD45neg CD31neg population: R1 = 3.1%, R2 = 4.62%, R3 = 14.34%, R4 = 0.46%. (C): Immunofluorescence staining of lung sections demonstrating the spatial localization of Sca-1-positive cells in the lung. Top row: Sca-1 (red) immunolocalization. Bottom row: overlay of Sca-1 staining with the nonciliated airway epithelial cell-specific marker CCSP (green). Images were taken at a magnification of ×200. Abbreviations: BADJ, bronchioalveolar duct junction; CCSP, Clara cell secretory protein; PE, phycoerythrin.
Sca-1 Expression in Clara Cell-Depleted Lungs
To further examine the population of lung epithelial cells that are positive for cell surface Sca-1 immunoreactivity, we used a transgenic approach to ablate CCSP-expressing cells of the airway epithelium. In this model, expression of herpes simplex virus thymidine kinase under the mouse CCSP promoter allows ablation of the entire CCSP-expressing population following GCV treatment. We have previously demonstrated that GCV exposure of CCSP-HSVtk transgenic mice results in ablation of both the abundant facultative TA (Clara) cell population and rare bronchiolar stem cells [7]. At day 6 post-GCV treatment, CCSP-expressing cells were significantly depleted (supporting information Fig. 2). Isolated lung cells recovered from either untreated or GCV-treated mice were interrogated by flow cytometry. After exclusion of dead and CD45pos CD31pos cells, Sca-1 and CD34 expression was analyzed and compared between treated and untreated animals (Fig. 2A). In the absence of CCSP-expressing cells, the size of the CD45neg CD31neg Sca-1pos CD34pos population did not change, whereas the size of the CD45neg CD31neg Sca-1pos CD34neg population increased, suggesting that other cells in the lung have the ability to express and upregulate Sca-1 in response to this mode of injury (Fig. 2A, 2C).
Figure 2.

Sca-1 and CD34 expression following depletion of Clara cell secretory protein (CCSP)-expressing cells. (A): Flow cytometry analysis of Sca-1 and CD34 expression on the surface of CD45neg CD31neg live cells isolated from untreated mice (top row) or CCSP-HSVtk transgenic mice recovered for 6 days post-GCV treatment (bottom row). Left column represents unstained samples, and right column represents Sca-1 CD34 dual-stained sample. (B): Overlay of the autofluorescence (AF) profile (unstained sample) of CD45neg CD31neg live cells isolated from untreated mice (purple contour) or CCSP-HSVtk transgenic mice recovered for 6 days post-GCV treatment (black contour), demonstrating the disappearance of the AFhigh population following ablation of CCSP-expressing airway epithelial cells. (C): Quantification of data in (A). The percentages of cells in each of the three gates (Sca-1pos CD34pos, Sca-1neg CD34pos, and Sca-1pos CD34neg) were compared between untreated (dark gray bars) and GCV-treated (light gray bars) samples. (D): Intracellular staining for CCSP of cells isolated from a wild-type animal showing the presence of CCSP-immunoreactive cells in the AFhigh population. The panels represent unstained sample (top left), no primary control (top right), CCSP-stained sample (bottom left), and overlay of the AF profiles of the CCSP-positive (red contour) and no primary control (black contour plot) samples (bottom right). Abbreviations: GCV, ganciclovir; PE, phycoerythrin; Pe, phycoerythrin.
Ganciclovir treatment resulted in loss of the AFhigh population (unstained sample in Fig. 2A, 2B), suggesting that some or all CCSP-expressing cells have the property of high autofluorescence. To further explore this possibility, isolated cells from wild-type animals were analyzed by flow cytometry for intracellular CCSP expression, and their autofluorescence characteristics were determined (Fig. 2D). Overlay of the autofluorescence profiles of the CCSP-immunoreactive population and the control sample with no primary antibody demonstrates that the majority of the CCSP-expressing cells belong to the AFhigh population. Importantly, a less abundant fraction of CCSP-expressing cells were localized to the AFlow population, suggesting that there are distinct subpopulations of CCSP-expressing cells in the airway epithelium. In conclusion, these data confirm that Sca-1 expression is not unique to the airway epithelium and that the AFhigh population contains a subpopulation of secretory cells. By inference, these data suggest that an abundant CCSP-expressing cell population with the characteristics of high autofluorescence accounts for a significant fraction of Sca-1low CD34neg cells identified within the CD45neg CD31neg live fraction of lung epithelial cells.
The Cell-Surface Phenotype of Lineage-Tagged Airway Epithelial Cells
To clarify the cellular origins of different populations of isolated lung cells, a lineage tracing mouse model was used. In these mice, tagging of secretory cells and their progeny was achieved by CCSP-Cre-mediated recombination of a floxed stop sequence knocked into the Rosa 26 locus (ROSA-LSL-eYFP). Excision of the stop sequence allowed expression of eYFP protein [13]. Analysis of eYPF expression within CCSP-Cre/ROSA-LSL-eYPF demonstrated a pattern of recombination that was airway-specific and accounted for approximately 90% of bronchiolar epithelial cells, as has been reported previously for other Cre substrates (supporting information Fig. 3; [12]). Lineage-tagged bronchiolar cells included both CCSP-expressing cells and ciliated cells, consistent with the known lineage relationship between these cell populations [2]. Flow cytometry analysis of isolated lung cells from CCSP-Cre ROSA-LSL-eYFP mice and analysis for autofluorescent characteristics identified two distinct populations of eYFPpos cells: one showing high autofluorescence and one showing low autofluorescence (Fig. 3A).
Figure 3.

Lineage tracing to identify cell types contributing to cell fractions defined by cell surface Sca-1 and CD34. (A): Flow cytometry analysis of the lineage tag (eYFP) in cells isolated from Clara cell secretory protein-Cre Rosa26-LSL-eYFP lungs. Left panel shows eYFP expression in the live CD45 CD31-negative population. Two distinct eYFPpos populations were identified (eYFPpos AFhi and eYFPpos AFlo). Overlay of the autofluorescence profiles of the two populations (eYFPpos AFhi, red contour; eYFPpos AFlo, green contour) demonstrates that they correspond to the AFhi and AFlo populations, respectively (right panel). (B): Sca-1 and CD34 expression among isolated, lineage-tagged airway epithelial cells. Left column, Sca-1 and CD34 staining; right column, eYFP analysis of cells in the indicated gates. Unstained, eYFP expression in an unstained sample; Sca-1 low versus high, eYFP expression in the Sca-1low population (R3, red contour) and the Sca-1high population (R4, green contour) in a Sca-1-stained sample; CD34-positive, eYFP expression in the CD34-positive population (R2, red contour) of a CD34-stained sample; Sca-1/CD34 dual-positive, eYFP expression in the Sca-1pos CD34pos population (R5, red contour). In all experiments dead and CD45pos CD31pos cells were excluded from the analysis. Abbreviations: AFhi, high autofluorescence; AFlo, low autofluorescence; PE, phycoerythrin.
To address the lineage relationship between the two populations of CD45neg CD31neg Sca-1pos cells, we analyzed eYFP expression in the Sca-1low and Sca-1high populations (Fig. 3B). The Sca-1low population (12.74% of the CD45negCD31neg live population) had 23.6% eYFPpos cells, whereas the Sca-1high population (1.85% of the CD45negCD31neg live population) had very few eYFPpos cells (6.97%). These data demonstrate that Sca-1low but not Sca-1high cells contain CCSP-expressing cells and their derivatives. Analysis of CD45neg CD31neg CD34pos cells indicated that the CD34pos population did not belong to the bronchiolar lineage, as the vast majority of CD34pos cells were eYFP-negative. Moreover, in the Sca-1 CD34-stained sample, the CD45neg CD31neg Sca-1pos CD34pos population lacked eYFPpos cells, demonstrating that cell surface staining for both Sca-1 and CD34 identified a population of cells independent of the bronchiolar lineage (Fig. 3B). Together these data demonstrate that expression of Sca-1 and CD34 is not a characteristic of airway epithelial cells but that low levels of cell surface Sca-1 define two populations of bronchiolar epithelial cells that can be segregated according to their autofluorescence characteristics.
Bronchiolar Stem Cells Are Defined by Their CD45neg CD31neg CD34neg Sca-1low Cell Surface Phenotype and Low Autofluorescence
The absence of definitive assays to identify bronchiolar stem cells in isolated cell preparations required the use of genetic models of stem cell expansion to verify their molecular phenotype. We have previously shown that stabilization of β-catenin in the airway epithelium late in lung development resulted in the appearance of supernumerary bronchiolar stem cells, based on the resistance of these cells to naphthalene, their proliferative potential, and expression of putative stem cell markers [12]. Histologically the lungs of CatnnbfloxE3/floxE3ΔE3 mice have significantly more CCSP/Pro-SPC dual-positive cells compared with wild-type lungs ([12]; Fig. 4A). Single-cell suspensions were prepared from lungs of wild-type and ΔE3 mice, and the properties of bronchiolar epithelial cells were interrogated by flow cytometry. No differences were observed between cell preparations isolated from wild-type and ΔE3 mice in the abundance of Sca-1pos CD34pos cells within the CD45neg CD31neg live fraction (Fig. 4B, 4C). These data are consistent with our earlier demonstration that cells of the bronchiolar lineage exhibit a Sca-1pos CD34neg phenotype (Fig. 3B). To confirm that airway epithelial cells are not found in the Sca-1pos CD34pos fraction but mostly in the Sca-1pos CD34neg fraction, cells were sorted according to their Sca-1 and CD34 expression profiles into four fractions: CD45neg CD31neg Sca-1pos CD34pos, CD45neg CD31neg Sca-1pos CD34neg, CD45neg CD31neg Sca-1neg CD34pos, and CD45neg CD31neg Sca-1neg CD34neg. Immunofluorescence analysis of epithelial markers CCSP and Pro-SPC revealed that the CD45neg CD31neg Sca-1pos CD34neg fraction had 57.74% CCSP Pro-SPC dual-positive cells in wild-type and 51.18% in ΔE3 fraction (Fig. 5B). Morphologically, CD45neg CD31neg Sca-1pos CD34neg cells isolated from ΔE3 mice were smaller and had a lower cytoplasmic/nucleus ratio compared with wild-type cells (Fig. 5A). This finding was consistent with our previous demonstration that potentiation of β-catenin in bronchiolar cells of ΔE3 mice results in cells that lack cytoplasmic organelles typical of the facultative TA (Clara) cells [12]. We noted that in both genotypes the majority of CCSP-positive cells were also Pro-SPC-positive. This unexpected finding was in contrast to the molecular phenotype of cells characterized in situ, in which only ΔE3 mice show expansion of the bronchiolar pool of CCSP/Pro-SPC dual-positive cells. The CD45neg CD31neg Sca-1pos CD34pos fraction contained a population of large cells that were positive only for Pro-SPC, suggesting that mature alveolar type II cells localized to this fraction. In conclusion, we show that Sca-1 and CD34 together failed to capture the increase in stem cell pool size in ΔE3 animals. This finding is consistent with our earlier analysis in which cells of the bronchiolar lineage were localized exclusively to the CD34neg fraction.
Figure 4.

The cell surface phenotype of WT and ΔE3 cells. (A): Immunofluorescence analysis of Pro-SPC (red) and CCSP (green) in lung section from WT (left column) and CCSP-Cre, CatnnbfloxE3/floxE3 (ΔE3) animals (right column). The top row shows Pro-SPC staining, and the bottom row represents merged images of Pro-SPC and CCSP staining (magnification, ×200). Insets represent higher-magnification images of the outlined areas. (B): Sca-1 and CD34 expression on the surface of cells isolated from WT (top row) and ΔE3 animals (bottom row). In left-to-right order, the columns represent unstained, Sca-1-stained, CD34-stained, and Sca-1- and CD34 dual-stained samples, respectively. Dead and CD45pos CD31pos cells were excluded from this analysis. (C): Quantification of data in (B), comparing the percentages of cells in each population of Sca-1/CD34 dual-stained samples from WT (gray bars) and ΔE3 (white bars) mice. Abbreviations: CCSP, Clara cell secretory protein; PE, phycoerythrin; Pro-SPC, pro-surfactant protein C; WT, wild-type.
Figure 5.

CCSP/Pro-SPC staining of cells sorted according to their Sca-1/CD34 cell surface expression. Cells in each of the Sca-1neg CD34pos, Sca-1neg CD34neg, Sca-1pos CD34neg, and Sca-1pos CD34pos populations were sorted. Cytospins from each fraction were stained with CCSP (green) and Pro-SPC. (A): Images (magnification, ×400) of CCSP/Pro-SPC-stained cytospins demonstrating the presence of CCSP Pro-SPC dual-positive cells in the Sca-1pos CD34neg fraction. (B): Cells in each fraction were counted and classified either as CCSP-positive (upper left graph), Pro-SPC-positive (upper right graph), or CCSP and Pro-SPC dual-positive (lower graph). The graphs represent data from three WT animals (black bars) and three ΔE3 animals (hatched bars). Abbreviations: CCSP, Clara cell secretory protein; Pro-SPC, pro-surfactant protein C; WT, wild-type.
Since autofluorescence characteristics of bronchiolar cells represented another parameter allowing their segregation, we sought to test whether inclusion of this parameter provided a tool for identification of stem cells. We speculated that autofluorescence might relate to the high metabolic activity of facultative TA (Clara) cells and, as such, represents a physical property that distinguishes Clara cells from bronchiolar stem cells. Comparison of the autofluorescence characteristics of the CD45neg CD31neg live cells isolated from wild-type and ΔE3 mice revealed a dramatic decrease in the size of the autofluorescent population recovered from ΔE3 mice (Fig. 4B, unstained sample; Fig. 6A). To determine whether the combination of positive and negative markers that we determined so far is instrumental in revealing the increase in stem cell numbers in the ΔE3 mice, we further interrogated cell surface expression of CD34 and Sca-1 as a function of autofluorescence levels within the CD45neg CD31neg live population (Fig. 6). Comparison of the Sca-1pos population between wild-type and ΔE3 cells demonstrated a slight increase in Sca-1pos cells in the ΔE3 genotype (Fig. 6B vs. 6E, 17.94% vs. 22.94% respectively). Exclusion of CD34pos cells and AFhigh cells allowed us to compare the CD45neg CD31neg CD34neg Sca-1low AFlow population between wild-type and ΔE3 genotypes (Fig. 6C, 6F). The ΔE3 CD45neg CD31neg CD34neg Sca-1low AFlow population was dramatically expanded over its wild-type counterpart (Fig. 2G, 18.04% vs. 9.2% of live CD45neg CD31neg, respectively; p = .0002), reflecting the stem cell expansion associated with stabilization of β-catenin. Since our previous analysis of this fraction of cells indicated that both epithelial and nonepithelial cell types are represented, the approximately twofold increase in cells localizing to this population underrepresented the actual change in stem cell pool size that accompanies potentiation of β-catenin in ΔE3 mice. These data strongly suggest that bronchiolar stem cells can be distinguished from the more abundant pool of facultative TA (Clara) cells on the basis of their low-autofluorescent characteristics and provide a strategy for their prospective fractionation and analysis.
Figure 6.
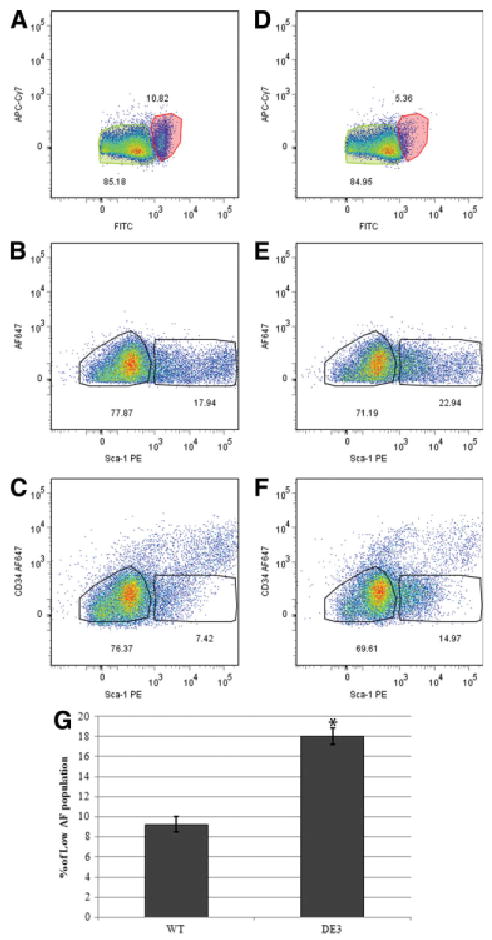
Expansion of the stem cell population in the ΔE3 mice results in an increase in the number of AFlow, Sca-1pos cells. Cells were isolated from either WT (A–C) or ΔE3 (D–F) mice and depleted of dead and CD45pos CD31pos cells, and Sca-1 expression was analyzed in the AFlow population (green shaded gate). (A, D): The AFhigh (red) and AFlow (green) populations of cells from WT and ΔE3 mice were identified in the FITC/APC-Cy7 channels. (B, E): Expression of Sca-1 in the AFlow population of Sca-1-stained samples was analyzed and compared between genotypes. (C, F): Sca-1pos CD34neg populations in the AFlow population of Sca-1 CD34-stained samples was compared between genotypes. (G): Quantification of data in (C) and (F). The number of cells in the Sca-1pos CD34neg population of six WT and six ΔE3 animals from three independent experiments show the enrichment in Sca-1-positive cells in the ΔE3 phenotype (p < .0005). Abbreviations: AF, autofluorescence; APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; WT, wild-type.
Discussion
We show that Sca-1, a previously described cell surface marker for the bronchioalveolar stem cell [10], is a common cell surface marker for a broad population of bronchiolar epithelial progenitor cells that include the abundant pool of facultative TA (Clara) cells and naphthalene-resistant bronchiolar stem cells. We found that bronchiolar progenitor cells could be distinguished from many other epithelial and mesenchymal cell types on the basis of their Sca-1low phenotype. Bronchiolar cells could be further subdivided on the basis of their AF characteristics: facultative TA (Clara) cells displayed an AFhigh phenotype, whereas bronchiolar stem cells exhibited an AFlow phenotype. This fractionation approach was validated using strategies involving targeted cell ablation, lineage tagging, and stem cell expansion and provides a robust set of criteria for further investigation of isolated bronchiolar stem cells at molecular and functional levels.
Our demonstration that bronchiolar stem cells can be enriched for within dissociated mouse lung preparations on the basis of their CD45neg CD31neg CD34neg Sca-llow AFlow phenotype differs from the previously described characteristics of isolated bronchioalveolar stem cells detailed by Kim et al. [10]. Differences between our findings are that in our study Sca-1 did not distinguish between subsets of CCSP-expressing cells (facultative TA vs. stem cells), that the epithelial Sca-1low fraction was a relatively abundant component of the total cell preparation, and that epithelial cells defined by a CCSP-Cre activated lineage tag were negative for CD34. The basis for these differences may be related to methods used for cell isolation and/or antibodies used to define cell surface phenotype. Lung cell preparations used for analysis by Kim et al. were generated using dispase/collagenase digestion coupled with methods optimized for the isolation of alveolar type II pneumocytes [10]. In contrast, lung cell preparations used herein were generated through use of Elastase and methods optimized for inclusion of a broad population of epithelial cells from the conducting airway and alveolus [15]. Importantly, bronchiolar stem cells from both bronchioalveolar duct junction and neuroepithelial body microenvironments in addition to the abundant pool of facultative TA (Clara) cells should be represented among dissociated cell preparations used in this study. This would not be the case using preparations that largely exclude epithelial cells from conducting airways. Furthermore, differences in cell surface CD34 reactivity observed herein and that of Kim et al. [10] remain to be determined. The possibility that absence of CD34 reactivity among lung epithelial cell types in this study results from conditions used for proteolytic dissociation of cells would seem unlikely, as this antigen is preserved on the surface of other nonepithelial cell types.
Stem cells are commonly thought to exhibit a less-differentiated character than their transit-amplifying progeny. Whereas this distinction is difficult to make for rapidly renewing tissues such as the epithelium of the small intestine, for which both populations appear to proliferate frequently and lack characteristics of specialized epithelial cells [17], it is more apparent within the progenitor cell hierarchy of the bronchiolar epithelium. We demonstrate that Clara cells, an abundant facultative TA cell type of bronchiolar airways, exhibit the distinguishing characteristic of high autofluorescence. This characteristic of Clara cells is supported through (a) loss of the AFhigh fraction of lung cells following ablation of CCSP-expressing cells (including Clara and stem cells) in GCV treated CCSP-HSVtk transgenic mice, and (b) loss of AFhigh cells following airway potentiation of β-catenin signaling. Autofluorescence is a well-known characteristic of the airway epithelium [18]. In human patients autofluorescence bronchoscopy is used as a screening tool for lung cancer [18–20]. In lung cancer, a patient's malignant cells, which are poorly differentiated in character, are detected as low-level autofluorescence fields within a high autofluorescent background of healthy epithelial cells [18–20]. Collectively, these data argue that stem cells can be distinguished from their more differentiated derivatives on the basis of autofluorescence characteristics and that this property is common between airway stem cells and tumor cells.
Bronchiolar epithelial cells observed in airways of mice following constitutive potentiation of β-catenin signaling lack differentiated features (cytoplasmic organelles and differentiation markers) typical of facultative TA (Clara) cells, are resistant to naphthalene injury, and show a CCSP/Pro-SPC dual-expressing phenotype, all characteristics of the bronchiolar stem cell [10, 12]. However, even though coexpression of CCSP and Pro-SPC has the potential to distinguish bronchiolar stem cells from more abundant facultative TA (Clara) cells in vivo, our data suggest that this may not be the case following enzymatic dissociation and sorting of lung cells. We find that all CCSP-immunoreactive cells present within the Sca-1low fraction (both AFhigh and AFlow populations) show a Pro-SPC-immunoreactive phenotype. These data suggest that Pro-SPC immunoreactivity does not distinguish between facultative TA (Clara) cells and bronchiolar stem cells following enzymatic dissociation of lung tissue and subsequent fractionation of isolated cells. This is consistent with our observation of a similar frequency of CCSP/Pro-SPC dual-positive cells between isolated wild-type and ΔE3 cell preparations. We conclude that use of CCSP/Pro-SPC dual positivity alone as a basis to investigate the impact of signaling pathways on isolated bronchiolar epithelial cells does not provide a basis for discrimination between rare bronchiolar stem cells and abundant facultative progenitor (Clara) cells [21–23]. We show that despite upregulation of Pro-SPC within mature Clara cells, their distinguishing morphological features coupled with unique high autofluorescence characteristics provide a basis for their separation from low-autofluorescent bronchiolar stem cells.
Standard methods in stem cell biology include in vivo and in vitro assays to demonstrate self-renewal and differentiation potential of the proposed stem cell population. Even though in vitro models have been developed allowing propagation of tracheobronchial epithelial cells [24], bronchiolar epithelial cells are extremely difficult to maintain and propagate in vitro. Moreover, no in vivo transplantation studies have been reported that allow faithful establishment of bronchiolar epithelium from fractionated preparations of bronchiolar cells. Fractionation methods allowing enrichment of bronchiolar stem cells described herein will allow further analysis of gene expression to define a unique molecular phenotype and will provide a basis upon which to build in vitro and transplantation models to assess mechanisms of self-renewal. Together with transgenic animal models for identification and manipulation of the stem cell compartment, these assays will provide critical tools to unravel the bronchiolar stem cell phenotype and mechanisms governing the behavior of these cells in normal and diseased lung.
Conclusion
Our data suggest that bronchiolar progenitor cells including stem cells and Clara cells are Sca1low, CD34neg, CD45neg, CD31neg, that Pro-SPC immunoreactivity is acquired by Clara cells during their isolation, and that bronchiolar stem cells can be distinguished from Clara cells based upon low versus high autofluorescence, respectively. Use of Sca1pos, CD45neg, CD31neg alone to enrich bronchiolar progenitor cells (both Clara and stem cells) yields CCSP/Pro-SPC dual positive epithelial cells that are contaminated by CD34pos cells of a Sca1high phenotype and not of the bronchiolar lineage.
Supplementary Material
Acknowledgments
We thank Lynda Guzik from the University of Pittsburgh for technical support with flow cytometry data acquisition and fluorescence-activated cell sorting (FACS). Flow cytometry was also performed in the Duke Human Vaccine Institute Research Flow Cytometry Core Facility, which is supported in part by NIH Grants AI051445 and AI058607. We thank Dawn Jones Marshall, Letealia M. Oliver, and Patrice M. McDermott for technical support with flow cytometry data acquisition and FACS at Duke University. Research costs associated with this publication were supported by NIH Grants HL064888 and HL090146.
Footnotes
Author contributions: R.M.T.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; E.L. and J.F.W.: collection and/or assembly of data, data analysis and interpretation; B.R.S.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
See www.StemCells.com for supporting information available online.
References
- 1.Evans MJ, Johnson LV, Stephens RJ, et al. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest. 1976;35:246–257. [PubMed] [Google Scholar]
- 2.Evans MJ, Cabral-Anderson LJ, Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest. 1978;38:648–653. [PubMed] [Google Scholar]
- 3.Randell SH. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3:718–725. doi: 10.1513/pats.200605-117SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stripp BR. Hierarchical organization of lung progenitor cells: Is there an adult lung tissue stem cell? Proc Am Thorac Soc. 2008;5:695–698. doi: 10.1513/pats.200801-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: Privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 6.Rawlins EL, Ostrowski LE, Randell SH, et al. Lung development and repair: Contribution of the ciliated lineage. Proc Natl Acad Sci U S A. 2007;104:410–417. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong KU, Reynolds SD, Giangreco A, et al. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 8.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchioalveolar duct junction. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stripp BR, Maxson K, Mera R, et al. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol. 1995;269:L791–L799. doi: 10.1152/ajplung.1995.269.6.L791. [DOI] [PubMed] [Google Scholar]
- 10.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds SD, Hong KU, Giangreco A, et al. Conditional clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:L1256–L1263. doi: 10.1152/ajplung.2000.278.6.L1256. [DOI] [PubMed] [Google Scholar]
- 12.Reynolds SD, Zemke AC, Giangreco A, et al. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells. 2008;26:1337–1346. doi: 10.1634/stemcells.2008-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 15.Chichester CH, Philpot RM, Weir AJ, et al. Characterization of the cytochrome P-450 monooxygenase system in nonciliated bronchiolar epithelial (Clara) cells isolated from mouse lung. Am J Respir Cell Mol Biol. 1991;4:179–186. doi: 10.1165/ajrcmb/4.2.179. [DOI] [PubMed] [Google Scholar]
- 16.Kotton DN, Summer RS, Sun X, et al. Stem cell antigen-1 expression in the pulmonary vascular endothelium. Am J Physiol Lung Cell Mol Physiol. 2003;284:L990–L996. doi: 10.1152/ajplung.00415.2002. [DOI] [PubMed] [Google Scholar]
- 17.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 18.Ikeda N, Hayashi A, Iwasaki K, et al. Comprehensive diagnostic bronchoscopy of central type early stage lung cancer. Lung Cancer. 2007;56:295–302. doi: 10.1016/j.lungcan.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Leonhard M. New incoherent autofluorescence/fluorescence system for early detection of lung cancer. Diagn Ther Endosc. 1999;5:71–75. doi: 10.1155/DTE.5.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam B, Wong MP, Fung SL, et al. The clinical value of autofluorescence bronchoscopy for the diagnosis of lung cancer. Eur Respir J. 2006;28:915–919. doi: 10.1183/09031936.06.00131405. [DOI] [PubMed] [Google Scholar]
- 21.Ventura JJ, Tenbaum S, Perdiguero E, et al. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39:750–758. doi: 10.1038/ng2037. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Goss AM, Cohen ED, et al. A Gata6-Wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y, Iwanaga K, Raso MG, et al. Phosphatidylinositol 3-kinase mediates bronchioalveolar stem cell expansion in mouse models of oncogenic K-ras-induced lung cancer. PLoS ONE. 2008;3:e2220. doi: 10.1371/journal.pone.0002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.You Y, Richer EJ, Huang T, et al. Growth and differentiation of mouse tracheal epithelial cells: Selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1315–L1321. doi: 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


