Abstract
The discovery of mirror neurons in macaque frontal cortex has sparked a resurgence of interest in motor/embodied theories of cognition. This critical review examines the evidence in support of one of these theories, namely that the mirror neurons provide the basis of action understanding. It is argued that there is no evidence from monkey data that directly tests this theory, and evidence from humans makes a strong case against the position.
“… we understand action because the motor representation of that action is activated in our brain.”
“The [motor] theory is so simple and so easy to present that every one is glad to believe it. The only question that any one cares to raise is how much of it will the known facts permit one to accept.”
-Walter B. Pillsbury (1911) p. 84
Motor theories of cognition have a long history in psychology (Scheerer, 1984), dating back at least to Berkeley’s motor interpretation of depth perception (Berkeley, 1709), and have been proposed as explanations for a wide range of mental processes. For example, in the early part of the 20th century, Margaret Floy Washburn proposed a motor theory of mental imagery (Washburn, 1914, 1916), and John B. Watson explained thought as nothing more than speech-related sensory-motor processes: “according to my view, thought processes are really motor habits in the larynx” (p. 174) (Watson, 1913). As early as 1910, in the Presidential Address at the American Psychological Association meeting in Minneapolis, Walter B. Pillsbury summarized the prevalence of motor theories in the history of psychology succinctly, “… there is nothing in the mind that has not been explained in terms of movement” (Pillsbury, 1911) (p. 84). He also highlighted the widespread popularity of motor theories in his own time, commenting that, “A reader of some of the texts lately published would be inclined to believe that there was nothing in consciousness but movement, and that the presence of sense organs, or of sensory and associatory tracts in the cortex was at the least a mistake on the part of the Creator” (Pillsbury, 1911) (p. 83).
The mirror neuron theory of action understanding (di Pellegrino, Fadiga, Fogassi, Gallese, & Rizzolatti, 1992; Gallese, Fadiga, Fogassi, & Rizzolatti, 1996; Rizzolatti & Craighero, 2004; Rizzolatti, Fogassi, & Gallese, 2001) is the latest in this long line of motor theories -- the motor theory of speech perception (Liberman, Cooper, Shankweiler, & Studdert-Kennedy, 1967) being a prominent mid-century representative -- and as with motor theories of the past, seems to have a firm grasp on the field. In fact, judging from the frequency of appearance of mirror neuron-related publications in prominent journals, and the range of abilities and disorders to which the theory has been extended (e.g., speech perception, music perception, empathy, altruism, emotion, theory of mind, imitation, autism spectrum disorder, among others), the comments of Pillsbury, appropriately updated, are equally applicable today as they were a century ago.
Pillsbury’s goal in his address was “to attempt a critical if sympathetic survey of the different formulations of the theory and to compare it with the facts” (p. 84). My goal here with respect to mirror neuron theory is the same. Mirror neurons are an interesting class of cells that deserve to be thoroughly investigated and their function fully understood. My view is that the intense focus on one interpretation of mirror neuron function, that of action understanding, has impeded progress on mirror neuron research. Although the action understanding hypothesis is interesting and worthy of investigation, I will argue that it fails dramatically on empirical examination (Mahon & Caramazza, 2005; Negri et al., 2007). I will start by providing a brief review of the properties of mirror neurons, followed a discussion of eight problems for the mirror neuron theory of action understanding.
Mirror neurons: the data
Mirror neurons, which famously respond both when the monkey makes active movements and when it observes the experimenter making meaningful movements, were discovered in frontal area F5 of the macaque monkey (macaca nemestrina) (di Pellegrino et al., 1992; Gallese et al., 1996). Studies of F5 before the discovery of mirror neurons revealed that most cells in that region respond during the execution of motor acts such as grasping, holding, and tearing, and a fraction of these also respond to passive somatosensory (~40%) or visual (~17%) stimulation in the absence of action (Rizzolatti et al., 1988). Accordingly, the region’s function was interpreted as supporting a motor “vocabulary where proximal and distal movement necessary for reaching, grasping, holding and bringing food to the mouth are represented” (Rizzolatti et al., 1988)(p. 506). In this context, responses to visual objects or somatosensory stimulation were interpreted not as the neural basis of object or tactile understanding, but as a mechanism for sensory stimulation to access various motor acts (Rizzolatti et al., 1988). Since the discovery of mirror neurons, interpretation of non-mirror neurons in F5 has not changed among most F5 experts. For example, with respect to “canonical” (i.e., non-mirror) object-responsive neurons in F5, Nelissen et al. (Nelissen, Luppino, Vanduffel, Rizzolatti, & Orban, 2005) state, “These neurons are known to play an important role in the visuomotor transformation for grasping, but they do not appear to have any role in objects’ identification” (p. 334).1
According to the most detailed early study (Gallese et al., 1996) mirror neurons comprised 17% of sampled cells in the portion of F5 that was examined, and exhibit the following properties. The cells were activated when the monkey observed hand and/or mouth movements that were directed toward objects (“goal-directed” actions). Roughly half (55%) were selective for one type of action, with grasping the most frequently represented movement across the population of cells (75% of cells). The majority of cells were either strictly or broadly congruent with their action execution response properties. The cells did not respond to visually presented objects or food items, faces, non-goal-directed body movements, goal-directed actions made using tools (although see (Ferrari, Rozzi, & Fogassi, 2005)), mimicking of grasping in the absence of an object (pantomime), or gestures having emotional meaning. The cells do not exhibit movement preparation activity: they discharge when the monkey observes an action, stop firing when the action terminates, and remain quiet even if the object is moved toward the monkey, firing again only when the monkey initiates its own action. This is an important fact as this property distinguishes mirror neurons from well-known “set-related” neurons in nearby monkey area 6 that discharge before movement onset (Weinrich, Wise, & Mauritz, 1984; Wise & Mauritz, 1985). As important controls for the possibility that “mirror activity” reflected some form of covert movement, Gallese et al. (1996) recorded from the hand area of primary motor cortex (F1 or M1), and recorded EMG activity from several hand and mouth muscles during action observation. No M1 cells fired, and no EMG activity was elicited in response to action observation. On the basis of this evidence, mirror neurons were hypothesized to support “action understanding.”
Since these early studies, mirror neurons have also been found in monkey parietal cortex (Gallese, Fogassi, Fadiga, & Rizzolati, 2002), and problematically (see below), in M1 (Tkach, Reimer, & Hatsopoulos, 2007).
Mirror neurons: The theory
Unlike the majority of the (non-mirror) neurons in macaque area F5, which are argued to support a “motor vocabulary” (Rizzolatti et al., 1988), mirror neurons are claimed to support “action understanding” (di Pellegrino et al., 1992; Gallese et al., 1996; Rizzolatti & Craighero, 2004; Rizzolatti et al., 2001). “Action understanding” is defined somewhat differently in various papers. Gallese et al. (1996) define it as “the capacity to recognize that an individual is performing an action, to differentiate this action from others analogous to it, and to use this information in order to act appropriately” (p. 606). Rizzolatti et al. (2001) propose that action understanding is, “the capacity to achieve the internal description of an action and to use it to organize appropriate future behaviour” (p. 661). Rizzolatti and Craighero (2004) claim, “Each time an individual sees an action done by another individual, neurons that represent that action are activated in the observer’s premotor cortex. This automatically induced, motor representation of the observed action corresponds to that which is spontaneously generated during active action and whose outcome is known to the acting individual. Thus, the mirror system transforms visual information into knowledge” (p. 172). Nelissen et al. (Nelissen et al., 2005) state that “A mere visual representation [of an action], without involvement of the motor system, provides a description of the visible aspects of the movement of the agent, but does not give information critical for understanding action semantics, i.e., what the action is about, what its goal is, and how it is related to other actions” (p. 332). The notion “action understanding” has been generalized in humans to include speech perception (Gallese et al., 1996; Rizzolatti & Arbib, 1998; Wilson, Saygin, Sereno, & Iacoboni, 2004).
It is not obvious from the definitions quoted above what “action understanding” means. For example, with respect to the first definition, upon seeing an individual producing meaningless, non-goal-directed actions (e.g., flailing the arms, which should yield no mirror neuron activity), one could presumably “recognize” that actions are being performed, “differentiate” such actions from other actions (e.g., swinging the arms rhythmically), and “act appropriately” in response (walk away, or call 911), all without “understanding” the meaning of the actions in the goal-directed sense. The nature of the “internal description” in the second definition is itself undefined and therefore adds little clarity to the nature of “action understanding.” In the third definition, the idea that understanding is achieved by knowing the “outcome” is also somewhat vague, because “outcome” is not defined. The fourth definition also includes concepts that are underspecified: what is the action of grasping a peanut “about”? What is the “goal” of such an action? And on what level of analysis is “relation” between actions defined?
The most reasonable interpretation (in my mind) is that what is being “understood” by mirror neurons while observing peanut-grasping is something closer to the concept, ‘grasping-with-the-hand.’ However, Nelissen et al. suggest that mirror neurons are coding more than the “essence of grasping” (p. 334) which they believe is coded in a more anterior region of F5 (Nelissen et al., 2005). In short, the concept of “action understanding” has been evolving, but at its core is the idea that self-generated actions have an inherent semantics and that observing the same action in others affords access to this action semantics.
The existence of mirror neurons has been inferred to exist in humans, beginning with the earliest mirror neuron reports (di Pellegrino et al., 1992; Gallese et al., 1996). These early claims (Gallese et al., 1996) were based on (i) the fact that pantomime recognition deficits exist in aphasia (Gainotti & Lemmo, 1976), (ii) a PET study in humans showing activation in Broca’s region during action observation, (Rizzolatti et al., 1996), and (iii) a transcranial magnetic stimulation (TMS) study that showed enhanced distal muscle motor-evoked potentials (MEPs) during action observation (Fadiga, Fogassi, Pavesi, & Rizzolatti, 1995). However, the empirical basis for the generalization of the mirror neurons to humans was dubious from the start based on the very data that was claimed to support it: (i′) Mirror neurons do not respond to pantomimed actions and so pantomime recognition should not rely on the mirror system. Further, pantomime recognition deficits were not associated with frontal lesions, but rather were predominantly associated with posterior lesions (Heilman, Rothi, & Valenstein, 1982). (ii′) The PET study showing Broca’s region activation during action observation failed to show overlapping activation during grasping production (Rizzolatti et al., 1996), in contrast to the central mirror neuron observation. And (iii′) the TMS finding of peripheral motor activation during action observation directly contradicted the early demonstration in monkeys that M1 and the peripheral motor system did not exhibit mirror properties (Gallese et al. 1996). Mirror neuron findings were also quickly generalized to speech (di Pellegrino et al., 1992; Gallese et al., 1996) on the basis of analogy to the motor theory of speech perception (Liberman et al., 1967). But despite its mirror neuron-led resurgence in popularity among non-speech scientists, the motor theory of speech perception “has few proponents within the field of speech perception” (p. 361) (Galantucci, Fowler, & Turvey, 2006). Thus, the theoretical grounding of mirror neuron theory in the speech domain was not particularly strong.
Mirror neurons have also been generalized to explain imitation (Rizzolatti & Craighero, 2004). This function of mirror neurons, however, has been restricted to humans because macaques (at least adult macaques (Ferrari et al., 2006)) do not imitate (Visalberghi & Fragaszy, 2001). This means that mirror neuron function, as it is studied in macaque monkeys, cannot be the basis of imitation. Rizzolatti and Craighero (2004) emphasize that, “the primary function of mirror neurons cannot be action imitation” (p. 172). Any evidence regarding the neural basis of imitation in humans, therefore, cannot be empirically linked to mirror neurons.
Although the “mirror system” has been used as the basis for understanding a range of behaviors, we will focus our attention on the core function supposed to hold across species, namely action understanding. If mirror neuron theory fails to stand up empirically with respect to its core claim, as I will argue, then linkage between mirror neurons and the many systems and disorders linked to their function is highly dubious.
The perception of a graspable object is sufficient to trigger the activation of cells in motor area F5 (Rizzolatti et al., 1988). Most mirror neuron theorists do not endow cells that respond to the perception of objects with an object semantics. Instead they propose that F5 contains a motor vocabulary, and that sensory (object) responses in F5 cells reflect a means for grasping-related sensory information to access that vocabulary.2 When considering mirror neuron function, it is helpful to adopt this view of F5 function as the null hypothesis. Namely, that F5 is fundamentally a motor area that is capable of supporting sensory-motor associations. In order to make a serious case for mirror neurons as the basis of action understanding, one has to show that they are qualitatively different from other sensory-motor cells in F5, specifically, that they are coding more than just a sensory-motor association (they have a semantics that other sensory cells in F5 do not). In what follows, I will detail eight problems that undermine the claim that mirror neurons go beyond other sensory-motor cells in F5 and support action understanding.
1. There is no evidence in monkeys that mirror neurons support action understanding
The mirror neuron theory of action understanding predicts that disruption of motor areas in F5 should produce deficits in action perception. While functional disruption of macaque area F5 has been shown to disrupt grasping behavior (Fogassi et al., 2001), the predicted corresponding decrement in action perception has never been reported. Rizzolatti and Craighero (2004) argue that such studies are not feasible. This is because (i) the mirror system is bilateral, and involves parietal structures, (ii) there are other mechanisms that mediate action recognition, and (iii) if one lesioned the entire mirror neuron system, more general cognitive deficits would result, making interpretation difficult (Rizzolatti and Craighero, 2004). However, if the claim is that motor systems underlie action understanding, and if it is possible to impair motor behavior by disruption of motor systems in F5 (Fogassi et al., 2001), then it should follow that action understanding will be commensurately impaired. If, on the other hand, motor behavior and action understanding dissociate in macaque following F5 disruption, this would constitute evidence against a critical role for motor systems (and area F5) in action understanding, independently of whether the mirror system extends beyond F5 or not.
In place of the standard lesion method, three studies are held up as evidence that mirror neurons in monkeys support action understanding. One involves the demonstration that some mirror neurons (15%) respond to action-associated sounds presented in isolation (cracking peanut shell, ripping paper) (Kohler et al., 2002). The logic here is that “If mirror neurons mediate action understanding, their activity should reflect the meaning of the observed action, not its visual features” Rizzolatti and Craighero (2004) (p. 173). According to this logic, Kohler et al.’s findings indicate that 85% of mirror neurons do not mediate action understanding because their activity does not reflect the meaning of the perceived action3. But still this leaves a population of 15% of mirror neurons -- the audiovisual type -- that may code the meaning of actions. Does the existence of these audiovisual mirror neurons prove that they are coding meaning? No. A more straightforward interpretation of this result is that sounds can be associated with actions in F5 neurons, just as objects can be associated with actions in F5 neurons (Rizzolatti et al., 1988). Framed in terms of a priming explanation, we might argue that the animal has associated the action of breaking a peanut with the sound of breaking a peanut, and when hearing only the sound, the activation spreads to F5; a form of partial cue retrieval. Right or wrong, the point is that we don’t need to endow these cells with semantic properties to explain the finding.
The second experiment showed that while mirror neurons don’t respond to pantomimed actions (actions without the object present), they do respond if an action is directed toward an object that is hidden behind a screen such that the monkey knows it is there (Umiltà et al., 2001). In this scenario, more than half of the mirror neurons that were tested, also responded in the hidden condition. The logic here is the same, that it is not the physical features of the action that drives the response, but rather the knowledge of the “meaning” of the action. Again, following the logic, the results of the study indicate that half of all mirror neurons are not coding action meaning, and again there is a simpler explanation. The monkey can represent the object in working memory which, according to popular views, involves the same systems that represent the object when it is physically present (Fuster, 1995; Pasternak & Greenlee, 2005; Postle, 2006; Ruchkin, Grafman, Cameron, & Berndt, 2003). This information can then be used in the normal manner as if the object were visible.
Rizzolatti and Craighero (2004) claim that these studies show that “the activity of mirror neurons correlates with action understanding” (p. 174). However, “action understanding” was never actually measured, and there is a simpler explanation of both results, one that fits well with the hypothesized function of the non-mirror neurons in F5, namely that perceptual information --including objects, tactile stimulation, sounds, and actions -- can be associatively linked to, and can prime a “motor vocabulary” in F5 (Rizzolatti et al., 1988).
The third study by Fogassi et al. (Fogassi et al., 2005) uses a different approach to argue for abstract, action understanding properties of mirror neurons. These authors present very interesting data from the inferior parietal lobule (IPL) of monkeys, which also contains mirror neurons, as noted above. Monkeys were trained either to grasp a piece of food and put it in his (the monkey’s) mouth, or to pick up an object and put it in a container. In some conditions, the container was next to the monkey’s mouth such that the mechanics of the movement were very similar between grasping-to-eat and grasping-to-place. In addition, a condition was also implemented in which the monkey grasped and placed a piece of food in the container to control for differences between food items and objects, both visually and tactilely. In all variants of the experiment, the authors report that some IPL cells preferentially responded to the goal of the action: grasping-to-eat vs. grasping-to-place. Again, this was true even when the placing-action terminated in close proximity to the mouth and involved grasping a piece of food. Some of these cells also responded selectively and congruently during the observation of grasping-to-eat and grasping-to-place. So both in perception and action, there are IPL cells that seem to be selective for the specific goal of an action rather than the sensory or motor features of an action -- a very intriguing result. Fogassi et al. discuss their motor findings in the context of “intentional chains” in which different motor acts forming the entire action are linked in such a way that each act is facilitated in a predictive and goal-oriented fashion by the previous ones. They give an example of IPL neurons observed in another unpublished study that respond to flexion of the forearm, have tactile receptive fields around the mouth, and respond during grasping actions of the mouth and suggest that, “these neurons appear to facilitate the mouth opening when an object is touched or grasped” (p. 665). Regarding the action perception response properties of the IPL neurons in their study, Fogassi et al. all conclude, “that IPL mirror neurons, in addition to recognizing the goal of the observed motor act, discriminate identical motor acts according to the action in which these acts are embedded. Because the discriminated motor act is part of a chain leading to the final goal of the action, this neuronal property allows the monkey to predict the goal of the observed action and, thus, to ‘read’ the intention of the acting individual” (p. 666).
According to Fogassi et al., IPL mirror neurons code action goals and can “read the intention” of the acting individual. Perhaps Fogassi et al.’s notion of predictive coding and their example of the IPL neuron with receptive fields on the face can provide a simpler explanation. Suppose the abstract goal of an action and/or it’s meaning is coded outside of the motor system. And suppose that Fogassi et al. are correct in that a complex motor act leads to some form of predictive coding (anticipatory opening of the mouth, salivation, perhaps even forward modeling of the expected somatosensory consequences of the action). The predictive coding in the motor system is now going to be different for the grasping-to-eat versus grasping-to-place actions. For eating, there may be anticipatory opening of the mouth, salivation, perhaps even forward modeling of the expected somatosensory consequences of the action. For placing, there will be no mouth-related coding, but there may be other kinds of coding such as expectations about the size, shape or feel of the container, or the sound that will result if the object is placed in it. If cells in IPL differ in their sensitivity to feedback from these different systems, then it may look like the cells are coding goals, when in fact they are just getting differential feedback input from the forward models. Observing an action may activate this system with similar electrophysiological consequences, not because it is reading the intention of the actor, but simply because the sensory event is associated with particular motor acts.
2. Action understanding can be achieved via non-mirror neuron mechanisms
Rizzolatti and Craighero (2004) noted that the mirror neuron system may not be the only mechanism that can support action understanding. Rizzolatti et al. (2001) also emphasize that “these [mirror neuron] findings do not exclude the possibility that other areas are involved in the description of biological movement and the understanding of action” (p. 662). The existence of other mechanisms for action understanding is a problem for the mirror neuron theory of action understanding for two reasons. One, it places action understanding on par with “object understanding.” Object responses in F5 are not generally interpreted as the neural basis for object understanding (Rizzolatti et al., 1988), presumably because other neural systems in the ventral visual stream support object recognition/understanding. Object information, processed for “meaning” in the temporal lobe, can gain access to motor programs as appropriate for behaviors like grasping, thus explaining the object response properties of F5 cells, even though the meaning of the objects is not coded in these motor areas (Nelissen et al., 2005). If there is a neural network outside of the mirror system that can support action understanding, as Rizzolatti and colleagues suggest, then we can propose an identical form of interaction. Actions are processed for “meaning” in this other system, which via the same associative mechanisms can gain access to motor programs in F5, thus producing “mirror” responses, analogous to object responses.
A candidate region for an action understanding alternative to mirror neurons is the superior temporal sulcus (STS). Cells in portions of the macaque STS respond to a wide range of actions in a manner that appears more sophisticated than that found in mirror neurons. STS neurons respond to actions such as walking towards or away, head turning, movement into or out of view, arm movements, and hand-object interaction where there is selectivity for specific actions including reaching, retrieving, manipulating, picking, tearing, presenting to the monkey, and holding (Perrett, Mistlin, Harries, & Chitty, 1990; Perrett et al., 1985). These cells do not have motor properties in that they don’t appear to fire during action execution (although this hasn’t been investigated thoroughly). Interestingly, the region of the inferior parietal cortex that contains mirror neurons (PF), and which projects to F5, receives input from the STS (Rizzolatti & Craighero, 2004). This would seem to be an ideal circuit for representing actions (STS) and coordinating their interaction (PF) with the motor system (F5).
3. M1 contains mirror neurons
It was recently observed that mirror neurons exist in primary motor cortex of macaque monkeys (Tkach et al., 2007). While this is consistent with the motor evoked potential work in humans (Fadiga et al., 1995), it undermines an important control observation in the original mirror neuron reports. Recall that the lack of mirror neurons in M1 was taken as evidence against the possibility that the monkeys were covertly generating movement responses during the perception of actions. In other words, it ruled out the possibility that “mirror” responses were merely some kind of unimplemented motor command, and opened the door to a more interesting, higher-level function. Now with the demonstration of “mirror” responses in low-level motor circuitry (M1 in macaque, and distal muscles in humans, as demonstrated with TMS), it is entirely possible that “mirror” responses are nothing more than the facilitation of the motor system via learned associations. Tkach et al. (2007) suggest a similar interpretation of their data, namely, “that the neural activity during observation is attributable to the covert generation of a motor command and that [the reason] we observe congruent neural activity during observation [is] because the visual goal, and thus the motor command generated, is the same as during active movement” (p. 13247).
4. The relation between macaque mirror neurons and the “mirror system” in humans is either non-parallel or undetermined
As noted above, mirror neuron function has been generalized to a wide range of human behaviors. Indeed, much of the excitement over mirror neurons is directly related to their potential to explain complex human capacities and disorders. A statement by Oberman et al. (Oberman et al., 2005) illustrates both the extent of the generalization and the excitement: “Mirror neurons are primarily thought to be involved in perception and comprehension of motor actions, but they may also play a critical role in higher order cognitive processes such as imitation, theory of mind, language, and empathy, all of which are known to be impaired in individuals with autism spectrum disorders” (p. 190–191, citation numbers omitted).
The problem with statements such as this, and many like it, is that the species that has been shown to possess mirror neurons does not, to our knowledge, possess any of these higher order cognitive processes, and the species that possesses the higher order cognitive processes, has not been shown conclusively to possess mirror neurons (Chong, Cunnington, Williams, Kanwisher, & Mattingley, 2008; Dinstein, 2008; Dinstein, Hasson, Rubin, & Heeger, 2007; Dinstein, Thomas, Behrmann, & Heeger, 2008). To be sure, there have been a host of studies aimed at investigating the “mirror system” in humans, but much of this work has investigated behaviors that mirror neurons could not possibly support given their response properties in monkeys4, and therefore the connection between these behaviors and mirror neurons is tenuously based on a chain of assumptions: mirror neurons exist in humans (there are individual cells that respond both during action execution and action perception), mirror neurons have evolved to support functions in humans that they do not support in monkeys, this evolution has conserved the functional properties found in monkeys, and mirror neurons are responsible for the behavior in question. There is nothing wrong with using animal models to generate testable hypotheses in humans -- indeed, this is a productive and important research strategy. The problem in the case of mirror neurons is that the system has been generalized to humans without systematic validation, and with the wholesale adoption of the mirror neuron doctrine concerning action understanding. When a human study starts with the assumption that mirror neurons support action understanding (see above quote from Oberman et al.), and that a homologous and functionally enriched system exists in humans, it is then an easy and prima facie logical inference that the human mirror system can support higher-order functions such as language and empathy. But this inference falls apart if any of the assumptions about mirror neurons are incorrect. Thus, my caution here is not that we can’t or even shouldn’t use mirror neurons to guide human research, but that we have to first validate our assumptions before making inferences regarding human behaviors, especially those that don’t exist in monkeys.
Let me illustrate the problem with an abstract argument. Suppose that Rizzolatti and colleagues are correct, namely that mirror neurons in monkeys are the basis for action understanding, but not imitation (because adult macaques don’t imitate). In humans, the “mirror system” behaves differently than in monkeys such that it appears to support imitation (Rizzolatti & Craighero, 2004): it activates during the perception and execution of even meaningless movements (Iacoboni et al., 1999). This observation has led some mirror neuron theorists to argue that the mirror system in humans has evolved to support not only action understanding (based on inferences from monkey data), but also imitation (based on human data) (Rizzolatti & Craighero, 2004). The assumption made by these authors is that in the evolution of this system, old properties of mirror neurons are fully conserved. But what if the mirror system evolved in humans such that it now supports imitation but no longer supports action understanding? Perhaps humans evolved a more sophisticated semantic system, distinct from the motor system, that freed the mirror system to support imitation. Possibilities such as this are not considered in mainstream mirror neuron theorizing. Instead, monkey data and theories are typically imported to human work without empirical validation of the assumptions.
Here is a concrete example of how monkey data are assumed to hold, problematically, in human work. In the context of studying the human “mirror system,” a number of functional imaging experiments have investigated the perception of meaningless gestures, pantomimed gestures, and imitation (Decety et al., 1997; Grezes, Costes, & Decety, 1998; Iacoboni et al., 1999; Koski, Iacoboni, Dubeau, Woods, & Mazziotta, 2003; Koski et al., 2002). These studies, which often implicate portions of the inferior frontal gyrus and the inferior precentral gyrus, are cited as evidence for the existence of a human “mirror system” that has evolved to support imitation (Rizzolatti & Craighero, 2004). But this is not the only interpretation. There are at least three logical possibilities. (i) Mirror neurons do not exist in humans, and the activation in these studies results from the function of some other system. (ii) Mirror neurons exist in humans exactly as they do in monkeys (with the same properties), and the activations in these studies result from the function of some other system. (iii) Mirror neurons exist in humans, but have evolved such that they now support pantomime recognition and imitation. The third interpretation is typically adopted while the other possibilities are not even considered. Why? In monkey mirror neuron research other possibilities were considered. Gallese et al. (1996) considered both the possibility that mirror neurons reflected “set-related” responses and the possibility that mirror neurons were reflecting a non-implemented motor plan (see above). Because these possibilities were ruled out empirically in monkeys, it is assumed (probably implicitly) that there is no need to rule them out in humans. But this is faulty logic. If mirror neurons exist in humans as is claimed, the system is demonstrably different from that in the monkey. One therefore cannot assume that monkey data will hold in the human system. The alternative possibilities have to be ruled out empirically again. Consider in this respect a highly cited study of imitation in the human mirror system (Iacoboni et al., 1999), which found equivalent activation during the passive perception of an action (a moving hand), a static hand, and a rectangle with a spatial cue (to which subjects were previously trained to make a hand movement). The authors explain the activation to the latter non-action stimulus is this way: “During all scans the participants knew that the task was either to move a finger or to refrain from moving it. Thus mental imagery of their finger (or of the finger movement) should have been present even during simple observation.” (Iacoboni, et al. 1999, p. 2526–2527). This would suggest that it is not action perception that is driving these “mirror activations,” but simply the internal activation of a motor act. Indeed, there is evidence that human area 44, a presumed component of the human mirror system, is involved in movement preparation (Krams, Rushworth, Deiber, Frackowiak, & Passingham, 1998).
There are also a number of studies that have investigated the human mirror system using behaviors that do hold of mirror neurons, namely object-directed actions. A recent meta-analyses of fMRI studies of the human “mirror system” (Morin & Grezes, 2008) has suggested that human BA 44 is not the homologue of the macaque mirror neuron F5 region, as this region is insensitive to the presence or absence of target objects in action perception. That is, BA 44 does not distinguish between object-directed actions and actions that are non-object directed. Instead Morin and Grezes (2008) point out that a more posterior region, ventral premotor cortex (BA6), is activated significantly more often during the perception of object-directed action than actions without object goals. Accordingly, these authors propose ventral BA 6 as the human homologue of the mirror system.
While Morin and Grezes’ hypothesis is quite reasonable and based on direct parallels with the macaque mirror neuron system, it remains to be experimentally verified. For example, a nontrivial fraction (36%) of the studies reviewed by Morin and Grezes reported that perception of non-object directed actions activated ventral BA 6. What drove these activations? Also, it will be important to confirm that this area has overlapping sensory-motor response properties. Surprisingly, many investigations of the mirror system fail to confirm this fundamental property of mirror neurons -- another example of unverified generalization from monkey to human work. Indeed, Morin and Grezes’ review, which aimed explicitly to identify the human homologue of the macaque mirror neuron system, focused exclusively on perceptual responses. It will be important to determine whether the response properties of this ventral BA 6 region can be linked directly to action processing, or whether it might be performing a more general function, on which action processes rely. For example, recent fMRI and lesion evidence has implicated this region in predicting sequences of abstract non-biological stimuli, suggesting a more general functional role involving sequence processing (Schubotz, Sakreida, Tittgemeyer, & von Cramon, 2004; Schubotz & von Cramon, 2004).
Other evidence often cited as support for the existence of a human “mirror system” is the demonstration that viewing actions can result in peripheral motor potentiation. TMS stimulation of motor cortex produces motor evoked potentials in distal muscles. The amplitude of MEPs in distal muscles is enhanced during action observation (Fadiga et al., 1995), which as been interpreted as evidence for a human “mirror system.” While this work shows clearly that associations between observed actions and motor execution systems exist, it does not indicate that these associations are mediated by anything like macaque mirror neurons. For example, TMS/MEP data cannot rule out the possibility that the link between action observation and action execution could be mediated by a non-motor conceptual representation.
The relation between the macaque mirror neuron system and the hypothesized human homologue remains to be elucidated (for recent discussion and evidence on this debate see (Chong et al., 2008; Dinstein et al., 2007; Dinstein et al., 2008)). This in itself, however, is not an argument against the mirror neuron theory of action understanding. By the same token, even if a network with mirror-neuron like properties can be fully outlined in humans, this in itself would not be an argument for the mirror neuron theory of action understanding. Such as argument requires a different sort of evidence; this is the topic of the next section.
5. Action understanding in humans dissociates from neurophysiological indices of the human “mirror system”
There are examples in the human “mirror system” literature of dissociations between action understanding and “mirror system” function. One study (Buccino et al., 2004) examined functional activations during the perception of biting actions or communicative gestures performed by a human, a monkey, or a dog. Independent of the species performing the action, viewing biting actions activated regions thought to be part of the human “mirror system,” the left inferior frontal gyrus and precentral gyrus (among other areas). Viewing communicative gestures elicited activation of these frontal “mirror systems” for actions performed by a human (lip-reading) and a monkey (lip smacking), but not a dog (barking). On the assumption that the study participants “understood” all three communicative actions, it is interesting that only the human and monkey actions resulted in “mirror system” activation. (At least subjects very likely understood that the lip-reading action was associated with speech and the barking action was associated with barking. The monkey action arguably contained less semantic information.) This result clearly shows that actions can be understood without the “mirror system,” or more to the point, that “mirror system” activity is not particularly correlated with action understanding. Of interest is that the STS was activated across all conditions.
Another demonstration of the dissociability of the “mirror system” from action understanding comes from a TMS/MEP study (Catmur, Walsh, & Heyes, 2007). The authors used TMS to induce motor evoked potentials in the abductor muscles of the hand. When subjects watched a video of a hand with index finger abduction, the MEPs were greater in the subjects own index finger, whereas when the video showed movement of a hand with little finger abduction, MEPs were greater in the little finger of the observer. This is the standard “mirror” MEP effect. The investigators then trained subjects to move their fingers in a manner incongruent with the hand in the video: move little finger when index finger movement is shown and vise versa. After training, MEPs were greater in the little finger when index finger movement was observed, and vise versa. “Mirror” effects can be trained simply by sensory-motor association. The important implication of this result is that study participants who exhibited incongruent MEP responses, presumably did not mistake the perception of index finger movement for little finger movement and vise versa. This indicates that a prominent indicator of human “mirror system” activity (Fadiga et al., 1995) dissociates from action understanding.
It should not be surprising that measures of human “mirror system” function dissociate from action understanding, as we are fully capable of understanding actions we have never produced. For example, musically untrained people can recognize, say, saxophone playing even if they’ve never touched the instrument, just as one can recognize actions of non-conspecifics (barking, flying). Similarly, it would be surprising, maladaptive even, if all observed actions resulted in the activation of the exact same motor program in the observer. Indeed, most sports would be impossible to play, as the observation of an object-directed action (throwing a ball) would result in the activation of the same action in the observer when a very different action is required (catching or blocking). The same is arguably true in many daily activities. The results of Catmur et al. show that presumed “mirror system” activity isn’t mirroring anything, but rather reflects adaptive task-dependent sensory-motor associations.
6. Action understanding and action production dissociate
As noted above, there is no evidence that deactivation of the monkey mirror system disrupts action understanding. The issue has been taken up in human research, however, where there are now several published studies that investigate action recognition. This work is suitable for testing several predictions of the (human extrapolated) mirror neuron theory of action understanding. One such prediction is that action understanding and action production should be strongly correlated. While it has been found that these two abilities can be correlated in group studies, there is strong evidence that they also are quite dissociable.
Several recent studies have investigated the issue. One assessed a sample of 21 patients with limb apraxia and found a strong correlation between gesture production (imitation of meaningful gestures) and gesture recognition (determining which of two sequentially presented gestures match a gesture name) (Buxbaum, Kyle, & Menon, 2005). However, because the production measure has a perceptual component, a deficit affecting only perception could lead to correlated deficits on the recognition and production tasks. Further, the recognition task involved some form of working memory: subjects had to remember the gesture name (an auditorily and visually presented verb, eg., “hammering”), and two gestures that were presented sequentially with a 2 second inter-stimulus interval. If working memory for gestures recruits some form of motor-related rehearsal component, as is the case for speech (Baddeley, 1992), then both tasks shared a production component, which may also have contributed to the correlation.
Another study (Pazzaglia, Smania, Corato, & Aglioti, 2008) also tested a sample of 21 patients with limb apraxia and found a correlation (r ≈ .5) between a gesture discrimination task (judging whether or not an action is performed correctly) and a gesture production task (asking subjects “to perform seven complex actions that required the use of real objects” p. 3031). However, a cluster analysis showed that while 14 of the 21 patients with limb apraxia had “a severe gesture recognition deficit” 7 patients “presented with no deficit” (p. 3034) indicating that the two abilities are dissociable.
A third study (Tessari, Canessa, Ukmar, & Rumiati, 2007) of unselected left hemisphere damage patients (n=22) reported a weaker correlation between gesture imitation and action (pantomime) recognition (r = .32; again, not surprising because imitation involves a recognition component), but no correlation between action recognition and real object use (r = −0.13), which arguably provides a better assessment of “mirror system” function. Importantly, double-dissociations were evident across patients in the latter relation: Case 23 performed at 20% accuracy on action recognition, but 100% on object use, whereas Case 15 performed at 100% accuracy on object recognition and 57% on object use. Other cases showed similar dissociations.
Similar findings of group-level gesture perception-production correlation, but case-level dissociations were obtained by Negri and co-workers (Negri et al., 2007). This study tested an unselected group of 37 patients with unilateral brain lesions on several tasks including pantomime recognition, pantomime imitation, object use, and object recognition. Significant correlations were found between object use and pantomime recognition (r = .58), object use and object recognition (r = .37), pantomime imitation and recognition (r = .59), and pantomime imitation and object use (r = .79). However, despite these group trends, subsets of patients demonstrated dissociations between each of the correlated pairs of tests including double-dissociations between object use and pantomime recognition, and object use and object recognition. The authors of this study conclude that “…(a) The ability to use objects is not necessary in order to be able to recognize object-associated pantomimes; (b) the ability to imitate pantomimes is not necessary in order to be able to recognize object-associated pantomimes; and (c) the ability to use objects is not necessary in order to be able to recognize objects” (p. 806).
Sign language provides additional evidence for the dissociation between action production and action understanding. For example, case “Gail D.” presented with very severe deficits in sign language production associated with a large left frontal lobe lesion, yet her comprehension of sign language was well-preserved (Poizner, Klima, & Bellugi, 1987).
In summary, while gesture production and gesture recognition can be correlated in groups of both apraxic and unselected patients with focal brain lesions, these abilities double-dissociate, contrary to the prediction of the mirror neuron theory of action understanding.
7. Damage to the inferior frontal gyrus is not correlated with action understanding deficits
If the human homologue of F5 is BA44/6, then damage to this region should result in action understanding deficits. Available evidence does not support this prediction. For example, based on earlier research, Heilman and colleagues (Heilman et al., 1982) have argued that lesions to the parietal lobe are associated with both production and comprehension deficits, whereas frontal lesions produce only production deficits. A mirror neuron proponent may counter than the parietal lobe also contains mirror neurons, and thus the association between parietal lobe damage and action understanding deficits could be viewed as consistent with “mirror system” claims. Following this line of argument one would have to conclude that portions of the mirror system that are more closely aligned with the motor system, BA44/6, do not support action understanding. This is clearly contrary to the central claim of Rizzolatti and colleagues that it is motor representations that underlie action understanding.
More recent studies using modern lesion analysis methods have provided mixed results regarding the anatomical correlate of action understanding deficits. One such study (Buxbaum et al., 2005) confirmed earlier observations showing an association between deficits in object-related gesture recognition and lesions to the inferior parietal lobe, whereas another study (Saygin, Wilson, Dronkers, & Bates, 2004) reported that action comprehension is associated with lesions to BA44/6/4. However, this latter study examined a sample of aphasic patients which may have biased their findings compared to studies that use unselected patients or patients selected on the basis of gesture-related deficits. Further, Saygin et al. did not use dynamic actions for their stimuli, but rather static pictures of pantomimed actions (the subject then pointed to the pictured object that best fit the action). The relation between action understanding in dynamic actions and static actions is unknown, so interpretation of this study is further compromised. However, it is relevant that deficits in the understanding of linguistically specified actions (written phrases such as, ‘She is sweeping the…’ followed by the same picture choices used in the “action” condition) dissociated behaviorally from understanding of pictured actions, and were not associated with lesions to BA44/6/4, but with portions of the superior temporal gyrus, insula, and inferior parietal lobe. One can conclude from the behavioral and neural dissociation between pictured actions and linguistically specific actions, that what is being mapped in this study, and associated with BA44/6/4 in the picture condition, is not “action semantics,” as access to this information is available via other routes. Thus, this study provides evidence against the view that the meaning of actions is encoded in motor representations in motor cortex.
Another recent study (Pazzaglia et al., 2008) appears to provide compelling evidence for an association between IFG damage and deficits in action understanding. Lesions in patients with limb apraxia and gesture discrimination deficits were compared with lesions in patients with limb apraxia but without gesture discrimination deficits. Subtraction of the lesions in these two groups of patients identified left IFG as being associated with the limb apraxia plus gesture discrimination deficits. A voxel-based lesion-symptom mapping analysis showed the same result. However, an examination of the relation between the amount of damaged tissue in the IFG and gesture discrimination scores in the group of patients who had gesture discrimination deficits showed no relation (Figure 1, circles). For example, the four patients with the most IFG involvement (Figure 1, right solid rectangle) had gesture discrimination scores that are indistinguishable from the three patients with the least IFG involvement (left solid rectangle), and the latter are themselves well within the distribution of patients without gesture discrimination deficits (left dashed rectangle) in terms of the amount of IFG involvement.
Figure 1.
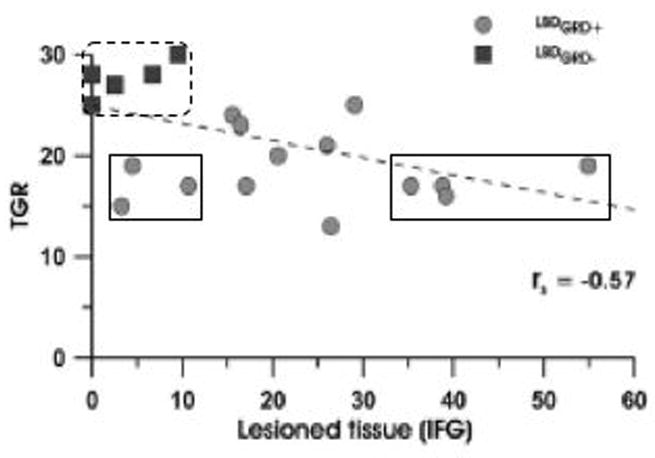
Scatterplot showing the relation between gesture recognition and the amount of lesioned tissue in the inferior frontal gyrus left hemisphere damaged patients with apraxia. Dark square points are patients without gesture recognition deficits; lighter circle points are patients with gesture recognition deficits. Solid rectangles are aligned on the y-axis and show that patients at the extremes of the distribution of IFG tissue damage have indistinguishable scores on gesture recognition. Dotted rectangle outlines patients without gesture recognition deficits for comparison. Figure modified from (Pazzaglia et al., 2008).
Clearly, IFG involvement is not predicting gesture discrimination performance. It is unclear why the lesion subtraction and voxel-based mapping analyses identified the IFG in this study, but the fact that these analyses were calculated using a measure that was not corrected for response bias may be a factor (the task was implemented using a signal detection paradigm, but percent correct rather than the bias-corrected d′ statistic was used for lesion analyses).
Two recent rTMS experiments have studied the effects of functional disruption of ventral premotor cortex (vPMc) on visual discrimination of action-related pictures (Urgesi, Calvo-Merino, Haggard, & Aglioti, 2007; Urgesi, Candidi, Ionta, & Aglioti, 2007). In both of these studies, subjects were asked to make two-choice, match-to-sample judgments: a picture of a body configuration was presented (the sample) followed by a mask (500msec), and then a picture of two body configurations; the subject was asked to indicate which of the two matched the sample. First, it is important to note that neither of these studies actually tested action understanding. That is, discrimination performance did not depend on understanding the meaning of the actions, but could be performed based on configural information alone. One study (Urgesi, Candidi et al., 2007) compared the effects of interference stimulation of vPMc with interference stimulation of a ventral temporal-occipital location (the extrastriate body area, EBA) during action discrimination (which action matches the sample?) versus form discrimination (which actor matches the sample, independent of action?). For action judgments vPMc stimulation yielded longer reaction times that EBA stimulation, and the reverse held for form judgments, longer reaction times for EBA stimulation than vPMc stimulation. Stimulation had no effect on accuracy. In the other study (Urgesi, Calvo-Merino et al., 2007), which seemed to involve more difficult stimuli and only asked subjects to judge body configuration, an effect of accuracy was observed with vPMc stimulation associated with more errors on the configuration matching task than with EBA stimulation. Oddly, there were no reaction time effects.
So two studies show that interference stimulation to vPMc negatively affects performance on a body configuration delayed matched-to-sample task. Again, because these studies did not assess action understanding, they cannot speak to the question of whether the mirror system supports action understanding. However, they do suggest that processing of body configurations at least in the delayed match-to-sample task involves vPMc to some extent. Given that the tasks involved working memory, it seems possible that this region may support some sort of working memory for body configurations. This is consistent with many claims regarding the sensory-motor nature of working memory systems (Buchsbaum & D’Esposito, 2008; Hickok, Buchsbaum, Humphries, & Muftuler, 2003; Pa, Wilson, Pickell, Bellugi, & Hickok, in press; Postle, 2006; Ruchkin et al., 2003; Wilson, 2001).
More work is needed to characterize the neural basis of “action understanding.” Available evidence, however, leads us to conclude that the inferior frontal gyrus does not play a central role.
8. Generalization of the mirror system to speech recognition fails on empirical grounds
Mirror neuron function has been generalized to speech perception from the earliest reports (Gallese et al., 1996; Rizzolatti & Arbib, 1998). Basing their speculation on the motor theory of speech perception (Liberman et al., 1967; Liberman & Mattingly, 1985), Rizzolatti and colleagues suggested that mirror neurons may underlie the perception of speech gestures. The motor theory of speech perception had been all but abandoned among the majority of speech scientists when mirror neurons were discovered, but has enjoyed a healthy revival since. However, there is exceptionally strong evidence against the motor theory of speech perception, and consequently the mirror neuron generalization of action understanding to the speech domain.
A motor theory of speech perception makes a very clear and strong prediction. Damage to the motor speech areas should produced deficits in speech recognition. In fact, damage to motor speech areas, evidenced in many cases by large left frontal lesions and severe speech production deficits, do not typically lead to speech recognition deficits. Paul Broca’s original case, Leborne, is representative of this pattern in that the patient could produce little more than the syllable ‘tan’ yet “understood almost all that was said to him” (p. 63) (Broca, 1861/1960). Much subsequent work has confirmed the pattern at least at the single word level (Goodglass, 1993; Goodglass, Kaplan, & Barresi, 2001)5. For example, a recent study reported that Broca’s aphasics (n=9) were indistinguishable from control subjects on an auditory word comprehension test involving 236 items (Moineau, Dronkers, & Bates, 2005). Lesions associated with Broca’s aphasia tend to be relatively large, involving most of the lateral frontal lobe, motor cortex, and anterior insula but often also extend posteriorly to include the parietal lobe (Damasio, 1992; Damasio, 1991; Dronkers, Redfern, & Knight, 2000); thus the entire left hemisphere “mirror system” can be affected in Broca’s aphasia. A motor theory of speech recognition has no explanation for the existence of a syndrome such as Broca’s aphasia.
While proponents of motor theories of speech recognition typically completely ignore the speech comprehension abilities of Broca’s aphasics -- e.g., a recent review of the motor theory of speech perception failed to even mention the syndrome (Galantucci et al., 2006) -- it is more often noted by motor theorists that Broca’s aphasics can be impaired on syllable discrimination tasks, i.e., the ability to judge whether pairs of non-sense syllables are the same (/ba/ - /ba/) or different (/ba/ - /da/) (Blumstein, 1995). Although this would appear to provide evidence favoring a motor theory of speech recognition, it does not. Performance on such tasks doubly dissociates from measures of auditory comprehension. For example, Miceli et al. (Miceli, Gainotti, Caltagirone, & Masullo, 1980) report 19 patients who were impaired relative to controls on a syllable discrimination task, yet performed at 100% accuracy in matching a spoken word (e.g., “bear”) to a picture presented along with three foil pictures one phonemically related (e.g., PEAR), one semantically related (e.g., MOOSE), and one unrelated (e.g., GRAPES). Another nine patients in that study showed the reverse pattern of performance; they were impaired relative to controls on the auditory comprehension task, but performed normally on the syllable discrimination task. Patients with non-fluent speech production deficits, such as Broca’s aphasics, are typically the most impaired on syllable discrimination tasks (Basso, Casati, & Vignolo, 1977) (Figure 2). This indicates that syllable discrimination tasks are tapping some ability, or abilities (e.g., working memory, executive, or attentional processes) that is/are not necessary for normal, ecologically valid speech recognition, and therefore are not valid measures for assessing speech recognition (see (Hickok & Poeppel, 2000, 2004, 2007) for review and extensive discussion).
Figure 2.
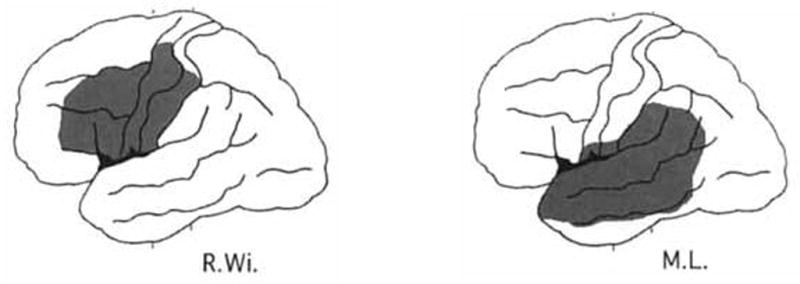
Reconstructed brain lesions in two patients from Caplan et al. (Caplan, Gow, & Makris, 1995). Case R.Wi. is a Broca’s aphasic with good auditory comprehension (by definition) whereas Case M.L. is a Wernicke’s aphasic with relatively poor comprehension (by definition). On a syllable discrimination task R.Wi. performed at 72% correct (A′ = .72) whereas M.L. performed much better at 90% correct (A′ = .90). Figure modified from (Caplan et al., 1995).
Another syndrome that clearly demonstrates the dissociability of motor-speech functions and speech understanding is mixed transcortical aphasia, sometimes referred to as “isolation of the speech zone” (Bogousslavsky, Regli, & Assal, 1988; Geschwind, Quadfasel, & Segarra, 1968) This syndrome is characterized by a severe deficit in the comprehension of speech, despite the well-preserved ability to repeat heard speech, sometimes compulsively. The syndrome is associated with damage to left frontal and posterior parietal regions but with sparing of perisylvian speech-related areas such as Broca’s area, superior temporal gyrus, and the tissue in between. This pattern of damage leaves sensory-motor functions of speech intact (explaining repetition ability), while apparently disrupting systems involved in mapping speech onto conceptual-semantic representations. This is the reverse dissociation compared to Broca’s aphasia, and indicates that preservation of motor speech functions is neither necessary nor sufficient for speech understanding.
In short, data from lesion studies of speech processing unequivocally demonstrate that the motor theory/mirror neuron theory of speech perception is incorrect in any strong form. This is not to say that sensory-motor circuits cannot contribute to speech recognition. Top-down processes initiated in any frontal circuit (not just motor) may be able to influence speech recognition to some extent via sensory-motor circuits. This may be particularly useful under noisy listening conditions (Moineau et al., 2005). But this influence is modulatory, not primary.
What role does the “mirror system” play in action understanding?
The evidence reviewed above shows that action understanding can doubly-dissociate from “mirror system” function, both in the domain of manual gesture and speech. Clearly then, the “mirror system” is not the basis for action understanding.
What does the “mirror system” reflect? There are two possibilities, as suggested by Mahon and Caramazza in their critical analysis of “embodied cognition” hypotheses, generally (Mahon & Caramazza, 2008). One is that it reflects pure Pavlovian association. Pair a tone with a puff of air to the eye and pretty soon the tone alone will elicit a blink response. This motor response to a sensory event does not indicate that the blink response is coding the meaning of the tone. Perhaps the activity of mirror neurons simply reflects sensory-motor pairings. The fact that “mirror system” activity can be dynamically re-mapped with training (Catmur et al., 2007), lends some support for this idea. Mahon and Caramazza suggest another possibility. Concepts, including action-related concepts, involve an abstract level of representation that is distinct from sensory-motor systems. These abstract representations are sufficient for recognition, but can be associated with related sensory-motor information that “colors conceptual processing, enriches it, and provides it with a relational context” (p. 68). So one can have a perfectly viable concept of ‘saxophone playing’ without ever having touched such an instrument, and without the concept being tied to a specific sensory-motor situation (e.g., the concept may apply equally well to playing an alto or tenor sax, a toy instrument, or to mimicking saxophone playing on some other object). However, knowledge of how to grasp a saxophone, finger the keys, and position one’s mouth on the mouthpiece, can, according to Mahon and Caramazza, augment the abstract concept by providing a specific sensory-motor association. This information might even lead to a different “understanding” of a saxophone-playing action; for example, in a situation where the player is holding the instrument improperly, the observer with sensory-motor experience with a saxophone might recognize that the player is not an expert, whereas someone without such experience may not be able to access this “enriched” knowledge. Or in other situations, sensory-motor knowledge may allow the observer to generate predictions about subsequent actions that could influence sensory systems in a top-down fashion and facilitate subsequent perceptual recognition. The view promoted by Mahon and Caramazza admits that motor knowledge can influence or augment action “understanding” to some degree, but without committing to the empirically untenable position that action understanding is dependent on the motor system. This is a desirable result and deserves empirical evaluation.
While is seems entirely possible that motor experience can augment conceptual understanding in some situations, in others, mirror-like activity appears to reflect sensory-motor associations that are devoid of meaningful conceptual content. “Mirror system” activity that has been observed during the imitation of meaningless gestures (Iacoboni et al., 1999) is one such situation. The demonstration that “mirror activity” associated with viewing actions can be re-mapped such that it becomes associated with a completely different action (Catmur et al., 2007) is another. Thus, perhaps both of the possibilities raised by Mahon and Caramazza apply to the “mirror system.”
Conclusion
Mirror neurons are a fascinating class of cells that deserve to be thoroughly investigated in the monkey, and explored systematically for possible homologues in humans. The early hypothesis that these cells underlie action understanding is likewise an interesting and prima facie reasonable idea. However, despite its widespread acceptance, the proposal has never been adequately tested in monkeys, and in humans there is strong empirical evidence, in the form of physiological and neuropsychological (double-) dissociations, against the claim.
Why does the hypothesis remain prominent, indeed all but accepted as fact, despite solid evidence to the contrary? I suggest that Pillsbury was right. Motor theories are simple and easy to understand: “… we understand action because the motor representation of that action is activated in our brain” Rizzolatti, Fogassi, and Gallese (2001) p. 661. We see someone pouring liquid from a bottle into a glass; this activates a motor representation associated with our own liquid-pouring experiences, and voilà, we have understanding. But scratch the surface of action understanding and it is immediately clear that the problem is not that simple (Pinker, 1989, 2007). For example, the motor act of pouring liquid from a bottle into a glass could be understand as pouring, filling, emptying, tipping, rotating, inverting, spilling (if the liquid missed its mark), defying/ignoring/rebelling (if the pourer was instructed not to pour), and so on. A motor representation can’t distinguish between the range of possible meanings associated with such an action. A mirror neuron theorist might protest that it is the goal or intention that is coded by mirror neurons not the specific actions (Fogassi et al., 2005). But a goal, say to fill a glass with water, can be accomplished with any number of individual actions or sequence of actions: pouring from a pitcher, turning a spigot, dipping the glass in a lake, setting the glass in the rain, positioning an array of leaves to collect and funnel dew into the glass, digging a well and pumping water into the glass, or even commanding someone else to do any of these! Given the range of meanings associated with a specific action and the range of actions that can achieve a specific goal, there must be a clear distinction between goals and the motor routines that are implemented in a given circumstance to achieve those goals. If mirror neurons are reflecting goals and not actions, then a statement about mirror neurons such as, “… we understand action because the motor representation of that action is activated in our brain” (Rizzolatti, Fogassi, and Gallese (2001) p. 661) is either false because mirror neurons do not code actions, or it is false because motor representations are not the basis of action understanding.
Unfortunately, more than 10 years after their discovery, little progress has been made in understanding the function of mirror neurons. I submit that this is a direct result of an overemphasis on the action understanding theory, which has distracted the field away from investigating other possible (and potentially equally important) functions.
Acknowledgments
I would like to thank Richard Ivry, Steven Pinker, David Poeppel, and Stephen Wilson for invaluable comments on an earlier draft of this paper. This work was supported by NIH grant #DC0361.
Footnotes
Some theorists have suggested that object recognition is dependent on action-related motor systems (Gallese & Lakoff, 2005). This theoretical position will not be discussed here, but see Mahon & Caramazza (2005) and Negri et al. (2007) for a critical evaluation.
As Richard Ivry has pointed out (personal communication), the “sensory” activity in F5 may be explained rather straightforwardly in terms of motor priming. Suppose action concepts are represented upstream to F5. In self-generated movement, the links between an action concept and its associated motor code in F5 become activated. During object or action observation these links are automatically re-activated, primed, as a result of their prior association. So on this view, “sensory” activity in F5 cells need not even involve a mechanism to access a motor vocabulary, but rather may be the motor reflection of that access process.
A reviewer suggested that this argument is “totally nonsense” and suggested instead that the finding may indicate “that only 15% of mirror neurons code the meaning of the perceived action also on the basis of its sound and not only on the basis of its visible outcome.” Let me be clear: this is not my argument. It is Rizzolatti and colleagues’. If the claim is that the 15% of mirror neurons respond to action-related sounds because they are coding the abstract meaning of the action irrespective of the sensory input -- “audiovisual mirror neurons code abstract contents -- the meaning of actions” (Kohler et al. 2002) (p. 846) -- then it follows that the remaining 85% are not coding the abstract meaning, but rather something sensory specific.
For example, the strongest evidence to date for the existence of mirror neurons in humans comes from a study (Chong et al., 2008) that used fMRI to assess adaptation (repetition suppression) across gesture execution-observation tasks. This study reported an adaptation effect in the right parietal lobe using pantomimed gestures -- a stimulus that doesn’t activate macaque mirror neurons. The location of the effect is also puzzling in that it is inconsistent with human data from apraxia which typically is associated with left hemisphere disease (see below).
Broca’s patients often have some comprehension difficulty at the sentence level. However, these deficits are primarily restricted to sentences in which successful comprehension depends on accurate syntactic analysis (e.g., “He showed her the baby pictures” vs. “He showed her baby the pictures”). If lexical information provides clues to correct interpretation (“The apple that the boy ate was red”), Broca’s aphasics usually perform well in comprehension assessments, providing further evidence for well-preserved word-level comprehension (Caramazza & Zurif, 1976).
References
- Baddeley AD. Working memory. Science. 1992;255:556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Basso A, Casati G, Vignolo LA. Phonemic identification defects in aphasia. Cortex. 1977;13:84–95. doi: 10.1016/s0010-9452(77)80057-9. [DOI] [PubMed] [Google Scholar]
- Berkeley G. An essay towards a new theory of vision. Dublin: Pepyat; 1709. [Google Scholar]
- Blumstein S. The neurobiology of the sound structure of language. In: Gazzaniga MS, editor. The cognitive neurosciences. Cambridge, MA: MIT Press; 1995. pp. 913–929. [Google Scholar]
- Bogousslavsky J, Regli F, Assal G. Acute transcortical mixed aphasia. A carotid occlusion syndrome with pial and watershed infarcts. Brain. 1988;111(Pt 3):631–641. doi: 10.1093/brain/111.3.631. [DOI] [PubMed] [Google Scholar]
- Broca P. Remarks on the seat of the faculty of articulate language, followed by an observation of aphemia. In: von Bonin G, editor. Some papers on the cerebral cortex. Oxford: Blackwell Scienfitic Publications; 18611960. [Google Scholar]
- Buccino G, Lui F, Canessa N, Patteri I, Lagravinese G, Benuzzi F, Porro CA, Rizzolatti G. Neural circuits involved in the recognition of actions performed by nonconspecifics: an FMRI study. J Cogn Neurosci. 2004;16(1):114–126. doi: 10.1162/089892904322755601. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, D’Esposito M. The search for the phonological store: from loop to convolution. J Cogn Neurosci. 2008;20(5):762–778. doi: 10.1162/jocn.2008.20501. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Kyle KM, Menon R. On beyond mirror neurons: internal representations subserving imitation and recognition of skilled object-related actions in humans. Brain Res Cogn Brain Res. 2005;25(1):226–239. doi: 10.1016/j.cogbrainres.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Caplan D, Gow D, Makris N. Analysis of lesions by MRI in stroke patients with acoustic-phonetic processing deficits. Neurology. 1995;45:293–298. doi: 10.1212/wnl.45.2.293. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Zurif EB. Dissociation of algorithmic and heuristic processes in sentence comprehension: Evidence from aphasia. Brain and Language. 1976;3:572–582. doi: 10.1016/0093-934x(76)90048-1. [DOI] [PubMed] [Google Scholar]
- Catmur C, Walsh V, Heyes C. Sensorimotor learning configures the human mirror system. Curr Biol. 2007;17(17):1527–1531. doi: 10.1016/j.cub.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Chong TT, Cunnington R, Williams MA, Kanwisher N, Mattingley JB. FMRI adaptation reveals mirror neurons in human inferior parietal cortex. Curr Biol. 2008;18(20):1576–1580. doi: 10.1016/j.cub.2008.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR. Aphasia. New England Journal of Medicine. 1992;326:531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- Damasio H. Neuroanatomical correlates of the aphasias. In: Sarno M, editor. Acquired aphasia. 2. San Diego: Academic Press; 1991. pp. 45–71. [Google Scholar]
- Decety J, Grezes J, Costes N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F. Brain activity during observation of actions: influence of action content and subject’s strategy. Brain. 1997;120:1763–1777. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91(1):176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Dinstein I. Human cortex: reflections of mirror neurons. Curr Biol. 2008;18(20):R956–959. doi: 10.1016/j.cub.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Dinstein I, Hasson U, Rubin N, Heeger DJ. Brain areas selective for both observed and executed movements. J Neurophysiol. 2007;98(3):1415–1427. doi: 10.1152/jn.00238.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Thomas C, Behrmann M, Heeger DJ. A mirror up to nature. Curr Biol. 2008;18(1):R13–18. doi: 10.1016/j.cub.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers NF, Redfern BB, Knight RT. The neural architecture of language disorders. In: Gazzaniga MS, editor. The new cognitive neurosciences. Cambridge, MA: MIT Press; 2000. pp. 949–958. [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73(6):2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Rozzi S, Fogassi L. Mirror neurons responding to observation of actions made with tools in monkey ventral premotor cortex. J Cogn Neurosci. 2005;17(2):212–226. doi: 10.1162/0898929053124910. [DOI] [PubMed] [Google Scholar]
- Ferrari PF, Visalberghi E, Paukner A, Fogassi L, Ruggiero A, Suomi SJ. Neonatal imitation in rhesus macaques. PLoS Biol. 2006;4(9):e302. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308(5722):662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey: A reversible inactivation study. Brain. 2001;124(Pt 3):571–586. doi: 10.1093/brain/124.3.571. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Memory in the cerebral cortex. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Gainotti G, Lemmo M. Comprehenion of symbolic gestures in aphasia. Brain and Language. 1976;3:451–460. doi: 10.1016/0093-934x(76)90039-0. [DOI] [PubMed] [Google Scholar]
- Galantucci B, Fowler CA, Turvey MT. The motor theory of speech perception reviewed. Psychon Bull Rev. 2006;13(3):361–377. doi: 10.3758/bf03193857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119(Pt 2):593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fogassi L, Fadiga L, Rizzolati G. Action representation and the inferior parietal lobule. In: Prinz W, Hommel B, editors. Attention & Performance XIX. Common Mechanisms in Perception and Action. Oxford: Oxford University Press; 2002. [Google Scholar]
- Gallese V, Lakoff G. The brain’s concepts: The role of the sensory-motor system in conceptual knowledge. Cogn Neuropsychol. 2005;22:455–479. doi: 10.1080/02643290442000310. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Quadfasel FA, Segarra JM. Isolation of the speech area. Neuropsychologia. 1968;6:327–340. [Google Scholar]
- Goodglass H. Understanding aphasia. San Diego: Academic Press; 1993. [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. The assessment of aphasia and related disorders. 3. Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Grezes J, Costes N, Decety J. Top-down effect of strategy on the peception of human biological motion: A PET investigation. Cognitive Neuropsychology. 1998;15:553–582. doi: 10.1080/026432998381023. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Rothi LJ, Valenstein E. Two forms of ideomotor apraxia. Neurology. 1982;32(4):342–346. doi: 10.1212/wnl.32.4.342. [DOI] [PubMed] [Google Scholar]
- Hickok G, Buchsbaum B, Humphries C, Muftuler T. Auditory-motor interaction revealed by fMRI: Speech, music, and working memory in area Spt. Journal of Cognitive Neuroscience. 2003;15:673–682. doi: 10.1162/089892903322307393. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286(5449):2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: action representation in mirror neurons. Science. 2002;297(5582):846–848. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- Koski L, Iacoboni M, Dubeau MC, Woods RP, Mazziotta JC. Modulation of cortical activity during different imitative behaviors. J Neurophysiol. 2003;89(1):460–471. doi: 10.1152/jn.00248.2002. [DOI] [PubMed] [Google Scholar]
- Koski L, Wohlschlager A, Bekkering H, Woods RP, Dubeau MC, Mazziotta JC, Iacoboni M. Modulation of motor and premotor activity during imitation of target-directed actions. Cereb Cortex. 2002;12(8):847–855. doi: 10.1093/cercor/12.8.847. [DOI] [PubMed] [Google Scholar]
- Krams M, Rushworth MF, Deiber MP, Frackowiak RS, Passingham RE. The preparation, execution and suppression of copied movements in the human brain. Exp Brain Res. 1998;120(3):386–398. doi: 10.1007/s002210050412. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Cooper FS, Shankweiler DP, Studdert-Kennedy M. Perception of the speech code. Psychol Rev. 1967;74(6):431–461. doi: 10.1037/h0020279. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Mattingly IG. The motor theory of speech perception revised. Cognition. 1985;21:1–36. doi: 10.1016/0010-0277(85)90021-6. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. The orchestration of the sensory-motor systems: Clues from neuropsychology. Cogn Neuropsychol. 2005;22:480–494. doi: 10.1080/02643290442000446. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. J Physiol Paris. 2008;102(1–3):59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Miceli G, Gainotti G, Caltagirone C, Masullo C. Some aspects of phonological impairment in aphasia. Brain and Language. 1980;11:159–169. doi: 10.1016/0093-934x(80)90117-0. [DOI] [PubMed] [Google Scholar]
- Moineau S, Dronkers NF, Bates E. Exploring the processing continuum of single-word comprehension in aphasia. J Speech Lang Hear Res. 2005;48(4):884–896. doi: 10.1044/1092-4388(2005/061). [DOI] [PubMed] [Google Scholar]
- Morin O, Grezes J. What is “mirror” in the premotor cortex? A review. Neurophysiol Clin. 2008;38(3):189–195. doi: 10.1016/j.neucli.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Negri GA, Rumiati RI, Zadini A, Ukmar M, Mahon BZ, Caramazza A. What is the role of motor simulation in action and object recognition? Evidence from apraxia. Cogn Neuropsychol. 2007;24(8):795–816. doi: 10.1080/02643290701707412. [DOI] [PubMed] [Google Scholar]
- Nelissen K, Luppino G, Vanduffel W, Rizzolatti G, Orban GA. Observing others: multiple action representation in the frontal lobe. Science. 2005;310(5746):332–336. doi: 10.1126/science.1115593. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cognitive Brain Research. 2005;24:190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Pa J, Wilson SM, Pickell B, Bellugi U, Hickok G. Neural organization of linguistic short-term memory is sensory modality-dependent: Evidence from signed and spoken language. Journal of Cognitive Neuroscience. doi: 10.1162/jocn.2008.20154. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak T, Greenlee MW. Working memory in primate sensory systems. Nature Reviews Neuroscience. 2005;6:97–107. doi: 10.1038/nrn1603. [DOI] [PubMed] [Google Scholar]
- Pazzaglia M, Smania N, Corato E, Aglioti SM. Neural underpinnings of gesture discrimination in patients with limb apraxia. J Neurosci. 2008;28(12):3030–3041. doi: 10.1523/JNEUROSCI.5748-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrett DI, Mistlin AJ, Harries MH, Chitty AJ. Understanding the visual appearance and consequence of hand actions. In: Goodale MA, editor. Vision and action: The control of grasping. Norwood, NJ: Ablex; 1990. pp. 163–180. [Google Scholar]
- Perrett DI, Smith PAJ, Mistlin AJ, Chitty AJ, Head AS, Potter DD, Broennimann R, Milner AD, Jeeves MA. Visual analysis of body movements by neurones in the temporal cortex of the macaque monkey: a preliminary report. Behav Brain Res. 1985;16:153–170. doi: 10.1016/0166-4328(85)90089-0. [DOI] [PubMed] [Google Scholar]
- Pillsbury WB. The place of movement in consciousness. Psychological Review. 1911;18(2):83–99. [Google Scholar]
- Pinker S. Learnability and cognition: The acquisition of argument structure. Cambridge, MA: MIT Press; 1989. [Google Scholar]
- Pinker S. The stuff of thought: Language as a window into human nature. New York: Viking; 2007. [Google Scholar]
- Poizner H, Klima ES, Bellugi U. What the hands reveal about the brain. Cambridge, MA: MIT Press; 1987. [Google Scholar]
- Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience. 2006;139:23–38. doi: 10.1016/j.neuroscience.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib M. Language within our grasp. Trends in Neurosciences. 1998;21:188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino G, Matelli M. Functional organization of inferior area 6 in the macaque monkey. II. Area F5 and the control of distal movements. Exp Brain Res. 1988;71(3):491–507. doi: 10.1007/BF00248742. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, Fazio F. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp Brain Res. 1996;111(2):246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2(9):661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Grafman J, Cameron K, Berndt RS. Working memory retention systems: a state of activated longt-term memory. Behav Brain Sci. 2003;26:709–777. doi: 10.1017/s0140525x03000165. [DOI] [PubMed] [Google Scholar]
- Saygin AP, Wilson SM, Dronkers NF, Bates E. Action comprehension in aphasia: linguistic and non-linguistic deficits and their lesion correlates. Neuropsychologia. 2004;42(13):1788–1804. doi: 10.1016/j.neuropsychologia.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Scheerer E. Motor theories of cognitive structure: A historical review. In: Prinz W, Sanders AF, editors. Cognition and Motor Processes. Berlin: Springer-Verlag; 1984. pp. 77–98. [Google Scholar]
- Schubotz RI, Sakreida K, Tittgemeyer M, von Cramon DY. Motor areas beyond motor performance: deficits in serial prediction following ventrolateral premotor lesions. Neuropsychology. 2004;18(4):638–645. doi: 10.1037/0894-4105.18.4.638. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, von Cramon DY. Sequences of abstract nonbiological stimuli share ventral premotor cortex with action obsevation an imagery. Journal of Neuroscience. 2004;24:5467–5474. doi: 10.1523/JNEUROSCI.1169-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessari A, Canessa N, Ukmar M, Rumiati RI. Neuropsychological evidence for a strategic control of multiple routes in imitation. Brain. 2007;130(Pt 4):1111–1126. doi: 10.1093/brain/awm003. [DOI] [PubMed] [Google Scholar]
- Tkach D, Reimer J, Hatsopoulos NG. Congruent activity during action and action observation in motor cortex. J Neurosci. 2007;27(48):13241–13250. doi: 10.1523/JNEUROSCI.2895-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umiltà M, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G. I know what you are doing. a neurophysiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Calvo-Merino B, Haggard P, Aglioti SM. Transcranial magnetic stimulation reveals two cortical pathways for visual body processing. J Neurosci. 2007;27(30):8023–8030. doi: 10.1523/JNEUROSCI.0789-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urgesi C, Candidi M, Ionta S, Aglioti SM. Representation of body identity and body actions in extrastriate body area and ventral premotor cortex. Nat Neurosci. 2007;10(1):30–31. doi: 10.1038/nn1815. [DOI] [PubMed] [Google Scholar]
- Visalberghi E, Fragaszy D. Do monkeys ape? Ten years after. In: Dautenhahn K, Nehaniv CL, editors. Imitation in animals and artifacts. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- Washburn MF. The function of incipient motor processes. Psychological Review. 1914;21(5):376–390. [Google Scholar]
- Washburn MF. Movement and mental imagery: Outlines of a motor theory of the complexer mental processes. Boston: Houghton Mifflin; 1916. [Google Scholar]
- Watson JB. Psychology as the behaviorist views it. Psychological Review. 1913;20(2):158–177. [Google Scholar]
- Weinrich M, Wise SP, Mauritz KH. A neurophysiological study of the premotor cortex in the rhesus monkey. Brain. 1984;107(Pt 2):385–414. doi: 10.1093/brain/107.2.385. [DOI] [PubMed] [Google Scholar]
- Wilson M. The case for sensorimotor coding in working memory. Psychonomic Bulletin & Review. 2001;8:44–57. doi: 10.3758/bf03196138. [DOI] [PubMed] [Google Scholar]
- Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nat Neurosci. 2004;7:701–702. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- Wise SP, Mauritz KH. Set-related neuronal activity in the premotor cortex of rhesus monkeys: effects of changes in motor set. Proc R Soc Lond B Biol Sci. 1985;223(1232):331–354. doi: 10.1098/rspb.1985.0005. [DOI] [PubMed] [Google Scholar]


