Abstract
The prevalence of diabetes has been accelerating at an alarming rate in the last decade; some describe it as an epidemic. Diabetic eye complications are the leading cause of blindness in adults aged 25–74 in the United States. Early diagnosis and development of effective preventatives and treatments of diabetic retinopathy are essential to save sight. We describe efforts to establish functional indicators of retinal health and predictors of diabetic retinopathy. These indicators and predictors will be needed as markers of the efficacy of new therapies. Clinical trials aimed at either prevention or early treatments will rely heavily on the discovery of sensitive methods to identify patients and retinal locations at risk, as well as to evaluate treatment effects.
We report on recent success in revealing local functional changes of the retina with the multifocal electroretinogram (mfERG). This objective measure allows the simultaneous recording of responses from over 100 small retinal patches across the central 45 degree field. We describe the sensitivity of mfERG implicit time measurement for revealing functional alterations of the retina in diabetes, the local correspondence between functional (mfERG) and structural (vascular) abnormalities in eyes with early nonproliferative retinopathy, and longitudinal studies to formulate models to predict the retinal sites of future retinopathic signs. A multivariate model including mfERG implicit time delays and ‘person’ risk factors achieved 86% sensitivity and 84% specificity for prediction of new retinopathy development over one year at specific locations in eyes with some retinopathy at baseline. A preliminary test of the model yielded very positive results. This model appears to be the first to predict, quantitatively, the retinal locations of new nonproliferative diabetic retinopathy development over a one-year period. In a separate study, the predictive power of a model was assessed over one- and two-year follow-ups. This permitted successful prediction of new retinopathy development in eyes with and without retinopathy at baseline. Finally, we briefly describe our current research efforts to (a) locally predict future sight-threatening diabetic macular edema, (b) investigate local retinal function change in adolescent patients with diabetes, and (c) better understand the physiological bases of the mfERG delays.
The ability to predict the retinal locations of future retinopathy based on mfERG implicit time provides clinicians a powerful tool to screen, follow up, and even consider early prophylactic treatment of the retinal tissue in diabetic patients. It also aids identification of 'at risk' populations for clinical trials of candidate therapies, which may greatly reduce their cost by decreasing the size of the needed sample and the duration of the trial.
1. Introduction
Currently, more than 194 million people live with diabetes world-wide and this number is expected to exceed 333 million by the year 2025 (International Diabetes Federation, 2003). To put these numbers into perspective, it is estimated that one in 20 people worldwide have diabetes. The Centers for Disease Control (CDC) and Prevention estimate that approximately 14.6 million people in the United States have been diagnosed with diabetes and another 6.2 million are living with undiagnosed diabetes (CDC, 2005). Diabetes-related eye disease is the greatest cause of loss of vision in people of working age (20–74 years) in the United States. The total cost of diabetes in the U.S. in 2002 was estimated to be $132 billion (CDC, 2003). The socio-economic impact of diabetes-related vision loss and its effect on quality-of-life issues make it a top priority in efforts to develop effective treatments and preventative measures.
Our group has studied vision changes produced by diabetes for more than two decades. Initially our research efforts focused on increasing the understanding of the effects of diabetes on human vision, particularly in its early stages. More recently, we have been applying the multifocal electroretinogram (mfERG), an objective and non-invasive method to measure retinal responses to visual stimulation from small, essentially discrete patches of human retina. Using this method we have formulated quantitative models for predicting the development of local retinal patches of diabetic retinopathy.
In this article we first briefly review the disease known commonly as diabetes, then present an overview of the changes in vision function in the various stages of this disease, as measured using psychophysical and “conventional” (non-local) electrophysiological methods. Next, the mfERG technique is briefly introduced, and its recent role in diabetes research is discussed. Finally, we describe our progress in the prediction of the development of diabetic retinopathy, the formulation of quantitative predictive models based on the mfERG, the implications of our recent findings, and the directions that our research efforts are aimed at present and over the long term.
2. Overview of Diabetes
2.1 The Disease
Blood glucose, a primary source of energy for cellular metabolism, is regulated by pancreatic hormone secretion of either glucagon or insulin. In conditions of low blood glucose (hypoglycemia), glucagon is produced by pancreatic alpha-cells and is responsible for stimulating the liver to release stored glucose into the bloodstream. In conditions of elevated blood glucose, (hyperglycemia) insulin is up-regulated by the pancreatic beta-cells. The hormone insulin is a key mediator responsible for the uptake of glucose from the bloodstream and its absorption for cellular metabolism or storage.
Diabetes, more formally referred to as diabetes mellitus, is a disorder characterized by uncontrolled concentrations of glucose in the blood due to insulin-related abnormalities. These widely fluctuating and typically abnormally elevated blood glucose concentrations can be due to two main types of diabetes. Type 1 diabetes, which is sometimes referred to as juvenile-onset or insulin-dependent diabetes, accounts for 5–10% of all diagnosed cases and is associated with inadequate insulin production. This form of diabetes is treated primarily with insulin delivered by injection or a pump. Type 2 diabetes, which is sometimes referred to as adult-onset or non-insulin-dependent diabetes, accounts for 90–95% of the diagnosed cases. It is characterized primarily by an insensitivity of cellular membranes to insulin which interferes with the absorption of glucose, and it is treated primarily with medications that either increase cellular sensitivity to insulin or increase insulin production. In advanced cases of Type 2 diabetes, pancreatic dysfunction can lead to inadequate insulin production.
Treatments that control blood glucose concentrations (e.g., insulin injection or sulfonylureas) prolong and improve the lives of individuals with this otherwise debilitating disease. However, chronic blood glucose elevations and/or wide fluctuations in both types of diabetes can lead to a number of complications, including increased risk of cardio-vascular disease, kidney disease (nephropathy), neural damage (neuropathy), and retinal disease (diabetic retinopathy).
In addition to the 14.6 million diagnosed diabetics in the U.S., the CDC estimates that there are 6.2 million undiagnosed cases, bringing the total to approximately 20.8 million diabetic individuals (about 7% of the country’s population). Approximately 60% of the 14.6 million individuals in the United States diagnosed with diabetes through the year 2004 were younger than 65 years of age (CDC, 2005). Among people aged 20 years or older in the U.S., 8.7% of all non-Hispanic whites and 13.3% of all non-Hispanic blacks have diabetes (CDC, 2005). The CDC estimates that Mexican Americans, the largest Hispanic/Latino subgroup in the U.S., are 1.7 times as likely to have diabetes as non-Hispanic whites after adjusting for age differences in the populations. For Native Americans, the prevalence is even higher. Type 2 diabetes has been increasingly characterized as a growing epidemic. It is associated with obesity, poor diet and sedentary life style and is even diagnosed in children and adolescents at an accelerating rate.
2.2 Diabetes-related Eye Disease
Diabetic retinopathy causes up to 24,000 new cases of blindness each year in the United States (CDC, 2003). According to CDC statistics, 3 million people in the U.S. over 18 years of age had uncorrectable visual impairments related to diabetes (CDC, 2003). Epidemiological studies have established that good metabolic control (i.e., preventing abnormally elevated blood glucose) significantly reduces the risk of development and progression of ocular and visual complications of both Type 1 and Type 2 diabetes (Stratton, et al., 2001; The Diabetes Control and Complications (DCCT) Research Group, 1995; UKPDS, 1998) However, good metabolic control is difficult to achieve and maintain. In addition, sight-threatening diabetic retinopathy occurs at a substantial rate even among those who manage their diabetes well (The Diabetes Control and Complications (DCCT) Research Group, 1995).
Diabetic retinopathy has classically been defined as pathology of the microvasculature, primarily of the inner retina (Gardner, et al., 2000). The earliest form is nonproliferative diabetic retinopathy (NPDR), also referred to as background or simple diabetic retinopathy. In this form, there is abnormal dilation of blood vessels, leakage and bleeding of the blood vessels, and fluid accumulation within the retina. A more advanced form, proliferative diabetic retinopathy, is more sight-threatening. It is characterized by neovascularization, the formation of abnormal new blood vessels that are fragile and leaky. Proliferative diabetic retinopathy is the primary cause of severe vision loss in Type 1 diabetes (Aiello, et al., 1998; Cunha-Vaz and Bernardes, 2005). The retinas of both types of diabetics are also at risk for the development of edema. Edema results from the breakdown of the blood-retinal barrier and leakage of plasma constituents into the middle retinal layers, and can be focal (cystoid) and/or diffuse. Diabetic retinopathy and clinically significant macular edema (CSME) are largely responsible for the irreversible, debilitating visual consequences of diabetes (Early Treatment Diabetic Retinopathy Study Research Group (ETDRS), 1985; Early Treatment Diabetic Retinopathy Study Research Group (ETDRS), 1991). In addition, there is a large body of evidence suggesting that some of the diabetes-related visual abnormalities are due to neurodegeneration that may occur independent of microvasculature pathology (Barber, 2003). One example of neuropathy affecting ocular function is the inability of the pupil to dilate normally (Cahill, et al., 2001; Pittasch, et al., 2002; Sharma, et al., 1997). In addition to the vascular and neural abnormalities, there are other changes to the eye that are associated with diabetes. These include increased risk of glaucoma, reductions in the clarity and spectral transmission curve of the crystalline lens (cataract), and changes in refraction (Fledelius, 1987; Sparrow, 1990). However, the retinal complications of diabetes are the most devastating.
2.3 The Value of Early Detection of Retinal Dysfunction
Current treatments of diabetic retinopathy are aimed at slowing progression of sight loss once structural damage to the retina is funduscopically obvious. These treatments include focal and pan-retinal laser photocoagulation, both of which destroy retinal tissue. In the future, the key to preventing all or most of the impact of diabetes on vision will be the development of agents or treatments that prevent the development of early retinopathy and/or the sub-clinical pathological vascular and neural changes that underlie the early functional changes. Efficient conduct of clinical trials of such putative agents or treatments will necessarily rely on the use of surrogate (early functional) markers of diabetic retinopathy and identification of a subpopulation with a high risk for the development of retinopathy. Early assessment and intervention are needed.
Functional abnormalities of the retina and vision can occur before clinical signs of retinopathy appear in diabetes. As we will describe below, some of these functional abnormalities appear to become more severe with the progression of retinopathy and, more importantly, some appear to predict the appearance or worsening of retinopathy. Taken together, these simple observations suggest that utilizing objective tests for early detection of retinal dysfunction and prediction of sight-threatening diabetic retinopathy would be of great value.
3. Vision and Retinal Function Changes Related to Diabetes
3.1 Changes in Vision
Alterations in spatial, color and dynamic vision have been observed in eyes with normal visual acuity and no clinical signs of diabetic retinopathy. Contrast sensitivity is impaired (Di Leo, et al., 1992; Hyvarinen, et al., 1983; Liska and Dostalek, 1999; Sokol, et al., 1985; Stavrou and Wood, 2003; Verrotti, et al., 1998), as is contrast sensitivity for motion detection (Kawasaki, et al., 1986). Recovery from glare (retinal photo stress) is delayed and dark-adapted thresholds are elevated (Greenstein, et al., 1993; Parisi, et al., 1994; Spafford and Lovasik, 1986).
There is a relatively long history of publications documenting changes in color discrimination that are associated with diabetes. In general these studies suggested a tritan or blue-yellow form of loss involving the short-wavelength sensitive (S-cone) pathway. We reported such S-cone pathway loss in the early 1980’s (Adams, 1982; Adams, et al., 1987a; Zisman and Adams, 1982). Changes in this pathway are not only an early event in diabetic eye disease but, importantly, the severity of these color defects correlate with both glycemic control and severity of retinopathy and are greater when macular edema is present (Adams, 1982; Adams, et al., 1987a; Adams, et al., 1987b; Bresnick, et al., 1985; Daley, et al., 1987; Greenstein, et al., 1990; Kurtenbach, et al., 2002; Lutze and Bresnick, 1994; Muntoni, et al., 1982; Volbrecht, et al., 1994; Zisman and Adams, 1982).
Our laboratory developed a rapid method to evaluate the sensitivity of the foveal S-cone pathway for subjects with diabetes. The test consisted of short-wavelength (blue) test flashes superimposed on a bright yellow background that suppresses L- and M-cone sensitivity (Adams, et al., 1987a; Adams, et al., 1982). This test was also later applied to subjects with glaucoma both for foveal and non-foveal measures across the central 20 degrees of the retina (Heron, et al., 1987), and was the fore-runner of short-wavelength automated perimetry - SWAP (Johnson, et al., 1989; Sample, et al., 1996).
We and others have documented widespread abnormalities with SWAP in eyes of diabetic individuals without retinopathy that have normal achromatic visual fields (Afrashi, et al., 2003; Han, et al., 2004a), though some have not seen these changes until some retinopathy is present (Nomura, et al., 1999; Remky, et al., 2003). In diabetes without retinopathy, approximately 20% of SWAP locations tested were abnormal compared to about 40% in the presence of mild-to-moderate retinopathy (Han, et al., 2004a). Although we have not yet discussed the mfERG in detail, it is of interest here to note that, in the absence of retinopathy, there was no spatial association between SWAP and mfERG response abnormalities. This is consistent with the fact that the two measures assess distinct mechanisms/pathways which are affected differently in early diabetic eye disease. It has been reported that the location of SWAP abnormalities corresponds to that of macular edema, although the spatial extent of functional loss is greater than that of the edema (Hudson, et al., 1998).
3.2 Functional Changes Measured with “Conventional” Electrophysiology
Recent reviews (Shirao and Kawasaki, 1998; Tzekov and Arden, 1999) have discussed electrophysiological investigations of diabetes-induced alterations of retinal function. A brief review of the “conventional” retinal electrophysiology work that did not employ multifocal techniques is relevant to our later discussion of the mfERG.
The amplitude of the b-wave of the scotopic full-field (flash) ERG, reflecting largely the activity of the bipolar cells, and the implicit time of the oscillatory potentials (OPs), manifestations of feedback between the amacrine and bipolar cells and/or feedback from ganglion cells to amacrine cells, are abnormal in diabetes in the absence of visible fundus signs of retinopathy (Coupland, 1987; Hardy, et al., 1995; Juen and Kieselbach, 1990; Levin, et al., 1982; Lovasik and Spafford, 1988; Shirao, et al., 1991; Yonemura and Kawasaki, 1978). In particular, the implicit time of the initial OP (OP1) is consistently reported to be delayed in diabetes prior to retinopathy development (Bresnick and Palta, 1987; Shirao, et al., 1991; Simonsen, 1965; Yonemura and Kawasaki, 1978; Zaharia, et al., 1987). These findings suggest that function of neural components of the middle and inner retinal layers is altered in diabetes prior to the development of retinopathy.
In eyes with minimal to moderate NPDR, additional ERG measures are altered. These include an increased scotopic b-wave implicit time, nonselective reduction of scotopic OP amplitudes, and increased implicit time and decreased amplitude of the photopic 30 Hz flicker response, reflecting cone dysfunction (Holopigian, et al., 1992; Van der Torren and Mulder, 1993; Weiner, et al., 1997). These findings suggest that photoreceptor abnormalities occur in the presence of retinopathy.
There is evidence from studies using the electrooculogram (EOG) that disruption of the outer blood retinal barrier (BRB) is an early manifestation of diabetic eye disease. The outer BRB is comprised of the retinal pigment epithelium (RPE), a single layer of cells lying behind the photoreceptors. The RPE is electrically polarized, and this trans-epithelial potential comprises the major component of the corneo-retinal potential, which can be measured non-invasively as the EOG. It has been reported that the light peak response (measured as the Arden ratio) is relatively insensitive to diabetes and is not correlated with diabetic retinopathy (Moloney and Drury, 1982; Shirao and Kawasaki, 1998). However, it has also been reported that the fast oscillation ratio of the EOG differed significantly among groups of subjects who were non-diabetic, diabetic without retinopathy, and diabetic with mild retinopathy despite the fact that they had normal Arden ratios (Schneck, et al., 2001). The non-photic bicarbonate response is more sensitive to diabetic eye disease: it is abnormal in 40% of eyes of diabetics without retinopathy, increasing in frequency of abnormality as retinopathy appears and progresses (Shirao and Kawasaki, 1998). Injection of glucose in non-diabetic volunteers produces a significant increase in the Arden ratio (Balik and van Lith, 1970), and in non-diabetic normal subjects the fast oscillation of the EOG is sensitive to acute fluctuations of blood glucose within the physiological range (Schneck, et al., 2000). This suggests that the ion pumps of the RPE are susceptible to blood glucose fluctuations and likely to be adversely affected by chronic hyperglycemia.
3.3 Interpretations
The psychophysical and “conventional” electrophysiological studies of visual function in individuals with diabetes demonstrate that functional alterations are present even in the absence of vascular changes assessed by retinal photography and ophthalmic examination. The implication of these findings is that, in addition to retinopathy-associated vision loss, diabetes induces changes in vision function that are not secondary to vascular damage. The view that diabetic retinopathy is a neurosensory as well as a vascular disorder is solidly supported and gaining widespread affirmation (Barber, 2003; Bresnick, 1986; Frank, 1984; Layton, et al., 2006; Shirao and Kawasaki, 1998). First, early retinopathy occurs in discrete local regions, typically away from the fovea. Foveal measures (such as color vision) and global measures (e.g. the flash ERG) nonetheless are abnormal in retinopathy-free eyes. In addition, as we describe below, local measures often show different spatial loss profiles in retinopathy-free retinas, with overlap increasing in the presence of retinopathy.
Though functional changes can occur in the absence of retinopathy, this does not mean that function is not related to retinopathy. Clearly, advanced retinopathy and edema are responsible for the severe vision loss that occurs in diabetes. Moreover, many of the functional changes observed in early diabetic eye disease become more pronounced as retinopathy progresses; there is a significant correlation between retinopathy severity and the magnitude of the functional loss.
Given the local nature of early-to-moderate nonproliferative diabetic retinopathy, the association between retinopathy and functional status would best be established using local functional measures. One might expect that global measures can “miss” local abnormalities because the remaining healthy retina predominates the functional measure. Of the measures discussed thus far, only perimetry maps function locally. Alternative, objective mapping procedures would be highly advantageous.
The known risk factors (e.g., metabolic control, age, disease duration, lipid levels) are not sufficient to make predictions of retinopathy development in specific retinal locations. Functional measures such as full-field ERGs, conventional OPs of the ERG and tritan color defects, although they are significant predictors of retinopathy progression (Aspinall, et al., 1983; Bresnick, et al., 1984; Bresnick and Palta, 1987), also do not permit local assessment across the retina. The visual consequence of a retinopathic event, such as focal edema, depends on its retinal location. Thus, there is a need to identify a measure that maps local function and predicts the development of retinopathy at specific locations. The mfERG is such a measure (Bearse and Sutter, 1996; Hood, 2000; Marmor, et al., 2003; Sutter and Tran, 1992). Below we present an overview of the application of the mfERG to diabetic eye disease. The mfERG has proven to sensitively detect early functional change, to provide an index of retinal status, and predict not only which eyes but also which retinal locations will develop new retinopathy signs in the near future.
4. The Multifocal ERG (mfERG) Technique
4.1 Rationale for Its Application
The noninvasive mfERG technique allows for the extraction of retinal responses that are generated by up to hundreds of discrete retinal locations. We believe that local testing of the functional status of the diabetic retina is important for a number of reasons. First, as in many retinal diseases, it is reasonable to expect that the functional changes produced by diabetes will be non-uniform across the retina. In fact, the fundus signs of early retinopathy tend to be in localized, isolated patches where small vascular abnormalities (microaneurysms and dot hemorrhages), cotton wool spots, exudates and/or edema appear. As mentioned earlier the use of stimuli with large spatial extent relative to localized, sparse abnormalities can result in a loss of sensitivity to dysfunction because the recorded response will be produced by activity generated within both relatively healthy and abnormal retinal locations. This can also be true if responses evoked from small retinal patches are combined or averaged over relatively large retinal areas.
A second consideration is that local mfERG measurement allows for the mapping of retinal function, and the location of dysfunction in the retina can be indicative of its future potential impact on vision. For example, if localized dysfunction that was known to be associated with subsequent edema development occurred close to the fovea, it would be considered more clinically important than if it occurred in the periphery. In addition, both the location and the spatial extent of dysfunction can be established.
Third, it is possible that abnormalities that are restricted to relatively small patches of retina indicate something different than abnormalities that are relatively large in extent. For example, dysfunction restricted to a small patch of retina may suggest that the probability is lower at that location for function to degrade further or for retinopathy to subsequently develop than in a case where dysfunction covers a larger patch. This could be expected because, if the mfERG abnormalities are due primarily to circulatory deficiencies and tissue hypoxia, the spatial extent of hypoxia is less in cases of localized abnormalities, and greater in cases of extensive abnormalities.
Fourth, various components of the mfERG are believed to be associated with quite specific functions (e.g., fast adaptation) and layers (e.g., inner retina) within the retina (Hood, 2000; Hood, et al., 2001; Hood, et al., 2002; Sutter, 2001; Sutter and Bearse, 1999). To the degree to which there is a solid foundation for assigning retinal generators to the mfERG components, there is considerable potential to examine these functions and putative cellular mechanisms in diabetic eyes at local geographical sites across the retina.
4.2 Stimulation and Recording Considerations
We record mfERGs using the Visual Evoked Response Imaging System (VERIS 4.3, EDI, Redwood City, California, USA). Pupils are maximally dilated with 1.0% tropicamide and 2.5% phenylephrine and the cornea is anesthetized with 0.5% proparacaine. Retinal signals are acquired with a bipolar contact lens electrode (Hansen Ophthalmic, Solon City, Iowa, USA) filled with 1% carboxymethylcellulose sodium (Refresh Celluvisc, Allergan Inc., Irvine, California, USA) and a ground electrode is clipped to the right earlobe. The fellow eye is occluded with light pressure to prevent blinking and the electrical artifacts it can introduce.
Most of our mfERG recordings, and all of those used to date in our predictive modeling studies, are performed using what is often referred to as the “standard” stimulus paradigm. Other, “non-standard” mfERG paradigms, such as the slow-flash paradigm, will be described later when appropriate. The “standard” visual stimuli are comprised of an array of 103 hexagonal elements displayed on a monochrome CRT (part of an eye camera/display/refractor unit) at a 75 Hz frame rate. The spatial layout of the multifocal stimulus array, and how it maps onto the fundus, are depicted in Fig. 1. The hexagons, which are scaled with eccentricity to evoke responses of approximately equal amplitude, are modulated pseudorandomly between white (200 cd/m2) and black (< 3 cd/m2) according to an m-sequence during the 7.5 min recordings. In each video frame, each stimulus element has an equal probability of being white or black, maintaining the overall mean luminance of the stimulus display at a fairly constant value (approximately 100 cd/m2).
Figure 1.
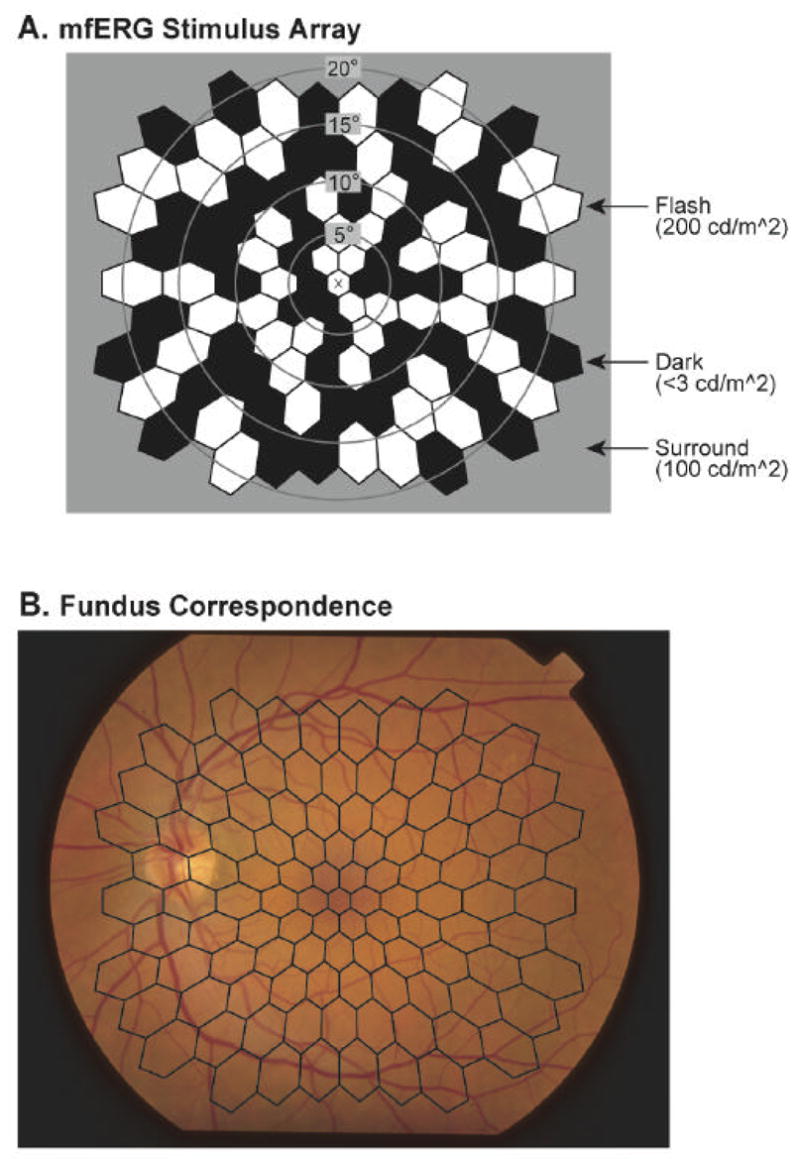
The multifocal stimulus. A: The stimulus array is comprised of 103 hexagonal elements that are scaled with retinal eccentricity. The X in the center is the fixation target. The stimulus elements are modulated pseudorandomly between black (< 3 cd/m2) and white (200 cd/m2) according to an m-sequence. B: Spatial correspondence between the stimulus array and the retina is shown. The fundus photograph is of the left eye of a diabetic patient with no retinopathy.
Recordings are made in sixteen 25-second long segments and, if necessary, observers adjust the stimulus unit for best focus of a central fixation cross before each segment. Recording quality is monitored by observation of the real-time signal voltage, and stability of fixation and eye movements are monitored with the in-line infrared eye camera. Recording segments contaminated by either electrical artifacts (significant noise or saturation) or loss of fixation are discarded and repeated. Retinal signals are filtered 10–100 Hz (half-height amplitude) and amplified 100,000 times based the on the results of our earlier study showing the advantages of 10–100 Hz over 10–300 Hz filtering (Han, et al., 2004b). MfERGs are processed in the usual way with one iteration of artifact removal and spatial averaging with 1/6 of the surrounding responses.
There are three points regarding the multifocal technique that are important to note. First, the mathematical properties of the optimized m-sequence make it possible to extract the retinal responses generated within discrete retinal patches (ultimately limited, of course, by factors such as light scatter). Second, the small electrical signals generated within the hexagonal retinal patches can be extracted with high signal-to-noise ratios by virtue of the fact that a very large number of stimulus events are “averaged”: More than 16,300 ((215-1)/2 ± 1) local flashes occur within each retinal patch during a 7.5 min recording of the “standard” mfERG. Third, the technique not only isolates a mean flash response (referred to as the first-order response kernel) but, also, temporal (e.g., two-flash) interactions in the form of higher-order response kernels at each stimulated location. These higher-order kernels provide information about fast adaptation that have proven useful in the study of diabetes (as discussed later) and other retinal disorders. More extensive descriptions of the technique can be found elsewhere (Hood, 2000; Keating, et al., 2002; Sutter and Tran, 1992).
5. Detection of Local mfERG Abnormalities in Diabetes
A fairly large number of mfERG studies have examined retinal function in diabetes after averaging the local responses over relatively large retinal areas including retinal quadrants and concentric rings around the fovea (Klemp, et al., 2004; Kurtenbach, et al., 2000; Onozu and Yamamoto, 2003; Palmowski, et al., 1997; Shimada, et al., 2001; Tyrberg, et al., 2005; Yamamoto, et al., 2001). This approach can provide information about regional retinal function. However, as noted earlier, the obvious limitation imposed by spatially averaging local mfERG waveforms over large retinal areas is that information about more localized retinal abnormalities is potentially lost. Therefore, in the following sections we focus on mfERG studies where only very limited local response combination and/or spatial averaging was performed.
5.1 Is Local Retinal Dysfunction Associated with Diabetic Retinopathy?
Spatial associations between mfERG abnormalities and early-stage retinopathic lesions should exist if the mfERG is sensitive to the consequences (or underlying causes) of local retinopathy development. With the mfERG, it is possible to answer the question, “Is retinal function more abnormal at locations where fundus signs of retinopathy are present, compared to locations where fundus signs are absent?”
In our first study to address this question (Fortune, et al., 1999), we used the “standard” mfERG paradigm and the template-stretching technique of Hood & Li (Hood and Li, 1997) to examine local response amplitudes and implicit times. We examined 16 normal control subjects, 8 diabetic subjects without retinopathy, and 8 diabetic subjects with nonproliferative diabetic retinopathy (NPDR). Within retinal locations where clinical signs of mild-to-moderate NPDR existed (e.g., microaneurysms and focal edema), mfERG implicit times were significantly delayed by up to 6 standard deviations compared to the control eyes. Furthermore, the abnormal implicit time delays became larger with increasing local retinopathy grade, as shown in Fig. 2. In this figure, grade 1 corresponds to the ETDRS level 10 (retinopathy absent), grade 2 to ETDRS level 20 (only microaneurysms), grade 3 to ETDRS level 35 (mild NPDR; microaneurysms plus other mild lesions), and grade 4 to ETDRS level 43 (moderate NPDR; moderate to severe hemorrhages or microaneurysms, or intraretinal microvascular abnormalities definitely present) (ETDRS, 1991).
Figure 2.
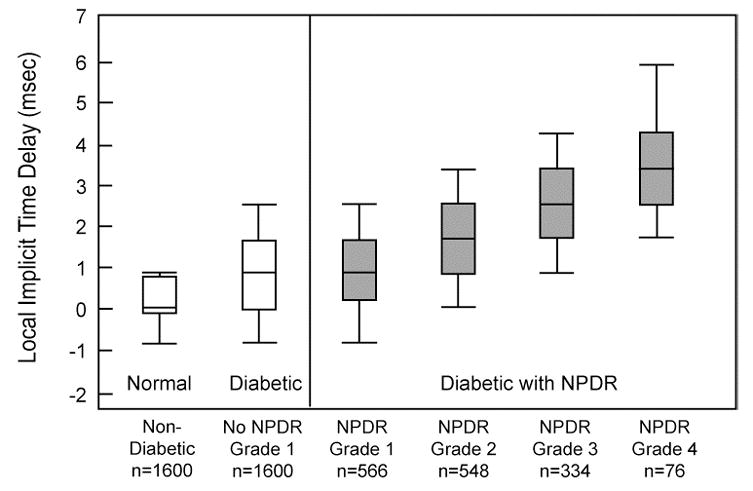
Distributions of local mfERG implicit time delays. Delays were calculated at each retinal location by subtracting the mean normal implicit time at that location from the individual implicit times. The leftmost white box-and-whiskers represent the distribution of normal delays and the white box-and-whiskers on its right represent the distribution of delays in eyes of diabetic subjects without retinopathy. The gray distributions were obtained from diabetic subjects with NPDR. Each box shows the 25th and 75th percentiles, the line inside it represents the median, and the whiskers represent the 5th and 95th percentiles. Local retinopathy grades (defined in the text) and the number of mfERG measurements in each distribution are given below each box. The local mfERG delays are progressively more abnormal (longer) with increasing severity (grade) of retinopathy. (Adapted from Fortune, et al., 1999.)
Interestingly, responses recorded from adjacent retinal locations that were clinically normal in appearance (labeled NPDR Grade 1) were also delayed relative to the normal subjects, albeit to a smaller extent than they were in locations with retinopathy. However, response amplitudes were much more normal than implicit times, and the local mfERG amplitudes, in contrast to the implicit times, were not consistently spatially associated with diabetic retinopathy in eyes that had it. This is a consistent finding in all of our subsequent studies; amplitude is not reliably abnormal in diabetes. In contrast, mfERG implicit time abnormalities are locally associated with retinopathy and are also related to the severity of local retinopathy.
To investigate local retinal function in earlier-stage retinopathy, we then examined 15 diabetic subjects with considerably milder (only a few scattered early local retinal changes) diabetic retinopathy (Schneck, et al., 2004). Even in these eyes, 29% of the local mfERGs had abnormal implicit times, where abnormality was defined as implicit time greater than or equal to 2 standard deviations above the control mean (Z-score ≥ 2; P < 0.023). Based on the standard mfERG stimulus layout (Fig. 1), “retinopathy zones” were formed that included the stimulated retinal patch with the retinopathic lesion and the three to six stimulated retinal patches surrounding it. In these retinopathy zones, approximately 49% of the local mfERGs had abnormal implicit times, whereas only 20% of the local implicit times were abnormal in areas that did not have signs of retinopathy. In this group of patients, we verified that mfERG implicit time abnormalities are more frequently observed with increasing lesion severity: Whereas 64% of the implicit times were abnormal in zones containing small patches of edema, approximately 48% were abnormal in zones containing microaneurysms, dot hemorrhages or hard exudates. It also has been reported that “standard” mfERG implicit time abnormalities are associated with sites of macular edema in more severely affected diabetic patients, although these functional abnormalities appear to have much larger spatial extents than the pathology (Greenstein, et al., 2000).
More recently, we observed that not only are the “standard” first-order mfERG implicit times spatially associated with sites of diabetic retinopathy, but these associations also exist for the amplitude of the second order kernel of the mfERG measured using a signal-to-noise ratio (Han, et al., 2005). Since the second order kernel of the mfERG reflects the activity of local fast adaptive mechanisms in the retina, these results suggest that mechanisms underlying fast adaptation are abnormal at retinal sites where clinical signs of diabetic retinopathy are located.
5.2 Are There Local mfERG Abnormalities in Diabetic Eyes Without Retinopathy?
For the implicit time of the mfERG to have potential value as a predictor of local diabetic retinopathy, it must be delayed relative to normal in retinal locations and eyes where retinopathy is not yet present. In our initial mfERG study of diabetics, we found that local mfERG implicit times were significantly prolonged, compared to normal control subjects, in the eyes of diabetic subjects without retinopathy (Fortune, et al., 1999). These abnormalities were less severe than those observed within retinal areas with NPDR (Fig. 2).
In a second study, we examined retinal function in 17 diabetic subjects with predominantly mild diabetic retinopathy and 12 diabetic subjects who had no funduscopic signs of retinopathy (Han, et al., 2004b). “Standard” mfERGs recorded from one eye of each subject were examined and normative data was obtained from a group of 20 age-similar control subjects. In this study, mfERG implicit time abnormality was defined as implicit time Z-score >= 2. In the eyes of diabetic subjects without retinopathy, 17% of the implicit times were abnormal, compared to 29% of those obtained from the subjects with early NPDR. For both groups of diabetic subjects, the proportion of abnormal implicit times was significantly greater than either the proportion expected on the basis of chance alone (2.3%) or the proportion observed in the control subjects (1.4%). These results confirm that local retinal function in diabetic subjects without retinopathy is abnormal, although not to the same degree as that observed in subjects with NPDR.
We have also longitudinally followed a sample of 22 diabetic subjects over a one year study period (Han, et al., 2004c). MfERGs were recorded and fundus photographs were taken at both the baseline (first) visit and one-year follow-up visit. At baseline, 11 of the diabetic subjects had no diabetic retinopathy and 11 had some, predominantly mild, NPDR. The distributions of the implicit time Z-scores from retinal areas without retinopathic lesions are shown in Fig. 3, where the horizontal dashed line indicates the Z-score = 2 criterion for abnormality. The distribution of implicit time Z-scores obtained from the subjects without retinopathy (“No NPDR”) is centered between those of the normal control group and those of the subjects with some baseline diabetic retinopathy. Over the study period, subjects without baseline retinopathy did not develop any retinopathy and their mfERG implicit times did not change significantly; 15% of the local responses were abnormal at baseline and 12% were abnormal at follow-up. These results suggest that retinal function is abnormal but fairly stable in the eyes of diabetic subjects who do not have baseline NPDR and do not develop it over a one year period.
Figure 3.
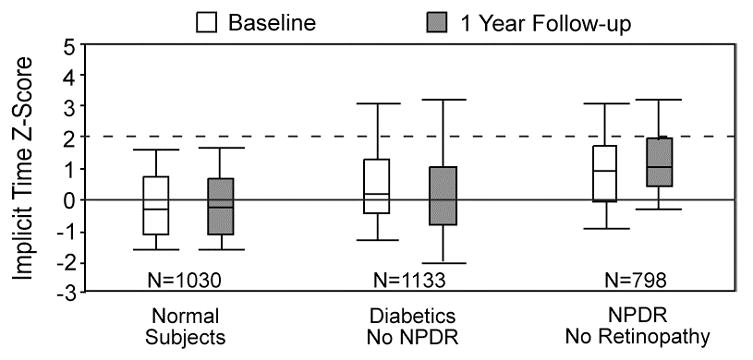
Local mfERG implicit time Z-score distributions at baseline and one-year follow-up. Results are shown for normal subjects, subjects with diabetes and no retinopathy (“Diabetics No NPDR”), and from retinopathy-free areas in the eyes of diabetic subjects with some NPDR (“NPDR No Retinopathy”). The line at Z-score = 0 indicates the mean normal value and the dashed line indicates the limit of normal. The distribution of implicit times of diabetic subjects without retinopathy is centered between those of the normal and NPDR groups, and did not change significantly over the one-year period. (Adapted from Han, et al., 2004c.)
An example of the advantages afforded by analyzing local mfERGs rather than measuring waveforms derived from averaging responses over large retinal areas can be seen in Fig. 4. The maps in this figure show retinal distributions of implicit time abnormalities for the P1 component of the “slow flash” mfERG (sf-mfERG), with darker shades indicating higher frequencies of abnormality across subjects. In the sf-mfERG paradigm, focal flashes are separated by a minimum of 53.3 ms (4 video frames), allowing the local responses to develop and decay before subsequent flashes occur. Abnormalities were defined conservatively (P < 0.01) in this study (Bearse, et al., 2004a). There are two main points to note in Fig. 4. First, P1 implicit time abnormalities occur most frequently in the diabetic subjects with predominantly mild NPDR (rightmost panel) as compared to control subjects (leftmost panel) and diabetic subjects without retinopathy (middle panel). Second, and more important, the abnormalities are not uniformly distributed. Instead, in both groups of diabetic subjects, they occur more frequently in the inferior retina (the lower half of each map) than in the superior retina. This bias may be related to the lower vasodilator reserve and greater susceptibility for ischemia reported for the inferior retina compared to the superior retina (Chung, et al., 1999; Robinson, et al., 1986). These results emphasize the point that the retinal topography of abnormalities can be obscured by grouping local responses within areas such as rings.
Figure 4.
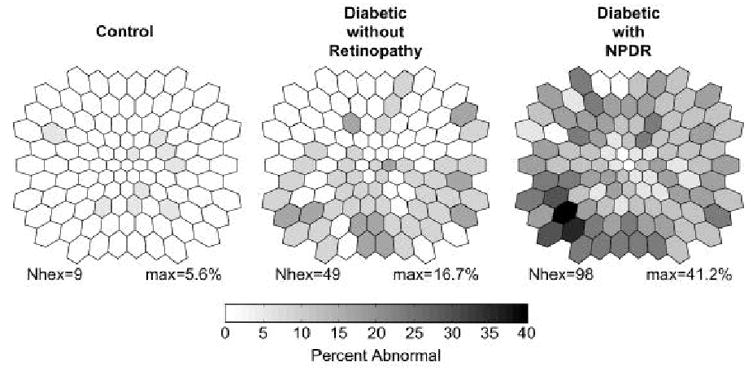
Frequency of sf-mfERG P1 implicit time abnormalities at each of the 103 retinal locations. Results are shown for control subjects (left), diabetic subjects without retinopathy (middle), and diabetic subjects with mild or moderate NPDR (right). Each frequency map is plotted as a left eye observed in retinal view. For each retinal map, “Nhex” is the number of locations with at least one abnormality and “max” is the maximum frequency of abnormality. In both groups of diabetic subjects, abnormal P1 implicit times are not uniformly distributed, occurring more frequently within the inferior retina. (Adapted from Bearse, et al., 2004a.)
5.3 Are Local “Inner” Retinal Response Contributions Abnormal?
Many electrophysiological studies of diabetes have suggested that the inner retina is most affected early in the disease. Specifically, full-field flash ERG studies have shown the high frequency oscillatory potentials (OPs), which are thought to be largely generated by the activity of amacrine cells, are preferentially affected by diabetes and diabetic retinopathy (Bresnick, et al., 1984; Bresnick and Palta, 1987; Tzekov and Arden, 1999; Yonemura, et al., 1962; Yoshida, et al., 1991). The sf-mfERG stimulus evokes local responses with high frequency components similar to full-field flash OPs (Bearse, et al., 2000; Fortune, et al., 2003; Hood, et al., 1997; Rangaswamy, et al., 2003; Wu and Sutter, 1995). These are referred to as multifocal OPs or mfOPs.
The difficulty in isolating local mfOPs from human subjects is that they are a small part of a small retinal response, ordinarily requiring either very long recording times or spatial averaging over relatively large retinal areas to achieve reasonable signal-to-noise ratios. We recently developed a technique to enhance the signal-to-noise ratios of the local higher-order mfOPs by combining digital filtering (90–225 Hz) with summation of second-order mfOPs and induced mfOPs in the first-order sf-mfERG kernels (Bearse, et al., 2004b). This allows us to investigate, in recordings of less than eight minutes, enhanced higher-order mfOPs generated within 35 relatively small contiguous patches covering the central 45 degrees of the retina. The layout of the 35 analysis groups is shown in Fig. 5A, and an example of the corresponding enhanced mfOPs recorded from a control subject is shown in Fig. 5B.
Figure 5.
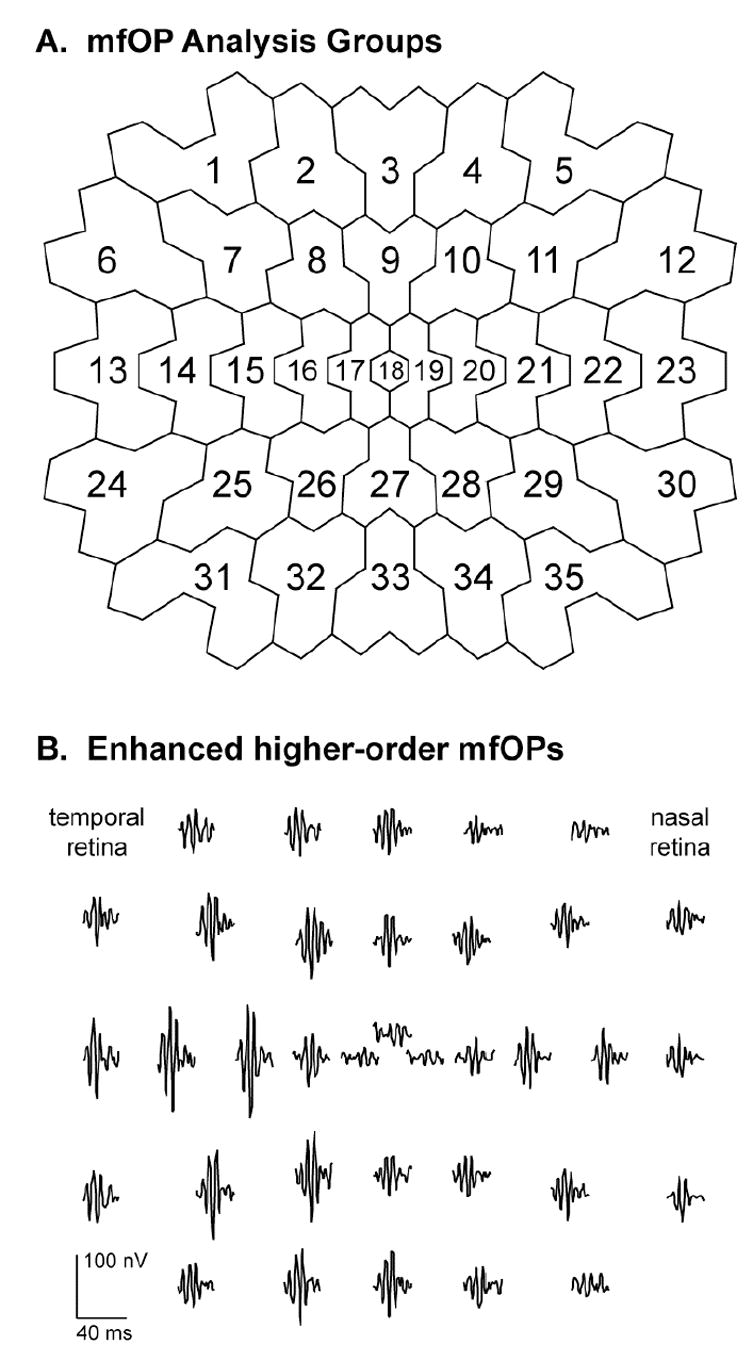
Local multifocal oscillatory potentials (mfOPs) isolated from the sf-mfERG. A: The 35 retinal zones used for mfOP analysis. B: Example waveforms of the 35 enhanced higher-order mfOPs obtained from a normal control subject. The small central waveform is displaced upwards for visibility. MfOP amplitudes are characteristically larger in the temporal retina than in the nasal retina. (Adapted from Bearse, et al., 2004b.)
We have examined the signal-to-noise ratios (SNRs) of enhanced higher-order mfOPs in 16 diabetic subjects without retinopathy and 16 diabetic subjects with early, predominantly mild NPDR (Bearse, et al., 2004b). In this study, abnormality was defined as a mfOP SNR below the 95th percentile of normal control subjects. The mean SNR of the enhanced mfOPs were abnormal in approximately 25% of the eyes of diabetics without retinopathy and in 62% of the eyes with NPDR. Interestingly, in individual eyes the retinal distributions of the abnormal first-order and the enhanced higher-order mfOPs differed significantly. Furthermore, abnormalities of the enhanced higher-order mfOPs were spatially associated with the presence of NPDR lesions but the first-order mfOP abnormalities were not. Similar to the difference between the first- and second-order kernels of the “standard” mfERG discussed earlier, higher-order mfOPs, in contrast to the first-order mfOPs, reflect the effects of fast adaptive retinal mechanisms. These findings, therefore, provide additional evidence that abnormalities of fast adaptive mechanisms are spatially associated with retinal sites containing fundus signs of early diabetic retinopathy.
5.4 Why Does Diabetes Affect mfERG Implicit Time more than Amplitude?
We have noted above that, compared to response amplitude, implicit time of the “standard” local first-order mfERG is more frequently abnormal in both eyes with diabetic retinopathy and eyes without retinopathy (Fortune, et al., 1999; Han, et al., 2004b; Han, et al., 2004c). Abnormal implicit times, not abnormal amplitudes, are also spatially associated with mild and moderate NPDR in eyes with NPDR (Fortune, et al., 1999). Furthermore, to anticipate the modeling results described below, implicit times are predictive of the local development of diabetic retinopathy. Why is local response implicit time affected to a greater degree than local response amplitude?
One reason for the greater sensitivity of implicit time compared to amplitude is primarily statistical in nature. The inter-subject variability of local mfERG implicit time measurements is lower than it is for local amplitude measurements among normal control subjects (Fortune, et al., 1999; Han, et al., 2004c). Figure 6 shows the distributions of amplitude and implicit time coefficients of variation for the 103 local “standard” mfERGs recorded from a group of 30 normal control subjects. The difference between the two response measures is striking (P < 0.001). The lower inter-subject variability for local implicit times in normal subjects provides smaller confidence intervals for that response measure than those obtained for the local mfERG amplitudes. Consequently, for abnormalities to reach statistical significance, the relative deviation from “normal” must be greater for amplitude measurements than for implicit time measurements.
Figure 6.
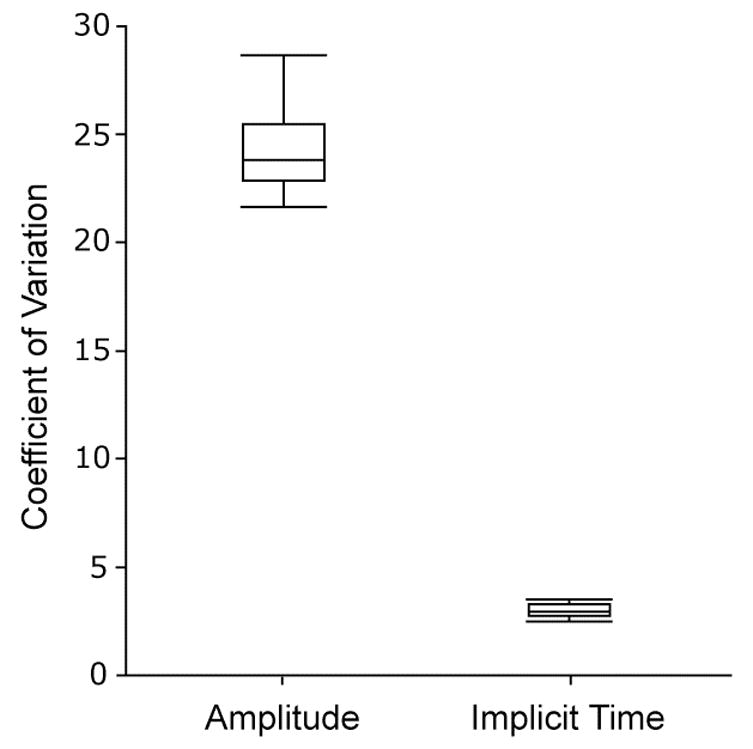
Distributions of “standard” mfERG amplitude and implicit time coefficients of variation, calculated across 30 normal subjects at each of the 103 stimulated retinal locations. Implicit time (median = 3.0%) is much less variable than amplitude (median = 23.8%) at each location (P < 0.001), contributing to smaller normative confidence intervals for implicit time.
A more interesting factor that is likely playing an important role is the type of pathology underlying the retinal dysfunction that we have investigated. In diabetes without retinopathy and in cases where only early, mild lesions such as a few scattered dot hemorrhages or microaneurysms are present, it is reasonable to expect that the retinal tissue is not necrotic even in the vicinity of small lesion sites. Thus, we would expect that the primary generators of the mfERG, the bipolar cells (Hare and Ton, 2002; Hood, et al., 2002), would be functioning abnormally but not completely silenced. Given the prevailing view that early diabetic retinal disease first affects the microvasculature supplying inner retinal neurons including ganglion, amacrine and bipolar cells (and the Muller cells to some degree), then the time course of signal generation and signal propagation through the retinal circuitry will be abnormally prolonged but the responses will not be extinguished (Hare and Ton, 2002; Hood, et al., 2002; Hood, et al., 1999). With these considerations, we would expect that the local retinal responses would be abnormally delayed but not necessarily reduced in amplitude. This is consistent with the interpretation that, in early diabetic eye disease, post-receptoral factors appear to primarily underlie response abnormalities. In contrast, in diseases where the outer retina is definitely affected, such as retinitis pigmentosa and progressive cone dystrophy, the mfERG is typically reported to be both decreased in amplitude and delayed in implicit time (Holopigian, et al., 2002; Hood, et al., 1998; Kondo, et al., 1995; Seeliger, et al., 1998).
If the time course of signal propagation through the retina is abnormally prolonged in diabetes, then it is likely that fast adaptive mechanisms should also be affected. Consistent with this view, it has been proposed that “standard” first-order mfERGs with normal amplitudes and abnormally delayed implicit times are associated with abnormally small second-order mfERG kernels. This association has been observed in “atypical” mfERGs recorded from patients with retinitis pigmentosa, progressive cone dystrophy and NPDR with clinically significant macular edema (CSME), and is believed to originate from dysfunction in the outer plexiform layer (Greenstein, et al., 2004). Recently, we examined this association in a preliminary study of two groups of diabetics: 6 subjects with NPDR and no CSME, and 20 subjects without NPDR in either eye (Bronson-Castain, et al., 2006). In patients without retinopathy, within retinal areas with normal first-order amplitudes and delayed first-order implicit times, no association was found between first-order implicit time and second-order amplitude (R2 = 0.02; P > 0.05). However, there was a weak but statistically significant association in the patients with mild or moderate NPDR (R2 = 0.13; P < 0.001). These results suggest that it is not until after fundus signs of diabetic retinopathy are present that first order implicit time delays and second order amplitudes are significantly associated. Furthermore, even in the presence of retinopathy it appears that abnormalities of fast adaptive processes are not strongly associated with local mfERG delays in diabetes (except, perhaps, in the presence of CSME). The reason(s) for the first-order mfERG implicit time delays observed in diabetes remain to be fully understood.
6. Predicting the Development of Local Diabetic Retinopathy
Our first goal was to establish whether mfERG implicit time abnormalities are more likely to occur in retinal locations where new NPDR subsequently develops than they are to occur in other retinal areas. Our initial efforts have concentrated on investigating the development of new, predominantly mild, NPDR. While the term “prediction” could be interpreted to imply a stronger meaning than we intend, for lack of a better term we will use it when referring to the fact that abnormally delayed local mfERG implicit time often precedes (i.e., “predicts”) the appearance of diabetic retinopathy in corresponding local retinal patches.
6.1 Do mfERG Abnormalities Precede Retinopathy Appearance?
Prior to consideration of quantitative models formulated to predict the appearance of new diabetic retinopathy in retinal locations displaying abnormal function, it is first necessary to establish that mfERG implicit time abnormalities do indeed occur at retinal sites where new retinopathy subsequently develops. To do this, we performed a study in which one eye of 11 diabetic patients with predominantly mild NPDR and 11 diabetic patients without retinopathy were tested at baseline and then retested 12 months later (Han, et al., 2004c). The template-stretching method was used to measure local mfERG implicit times (Hood and Li, 1997). After one year, two-thirds of the eyes that had some diabetic retinopathy at baseline developed new retinopathy in zones that were free of retinopathy at baseline. In these eyes, abnormal baseline mfERG implicit times (defined as implicit time Z-scores ≥ 2) occurred within 35% of the retinal zones that were free of retinopathy at baseline.
Of the 63 initially retinopathy-free zones with abnormal baseline implicit times, 22 (35%) developed new diabetic retinopathy at follow-up. In contrast, only 2% of the retinal zones with normal baseline implicit times developed new diabetic retinopathy. Table 1 summarizes these results. Development of new retinopathy within one year was approximately 21 times more likely in retinal zones with abnormal baseline mfERG implicit times than it was in zones with normal baseline implicit times. The odds ratio for developing new diabetic retinopathy in the zones with abnormal baseline mfERG implicit times was 31.4 (P < 0.001). These results established that localized functional abnormalities of the retina, evident as abnormally prolonged implicit times of the “standard” mfERG, precede the development of new retinopathy observed one year later in specific corresponding retinal locations (Han, et al., 2004c).
Table 1.
Association between new retinopathy development and baseline implicit time status.
| NPDR Development at 1 Yr. Follow-up | |||
|---|---|---|---|
| T0 mfERG Zone | Yes | No | Total |
| Abnormal | 22 | 41 | 63 |
| Normal | 2 | 117 | 119 |
| Total | 24 | 158 | 182 |
Odds ratio = 31.4 (P < 0.001).
“T0 mfERG Zone” is the retinal zone’s implicit time status at baseline.
6.2 Formulation of Quantitative Predictive Models
Most previous studies that investigated the prediction of the onset of diabetic retinopathy have developed multivariate models (which utilize multiple risk factors) based on patient health information including the duration of diabetes, cholesterol level, blood glucose level, and the presence or absence of microalbuminuria (Donaghue, et al., 2003; Liu, et al., 1993; Nguyen, et al., 1996; Wirta, et al., 1999). Measures of visual function have also been examined, including the OPs of the conventional full-field ERG and blue-yellow color discrimination (Aspinall, et al., 1983; Bresnick, et al., 1984; Simonsen, 1980). This approach is inherently limited. Whereas diabetic retinopathy is a disease that occurs non-uniformly in patches across the retina, these predictive models deal with the entire retina as a unit at risk; they predict eyes, rather than retinal locations, at risk.
We have seen that abnormally delayed mfERG implicit times locally precede the appearance of new lesions in eyes with mild and moderate NPDR. Consequently, formulation of a predictive model based on mfERG implicit time can provide prognostic information related to spatial location. The correlation of implicit times within individual retinas can be estimated and compensated for in the construction of a univariate model based on mfERG implicit time as the sole predictive variable. Furthermore, additional factors that could contribute to the risk of developing early diabetic retinopathy can be evaluated and incorporated into a multivariate model with local mfERG implicit time providing spatial information. Although the mfERG is our only functional measure that allows for the prediction of the appearance of fundus signs of retinopathy in specific retinal locations, the addition of subject health factors such as the blood glucose concentration at the time of testing and the duration of diabetes could increase the predictive power of formal models. We therefore examined both univariate (mfERG implicit time alone) and multivariate models, both incorporating estimation and adjustment for within-eye correlations of local implicit times.
6.2.1 Methods for Model Building
In the first of our model-building efforts, 28 eyes of 28 diabetic subjects (12 with some baseline NPDR and 16 without baseline retinopathy) were studied during an initial and one-year follow-up examination (Han, et al., 2004d). All of the diabetic subjects we have studied had 20/25 or better visual acuity, no media opacity, no history of ocular surgery, and no other eye disease except mild or moderate initial NPDR. During the initial examination, medical history was recorded, blood glucose concentrations were measured, and “standard” mfERGs were recorded from 103 retinal locations. Dilated eye examinations were performed and 50-degree stereoscopic fundus photographs were taken to image the tested retinal area. After one year (± 1.7 months) a follow-up examination was performed that was identical to the initial exam. The fundus photographs were graded by a retinal specialist who was masked to all examination data including the mfERG results.
Figure 7 shows diagrammatically how the baseline mfERGs are analyzed and placed into spatial registration with the follow-up fundus photographs, using as an example the results obtained from the left eye of a diabetic subject with NPDR. The 103 local mfERGs (Fig. 7A) are measured using the template-stretching method (Hood and Li, 1997), then converted to implicit time Z-scores based on normative results obtained from control subjects. Retinal patches with abnormal implicit time Z-scores (>= 2) are colored red in Fig. 7B and patches with normal Z-scores are colored white. The fundus photograph (Fig. 7C) is obtained at follow-up and graded. Next, the 103 local implicit time Z-scores are arranged into 35 fixed “zones” comprised of either two or three adjacent stimulated retinal patches (Fig. 7D). All but two of the zones contain three patches. Each retinal zone is assigned the maximum of the two or three individual Z-scores falling within it (zones with abnormal implicit time Z-scores are colored red in Fig. 7D). Finally, the zones are superimposed over the graded fundus photograph and the retinopathic lesions are mapped onto them (lesion locations are represented by the black dots in Fig. 7D). In this example, four of the five retinopathic lesions fall within zones with abnormal baseline mfERG implicit times and the fifth lesion, a microaneurysm, developed in a zone neighboring implicit time abnormalities.
Figure 7.
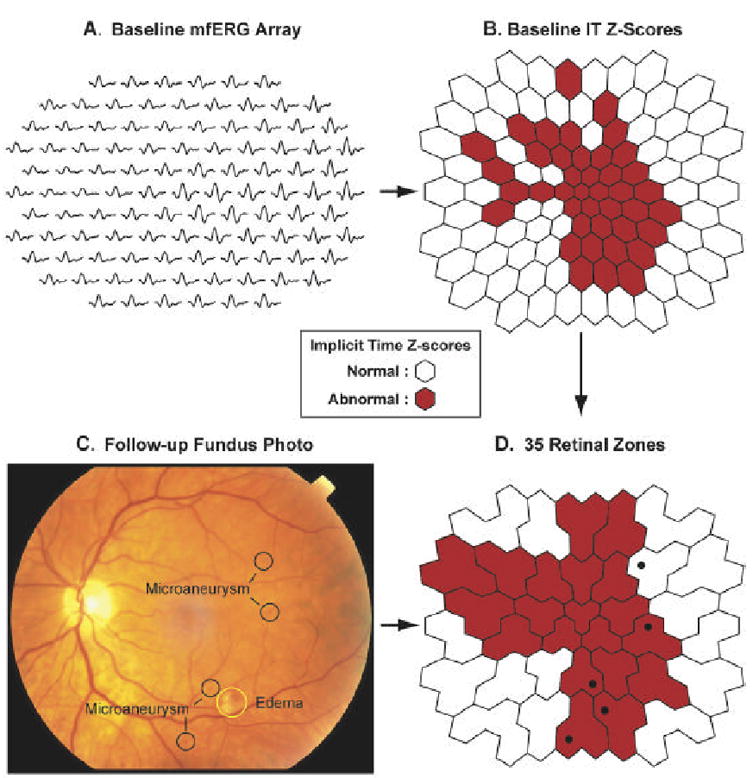
Mapping the baseline mfERG implicit times and follow-up retinopathic lesions onto the 35 retinal zones used in the modeling studies. These data were obtained from the left eye of a diabetic subject with NPDR. A: The array of 103 “standard” baseline mfERGs are plotted in retinal view orientation. B: Baseline implicit time (IT) Z-scores are calculated for the 103 mfERG on the basis of normative data collected from 30 control subjects. Abnormal Z-scores (Z >= 2) are indicated by red hexagons, and normal Z-scores by white. C: The fundus photograph, taken at follow-up, is graded blind to the other examination results. In this eye five lesions were noted within the stimulated retinal area. D: Baseline implicit times and retinopathic lesions are analyzed within the 35 retinal zones. Each zone is assigned the largest Z-score. Zones with abnormal implicit times are colored red and black dots signify the locations of the retinopathic lesions. In this example, four of the five lesions occur within zones having abnormal baseline implicit times, and the fifth (a microaneurysm) is located adjacent to abnormal zones.
The retinal zones are constructed for a number of reasons. First, the retinal lesion identified in a fundus photograph could be smaller in appearance than the actual extent of the anatomical lesion. Second, the visible lesion might not lie directly over the actual location of the anatomical lesion. Third, the use of zones helps to reduce the effects of possible mismatches between the retinal locations of the mfERG stimulus array and the fundus photographs/gradings. It is critical to note that, in all of our predictive modeling studies, retinal zones with pre-existing (baseline) retinopathy were excluded from further analysis.
We examine the association between baseline mfERG implicit time (and other risk factors) and the incidence of new signs of retinopathy using logistic regression (Stata 9.1, StataCorp, College Station, TX, USA). Logistic regression is widely used in studies with outcome measures involving binary disease states (e.g., retinopathy develops or does not) and continuous or binary risk factors. Additional potential risk factors that we have examined are duration of diabetes, age, gender, blood glucose concentration, diabetes type, and baseline retinopathy status (presence or absence of retinopathy). Generalized estimating equations are applied with corrected (robust) estimation of the variance-covariance matrix for model coefficient estimates to allow for within-eye correlation of mfERG implicit times among retinal zones (Zeger, et al., 1988). A compound symmetric covariance structure that assumes common covariance among the mfERG zones within an eye and independence between subjects is used to estimate the regression coefficient associated with each risk factor.
In the formulation of multivariate models, the association of each variable (potential risk factor) with subsequent retinopathy development is first examined alone using logistic regression (a univariate analysis). Next, a preliminary multivariate model is constructed based on the variables that were observed to be significantly associated with retinopathy development in the univariate analyses. Finally, the remaining variables are added to the preliminary multivariate model one at a time, in ascending probability order, to assess their additional contributions to the model. For further details of the model-building methods, please refer to (Han, et al., 2004d).
6.2.2 Quantitative Models Predicting Local Retinopathy Over One Year
In our initial model-building efforts (Han, et al., 2004d), we examined 980 retinal zones (35 retinal zones per eye in 28 subjects with diabetes). Characteristics of these diabetic subjects are given in Table 2. Of the 980 zones, 61 (6%) had pre-existing retinopathy at baseline and, therefore, those zones were excluded from further analysis since they have no predictive value. New diabetic retinopathy developed in 11 of the 12 eyes that had some baseline NPDR and in one of the 16 eyes that had no baseline retinopathy over the one-year study period. Of the 919 retinal zones that did not have baseline retinopathy, 64 (7%) had developed new retinopathy at follow-up. Of these, 57 (89%) of the zones developed microaneurysms or dot hemorrhages, 6 (9%) developed hard exudate, 2 (3%) developed cotton wool spots, and one zone developed a small patch of edema.
Table 2.
Characteristics of subjects with diabetes in the initial one-year modeling study.
| T0 Status | Subjects (n) | Gender (M/F) | Age ± S.D. (years) | DM Type (Type: n) | DM Duration ± S.D. (years) | Blood glucose ± S.D. (mg/dL) |
|---|---|---|---|---|---|---|
| NPDR | 12 | M: 7
F: 5 |
52.5 ± 7.4 | 1: 2
2: 10 |
10.2 ± 6.1 | 191 ± 74 |
| No DR | 16 | M: 7
F: 9 |
50.1 ± 10.1 | 1: 3
2: 13 |
5.2 ± 2.3 | 146 ± 51 |
T0 status: the eye had some retinopathy (NPDR) or no retinopathy (No DR) at baseline.
The first step was to examine the mfERG implicit time as the sole risk factor by using a generalized estimating equation based on logistic regression to formulate a univariate predictive model of retinopathy development over the one-year period. The general form of a univariate logistic regression model is:
where is the log odds (logit) of outcome occurrence, x is a risk factor of interest, the regression coefficient b is the log odds ratio for a unit change in x, and a is the log odds of outcome occurrence when the risk factor x equals 0. The odds ratio for the risk factor x equals eb. In a multivariate analysis, multiple risk factors are examined, producing equations that are similar to the general form of the univariate model shown above but with the addition of multiple terms and additional regression coefficients (e.g., a + bx + cy + dz). An alternate form of the general univariate logistic regression model that provides direct access to the probability of the outcome is:
Utilizing these techniques, univariate analysis of mfERG implicit time resulted in the following model:
which is equivalent to
where pret is the probability of developing new diabetic retinopathy in a zone at follow-up and mfergIT is the baseline mfERG implicit time Z-score of the zone. The regression coefficient value is 0.21 (significant at P = 0.03), indicating that longer baseline mfERG implicit times are associated with higher probabilities of developing new retinopathy within retinal zones within one year. The odds ratio for this coefficient, e0.21 = 1.23, is also an approximation of the relative risk of developing new retinopathy. In other words, there is a 23% increase in the risk for development of new diabetic retinopathy associated with a unit increase in baseline mfERG implicit time Z-score.
The accuracy of a predictive model can be assessed by constructing a receiver operating characteristic (ROC) curve. An ROC curve describes the relationship between sensitivity (the probability of correctly predicting the development of new retinopathy) and 1-specificity (the probability of incorrectly predicting retinopathy development where none occurs) at various criterion pret values obtained from the alternate form of the logistic regression model. The area under the curve (AUC) for an ROC curve can range from 0.5, indicating chance performance of the model, to 1.0, indicating perfect predictive performance. For AUCs, we determined standard errors, constructed binomial exact confidence intervals, and conducted hypothesis tests where possible (DeLong, et al., 1988).
The ROC curve for our one-year univariate model is shown in Figure 8A. The AUC for this model is 0.80 (95% CI = 0.78–0.83), indicating that mfERG implicit time is a significant predictor of retinopathy development. For this model, a criterion pret of 0.1 yields relatively high values of 73% sensitivity and 77% specificity. Overall, this model, based solely on mfERG implicit time, performs quite well at predicting the development of new local diabetic retinopathy.
Figure 8.
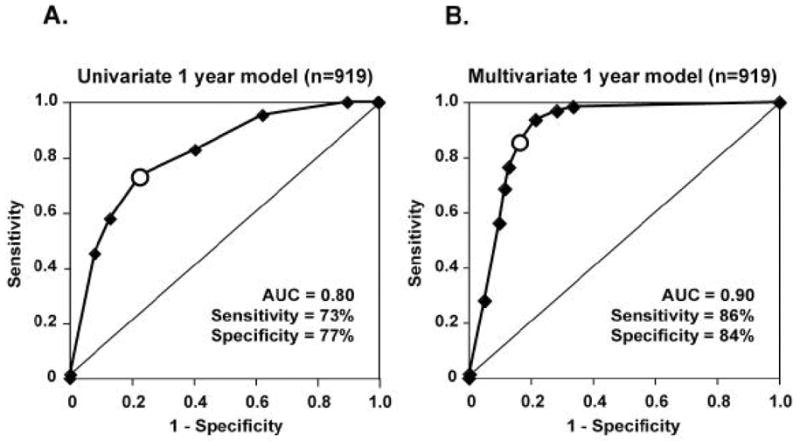
Receiver operating characteristic (ROC) curves describing the performances of the univariate and multivariate one-year predictive models, both based on 919 retinal locations that did not have baseline retinopathy. Each ROC curve is generated by plotting sensitivity and 1-specificity at various criterion probabilities for retinopathy development. Area under the curve (AUC), a measure of overall model accuracy, can vary from a minimum of 0.5 (diagonal line in each graph) to 1.0 (perfect performance). A: The univariate one-year model has an AUC = 0.80 and, at a 0.1 criterion probability (open symbol on curve), sensitivity = 73% and specificity = 77%. B: The multivariate model performed better than the univariate model, with AUC = 0.90 and, at a 0.4 criterion probability (open symbol on curve), sensitivity = 86% and specificity = 84%. (Adapted from Han, et al., 2004d.)
Now let us consider whether the inclusion of known or suspected risk factors, which are not themselves localized with regard to the retina, improves the local predictive power of the mfERG implicit time model. The six additional factors we examined for this purpose were age, gender, baseline retinopathy status, duration of diabetes, type of diabetes, and blood glucose concentration at the time of mfERG recording. The factors age (age; units = years), duration of diabetes (dmDuration; units = years) and blood glucose concentration (bloodGlucose; units = mg/dL) are continuous variables on interval scales. The factors gender (gender), baseline retinopathy status (hasRet) and type of diabetes (diabType) are binary variables and a value of 1 is arbitrarily assigned to each of female gender, the presence of baseline retinopathy, and Type 1 diabetes.
The results obtained for the association of each variable alone with the development of diabetic retinopathy are summarized in Table 3. Whereas hasRet, dmDuration and mfergIT have significant power to predict the development of new retinopathy, the variables age, gender, diabType and bloodGlucose are not, by themselves, significant predictors. Regression coefficients for hasRet and dmDuration are positive, as expected, indicating that the presence of baseline retinopathy (in zones not involved in actual model making) and longer duration of diabetes increase the probability of new retinopathy development within one year.
Table 3.
Univariate analysis of potential variables for use in the one-year multivariate model.
| Variable | P-value | Regression Coefficient |
|---|---|---|
| Age | 0.57 | 0.02 |
| Gender | 0.86 | 0.14 |
| hasRet | < 0.001 * | 4.83 |
| dmDuration | < 0.001 * | 0.27 |
| diabType | 0.58 | 0.62 |
| bloodGlucose | 0.39 | 0.01 |
| mfergIT | 0.03 * | 0.21 |
= statistically significant.
A preliminary multivariate model was then formulated based on the variables that were significantly associated (P < 0.05) with the development of retinopathy in the univariate analysis: mfergIT; hasRet; dmDuration. Next, the other variables were added to the preliminary multivariate model one at a time, in the order of increasing P-values obtained in the univariate analyses, to formulate a final multivariate model. The criterion we used for allowing a variable to remain in the final multivariate model was P < 0.20 for each newly added variable (Jewell, 2003). We found that whereas bloodGlucose (P = 0.17) met this criterion, age (P = 0.75), gender (P = 0.96) and diabType (P = 0.61) did not provide significant predictive information to the model.
Our one-year multivariate model was, therefore, formulated as:
Table 4 shows the coefficients, P-values and odds ratios for the four variables in this model. All four variables are positively correlated with the probability of developing new diabetic retinopathy, as indicated by the fact that their odds ratios are greater than 1.0. Note, however, that whereas the variable bloodGlucose contributes to the predictive power of the model, its odds ratio does not reach statistical significance.
Table 4.
Parameters of the one-year multivariate predictive model.
| Variable (units) | Coefficient | P-value | Odds Ratio (95% CI) |
|---|---|---|---|
| mfergIT (per z-unit score) | 0.32 | 0.038 | 1.38 (1.02–1.86) |
| hasRet (yes/no) | 3.84 | < 0.001 | 46.36 (5.97–359.78) |
| dmDuration (per year) | 0.14 | 0.025 | 1.15 (1.02–1.30) |
| bloodGlucose (per mg/dL) | 0.005 | 0.175 | 1.03 (0.94–1.12) |
We can interpret the variables in the model in the following way. With all the other variables fixed in value, the odds ratio for the development of new retinopathy is 1.15 for each one-year increment in the duration of diabetes (variable dmDuration), indicating a 15% increase in annual probability for developing retinopathy. Likewise, there is a 38% increase in the probability of retinopathy development at a specific retinal location over a one year interval for each unit increase in mfERG implicit time Z-score (variable mfergIT) with all other variables held fixed. The largest odds ratio, 46.36, is seen to occur for hasRet. Whereas at first glance this could be interpreted to indicate that the presence of baseline diabetic retinopathy somewhere in an eye is a very strong predictor of future new retinopathy development elsewhere in the eye over a one-year period, this interpretation should be tempered with caution due to its very large 95% confidence interval.
Figure 8B shows the ROC curve for the one-year multivariate model; it performs better than the univariate model (Fig. 8A) which was based on mfERG implicit time alone. The ROC curve of the multivariate model has an AUC of 0.90 (95% CI = 0.87–0.91), which is significantly (P < 0.001) greater than the AUC of 0.80 (95% CI = 0.78–0.83) obtained for the univariate model. At a criterion pret of 0.4, the multivariate model has a sensitivity of 86% and a specificity of 84%, both of which are higher than the model using mfERG implicit time as its sole predictive variable. Thus, the multivariate model predicts the appearance of diabetic retinopathy at specific new retinal locations with better sensitivity and accuracy than the univariate model.
6.2.3 Preliminary Testing of the One-Year Multivariate Model
In order to test the one-year multivariate predictive model, we examined a sample of 12 eyes (one eye per subject) that were not included in the model-making process (Han, et al., 2004d). Four of the 12 eyes belonged to four new subjects with diabetes, one with NPDR and three without retinopathy. The other eight eyes (four with NPDR and four without retinopathy) were fellow eyes of those that were part of the model-making group. To further separate the model-making and model-testing samples, the model-testing data were obtained from these fellow eyes one year after the model-making data had been obtained from the other eye. The scheme of 35 retinal zones that was previously used to formulate the predictive models was also used to test the model.
In the model-testing eyes, 10 (2.3%) of the 420 retinal zones had diabetic retinopathy at baseline: eight had microaneurysms and/or dot hemorrhages and two had hard exudates. New retinopathy was noted at the one year follow-up within four of the eyes with baseline NPDR and in one of the eyes without baseline retinopathy. Forty-seven (11.5%) of the 410 zones without baseline retinopathy developed new fundus signs: 34 zones with microaneurysms and/or dot hemorrhages; 11 zones with hard exudates; and two zones with cotton wool spots.
Table 5 summarizes the model-testing performance of the multivariate model. Using the same criterion probability of new retinopathy development that had been used in model-making, pret = 0.4, the multivariate model performed well, correctly predicting the development of new retinopathy in 42 of the 47 zones. This corresponds to a sensitivity of 89% (95% CI = 80.6–98.2%). The instances of new retinopathy development that were not predicted were all relatively minor lesions (microaneurysms and/or dot hemorrhages) and included three zones in the eye that did not have baseline retinopathy. At the pret = 0.4 criterion, the model had a specificity of 86% (95% CI = 82.1–89.3%).
Table 5.
Results obtained from testing of the one-year multivariate model.
| Model Prediction | Developed New Retinopathy | Remained Free of Retinopathy | Total Zones |
|---|---|---|---|
| Develop new Retinopathy | 42 | 52 | 94 |
| Remain Retinopathy-Free | 5 | 311 | 316 |
| Total Zones | 47 | 363 | 410 |
The sensitivity and specificity of this multivariate predictive model was high when applied to the model-testing data, and similar to the predictive performance that was expected on the basis of the model-making accuracy. It should be noted that, although care was taken to minimize overlap of the model-making and model-testing patient samples, a more rigorous approach to testing the predictive model could be undertaken in the future. Ideally, validation of the model would be performed on entirely new samples of subjects by independent laboratories. Nonetheless, the model is promising in that it appears to be the first to quantitatively predict the retinal locations of new NPDR development over a one-year period.
6.2.4 Formulation and Comparison of Models for Prediction over One and Two Years
More recently we followed 20 eyes of 20 subjects with diabetes over a two-year period (Ng, et al., 2006). Six of these subjects had some mild-to-moderate baseline NPDR and 14 had no baseline retinopathy. Characteristics of these subjects are given in Table 6. MfERG recording, fundus photographs, medical history and blood glucose concentration were obtained from each subject at baseline (T0), one-year follow-up (T1), and two-year follow-up (T2). As in our previous studies, local mfERG implicit times were measured using the template-stretching method (Hood and Li, 1997) and the results were once again analyzed within the 35 retinal zones as depicted in Fig 7. In this work, implicit time Z-scores were calculated on the basis of results obtained from a group of 30 normal subjects.
Table 6.
Characteristics of diabetic subjects in the two-year predictive modeling study.
| Baseline Status | Subjects (n) | Gender (M/F) | Age ± S.D. (years) | DM Type (Type: n) | Duration ± S.D. (years) | Blood glucose ± S.D. (mg/dL) |
|---|---|---|---|---|---|---|
| NPDR | 6 | M: 4
F: 2 |
53.6 ± 6.3 | 1: 1
2: 5 |
10.8 ± 7.5 | 207 ± 76 |
| No DR | 14 | M: 8
F: 6 |
51.2 ± 7.7 | 1: 2
2:12 |
4.5 ± 2.7 | 171 ± 74 |
NPDR = nonproliferative diabetic retinopathy; No DR = no diabetic retinopathy
Using the model-making procedures described earlier, univariate and multivariate models were formulated to locally predict the development of new NPDR. Both one- and two-year models were formulated to compare the accuracy of the predictions over different time periods. For the two-year predictive models, new retinopathy occurring at either T1 or T2 was considered to be a positive outcome (i.e., local retinopathy status was cumulatively measured).
At baseline, NPDR was present in 42 (6%) of the 700 (20 eyes X 35 zones per eye) total retinal zones. These zones were excluded from further analysis. New retinopathy appeared within 21 (3%) of the 658 remaining zones by T1 and within 47 (7%) of the zones by T2 (including those which developed retinopathy by T1). This new retinopathy occurred in 7 of the 20 eyes (3 with no baseline retinopathy and 4 with some baseline retinopathy).
First, let us consider one- and two- year models based on mfERG implicit time alone. The resulting formula for the one-year (T0 to T1) univariate predictive model is:
and the formula for the two-year (T0 to T2) model, derived from the same eyes, is:
The odds ratio for the mfERG implicit time (mfergIT) term is 1.46 (P < 0.001) for the one-year model and 1.38 (P = 0.025) for the two-year model. These can be interpreted as approximations for relative risks. Thus, the increase in risk for the development of new local diabetic retinopathy for each unit increase in mfERG implicit time Z-score is 46% for the one-year univariate model and 38% for the two-year univariate model.
The appearances of their ROC curves (Fig. 9) suggest that the one-year univariate model is more accurate than the two-year model. In fact, this is true. The AUC for the one-year univariate model is 0.86 (95% CI = 0.84–0.89), compared to 0.75 (95% CI = 0.71–0.78) for the two-year model. At the criterion P-value indicated by the large dot on each ROC curve, the sensitivity and specificity for the one-year model are 86% and 76%, respectively, whereas they are 72% and 69%, respectively, for the two-year model.
Figure 9.
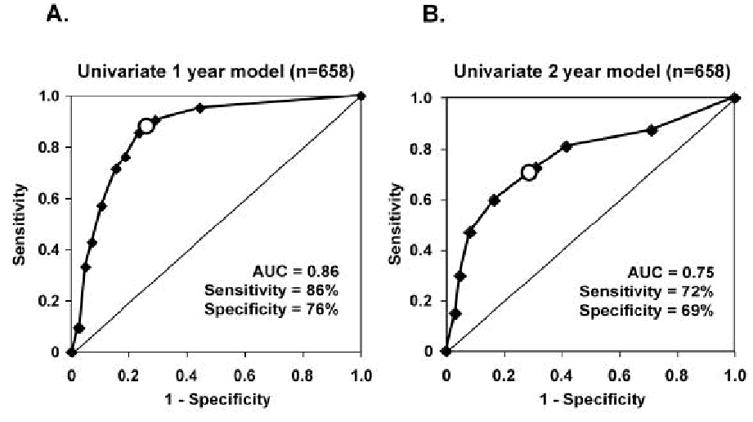
ROC curves for the univariate one-year (A) and two-year (B) models based on 658 retinal locations within the same eyes that did not have retinopathy at baseline. The one-year univariate model, had AUC = 0.86, sensitivity = 86% and specificity = 76%. For the two-year univariate model, AUC = 0.75, sensitivity = 72% and specificity = 69%. All values are higher for the one-year model than for the two-year model.
Given that the incorporation of additional risk factors improved the performance of the multivariate compared to the univariate models in our initial modeling study that was described earlier, we expected to see similar improvement in this study. The risk factors we examined in the formulation of multivariate models in the present study were mfERG implicit time Z-score (mfergIT), duration of diabetes in years (dmDuration), blood glucose concentration in mg/dl (BGC), age, and gender. In contrast to the one-year multivariate model described earlier, in this study we did not examine “some retinopathy at baseline” as a risk factor due to the bias introduced by the unequal numbers of subjects with and without baseline retinopathy (6 and 14, respectively).
The form of the one- and two-year multivariate models is:
and the parameters for the one- and two-year models are shown in Table 7. The estimated odds ratio (“OR” in Table 7) for each variable is greater than 1.0 in both models, indicating that each variable is positively associated with the development of new diabetic retinopathy within a retinal zone. All of the variables and their odds ratios reach statistical significance except for mfERG implicit time (mfergIT) in the two-year model. Counterintuitively this suggests that the mfERG implicit time has less predictive power over the two-year period than it does over the one-year period. We will consider this further below. However, it is important to note that, because the mfERG is the only local retinal measure in the model, the variable mfergIT is essential for prediction of retinopathy within specific retinal zones (patches).
Table 7.
Parameters of the one- and two-year predicitve models.
| One-Year Model | Two-Year Model | |||||
|---|---|---|---|---|---|---|
| Variable (units) | Coeff. | P-value | OR (95% CI) | Coeff. | P-value | OR (95% CI) |
| mfergIT (Z-score) | b = 0.48 | 0.026 | 1.62 (1.06–.48) | b = 0.25 | 0.188 | 1.28 (0.89–.86) |
| dmDuration (years) | c = 0.49 | 0.009 | 1.64 (1.13–.38) | c = 0.26 | <0.001 | 1.29 (1.14–.45) |
| BGC (mg/dl) | d = 0.02 | 0.016 | 1.02 (1.004–.04) | d = 0.01 | 0.004 | 1.01 (1.004–.02) |
Coeff. = coefficient; OR = odds ratio
The constants (a) for the one- and two-year models are −5.35 and −7.71, respectively.
The ROC curves shown in Fig. 10 summarize the performances of the one-year and two-year multivariate models formulated in this study. Whereas both models predict retinopathy with high accuracy, the performance of the one-year model is especially impressive. The AUC for the one-year model (0.95, 95% CI = 0.93–0.97) and that for the two-year model (0.88, 95% CI = 0.87–0.92) are significantly different (each AUC is outside the other model’s 95% confidence interval). At the criterion P-value indicated by the large dot on each of the ROC curves, the sensitivity and specificity are, respectively, 95% and 93% for the one-year multivariate model, and 81% and 82% for the two-year multivariate model.
Figure 10.
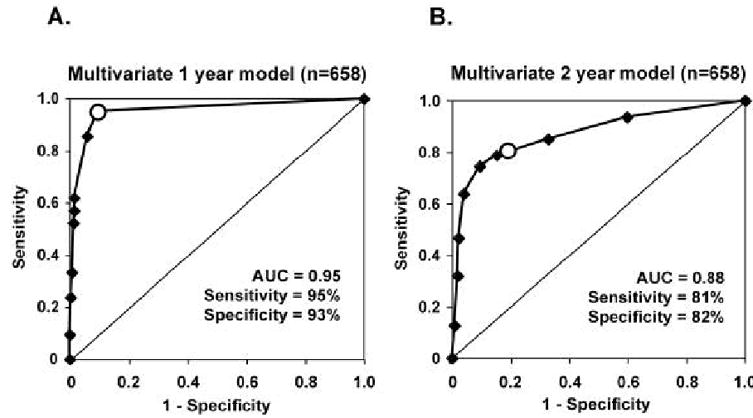
ROC curves for the multivariate one-year (A) and two-year (B) models based on the 658 retinal locations that did not have retinopathy at baseline. The one-year model had AUC = 0.95, sensitivity = 95% and specificity = 93%. For the two-year model, AUC = 0.88, sensitivity = 81% and specificity = 82%. As was found for the univariate models, all values are higher for the one-year model than for the two-year model.
As we expected, the multivariate models predicted the development of new retinopathy with higher accuracy than the univariate (mfERG implicit time alone) models. This is true for both the one-year and two-year models. On the other hand, both the univariate and multivariate one-year models performed better than the corresponding two-year models.
What could account for the observation that the one-year models performed better than the two-year models? A possible key factor is their difference in temporal proximity (closeness in time) between the measurement of baseline risk factors and the subsequent determination of local retinopathy development. If the predictive risk factors measured at baseline are unchanged over the study period, one might expect that the one-year and two-year models would perform at least equally well. However, the functional status of the retina, as reflected in mfERG implicit times, and other risk factors can change as time passes, making predictions over increasingly longer intervals more uncertain and potentially less accurate. For example, at a specific retinal location, conversion of a normal to an abnormally delayed implicit time after one year might itself be predictive of the development of retinopathy before the end of the second year, although the baseline implicit time that is used in the predictive model would have been normal. In this case, the two-year model, which uses baseline mfERG implicit time Z-scores, would be less predictive than a one-year model.
To investigate this possibility, we examined the mfERG implicit times within the retinal zones that developed new retinopathy between the first-year (T1) and second-year (T2) follow-ups. If the functional status of these zones changed between the measurement of baseline implicit times and the second year follow-up, the accuracy of predictions using the baseline measures could be adversely affected. As can be seen in Fig. 11, the implicit time Z-scores of these zones at baseline (T0; mean = 1.8) were lower (P = 0.02) than they were at the one-year follow-up (T1; mean = 2.3). These results suggest two important points. First, these zones became functionally more abnormal before the development of retinopathy occurred between T1 and T2. In fact, we have recently observed this in other diabetic subjects (Bearse, et al., 2006). Second, baseline implicit times underestimated the functional abnormality that actually preceded the development of retinopathy in the second year. In other words, increasing the time span between the functional measurement and outcome determination from one to two years made the mfERG implicit time less informative as a predictor. This is probably also true, to varying degrees, for the other predictive variables in the multivariate models. Therefore, it appears that periodic (perhaps annual) measurement of risk factors will maximize the predictive accuracy of these models.
Figure 11.
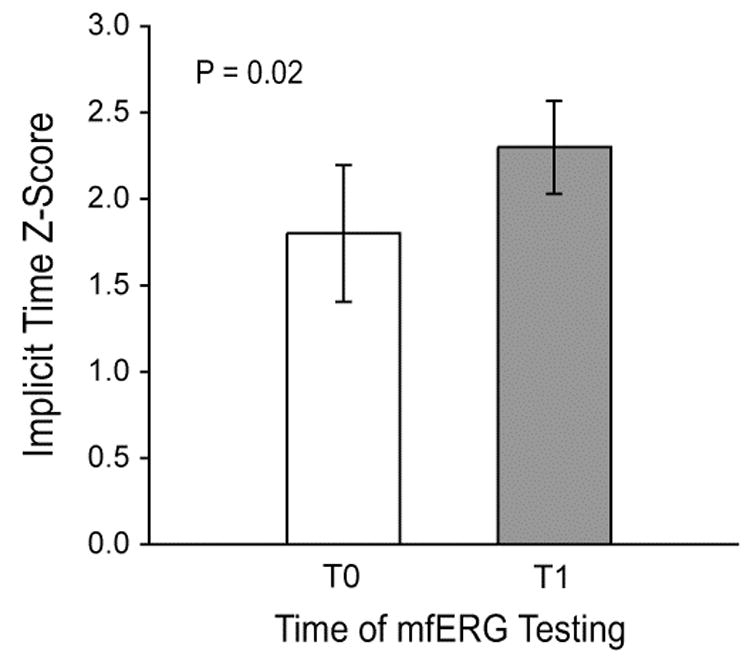
Mean mfERG implicit time Z-scores of retinal zones that developed new NPDR between the first (T1) and second (T2) follow-up examinations. The Z-scores were less abnormal at baseline (T0; mean = 1.8) than at the year one follow-up (T1; mean = 2.3) preceding retinopathy development (P = 0.02). The underestimation of dysfunction that actually preceded the development of retinopathy adversely affected the two-year models that used T0 Z-scores to predict retinopathy development.
6.2.5 Comparison of the “Standard” mfERG with Other Methods
As noted earlier, we studied blue cone pathway abnormalities across the retina using SWAP in the same diabetic subjects in whom we also measured mfERGs (Han, et al., 2004a). We noted approximately the same percentage of abnormalities for both SWAP thresholds and mfERG implicit times in this group, about 20% and 40% for diabetics without and with retinopathy, respectively. However, there was relatively poor spatial correlation of the local abnormalities for these two measures. SWAP thresholds, as we described earlier, are determined by the sensitivity of the entire S-cone pathway. In contrast, under the “standard” photopic recording conditions, the mfERG responses are generated predominantly by the more numerous L- and M-cones and their associated retinal circuitry. Since SWAP taps quite different neural pathways than the mfERG, we conclude that diabetes impacts both pathways and in some respect each measure underestimates the full loss of retinal integrity as a complication of diabetes. The advantage of the mfERG is that local implicit time abnormalities are significantly associated with early (mild) retinopathic lesions but local SWAP abnormalities are not (unpublished, J Chow, OD Thesis, UCB School of Optometry).
We have also compared, within the same 12 diabetic subjects with predominantly mild NPDR, the predictive performances of the “standard” mfERG with the previously described sf-mfERG (Bearse, et al., 2005). Techniques similar to those described earlier were used for both mfERG methods: measurement of local P1 implicit times using the template-stretching technique and analysis within 35 retinal zones. Although a larger proportion of implicit times was abnormal for the sf-mfERG paradigm (48%) compared to the “standard” mfERG (36%), the predictive performance of the “standard” (sensitivity = 78% and specificity = 74%) was slightly better than that of the sf-mfERG (sensitivity = 74% and specificity = 66%). Since the “standard” mfERG has approximately twice the signal-to-noise ratio of the sf-mfERG for the same recording length, it appears that it has an advantage as a predictor of diabetic retinopathy.
7. Future Directions
One of the most important issues that our initial modeling studies has raised is whether refinement and expansion of these models could provide tools that will identify eyes and retinal sites at risk for forms of retinopathy that are more likely to be immediately sight-threatening, including clinically significant macular edema. To date, our studies have focused on eyes with either early or no fundus signs of diabetic retinopathy. Because of this, the retinopathy that developed during our periods of study consisted primarily of dot hemorrhages and microaneurysms, with relatively few instances of edema and cotton wool spots. To determine whether models can be formulated to predict the local development of edema, we have begun to study diabetic patients at risk for the development of edema. In an effort to improve the predictive performance of the future models, we now obtain, in addition to the measurements of blood glucose concentrations, HbA1c measurements at the time of mfERG testing to establish a longer-term index of blood glucose control. Both measures of blood glucose control will be examined as candidate predictive variables in the formulation of future models. Further model expansion will include the examination of higher-order kernel and mfOP measurements as additional candidate predictive variables. SWAP thresholds will also be considered for inclusion as predictors since, although they are not spatially correlated with mild retinopathic lesions, they are associated with retinal edema.
There is still much to be learned about the relationships between functional abnormality, as reflected in the mfERG implicit time, and retinopathic lesion appearance. We now know that implicit time abnormalities are locally predictive of new diabetic retinopathy development and that these abnormalities vary in degree according to the severity of the lesion type. However, we do not yet know definitively whether mfERG implicit time, and the local functional status of the retina that it represents, changes when local retinopathy status changes. It is important, for example, to establish whether retinal function continues to worsen, becomes stable or even improves after the resolution of early retinopathic lesions. This knowledge would change the interpretation of lesion resolution, whether the resolution is spontaneous or the result of therapeutic intervention. An example of spontaneous resolution is the observation that approximately 42% of microaneurysms can disappear over a one-year period and 52% over a two-year period (Hellstedt and Immonen, 1996). We recently analyzed data that suggests local retinal function (mfERG implicit time) does not significantly improve when mild or moderate NPDR lesions spontaneously resolve (Bearse, et al., 2006).
Another question that we are currently addressing is whether the mfERG abnormalities we see in adult diabetic subjects also exist in adolescent diabetic individuals. This is important for a number of reasons. It is estimated that there are 206,000 people under the age of 20 that have diabetes and approximately 1 in 6 overweight adolescents have pre-diabetes (CDC, 2005). Type 2 diabetes now accounts for up to 20% of all newly diagnosed adolescent cases, and the number of adolescents diagnosed with Type 2 diabetes in the U.S. is estimated to be 39,000 (American Diabetes Association (ADA), 2000; Duncan, 2006; Rosenbloom and Silverstein, 2003). With the increase of Type 2 diabetes in adolescents and the associated obesity, hypertension and dyslipidemia, this population is at particular risk of developing diabetes-related eye disease (Berry, et al., 2006; Matthews and Wallace, 2002). The prevalence of NPDR in adolescents with diabetes is 14.5% and 2.3% of the patients exhibit signs of proliferative and preproliferative retinopathy (Kernell, et al., 1997). The prevalence of retinopathy during the first five years following diagnosis of diabetes is 10% in children diagnosed before the age of 13 and those diagnosed during adolescence (Klein, et al., 1985). This prevalence increases to 70% when duration is between 5 and 10 years (Klein, et al., 1985). There is a marked absence of studies of local neural retinal functions in adolescents, especially in Type 2 diabetes. To address these issues, we have initiated a project examining local mfERG abnormalities in adolescents with diabetes.
Finally, we are examining the nature and possible mechanisms underlying the large first-order mfERG implicit time delays observed in, and predictive of, diabetes. Based on the results of our preliminary study (Bronson-Castain, et al., 2006), local implicit time delays and abnormal fast adaptive mechanisms (as reflected in the second-order mfERG kernel amplitude) are only weakly associated. We are currently examining the local retinal relationships between abnormal implicit time and abnormal adaptation in larger samples of diabetics with and without retinopathy. In the near future, using long-duration multifocal stimuli, we will also examine possible contributions of ON and OFF response abnormalities to these first-order mfERG implicit time delays in diabetes.
8. Conclusions
Analysis of implicit times revealed that local “standard” mfERGs are abnormal in eyes of diabetic subjects without retinopathy and, to a greater degree, in eyes with mild or moderate NPDR. Abnormal mfERG implicit times are locally predictive of the development of new diabetic retinopathy over one and two years, and these functional abnormalities are spatially associated with retinopathy once it is present. The presence of abnormal mfERG implicit times in the absence of clinical fundus signs of diabetic retinopathy adds to the large body of electrophysiological and psychophysical literature documenting functional abnormalities prior to the appearance of overt pathological signs. More important is the ability of this mfERG measure to identify specific retinal sites of abnormality in the retinas of diabetic individuals.
These observations also contribute to a new view of the local aspects of the retinal effects of diabetes. First, the neural abnormalities that are responsible for the abnormally long implicit times are not uniformly distributed within the retinas of diabetic individuals. The retinal sites of these abnormalities are believed to be the outer plexiform layer and the bipolar cells, the major generators of the P1 component of the first-order mfERG (Hare and Ton, 2002; Hood, et al., 2002; Hood, et al., 1999). Second, these functional abnormalities appear before, not as a consequence of, spatially associated diabetic retinopathy. Third, the implicit times of local responses tend to become more abnormal with increasing severity of retinopathy. Thus, the implicit time of the local mfERG reveals local functional abnormalities of the retina that precede and predict the appearance of, and are correlated with the presence of, clinical signs of diabetic retinopathy. These observations are consistent with the view that retinal dysfunction in early diabetic retinopathy is primarily due to neuropathy or neurovasculopathy rather than microvascular pathology alone.
Models for the local prediction of development of new diabetic retinopathy such as those we have formulated can be useful in a number of applications. Because they identify eyes and retinal locations at risk for the retinopathy development, they could be used to identify diabetic individuals who should be followed more closely in clinical care, and also to identify patients for possible inclusion in studies assessing new prophylactic treatments. Furthermore, the fact that mfERG implicit times are predictive over a one-year period suggests that it will be useful even in clinical trials of this relatively short duration. Refinement and expansion of these models should also provide tools for the identification of eyes and retinal sites at risk for forms of retinopathy that are more immediately sight-threatening, including clinically significant macular edema.
Acknowledgments
We are grateful to Brad Fortune, Donald Hood and Nicholas Jewell for their contributions, insightful discussions and valuable assistance during many phases of this research. We also thank Ken Huie and Carl Jacobsen for their technical assistance, and Jenny Myung and Ellen Kim for their assistance in data management and analysis. This work was supported by National Eye Institute Grants EY02271 (AJA) and 5T32TY07043 (JN, KBC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams AJ. Chromatic and luminosity processing in retinal disease. Am J Optom Physiol Opt. 1982;59:954–960. doi: 10.1097/00006324-198212000-00004. [DOI] [PubMed] [Google Scholar]
- Adams AJ, Schefrin B, Huie K. New clinical color threshold test for eye disease. Am J Optom Physiol Opt. 1987a;64:29–37. doi: 10.1097/00006324-198701000-00005. [DOI] [PubMed] [Google Scholar]
- Adams AJ, Zisman F, Rodic R, Cavender JC. Chromaticity and Luminosity Changes in Glaucoma and Diabetes. Docum Ophthal Proc Series. 1982;33:413–418. [Google Scholar]
- Adams AJ, Zisman F, Ai E, Bresnick G. Macular edema reduces B cone sensitivity in diabetics. Appl Opt. 1987b:1455. doi: 10.1364/AO.26.001455. [DOI] [PubMed] [Google Scholar]
- Afrashi F, Erakgun T, Kose S, Ardic K, Mentes J. Blue-on-yellow perimetry versus achromatic perimetry in type 1 diabetes patients without retinopathy. Diabetes Res Clin Pract. 2003;61:7–11. doi: 10.1016/s0168-8227(03)00082-2. [DOI] [PubMed] [Google Scholar]
- Aiello LP, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 1998;21:143–156. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (ADA) Type 2 diabetes in children and adolescents. Diabetes Care. 2000;23:381–389. doi: 10.2337/diacare.23.3.381. [DOI] [PubMed] [Google Scholar]
- Aspinall PA, Kinnear PR, Duncan LJ, Clarke BF. Prediction of diabetic retinopathy from clinical variables and color vision data. Diabetes Care. 1983;6:144–148. doi: 10.2337/diacare.6.2.144. [DOI] [PubMed] [Google Scholar]
- Balik J, van Lith GH. The influence of glucose loading on the electrooculographic ratio (EOG) in normal subjects and diabetics. Acta Ophthalmol (Copenh) 1970;48:1097–1101. doi: 10.1111/j.1755-3768.1970.tb06589.x. [DOI] [PubMed] [Google Scholar]
- Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- Bearse MA, Han Y, Schneck ME, Barez S, Jacobsen C, Adams AJ. Comparison of Fast and Slow Flash Multifocal Electroretinogram Techniques: Prediction of Nonproliferative Diabetic Retinopathy Development. Invest Ophthalmol Vis Sci. 2005;46:E-Abstract 4761. [Google Scholar]
- Bearse MA, Han Y, Schneck ME, Bronson-Castain K, Ng J, Barez S, Jacobsen C, Adams AJ. Spatial and temporal relationships between changes in retinal function and diabetic retinopathy. Invest Ophthalmol Vis Sci. 2006;47:ARVO E-Abstract 4732. [Google Scholar]
- Bearse MA, Jr, Han Y, Schneck ME, Adams AJ. Retinal function in normal and diabetic eyes mapped with the slow flash multifocal electroretinogram. Invest Ophthalmol Vis Sci. 2004a;45:296–304. doi: 10.1167/iovs.03-0424. [DOI] [PubMed] [Google Scholar]
- Bearse MA, Jr, Han Y, Schneck ME, Barez S, Jacobsen C, Adams AJ. Local multifocal oscillatory potential abnormalities in diabetes and early diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004b;45:3259–3265. doi: 10.1167/iovs.04-0308. [DOI] [PubMed] [Google Scholar]
- Bearse MA, Jr, Shimada Y, Sutter EE. Distribution of oscillatory components in the central retina. Doc Ophthalmol. 2000;100:185–205. doi: 10.1023/a:1002783719958. [DOI] [PubMed] [Google Scholar]
- Bearse MA, Jr, Sutter EE. Imaging localized retinal dysfunction with the multifocal electroretinogram. J Opt Soc Am A. 1996;13:634–640. doi: 10.1364/josaa.13.000634. [DOI] [PubMed] [Google Scholar]
- Berry D, Urban A, Grey M. Management of type 2 diabetes in youth (part 2) J Pediatr Health Care. 2006;20:88–97. doi: 10.1016/j.pedhc.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Bresnick G. Diabetic retinopathy viewed as neurosensory disorder. Arch Ophthalmol. 1986;104:989–990. doi: 10.1001/archopht.1986.01050190047037. [DOI] [PubMed] [Google Scholar]
- Bresnick GH, Condit RS, Palta M, Korth K, Groo A, Syrjala S. Association of hue discrimination loss and diabetic retinopathy. Arch Ophthalmol. 1985;103:1317–1324. doi: 10.1001/archopht.1985.01050090069034. [DOI] [PubMed] [Google Scholar]
- Bresnick GH, Korth K, Groo A, Palta M. Electroretinographic oscillatory potentials predict progression of diabetic retinopathy. Preliminary report. Arch Ophthalmol. 1984;102:1307–1311. doi: 10.1001/archopht.1984.01040031057023. [DOI] [PubMed] [Google Scholar]
- Bresnick GH, Palta M. Oscillatory potential amplitudes. Relation to severity of diabetic retinopathy. Arch Ophthalmol. 1987;105:929–933. doi: 10.1001/archopht.1987.01060070065030. [DOI] [PubMed] [Google Scholar]
- Bronson-Castain K, Bearse M, Schneck M, Han Y, Adams AJ. Are abnormal multifocal electroretinogram (mfERG) implicit times locally related to abnormal adaptation in diabetes? Invest Ophthalmol Vis Sci. 2006;47:ARVO E-Abstract 1656. [Google Scholar]
- Cahill M, Eustace P, de Jesus V. Pupillary autonomic denervation with increasing duration of diabetes mellitus. Br J Ophthalmol. 2001;85:1225–1230. doi: 10.1136/bjo.85.10.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2003. Atlanta, GA: 2003. [Google Scholar]; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. 2004. [Google Scholar]
- CDC. National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2005. [Google Scholar]
- Chung HS, Harris A, Halter PJ, Kagemann L, Roff EJ, Garzozi HJ, Hosking SL, Martin BJ. Regional differences in retinal vascular reactivity. Invest Ophthalmol Vis Sci. 1999;40:2448–2453. [PubMed] [Google Scholar]
- Coupland SG. A comparison of oscillatory potential and pattern electroretinogram measures in diabetic retinopathy. Documenta Ophthalmologica. 1987;66:207–218. doi: 10.1007/BF00145234. [DOI] [PubMed] [Google Scholar]
- Cunha-Vaz J, Bernardes R. Nonproliferative retinopathy in diabetes type 2. Initial stages and characterization of phenotypes. Prog Retin Eye Res. 2005;24:355–377. doi: 10.1016/j.preteyeres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Daley ML, Watzke RC, Riddle MC. Early loss of blue-sensitive color vision in patients with type I diabetes. Diabetes Care. 1987;10:777–781. doi: 10.2337/diacare.10.6.777. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Di Leo MA, Caputo S, Falsini B, Porciatti V, Minnella A, Greco AV, Ghirlanda G. Nonselective loss of contrast sensitivity in visual system testing in early type I diabetes. Diabetes Care. 1992;15:620–625. doi: 10.2337/diacare.15.5.620. [DOI] [PubMed] [Google Scholar]
- Donaghue KC, Fairchild JM, Craig ME, Chan AK, Hing S, Cutler LR, Howard NJ, Silink M. Do all prepubertal years of diabetes duration contribute equally to diabetes complications? Diabetes Care. 2003;26:1224–1229. doi: 10.2337/diacare.26.4.1224. [DOI] [PubMed] [Google Scholar]
- Duncan GE. Prevalence of diabetes and impaired fasting glucose levels among US adolescents: National Health and Nutrition Examination Survey, 1999–2002. Arch Pediatr Adolesc Med. 2006;160:523–528. doi: 10.1001/archpedi.160.5.523. [DOI] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group (ETDRS) Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–1806. [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group (ETDRS) Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98:823–833. [PubMed] [Google Scholar]
- ETDRS. Fundus photographic risk factors for progression of diabetic retinopathy. Ophthalmology. 1991;98:823–833. [PubMed] [Google Scholar]
- Fledelius H. Refractive change in diabetes mellitus around onset or when poorly controlled: a clinical study. Acta Ophthalmologica. 1987;65:53–57. doi: 10.1111/j.1755-3768.1987.tb08491.x. [DOI] [PubMed] [Google Scholar]
- Fortune B, Schneck ME, Adams AJ. Multifocal electroretinogram delays reveal local retinal dysfunction in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1999;40:2638–2651. [PubMed] [Google Scholar]
- Fortune B, Wang L, Bui BV, Cull G, Dong J, Cioffi GA. Local ganglion cell contributions to the macaque electroretinogram revealed by experimental nerve fiber layer bundle defect. Invest Ophthalmol Vis Sci. 2003;44:4567–4579. doi: 10.1167/iovs.03-0200. [DOI] [PubMed] [Google Scholar]
- Frank RN. On the pathogenesis of diabetic retinopathy. Ophthalmology. 1984;91:626–634. doi: 10.1016/s0161-6420(84)34258-0. [DOI] [PubMed] [Google Scholar]
- Gardner TW, Antonetti DA, Barber AJ, LaNoue KF, Nakamura M. New insights into the pathophysiology of diabetic retinopathy: potential cell-specific therapeutic targets. Diabetes Technol Ther. 2000;2:601–608. doi: 10.1089/15209150050502023. [DOI] [PubMed] [Google Scholar]
- Greenstein V, Sarter B, Hood D, Noble K, Carr R. Hue discrimination and S cone pathway sensitivity in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1990;31:1008–1014. [PubMed] [Google Scholar]
- Greenstein VC, Chen H, Hood DC, Holopigian K, Seiple W, Carr RE. Retinal function in diabetic macular edema after focal laser photocoagulation. Invest Ophthalmol Vis Sci. 2000;41:3655–3664. [PubMed] [Google Scholar]
- Greenstein VC, Holopigian K, Seiple W, Carr RE, Hood DC. Atypical multifocal ERG responses in patients with diseases affecting the photoreceptors. Vision Res. 2004;44:2867–2874. doi: 10.1016/j.visres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Greenstein VC, Shapiro A, Hood DC, Zaidi Q. Chromatic and luminance sensitivity in diabetes and glaucoma. J Opt Soc Am A. 1993;10:1785–1791. doi: 10.1364/josaa.10.001785. [DOI] [PubMed] [Google Scholar]
- Han Y, Adams AJ, Bearse MA, Jr, Schneck ME. Multifocal electroretinogram and short-wavelength automated perimetry measures in diabetic eyes with little or no retinopathy. Arch Ophthalmol. 2004a;122:1809–1815. doi: 10.1001/archopht.122.12.1809. [DOI] [PubMed] [Google Scholar]
- Han Y, Bearse MA, Jr, Schneck ME, Barez S, Jacobsen C, Adams AJ. Towards optimal filtering of "standard" multifocal electroretinogram (mfERG) recordings: findings in normal and diabetic subjects. Br J Ophthalmol. 2004b;88:543–550. doi: 10.1136/bjo.2003.026625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Bearse MA, Jr, Schneck ME, Barez S, Jacobsen CH, Adams AJ. Multifocal electroretinogram delays predict sites of subsequent diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004c;45:948–954. doi: 10.1167/iovs.03-1101. [DOI] [PubMed] [Google Scholar]
- Han Y, Bearse MA, Schneck ME, Barez S, Jacobsen CH, Adams AJ. Comparison of Multifocal Electroretinogram (mFERG) First and Second Order Kernel Responses in Diabetic Patients. Invest Ophthalmol Vis Sci. 2005;46:E-Abstract 3426. [Google Scholar]
- Han Y, Schneck ME, Bearse MA, Jr, Barez S, Jacobsen CH, Jewell NP, Adams AJ. Formulation and evaluation of a predictive model to identify the sites of future diabetic retinopathy. Invest Ophthalmol Vis Sci. 2004d;45:4106–4112. doi: 10.1167/iovs.04-0405. [DOI] [PubMed] [Google Scholar]
- Hardy KJ, Fisher C, Heath P, Foster DH, Scarpello JHB. Comparison of colour discrimination and electroretinography in evaluation of visual patway dysfuction in aretinopathic IDDM patients. British journal of Ophthalmology. 1995;79:35–37. doi: 10.1136/bjo.79.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare WA, Ton H. Effects of APB, PDA, and TTX on ERG responses recorded using both multifocal and conventional methods in monkey. Effects of APB, PDA, and TTX on monkey ERG responses. Doc Ophthalmol. 2002;105:189–222. doi: 10.1023/a:1020553020264. [DOI] [PubMed] [Google Scholar]
- Hellstedt T, Immonen I. Disappearance and formation rates of microaneurysms in early diabetic retinopathy. Br J Ophthalmol. 1996;80:135–139. doi: 10.1136/bjo.80.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron G, Adams AJ, Husted R. Foveal and non-foveal measures of short wavelength sensitive pathways in glaucoma and ocular hypertension. Ophthalmic Physiol Opt. 1987;7:403–404. [PubMed] [Google Scholar]
- Holopigian K, Seiple W, Greenstein VC, Hood DC, Carr RE. Local cone and rod system function in progressive cone dystrophy. Invest Ophthalmol Vis Sci. 2002;43:2364–2373. [PubMed] [Google Scholar]
- Holopigian K, Seiple W, Lorenzo M, Carr R. A comparison of photopic and scotopic electroretinographic changes in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1992;33:2773–2780. [PubMed] [Google Scholar]
- Hood DC. Assessing retinal function with the multifocal technique. Prog Retin Eye Res. 2000;19:607–646. doi: 10.1016/s1350-9462(00)00013-6. [DOI] [PubMed] [Google Scholar]
- Hood DC, Bearse MA, Jr, Sutter EE, Viswanathan S, Frishman LJ. The optic nerve head component of the monkey's (Macaca mulatta) multifocal electroretinogram (mERG) Vision Res. 2001;41:2029–2041. doi: 10.1016/s0042-6989(01)00010-4. [DOI] [PubMed] [Google Scholar]
- Hood DC, Frishman LJ, Saszik S, Viswanathan S. Retinal origins of the primate multifocal ERG: implications for the human response. Invest Ophthalmol Vis Sci. 2002;43:1673–1685. [PubMed] [Google Scholar]
- Hood DC, Greenstein V, Frishman L, Holopigian K, Viswanathan S, Seiple W, Ahmed J, Robson JG. Identifying inner retinal contributions to the human multifocal ERG. Vision Res. 1999;39:2285–2291. doi: 10.1016/s0042-6989(98)00296-x. [DOI] [PubMed] [Google Scholar]
- Hood DC, Holopigian K, Greenstein V, Seiple W, Li J, Sutter EE, Carr RE. Assessment of local retinal function in patients with retinitis pigmentosa using the multifocal ERG technique. Vision Res. 1998;38:163–179. doi: 10.1016/s0042-6989(97)00143-0. [DOI] [PubMed] [Google Scholar]
- Hood DC, Li J. A technique for measuring individual multifocal ERG records. In: Y D, editor. Trends in Optics and Photonics. Vol. 11. Optical Society of America; Washington, DC: 1997. pp. 280–283. [Google Scholar]
- Hood DC, Seiple W, Holopigian K, Greenstein V. A comparison of the components of the multifocal and full-field ERGs. Vis Neurosci. 1997;14:533–544. doi: 10.1017/s0952523800012190. [DOI] [PubMed] [Google Scholar]
- Hudson C, Flanagan JG, Turner GS, Chen HC, Young LB, McLeod D. Short-wavelength sensitive visual field loss in patients with clinically significant diabetic macular oedema. Diabetologia. 1998;41:918–928. doi: 10.1007/s001250051008. [DOI] [PubMed] [Google Scholar]
- Hyvarinen L, Laurinen P, Rovamo J. Contrast sensitivity in evaluation of visual impairment due to diabetes. Acta Ophthalmol (Copenh) 1983;61:94–101. doi: 10.1111/j.1755-3768.1983.tb01399.x. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation. Diabetes Atlas 2003. 2. Imprimerie L Vanmelle SA, Gent/Mariakerke; Brussels, Belgium: 2003. p. 360. [Google Scholar]
- Jewell N. In: Statistics for Epidemiology. J N, editor. CRC Press: Boca Raton,FL; 2003. pp. 246–250. [Google Scholar]
- Johnson CA, Adams AJ, Lewis RA, Heijl Automated perimetry of short-wavelength-sensitive mechanisms in glaucoma and ocular hypertension; preliminary findings; In Proceedings of the VIIIth International Perimetric Society Meeting; Amsterdam: Kuglrer & Ghedini Publications; 1989. pp. 31–37. [Google Scholar]
- Juen S, Kieselbach GF. Electrophysiological changes in juvenile diabetics without retinopathy. Arch Ophthalmol. 1990;108:372–375. doi: 10.1001/archopht.1990.01070050070033. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Yonemura K, Yokogawa Y, Saito N, Kawakita S. Correlation between ERG oscillatory potential and psychophysical contrast sensitivity in diabetes. Doc Ophthalmol. 1986;64:209–215. doi: 10.1007/BF00159995. [DOI] [PubMed] [Google Scholar]
- Keating D, Parks S, Smith D, Evans A. The multifocal ERG: unmasked by selective cross-correlation. Vision Res. 2002;42:2959–2968. doi: 10.1016/s0042-6989(02)00359-0. [DOI] [PubMed] [Google Scholar]
- Kernell A, Dedorsson I, Johansson B, Wickstrom CP, Ludvigsson J, Tuvemo T, Neiderud J, Sjostrom K, Malmgren K, Kanulf P, Mellvig L, Gjotterberg M, Sule J, Persson LA, Larsson LI, Aman J, Dahlquist G. Prevalence of diabetic retinopathy in children and adolescents with IDDM. A population-based multicentre study. Diabetologia. 1997;40:307–310. doi: 10.1007/s001250050679. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. Retinopathy in young-onset diabetic patients. Diabetes Care. 1985;8:311–315. doi: 10.2337/diacare.8.4.311. [DOI] [PubMed] [Google Scholar]
- Klemp K, Larsen M, Sander B, Vaag A, Brockhoff PB, Lund-Andersen H. Effect of short-term hyperglycemia on multifocal electroretinogram in diabetic patients without retinopathy. Invest Ophthalmol Vis Sci. 2004;45:3812–3819. doi: 10.1167/iovs.03-1260. [DOI] [PubMed] [Google Scholar]
- Kondo M, Miyake Y, Horiguchi M, Suzuki S, Tanikawa A. Clinical evaluation of multifocal electroretinogram. Invest Ophthalmol Vis Sci. 1995;36:2146–2150. [PubMed] [Google Scholar]
- Kurtenbach A, Flogel W, Erb C. Anomaloscope matches in patients with diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2002;240:79–84. doi: 10.1007/s00417-001-0385-3. [DOI] [PubMed] [Google Scholar]
- Kurtenbach A, Langrova H, Zrenner E. Multifocal oscillatory potentials in type 1 diabetes without retinopathy. Invest Ophthalmol Vis Sci. 2000;41:3234–3241. [PubMed] [Google Scholar]
- Layton CJ, Becker S, Osborne NN. The effect of insulin and glucose levels on retinal glial cell activation and pigment epithelium-derived fibroblast growth factor-2. Mol Vis. 2006;12:43–54. [PubMed] [Google Scholar]
- Levin RD, Kwaan HC, Dobbie JG, Fetkenhour CL, Traisman HS, Kramer C. Studies of retinopathy and the plasma co-factor of platelet hyperaggregation in type 1 (insulin-dependent) diabetic children. Diabetologia. 1982;22:445–449. doi: 10.1007/BF00282588. [DOI] [PubMed] [Google Scholar]
- Liska V, Dostalek M. Are contrast sensitivity functions impaired in insulin dependent diabetics without diabetic retinopathy? Acta Medica (Hradec Kralove) 1999;42:133–138. [PubMed] [Google Scholar]
- Liu QZ, Pettitt DJ, Hanson RL, Charles MA, Klein R, Bennett PH, Knowler WC. Glycated haemoglobin, plasma glucose and diabetic retinopathy: cross-sectional and prospective analyses. Diabetologia. 1993;36:428–432. doi: 10.1007/BF00402279. [DOI] [PubMed] [Google Scholar]
- Lovasik JV, Spafford MM. An electrophysiological investigation of visual function in juvenile insulin-dependent diabetes mellitus. Am J Optom Physiol Opt. 1988;65:236–253. doi: 10.1097/00006324-198804000-00002. [DOI] [PubMed] [Google Scholar]
- Lutze M, Bresnick GH. Lens-corrected visual field sensitivity and diabetes. Invest Ophthalmol Vis Sci. 1994;35:649–655. [PubMed] [Google Scholar]
- Marmor MF, Hood DC, Keating D, Kondo M, Seeliger MW, Miyake Y. Guidelines for basic multifocal electroretinography (mfERG) Doc Ophthalmol. 2003;106:105–115. doi: 10.1023/a:1022591317907. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Wallace TM. Children with type 2 diabetes: the risks of complications. Horm Res. 2002;57(Suppl 1):34–39. doi: 10.1159/000053310. [DOI] [PubMed] [Google Scholar]
- Moloney J, Drury MI. Retinopathy and retinal function in insulin-dependent diabetes mellitus. Br J Ophthalmol. 1982;66:759–761. doi: 10.1136/bjo.66.12.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntoni S, Serra A, Mascia C, Songini M. Dyschromatopsia in diabetes mellitus and its relation to metabolic control. Diabetes Care. 1982;5:375–378. doi: 10.2337/diacare.5.4.375. [DOI] [PubMed] [Google Scholar]
- Ng JS, Bearse JMA, Han Y, Schneck ME, Barez S, Jacobsen C, Adams AJ. Modeling the development of non–proliferative diabetic retinopathy over 2 years. Invest Ophthalmol Vis Sci. 2006;47:E-Abstract 988. [Google Scholar]
- Nguyen HT, Luzio SD, Dolben J, West J, Beck L, Coates PA, Owens DR. Dominant risk factors for retinopathy at clinical diagnosis in patients with type II diabetes mellitus. J Diabetes Complications. 1996;10:211–219. doi: 10.1016/1056-8727(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Nomura R, Terasaki H, Miyake Y. Blue-on-yellow perimetry to evaluate S cone sensitivity in diabetics. Ophthal Res. 1999:69–72. doi: 10.1159/000055592. [DOI] [PubMed] [Google Scholar]
- Onozu H, Yamamoto S. Oscillatory potentials of multifocal electroretinogram retinopathy. Doc Ophthalmol. 2003;106:327–332. doi: 10.1023/a:1022903627332. [DOI] [PubMed] [Google Scholar]
- Palmowski AM, Sutter EE, Bearse MA, Jr, Fung W. Mapping of retinal function in diabetic retinopathy using the multifocal electroretinogram. Invest Ophthalmol Vis Sci. 1997;38:2586–2596. [PubMed] [Google Scholar]
- Parisi V, Uccioli L, Monticone G, Parisi L, Menzinger G, Bucci MG. Visual evoked potentials after photostress in insulin-dependent diabetic patients with or without retinopathy. Graefes Arch Clin Exp Ophthalmol. 1994;232:193–198. doi: 10.1007/BF00184004. [DOI] [PubMed] [Google Scholar]
- Pittasch D, Lobmann R, Behrens-Baumann W, Lehnert H. Pupil signs of sympathetic autonomic neuropathy in patients with type 1 diabetes. Diabetes Care. 2002;25:1545–1550. doi: 10.2337/diacare.25.9.1545. [DOI] [PubMed] [Google Scholar]
- Rangaswamy NV, Hood DC, Frishman LJ. Regional variations in local contributions to the primate photopic flash ERG: revealed using the slow-sequence mfERG. Invest Ophthalmol Vis Sci. 2003;44:3233–3247. doi: 10.1167/iovs.03-0009. [DOI] [PubMed] [Google Scholar]
- Remky A, Weber A, Hendricks S, Lichtenberg K, Arend O. Short-wavelength automated perimetry in patients with diabetes mellitus without macular edema. Graefes Arch Clin Exp Ophthalmol. 2003 doi: 10.1007/s00417-003-0666-0. [DOI] [PubMed] [Google Scholar]
- Robinson F, Riva CE, Grunwald JE, Petrig BL, Sinclair SH. Retinal blood flow autoregulation in response to an acute increase in blood pressure. Invest Ophthalmol Vis Sci. 1986;27:722–726. [PubMed] [Google Scholar]
- Rosenbloom AL, Silverstein J. Type 2 diabetes in children and adolescents: A clinicians guide to diagnosis, epidemiology, pathogenesis, prevention, and treatment. NY: McGraw-Hill Companies; New York: 2003. [Google Scholar]
- Sample PA, Johnson CA, Haegerstrom-Portnoy G, Adams AJ. Optimum parameters for short-wavelength automated perimetry. J Glaucoma. 1996;5:375–383. [PubMed] [Google Scholar]
- Schneck ME, Bearse MA, Jr, Han Y, Barez S, Jacobsen C, Adams AJ. Comparison of mfERG waveform components and implicit time measurement techniques for detecting functional change in early diabetic eye disease. Doc Ophthalmol. 2004;108:223–230. doi: 10.1007/s10633-004-8745-z. [DOI] [PubMed] [Google Scholar]
- Schneck ME, Fortune B, Adams AJ. The fast oscillation of the electrooculogram reveals sensitivity of the human outer retina/retinal pigment epithelium to glucose level. Vision Res. 2000;40:3447–3453. doi: 10.1016/s0042-6989(00)00173-5. [DOI] [PubMed] [Google Scholar]
- Schneck ME, Shupenko LA, Verdon WA, Adams AJ. The fast and slow oscillations of the electrooculogram in diabetes. Invest Ophthalmol Vis Sci. 2001;42:S806. [Google Scholar]
- Seeliger M, Kretschmann U, Apfelstedt-Sylla E, Ruther K, Zrenner E. Multifocal electroretinography in retinitis pigmentosa. Am J Ophthalmol. 1998;125:214–226. doi: 10.1016/s0002-9394(99)80094-4. [DOI] [PubMed] [Google Scholar]
- Sharma S, Hoskin-Mott A, Benstead T, Maxner C. Correlation of the pilo-pupil ratio average, a new test for autonomic denervation, to the severity of diabetic retinopathy. Can J Ophthalmol. 1997;32:170–174. [PubMed] [Google Scholar]
- Shimada Y, Li Y, Bearse MA, Jr, Sutter EE, Fung W. Assessment of early retinal changes in diabetes using a new multifocal ERG protocol. Br J Ophthalmol. 2001;85:414–419. doi: 10.1136/bjo.85.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirao Y, Kawasaki K. Electrical responses from diabetic retina. Prog Retin Eye Res. 1998;17:59–76. doi: 10.1016/s1350-9462(97)00005-0. [DOI] [PubMed] [Google Scholar]
- Shirao Y, Okumura T, Ohta T, Kawasaki K. Clinical importance of electroretinographic oscillatory potentials in early detection and objective evaluation for diabetic retinopathy. Clin Vis Sci. 1991;6:445–450. [Google Scholar]
- Simonsen SE. Electroretinographic study of diabetes: Preliminary report. Acta Ophthalmol. 1965;43:841–843. [Google Scholar]
- Simonsen SE. The value of the oscillatory potential in selecting juvenile diabetics at risk of developing proliferative retinopathy. Acta Ophthalmol (Copenh) 1980;58:865–878. doi: 10.1111/j.1755-3768.1980.tb08312.x. [DOI] [PubMed] [Google Scholar]
- Sokol S, Moskowitz A, Skarf B, Evans R, Molitch M, Senior B. Contrast sensitivity in diabetics with and without background retinopathy. Arch Ophthalmol. 1985;103:51–54. doi: 10.1001/archopht.1985.01050010055018. [DOI] [PubMed] [Google Scholar]
- Spafford MM, Lovasik JV. Clinical evaluation of ocular and visual functions in insulin-dependent juvenile diabetics. Am J Optom Physiol Opt. 1986;63:505–519. doi: 10.1097/00006324-198607000-00003. [DOI] [PubMed] [Google Scholar]
- Sparrow J. Biometry of the crystalline lens in early-onset diabetes. British Journal of Ophthalmology. 1990;74:654–660. doi: 10.1136/bjo.74.11.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrou EP, Wood JM. Letter contrast sensitivity changes in early diabetic retinopathy. Clin Exp Optom. 2003;86:152–156. doi: 10.1111/j.1444-0938.2003.tb03097.x. [DOI] [PubMed] [Google Scholar]
- Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, Matthews DR. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156–163. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- Sutter EE. Imaging visual function with the multifocal m-sequence technique. Vision Res. 2001;41:1241–1255. doi: 10.1016/s0042-6989(01)00078-5. [DOI] [PubMed] [Google Scholar]
- Sutter EE, Bearse MA., Jr The optic nerve head component of the human ERG. Vision Res. 1999;39:419–436. doi: 10.1016/s0042-6989(98)00161-8. [DOI] [PubMed] [Google Scholar]
- Sutter EE, Tran D. The field topography of ERG components in man--I. The photopic luminance response. Vision Res. 1992;32:433–446. doi: 10.1016/0042-6989(92)90235-b. [DOI] [PubMed] [Google Scholar]
- The Diabetes Control Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47:1703–1720. doi: 10.1038/ki.1995.236. [DOI] [PubMed] [Google Scholar]
- Tyrberg M, Ponjavic V, Lovestam-Adrian M. Multifocal electroretinography (mfERG) in insulin dependent diabetics with and without clinically apparent retinopathy. Doc Ophthalmol. 2005;110:137–143. doi: 10.1007/s10633-005-4187-5. [DOI] [PubMed] [Google Scholar]
- Tzekov R, Arden GB. The electroretinogram in diabetic retinopathy. Surv Ophthalmol. 1999;44:53–60. doi: 10.1016/s0039-6257(99)00063-6. [DOI] [PubMed] [Google Scholar]
- UKPDS. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. Bmj. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- Van der Torren K, Mulder P. Comparison of the second and third oscillatory potentials with oscillatory potential power in early diabetic retinopathy. Doc Ophthalmol. 1993;83:111–118. doi: 10.1007/BF01206209. [DOI] [PubMed] [Google Scholar]
- Verrotti A, Lobefalo L, Petitti MT, Mastropasqua L, Morgese G, Chiarelli F, Gallenga PE. Relationship between contrast sensitivity and metabolic control in diabetics with and without retinopathy. Ann Med. 1998;30:369–374. doi: 10.3109/07853899809029936. [DOI] [PubMed] [Google Scholar]
- Volbrecht VJ, Schneck ME, Adams AJ, Linfoot JA, Ai E. Diabetic short-wavelength sensitivity: variations with induced changes in blood glucose level. Invest Ophthalmol Vis Sci. 1994;35:1243–1246. [PubMed] [Google Scholar]
- Weiner A, Christopoulos VA, Gussler CH, Adams DH, Kaufman SR, Kohn HD, Weidenthal DT. Foveal cone function in nonproliferative diabetic retinopathy and macular edema. Invest Ophthalmol Vis Sci. 1997;38:1443–1449. [PubMed] [Google Scholar]
- Wirta O, Pasternack A, Mustonen J, Laippala P, Lahde Y. Retinopathy is independently related to microalbuminuria in type 2 diabetes mellitus. Clin Nephrol. 1999;51:329–334. [PubMed] [Google Scholar]
- Wu S, Sutter EE. A topographic study of oscillatory potentials in man. Vis Neurosci. 1995;12:1013–1025. doi: 10.1017/s0952523800006696. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Yamamoto T, Hayashi M, Takeuchi S. Morphological and functional analyses of diabetic macular edema by optical coherence tomography and multifocal electroretinograms. Graefes Arch Clin Exp Ophthalmol. 2001;239:96–101. doi: 10.1007/s004170000238. [DOI] [PubMed] [Google Scholar]
- Yonemura D, Aoki T, Tsuzuki K. Electroretinogram in diabetic retinopathy. Arch Ophthalmol. 1962;68:19–24. doi: 10.1001/archopht.1962.00960030023005. [DOI] [PubMed] [Google Scholar]
- Yonemura D, Kawasaki K. Electrophysiological study on activities of neuronal and non-neuronal retinal elements in man with reference to its clinical application. Jpn J Ophthalmol. 1978;22:198–213. [PubMed] [Google Scholar]
- Yoshida A, Kojima M, Ogasawara H, Ishiko S. Oscillatory potentials and permeability of the blood-retinal barrier in noninsulin-dependent diabetic patients without retinopathy. Ophthalmology. 1991;98:1266–1271. doi: 10.1016/s0161-6420(91)32144-4. [DOI] [PubMed] [Google Scholar]
- Zaharia M, Olivier P, Lafond G, Blondeau P, Brunette JR. Lobular delayed choroidal perfusion as an early angiographic sign of diabetic retinopathy: a preliminary report. Can J Ophthalmol. 1987;22:257–261. [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- Zisman F, Adams AJ. Spectral sensitivity of cone mechanisms in juvenile diabetics. In: V G, editor. In Doc Ophthalmol Proc Series 33. The Hague: DW Junk; 1982. pp. 127–131. [Google Scholar]


