Abstract
Estrogen has both rapid and longer-term direct effects on cardiovascular tissues mediated by the two estrogen receptors, ERα and ERβ. Previous work identified that estrogen regulates the expression of inducible nitric oxide synthase (iNOS) in vascular smooth muscle cells (VSMC). ERβ knockout mice have vascular dysfunction due to dysregulation of iNOS expression and these mice are hypertensive (Zhu et al, Science 2002;295:505-508). Here we report studies to examine the differential regulation of iNOS gene expression by ERα and ERβ. Immunoblotting and RT-PCR studies revealed that different VSMC lines expressed different levels of ERα and ERβ protein and mRNA. VSMC from different vascular beds were studied, including aortic VSMC expressing ERα and radial VSMC expressing ERβ. E2 inhibited NO production and iNOS protein expression in aortic VSMC. Human iNOS promoter reporter studies revealed suppression of iNOS reporter activity by E2 in aortic VSMC, and stimulation of iNOS reporter activity by E2 in radial arterial VSMC. In heterologous expression studies of COS-7 cells lacking endogenous ER, E2 treatment of COS-7 cells did not alter iNOS reporter activity in the presence of ERα, while reporter activity increased 2.3 fold in the presence of ERβ. Similar experiments in COS-7 cells using the selective estrogen receptor modulator raloxifene showed that raloxifene caused a reduction in iNOS reporter activity with ERα co-expression, and an increase with ERβ co-expression. Rat VSMC expressing ERβ but not ERα also showed increased iNOS reporter activity with E2 treatment, an effect lost when ERα was introduced into the cells. Taken together, these data support that hiNOS transcription is positively regulated by ERβ and negatively regulated by ERα in VSMC, supporting differential actions of these two estrogen receptors on a physiologically relevant gene in vascular smooth muscle cells.
Keywords: vascular smooth muscle cell, hormone, estrogen receptor α, estrogen receptor β, nitric oxide synthase
Introduction
Physiological effects of estrogen on vascular smooth muscle cells are mediated by two intracellular estrogen receptors, ERα and β, which regulate transcription of target genes following ligand activation through binding to specific DNA target sequences (Kannel et al, 1976; Marcus et al, 1994; Gardin et al, 1995; Mendelsohn & Karas, 1999). Regulation of specific genes in VSMC by estrogen is still not well understood. Recent studies suggest that nitric oxide (NO) plays an important role in estrogen-mediated effects on vascular smooth muscle cells. NO is generated by a family of NO synthases (NOS) which catalyze the conversion of the amino acid L-arginine to citrulline. In vivo, estrogen reduces vasoconstriction in vessels from which the endothelium has been removed from both humans and wild-type animals (Mügge et al, 1997;Binko & Majewski. 1998), an effect that is blocked by pharmacologic inhibition of iNOS (Binko & Majewski. 1998). Estrogen has been shown to increase expression and activity of NOS in vascular smooth muscle cells (Zhu et al, 2002), and this effect is responsible for vascular dysfunction in mouse vessels from ERβ knockout mice.
Estrogen receptors bind to a specific DNA sequence, the estrogen response element (ERE), in the promoters of many estrogen-responsive genes. However, there are no full palindromic ERE sequence elements for the consensus vitellogenin ERE identified thus far in any of those genes identified to be regulated by estrogen in vascular tissue, including the human iNOS gene. Several studies have shown that selective ER modulators can modulate gene expression by mechanisms that are independent of binding to a classical ERE (Paech et al 1997; Ray et al 1997; Srivastava et al, 1999). There is also considerable evidence indicating that ERs may also function by interacting with other transcription factors, including AP-1, specificity protein-1, and nuclear factor-κB, bound to their specific response elements in target genes that do not contain EREs (Ray et al, 1997; Webb et al 1999; Sanches et al, 2002). Moreover, it is evident that estrogen effects are not only dependent on the target gene but also reliant on the cell context. In a similar fashion, the cAMP regulatory element (CRE) motif of corticotrophin-releasing hormone promoter region can be affected by the action of estrogen receptor in human placental syncytiotrophoblast cells (Ni et al, 2004). Nitric oxide production from VSMC can be pathophysiologic or physiologic, depending in part on the degree of iNOS activation in a given setting. For example, the general hypotension occurring in septic shock is a result of overwhelming vasodilatory actions of NO released by iNOS in the context of septicemia. In contrast, in atherosclerosis and balloon-induced injury, increased production of NO by cGMP may provide beneficial effects by inhibiting platelet and leukocyte adhesion as well as the proliferation of VSMC (Galea &. Feinstein, 1999). In this study we examined the molecular mechanisms for estrogen-stimulated changes in iNOS gene expression and the roles of ERα and ERβ. We found that transcriptional induction of iNOS by estradiol was promoted by ERβ but attenuated by ERα in several VSMC and in heterologous expression studies.
Materials and Methods
Materials
17 β-estradiol (E2) was purchased from Sigma Chemical Co. (St. Louis, MO). ICI182,780 was obtained from Tocris (Ballwin, MO). COS-7 cell line, which is an African green monkey fibroblast-like cell line, and A10 cell line, which was derived from the thoracic aorta of embryonic rats, were obtained from American Type Culture Collection (Rockville, MD). Rabbit polyclonal anti-ERβ antibody was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell culture
1. Human Aortic (AO), Coronary (Cor) and Radial (Rad) arterial vascular smooth muscle cells were cultured in our laboratory as previously described. AO and Cor arterial smooth muscle cells were immortalized, and Rad arterial smooth muscle cells were primary culture. These arterial smooth muscle cells were harvested from human surgical and autopsy specimens as described (Karas et al, 1994). These vascular smooth muscle cells were developed in our laboratory by the explants method and characterized by both morphology and by immunohistochemical studies of expression of smooth muscle cell-specific α-actin. Ao and Cor arterial smooth muscle cells were immortalized by retroviral constructs containing the E6 and E7 human papillomavirus proteins as we have reported previously (Pace et al, 1999). Ao and Rad arterial smooth muscle cells were derived from male donors, and Cor arterial smooth muscle cells were derived from 26-year-old female. COS-7 cells were used to selectively express transfected ERs as this cell line expresses neither ERα nor ERβ. A10 rat vascular smooth muscle cells were used as this cell line expresses only ERβ (Takahashi et al, 2003). These cells were maintained in phenol-red free Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Grand Island, NJ, USA) supplemented with 10% charcoal-stripped, estrogen-free fetal calf serum, and 0.5% penicillin-streptomycin (Gibco BRL) at 37°C, in humidified incubator at 5% CO2 with changed every other day.
Nitrite determination
NO in the culture medium was measured by using the Griess diazotization reaction (Griess Reagent Kit for Nitrite Determination, Molecular Probes, Eugene, OR) to detect nitrite formed by the spontaneous oxidation of NO.
Western blot analysis
To prepare whole-cell lysates, cells were lysed in cell lysis buffer (50 mM Tris, 150 mM NaCl, 1 mM Phenylmethylsulfonyl fluoride (PMSF), 10 μM/ml leupeptin, 10 μg/ml aprotinin, and 1 mM sodium orthovanadate). The insoluble material was excluded by centrifugation. The protein concentration in the supernatant of tissue homogenate was determined using DC protein assay reagent (Bio-Rad, Hercules, CA), and adjusted the same concentaration with cell lysis buffer. The resulting supernatant was mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and was subjected to SDS-PAGE. Cellular lysates were fractionated by 10% SDS-PAGE, electrophoreically transferred to nitrocellulose membrane, and then analyzed for immunoreactivity with a rabbit anti iNOS antibody (BD transduction laboratories, San Jose, CA). Following incubation with the primary anbibody, the membrane was exposed to a horseradish peroxidase-conjugated secondary anti-rabbit antibody (GE Healthcare, Buckinghamshire, UK), subjected to ECL plus (GE Healthcare), and exposed to film. Equal loading was verified by stripping and reprobing the blots with mouse anti β-actin (GE Healthcare).
Reverse Transcriptase-polymerase Chain Reaction (RT-PCR)
Cells were starved for 24 hours and then stimulated in absence or presence of 10 nM 17β-estradiol (E2) or 100 mg/ml lipopolysaccharide (LPS: Sigma, St. Louis, MO) for 24 hours before harvest. Total RNA was isolated from vascular smooth muscle cells with RNeasy (Qiagen, Valencia, CA). Total RNA of 1.0 μg was used as a template for first-strand cDNA syntesis using random hexamer as primer in the presence of Superscript reverse transcriptase (Invitrogen, Carlsbad, CA). One milliliter of cDNA was subsequently used as a template for PCR amplification using Taq polymerase (New England Biolabs, Ipswich, MA) using specific primers of iNOS, β-actin, ERα and ERβ, respectively.
Plasmid constructs
2. The plasmids pGL3-8,296, contained the full-length hiNOS promoter was kind gift from Dr. Joel Moss (The Pulmonary-Critical Care Medical Branch, NHLBI, National Institutes of Health, Bethesda, MD) (Arnold et al, 2001). The human estrogen receptor α (ER α) expression vector was a kind gift from Dr. Pierre Chambon (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Collège de France, Illkirch Cedex, France).
Transient transfection
Transient transfection was performed with 1.0 μg reporter plasmid DNA for wild-type hiNOS promoter-directed luciferase expression per well using the Lipofectamine Plus transfection reagent (Invitorgen, Carlsbad, CA) according the manufacturer's protocol. In some experiments, ERα or ERβ expression plasmed was co-transfected to detect alteration of hiNOS promoter activity.
Pharmacological treatment and luciferase assay
At 24 h after transfection, cells were treated with E2 dissolved in ethanol; the latter did not exceed 0.01% final concentration. Controls received the same amount of ethanol. The cells were then further incubated for 24 h under the same conditions.
After incubation, the cells were harvested and luciferase assays were performed with the PicaGene dual seapansy system (Toyo Ink, Tokyo, Japan) following the manufacturer's protocol. Firefly-luciferase activity and seapansy-luciferase activity were measured as relative light units with a luminometer (Lumat LB9507, EG&G, Berthold, Bad Wildbad, Germany). The firefly-luciferase activity was then normalized relative to the sea-pansy-luciferase activity to determine the transfection efficiency.
Statistical analysis
Statistical significance of the results was determined by Student's t-test for experiments with two groups or by performing an ANOVA followed by Fisher's least significance difference test for experiments with more than two groups; p <0.05 was accepted as statistically significant.
Results
Effect of estradiol E2, ICI182,780, and LPS on NO production in aortic vascular smooth muscle cells
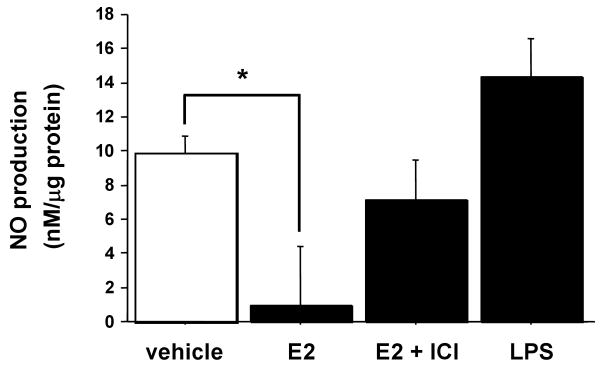
E2 stimulation of NO production in VSMC was first examined in cultured aortic VSMC (Ao184 cells) in the absence or presence of the estrogen receptor antagonist ICI182,780, with LPS used as a positive control (Figure 1). NO synthesis was determined by assaying the culture supernatants for nitrite, using Griess reagent. NO production was suppressed by E2 (10 nM), and this effect was significantly reversed by ICI182,780 (1 μM).
Figure 1.
The effects of E2, ICI and LPS on NO production in aortic vascular smooth muscle cells. Aortic VSMC was treated with E2 (10 nM), ICI 182,780 (1 μM) and LPS (100 μg/ml). NO synthesis was determined by assaying the culture supernatants for nitrite, using Griess reagent. Values are expressed as means ±S.E. of three individual experiments, performed in triplicate. * P<0.05, significantly different from the vehicle.
Western blot analysis of iNOS protein in VSMCs
To explore whether the effects of NO production by E2 were different between aortic vascular smooth muscle cells (Ao184) and coronary arterial vascular smooth muscle cells (Co396), western blot analysis of iNOS protein following estrogen treatment was studied (Figure 2). Protein was extracted from VSMCs treated with vehicle (0.1% ethanol), E2 (10 nM) or LPS (100 mg) for 24 hours. Mouse macrophage cell lysate stimulated with IFNγ (10 ng/ml) and LPS (100 μg/ml) for 12 hours, served as a positive control. Although E2 attenuated iNOS protein expression in Ao184 cells (cf. Fig 1), E2 did not affect iNOS protein expression in Co396 cells.
Figure 2.
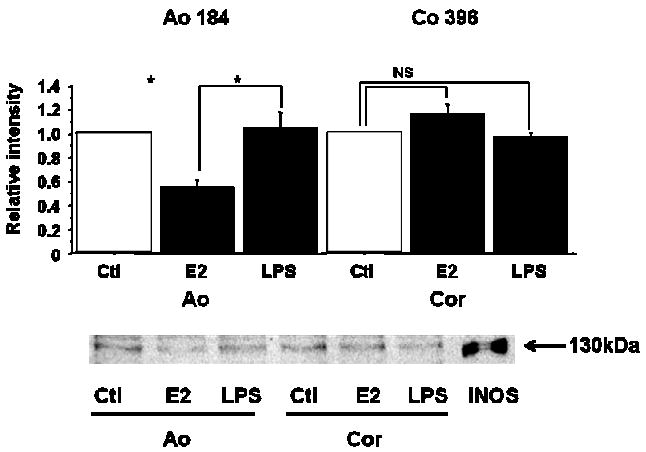
Western blot analysis of iNOS protein in VSMCs.
Protein was extracted from VSMCs (Ao, Cor) treated with vehicle, E2 (10 nM) or LPS (100 μg/ml) for 24 h. Mouse macrophage cell lysate that was stimulated with IFNγ (10 ng/ml) and LPS (1 μg/ml) for 12 h, was served as a positive control. Values are expressed as means ±S.E. of three individual experiments, performed in triplicate. *P<0.05, significantly different from the vehicle.
The effects of E2 on ERs and iNOS mRNA expression
To examine potential mechanisms for the difference in iNOS induction in different VSMC, the distribution of ERs among various VSMCs (Ao, Cor and Rad) was studied using semiquantitative RT-PCR (Figure 3A). In Ao, ERα mRNA was significantly more abundant than ERβ mRNA. In radial arterial VSMC, the converse was seen: ERβ mRNA was significantly more abundant. However, in coronary arterial smooth muscle cells, no significant difference was detected between ERα and ERβ mRNA expression (Figure 3). Potential differences in iNOS expression following E2 were next evaluated (Figure 3B-D). In Ao, which express mainly ERα, iNOS mRNA expression was significantly suppressed by 10 nM E2. In contrast, iNOS mRNA expression was increased by E2 in radial artery VSMC, which express predominantly ERβ. No significant changes in iNOS expression were detected following E2 treatment of coronary VSMC. These data support the hypothesis that the relative level of ERα and ERβ expression may regulate expression of iNOS.
Figure 3.
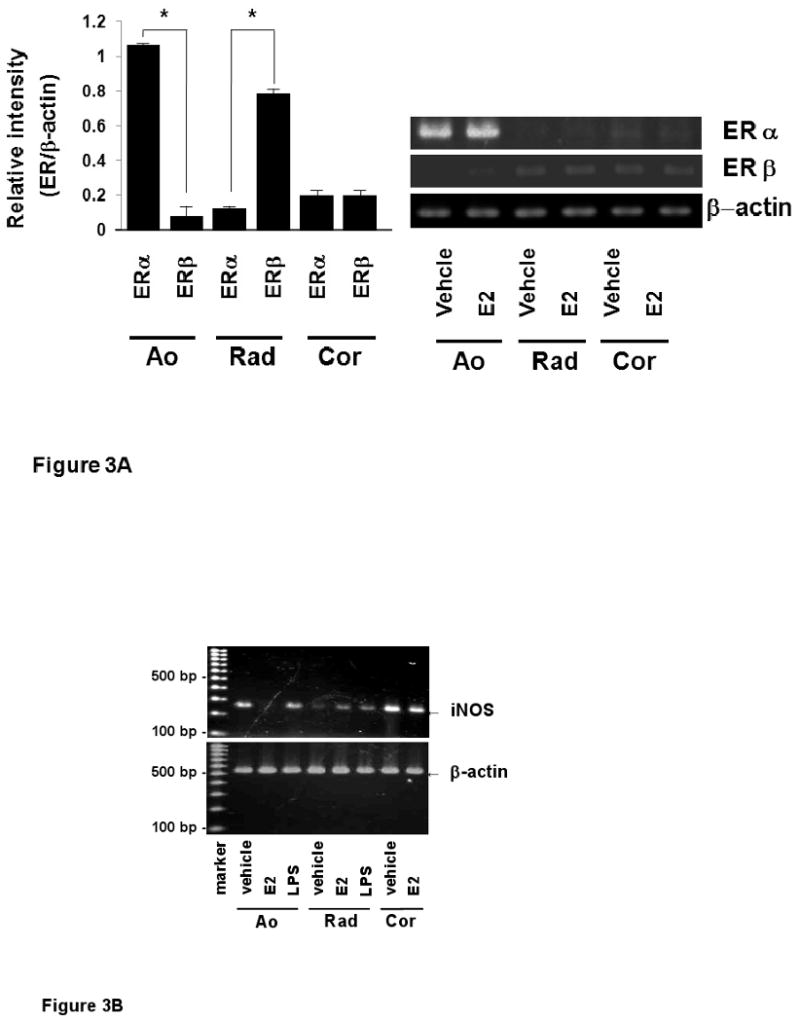
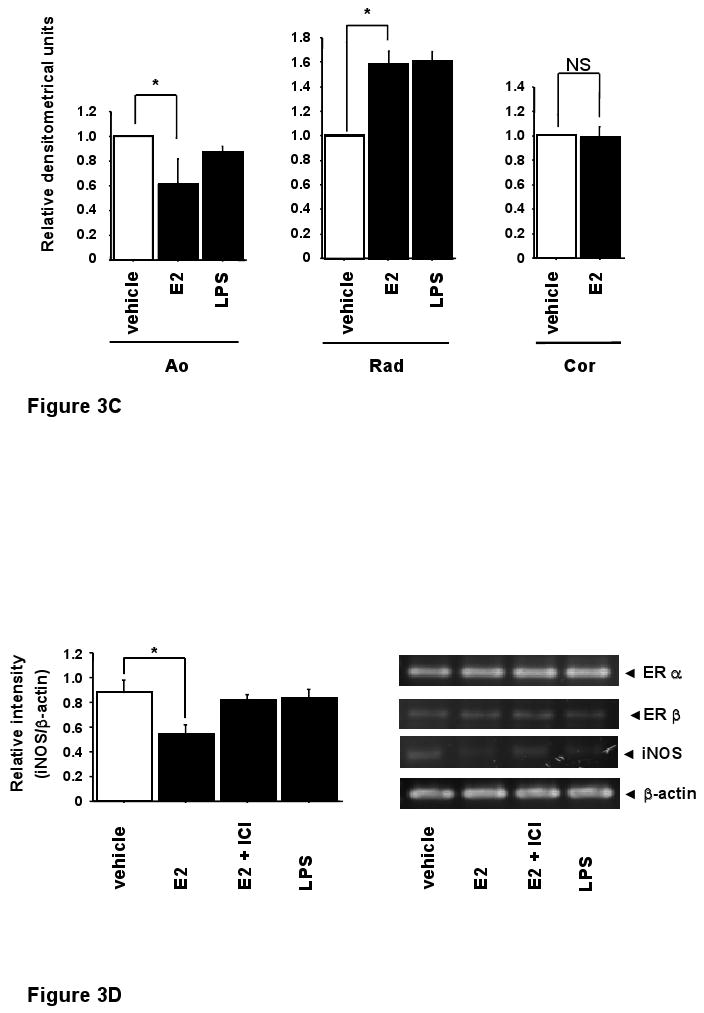
The effects of E2 on ERs and iNOS mRNA expression.
A, mRNA expressions of estrogen receptor α (ERα) and β (ERβ) were measured by RT-PCR in various vascular smooth muscle cells. Ao: aortic vascular smooth muscle cells, Rad: radial arterial vascular smooth muscle cells, Cor: coronary arterial vascular smooth muscle cells). B, C, iNOS mRNA expressions was examined by RT-PCR. mRNA was extracted from VSMCs (Ao, Rad, Cor) treated with vehicle, E2 (10 nM) or LPS (100 μg/ml) for 24 h, respectively. D, RT-PCR analysis for estrogen receptors and iNOS mRNA expression in aortic vascular smooth muscle cells. Values are expressed as means ±S.E. of three individual experiments, performed in triplicate. *P<0.05, significantly different from the vehicle.
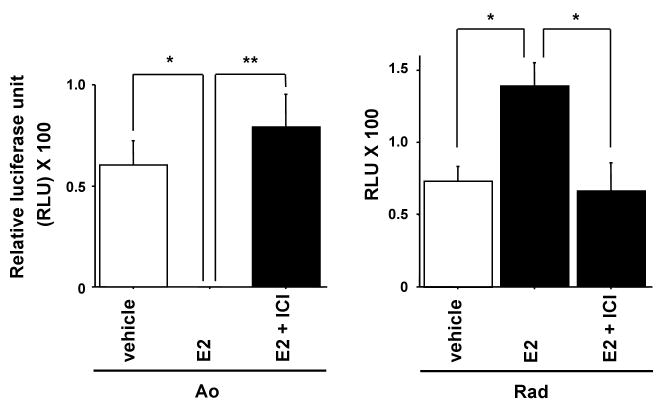
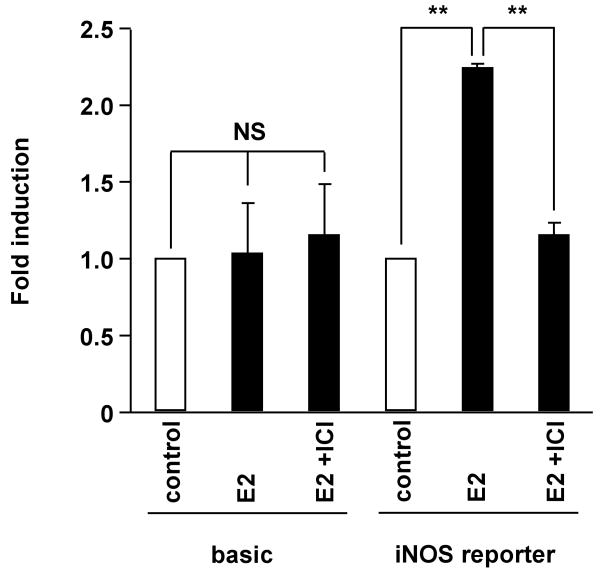
iNOS reporter activity in aortic vascular smooth muscle cells and radial arterial vascular smooth muscle cells
To confirm whether the difference of distribution of ERs affects transcriptional regulation of iNOS, iNOS reporter activities were examined in Ao and Rad VSMCs. Luciferase assay of the iNOS reporter construct pGL3-hiNOS 8,296 bp was performed (Figure 4). In Ao, iNOS reporter activity was suppressed by 10 nM E2, and 1 μM ICI182,780 reversed the effect. In contrast, in radial arterial VSMC, iNOS reporter activity was increased by E2, and the effect of E2 was reversed by ICI 182,780. These results support that iNOS reporter activity is negatively regulated by ERα and positively regulated by ERβ.
Figure 4.
iNOS reporter activity in aortic vascular smooth muscle cells and radial arterial vascular smooth muscle cells.
Luciferase assay of the iNOS reporter construct pGL3-hiNOS 8,296 bp in aortic vascular smooth muscle cells (Ao) and radial arterial vascular smooth muscle cells (Rad) with vehicle, E2 (10 nM) and ICI 182, 780 (1 μM) for 24 h. Values are expressed as means ±S.E. of three individual experiments, performed in triplicate. *P<0.05, **P<0.01, significantly different from the vehicle.
iNOS reporter activity in COS-7 cells
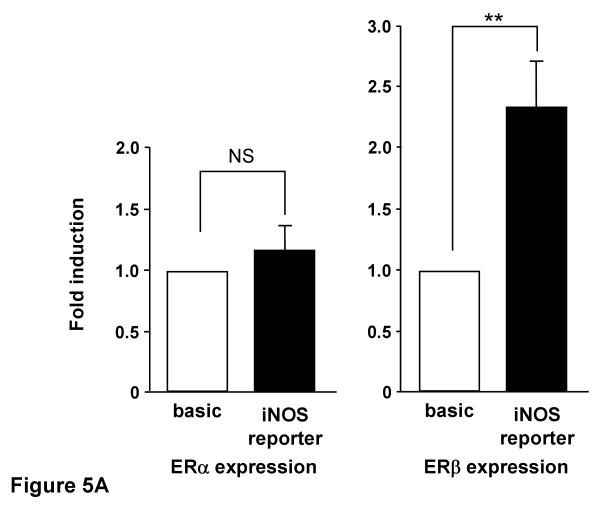
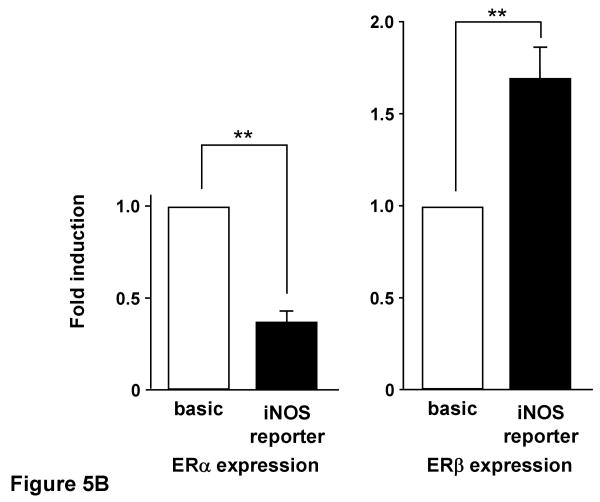
To further explore the role of ERs on iNOS transcriptional regulation, we also conducted experiments in COS-7 cells, which lack endogenous ER, by co-transfecting iNOS reporter plasmid and ER expression plasmid. E2 treatment of COS-7 cells did not alter iNOS reporter activity in the presence of ERα co-expression, while iNOS reporter activity increased 2.3 fold when ERβ was co-expressed with the iNOS reporter (Figure 5-A). We performed similar experiments using the selective estrogen receptor modulator raloxifene (Ral) (Figure 5-B). 10 nM of Ral led to a reduction in iNOS reporter activity to 40% of control in the presence of ERα expression, while iNOS reporter activity was significantly increased in the presence of ERβ co-expression in COS-7 cells.
Figure 5.
A, iNOS reporter activity with estrogen receptor α and β in the presence of E2 in COS-7 cells. COS-7 cells co-transfected with the iNOS reporter and estrogen receptor (ER) expression plasmid were incubated for 24 h with or without 10 nM E2 before assay of luciferase activity. B, iNOS reporter activity with estrogen receptor α and β in the presence of raloxifene in COS-7 cells. COS-7 cells co-transfected with the iNOS reporter and estrogen receptor (ER) expression plasmid were incubated for 24 h with or without 10 nM raloxifene before assay of luciferase activity. Data are the means of values from four experiments with assays in triplicate, expressed relative to luciferase activity of pGL3-basic. **P<0.01, significantly different from basic plasmid.
iNOS reporter activity in COS-7 cells
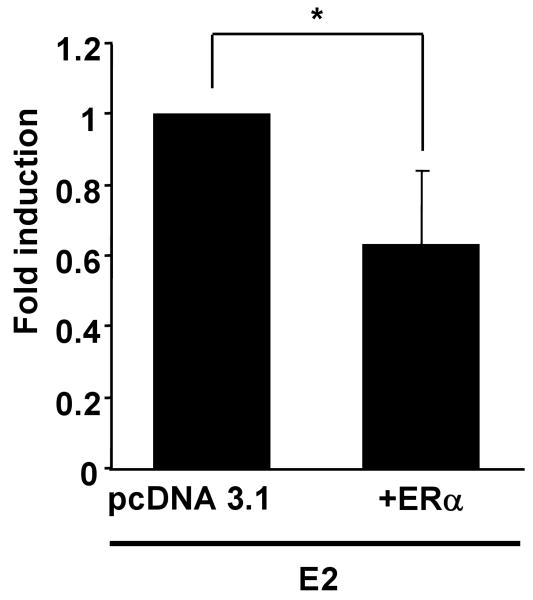
Rat vascular smooth muscle cells (A10 cells), which express ERβ but not ERα, were transfected with the reporter construct for full legth hiNOS promoter (-8,296) with or without the expression plasmid for human ERα. Treatment with 10 nM of E2 increased iNOS reporter activity in A10 cells (Figure 6). Control experiments using ICI182,780 (1 μM) demonstrated a complete inhibition of E2-mediated activation of hiNOS reporter construct supporting that the effect was mediated by ERβ. Transfection of ERα into A10 cells led to inhibition of iNOS reporter activity in the presence of E2 (Figure 7).
Figure 6.
Luciferase activity in A10 rat thoracic arterial vascular smooth muscle cells transfected with iNOS reporter plasmid and treated with 10 nM E2, or 10 nM E2 and 1μM ICI 182,780 for 24 h. reporter activity is shown relative to the vehicle control. Values represent the mean ± SEM of experiments performed in triplicate. **, P <0.01, compared with vehicle controls.
Figure 7.
E2 mediates negative transcriptional regulation of the hiNOS gene via ERα in a dose-dependent manner. A10 cells were transfected with the pGL3-hiNOS reporter construct and ERα expression vector or its backbone vector (pcDNA3.1+). The ratios of reporter construct to expression vector are 1:1, 2:1 and 10:1, respectively. After 24 h, the cells were treated with 10 nM E2 in ethanol, and incubated for an additional 24 h. Cells were then harvested, and luciferase activity was determined and normalized to seapansy-luciferase activity. Relative luciferase activity is shown as fold induction, with the mean for the group (backbone vector) taken as 1. Values are expressed as means ±S.E. of three individual experiments, which were performed in triplicate. *, P <0.05, significantly different from the vehicle.
Discussion
This study shows that estrogen-dependent transcriptional regulation of the human iNOS gene is dependent on the specific subtype present in the cell. The data in both native human VSMC and in heterologous cells, as well as murine VSMC, are consistent with ERβ-mediated increases in iNOS gene expression and ERα-mediated suppression of the iNOS gene.
Differential effects of ERα and ERβ have also been observed for the cyclin D1 gene in rat vascular A10 cells, for which estrogen inhibited cyclin D1 expression when ERα was expressed but did have an effect in parental A10 cells that express only ERβ (18). Differential and opposing regulation of promoter activity by estradiol with ERα and ERβ also was reported recently with the plasminogen activator inhibitor-1 (PAI-1) promoter in endothelial cells. ERα increased PAI-1 promoter activity in bovine aortic endothelial cells by an estrogen-dependent mechanism, whereas ERβ suppressed PAI-1 promoter activity by an estrogen-independent mechanism (Smith et al, 2004).
Although both ER subtypes are highly homologous (96%) in their DNA-binding domains, allowing both receptors to in theory bind the same EREs within the promoter of the target genes, the transcriptional activity of ERs is mediated by both a ligand-independent transcriptional-activation function (TAF-1) located within the amino-terminal region and a hormone-dependant transcriptional-activation function (TAF-2) located within carboxyl-terminal region (Mendelsohn & Karas, 1999). Many nuclear receptor coregulators interacting with the TAF-2 domain of various receptors interact equally well with ERα and ERβ, including RIP 140, TIF-2, SRC-1 and SHP (short heterodimer partner) (Smith et al, 2004; Seol et al, 1998). However, the two receptor isoforms show quite significant differences in their amino-terminal (TAF-1) domains, and it is possible that the two receptors interact with different sets of coactivator or co-repressor proteins as one explanation for the differential regulation observed in this study. The ligand–binding domains are relatively conserved (55%) between ERα and ERβ, and are reported to display a similar affinity for E2 (Kuiper et al, 1998). It remains to be determined how further varying the ratio of ERα and ERβ could affect the response of the hiNOS promoter, especially as ERs can form both homodimers and heterodimers.
Differential expression and functions of ERα and ERβ also are observed in vivo (Kuiper et al, 1998). ERβ is found in many tissues including the prostate, uterus, ovary, testis, bladder, lungs, and brain, but not in liver. Although ERβ is also expressed in the mammary gland, it appears that ERα is more important estrogen receptor in this particular tissue. In the uterus, ERα is more abundant than ERβ, while ovarian folliculogenesis is predominantly regulated by ERβ. These differences in tissue distribution and function are of potential importance from the pharmaceutical point of view. In syncytiotrophoblast cells transfected with ERα expression and corticotropin-releasing hormone promoter reporter vectors, E2 significantly decreased the ERα/CRE-dependent reporter activity. This inhibition of reporter activity via ERα might be mediated by a repressor domain in the amino-terminal region of ERα comparable to TAF-1 of ERβ (Gustafsson, 1999). Alternatively, the ligand-activated ERα or ERβ/CRE complex may enhance interactions with co-regulator protein(s). Several recent studies have indicated an interaction between the cAMP-mediated signaling and response to estrogen. Increased cAMP is reported to stimulate the transcriptional activity of ER. The extent of stimulation varied depending on the promoter context (Hall & McDonnell, 1999). Phosphorylation of ERs may be involved in this response, because ERs contain a number of potential protein kinase A phosphorylation sites. Alternatively, phosphorylation of the steroid receptor co-activator (SRC1) has been proposed to promote a more stable interaction between SRC1 and p300/CBP, facilitating their functional synergism (Coleman et al, 2003).
In summary, our findings support that estrogen-mediated effects on the inducible iNOS gene, a physiologically relevant target of estrogen in vascular smooth muscle, are differentially regulated, induced by ERβ and inhibited by ERα. These findings are particularly interesting and somewhat unusual in the context of our recent observation that ERα is in general an activator of vascular gene expression in response to estrogen, while the majority (80 percent or more) of genes regulated by ERβ following estrogen are inhibited in their expression (O'lone et al, 2007). These results suggest that ERα selective compounds for ERα or ERβ might have therapeutic potential for cardiovascular disease. These results may contribute to our understanding of how estrogens can modulate regulation of cardiovascular systems by other effectors.
Acknowledgments
We are grateful to Joel Moss for providing the human iNOS reporter plasmid used in this study. This work was supported in part by NIH RO1 HL50569 (MEM), and Grants-in-Aid for Scientific Research No. 18591822 (to K.T.) and No. 17390445 (to H.K.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and in part by Grants-in-Aid for the 21st Century Center of Excellence (COE) Program from the Japan Society for the Promotion of Science.
References
- Binko J, Majewski H. 17 β-Estradiol reduces vasoconstriction in endothelium-denuded rat aortas through inducible NOS. Am J Physiol Heart Circ Physiol. 1998;274:H853. doi: 10.1152/ajpheart.1998.274.3.H853. [DOI] [PubMed] [Google Scholar]
- Coleman KM, Dutertre M, El-Gharbawy A, Rowan BG, Weigel NL, Smith CL. Mechanistic differences in the activation of estrogen receptor-alpha (ER alpha)- and ER beta-dependent gene expression by cAMP signaling pathway(s) J Biol Chem. 2003;278:12834–12845. doi: 10.1074/jbc.M212312200. [DOI] [PubMed] [Google Scholar]
- Galea E, Feinstein DL. Regulation of the expression of the inflammatory nitric oside synthase (NOS2) by cyclic AMP. The FASEB J. 1999;13:2125–2137. doi: 10.1096/fasebj.13.15.2125. [DOI] [PubMed] [Google Scholar]
- Gardin JM, Wagenknecht LE, Anton-Culver H, Flack J, Gidding S, Jurosaki T, Wong ND, Manolio TA. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92:380–387. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- Gustafsson JÅ. Estrogen receptor B – a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163:379–383. doi: 10.1677/joe.0.1630379. [DOI] [PubMed] [Google Scholar]
- Hall JM, McDonnell DP. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- Johansson L, Thomsen JS, Damdimopoulos AE, Spyrou G, Gustafsson JÅ, Treuter E. The orphan nuclear receptor SHP inhibits agonist-dependent transcriptional activity of estrogen receptors ERα and ERβ. J Biol Chem. 1999;247:345–353. doi: 10.1074/jbc.274.1.345. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85:447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- Karas RH, Patterson BL, Mendelsohn ME. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation. 1994;89:1943–50. doi: 10.1161/01.cir.89.5.1943. [DOI] [PubMed] [Google Scholar]
- Kristof AS, Marks-Konczalik J, Moss J. Mitogen-activated Protein Kinases Mediate Activator Protein-1-dependent Human Inducible Nitric-oxide Synthase Promoter Activation. J Biol Chem. 2001;276(11):8445–52. doi: 10.1074/jbc.M009563200. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JÅ. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Marcus R, Krause L, Weder AB, Dominguez-Meja AN, Schork D, Julius S. Sex-specific determinants of increased left ventricular mass in the Tecumseh Brood Pressure Study. Circulation. 1994;90:928–936. doi: 10.1161/01.cir.90.2.928. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. New Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs A. The L-arginine-nitric oxide pathway. New Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Mügge A, Barton M, Fieguth HG, Riedel M. Contractile responses to histamine, serotonin, and angiotensin II are impaired by 17 beta-estradiol in human internal mammary arteries in vitro. Pharmacology. 1997;54:162. doi: 10.1159/000139483. [DOI] [PubMed] [Google Scholar]
- Ni X, Hou Y, King BR, Tang X, Read MA, Smith R, Nicholson RC. Estrogen Receptor-Mediated Down-Regulation of Corticotropin-Releasing Hormone Gene Expression Is Dependent on a Cyclic Adenosine 3′,5′-Monophosphate Regulatory Element in Human Placental Syncytiotrophoblast Cells. J Clin Endocrinol Metab. 2004;89:2312–2318. doi: 10.1210/jc.2003-030948. [DOI] [PubMed] [Google Scholar]
- O'lone R, Knorr K, Jaffe IZ, Schaffer ME, Martini PG, Karas RH, Bienkowska J, Mendelsohn ME, Hansen U. Estrogen receptors alpha and beta mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol Endocrinol. 2007;21:1281–1296. doi: 10.1210/me.2006-0497. [DOI] [PubMed] [Google Scholar]
- Pace MC, Chambliss KL, German Z, Yuhanna IS, Mendelsohn ME, Shaul PW. Establishment of an immortalized fetal intrapulmonary artery endothelial cell line. Am J Phisiol. 1999;277:L106–12. doi: 10.1152/ajplung.1999.277.1.L106. [DOI] [PubMed] [Google Scholar]
- Paech K, Webb P, Kuipper GG, Nilsson S, Gustafsson J, Kushner PJ, Scanlan TS. Diffential ligand activation of estrogen receptors ERalpha and ERbeta at AP1 sites. Science. 1997;277:1508–1510. doi: 10.1126/science.277.5331.1508. [DOI] [PubMed] [Google Scholar]
- Ray P, Ghosh SK, Zhang DH, Ray A. Repression of interleukin-6 gene expression by 17 β-estradiol: inhibition of the DNA binding activity of the transcription factors NF-IL6 and NFκ-B by the estrogen receptor. FEBS Lett. 1997;409:79–85. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- Rowan BG, Garrison N, Weigel NL, O'malley BW. 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol Cell Biol. 2000;20:8720–8730. doi: 10.1128/mcb.20.23.8720-8730.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanches R, Nguyen D, Rocha W, White JH, Mader S. Diversity in the mechanisms of gene regulation by estrogen receptors. Bioassays. 2002;24:244–254. doi: 10.1002/bies.10066. [DOI] [PubMed] [Google Scholar]
- Seol W, Hanstein B, Brown M, Moore DD. Inhibition of estrogen receptor action by the orphan receptor SHP (Short Heterodimer Partner) Mol Encocrinol. 1998;12:1551–1557. doi: 10.1210/mend.12.10.0184. [DOI] [PubMed] [Google Scholar]
- Smith LH, Coats SR, Qin H, Petrie MS, Covington JW, Su M, Eren M, Vaughan DE. Differential and Opposing Regulation of PAI-1 Promoter Activity by Estrogen Receptor α and Estrogen Receptor β in Endothelial Cells. Circ Res. 2004;95:269–275. doi: 10.1161/01.RES.0000136521.70093.f1. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Weitzmann MN, Cenci S, Ross FP, Adler S, Pacifici R. Estrogen decrease TNF gene expression by blocking JNK activity and the resulting production of c-Jun and JunD. J Clin Invest. 1999;104:503–513. doi: 10.1172/JCI7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Ohmichi M, Yoshida M, Hisamoto K, Mabuchi S, Arimoto-Ishida E, Mori A, Tsutsumi S, Tasaka K, Murata Y, Kurachi H. Both estrogen and raloxifene cause G1 arrest of vascular smooth muscle cells. J Endocrinol. 2003;178(2):319–29. doi: 10.1677/joe.0.1780319. [DOI] [PubMed] [Google Scholar]
- Webb P, Nguyen P, Valentine C, Lopez GN, Kwok GR, McInerney E, Katzenellenbogen BS, Enmark E, Gustafsson JA, Nilsson S, Kushner PJ. The estrogen receptor enhances AP-1 activity by two distinct mechanisms with different requirements for receptor transactivation functions. Mol Endocrinol. 1999;13:1672–1685. doi: 10.1210/mend.13.10.0357. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thorén P, Smithies O, Gustafsson JA, Mendelsohn ME. Abnormal Vascular Function and Hypertension in Mice Deficient in Estrogen Receptor B. Science. 2002;295:505–508. doi: 10.1126/science.1065250. [DOI] [PubMed] [Google Scholar]