Abstract
Habituation, as described in the landmark paper by Thompson and Spencer (1966), is a form of simple, nonassociative learning in which the magnitude of the response to a specific stimulus decreases with repeated exposure to that stimulus. A variety of neuronal and behavioral responses have been shown to be subject to habituation based on the criteria presented in that paper. It has been known for several decades that the magnitude of hypothalamic-pituitary-adrenal (HPA) activation occurring in response to a stressor declines with repeated exposure to that same stressor. For some time this decline has been referred to as “habituation” in the stress neurobiology literature. However, how this usage compares to the definition proposed by Thompson and Spencer has not been systematically addressed. For this special issue, we review the stress neurobiology literature and examine the support available for considering declines in HPA response to repeated stress to be response habituation in the sense defined by Thompson and Spencer. We conclude that habituation of HPA activity meets many, but not all, important criteria for response habituation, supporting the use of this term within the context of repeated stress. However, we also propose that response habituation can, at best, only partially explain the phenomenon of HPA habituation which also involves well-known negative feedback mechanisms, activation of broad stress-related neural circuitry and potentially more complex associative learning mechanisms.
Introduction
The term “habituation” is understood by many in neuroscience to refer to any decrease in responsiveness to a repeated stimulus, a form of nonassociative learning. The simuli to which responses can habituate and the habituating responses themselves can both vary widely in complexity. Regardless of the paradigm, if the measured response demonstrates “decrement as a result of repeated stimulation” it may be an example of habituation as defined by Thompson and Spencer (1966) in their landmark review of the subject. To aid in distinguishing habituation from other, nonspecific declines in behavior, they noted a set of nine criteria common to various habituating responses, which could function as a kind of “operational definition” and be used to evaluate novel paradigms. These criteria were recently reaffirmed at a special workshop on habituation, the focus of Rankin et al. in this issue.
The term “habituation” in the field of stress neurobiology refers to the reduction in physiological responses elicited by an nth exposure to a repeated homotypic (same) stressor in comparison to the large responses elicited by acute exposure to that stressor. Habituation has been used in this sense in the stress neurobiology literature for several decades, with notably early use in the late 1970s (Hennessy & Levine, 1977; Pfister, 1979). The use of this term corresponded with the acknowledgement that reductions in stress responses to a repeated stressor could be habituation as defined by Thompson and colleagues and it came to supplant the more general term “adaptation” used in literature published at about the same time (Pfister & King, 1976). Subsequent literature directly tested some individual criteria of response habituation in one arm of the stress response, hypothalamic- pituitary-adrenal (HPA) activity, after repeated stress (De Boer, Koopmans, Slangen, & Van de Gugten, 1990; Natelson, Ottenweller, Cook, Pitman, McCarty, & Tapp, 1988; Pitman, Ottenweller, & Natelson, 1988). Despite these efforts, no discussion has systematically considered habituation of HPA activity or other stress responses in the light of all the criteria noted by Thompson and Spencer, leaving some question as to whether “habituation” is an appropriate term to describe the declines that occur with repeated stress exposure. For this special issue, we will examine the evidence available from the stress neurobiology literature to determine to what degree habituation of HPA activity to repeated stress meets Thompson and Spencer's criteria for response habituation. A number of stress-related endpoints habituate to repeated stress exposure such as struggling behavior (Grissom, Kerr and Bhatnagar, 2008) and sympathetic adrenomedullary activity (Costoli, Bartolomucci, Graiani, Stilli, Laviola, Sgoifo, 2004; Schommer, Hellhammer, & Kirschbaum, 2003). However, we will focus on the HPA axis as the primary physiological endpoint of the response to stress because of the wealth of literature on HPA responses to stress and because of the crucial role that this axis plays in an organism's response to stress, described in the next section.
The nine criteria identified by Thompson and Spencer as forming an operational definition of response habituation are:
1) Given that a particular stimulus elicits a response, repeated applications of that stimulus result in decreased response (habituation).
2) If the stimulus is withheld, the response tends to recover over time (spontaneous recovery).
3) If repeated series of habituation training and spontaneous recovery are given, habituation becomes successively more rapid (this might be called potentiation of habituation).
4) The more rapid the frequency of stimulation, the more rapid and/or more pronounced is habituation.
5) The weaker the stimulus, the more rapid and/or more pronounced is habituation. Strong stimuli may yield no significant habituation.
6) The effects of habituation training may proceed beyond the zero or asymptotic response level.
7) Habituation of a response to a given stimulus exhibits stimulus generalization to other stimuli.
8) Presentation of another (usually strong) stimulus results in recovery of the habituated response (dishabituation).
9) Upon repeated application of the dishabituatory stimulus, the amount of dishabituation produced habituates (this might be called habituation of dishabituation).
For the purposes of discussing how these nine criteria apply to habituation of HPA activity we havegrouped these criteria into 4 overall themes based on similarity. These themes are:
A) Habituation occurs to repeated stimuli. This theme encompasses both Criterion 1, the basic phenomenon of habituation, and Criterion 9, habituation of responses to dishabituating stimuli, as the latter can be viewed as a special case of the former (Thompson and Spencer 1966).
B) Habituation is reversible. Criterion 2, spontaneous recovery, and Criterion 8, dishabituation, are both examples of the reversibility of habituation under different circumstances.
C) Habituation can be improved by modifying certain parameters. Habituation is stronger in magnitude and/or more rapid when it is relearned following a period of spontaneous recovery, (Criterion 3), when stimuli come with increasing frequency (Criterion 4), and when stimuli are relatively weak (Criterion 5).
D) Habituation can progress beyond experimental expectations. Habituation can progress to responses that are lower than baseline (Criterion 6), and can generalize to stimuli other than the original habituating stimulus (Criterion 7).
We will discuss each theme in turn during which we will address the individual criteria for that theme. We will first provide a brief introduction to HPA axis activity under conditions of stress.
The HPA axis
Through complex and stress-specific neural circuitry, stressful stimuli drive the paraventricular nucleus of the hypothalamus (PVN) to release corticotrophin releasing factor (CRF) and other secretagogues, notably arginine vasopressin (AVP), into the hypophyseal portal circulation. These secretagogues work in concert to activate the anterior pituitary to release adrenocorticotrophic hormone (ACTH) into the bloodstream. ACTH, in turn, stimulates the adrenal cortex to release glucocorticoids, with corticosterone being the primary glucocorticoid in rodents and cortisol the primary glucocorticoid in primates (de Kloet, 2000). Increased glucocorticoid release in the bloodstream provides negative feedback at the level of the brain and pituitary to inhibit further HPA activation (Dallman, Akana, Cascio, Darlington, Jacobson, & Levin, 1987). Glucocorticoids also exert wide-ranging physiological effects, including increasing glucose availability in the bloodstream and suppressing immune system activity, in order to divert metabolic resources to escaping or challenging the stressor at hand (Dallman, 2007). It is because of these widespread physiological and behavioral effects, useful during a short-term threat but potentially deleterious with prolonged exposure, that regulation of glucocorticoid release is of critical biological significance to the organism.
It is important to note that in addition to stress-induced stimulation of HPA activity, the axis is also under circadian regulation, with peak levels of ACTH and glucocorticoid occurring slightly before the time an organism becomes active after a period of rest (morning for diurnal organisms, evening for nocturnal). HPA activity can also be increased by activities not typically considered stressful, such as food intake as part of a nonrestricted diet (Dallman et al., 1987). The regulation of HPA activity with regards to these mechanisms is qualitatively different from the stimulated activation caused by exposure to a stressor and thus these drives to the HPA axis are not included when discussing habituation below.
Stressors can vary in the degree to which they are psychologically stressful and physically stressful and most stressors studied in animals are a mixture of both. By studying stressors with primarily psychological qualities, also called processive stressors (for example, restraint or novel environment) versus those with primarily physical qualities, also called physiological or systemic stressors (for example, ether exposure or hypoglycemia) (Herman & Cullinan, 1997), it has become apparent that there are important differences captured by the distinction between psychological and physiological stressors. Psychological stress activates the PVN primarily via limbic pathways whereas physical stress can more rapidly activate the PVN via brainstem nuclei without significantly involving limbic brain circuitry (Emmert & Herman, 1999). This is crucial to our discussion as HPA activity habituates to predominantly processive stressors and not to systemic stressors that involve an immediate physical threat (see Theme C, Criterion 5). Because restraint is widely used in studies of habituation of HPA activity, we will use it as an example to illustrate the criteria discussed below.
Animals are able to produce strong HPA responses to unfamiliar stress, an important component of appropriate coping with potentially threatening changes in environment. In this context, the ability to habituate to repeated exposure to the same type of stressors has strong evolutionary utility as it conserves energy and resources by dampening responses to stressors that are not life-threatening (Nesse, Bhatnagar, & Young, 2006). In the extreme, a lack of habituation to repeated exposure to psychological stressors in humans may be related to stress-related disease such as major depression or PTSD (Golier, Schmeidler, Legge, & Yehuda, 2007; Simeon, Knutelska, Yehuda, Putnam, Schmeidler, & Smith, 2007; Thomson & Craighead, 2007; Yehuda, Teicher, Trestman, Levengood, & Siever, 1996), providing further evidence for the importance of understanding how and under what circumstances organisms habituate to repeated stress.
Theme A: Habituation occurs to repeated stimuli
The first criterion described by Thompson and Spencer (1966) outlines the basic phenomenon of habituation, and the last criterion can be viewed as a special case of the first. The criteria in other themes serve to distinguish habituation from other decrements in response and highlight parametric manipulations which affect whether habituation is observed and to what degree it is observed.
Criterion 1) Given that a particular stimulus elicits a response, repeated applications of the stimulus result in decreased response (habituation).
Applied to HPA responses to repeated stress, the first criterion hypothesizes that if a given stimulus elicits an HPA response, repeated exposure to that stimulus will elicit a progressively reduced response. For example, HPA activity to a first restraint should be higher than HPA activity to the nth restraint exposure. Habituation of HPA activity to repeated exposure to a number of different stressors has been observed in both animals and humans. For example, habituation is consistently observed in animals to such diverse stressors as restraint (Bhatnagar, Huber, Nowak, & Trotter, 2002; Cole, Kalman, Pace, Topczewski, Lowrey, & Spencer, 2000; Girotti, Pace, Gaylord, Rubin, Herman, & Spencer, 2006; Gomez, Houshyar, & Dallman, 2002; Grissom, Iyer, Vining, & Bhatnagar, 2007; Jaferi, Nowak, & Bhatnagar, 2003; Jaferi & Bhatnagar, 2006; Jaferi & Bhatnagar, 2007; Keim & Sigg, 1976; Lunga & Herbert, 2004; Ma, Lightman, & Aguilera, 1999; McQuade, Tamashiro, Wood, Herman, McEwen, Sakai, Zhang, & Xu, 2006; Natelson et al., 1988; Simpkiss & Devine, 2003; Stamp & Herbert, 1999), exposure to cold (Bhatnagar & Meaney, 1995; Kant, Bunnell, Mougey, Pennington, & Meyerhoff, 1983), novel environment (Bassett, Cairncross, & King, 1973; Johnson & Moberg, 1980; Muir & Pfister, 1987; Pfister, 1979), immobilization (Garcia, Marti, Valles, Dal-Zotto, & Armario, 2000; Giralt, Garcia-Marquez, & Armario, 1987; Hauger, Lorang, Irwin, & Aguilera, 1990), water immersion without swimming (De Boer et al., 1990), noise (Armario, Castellanos, & Balasch, 1984; Armario, Lopez-Calderon, Jolin, & Balasch, 1986; Borrell, Torrellas, Guaza, & Borrell, 1980; De Boer, Van der Gugten, & Slangen, 1989), handling (Dobrakovova & Jurcovicova, 1984; Dobrakovova, Kvetnansky, Oprsalova, & Jezova, 1993), and repeated ethanol injection (Spencer & McEwen, 1990). Habituation in humans has been demonstrated to repeated psychosocial stress (Gerra, Zaimovic, Mascetti, Gardini, Zambelli, Timpano, Raggi, & Brambilla, 2001; Gunnar, Connors, & Isensee, 1989; Kirschbaum, Prussner, Stone, Federenko, Gaab, Lintz, Schommer, & Hellhammer, 1995; Schommer et al., 2003; Wust, Federenko, van Rossum, Koper, & Hellhammer, 2005) and to repeated parachute jumps (Deinzer, Kirschbaum, Gresele, & Hellhammer, 1997). Furthermore, habituation of HPA activity seems to increase progressively with each exposure to stress (Deinzer et al., 1997; Gomez et al., 2002; Keim & Sigg, 1976; Pfister, 1979). It should be noted that the majority of the studies cited above were conducted in male rodents. Habituation of HPA activity in female rodents has been demonstrated (Lunga & Herbert, 2004; McQuade et al., 2006) but may not be consistently observed (Bhatnagar, Lee, Vining, 2005) due to the particularly rapid estrous cycle of rodent females which can exert additional influence on HPA activity. Overall, substantial evidence over the last three decades demonstrates habituation of HPA responses to repeated exposure to a variety of stressors.
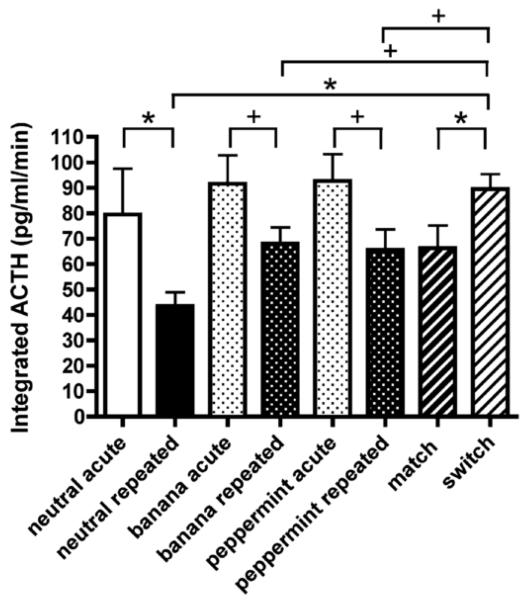
In the face of this overwhelming evidence that HPA activity habituates to repeated homotypic stress, there are occasional reports of the failure of HPA activity to habituate to repeated restraint (Marin, Cruz, & Planeta, 2007; Pitman et al., 1988) or repeated exposure to a novel environment (Hennessy, Levin, & Levine, 1977), for example. It is difficult to determine the exact cause of these discrepancies in each specific case, but there are certain variables that are known to enhance or diminish HPA habituation to a homotypic stressor. For example, habituation of HPA activity can vary based on individual differences in both animals and humans (Bhatnagar & Meaney, 1995; Wust et al., 2005; Bhatnagar et al,. 2005). Stressor predictability appears to be required (De Boer et al., 1989; Marti & Armario, 1997; Muir & Pfister, 1987) and stressor controllability seems potentially able to influence HPA habituation since it modulates HPA activation to acute stress (Misslin, Herzog, Koch, & Ropartz, 1982). Recently, we explicitly tested the role of context in habituation of HPA responses to repeated restraint by manipulating the odor animals were exposed to during repeated restraint (Grissom et al., 2007). For the first seven days, animals were restrained for 30 minutes per day while being exposed to either banana or peppermint odor. On day eight, HPA responses to 30 minutes of restraint were assessed during exposure to the same odor or the alternate odor. Animals who were exposed to the different odor during the 8th restraint exposure showed elevated HPA responses to the otherwise familiar restraint as compared to animals exposed to the matching odor (Figure 1). Therefore, a change in the context in which stress is experienced can diminish the magnitude of the habituated HPA response (discussed further below under Criterion 7). That manipulation of contextual variables and stressor predictability can affect HPA habituation is of experimental interest in its own regard and should be a focus for further investigation. Other variables that are not usually systematically manipulated can also modify habituation, including incidental noise and construction (Dallman, Akana, Bell, Bhatnagar, Choi, Chu, Gomez, Laugero, Soriano, & Viau 1999), exposure to a novel experimenter during repeated handling (Dobrakovova et al., 1993), and invasive manipulations such as surgery or repeated injections (Hodges & Mitchley, 1970; Kant et al., 1983). That such variables as context, predictability, and novel experimenters can have a significant impact on the habituation of HPA activity may be a result of the limited environment of the laboratory rodent. The relative lack of complexity of this environment may enhance the salience of these variables. The natural habitats of rodents provide much richer and more complex stimuli and a wider range of stress experiences. When repeatedly exposed to the same type of stress in their natural habitats or more complex environments, the impact of these variables is likely diminished.
Figure 1.
Habituation of HPA responses to repeated restraint is disrupted by exposure to an unfamiliar odor during restraint. Eight groups of animals were either unstressed or repeatedly restrained for 30min per day for 7 days prior to a test 30 minute restraint on day 8. Repeatedly restrained animals which were not exposed to a specific odor during prior restraint (other than the neutral odor of the housing room; “neutral repeated” group) exhibited significantly habituated integrated ACTH as compared to acutely restrained animals which were not exposed to a specific odor (“neutral acute” group), as expected. Repeatedly restrained animals exposed to only one of either banana odor (“banana repeated” group) or peppermint odor (“peppermint repeated” group) during all 8 days of prior restraint exposures exhibited significantly habituated HPA activity to the 8th restraint as compared to acutely restrained animals exposed to banana (“banana acute” group) or peppermint (“peppermint acute” group). Critically, repeatedly restrained animals exposed on day 8 to restraint with the same odor as the previous seven days (“match” group) had significantly lower HPA activity than repeatedly restrained animals exposed to the alternate odor during restraint on day 8 (“switch” group). This finding demonstrates the apparent context specificity of habituated HPA responses (see Criteria 1 and 7). * indicates p ≤ 0.05; + indicates p ≤ 0.09. Data are expressed as mean ± SEM. From Grissom et al., 2007, with permission.
Criterion 9) Upon repeated application of a dishabituatory stimulus, the amount of dishabituation produced habituates (this might be called habituation of dishabituation)
This criterion may be considered a special case of the overall concept of habituation, as it states that habituation of a response to one stimulus does not preclude habituation of that response to an additional, different stimulus, even if the latter stimulus was originally dishabituating. For example, this criterion predicts that in animals that have habituated to repeated cold and that then show a facilitated HPA response to novel restraint, further exposure to restraint should now also produce habituated HPA activity to the once-novel restraint. Although we know of no direct tests of this criterion with regards to the habituation of HPA activity, it seems intuitively likely that habituation of HPA activity to a formerly dishabituating stressor would be readily seen. For instance, as in the above example, novel restraint has been shown to produce dishabituated, or facilitated (Dallman & Jones, 1973) HPA responses in animals which have habituated to cold (Bhatnagar & Meaney, 1995; Kant et al., 1983; Bhatnagar and Dallman, 1998; see Criterion 8) and, as described for Criterion 1, animals readily habituate to repeated restraint. Therefore, though direct demonstration of this criterion is not available, the literature suggests that it is likely that this criterion would be supported if tested.
Theme B: Habituation is reversible
The two criteria included in this theme are arguably the most important for determining whether a given response decrement is habituation. Indeed, Thompson and Spencer noted that “habituation can usually be distinguished…because it is reversible.” (1966 p. 17) In their review, responses which habituate were found to recover, either spontaneously at some point in time after the cessation of the stimulus, or by being dishabituated by a novel stimulus.
Criterion 2) If the stimulus is withheld, the response tends to recover over time (spontaneous recovery)
Thompson and Spencer (1966) stated that spontaneous recovery of a response to the level seen at the start of an experiment had “become the most common method of demonstrating that a given response decrement is an example of habituation,” (p. 18) as opposed to nonspecific declines in response. However, they were careful to note that defining habituation based on spontaneous recovery alone would not be useful as a number of variables can greatly affect whether and when spontaneous recovery is observed.
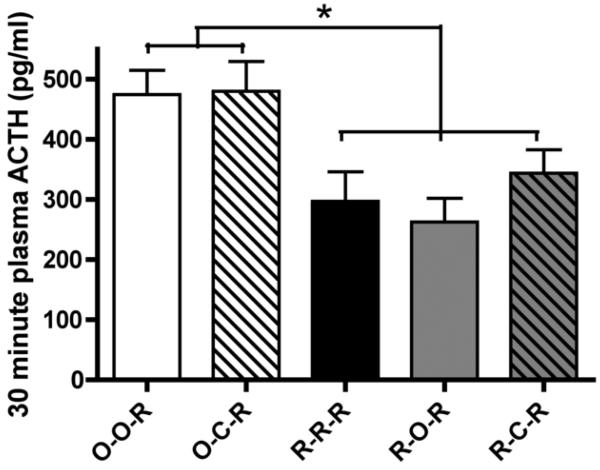
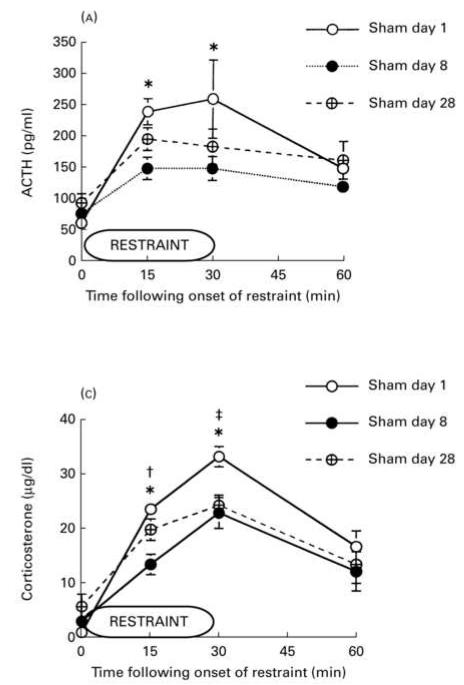
There are few studies examining HPA responses to the homotypic stressor at some point in time after the repeated stress has been terminated. One study found higher corticosterone responses at the end of three weeks of repeated stress than were seen upon re-exposure to the stressor after an additional three weeks of no treatment (Vogel & Jensh, 1988), indicating that three weeks without exposure to stress allowed a sort of “spontaneous reduction”, a finding that could be interpreted as the opposite of spontaneous recovery. Recently, we conducted an experiment designed to directly test some aspects of this and other criteria of habituation. Adult male rats were divided into 5 groups. Two groups functioned as acute restraint controls, one group had no treatment other than acute restraint on day 8 and the other group was exposed to acute 4°C cold for two hours on day 7 followed by acute restraint on day 8. In three other groups of rats, HPA responses to restraint were tested on day 8 after three patterns of repeated stress: repeated restraint on days 1-7, repeated restraint on days 1-6 with no treatment on day 7, or repeated restraint on days 1-6 with 2 hours of novel cold stress on day 7. In response to restraint on day 8, ACTH concentrations were similar in rats exposed to 7 previous days of restraint compared to repeatedly restrained rats that had no stress exposure on day 7 (Figure 2). We conclude that no recovery from habituation was seen in repeatedly restrained animals that skipped restraint on day 7 as compared to those who were restrained on day 7 (see also Criterion 8). In another study, adult male rats were restrained for 8 days, left undisturbed for 20 days, and restrained again on day 28. Peak ACTH (15 and 30 minutes into restraint) and corticosterone (30 minutes into restraint) concentrations on day 28 were either significantly lower than day one (acute) HPA activity or not different from either the acute (day one) responses and the habituated (day 8) responses (Bhatnagar et al., 2002; Figure 3). These results indicate that reductions in HPA activity are at least partially maintained to repeated restraint three weeks after the last stress. However, it may be the case that complete spontaneous recovery of HPA activity to a familiar stressor requires a long recovery period (see Criterion 6, Theme D). Therefore, the current findings, at best, provide limited support for spontaneous recovery of habituated HPA activity after a period without stress.
Figure 2.
Five groups of adult male rats were treated as follows. Two groups were acutely restrained on day 8; one of these groups received no treatment prior to restraint on day 8 (O-O-R) and one group was only exposed to 4°C cold for 2 hours on day 7 followed by acute restraint on day 8 (O-C-R). Three other groups of rats were repeatedly restrained. One group received daily restraint on days 1-8 (R-R-R). One group tested the effect of missing one day of restraint on day 7 on the maintenance of habituated HPA responses on day 8 (R-O-R). The last group tested the effect of a novel, dishabituating stressor (novel cold stress) on day 7 on habituation to restraint on day 8 (R-C-R). Overall, the two groups receiving acute restraint displayed similar levels of HPA activity in response to restraint. Importantly, the three groups that received repeated restraint demonstrated significantly habituated ACTH levels at the end of 30 minute restraint on day 8, regardless of treatment on day 7. Therefore, withholding restraint for one day did not result in spontaneous recovery of the habituated HPA response (see Criterion 2) and exposure to novel, presumably dishabituating cold stress did not alter the habituated response to familiar restraint (see Criterion 8). * indicates significantly different at p ≤ 0.05. Data are expressed as mean + SEM.
Figure 3.
Habituation of HPA activity to repeated restraint does not fully recover after three weeks without restraint. As part of a larger experiment investigating the role of the paraventricular thalamus on the production of habituated responses to HPA activity, the maintenance of habituation in sham-lesioned animals was tested three weeks after the last restraint exposure. Animals were sampled during days 1 and 8 of 8 daily restraints, then were undisturbed from day 9 until day 27. On day 28, all rats were once again restrained and sampled. Overall, animals demonstrated significant habituation by day 8 of restraint as compared to day 1. When animals were re-restrained on day 28, ACTH concentrations during restraint on day 28 were either significantly lower than day 1 responses nor significantly higher than day 8 responses, indicating neither significant habituation nor significant recovery. Corticosterone concentrations during restraint on day 28 were significantly different from day 8 values at 15 minutes and significantly different than day 1 values at 30 minutes. Overall, these results do not demonstrate clear spontaneous recovery of a previously habituated HPA response (Criterion 2). * p ≤ 0.05; day 1 significantly different from day 8. ‡ p ≤ 0.05; day 1 significantly different from day 28. † p ≤ 0.05; day 8 significantly different from day 28. Data are expressed as mean ± SEM. Adapted with permission from Bhatnagar et al., 2002.
As mentioned above, Thompson and Spencer (1966) noted that the length of time necessary to observe spontaneous recovery was extremely variable even for responses that were known to recover. Thus, although the reversibility of habituation to a particular stimulus is crucial to distinguishing it from nonspecific decrements in response, spontaneous recovery is not in itself sufficiently reliable to establish habituation. We believe that the overall lack of evidence supporting spontaneous recovery of HPA activity taken by itself cannot speak strongly for or against the idea of HPA habituation as anexample of response habituation. Therefore, Criterion 8 is a critical test of whether the habituation of HPA activity is an example of response habituation.
Criterion 8: Presentation of another (usually strong) stimulus results in recovery of the habituated response (dishabituation)
In contrast to the reversal of habituation due to spontaneous recovery, which is by definition spontaneously generated and as a result difficult to reliably observe, the reversal of habituation via the application of a novel, dishabituating stimulus should be subject to reliable provocation by the experimenter. As such, the demonstration of dishabituation with a novel stimulus may be one of the most important criteria to meet in order to argue that habituation of HPA activity is an example of response habituation.
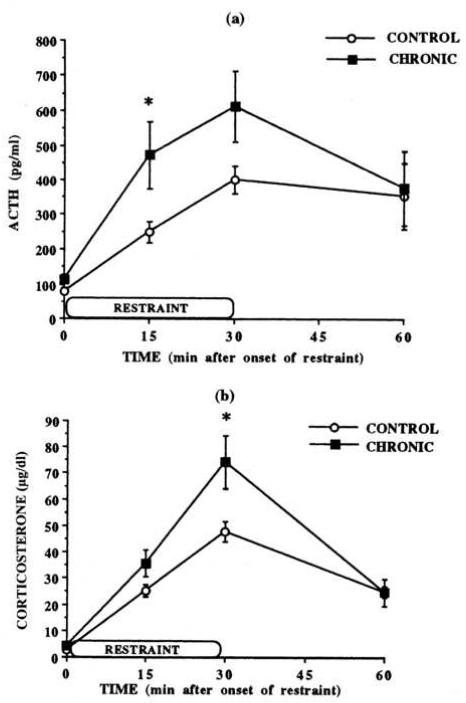
The criterion of dishabituation as defined by Thompson and Spencer requires that a formerly habituated response be returned to at least baseline response levels by a novel stimulus. Thus, dishabituated HPA activity in a previously habituated animal must result in ACTH and/or corticosterone levels that either meet or exceed the HPA activity in a naïve animal exposed to the dishabituating stressor. We believe that the phenomenon of dishabituation is akin to the phenomenon of “facilitation” in the stress literature, first described by Dallman and Jones (1973). Repeated intermittent exposure to a cold environment, repeated social stress, or repeated forced swim all lead to facilitated HPA responses to the novel stress of restraint (Akana, Hanson, Horsley, Strack, Bhatnagar, Bradbury, Milligan, & Dallman, 1996; Bhatnagar & Meaney, 1995; Bhatnagar & Dallman, 1998; Bhatnagar & Vining, 2003; Grissom, Kerr, & Bhatnagar, 2008; see Figure 4). In Figure 4 we include an example of facilitated HPA responses from the stress literature. As compared to naïve animals exposed to restraint stress (control), animals that have previously been exposed to 7 days of exposure to a cold environment produce significantly elevated concentrations of ACTH and corticosterone (chronic). While reduction of HPA responses to the cold environment was not specifically tested in this study, it has been previously found to occur (Bhatnagar & Meaney, 1995; see Criterion 1), indicating the elevated response to restraint seen in Figure 4 is in the context of previous habituation to repeated intermittent cold stress. However, we note the caveat that the above experiments do not explicitly demonstrate habituation prior to testing the facilitating effect of a novel stressor on HPA activity. In fact, prior habituation of HPA activity to the repeated stressor is not required to produce facilitated responses to a novel stressor, as in the example of HPA facilitation after repeated forced swim (Grissom, Kerr, & Bhatnagar, 2008) to which HPA activity does not habituate (Dal-Zotto, Marti, & Armario, 2000). This caveat makes it impossible to argue definitively that previously reported examples of HPA facilitation are true examples of dishabituation in the sense indicated by Thompson and Spencer. Overall, the phenomenon of facilitation of HPA activity to novel stress after prior stress exposure is well established, and we believe in some cases it can be considered a cognate of dishabituation as described by Thompson and Spencer.
Figure 4.
HPA responses are facilitated by exposure to a novel stressor after repeated prior stress. HPA responses have been previously shown to habituate to repeated cold stress (see Criterion 1). When acute restraint is adminstered on day 8 after 7 daily exposures to 4°C environment for 4 hours (CHRONIC), marked elevations in HPA activity are seen above the level seen in naïve animals (CONTROL) exposed to acute restraint. Therefore, HPA responses are dishabituated or facilitated to novel stress in habituated animals (see Criterion 8). * indicates significantly different at p ≤ 0.05. Data are expressed as mean ± SEM. From Bhatnagar and Dallman, 1998, with permission.
On a related note, Thompson and colleagues demonstrated the interesting finding that habituation is maintained in the face of dishabituation by a novel stimulus. Habituation a separate phenomena from dishabituation, in that habituation is not erased by dishabituation, but merely temporarily overridden (Groves & Thompson, 1970), indicating that mere exposure to a dishabituating stimulus is not sufficient to eliminate the habituated response. Recent evidence indicates that habituation of HPA activity develops prior to the development of the facilitating response (Weinberg & Spencer, personal communication), indicating that HPA habituation and facilitation operate as separate phenomena. We have recently tested the effect of a novel, presumably dishabituating stimulus on HPA activity to a repeated stressor. As described above (see Criteria 2), three groups of animals were repeatedly restrained on days 1-6, and either received no treatment, restraint, or novel cold on day 7 prior to a test restraint on day 8. Those animals who received novel cold on day 7, a presumably dishabituating stressor (although unfortunately this was not directly tested), displayed habituated HPA activity when re-exposed to restraint on day 8 (unpublished observations, Figure 2). This result indicates that habituation of HPA responses to familiar stress is maintained in the face of facilitation by novel stress, paralleling Thompson and colleagues earlier work demonstrating the maintenance of habituated responses despite dishabituation, and providing further evidence to support Criterion 8 with regards to HPA activity.
Theme C: Habituation can be enhanced by modifying certain parameters
As habituation is the reduction of response to a repeated stimulus, it is expected that manipulating variables having to do with the repeated stimulus might modify habituation, either in magnitude or in speed of development, or both. These variables include the number of habituation trials, the frequency of trials, and the severity of the habituating stimulus.
Criterion 3: If repeated series of habituation training and spontaneous recovery are given, habituation becomes successively more rapid (this might be called potentiation of habituation)
The third criterion as defined by Thompson and Spencer, potentiation of habituation, is the enhancement of habituation to a test stimulus after repeated bouts of habituation training followed by spontaneous recovery. This phenomenon requires repeated habituation training interspersed by periods of nondisturbance to allow for spontaneous recovery. A potential design to test this criterion with regards to stress is as follows: repeated restraint followed by a period of no treatment, followed by repeated restraint, and testing the rate of HPA habituation between the first set of repeated restraint exposures and the second. We presume that it is necessary to confirm spontaneous recovery of the response prior to beginning the next period of habituation training. If so, the lack of dependable observance of spontaneous recovery might render experimental demonstration of this criterion problematic – in the above example, it would be necessary to show fully recovered HPA activity prior to beginning the second set of restraint exposures, a result that has yet to be demonstrated empirically. Overall, there is no evidence to support this criterion in the stress literature.
Criterion 4: The more rapid the frequency of stimulation, the more rapid and/or more pronounced is habituation.
Thompson and Spencer noted that frequency can be defined both in terms of real time and in terms of number of stimuli delivered. In other words, habituation should be enhanced both by stimulating an absolute number of times more rapidly (for instance 8 restraints over 4 days versus over 8 days) or by stimulating more frequently within a set period of time (for instance 16 restraints versus 8 restraints over 8 days). Increasing frequency of stress in either way enhances habituation of HPA activity to repeated stress. Animals exposed 5 times to water-restraint habituated more fully when the 5 exposures were given over the course of 24 hours as compared to 72 hours (De Boer et al., 1990). In another study, rats restrained over a period of 15-16 days were exposed to a number of different rates of stress which lead to “dose-dependent” reductions in corticosterone levels elicited by a test restraint at the end of the experiment (Ma and Lightman, 1998; Figure 5). The greatest elevation over basal corticosterone levels was seen in acutely restrained rats, followed by levels seen in animals previously restrained once every seven days (a total of two restraints prior to day 15), followed by rats restrained once every three days (a total of 5 restraints prior to day 16), followed by restraint every other day (totaling 7 restraints prior to day 15). Daily restraint led to no significant elevation in corticosterone over basal levels during the test restraint (Figure 5). Overall, these studies show that both rapidity of presentation and absolute number of stress presentations can alter HPA activity, supporting this criterion with regards to HPA habituation.
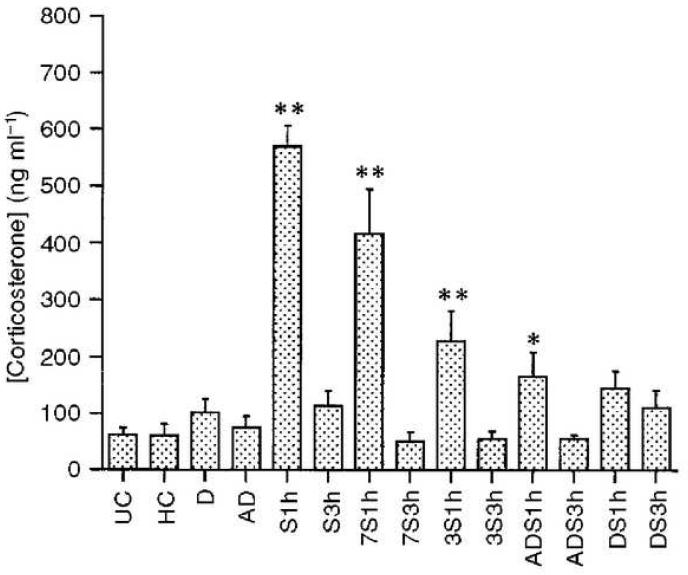
Figure 5.
HPA responses become more habituated with increasing numbers of stress exposures within a given period of time (Criterion 4). HPA responses were examined in a total of 14 groups of rats. Four groups were sampled under unstressed, basal conditions on day 15 after 14 prior days of either no treatment (UC, unhandled control), daily handling (HC), alternate day restraint (AD), or daily restraint (D). These groups, displayed on the far left, did not show any significant difference from the UC group, which was used as the primary control group. The remaining ten groups were restrained on day 15 or 16 and sampled either at the end of 1 hour restraint (1h groups) or 2 hours after the end of one hour restraint (3h groups) at which time responses for all groups had all returned to baseline (as indicated by no significant differences seen between any 3h group and UC). The largest significant elevations in corticosterone concentrations over UC animals were seen in naïve animals acutely restrained on day 15 (S1h). The next largest elevations were seen in animals exposed to restraint once every 7 days prior to restraint on day 15 (7S1h). The next largest elevations were seen in animals restrained once every three days prior to restraint on day 16 (3S1h). A slight, but significant elevation in corticosterone concentrations was seen in animals restrained every other day prior to restraint on day 15 (ADS1h) and no significant elevation in corticosterone over baseline was seen in animals restrained daily (DS1h). Thus, the most frequent presentation of restraint, daily restraint, produced the strongest habituation and habituation was weakened with less frequent stress exposure. This pattern of results supports Criterion 4, which states that habituation should be enhanced by stimulating more rapidly over a given period of time. ** p ≤ 0.01, as compared to UC; * p ≤ 0.05 as compared to UC. Data are expressed as mean + SEM. From Ma and Lightman, 1998, with permission.
Criterion 5: The weaker the stimulus, the more rapid and/or more pronounced is habituation. Strong stimuli may yield no significant habituation.
Noted in the original discussion of habituation was the observation that habituation was, in general, more rapid or more pronounced to weaker stimuli than to stronger stimuli. Thompson and Spencer noted that this pattern was characteristic of most habituating responses. In the stress literature, support has been found for this phenomenon despite the problematic aspects of attempting to compare the strength or severity of different stress modalities. First, as mentioned during the earlier discussion of the HPA axis under conditions of stress, there is a distinction between more processive stressors, which generally produce habituation, and more systemic stressors, which generally do not produce habituation upon repeated exposure. Stressors that fall clearly into the latter category include hemorrhage (Smith, Lovelock, Owens, Chan, & Falconer, 1988), hypoglycemia (De Goeij, Binnekade, & Tilders, 1992), water deprivation (Arnhold, Wotus, & Engeland, 2007) forced running (Kant et al., 1983), and forced swimming (Dal-Zotto, Marti, & Armario, 2000). These might be considered some of the strongest stimuli to the HPA axis. Highlighting the distinction between more processive and more systemic stressors, HPA activity has been shown to habituate to repeated water immersion+restraint (see Criteria 1 and 4), during which swimming is unnecessary and impossible because the animal is restrained, but does not habituate to forced swimming (Dal-Zotto, Marti, & Armario, 2000) during which constant physical activity, and therefore increased glucose availability provided by glucocorticoid release, is required to survive.
Within the set of stressors that do produce habituation of HPA activity, habituation appears in general to be more rapid to weaker stimuli than to stronger stimuli. A nice demonstration of this criterion using a semi-parametric approach compared prone restraint to supine restraint, the latter considered more stressful as evidenced by increased gastrointestinal pathology in rats given supine restraint. More rapid habituation in corticosterone activity was observed to prone restraint than to supine restraint although habituation eventually occurred in both groups (Natelson et al., 1988). Habituation of HPA activity is frequently observed to repeated restraint (see Criterion 1) in which an animal is confined in a tube or similar container allowing slight movement, but is less rapid and complete during the more severe stress of repeated immobilization in which an animal may be completely restricted in movement or have individual limbs restrained (Garcia et al., 2000; Giralt et al., 1987; Hauger et al., 1990; Vogel & Jensh, 1988). Overall, current evidence suggests that HPA activity more readily habituates to milder stressors than to more severe stressors.
Theme D: Habituation can progress beyond experimental expectations
While examples of habituation should conform to all of the criteria above, Thompson and Spencer also noted examples of habituation occurring to parameters beyond those expected by the experiment. These include the reduction of habituated responses below the original baseline (Criterion 6) and the generalization of the habituated response to stimuli other than the one used to originally generate habituation (Criterion 7).
Criterion 6: The effects of habituation training may proceed beyond the zero or asymptotic response level.
This criterion appears to be the most difficult to test for HPA activity. The simplest test would be to expose an animal to repeated stress, restraint for example, and continue sampling until HPA responses to restraint were lower than HPA responses in naïve nonstressed controls. However, as was discussed above, the HPA axis is stimulated not only by stressors but is under circadian regulation. During the circadian trough, when stress studies are typically carried out (see Bradbury, Cascio, Scribner, & Dallman, 1991), ACTH and corticosterone are constitutively secreted at low levels in unstressed animals (Dallman et al., 1987). At the circadian peak HPA activity is elevated, theoretically permitting reductions below baseline, but even in this case it seems unlikely that any stimulus to HPA activity could provoke ACTH and corticosterone levels to be lower than what is required by the overwhelming circadian drive. Thus the simplest method of testing this criterion, by observing HPA habituation to lower than basal levels, would probably be difficult to detect in intact animals at the circadian trough and/or may not be physiologically possible at any time.
However, Thompson and Spencer also proposed this criterion to be an extension of the idea that more stimulus presentations lead to more complete habituation (Criterion 4). Therefore, Criterion 6 also implies that if stimulation is continued beyond the point of stable habituation, slower recovery from the habituated response would ensue when the stimulation was eventually ended. The design of a potential experiment to test this interpretation could be the following: repeated restraint exposure for a period of time longer than what is necessary to produce no significant response to restraint followed by demonstration of delayed spontaneous recovery in these animals. However, this design is predicated on finding spontaneous recovery of HPA activity, which has not been clearly demonstrated (see Criterion 2). It is possible that in the few studies that assessed spontaneous recovery, repeated stress had perhaps gone over the point of stable habituation as described by Criterion 6, accounting for the lack of spontaneous recovery observed. For example, perhaps in Figure 3 (Bhatnagar et al., 2002), 8 repeated restraints might have been more than was necessary to produce stable habituation, thereby leading to a lack of spontaneous recovery three weeks later. In this case, Criterion 6 predicts that repeated exposure to fewer restraints (3 or 5 restraints as opposed to the 8 used in this paradigm) would allow spontaneous recovery three weeks later. We find this possibility unlikely, however. In this experiment animals still produced ACTH and corticosterone responses to an 8th restraint that were significantly greater than baseline (Figure 3) suggesting that complete habituation had not occurred. Therefore, the lack of spontaneous recovery in this experiment does not seem to be due to stress administration that had continued past the point of stable habituation. Indeed, other studies indicate that greater than 8 exposures to restraint are required for complete habituation (Ma and Lightman, 1998; Figure 5). Nevertheless, looking for spontaneous recovery after fewer stress exposures could be tested to address both this criterion and the idea of spontaneous recovery itself. At this time, however, there is no evidence that this criterion is supported.
Criterion 7: Habituation of a response to a given stimulus exhibits stimulus generalization to other stimuli
We believe that in order to fulfill the criterion of stimulus generalization, a study would have to demonstrate habituated levels of HPA activity to a first exposure to a stressor that is similar, though not identical, to the original habituating stressor. An experiment to test this criterion would have to identify two stressors that did not differ significantly in modality while still being somehow different, not an easy task. Our experiment looking at the effect of contextual stimuli on habituation to repeated restraint (see Criterion 1) may be one way of addressing this criterion. As mentioned above, after exposure to repeated restraint with one of two odors, exposure to a matching scent during a test restraint led to lower ACTH and corticosterone levels compared to those found in animals exposed to the alternate (“switch”) scent during the test restraint (Grissom et al., 2007; see Figure 1). This finding is not predicted by stimulus generalization, which would predict equivalent HPA activity in both groups, and so does not support this criterion. Other than this finding, the preponderance of literature indicates that exposure to any heterotypic stress after HPA habituation to a homotypic stressor does not elicit cross-stressor habituation but instead facilitation of HPA activity (Armario, Hidalgo, & Giralt, 1988; see Criterion 8). In these cases the heterotypic stressors differed widely in modality from the habituated stressor. A potentially interesting way of more specifically testing this criterion would be to make use of the differences between prone and supine restraint found by Natelson and colleagues (Natelson et al., 1988). Animals could be exposed to repeated prone restraint until habituation was observed, at which point HPA responses to supine restraint could be obtained and compared to naïve animals exposed to acute supine restraint. If responses to supine restraint were lower in animals habituated to prone restraint, support would be lent to Criterion 7; if not, it would provide further evidence that this criterion is not supported with regards to HPA habituation.
Unfortunately, it is unclear what characteristics make stimuli similar enough to each other to lead to generalization of habituation and what characteristics make stimuli sufficiently distinct from one another to elicit dishabituation/facilitation. This makes it difficult to determine whether the appropriate experiments have yet been conducted to test for the existence of stimulus generalization in repeated stress paradigms. Overall, this criterion is not supported with regards to HPA habituation but may not have been appropriately tested.
Discussion and conclusions
It is important to identify the neural mechanisms involved in changes in HPA activity to repeated stress for a number of reasons. Stress-induced HPA activation is a metabolically costly response system, with potentially deleterious effects if overactive. Therefore, it is adaptive for an organism to reduce HPA activity to a stressor that is not inherently harmful (Dallman et al., 1987; McEwen, 2004; Nesse et al., 2007). HPA responses are disrupted in persons suffering from psychopathology including major depression and post traumatic stress disorder (Golier et al., 2007; Simeon et al., 2007; Thomson & Craighead, 2007; Yehuda et al., 1996) and better understanding of the mechanisms that regulate HPA activity could likely aid in understanding the etiology and treatment of these and other disorders.
The process of habituation to repeated stress is, in part, regulated by corticosterone negative feedback mechanisms. Blockade of mineralocorticoid and/or glucocorticoid receptors systemically or centrally can block habituation of HPA activity (Cole et al., 2000; Dallman et al., 1987; Jaferi et al., 2003; Jaferi & Bhatnagar, 2006). However, negative feedback mechanisms cannot fully account for the phenomenon of HPA habituation because adrenalectomized animals, which lack stress-induced negative feedback produced by glucocorticoids, are able to habituate (Jaferi & Bhatnagar, 2006) and intact animals produce a facilitated response to a novel stressor in the face of increased negative feedback after repeated homotypic stress (see Criterion 8).
One possible explanation is that habituation of HPA activity is an example of response habituation that occurs with repeated presentation of a stimulus as described by Thompson and Spencer (De Boer et al., 1990; Natelson et al., 1988; Pitman et al., 1988). In this review we have attempted to assess the support for this hypothesis in the stress neurobiology literature using the original criteria forwarded by Thompson and Spencer (1966) as a “functional definition” of response habituation. Overall, a good deal of literature supports HPA habituation as an example of response habituation. In particular, Criteria 1, 4, 5, and 8 are supported, and Criterion 9 seems likely to be supported although it has not been directly tested. These criteria describe the well-documented phenomenon of habituation itself, its ability to be enhanced by increased frequency or number of presentations, variations in its induction related to the strength of stimulus, and its ability to be dishabituated by a novel stimulus. In terms of the themes that we elected to sort the criteria, support is found in general (i.e., for one or more criteria) for Themes A, B, and C. That is, HPA responses habituate (A), and this habituation can be reversed (B) and enhanced (C).
However, four remaining criteria are not supported. This is due largely to the lack of demonstration of spontaneous recovery seen in habituated HPA responses that is necessary to fulfill not only Criterion 2, but to test Criteria 3 and 6 which hinge on testing the rapidity of habituation after spontaneous recovery (3) or the rapidity of spontaneous recovery itself (6). The only criterion not requiring spontaneous recovery that has not been supported is Criterion 7, the generalization of habituation. The lack of support for Criteria 6 and 7 means there is no evidence supporting Theme D which encompasses the idea of habituation extending beyond the original baseline (6) or beyond the original habituating stimulus (7). Based on discussions regarding Theme D, habituation of HPA activity to repeated stress appears at this time to stay confined within the stressor modality that produced the original habituation. The lack of support for these criteria suggests that although mechanisms of response habituation may play an important role in the habituation of HPA activity, they cannot fully explain the phenomenon.
It is possible that changes in HPA activity after stress, in addition to hinging on mechanisms of negative feedback and response habituation, might be related to more complex learning mechanisms (see also Armario, 2006; Arnhold et al., 2007). Several pieces of evidence support this idea. Habituation of HPA activity can be disrupted by a change in context, suggesting contextual learning (as discussed earlier; Grissom et al., 2007). HPA activity in stressful environments is modified by conditioned inhibitors (Campeau, Falls, Cullinan, Helmreich, Davis, & Watson, 1997) and activated by a conditioned stimulus (Levine, Smotherman, & Hennessy, 1977). Furthermore, HPA activity has been shown to be reduced to only the second exposure to a stressor despite a long interval of time between the first and second exposures, suggesting some sort of memory trace produced by prior stress which can influence subsequent HPA activity (Armario, Valles, Dal-Zotto, Marquez, & Belda, 2004; Vogel & Jensh, 1988). Finally, habituation of HPA activity involves activation of distributed limbic circuitry (Herman, Figueiredo, Muller, Ulrich-Lai, Ostrander, Choi, and Cullinan, 2003) that overlaps with circuitry important for associative learning (Hunt, Fanselow, Richardson, Mauk, Freeman, & Stanton, 2007). Though direct tests of whether habituation of HPA activity relies on associative learning will be challenging to design, this idea must be addressed for a full understanding of adaptation of physiological and behavioral responses to repeated stress.
In sum, current evidence indicates that “habituation” of HPA activity is more complicated than its name implies. The adaptive reduction of responses to repeated stress appears to involve complex interactions between negative feedback mechanisms induced by repeated stress-induced release of glucocorticoids, response habituation mechanisms produced by repeated exposure to the stress stimulus, and possibly more complex learning and memory encoding information regarding previous stress exposures. A full understanding of habituation to repeated stress will likely depend on understanding the relationships between all of these mechanisms.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akana SF, Hanson ES, Horsley CJ, Strack AM, Bhatnagar S, Bradbury MJ, Milligan ED, Dallman MF. Clamped corticosterone (B) reveals the effect of endogenous B on both facilitated responsivity to acute restraint and metabolic responses to chronic stress. Stress (Amsterdam, Netherlands) 1996;1(1):33–49. doi: 10.3109/10253899609001094. [DOI] [PubMed] [Google Scholar]
- Akana SF, Shinsako J, Dallman MF. Relationships among adrenal weight, corticosterone, and stimulated adrenocorticotropin levels in rats. Endocrinology. 1983;113(6):2226–2231. doi: 10.1210/endo-113-6-2226. [DOI] [PubMed] [Google Scholar]
- Armario A. The hypothalamic-pituitary-adrenal axis: What can it tell us about stressors? CNS & Neurological Disorders Drug Targets. 2006;5(5):485–501. doi: 10.2174/187152706778559336. [DOI] [PubMed] [Google Scholar]
- Armario A, Castellanos JM, Balasch J. Adaptation of anterior pituitary hormones to chronic noise stress in male rats. Behavioral and Neural Biology. 1984;41(1):71–76. doi: 10.1016/s0163-1047(84)90745-3. [DOI] [PubMed] [Google Scholar]
- Armario A, Hidalgo J, Giralt M. Evidence that the pituitary-adrenal axis does not cross-adapt to stressors: Comparison to other physiological variables. Neuroendocrinology. 1988;47(3):263–267. doi: 10.1159/000124921. [DOI] [PubMed] [Google Scholar]
- Armario A, Lopez-Calderon A, Jolin T, Balasch J. Response of anterior pituitary hormones to chronic stress. the specificity of adaptation. Neuroscience and Biobehavioral Reviews. 1986;10(3):245–250. doi: 10.1016/0149-7634(86)90011-4. [DOI] [PubMed] [Google Scholar]
- Armario A, Valles A, Dal-Zotto S, Marquez C, Belda X. A single exposure to severe stressors causes long-term desensitization of the physiological response to the homotypic stressor. Stress (Amsterdam, Netherlands) 2004;7(3):157–172. doi: 10.1080/10253890400010721. [DOI] [PubMed] [Google Scholar]
- Arnhold MM, Wotus C, Engeland WC. Differential regulation of parvocellular neuronal activity in the paraventricular nucleus of the hypothalamus following single vs. repeated episodes of water restriction-induced drinking. Experimental Neurology. 2007;206(1):126–136. doi: 10.1016/j.expneurol.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett JR, Cairncross KD, King MG. Parameters of novelty, shock predictability and response contingency in corticosterone release in the rat. Physiology & Behavior. 1973;10(5):901–907. doi: 10.1016/0031-9384(73)90060-7. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84(4):1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Huber R, Nowak N, Trotter P. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. Journal of Neuroendocrinology. 2002;14(5):403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Meaney MJ. Hypothalamic-pituitary-adrenal function in chronic intermittently cold-stressed neonatally handled and non handled rats. Journal of Neuroendocrinology. 1995;7(2):97–108. doi: 10.1111/j.1365-2826.1995.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C. Facilitation of hypothalamic-pituitary-adrenal responses to novel stress following repeated social stress using the resident/intruder paradigm. Hormones & Behavior. 2003;43(1):158–165. doi: 10.1016/s0018-506x(02)00011-9. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Lee TM, Vining C. Prenatal stress differentially affects habituation of corticosterone responses to repeated stress in adult male and female rats. Hormones & Behavior. 2005;47(4):430–438. doi: 10.1016/j.yhbeh.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Borrell J, Torrellas A, Guaza C, Borrell S. Sound stimulation and its effects on the pituitary-adrenocortical function and brain catecholamines in rats. Neuroendocrinology. 1980;31(1):53–59. doi: 10.1159/000123050. [DOI] [PubMed] [Google Scholar]
- Bradbury MJ, Cascio CS, Scribner KA, Dallman MF. Stress-induced adrenocorticotropin secretion: Diurnal responses and decreases during stress in the evening are not dependent on corticosterone. Endocrinology. 1991;128(2):680–688. doi: 10.1210/endo-128-2-680. [DOI] [PubMed] [Google Scholar]
- Campeau S, Falls WA, Cullinan WE, Helmreich DL, Davis M, Watson SJ. Elicitation and reduction of fear: Behavioural and neuroendocrine indices and brain induction of the immediate-early gene c-fos. Neuroscience. 1997;78(4):1087–1104. doi: 10.1016/s0306-4522(96)00632-x. [DOI] [PubMed] [Google Scholar]
- Cole MA, Kalman BA, Pace TW, Topczewski F, Lowrey MJ, Spencer RL. Selective blockade of the mineralocorticoid receptor impairs hypothalamic-pituitary-adrenal axis expression of habituation. Journal of Neuroendocrinology. 2000;12(10):1034–1042. doi: 10.1046/j.1365-2826.2000.00555.x. [DOI] [PubMed] [Google Scholar]
- Costoli T, Bartolomucci A, Graiani G, Stilli D, Laviola G, Sgoifo A. Effects of chronic psychosocial stress on cardiac autonomic responsiveness and myocardial structure in mice. American Journal of Physiology. Heart and Circulatory Physiology. 2004;286(6):H2133–40. doi: 10.1152/ajpheart.00869.2003. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Modulation of stress responses: How we cope with excess glucocorticoids. Experimental Neurology. 2007;206(2):179–182. doi: 10.1016/j.expneurol.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Bell ME, Bhatnagar S, Choi S, Chu A, Gomez F, Laugero K, Soriano L, Viau V. Warning! nearby construction can profoundly affect your experiments. Endocrine. 1999;11(2):111–113. doi: 10.1385/ENDO:11:2:111. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: Variations on a Theme of B. Recent Progress in Hormone Research. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Jones MT. Corticosteroid feedback control of ACTH secretion: Effect of stress-induced corticosterone secretion on subsequent stress responses in the rat. Endocrinology. 1973;92(5):1367–1375. doi: 10.1210/endo-92-5-1367. [DOI] [PubMed] [Google Scholar]
- Dal-Zotto S, Marti O, Armario A. Influence of single or repeated experience of rats with forced swimming on behavioural and physiological responses to the stressor. Behavioural Brain Research. 2000;114(12):175–181. doi: 10.1016/s0166-4328(00)00220-5. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Koopmans SJ, Slangen JL, Van der Gugten J. Plasma catecholamine, corticosterone and glucose responses to repeated stress in rats: Effect of interstressor interval length. Physiology & Behavior. 1990;47(6):1117–1124. doi: 10.1016/0031-9384(90)90361-7. [DOI] [PubMed] [Google Scholar]
- De Boer SF, Van der Gugten J, Slangen JL. Plasma catecholamine and corticosterone responses to predictable and unpredictable noise stress in rats. Physiology & Behavior. 1989;45(4):789–795. doi: 10.1016/0031-9384(89)90296-5. [DOI] [PubMed] [Google Scholar]
- De Goeij DC, Binnekade R, Tilders FJ. Chronic stress enhances vasopressin but not corticotropin-releasing factor secretion during hypoglycemia. The American Journal of Physiology. 1992;263(2 Pt 1):E394–9. doi: 10.1152/ajpendo.1992.263.2.E394. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Stress in the brain. European Journal of Pharmacology. 2000;405(13):187–198. doi: 10.1016/s0014-2999(00)00552-5. [DOI] [PubMed] [Google Scholar]
- Deinzer R, Kirschbaum C, Gresele C, Hellhammer DH. Adrenocortical responses to repeated parachute jumping and subsequent h-CRH challenge in inexperienced healthy subjects. Physiology & Behavior. 1997;61(4):507–511. doi: 10.1016/s0031-9384(96)00465-9. [DOI] [PubMed] [Google Scholar]
- Dobrakovova M, Jurcovicova J. Corticosterone and prolactin responses to repeated handling and transfer of male rats. Experimental and Clinical Endocrinology. 1984;83(1):21–27. doi: 10.1055/s-0029-1210308. [DOI] [PubMed] [Google Scholar]
- Dobrakovova M, Kvetnansky R, Oprsalova Z, Jezova D. Specificity of the effect of repeated handling on sympathetic-adrenomedullary and pituitary-adrenocortical activity in rats. Psychoneuroendocrinology. 1993;18(3):163–174. doi: 10.1016/0306-4530(93)90001-2. [DOI] [PubMed] [Google Scholar]
- Emmert MH, Herman JP. Differential forebrain c-fos mRNA induction by ether inhalation and novelty: Evidence for distinctive stress pathways. Brain Research. 1999;845(1):60–67. doi: 10.1016/s0006-8993(99)01931-9. [DOI] [PubMed] [Google Scholar]
- Garcia A, Marti O, Valles A, Dal-Zotto S, Armario A. Recovery of the hypothalamic- pituitary-adrenal response to stress. effect of stress intensity, stress duration and previous stress exposure. Neuroendocrinology. 2000;72(2):114–125. doi: 10.1159/000054578. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Mascetti GG, Gardini S, Zambelli U, Timpano M, Raggi MA, Brambilla F. Neuroendocrine responses to experimentally-induced psychological stress in healthy humans. Psychoneuroendocrinology. 2001;26(1):91–107. doi: 10.1016/s0306-4530(00)00046-9. [DOI] [PubMed] [Google Scholar]
- Giralt M, Garcia-Marquez C, Armario A. Previous chronic ACTH administration does not protect against the effects of acute or chronic stress in male rats. Physiology & Behavior. 1987;40(2):165–170. doi: 10.1016/0031-9384(87)90202-2. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace TW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced cfos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138(4):1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Golier JA, Schmeidler J, Legge J, Yehuda R. Twenty-four hour plasma cortisol and adrenocorticotropic hormone in gulf war veterans: Relationships to posttraumatic stress disorder and health symptoms. Biological Psychiatry. 2007;62(10):1175–1178. doi: 10.1016/j.biopsych.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Gomez F, Houshyar H, Dallman MF. Marked regulatory shifts in gonadal, adrenal, and metabolic system responses to repeated restraint stress occur within a 3-week period in pubertal male rats. Endocrinology. 2002;143(8):2852–2862. doi: 10.1210/endo.143.8.8929. [DOI] [PubMed] [Google Scholar]
- Grissom N, Iyer V, Vining C, Bhatnagar S. The physical context of previous stress exposure modifies hypothalamic-pituitary-adrenal responses to a subsequent homotypic stress. Hormones and Behavior. 2007;51(1):95–103. doi: 10.1016/j.yhbeh.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Grissom N, Kerr W, Bhatnagar S. Struggling behavior during restraint is regulated by stress experience. Behavioral Brain Research. 2008 doi: 10.1016/j.bbr.2008.03.030. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: A dual-process theory. Psychological Review. 1970;77(5):419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Connors J, Isensee J. Lack of stability in neonatal adrenocortical reactivity because of rapid habituation of the adrenocortical response. Developmental Psychobiology. 1989;22(3):221–233. doi: 10.1002/dev.420220304. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Lorang M, Irwin M, Aguilera G. CRF receptor regulation and sensitization of ACTH responses to acute ether stress during chronic intermittent immobilization stress. Brain Research. 1990;532(12):34–40. doi: 10.1016/0006-8993(90)91738-3. [DOI] [PubMed] [Google Scholar]
- Hennessy JW, Levin R, Levine S. Influence of experiential factors and gonadal hormones on pituitary-adrenal response of the mouse to novelty and electric shock. Journal of Comparative and Physiological Psychology. 1977;91(4):770–777. doi: 10.1037/h0077368. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Levine S. Effects of various habituation procedures on pituitary-adrenal responsiveness in the mouse. Physiology & Behavior. 1977;18(5):799–802. doi: 10.1016/0031-9384(77)90186-x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: Central control of the hypothalamo-pituitary-adrenocortical axis. Trends in Neurosciences. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24(3):151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Mitchley S. The effect of ‘training’ on the release of corticotrophin in response to minor stressful procedures in the rat. The Journal of Endocrinology. 1970;47(2):253–254. doi: 10.1677/joe.0.0470253. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Fanselow MS, Richardson R, Mauk MD, Freeman JH, Jr, Stanton ME. Synapses, circuits, and the ontogeny of learning. Developmental Psychobiology. 2007;49(7):649–663. doi: 10.1002/dev.20250. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S. Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology. 2006;147(10):4917–4930. doi: 10.1210/en.2005-1393. [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S. Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Research. 2007;1186:212–223. doi: 10.1016/j.brainres.2007.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaferi A, Nowak N, Bhatnagar S. Negative feedback functions in chronically stressed rats: Role of the posterior paraventricular thalamus. Physiology & Behavior. 2003;78(3):365–373. doi: 10.1016/s0031-9384(03)00014-3. [DOI] [PubMed] [Google Scholar]
- Johnson LL, Moberg GP. Adrenocortical response to novelty stress in rats with dentate gyrus lesions. Neuroendocrinology. 1980;30(3):187–192. doi: 10.1159/000122998. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Bunnell BN, Mougey EH, Pennington LL, Meyerhoff JL. Effects of repeated stress on pituitary cyclic AMP, and plasma prolactin, corticosterone and growth hormone in male rats. Pharmacology, Biochemistry, and Behavior. 1983;18(6):967–971. doi: 10.1016/s0091-3057(83)80022-7. [DOI] [PubMed] [Google Scholar]
- Keim KL, Sigg EB. Physiological and biochemical concomitants of restraint stress in rats. Pharmacology, Biochemistry, and Behavior. 1976;4(3):289–297. doi: 10.1016/0091-3057(76)90244-6. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic Medicine. 1995;57(5):468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Levine S, Smotherman WP, Hennessy JW. Pituitary-adrenal hormones and learned taste aversion. Advances in Biochemical Psychopharmacology. 1977;17:163–177. [PubMed] [Google Scholar]
- Lunga P, Herbert J. 17Beta-oestradiol modulates glucocorticoid, neural and behavioural adaptations to repeated restraint stress in female rats. Journal of Neuroendocrinology. 2004;16(9):776–785. doi: 10.1111/j.1365-2826.2004.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140(8):3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiology & Behavior. 2007;90(1):29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Marti O, Armario A. Influence of regularity of exposure to chronic stress on the pattern of habituation of pituitary-adrenal hormones, prolactin and glucose. Stress (Amsterdam, Netherlands) 1997;1(3):179–189. doi: 10.3109/10253899709001107. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Annals of the New York Academy of Sciences. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- McQuade JM, Tamashiro KL, Wood GE, Herman JP, McEwen BS, Sakai RR, Zhang J, Xu M. Deficient hippocampal c-fos expression results in reduced anxiety and altered response to chronic stress in female mice. Neuroscience letters. 2006;403(12):125–130. doi: 10.1016/j.neulet.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Misslin R, Herzog F, Koch B, Ropartz P. Effects of isolation, handling and novelty on the pituitary--adrenal response in the mouse. Psychoneuroendocrinology. 1982;7(23):217–221. doi: 10.1016/0306-4530(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Muir JL, Pfister HP. Time course of the corticosterone and prolactin response following predictable and unpredictable novelty stress in rattus norvegicus. Physiology & Behavior. 1987;40(1):103–107. doi: 10.1016/0031-9384(87)90191-0. [DOI] [PubMed] [Google Scholar]
- Natelson BH, Ottenweller JE, Cook JA, Pitman D, McCarty R, Tapp WN. Effect of stressor intensity on habituation of the adrenocortical stress response. Physiology & Behavior. 1988;43(1):41–46. doi: 10.1016/0031-9384(88)90096-0. [DOI] [PubMed] [Google Scholar]
- Nesse RM, Bhatnagar S, Young E. The evolutionary origins and functions of the stress response. In: Fink G, editor. The Encyclopedia of Stress. Second Edition Academic Press; New York: 2007. [Google Scholar]
- Pfister HP. The glucocorticosterone response to novelty as a psychological stressor. Physiology & Behavior. 1979;23(4):649–652. doi: 10.1016/0031-9384(79)90154-9. [DOI] [PubMed] [Google Scholar]
- Pfister HP, King MG. Adaptation of the glucocorticosterone response to novelty. Physiology & Behavior. 1976;17(1):43–46. doi: 10.1016/0031-9384(76)90267-5. [DOI] [PubMed] [Google Scholar]
- Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: Chronic stress and habituation. Physiology & Behavior. 1988;43(1):47–55. doi: 10.1016/0031-9384(88)90097-2. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosomatic Medicine. 2003;65(3):450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J, Smith LM. Hypothalamic-pituitary-adrenal axis function in dissociative disorders, post-traumatic stress disorder, and healthy volunteers. Biological Psychiatry. 2007;61(8):966–973. doi: 10.1016/j.biopsych.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkiss JL, Devine DP. Responses of the HPA axis after chronic variable stress: Effects of novel and familiar stressors. Neuroendocrinology Letters. 2003;24(12):97–103. [PubMed] [Google Scholar]
- Smith R, Lovelock M, Owens PC, Chan EC, Falconer J. The effect of repetitive haemorrhage on plasma cortisol, beta-endorphin and N-terminal proopiomelanocortin in conscious sheep. Hormone and Metabolic Research. 1988;20(10):612–615. doi: 10.1055/s-2007-1010898. [DOI] [PubMed] [Google Scholar]
- Spencer RL, McEwen BS. Adaptation of the hypothalamic-pituitary-adrenal axis to chronic ethanol stress. Neuroendocrinology. 1990;52(5):481–489. doi: 10.1159/000125632. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94(4):1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: A model phenomenon for the study of neuronal substrates of behavior. Psychological Review. 1966;73(1):16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Thomson F, Craighead M. Innovative approaches for the treatment of depression: Targeting the HPA axis. Neurochemical Research. 2007 doi: 10.1007/s11064-007-9518-3. in press. [DOI] [PubMed] [Google Scholar]
- Vogel WH, Jensh R. Chronic stress and plasma catecholamine and corticosterone levels in male rats. Neuroscience Letters. 1988;87(12):183–188. doi: 10.1016/0304-3940(88)90167-x. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko IS, van Rossum EF, Koper JW, Hellhammer DH. Habituation of cortisol responses to repeated psychosocial stress-further characterization and impact of genetic factors. Psychoneuroendocrinology. 2005;30(2):199–211. doi: 10.1016/j.psyneuen.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: A chronobiological analysis. Biological Psychiatry. 1996;40(2):79–88. doi: 10.1016/0006-3223(95)00451-3. [DOI] [PubMed] [Google Scholar]