Abstract
Arginase induction was reported in several inflammatory lung diseases, suggesting that this may be a common feature underlying the pathophysiology of such diseases. As little is known regarding arginase expression in silicosis, the induction and cellular localization of arginase was elucidated in lungs of Sprague-Dawley rats 24 hr following exposure to varying doses of silica by intratracheal instillation. Arginase expression was evaluated by activity assay, quantification of arginase I and arginase II mRNA levels using real-time PCR, and immunohistochemistry. Analyses of cells and fluid obtained by bronchoalveolar lavage (BAL) showed that markers of pulmonary inflammation, tissue damage, activation of alveolar macrophages (AM) and NO production were significantly increased by all silica doses. Arginase activity was increased also in AMs isolated from BAL fluid of silica-treated rats. Silica produced 2- and 3-fold increases in arginase activity of whole lung at doses of 1 and 5 mg/100g body weight, respectively. Levels of arginase I mRNA, but not of arginase II mRNA, were similarly elevated. In control lungs, arginase I immunoreactivity was observed only in AMs sparsely dispersed throughout the lung; no iNOS immunoreactivity was detected. In silica-treated lungs, arginase I and iNOS were co-expressed in most AMs that were abundantly clustered at inflammatory foci. The rapid induction of arginase I expression in inflammatory lung cells, similar to induction of arginase in other inflammatory lung diseases, implicates elevated arginase activity as a factor in the development of lung damage following exposure to silica.
Keywords: iNOS, nitric oxide, macrophage, silicosis, inflammation, immunohistochemistry
Introduction
Silica inhalation through mining, tunneling, rockdrilling, sand blasting or working with concrete has been linked to silicosis, a pulmonary disease characterized by a severe decline in respiratory function and premature death (Banks 1996; Shi et al., 1998). In animal models, acute inhalation of silica produces sustained pulmonary inflammation, characterized by activated alveolar macrophages (AMs), increased number of polymorphonuclear (PMN) leukocytes in bronchoalveolar spaces, production of free radicals (Blackford et al., 1994; Huffman et al., 1998; Huffman et al., 2003; Sacks et al., 1998), and damage of lung tissue (Zhang et al., 1996). However, the precise mechanisms underlying many of the pathophysiologic changes remain unknown. Many studies of the pulmonary toxicity of silica focussed on the role of nitric oxide (NO) because of the greatly increased expression of inducible NO synthase (iNOS) and NO production in lung following exposure to silica (Blackford et al., 1994; Porter et al., 2002b; Srivastava et al., 2002; Zeidler et al., 2004; Zeidler et al., 2003). However, the recent discoveries of increased arginase expression in asthma (Meurs et al., 2002; Zimmermann et al., 2003), bleomycin-induced pulmonary fibrosis (Endo et al., 2003), and hyperoxic lung injury (Que et al., 1998) raised the possibility that changes in arginase expression may occur also in silicosis and thus play roles in the pathophysiology of this disease. Apart from a single recent report (Misson et al., 2004), little was known regarding the impact of silica exposure on arginase activity and expression in lung prior to the present study. In particular, nothing to our knowledge was known regarding the dose-dependence of silica-induced changes in arginase activity or localization of arginase-expressing cells within lungs following acute exposure to silica, nor had it been determined whether silica exposure affected expression of only one or both arginase isoforms.
Mammals express two arginase isozymes which are encoded by different genes and have different subcellular localizations, tissue distribution and patterns of regulation (Iyer et al., 1998; Morris 2002). Arginase I is a cytosolic enzyme that is highly expressed in the liver as a component of the urea cycle, while arginase II is a mitochondrial protein expressed in various nonhepatic tissues (Iyer et al., 1998; Morris 2002). The expression of both arginases is induced by a variety of cytokines and inflammatory stimuli in many cell types, including macrophages (Mori and Gotoh 2000; Morris 2000; Morris et al., 1998). Because both arginase and iNOS utilize L-arginine as substrate, elevated levels of arginase activity can inhibit NO production via substrate limitation (Mori and Gotoh 2000; Morris 2000; Morris et al., 1998). Under some circumstances, elevated arginase activity also inhibits iNOS expression (Lee et al., 2003). Ornithine produced from L-arginine by arginase is metabolized to polyamines and proline (Wu and Morris 1998), which are essential for cell proliferation and collagen synthesis, respectively. Thus, increased expression of the arginases during pulmonary inflammation might affect disease progression not only by reducing NO production due to substrate depletion but also by promoting long-term airway remodeling and fibrosis via enhanced synthesis of proline and polyamines (Meurs et al., 2003; Ricciardolo 2003).
Understanding early pulmonary responses to inhaled particulates may lead to prevention and intervention approaches to help eliminate or mitigate the development of irreversible occupational lung disease. Because early changes in arginase expression in lung may play roles in the initiation of silica-induced lung damage, in the present study experiments were performed to determine the dose-dependence of expression and cellular localization of the arginases in lungs of rats following acute exposure to silica.
METHODS
Animal Maintenance
Male Sprague-Dawley [Hla:(SD) CVF] rats (200–225 g) were obtained from Hilltop Lab Animals (Scottdale, PA). The animals were maintained as described previously (Porter et al., 2002a). The program of animal use was accredited by AAALAC International, and all procedures involving animals were performed under protocols approved by the NIOSH Institutional Animal Care and Use Committee (IACUC).
Intratracheal instillation
Prior to use, silica (MIN-U-SIL 5; U.S. Silica Corporation, Berkeley Springs, WV) was heated dry for 90 min at 160°C to inactivate any contaminating endotoxin. Suspensions of silica were prepared in 0.9% sterile saline (Baxter, Deerfield, IL). For intratracheal instillations, rats were anesthetized by i.p. injection of 30–40 mg/kg body weight (BW) sodium methohexital (Brevital, Eli Lilly and Company, Indianapolis, IN) and were intratracheally instilled using a 20-gauge 4-inch ball-tipped animal feeding needle. Rats received either 0.1, 1 or 5 mg silica/100 g BW dose or an equivalent volume of vehicle control.
Bronchoalveolar lavage
Rats were euthanized 24 hr post-intratracheal exposure with an i.p. injection of sodium pentobarbital (>100 mg/kg BW). After exsanguination by cutting the abdominal vena cava/abdominal aorta, a tracheal cannula was inserted and BAL was performed as described previously (Porter et al., 2001). Briefly, the first lavage (6 ml) was kept separate from the rest of the lavage fluid. Subsequent lavages used 8 ml of PBS until a total of 80 ml of lavage fluid was collected. BAL cells were isolated by centrifugation, and an aliquot of the acellular supernatant from the first BAL fluid was transferred to tubes for analysis of lactate dehydrogenase (LDH) activity and albumin levels. The acellular supernatants from the remaining lavage samples were discarded. BAL cells isolated from the first and subsequent lavages for the same rat were pooled after resuspension in HEPES-buffered medium (10 mM HEPES, 145 mM NaCl, 5.0 mM KCl, 1.0 mM CaCl2, 5.5 mM D-glucose; pH 7.4), centrifuged, and the BAL cell pellet was then resuspended in HEPES-buffered medium and placed on ice.
To assess pulmonary inflammation, cell counts of AMs and PMN cells were obtained using an electronic cell counter with a cell sizing attachment (Coulter Electronics, Hialeah, FL). Cytospin preparations of BAL cells were also made using 0.1 × 106 total phagocytes (AM + PMN cells) with a cytocentrifuge (Shandon Elliot Cytocentrifuge, London, UK) and stained with modified Wright-Giemsa stain (Hema-Tek 2000, Bayer Corporation, Elkhart, IN).
Analyses of BAL Fluid
BAL fluid albumin concentrations were determined as an indicator of the integrity of the air-blood barrier. BAL fluid albumin was measured colorimetrically at 628 nm, based on albumin binding to bromocresol green (Doumas et al., 1971) using a commercial assay kit (Sigma Chemical Company, St. Louis, MO) and a Cobas MIRA Analyzer (Roche Diagnostic Systems, Montclair, NJ). BAL fluid lactate dehydrogenase (LDH) activities were determined as a marker of cytotoxicity and were measured by monitoring the LDH-catalyzed oxidation of pyruvate coupled with the reduction of NAD at 340 nm (Gay et al., 1968) using a commercial assay kit and a Cobas MIRA Analyzer (both from Roche Diagnostic Systems).
Chemiluminescence Assays
Alveolar macrophage chemiluminescence was determined as described previously (Porter et al., 2002b). Briefly, resting AM chemiluminescence was determined by incubating 1.0 × 106 AM/ml at 37° C for 20 min, followed by the addition of luminol and the measurement of chemiluminescence. To determine zymosan-stimulated chemiluminescence, unopsonized zymosan was added immediately prior to the measurement of chemiluminescence. All chemiluminescence measurements were made with an automated luminometer (Berthold Autolumat LB 953, EG&G, Gaithersburg, MD) at 390–620 nm for 15 min. The integral of cpm versus time was calculated. Zymosan-stimulated chemiluminescence was calculated as the integral cpm in the zymosan-stimulated assay minus the integral cpm in the resting assay. NO-dependent chemiluminescence was determined by subtracting the zymosan-stimulated chemiluminescence from cells pre-incubated with 1 mM of the iNOS inhibitor 1400W (Axxora, San Diego, CA), from the zymosan-stimulated chemiluminescence without 1400W. The use of unopsonized zymosan in the chemiluminescence assay allowed only AM chemiluminescence to be measured, because unopsonized zymosan stimulates AM chemiluminescence (Castranova et al., 1987) but not PMN cell chemiluminescence (Allen 1977; Hill et al., 1977).
Separation of BAL AM and PMN cells
AMs and PMN cells were isolated by density gradient as described (Huffman et al., 2003). Briefly, at 24 hr after intratracheal exposure, rats were euthanized with an i.p. injection of sodium pentobarbital (>100 mg/kg BW), followed by exsanguination. BAL cell isolation and differentials were determined as described above. BAL cells from groups of three rats that received vehicle control were pooled. BAL cells from silica-exposed rats were not pooled. AM and PMN fractions from the density gradient were washed twice in PBS, and the final pellets were resuspended in PBS and aliquots removed for determination of cell differentials; the remaining cells were pelleted by centrifugation, snap frozen in liquid nitrogen and stored at −80°C.
The AM-enriched fraction of BAL cells isolated from saline-exposed rats was 94.7 ± 0.88% AM (n = 3). No PMN cell-enriched fraction was obtained from saline-exposed rats, consistent with the determination that saline-exposed rats have essentially no BAL PMN cells. For silica-exposed rats, the AM-enriched fraction was 64.3 ± 3.71% AM (n = 3) and the PMN cell-enriched fraction was 66.0 ± 1.53% PMN cells (n = 3). Within the AM-enriched cellular fraction, the major cellular contaminant was PMNs. Within the PMN-enriched cellular fraction, AMs were the major cellular contaminant.
Immunohistochemistry
Rats were euthanized 24 hr post-intratracheal exposure as described for BAL. Next, the lungs were removed and inflated via the trachea by instillation of 6 ml 2% paraformaldehyde in Ca2+ and Mg2+-free PBS. A suture was tied around the trachea to retain the fixative, and the lungs were placed in fixative for 4–6 hr. The lobes were separated, trimmed, rinsed in PBS and placed in 30% sucrose solution (w/v, prepared in PBS) overnight at 4°C. Each lobe was embedded in Optimal Cutting Temperature (OCT) compound (Tissue-Tek, Torrance, CA), frozen in dry-ice cooled 2-methylbutane (Fisher, Pittsburgh, PA), and stored at −80°C until sectioning. Sections of 10-μm thickness were cut in a cryostat (MICROM International GmbH, Walldorf, Germany) and pre-incubated with PBS containing 1% Triton X-100 (Fisher Scientific, Fair Lawn, NJ) for 10 min at room temperature. Tissue sections were washed and then blocked with 5% normal goat and/or donkey serum (both from Sigma) in PBS containing 0.15% glycine (Bio-Rad Laboratories, Hercules, CA) and 0.5% bovine serum albumin (BSA) (Sigma) for 45 min at room temperature. After washing, the sections were co-incubated with primary antibodies (all diluted with PBS containing 0.15% glycine and 0.5% BSA) in different combinations: chicken polyclonal antibody raised to recombinant human arginase I (1:500; (Morris et al., 1998)), rabbit polyclonal antibody raised to murine iNOS (1:1000; Santa Cruz Biotechnology, Inc. Santa Cruz, CA), rabbit polyclonal anti-human prosurfactant protein C (prosp-C, 1:2000; Chemicon International, Temecula, CA) or mouse monoclonal anti-rat CD68 (ED-1; 1:50; Serotec Ltd, Oxford, UK), for one hr in a moisture chamber at room temperature. The sections were rinsed and then incubated for one hr with Alexa488-conjugated goat anti-rabbit IgG (1:3000; Molecular Probes, Eugene, OR), Cy™5-conjugated donkey anti-chicken IgY (1:1000) and/or Cy™3-conjugated donkey anti-mouse IgG (1:3000; both from Jackson Immunoresearch Laboratories, Inc., West Grove, PA) in PBS containing 0.15% glycine and 0.5% BSA. Tissue sections were rinsed and mounted in gel/mount™ (Biomeda corp. Foster City, CA) to prevent fading. Control experiments established that no immunoreactivity was detected in sections incubated with only the secondary antibodies.
All micrographs of the immunolabeled sections were obtained using a digital camera system (Olympus Provis AX70 microscope and Olympus Digital camera Olympus America; Olympus Optical Co, Tokyo, Japan), and the pictures were captured using appropriate filter settings for Alexa488, Cy™5 and Cy™3. Adobe® Photoshop™ was used for image handling, and the three-color channels were handled separately. Only the background level, contrast and brightness of the entire image were changed in the final picture.
Arginase assay
In some experiments, BAL cells, lung and spleen tissue were isolated for analyses of arginase activity. For these studies, rats were exsanguinated by cutting the abdominal vena cava/abdominal aorta. The left lung lobe was then clamped off with a hemostat and was removed below the clamp. The spleen was also removed, and both lung and spleen samples were snap frozen in liquid nitrogen and stored at −80°C. Bronchoalveolar lavage of the right lung was done as described above except that all volumes of PBS used were halved. First, BAL fluid isolation and analyses, BAL cell isolation and BAL cell differentials were done as described above. After BAL cell differentials were determined, the remaining BAL cells were centrifuged (650 × g, 5 min, 4°C), the supernatant decanted and the cell pellet snap frozen in liquid nitrogen and stored at −80°C.
All arginase activity assays were performed on lysed BAL cells or on lung homogenates. Lungs were homogenized in 0.1% Triton-X-100 containing 2 mM Pefabloc (Boehringer Mannheim, Mannheim, Germany), 2 μg/ml pepstatin A, and 10 μg/ml leupeptin (both from Sigma) using a tapered tissue grinder (Wheaton, Millville, NJ). BAL cells were lysed on ice for 20 min in 0.1% Triton X-100 containing 2 mM Pefabloc, 2 μg/ml pepstatin A, and 10 μg/ml leupeptin. The lung homogenates or BAL cell lysates were centrifuged for 2 min at 13,600 × g at 4°C to remove insolubles. Supernatants were used for assays of protein concentration and arginase activity. Protein concentrations were determined by the bicinchoninic acid assay (Pierce, Rockford, IL). Arginase activity was determined as the conversion of [14C-guanidino]-L-arginine (50 Ci/mmole; American Radiolabeled Chemicals, Inc, St. Louis, MO) to [14C]urea, which was converted to 14CO2 by urease and trapped as Na2 14CO3 for scintillation counting as previously described (Morris et al., 1998).
Real-time PCR
For real-time PCR, rat lungs were isolated as described for arginase activity assay, and total RNA was isolated from snap frozen lungs. Briefly, lungs were homogenized in TRIzol reagent (Invitrogen Carlsbad, CA), using a Polytron homogenizer (Kinematica GmbH, Kriens, Switzerland). Following chloroform extraction and isopropanol precipitation, RNA was precipitated by incubation in 5 M NaCl in 100% ethanol. The RNA was washed with 70% ethanol and treated with RNase inhibitor (Applied Biosystems, Foster City, CA) for 45 min. Following heat inactivation treatmentat 65°C for 15 min, the RNA was cleaned and treated with deoxyribonuclease using the Qiagen RNA isolation kit, as directed by the manufacturer(Qiagen, Valencia, CA). Four μg of total RNA was reverse transcribed using random primers and Superscript II enzyme (both from Invitrogen Life Technologies), as directed by the manufacturer. For real-time PCR, the relative gene expression method (Liu and Saint 2002) was used. 18S rRNA was used as the normalizer, and RNA isolated from saline-treated lung served as the calibrator. Pre-designed Assays-on-Demand™ TaqMan® probes and primers for arginase I (NM 017134), arginase II (NM 019168), and eukaryotic 18S rRNA (X03205) were obtained from Applied Biosystems (Foster City, CA, USA). Real-time PCR was performed using TaqMan Universal PCR Master Mix (Applied Biosystems), and reactions were conducted in 50 μl volume. All samples were run in duplicate and with no-reverse-transcriptase controls on an ABI® Prism Sequence Detector 7700. Relative gene expression was calculated as 2−ΔΔCt, where ΔCt = Ctgene of interest − Ctnormalizer, and ΔΔCt = ΔCtsample − ΔCtcalibrator. Arginase mRNA levels are expressed relative to the levels for saline-treated lungs.
Statistical analysis
Data are expressed as mean ± standard error (SE). The effect of silica dose on each toxicological endpoint (Table 1 and Figure 1) was assessed using one-way analysis of variance using SAS Proc Mixed (SAS Institute, Cary, NC), with experiment used as a block effect. In all cases, tests were performed for homogeneity of variances between each dose level. In cases of unequal variances, analyses accounted for the heterogeneous variances enabling efficient inferences to be made. Pair-wise comparisons were performed for each dose level using the Tukey-Kramer method to adjust for multiple comparisons. Two-sided tests were used. All other data (Figures 2–4) were analyzed by ANOVA, followed by the Bonferroni post-hoc test for multiple comparisons. The criterion for significance was set at p<0.05.
TABLE 1.
Markers of pulmonary inflammation in control and silica-treated rats.
| Silica dose | BAL Cells | AM Chemiluminescence | ||
|---|---|---|---|---|
| Total | NO-dependent | |||
| (mg silica/100g body weight) | AM (×106/rat) | PMN (×106/rat) | cpm × 105/0.25 × 106 AM/15 min | cpm × 105/0.25 × 106 AM/15 min |
| 0 | 9.69 ± 0.86a | 0.15 ± 0.04a | 15.32 ± 2.98a | 2.43 ± 0.70a |
| 0.1 | 10.19 ± 1.25a | 6.20 ± 1.27b | 103.43 ± 18.72b | 55.34 ± 11.72b |
| 1 | 10.78 ± 1.35a | 18.42 ± 2.70c | 155.15 ± 17.75b | 75.37 ± 11.77b |
| 5 | 10.37 ± 0.94a | 20.13 ± 3.20c | 147.63 ± 23.66b | 57.50 ± 11.57b |
Data are expressed as means ± SE (n=7–8).
Four experiments, each consisting of two rats/dose, were conducted.
Values within the same column with different superscripts are significantly different.
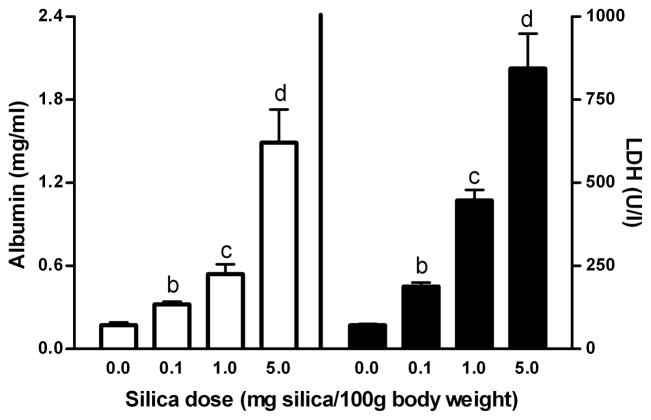
Figure 1. Albumin concentration and LDH activity in BAL fluid of rat lungs as a function of silica dose.
Scales on y-axes have been set to allow direct comparison of the magnitude of relative increases in albumin concentration (panel A) and LDH activities (panel B) with increasing silica dose. Within each panel, values (means ± SE; n = 6–7) identified by different lower case letters are significantly different (p<0.05)
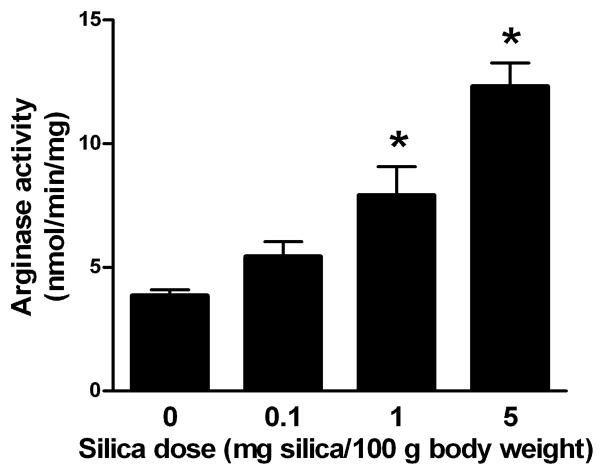
Figure 2. Arginase activity in rat lungs as a function of silica dose.
Rats were intratracheally instilled with the indicated amounts of silica or an equivalent volume of vehicle. After 24 hr, arginase activity was determined in lung homogenates. Values are expressed as mean ± SE (n = 5–22). *p<0.05.
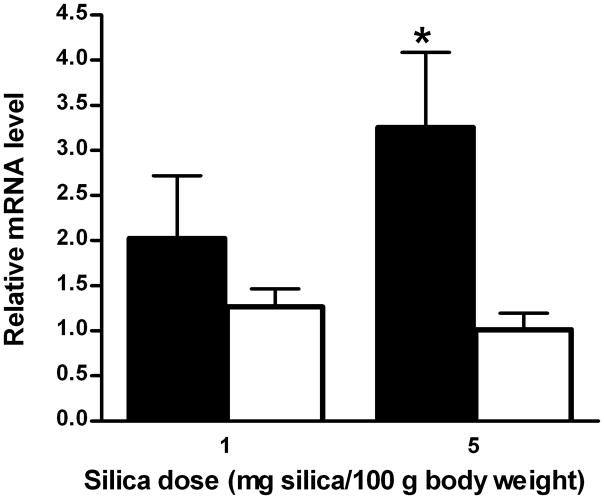
Figure 4. Levels of arginase I and II mRNAs in lungs of control and silica-treated rats.
Rats were intratracheally instilled with either 1 or 5 mg silica/100 g BW or an equivalent volume of vehicle. After 24 hr, total mRNA was isolated from rat lungs as described in Materials and Methods. Levels of arginase I (filled bars) and arginase II (open bars) mRNAs (mean ± SE; n = 5–8) are expressed relative to the levels in saline-treated lungs. *p<0.05.
RESULTS
Pulmonary inflammation following silica treatment
Several markers of pulmonary inflammation and tissue damage were quantified to evaluate the responses to varying amounts of silica. Although there were no changes in the number of AM in BAL fluid with increasing amounts of silica, AM became maximally activated at all doses of silica used in this study, as determined by chemiluminescence (Table I). In contrast to AM, there were marked increases in numbers of PMNs, which reached maximal levels by 1 mg silica/100 g BW (Table I). Cell and tissue damage, as indicated by levels of albumin and LDH activity in BAL fluid, showed progressive enhancement with increasing amounts of silica (Figure 1).
Arginase activity and gene expression in silica-treated lungs
Exposure to silica resulted in a dose-dependent increase in arginase activity in whole lung tissue, with statistically significant 2.1- and 3.2-fold rise at 1 and 5 mg silica/100 g BW, respectively (Figure 2). The profile of enhanced arginase activity with increasing amounts of silica is similar to the profiles of albumin and LDH activity in BAL fluid (Figure 1).
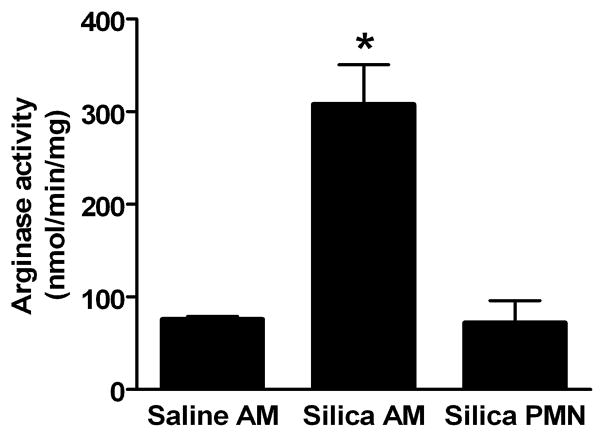
To determine which cell types may be contributing to the increased arginase activity in lungs of silica-treated rats, arginase activity was determined in AMs and PMNs isolated from BAL fluid of rats exposed to saline or 5 mg silica/100 g BW (Figure 3). Arginase specific activity was elevated 4.1-fold in AMs from silica-treated rats, relative to the specific activity in AMs from saline-treated rats. Owing to the very low abundance of PMNs in BAL fluid of saline-treated rats (Table I), it was not possible to isolate sufficient PMNs from control rats to measure arginase activity. Arginase specific activities in PMN from silica-treated rats were indistinguishable from the basal specific activities in AMs from saline-treated rats (Figure 3). Taking into account the arginase specific activities (AM activity 4-fold greater than PMN) and the total numbers of AM and PMN present in BAL fluid after silica treatment (PMN count twice that of AM), it was concluded that both cell types contribute significantly to total cellular arginase activity in BAL cells harvested from silica-treated rats.
Figure 3. Arginase activity in AMs and PMN cells isolated by BAL.
Arginase activity was determined in cell lysates of AMs and PMN cells isolated by BAL 24 hr after intratracheal instillation of vehicle or 5 mg silica/100 g BW. No PMN cells were obtained from control rats. Values are expressed as mean ± SE (n = 3). *p<0.05.
Arginase activity measurements reflect the sum of the activities of arginases I and II. In order to determine whether enhanced arginase activity in silica-treated lungs reflected increased expression of one or both arginase isozymes, the levels of arginase I and II mRNAs in whole lung were measured by real-time PCR. Paralleling the increases in arginase activity (Figure 2), 2-and 3.3-fold elevations in arginase I mRNA levels were observed in lungs of rats exposed to 1 and 5 mg silica/100 g BW, respectively, but there were no changes in levels of arginase II mRNA (Figure 4). These results indicate that elevated arginase I expression accounts for the increased arginase activity in lungs after acute exposure to silica.
Immunohistochemical analyses of silica-treated lungs
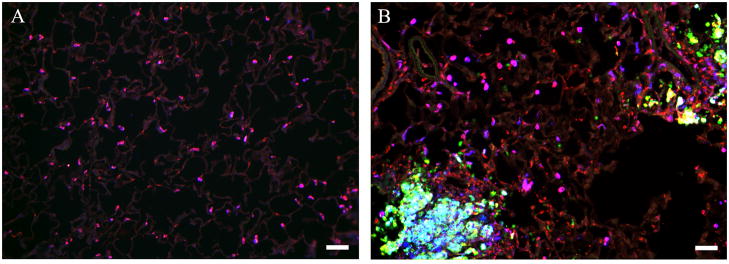
Within 24 hr of exposure to silica, multiple foci of inflammatory cells were seen in lungs of silica-treated rats (Figure 5B), but no such foci were found in lungs of saline-treated controls (Figure 5A). In control lungs, many, but not all, cells that were positive for the monocyte/macrophage marker ED-1 were also arginase I immunopositive (Figure 5A). The arginase I-positive AMs were mainly localized to individual alveoli (Figure 5A). No arginase I immunoreactivity was observed in alveolar type II epithelial cells (identified by immunostaining with antibody to pro-surfactant protein C; data not shown) nor were any iNOS-positive cells detected in saline-treated lungs (Figure 5A), consistent with previous reports (Blackford et al., 1994; Blackford et al., 1997; Porter et al., 2002b; Srivastava et al., 2002).
Figure 5. Immunohistochemical comparison of control and silica-treated rat lungs.
Immunohistochemistry was performed as described in Materials and Methods. Results are shown for rats treated with (A) vehicle or (B) 5 mg silica/100 g BW. Key to antibody staining: arginase I (blue), iNOS (green) and the macrophage marker ED-1 (red). Arginase I-positive macrophages are pink; iNOS-positive macrophages are yellow; cells expressing both arginase I and iNOS are turquoise. See Figure 6 for more detailed analysis. Photomicrographs are representative of multiple sections examined for each of 8 rats in both treatment groups. Scale bar = 50 μm.
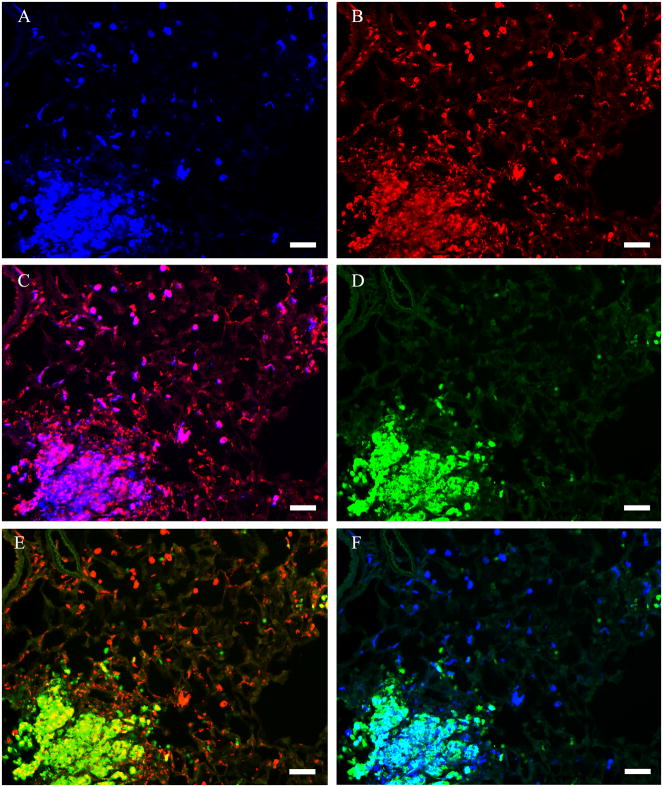
Numerous macrophages (identified by expression of the monocyte/macrophage marker ED-1 (Dijkstra et al., 1985)) were localized at focal sites of inflammation in lungs of silica-treated rats (Figure 6B). The number of inflammatory foci per lung rose with increasing amounts of silica treatment (data not shown), consistent with elevation in arginase activity (Figure 1). Within the foci, intense arginase I immunostaining (Figure 6A) was seen in ED-1 positive cells (Figure 6C) whereas iNOS staining was seen in both ED-1 positive and ED-1 negative cells (Figure 6D, E). Many, but not all, arginase I-immunopositive AMs in the inflammatory foci were also iNOS positive (Fig 6F). Only AMs immunopositive for arginase I but not for iNOS were observed in areas outside inflammatory foci in silica treated lungs (Fig 5B).
Figure 6. Identification of cells expressing arginase I and iNOS in silica-treated rat lung.
Results are shown for rats treated with 5 mg silica/100 g BW. The tissue section was stained with anti-arginase I (blue), anti-iNOS (green) and the macrophage marker ED-1 (red). (A) Identification of arginase I-positive cells. (B) Identification of macrophages with the macrophage marker ED-1. (C) Identification of arginase I-positive macrophages by colocalization of immunoreactivity for arginase I and ED-1 (pink). (D) Identification of iNOS-positive cells. (E) Identification of iNOS-positive macrophages by colocalization of immunoreactivity for iNOS and ED-1 (yellow). (F) Identification of cells expressing both arginase I and iNOS by colocalization of immunoreactivity for arginase I and iNOS (turquoise). Photomicrographs are representative of multiple sections examined for each of 8 silica-treated rats. Scale bar = 50 μm.
DISCUSSION
This is the first detailed analysis of arginase activity, expression, and cellular localization in rat lung following an acute exposure to silica. Arginase activity in whole lung increased in a dose-dependent manner within 24 hr of a single exposure to silica, similar to the dose-dependent rise in indices of lung damage (i.e., albumin concentration and LDH activity in BAL fluid) accompanying pulmonary inflammation. In contrast to the absence of any change in arginase II mRNA levels, increases in arginase I mRNA levels in whole lung correlated with elevation in arginase specific activity, indicating that increased expression of the arginase I isozyme accounted for enhanced activity. Arginase specific activity also increased in AMs isolated from BAL fluid following silica exposure, consistent with elevated arginase expression. Although arginase activity was present also in PMNs isolated from BAL fluid of silica-treated rats, it was not possible to determine whether this also represented increased expression because of the inability to obtain sufficient PMNs from BAL fluid of control rats for analysis. The presence of arginase activity in rat PMNs confirms previous reports of arginase activity and expression in PMNs (Munder et al., 2005; Reyero and Dorner 1975).
Arginase I-expressing cells in lungs of controls and silica-treated rats were almost exclusively AMs, based on colocalization of immunoreactivity with antibodies to arginase I and the macrophage marker ED-1. No colocalization of immunoreactivity with antibodies to arginase I and pro-surfactant protein C, a marker for alveolar type II epithelial cells, was observed in lungs of control or silica-treated rats. Small numbers of individual arginase I-positive cells were present throughout the lungs of saline-treated rats, mainly localized in the alveoli. In marked contrast to control lungs, large numbers of arginase I-positive cells were clustered at inflammatory foci in lungs of silica-treated rats. Most of the cells clustered at the inflammatory foci were macrophages, based on ED-1 immunoreactivity. Unlike the arginase I-positive cells in lungs of saline-treated rats, most of the arginase I-positive cells at inflammatory foci also expressed iNOS. Taking into account the fact that arginase specific activity was significantly greater in AMs isolated from BAL fluid of silica-treated rats, relative to AMs from saline-treated controls, the increased arginase activity in whole lung of silica-treated rats probably reflects elevated arginase I expression per cell as well as increased numbers of arginase I-expressing cells.
Prior to this report, little was known regarding effects of silica exposure on arginase expression in lung. A recent study examined some aspects of arginase activity and expression in mouse lung following exposure to a single large dose of silica (approx. 13 mg/100g BW) (Misson et al., 2004). As the earliest time examined was 3 day post treatment, it could not be ascertained whether increases in arginase activity and expression represented a rapid or delayed response to treatment. Although arginase activity of whole lung was not reported, arginase I mRNA levels were more than 2-fold above control values at 3 day post-treatment but returned to control levels at 30 and 60 day post-treatment. Significant increases in arginase activity and levels of both arginase I and iNOS mRNAs were found in AMs isolated from murine BAL fluid at 3 day after silica treatment, similar to results presented here. Unlike the present study, however, neither expression of arginase II nor immunohistochemical localization of arginase I and iNOS expression within the lung was determined. One study reported increased catabolism of arginine to urea in lungs of rats 3–14 day after exposure to approximately 16.7–20 mg silica/100g BW (Nelin et al., 2002), but enhanced catabolism correlated with increased pulmonary expression of arginine transporters and arginase activity was not determined. Consequently, it could not be determined whether the increased conversion of arginine to urea reflected enhanced arginine uptake, increased arginase activity, or some combination of the two.
The role of elevated arginase expression in inflammatory lung disease is still unresolved. One possibility is that increased arginase activity inhibits NO production (Mori and Gotoh 2000; Morris 2000; Morris et al., 1998) ---and possibly also iNOS expression (Lee et al., 2003) ---by reducing the availability of L-arginine, thus limiting NO-dependent cytotoxicity of activated macrophages. Arginase-dependent reductions in arginine availability may exacerbate cytotoxic effects of activated macrophages because NOS produces superoxide instead of NO when arginine is sufficiently limiting (Bronte et al., 2003; Xia et al., 1996).
It should be emphasized that the role of arginases in inflammatory lung diseases may not be simply to regulate availability of L-arginine for NOS but also to regulate polyamine and proline synthesis by producing L-ornithine, thus contributing to tissue remodeling and fibrosis (Meurs et al., 2003). Chronic silicosis develops over time, as retained intrapulmonary silica induces a sequence of events including inflammation, fibrogenesis and ultimately end-stage pulmonary fibrosis (Adamson 1992; Velan et al., 1993). Since lung tissues were analyzed only at 24 hr post-silica instillation, the role of arginase I expression in development of pulmonary fibrosis could not be investigated in the present study. However, increases in arginase activity or arginase I mRNA levels were not seen in a previous study of mouse lung or BAL cells at 30 and 60 day post-silica treatment, suggesting that sustained rise in arginase expression was not required for eventual development of pulmonary fibrosis in mice. However, it should be cautioned that this study did not rule out the possibilities that enhanced arginase activity and expression at early times (i.e., < 30 day) following silica exposure played an essential role in the eventual development of fibrosis or that increased activity of arginase I or II in a small subpopulation of cells within the lung occurred at longer times post-treatment. Therefore, further studies are warranted in order to better understand the roles of the arginases in silica-induced fibrosis.
Although it has not been determined whether the increased arginase activity in AMs from silica-treated lungs represents a direct response to the silica particles or an indirect response to inflammatory mediators produced in response to silica, the tight clustering of arginase I-positive cells at inflammatory foci, coupled with the previous finding that iNOS expression in rat AMs is induced by conditioned medium from a mixture of AMs and PMNs cultured in the presence of silica but not by silica itself (Castranova et al., 1998), suggests that induction of arginase I also reflects an indirect response via inflammatory mediators rather than a direct response to silica. This possibility is supported by the rather promiscuous induction of the arginases in response to a wide variety of cytokines and other inflammatory stimuli (Mori and Gotoh 2000; Morris 2000; Morris et al., 1998). Further studies will be required to identify the mediators involved in induction of arginase expression following exposure to silica.
In summary, this study demonstrated that a single exposure of rat lung to varying doses of silica results in dose-dependent increases in arginase activity within 24 hr in whole lung and in AMs isolated from BAL fluid and enhanced expression of arginase I but not of arginase II in lung. Dose-dependent elevation in arginase activity correlate well with dose-dependent increases in indices of cell and tissue damage. Our results indicate that the increased arginase activity in whole lung is probably due to greater numbers of arginase I-expressing cells as well as to increases in arginase I expression per cell. Immunohistochemistry demonstrated co-expression of arginase I and iNOS in macrophages localized at inflammatory foci in silica-treated lungs. The high concentration of arginase I-expressing cells at inflammatory foci raises the possibility that arginase may be inhibiting NO production at those sites. The increases in arginase activity and expression in silica-treated lungs resemble the elevation in arginase activity and expression reported for a wide variety of inflammatory lung diseases, suggesting that increases in arginase expression may play common roles in the pathophysiology of multiple lung diseases, particularly with regard to processes of airway remodeling and fibrosis.
Acknowledgments
The authors thank Dr. Robert Mercer for helpful discussion and Jason Devlin and Stuart Shand for assistance with microscopy. We thank Dr. Simon Watkins, Director of the Center for Biologic Imaging, for providing access to the instrumentation used for the immunohistochemical studies. This research was supported in part by NIH grants RO1 GM57384 and RO1 GM064509 to SMM.
Footnotes
Publisher's Disclaimer: The findings and conclusion in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Adamson IY. Radiation enhances silica translocation to the pulmonary interstitium and increases fibrosis in mice. Environ Health Perspect. 1992;97:233–238. doi: 10.1289/ehp.9297233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RC. Evaluation of serum opsonic capacity by quantitating the initial chemiluminescent response from phagocytizing polymorphonuclear leukocytes. Infect Immun. 1977;15:828–833. doi: 10.1128/iai.15.3.828-833.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks DE. Clinical features of silicosis. In: Castranova V, Vallyathan V, Wallace WE, editors. Silica and silica-induced lung diseases. Boca Raton, FL: CRC; 1996. pp. 23–37. [Google Scholar]
- Blackford JA, Jr, Antonini JM, Castranova V, Dey RD. Intratracheal instillation of silica up-regulates inducible nitric oxide synthase gene expression and increases nitric oxide production in alveolar macrophages and neutrophils. Am J Respir Cell Mol Biol. 1994;11:426–431. doi: 10.1165/ajrcmb.11.4.7522485. [DOI] [PubMed] [Google Scholar]
- Blackford JA, Jr, Jones W, Dey RD, Castranova V. Comparison of inducible nitric oxide synthase gene expression and lung inflammation following intratracheal instillation of silica, coal, carbonyl iron, or titanium dioxide in rats. J Toxicol Environ Health. 1997;51:203–218. doi: 10.1080/00984109708984022. [DOI] [PubMed] [Google Scholar]
- Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, Colombo MP, Zanovello P. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270–278. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- Castranova V, Lee P, Ma JYC, Weber KC, Pailes WH, Miles PR. Chemiluminescence from macrophages and monocytes. In: VanDyke K, Castranova V, editors. Cellular chemiluminescence. Boca Raton: CRC Press; 1987. pp. 4–19. [Google Scholar]
- Castranova V, Huffman LJ, Judy DJ, Bylander JE, Lapp LN, Weber SL, Blackford JA, Dey RD. Enhancement of nitric oxide production by pulmonary cells following silica exposure. Environ Health Perspect. 1998;106(Suppl 5):1165–1169. doi: 10.1289/ehp.98106s51165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology. 1985;54:589–599. [PMC free article] [PubMed] [Google Scholar]
- Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971;31:87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Endo M, Oyadomari S, Terasaki Y, Takeya M, Suga M, Mori M, Gotoh T. Induction of arginase I and II in bleomycin-induced fibrosis of mouse lung. Am J Physiol Lung Cell Mol Physiol. 2003;285:L313–L321. doi: 10.1152/ajplung.00434.2002. [DOI] [PubMed] [Google Scholar]
- Gay RJ, McComb RB, Bowers GN., Jr Optimum reaction conditions for human lactate dehydrogenase isoenzymes as they affect total lactate dehydrogenase activity. Clin Chem. 1968;14:740–753. [PubMed] [Google Scholar]
- Hill HR, Hogan NA, Bale JF, Hemming VG. Evaluation of nonspecific (alternative pathway) opsonic activity by neutrophil chemiluminescence. Int Arch Allergy Appl Immunol. 1977;53:490–497. doi: 10.1159/000231790. [DOI] [PubMed] [Google Scholar]
- Huffman LJ, Judy DJ, Castranova V. Regulation of nitric oxide production by rat alveolar macrophages in response to silica exposure. J Toxicol Environ Health A. 1998;53:29–46. doi: 10.1080/009841098159457. [DOI] [PubMed] [Google Scholar]
- Huffman LJ, Prugh DJ, Millecchia L, Schuller KC, Cantrell S, Porter DW. Nitric oxide production by rat bronchoalveolar macrophages or polymorphonuclear leukocytes following intratracheal instillation of lipopolysaccharide or silica. J Biosci. 2003;28(1):29–37. doi: 10.1007/BF02970129. [DOI] [PubMed] [Google Scholar]
- Iyer R, Jenkinson CP, Vockley JG, Kern RM, Grody WW, Cederbaum S. The human arginases and arginase deficiency. J Inherit Metab Dis 21 Suppl. 1998;1:86–100. doi: 10.1023/a:1005313809037. [DOI] [PubMed] [Google Scholar]
- Lee J, Ryu H, Ferrante RJ, Morris SM, Jr, Ratan RR. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci U S A. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Saint DA. A new quantitative method of real time reverse transcription polymerase chain reaction assay based on simulation of polymerase chain reaction kinetics. Anal Biochem. 2002;302:52–59. doi: 10.1006/abio.2001.5530. [DOI] [PubMed] [Google Scholar]
- Meurs H, Maarsingh H, Zaagsma J. Arginase and asthma: novel insights into nitric oxide homeostasis and airway hyperresponsiveness. Trends Pharmacol Sci. 2003;24:450–455. doi: 10.1016/S0165-6147(03)00227-X. [DOI] [PubMed] [Google Scholar]
- Meurs H, McKay S, Maarsingh H, Hamer MA, Macic L, Molendijk N, Zaagsma J. Increased arginase activity underlies allergen-induced deficiency of cNOS-derived nitric oxide and airway hyperresponsiveness. Br J Pharmacol. 2002;136:391–398. doi: 10.1038/sj.bjp.0704725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misson P, van den Brule S, Barbarin V, Lison D, Huaux F. Markers of macrophage differentiation in experimental silicosis. J Leukoc Biol. 2004;76:926–932. doi: 10.1189/jlb.0104019. [DOI] [PubMed] [Google Scholar]
- Mori M, Gotoh T. Relationship between arginase activity and nitric oxide production. In: Ignarro LJ, editor. Nitric Oxide Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 199–224. [Google Scholar]
- Morris SM., Jr . Regulation of arginine availability and its impact on NO synthesis. In: Ignarro LJ, editor. NitricOxide Biology and Pathobiology. San Diego: Academic Press; 2000. pp. 187–197. [Google Scholar]
- Morris SM., Jr Regulation of enzymes of the urea cycle and arginine metabolism. Annu Rev Nutr. 2002;22:87–105. doi: 10.1146/annurev.nutr.22.110801.140547. [DOI] [PubMed] [Google Scholar]
- Morris SM, Jr, Kepka-Lenhart D, Chen LC. Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. Am J Physiol. 1998;275:E740–E747. doi: 10.1152/ajpendo.1998.275.5.E740. [DOI] [PubMed] [Google Scholar]
- Munder M, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, Fuentes JM, Luckner C, Doschko G, Soler G, Eichmann K, Muller FM, Ho AD, Goerner M, Modolell M. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105:2549–2556. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- Nelin LD, Krenz GS, Chicoine LG, Dawson CA, Schapira RM. L-Arginine uptake and metabolism following in vivo silica exposure in rat lungs. Am J Respir Cell Mol Biol. 2002;26:348–355. doi: 10.1165/ajrcmb.26.3.4450. [DOI] [PubMed] [Google Scholar]
- Porter DW, Barger M, Robinson VA, Leonard SS, Landsittel D, Castranova V. Comparison of low doses of aged and freshly fractured silica on pulmonary inflammation and damage in the rat. Toxicology. 2002a;175:63–71. doi: 10.1016/s0300-483x(02)00061-6. [DOI] [PubMed] [Google Scholar]
- Porter DW, Millecchia L, Robinson VA, Hubbs A, Willard P, Pack D, Ramsey D, McLaurin J, Khan A, Landsittel D, Teass A, Castranova V. Enhanced nitric oxide and reactive oxygen species production and damage after inhalation of silica. Am J Physiol Lung Cell Mol Physiol. 2002b;283:L485–L493. doi: 10.1152/ajplung.00427.2001. [DOI] [PubMed] [Google Scholar]
- Porter DW, Ramsey D, Hubbs AF, Battelli L, Ma J, Barger M, Landsittel D, Robinson VA, McLaurin J, Khan A, Jones W, Teass A, Castranova V. Time course of pulmonary response of rats to inhalation of crystalline silica: histological results and biochemical indices of damage, lipidosis, and fibrosis. J Environ Pathol Toxicol Oncol. 2001;20(Suppl 1):1–14. [PubMed] [Google Scholar]
- Que LG, Kantrow SP, Jenkinson CP, Piantadosi CA, Huang YC. Induction of arginase isoforms in the lung during hyperoxia. Am J Physiol. 1998;275:L96–L102. doi: 10.1152/ajplung.1998.275.1.L96. [DOI] [PubMed] [Google Scholar]
- Reyero C, Dorner F. Purification of arginases from human-leukemic lymphocytes and granulocytes: study of their physicochemical and kinetic properties. Eur J Biochem. 1975;56:137–147. doi: 10.1111/j.1432-1033.1975.tb02216.x. [DOI] [PubMed] [Google Scholar]
- Ricciardolo FL. cNOS-iNOS paradigm and arginase in asthma. Trends Pharmacol Sci. 2003;24:560–561. doi: 10.1016/j.tips.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Sacks M, Gordon J, Bylander J, Porter D, Shi XL, Castranova V, Kaczmarczyk W, Van Dyke K, Reasor MJ. Silica-induced pulmonary inflammation in rats: activation of NF-kappa B and its suppression by dexamethasone. Biochem Biophys Res Commun. 1998;253:181–184. doi: 10.1006/bbrc.1998.9763. [DOI] [PubMed] [Google Scholar]
- Shi X, Castranova V, Halliwell B, Vallyathan V. Reactive oxygen species and silica-induced carcinogenesis. J Toxicol Environ Health B Crit Rev. 1998;1:181–197. doi: 10.1080/10937409809524551. [DOI] [PubMed] [Google Scholar]
- Srivastava KD, Rom WN, Jagirdar J, Yie TA, Gordon T, Tchou-Wong KM. Crucial role of interleukin-1beta and nitric oxide synthase in silica-induced inflammation and apoptosis in mice. Am J Respir Crit Care Med. 2002;165:527–533. doi: 10.1164/ajrccm.165.4.2106009. [DOI] [PubMed] [Google Scholar]
- Velan GM, Kumar RK, Cohen DD. Pulmonary inflammation and fibrosis following subacute inhalational exposure to silica: determinants of progression. Pathology. 1993;25:282–290. doi: 10.3109/00313029309066590. [DOI] [PubMed] [Google Scholar]
- Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Dawson VL, Dawson TM, Snyder SH, Zweier JL. Nitric oxide synthase generates superoxide and nitric oxide in arginine- depleted cells leading to peroxynitrite-mediated cellular injury. Proc Natl Acad Sci U S A. 1996;93:6770–6774. doi: 10.1073/pnas.93.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler P, Hubbs A, Battelli L, Castranova V. Role of inducible nitric oxide synthase-derived nitric oxide in silica-induced pulmonary inflammation and fibrosis. J Toxicol Environ Health A. 2004;67:1001–1026. doi: 10.1080/15287390490447296. [DOI] [PubMed] [Google Scholar]
- Zeidler PC, Roberts JR, Castranova V, Chen F, Butterworth L, Andrew ME, Robinson VA, Porter DW. Response of alveolar macrophages from inducible nitric oxide synthase knockout or wild-type mice to an in vitro lipopolysaccharide or silica exposure. J Toxicol Environ Health A. 2003;66:995–1013. doi: 10.1080/15287390306395. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Kusaka Y, He L, Zhang Z, Sato K. Dynamic changes of constituents in bronchoalveolar lavage fluid in experimental silicotic rats. Ind Health. 1996;34:379–388. doi: 10.2486/indhealth.34.379. [DOI] [PubMed] [Google Scholar]
- Zimmermann N, King NE, Laporte J, Yang M, Mishra A, Pope SM, Muntel EE, Witte DP, Pegg AA, Foster PS, Hamid Q, Rothenberg ME. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest. 2003;111:1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]