Abstract
The identification of nitric oxide (•NO) as an endogenously produced free radical mediator of endothelial-dependent relaxation and host defense has fundamentally changed concepts of cell signal transduction. Ligand-receptor oriented paradigms of cell signaling were originally centered on the concept of a high affinity and specific interaction between a ligand and its receptor, resulting in the activation of secondary signaling events such as gene expression or modulation of catalytic protein function. While •NO ligation of the heme iron of soluble guanylate cyclase is consistent with this perspective, the readily diffusible and broadly reactive •NO is increasingly appreciated to react with a vast array of target molecules that mediate paracrine vasodilator actions, inhibition of thrombosis and neointimal proliferation and both pro- and anti-inflammatory signaling reactions that are not affected by inhibitors of soluble guanylate cyclase. There is an expanding array of functionally significant “off target” collateral reactions mediated by •NO that are guanylate cyclase-independent and rather are dictated by anatomic distribution and the formation of secondary •NO-derived species. These reactions are a critical element of redox-regulated signaling and are addressed herein in the context of the oxidation of unsaturated fatty acids to vascular and inflammatory signaling mediators. Because of their abundance and the intrinsic reactivity of unsaturated lipid intermediates and eicosanoid metabolism enzymes with •NO and other oxides of nitrogen, lipid signaling mechanisms are a significant target for regulation by •NO in the vascular compartment. This convergence of •NO and lipid signaling pathways thus adds another level of regulation to physiological responses such as vasodilation, thrombosis and inflammation. Herein, interactions between •NO and lipid signaling events are placed in the context of cardiovascular regulation.
Keywords: nitric oxide, lipids, redox reaction, fatty acids, cardiovascular
Nitric Oxide – Synthesis and Biological Effects
Nitric oxide synthase isoforms
Nitric oxide (•NO) is generated by three distinct subtypes of •NO synthase (NOS) isoenzymes that oxidize L-arginine to L-citrulline. 1 These can be subdivided into the constitutively expressed endothelial NOS (eNOS) and neuronal NOS (nNOS) and the inducible isoenzyme iNOS. 2 iNOS is strongly induced by a series of inflammatory stimuli such as endotoxin, interleukin-1β, interferon γ and tumor necrosis factor α. Inducible NOS was first reported to be present in macrophages 3 but has subsequently also been detected in vascular cell types including endothelial cells, 4 smooth muscle cells, 5 platelets 6 and myocytes. 7 In addition to its inducibility, iNOS differs from other NOS isoenzymes in that it does not require an increase in intracellular Ca2+-concentration for catalytic activation. Although net extents of •NO production can exceed that of the constitutive isoenzymes up to 1000-fold it does not exhibit a greater rate of catalysis. Rather this phenomenon can be explained by higher protein concentrations of iNOS.8–10
Signal transduction by nitric oxide
Signaling reactions induced by •NO differ considerably from other signaling mediators. The predominant and best characterized physiological action of •NO is the formation of an Fe-nitrosyl intermediate with the heme iron of soluble guanylate cyclase (sGC), inducing sGC conformational changes and the activation of catalytic activity that in turn increases rates of production of cyclic guanosine monophosphate (cGMP). 11 The signaling actions of NOS enzymes are then propagated by activating cGMP-dependent ion channels, protein kinases and phosphodiesterases. 12 Via these effector proteins, •NO mediates alterations in intracellular Ca2+ levels, smooth muscle relaxation and the regulation of blood pressure. 13–15 In addition, this signaling axis mediates neurotransmission and inhibits both platelet aggregation and leukocyte function. 16
Non-sGC/cGMP-dependent signaling by •NO
Because of the small molecular radius of •NO, its unpaired electron and a propensity to undergo reactions with diverse metal centers, molecular oxygen and oxygen-derived species, signaling reactions of •NO can also be induced by metal ligation and the post-translational modification of a broad population of susceptible target proteins. Consequently, this redox-related •NO signaling will be conveyed by changes in local •NO concentration and the presence of other redox intermediates that •NO can react with. 17 This introduces a panoply of additional sGC/cGMP-independent mechanisms whereby •NO can regulate inflammatory and vascular function, via reactions that are influenced by the protein reactivity of •NO and its metabolites.
While the major oxidation products of •NO present in tissues and excreted in urine are nitrite (NO2−) and nitrate (NO3−), •NO also reacts with molecular oxygen at relatively low rates and much more rapidly with a variety of radical species possessing unpaired electrons, yielding oxides of nitrogen (NOx) sometimes referred to as reactive nitrogen species (RNS, Figure 1). Thus, •NO reacts with one of the two unpaired electrons of molecular oxygen to ultimately yield two molecules of nitrogen dioxide (•NO2), a reaction shown to be of relevance in vivo.18 Then, •NO can react further with •NO2 to form the unstable nitrosating (addition of an NO-group) and nitrating (addition of an NO2-group) species dinitrogen trioxide (N2O3) and dinitrogen tetroxide (N2O4). Dinitrogen trioxide yields nitrosonium ion (NO+) and other metabolic reactions of •NO can yield nitroxyl (HNO), both shown to exert significant bioactivity. 19 Finally, in the context of redox signaling, a key metabolite of •NO is peroxynitrite (ONOO−), formed by the kinetically fast reaction of •NO with superoxide (O2•−). 20 Of note, ONOO− is in itself a highly oxidizing species and has a relatively long aqueous half-life of about 1.0 second. Moreover, reactions of ONOO− with H+ or CO2 give rise to secondary species such as hydroxyl radical (•OH), carbonate radical (HCO3•) and •NO2.21
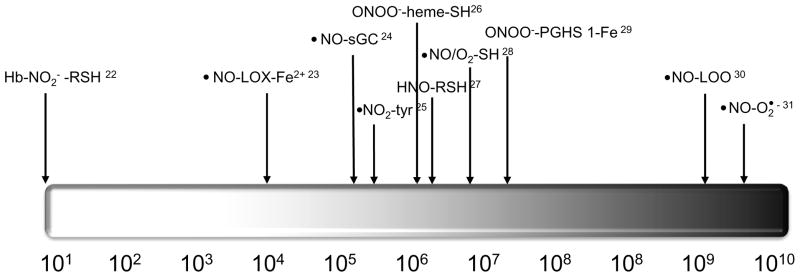
Figure 1. Reaction rate constants for critical reactions of •NO and •NO-derived species with key molecular targets that transduce cell signaling events.
Both •NO and •NO-derived RNS can transduce cell signaling by modulating the actions of biomolecules via a variety of chemical modifications, with the post-translational modification of proteins playing the most important role. Thus, S-nitrosation of cysteine thiols by •NO, a reaction mediated by a variety of •NO and thiol intermediates 32, 33 leads to a) the creation of a more stable reservoir for •NO and subsequent mitigation of sGC/cGMP-independent signaling and b) direct alterations in protein structure and function. 34 For example, S-nitrosation inhibits the activity of signaling cascades such as mitogen-activated protein kinase (MAPK), 35 Src kinases, 36 caspases, 37 protein tyrosine phosphatases 38 or the NOS-activity regulating enzyme dimethylarginine dimethylaminohydrolase (DDAH) 39 and modulates membrane receptors 40 and transcription factors, such as hypoxia-inducible factor-1α41 and nuclear factor κB (NFκB).42
Via different mechanisms, the •NO-derived oxidizing and nitrating species ONOO− induces a variety of structural modifications to protein targets. 17 This includes the oxidation of thiols and the nitration of tyrosine residues, resulting in a broad array of cell signaling consequences. For example, ONOO− inhibits NFκB signaling 43 and multiple phosphotyrosine-dependent signaling mechanisms including MAPK 44 and the protein kinase B (Akt) pathways. 45 Cysteine thiols are very susceptible to oxidation by ONOO−, as are Fe-S complexes and the ferrous heme of transition metal center containing proteins, all reactions that can contribute to the inhibition of mitochondrial respiratory chain activity 46 and iNOS. 47
In addition to multifaceted reactions with proteins, •NO and its products play an important role in the oxidation and downstream signaling actions of lipids. 48 For example, •NO potently terminates the propagation of lipid peroxidation reactions, 30, 49, 50 while concomitantly forming hydroperoxide, epoxide and nitrated byproducts. 30 Under other conditions where reactive oxygen species (ROS) are abundant, •NO can alternatively initiate lipid oxidation via the generation of ONOO−, 51, 52 resulting in the formation of lipid hydroperoxide, conjugated diene, aldehyde and isoprostane derivatives that can function as secondary signaling mediators.
Moreover, •NO and its products can regulate gene expression of enzymes of eicosanoid synthesis and, by direct protein interactions, modulate the enzymatic generation of lipid mediators while at the same time becoming catalytically consumed. The following sections expand on these events and underscore how the enzymatic and non-enzymatic generation of lipid mediators are modulated by •NO and •NO-derived species, resulting in altered vascular and inflammatory signaling.
Nitric Oxide Modulation of Enzymatically-Generated Lipid Mediators
Nitric oxide and prostaglandins
Prostanoid biosynthesis
Prostaglandins (PGs) are lipid mediators that play a key role in modulating vascular function. They are derived from the 20-carbon tetra-unsaturated fatty acid, arachidonic acid, by prostaglandin H synthase (PGHS) in a two-step reaction. Arachidonic acid is first converted into the unstable cyclic endoperoxide PGG2 by the cyclooxygenase activity of PGHS. Subsequently, the peroxidase activity of PGHS cleaves the peroxide, which leads to the unstable endoperoxide PGH2. Isomerization of this endoperoxide by specific enzymes finally yields multiple biologically active prostanoids such as thromboxanes and I, E, D F series prostaglandins. Physiologically, the signaling of vasorelaxant, antithrombotic prostanoids overrides those of their vasoconstricting, prothrombotic counterparts. Under different pathological conditions however, this balance can shift and the vasoconstrictive actions of thromboxane A2 and PGH2 can predominate. The two isoforms of PGHS, PGHS-1 and PGHS-2, both play an important role in the regulation of this balance. Accordingly, in spite of their structural and functional similarity, they exhibit substantial differences in expression and activity. Whereas PGHS-1 is considered to be the constitutively expressed isoform regulating vascular tone, PGHS-2 is induced by inflammatory stimuli such as cytokines. PGHS-2 expression is associated with several cardiovascular risk factors and the development of atherosclerosis. 53, 54
While both PGHS isoforms require the presence of lipid peroxides or ONOO− for activation, 55 PGHS-2 becomes activated at 10 times lower concentrations of lipid peroxides than PGHS-1. Consequently, independent activation of both isoenzymes is possible within one cell. Moreover, PGHS-2 displays a higher specific activity at lower arachidonate concentrations compared with PGHS-1, which also contributes to the differential activation of both enzymes.56
Interactions between •NO and prostaglandin H synthase
The first observation of an impact of •NO and NOx on prostaglandin biosynthesis was made 15 years ago when it was shown that LPS increased the production of •NO and prostaglandins by murine macrophages and that inhibition of NOS decreased rates and extents of prostaglandin production. 57 Moreover, LPS-induced formation of prostaglandins was blunted by inhibition of NOS in LPS-treated mice and conversely, •NO donor administration increased prostaglandin synthesis. 58 Subsequent biochemical studies of purified PGHS-1 however, did not support that •NO mediated either the activation or inhibition of PGHS-1. 59, 60 Rather, the reaction product of •NO and O2•−, ONOO−, was shown to activate both isoforms of PGHS. 55, 60 The mechanism proposed for this activation is similar to that appreciated for the PGHS “priming” function of lipid peroxides, wherein the formation of a FeIV=O radical (compound I) which is associated with a porphyrin pi cation radical or a nearby tyrosine or tryptophan radical and the subsequent intramolecular formation of a •Tyr-radical is initiated by the reaction of ONOO− with the FeIII-heme of PGHS. 55, 61 Recent investigations have demonstrated that activation of PGHS by this mechanism is triggered by nanomolar levels of endogenous ONOO−.62
Subsequent investigation revealed additional mechanisms whereby ONOO− reacts with PGHS. Purified PGHS-1 preparations and vascular smooth muscle cell studies revealed that ONOO− induces the nitration of Tyr385 of PGHS-1, leading to enzyme inactivation. 63 Nitrated PGHS-1 has been detected in human carotid endarterectomy samples and in atherosclerotic lesions of ApoE−/− mice, and is not detectable in ApoE−/−/iNOS−/− mice. 63, 64 These contradictory observations of both PGHS activation and inhibition by ONOO− can be clarified by considering experimental conditions. The activation of PGHS-1 by ONOO− was only observed in the presence of a peroxide scavenger (glutathione peroxidase) suggesting that, in the absence of peroxides, ONOO− stimulates catalytic activity of PGHS-1 by oxidation of the FeIII-heme 60 and thus provides the “peroxide tone” necessary for the activation of PGHS-1. 65 In the absence of a peroxide scavenger and in the inflammatory milieu of arteriosclerotic lesions, ONOO− can also inhibit PGHS-1 by nitration of Tyr385. These findings are also supported by a recent study demonstrating that the effect of ONOO− on PGHS is dependent on whether the enzyme is in the resting or active state. 29
S-nitrosation is another mechanism whereby •NO can modulate PGHS activity, an event, shown to be facilitated by selective binding of iNOS to PGHS-2 both in vitro and in macrophages. This leads to S-nitrosation of Cys526 of PGHS-2 and enzyme activation, 66 an event only observed for PGHS-2. 60, 66 In physiologic concentrations NO2− is also able to inhibit PGHS-2 in the presence of peroxides via heme-catalyzed oxidation to •NO2 and nitration of a tyrosine residue.67
Another impact of •NO on PGHS-2 but not PGHS-1 is the upregulation of PGHS-2 protein synthesis at both the post-transcriptional and post-translational level via •NO-dependent alterations in MAPK signaling. 68–70 In contrast, •NO also induces tyrosine nitration of PGHS-2 and inhibits its activity. 71 Thus, PGE2 production by fibroblasts from PGHS1-deficient mice is inhibited by •NO, while the same concentrations of •NO lead to an increase in PGE2 production in fibroblasts of PGHS-2-deficient mice. 72 Moreover, in murine lung-derived endothelial cells iNOS-dependent •NO generation increased prostaglandin production, whereas cell exposure to higher •NO concentrations inhibited prostaglandin biosynthesis. 73 In aggregate, there is a differential regulation of PGHS-1 and PGHS-2 by •NO and secondary NOx species, events that will be dictated by mediator concentrations and the underlying redox state of cells.
Nitric oxide and prostaglandin I2 synthase
Another interaction between •NO and prostaglandin signaling occurs downstream of PGHS, the inhibition of prostaglandin I2 synthase (PGI2S) by ONOO−. 74 The nitration of Tyr430 is induced by ONOO−, inhibiting substrate access to the catalytic site of PGI2S. 65, 75 Nitrated PGI2S has been detected in diverse animal models of disease, including diabetes, 76 ischemia-reperfusion, 77 nitroglycerin tolerance, 78 septic shock 79 and atherosclerosis. 80 Since prostaglandin I2 is a key vasodilatory prostanoid, its balance with platelet-derived thromboxane A2 is critical for maintaining vascular homeostasis. Consequently, the inhibition of PGI2S by •NO-derived species can decrease both rates of PGI2 formation and PGI2 concentration, resulting in the accumulation of PGH2 and activation of the thromboxane A2 receptor. 81 This underscores the critical cardiovascular actions of ONOO− where, by affecting PGI2S activity, there can be a shift in the balance of vasorelaxant and vasoconstrictive prostanoids.82
Nitric oxide and lipoxygenases
Lipoxygenases (LOX) are non-heme iron-containing enzymes that catalyze the oxygenation of polyunsaturated fatty acids to unstable hydroperoxides, a process termed lipoxygenation. These hydroperoxides are rapidly reduced and converted to secondary, more stable signaling mediators. 83, 84 During lipoxygenation, fatty acid radical intermediates are formed, which usually remain enzyme-bound, but under pathological conditions can also be released from the enzyme to induce further free radical reactions. 85 Multiple LOX isoforms are expressed by mammals, which were initially named according to the carbon atom number of arachidonic acid where they introduce oxygen. A phylogenetic nomenclature has been suggested recently, 86 that allows for species-related differences. Also, LOX isoforms can introduce oxygen into both shorter and longer chain unsaturated fatty acids, can oxidize both free and esterified fatty acids and can oxygenate fatty acids at more than one position. 87–89 In humans, 5-LOX (also 5-LOX in the phylogenetic nomenclature) and 15-LOX (12/15LOX, new nomenclature) are expressed in reticulocytes and, upon activation, in monocytes.
There is a significant contribution of LOX-derived species to the pathogenesis of vascular inflammatory diseases, 84 with 5-LOX being the best characterized isoenzyme. 5-LOX reaction with arachidonic acid yields 5-hydroperoxyeicosatetraenoic acid (5-HPETE), an intermediate in the biosynthesis of the pro-inflammatory signaling mediators leukotrienes. 5-LOX is abundantly expressed in both, rodent models of atherosclerotic disease and in atherosclerotic lesions of humans, 90, 91 where it can generate chemotactic products that induce inflammatory cell accumulation 92 and exert direct vasoconstrictor actions. 93, 94
15-LOX has been detected in monocytes, macrophages and platelets, with its expression induced by interleukin-4 and -13 with concomitant down-regulation of expression of 5-LOX. 91 Compared with 5-LOX, the physiological actions of 15-LOX are less apparent, although a role in antagonizing inflammatory responses has also been suggested. 95, 96
The contributions of 15-LOX-derived species to inflammatory vascular diseases is complicated by contradictory data. On the one hand, rodent models of atherosclerosis (15-LOX knock out and 15-LOX overexpressing mice) suggest a pro-atherogenic role of 15-LOX. 97–99 This is confirmed by the detection of a spectrum of regioisomers of fatty acid oxidation products in clinical specimens of early atherosclerotic lesions that are indicative of LOX-catalyzed reactions, rather than more random free radical events. 83, 85 On the other hand rabbits overexpressing 15-LOX exhibited a marked decrease in atherosclerotic lesion area. 100, 101 The reasons for these contradictory findings remain elusive, with concentration-dependent signaling responses to the HPETE products of 15-LOX suggested as an explanation. 83
LOX activation inhibits •NO signaling and conversely, •NO inhibits LOX enzymatic activity and product formation. 49, 102 The LOX-dependent oxidation of polyunsaturated fatty acids requires oxidation of the non-heme iron from the ferrous (Fe2+) to the ferric state (Fe3+). It was first proposed that •NO forms an inhibitory iron-nitrosyl complex with the enzyme in its ferrous form. 23, 103 This reaction requires very high and non-physiological concentrations of •NO to detect the iron-nitrosyl complex however, with LOX inhibition by •NO detectable at much lower concentrations. 102, 104 Current data support that •NO reacts with LOX-bound lipid peroxyl radicals to yield an organic peroxynitrite (LOONO) intermediate. This mechanism of inhibition is kinetically rapid (Figure 1), was observed to occur at physiological concentrations of •NO (nM) and ultimately yields a fatty acid hydroperoxide and nitrite as products. After the hydroperoxide is released by LOX however, the enzyme is left in an inactive ferrous form, requiring reactivation for another catalytic cycle. 49 These reactions with •NO have been defined for 15-LOX, but because of similar catalytic mechanisms of other LOX isoenzymes it can be assumed that these events are also applicable to 5-LOX. Accordingly, •NO-dependent suppression of 5-LOX metabolism has been shown in cell and animal models. 105, 106 The inhibitory effects of •NO and ONOO− on 5-LOX has also been associated with tyrosine nitration and S-nitrosylation of 5-LOX. 107, 108 These latter data stem from isolated enzyme and cell studies, with confirmatory studies in animal models remaining to be done.
Nitric oxide and cytochrome P 450 (CYP) enzymes
CYP enzymes are membrane-bound, heme-containing oxidases best known for catalyzing the disposition of xenobiotics. While most CYP enzymes are predominantly expressed in the liver, many CYP isoenzymes are present in extrahepatic locations such as endothelial and vascular smooth muscle cells and contribute to cardiovascular regulation. 109, 110 In this context the CYP-2 family of enzymes having epoxygenase activity and the CYP-4 family, which consists of ω-hydroxylases, are of particular significance.
The epoxygenases of the CYP-2 family catalyze the generation of different epoxide regioisomers from arachidonic acid, termed epoxyeicosatrienoic acids (EETs). Because of an ability to hyperpolarize endothelial and smooth muscle cells and mediate vasodilatation independently of •NO and PGI2, EETs are one component of a collection of species given the functional terminology endothelium-derived hyperpolarizing factor (EDHF). 110–112 The CYP-4 family, specifically the CYP-4A family, generates an essential mediator of angiotensin II and endothelin-1 signal transduction, 20-hydroxyeicosatetraenoic acid (20-HETE). 113, 114
Substantial evidence supports that •NO inhibits CYP isoenzymes, 115, 116 with different mechanisms proposed to account for this inhibition. First, CYP inhibition induced by formation of a reversible heme iron-nitrosyl-complex has been suggested 117 and supported by observations in a rat model exposed to LPS-induced, iNOS-derived •NO. 118 For some isoforms, CYP inactivation by ONOO−-induced tyrosine nitration has been reported, but the physiological relevance of these findings remain to be elucidated. 119, 120 Moreover, it was suggested that •NO also induces proteasomal degradation of CYP protein 121 and affects CYP transcription 122.
Overall, the cardiovascular impact of CYP inhibition remains to be defined due to contradictory observations. For example, the inhibition of HETE synthesis enhances the vasodilatory effects of •NO. 123 Alternatively, decreased •NO bioavailability enhances CYP-dependent synthesis of EET and thus serves as a “reserve” vasodilatory mechanism. 124, 125 Since •NO can impact both the synthesis of EET (CYP 2) and HETE (CYP-4A), 123, 126 these findings are in conflict and suggest a more complex interaction between •NO and CYP-derived lipid mediator formation and action. Similarly, studies assessing the effect of CYP-2C9 on acetylcholine-induced vasodilation of the forearm report conflicting results. 127, 128
CYP-derived fatty acid oxidation products also influence the bioavailability of •NO. For example, exposure of vascular endothelial cells to 5,6-EET stimulates •NO synthesis by increasing intracellular Ca2+ levels. 129 In contrast, CYPs can also accelerate the vascular generation of ROS. Specifically, porcine artery CYP-2C serves as a source of ROS that react with •NO and impair •NO-dependent vascular relaxation. 124 Recent findings also suggest an impact of 20-HETE on •NO-biosynthesis by uncoupling eNOS, enhancing eNOS-derived ROS production and the subsequent inhibition of •NO signaling. 130 Again, how these paradoxical findings can be integrated into the physiology of vascular function remains to be clarified in vessel and organ preparations and in in vivo models.
Nitric Oxide and Redox-Derived Lipid Mediators
Redox-derived metabolites of •NO
Nitric oxide exerts a pervasive influence on the generation and actions of lipid signaling mediators, by modulating both enzymatic- and free radical/oxidant-dependent unsaturated fatty acid oxygenation. When lipid oxidation is initiated by free radical/oxidant species, •NO and its byproducts can either inhibit or stimulate fatty acid oxidation, 131 yielding products that can display unique signaling actions. The net biochemical and physiological outcome when reactions of •NO and oxidizing lipids converge will be dictated by multiple conditions, including the specific composition and concentrations of the oxidative inflammatory milieu. This includes free radical and oxidizing species (e.g., O2•−, H2O2, •OH, ONOO−, •NO2 HOCl, metal catalysts), oxidant scavengers (e.g., tocopherols, glutathione, thioredoxin, catalase, superoxide dismutases, peroxidases), and other aspects of the local chemical milieu (e.g., pH, availability of fatty acids, relative lipophilicity). Oxidized lipid mediators not derived from PGHS, LOX and CYP450 typically stem from autocatalytic lipid peroxidation reactions occurring in membranes and lipoproteins. The kinetically favourable reaction of O2•− with •NO in particular gives a unique initiator of lipid peroxidation, ONOO−. Notably, ONOO−-induced lipid oxidation proceeds without metal catalysis, in contrast to H2O2. 131, 132 Moreover, since •NO is a radical itself, it readily reacts with lipid peroxyl radicals and can rapidly terminate chain propagation reactions of lipid oxidation. Thus, the extent and product profile of O2•− induced lipid oxidation will be a function of relative concentrations of •NO. The interactions of •NO with non-enzymatically generated lipid mediators are exemplified in the studies outlined below (see also Figures 1 and 2 for an overview of interactions between •NO and lipid mediators).
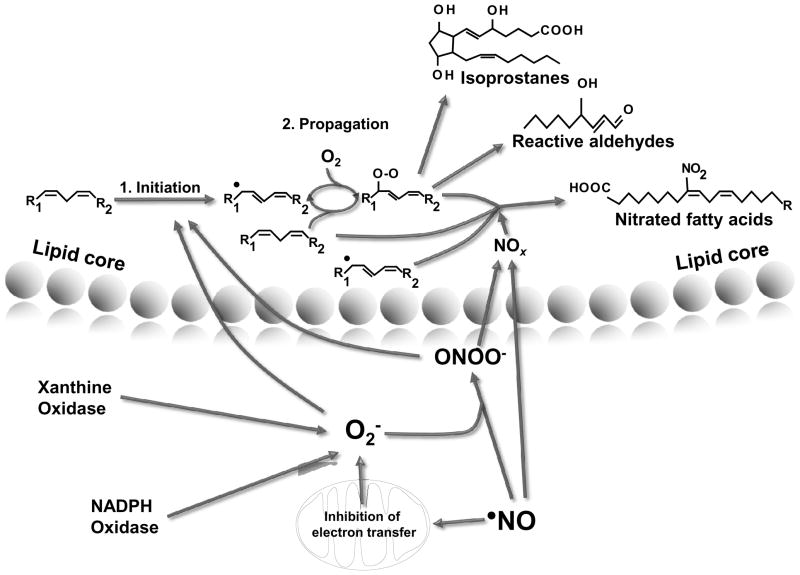
Figure 2. Formation of lipid signaling mediators from redox reactions within the lipid core of lipoproteins or cell membranes.
(1) Oxidation of membrane lipids by free radicals and oxidants initiate lipid peroxidation, by formation of lipid radical species that then react with molecular oxygen or other radicals to form lipid peroxyl radicals. Peroxyl radical species can then either further propagate lipid peroxidation events or they can (2) serve as precursors for the generation of isoprostanes and reactive aldehyde derivatives. Finally, both native fatty acids or oxidized fatty acids can react with nitrogen dioxide (•NO2) arising from secondary reactions of •NO and nitrite (NO2−) leading to the formation of nitro-fatty acid derivatives (3)
Nitric oxide and isoprostanes
Isoprostanes are prostaglandin-like compounds formed during free radical catalyzed auto-oxidation of polyunsaturated fatty acids that proceed independently of PGHS. 133 Isoprostanes are generated at increased rates during inflammatory states. Notably, iNOS-derived •NO contributes significantly to the formation of isoprostanes, with iNOS−/− mice displaying reduced levels of isoprostane formation. 134, 135 In support of a role for ONOO− as the proximal mediator of fatty acid oxidation to isoprostane derivatives, ONOO− will directly induce formation of F2-isoprostanes during the oxidation of lipoproteins and plasma lipids. 136 Consistent with its ability to terminate chain propagation reactions of lipid oxidation, •NO, when present in excess of O2•−, can also inhibit oxidant-induced F2-isoprostane formation. 137 Of note, there is an inverse correlation between net extents of •NO synthesis and free radical-induced 15-F2t-isoprostane production in subjects with type 1 diabetes, an event not observed in control subjects. This is consistent with the ability of •NO, under these conditions, to inhibit lipid peroxidation. 138
Several groups of isoprostanes are found in vivo, with F2-isoprostanes the most abundant. As in the case of prostaglandins the letter F indicates the type of cyclopentane ring, thus F2-isoprostanes share structural similarity with prostaglandin F2α. Correspondingly, D2, E2, G2, and H2 isoprostanes have been described, which are isomers of their respective prostaglandin. Each class of isoprostanes contains 64 isomers, made up of four regioisomers each and consisting of eight racemic diastereoisomers. 139 In addition to these isoprostane classes, two other groups have been described, the A2- and J2-isoprostanes, which have an electrophilic cyclopentenone ring instead of the cyclopentane ring, a property that influences their mode of signaling.
F2-isoprostanes are extremely robust biomarkers of oxidative stress-induced lipid peroxidation. 139–141 In vivo generation of F2-isoprostanes is most commonly assessed by measurement of the urinary metabolite of 15-F2t-isoprostane, 2,3-dinor-5,6-dihydro-15-F2t-isoprostane. 139, 142 However, F2-isoprostane derivatives can also be detected in plasma and measurement of esterified isoprostanes in plasma lipoproteins or tissue biopsy samples is also an option. 142, 143
Most studies concerning the signaling actions of isoprostanes have investigated F2-isoprostanes, especially 15-F2t-isoprostanes. In animal models, 15-F2t-isoprostanes are potent vasoconstrictors in vascular beds including renal arteries, 144 pulmonary arteries, 133 coronary arteries, 145 and cerebral arterioles. 146 Moreover, an involvement in obstructive pulmonary disease has been suggested. 147 Similarly, vasoconstriction of pulmonary 148 and renal arteries 149 as well as airway constriction 148 has been found for E2-isoprostanes, in particular 15-E2t-isoprostanes. In addition to these excitatory effects on smooth muscle cells, F2-isoprostanes induce platelet activation, 150 increase endothelin-1 expression 151 in endothelial cells and are generated by oxidizing LDL. 152 Accordingly, a role may exist for isoprostanes in the pathophysiology of atherosclerosis, with 15-F2t-isoprostanes detected at increased levels in ApoE and LDL-receptor-deficient mice. 153
The signaling actions of E2-and F2-isoprostanes are viewed to be receptor-mediated, however the specific receptors involved remain to be clarified clinically. Agonism of the thromboxane A2 receptor is proposed to be responsible for the vasoconstrictive and proatherogenic effects of F2-isoprostanes, from studies of transgenic mice. 154, 155 In contrast, in platelets F2-isoprostanes have been shown to act as an antagonist of this receptor. 156 While isoprostanes are also proposed to serve as peroxisome proliferator-activating receptor γ (PPARγ) ligands, 157 the evidence for a unique isoprostane receptor is limited to in vitro studies. 155, 158
Conversely, signal transduction by A2- and J2-isoprostanes is not ascribed to specific ligand-receptor interactions. Due to the α,β-unsaturated carbonyl group of the cyclopentenone ring, these compounds exhibit an electrophilic reactivity which enables a Michael addition with nucleophilic residues, typically thiol-containing biomolecules which in the case of proteins can result in an altered function of these proteins. 159 This type of posttranslational modification by electrophiles is emerging as a significant pathway of signal transduction, with the signaling actions of these derivatives differing substantially from the aforementioned isoprostane classes. Thus, 15-A2- and 15-J2-isoprostanes exhibit anti-inflammatory properties, such as inhibition of NF-κB-dependent expression of iNOS and PGHS2 in LPS-stimulated murine macrophages. 160 Overall, the signaling actions of isoprostanes and the impact of •NO appear to be tissue, regioisomer and species specific, thus extrapolation of individually-reported effects should be made with caution (see 139 for a recent, more detailed review on the biological effects of isoprostanes).
Nitric oxide and reactive aldehydes
Non-enzymatic oxidation of polyunsaturated fatty acids yields a variety of reactive aldehydes, which are derived from lipid hydroperoxides undergoing acyl chain cleavage. These products of lipid peroxidation are also sometimes useful markers of oxidative inflammatory conditions and include species such as malondialdehyde (MDA), glyoxal, acrolein, 4-hydroxy-2-nonenal (4-HNE) and 4-oxo-2-nonenal (ONE). While these byproducts have traditionally been regarded as cytotoxins, they are also increasingly recognized to manifest cell signaling actions.
The best characterized lipid aldehyde derivative is HNE, which contains an α,β-unsaturated carbonyl group and thus displays electrophilic reactivity towards thiols, histidine and other biological nucleophiles. At high concentrations, this reactivity can support cytotoxic reactions with DNA bases and other cellular components, while in lower concentrations α,β-unsaturated carbonyls can induce the posttranslational modification of a wide variety of proteins and indirectly act as anti-inflammatory signaling mediators.
For example, HNE induces a broad range of actions on protein kinase signaling by activating multiple isoforms of protein kinase C, 161, 162 several members of the MAP kinase family, 163, 164 and tyrosine kinase receptors, 165 as well as inhibiting most catalytic thiol-containing protein tyrosine phosphatases. 166, 167 HNE also inhibits mitochondrial aldehyde dehydrogenase-2 (ALDH-2) leading to cardioprotection in a rodent model of ischemia. 168
In terms of regulating transcription factor function, HNE inhibits NF-κB signaling by preventing the phosphorylation of IκB 169 and activates nuclear factor E2-related transcription factor (Nrf2)-regulated gene expression, 170 leading to increased expression of the antioxidant actions of heme oxygenase-1 (HO-1), peroxiredoxin and stress protein A 170.
As noted, •NO and •NO-derived reactive species can initiate and propagate membrane lipid peroxidation, and even inhibit this process when •NO is in “excess” of inciting oxidants. 51, 102 Hence, increased generation of reactive aldehyde products of lipid oxidation are to be expected in inflammatory states involving enhanced generation of ROS and elevated •NO concentrations as a consequence of increased iNOS expression. Indeed, it was shown in insulin-secreting RINm5F cells that •NO donors, as well as interleukin-1β induced iNOS expression leads to increased production of MDA and 4-HNE. This was inhibited by manganese superoxide dismutase (Mn-SOD), suggesting an involvement of ONOO− in stimulating lipid oxidation. 171 Furthermore, in a rat model of renal ischemia-reperfusion (I/R), post-I/R infusion of L-arginine into kidneys increased organ production of 4-HNE. 172 Similarly, in a model of DOCA-salt-induced hypertension and cardiac hypertrophy, iNOS−/−mice exhibited a significantly reduced myocardial production of 4-HNE, in comparison to wild-type mouse myocardium. 173 Finally, there is a contribution of iNOS in lipid radical generation and the formation of 4-HNE in streptozotocin-induced diabetes. 174
There is an opposing influence of 4-HNE on •NO-homeostasis. This is evidenced by a dose-dependent HNE inhibition of LPS/interferon-γ induced •NO production and iNOS-expression in vascular smooth muscle cells, an effect mediated by HNE inhibition of NF-κB signaling. 175 Moreover, it has been suggested that 4-HNE also reduces •NO production by modulation of methylarginine levels and DDAH activity. For example, bovine aortic endothelial cell exposure to 4-HNE decreases DDAH catalytic activity. In this model system, DDAH-overexpression and L-arginine supplementation partially reversed the inhibition of •NO production. 176 This multifaceted inhibition of •NO production can significantly influence the progression of inflammatory conditions. 177 Thus, the lipid oxidation product 4-HNE might serve as a negative feedback mechanism to counteract overt production of •NO by iNOS. How these findings are applicable in vivo to cardiovascular events remains to be defined.
Nitric oxide and fatty acid nitration
Nitro-fatty acids (NO2-FA) are endogenous signaling mediators, which exhibit anti-inflammatory signaling actions. NO2-FA are generated from the reaction of •NO-derived species with unsaturated fatty acids and constitute a biochemical convergence of reactants participating in •NO and lipid signaling. During peroxyl radical-induced fatty acid chain propagation reactions, NO2-FA emerge as termination products in the presence of •NO and its oxidative byproducts. 30, 102, 131, 178 Different biochemical mechanisms, reviewed elsewhere, 179 account for the generation of NO2-FA, with all having •NO2 in common as the proximal nitrating species. Basal plasma and tissue levels of NO2-FA are in the nM concentration range, with inflammatory events such as ischemia-reoxygenation leading to higher concentrations in specific compartments such as mitochondria or macrophages. 180–183
As for cyclopentenone isoprostanes and reactive aldehydes, the electrophilic adduction of biological targets is central to how NO2-FA transduce signaling actions. The robust electrophilic reactivity of NO2-FA is explained by the high electronegativity of the NO2 group, especially when bound to an olefinic carbon of unsaturated fatty acids. This facilitates Michael addition reaction between the alkenyl β-carbon of NO2-FA and nucleophiles such as protein histidine and cysteine residues, a process which in the case of NO2-FA is termed nitroalkylation. 184 By virtue of this reactivity, NO2-FA inhibit pro-inflammatory cytokine secretion in LPS-stimulated macrophages by repressing NF-κB dependent target gene expression, an effect which is induced by the nitroalkylation of functionally critical cysteines in the p65 subunit of NF-κB. 185 In addition, NO2-FA inhibit vascular smooth muscle cell proliferation by activation of Keap1/Nrf2-(Kelch-like ECH-associating protein 1/nuclear factor erythroid 2-related factor 2)-mediated signaling. Nitroalkylation of the cysteine-rich cytoplasmatic suppressor protein Keap1 leads to dissociation of Keap1 from the transcription factor Nrf2, allowing translocation of Nrf2 to the nucleus. 186 Another significant signaling action of NO2-FA is the ability to activate PPARγ and to a lesser extent PPARα and PPARδ. 187 NO2-FA have a receptor affinity exceeding virtually all putative endogenous PPARγ ligands, and that rivals the receptor affinity of synthetic thiazolidinedione ligands (e.g., Rosiglitazone). Similar to thiazolidinediones NO2-FA induce increased glucose uptake in adipocytes. 188 Of note, NO2-FA exhibit a reduced potential to induce adipogenesis and adipocyte triglyceride accumulation in comparison to rosiglitazone, a property currently viewed to be a consequence of the distinctive PPAR-coregulator protein interactions induced by NO2-FA.179, 187
Besides signal transduction reactions, which depend on electrophilic reactivity, NO2-FA signal via several other mechanisms. While not viewed to be clinically significant, NO2-FA release •NO and thus under certain circumstances might serve as reservoir for •NO. NO2-FA-dependent •NO release is mediated by a Nef-like reaction, involving the aqueous conversion of nitro compounds into carbonyls. The resulting radical is stabilized by conjugation with the alkene and by the hydroxy-group, a moiety known to stabilize radicals. 189, 190 As a consequence, NO2-FA mediate the cGMP-dependent relaxation of preconstricted rat aortic vessel rings. 191 The occurrence of this reaction in vivo however, awaits definitive confirmation, since NO2-FA are resistant to aqueous-mediated decay reactions when stabilized by membrane and lipoprotein lipids or upon protein adduction. 179 Other anti-inflammatory signaling actions of NO2-FA include inhibition of neutrophil and platelet activation via non-cGMP dependent mechanisms. Thus, nitrolinoleic acid (LNO2) inhibited O2•− production, azurophilic degranulation, calcium mobilization and CD 11b expression in activated polymorphonuclear neutrophils. 192, 193 Moreover, in platelets LNO2 inhibited thrombin-induced aggregation. 193 For both cell types it was suggested that these mechanisms are mediated by an induction of adenylate cyclase activity. A final mechanism whereby NO2-FA mediate anti-inflammatory signaling is via upregulation of endothelial and epithelial HO-1 gene expression. 194, 195 Induction of HO-1 expression by NO2-FA is independent of •NO, NF-κB and PPAR-regulated mechanisms. Instead, transcriptional regulation by the Keap1/Nrf2 and the cAMP-dependent response element has been proposed. 194 HO-1 upregulation is a central protective response during many inflammatory processes including sepsis, arteriosclerosis and transplant rejection. A recent study showed generation of an endogenous NO2-FA in Langendorff rat hearts subjected to ischemic preconditioning. Furthermore experiments on isolated cardiomyocytes subjected to anoxia/reoxygenation performed in this study suggest cardioprotective effects of these compounds. 183 Studies investigating the cardioprotective and anti-atherosclerotic actions of these compounds in animal studies are currently ongoing.
Conclusions
Nitric oxide and lipid signaling converge via multiple mechanisms, with the eventual biological outcome from these interactions depending to a great extent on the chemical equilibria of all potential reactants in a particular biological locus. This presents significant challenges in translating in vitro-based observations to more clinically relevant conditions. Multiple analytical and conceptual challenges also remain, as investigators strive to gain a detailed understanding of the myriad of signaling reactions mediated by the family of redox signaling mediators stemming from •NO. This includes the redox-related •NO byproduct ONOO−, partially reduced oxygen species (e.g., O2•−, H2O2 and •OH), oxidized halides (e.g., HOCl, HOBr), oxidized thiols, S-nitrosothiols and oxidized or nitrated fatty acids. Due to the broad reactivity of •NO and kinetic considerations, the individual byproducts discussed in this review are never formed exclusively, and they often display overlapping reactivities with target molecules. A case in point is when cells or tissues are exposed in vitro to reactive mediator concentrations not experienced in vivo. For example, a cell faced with a high concentration of •NO coming from external addition of •NO donors or transfection of NOS would be expected to undergo thiol S-nitrosation, the oxidation of thiols to disulfides and higher oxidation states and the formation of mixed disulfides with glutathione. Additionally, ONOO− formation, fatty acid oxidation or nitration and FeS complex oxidation would be expected. As the field of redox signaling moves forward, it remains imperative that we continue to incisively define not only discrete mechanisms of redox signaling, but also that we strive to translate these reactions into an understanding of how they impact cell, vascular and organ function. As we gain additional insight from both in vivo models and clinical measurement of the molecular events and physiological responses to redox mediators, we will also be better positioned for developing new drug strategies.
Acknowledgments
Sources of Funding: This work was supported by National Institutes of Health grants R01 HL58115 and R01 HL64937, and the Deutsche Herzstiftung.
Non-standard abbreviations and acronyms
- CYP
cytochrome P
- DDAH
dimethylarginine dimethylaminohydrolase
- 5-HPETE
5-hydroperoxyeicosatetraenoic acid
- NOS
NO synthase
- EET
epoxyeicosatrienoic acids
- EDHF
endothelium-derived hyperpolarizing factor
- eNOS
endothelial NO sythase
- iNOS
inducible NO synthase
- nNOS
neuronal NO synthase
- sGC
guanylate cyclase
- cGMP
cyclic guanosine monophosphate
- MAPK
mitogen-activated protein kinase
- NFκB
nuclear factor κB
- PG
prostaglandin
- PGHS
prostaglandin H synthase
- PGI2S
prostaglandin I2 synthase
- PPAR
peroxisome proliferator-activating receptor
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- LPS
lipopolysaccharids
- LOX
lipoxygenases
- MDA
malondialdehyde
- 4-HNE
4-hydroxy-2-nonenal
- ONE
4-oxo-2-nonenal
- HO-1
heme oxygenase-1
- NO2-FA
nitro-fatty acids
- Keap1
Kelch-like ECH-associating protein 1
- Nrf2
nuclear factor erythroid 2-related factor 2
- LNO2
9, 10, 12 and 13-nitro-linoleic acid
Footnotes
Disclosure: BAF acknowledges financial interest in Complexa, Inc.
References
- 1.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–12. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Higgs EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Invest. 1991;21:361–74. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 3.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proceedings of the National Academy of Sciences. 1997;94:6954–8. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balligand JL, Ungureanu-Longrois D, Simmons WW, Kobzik L, Lowenstein CJ, Lamas S, Kelly RA, Smith TW, Michel T. Induction of NO synthase in rat cardiac microvascular endothelial cells by IL-1 beta and IFN-gamma. Am J Physiol. 1995;268:H1293–303. doi: 10.1152/ajpheart.1995.268.3.H1293. [DOI] [PubMed] [Google Scholar]
- 5.MacNaul KL, Hutchinson NI. Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem Biophys Res Commun. 1993;196:1330–4. doi: 10.1006/bbrc.1993.2398. [DOI] [PubMed] [Google Scholar]
- 6.Chen LY, Mehta JL. Further evidence of the presence of constitutive and inducible nitric oxide synthase isoforms in human platelets. J Cardiovasc Pharmacol. 1996;27:154–8. doi: 10.1097/00005344-199601000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Satoh M, Nakamura M, Tamura G, Makita S, Segawa I, Tashiro A, Satodate R, Hiramori K. Inducible nitric oxide synthase and tumor necrosis factor-alpha in myocardium in human dilated cardiomyopathy. J Am Coll Cardiol. 1997;29:716–24. doi: 10.1016/s0735-1097(96)00567-0. [DOI] [PubMed] [Google Scholar]
- 8.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–8. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 9.Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–8. [PubMed] [Google Scholar]
- 10.Nathan C. Nitric oxide as a secretory product of mammalian cells. The FASEB Journal. 1992;6:3051–64. [PubMed] [Google Scholar]
- 11.Ignarro LJ. Haem-dependent activation of cytosolic guanylate cyclase by nitric oxide: a widespread signal transduction mechanism. Biochem Soc Trans. 1992;20:465–9. doi: 10.1042/bst0200465. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt H, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochimica et biophysica acta. Molecular cell research. 1993;1178:153–75. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- 13.Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB J. 1993;7:328–38. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- 14.Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989;86:3375–8. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 16.Bloodsworth A, O’Donnell VB, Freeman BA. Nitric oxide regulation of free radical-and enzyme-mediated lipid and lipoprotein oxidation. Arterioscler Thromb Vasc Biol. 2000;20:1707–15. doi: 10.1161/01.atv.20.7.1707. [DOI] [PubMed] [Google Scholar]
- 17.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Miller MJ, Joshi MS, Thomas DD, Lancaster JR. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci U S A. 1998;95:2175–9. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller TW, Cherney MM, Lee AJ, Francoleone NE, Farmer PJ, King SB, Hobbs AJ, Miranda KM, Burstyn JN, Fukuto JM. The effects of nitroxyl (HNO) on soluble guanylate cyclase activity: Interactions at ferrous heme and cysteine thiols. J Biol Chem. 2009 doi: 10.1074/jbc.M109.014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–4. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radi R, Denicola A, Freeman BA. Peroxynitrite reactions with carbon dioxide-bicarbonate. Methods Enzymol. 1999;301:353–67. doi: 10.1016/s0076-6879(99)01099-x. [DOI] [PubMed] [Google Scholar]
- 22.Gladwin MT, Grubina R, Doyle MP. The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase. Acc Chem Res. 2009;42:157–67. doi: 10.1021/ar800089j. [DOI] [PubMed] [Google Scholar]
- 23.Holzhütter HG, Wiesner R, Rathmann J, Stösser R, Kühn H. A kinetic model for the interaction of nitric oxide with a mammalian lipoxygenase. Eur J Biochem. 1997;245:608–16. doi: 10.1111/j.1432-1033.1997.00608.x. [DOI] [PubMed] [Google Scholar]
- 24.Bellamy TC, Garthwaite J. The receptor-like properties of nitric oxide-activated soluble guanylyl cyclase in intact cells. Mol Cell Biochem. 2002;230:165–76. [PubMed] [Google Scholar]
- 25.Prütz WA, Mönig H, Butler J, Land EJ. Reactions of nitrogen dioxide in aqueous model systems: oxidation of tyrosine units in peptides and proteins. Arch Biochem Biophys. 1985;243:125–34. doi: 10.1016/0003-9861(85)90780-5. [DOI] [PubMed] [Google Scholar]
- 26.Upmacis RK, Deeb RS, Hajjar DP. Oxidative alterations of cyclooxygenase during atherogenesis. Prostaglandins Other Lipid Mediat. 2006;80:1–14. doi: 10.1016/j.prostaglandins.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Miranda KM, Paolocci N, Katori T, Thomas DD, Ford E, Bartberger MD, Espey MG, Kass DA, Feelisch M, Fukuto JM, Wink DA. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc Natl Acad Sci U S A. 2003;100:9196–201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharitonov VG, Sundquist AR, Sharma VS. Kinetics of nitrosation of thiols by nitric oxide in the presence of oxygen. J Biol Chem. 1995;270:28158–64. doi: 10.1074/jbc.270.47.28158. [DOI] [PubMed] [Google Scholar]
- 29.Trostchansky A, O’Donnell VB, Goodwin DC, Landino LM, Marnett LJ, Radi R, Rubbo H. Interactions between nitric oxide and peroxynitrite during prostaglandin endoperoxide H synthase-1 catalysis: a free radical mechanism of inactivation. Free Radic Biol Med. 2007;42:1029–38. doi: 10.1016/j.freeradbiomed.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 30.O’Donnell VB, Chumley PH, Hogg N, Bloodsworth A, Darley-Usmar VM, Freeman BA. Nitric oxide inhibition of lipid peroxidation: kinetics of reaction with lipid peroxyl radicals and comparison with alpha-tocopherol. Biochemistry. 1997;36:15216–23. doi: 10.1021/bi971891z. [DOI] [PubMed] [Google Scholar]
- 31.Huie RE, Padmaja S. The reaction of NO with superoxide. Free Radic Res Commun. 1993;18:195–9. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 32.Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 33.Fukuto JM, Cho CH, Switzer CH, Ignarro LJ. Nitric oxide: biology and pathobiology. San Diego: Academic Press; 2000. The chemical properties of nitric oxide and related nitrogen oxides; pp. 23–40. [Google Scholar]
- 34.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–66. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 35.Haendeler J, Hoffmann J, Tischler V, Berk BC, Zeiher AM, Dimmeler S. Redox regulatory and anti-apoptotic functions of thioredoxin depend on S-nitrosylation at cysteine 69. Nat Cell Biol. 2002;4:743–9. doi: 10.1038/ncb851. [DOI] [PubMed] [Google Scholar]
- 36.Arnelle DR, Stamler JS. NO+, NO, and NO− donation by S-nitrosothiols: implications for regulation of physiological functions by S-nitrosylation and acceleration of disulfide formation. Arch Biochem Biophys. 1995;318:279–85. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 37.Mannick JB, Schonhoff C, Papeta N, Ghafourifar P, Szibor M, Fang K, Gaston B. S-Nitrosylation of mitochondrial caspases. J Cell Biol. 2001;154:1111–6. doi: 10.1083/jcb.200104008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xian M, Wang K, Chen X, Hou Y, McGill A, Zhou B, Zhang ZY, Cheng JP, Wang PG. Inhibition of protein tyrosine phosphatases by low-molecular-weight S-nitrosothiols and S-nitrosylated human serum albumin. Biochem Biophys Res Commun. 2000;268:310–4. doi: 10.1006/bbrc.2000.2117. [DOI] [PubMed] [Google Scholar]
- 39.Leiper J, Murray-Rust J, McDonald N, Vallance P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc Natl Acad Sci U S A. 2002;99:13527–32. doi: 10.1073/pnas.212269799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stamler JS, Lamas S, Fang FC. Nitrosylation The Prototypic Redox-Based Signaling Mechanism. Cell. 2001;106:675–83. doi: 10.1016/s0092-8674(01)00495-0. [DOI] [PubMed] [Google Scholar]
- 41.Sumbayev VV, Budde A, Zhou J, Brüne B. HIF-1 alpha protein as a target for S-nitrosation. FEBS Lett. 2003;535:106–12. doi: 10.1016/s0014-5793(02)03887-5. [DOI] [PubMed] [Google Scholar]
- 42.DelaTorre A, Schroeder RA, Kuo PC. Alteration of NF-kappa B p50 DNA binding kinetics by S-nitrosylation. Biochem Biophys Res Commun. 1997;238:703–6. doi: 10.1006/bbrc.1997.7279. [DOI] [PubMed] [Google Scholar]
- 43.Matata BM, Galiñanes M. Peroxynitrite is an essential component of cytokines production mechanism in human monocytes through modulation of nuclear factor-kappa B DNA binding activity. J Biol Chem. 2002;277:2330–5. doi: 10.1074/jbc.M106393200. [DOI] [PubMed] [Google Scholar]
- 44.Go YM, Patel RP, Maland MC, Park H, Beckman JS, Darley-Usmar VM, Jo H. Evidence for peroxynitrite as a signaling molecule in flow-dependent activation of c-Jun NH(2)-terminal kinase. Am J Physiol. 1999;277:H1647–53. doi: 10.1152/ajpheart.1999.277.4.H1647. [DOI] [PubMed] [Google Scholar]
- 45.Klotz LO, Schieke SM, Sies H, Holbrook NJ. Peroxynitrite activates the phosphoinositide 3-kinase/Akt pathway in human skin primary fibroblasts. Biochem J. 2000;352(Pt 1):219–25. [PMC free article] [PubMed] [Google Scholar]
- 46.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radical Biology and Medicine. 2002;33:1451–64. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 47.Hühmer AF, Nishida CR, Ortiz de Montellano PR, Schöneich C. Inactivation of the inducible nitric oxide synthase by peroxynitrite. Chem Res Toxicol. 1997;10:618–26. doi: 10.1021/tx960188t. [DOI] [PubMed] [Google Scholar]
- 48.O’Donnell VB, Freeman BA. Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circ Res. 2001;88:12–21. doi: 10.1161/01.res.88.1.12. [DOI] [PubMed] [Google Scholar]
- 49.O’Donnell VB, Taylor KB, Parthasarathy S, Kühn H, Koesling D, Friebe A, Bloodsworth A, Darley-Usmar VM, Freeman BA. 15-Lipoxygenase catalytically consumes nitric oxide and impairs activation of guanylate cyclase. J Biol Chem. 1999;274:20083–91. doi: 10.1074/jbc.274.29.20083. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi K, Noguchi N, Niki E. Action of nitric oxide as an antioxidant against oxidation of soybean phosphatidylcholine liposomal membranes. FEBS Lett. 1995;370:37–40. doi: 10.1016/0014-5793(95)00786-9. [DOI] [PubMed] [Google Scholar]
- 51.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: the cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–7. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 52.Patel RP, Diczfalusy U, Dzeletovic S, Wilson MT, Darley-Usmar VM. Formation of oxysterols during oxidation of low density lipoprotein by peroxynitrite, myoglobin, and copper. J Lipid Res. 1996;37:2361–71. [PubMed] [Google Scholar]
- 53.Baker CS, Hall RJ, Evans TJ, Pomerance A, Maclouf J, Creminon C, Yacoub MH, Polak JM. Cyclooxygenase-2 is widely expressed in atherosclerotic lesions affecting native and transplanted human coronary arteries and colocalizes with inducible nitric oxide synthase and nitrotyrosine particularly in macrophages. Arterioscler Thromb Vasc Biol. 1999;19:646–55. doi: 10.1161/01.atv.19.3.646. [DOI] [PubMed] [Google Scholar]
- 54.Schönbeck U, Sukhova GK, Graber P, Coulter S, Libby P. Augmented expression of cyclooxygenase-2 in human atherosclerotic lesions. Am J Pathol. 1999;155:1281–91. doi: 10.1016/S0002-9440(10)65230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 1996;93:15069–74. doi: 10.1073/pnas.93.26.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Swinney DC, Mak AY, Barnett J, Ramesha CS. Differential allosteric regulation of prostaglandin H synthase 1 and 2 by arachidonic acid. J Biol Chem. 1997;272:12393–8. doi: 10.1074/jbc.272.19.12393. [DOI] [PubMed] [Google Scholar]
- 57.Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric Oxide Activates Cyclooxygenase Enzymes. Proceedings of the National Academy of Sciences. 1993;90:7240–4. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salvemini D, Settle SL, Masferrer JL, Seibert K, Currie MG, Needleman P. Regulation of prostaglandin production by nitric oxide; an in vivo analysis. British Journal of Pharmacology. 1995;114:1171. doi: 10.1111/j.1476-5381.1995.tb13330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsai AL, Wei C, Kulmacz RJ. Interaction between nitric oxide and prostaglandin H synthase. Arch Biochem Biophys. 1994;313:367–72. doi: 10.1006/abbi.1994.1400. [DOI] [PubMed] [Google Scholar]
- 60.Upmacis RK, Deeb RS, Hajjar DP. Regulation of prostaglandin H2 synthase activity by nitrogen oxides. Biochemistry. 1999;38:12505–13. doi: 10.1021/bi983049e. [DOI] [PubMed] [Google Scholar]
- 61.Shimokawa T, Kulmacz RJ, DeWitt DL, Smith WL. Tyrosine 385 of prostaglandin endoperoxide synthase is required for cyclooxygenase catalysis. J Biol Chem. 1990;265:20073–6. [PubMed] [Google Scholar]
- 62.Schildknecht S, van der Loo B, Weber K, Tiefenthaler K, Daiber A, Bachschmid MM. Endogenous peroxynitrite modulates PGHS-1-dependent thromboxane A2 formation and aggregation in human platelets. Free Radic Biol Med. 2008;45:512–20. doi: 10.1016/j.freeradbiomed.2008.04.042. [DOI] [PubMed] [Google Scholar]
- 63.Deeb RS, Resnick MJ, Mittar D, McCaffrey T, Hajjar DP, Upmacis RK. Tyrosine nitration in prostaglandin H2 synthase. The Journal of Lipid Research. 2002;43:1718–26. doi: 10.1194/jlr.m200199-jlr200. [DOI] [PubMed] [Google Scholar]
- 64.Deeb RS, Shen H, Gamss C, Gavrilova T, Summers BD, Kraemer R, Hao G, Gross SS, Lainé M, Maeda N, Hajjar DP, Upmacis RK. Inducible nitric oxide synthase mediates prostaglandin h2 synthase nitration and suppresses eicosanoid production. Am J Pathol. 2006;168:349–62. doi: 10.2353/ajpath.2006.050090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bachschmid M, Schildknecht S, Ullrich V. Redox regulation of vascular prostanoid synthesis by the nitric oxide-superoxide system. Biochem Biophys Res Commun. 2005;338:536–42. doi: 10.1016/j.bbrc.2005.08.157. [DOI] [PubMed] [Google Scholar]
- 66.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–70. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 67.Schildknecht S, Heinz K, Daiber A, Hamacher J, Kavakli C, Ullrich V, Bachschmid M. Autocatalytic tyrosine nitration of prostaglandin endoperoxide synthase-2 in LPS-stimulated RAW 264.7 macrophages. Biochem Biophys Res Commun. 2006;340:318–25. doi: 10.1016/j.bbrc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 68.von Knethen A, Brüne B. Cyclooxygenase-2: an essential regulator of NO-mediated apoptosis. FASEB J. 1997;11:887–95. [PubMed] [Google Scholar]
- 69.Notoya K, Jovanovic DV, Reboul P, Martel-Pelletier J, Mineau F, Pelletier JP. The induction of cell death in human osteoarthritis chondrocytes by nitric oxide is related to the production of prostaglandin E2 via the induction of cyclooxygenase-2. J Immunol. 2000;165:3402–10. doi: 10.4049/jimmunol.165.6.3402. [DOI] [PubMed] [Google Scholar]
- 70.Cheng HF, Zhang MZ, Harris RC. Nitric oxide stimulates cyclooxygenase-2 in cultured cTAL cells through a p38-dependent pathway. Am J Physiol Renal Physiol. 2006;290:F1391–7. doi: 10.1152/ajprenal.00315.2005. [DOI] [PubMed] [Google Scholar]
- 71.Gunther MR, Hsi LC, Curtis JF, Gierse JK, Marnett LJ, Eling TE, Mason RP. Nitric oxide trapping of the tyrosyl radical of prostaglandin H synthase-2 leads to tyrosine iminoxyl radical and nitrotyrosine formation. J Biol Chem. 1997;272:17086–90. doi: 10.1074/jbc.272.27.17086. [DOI] [PubMed] [Google Scholar]
- 72.Clancy R, Varenika B, Huang W, Ballou L, Attur M, Amin AR, Abramson SB. Nitric oxide synthase/COX cross-talk: nitric oxide activates COX-1 but inhibits COX-2-derived prostaglandin production. J Immunol. 2000;165:1582–7. doi: 10.4049/jimmunol.165.3.1582. [DOI] [PubMed] [Google Scholar]
- 73.Davidge ST, Baker PN, Laughlin MK, Roberts JM. Nitric oxide produced by endothelial cells increases production of eicosanoids through activation of prostaglandin H synthase. Circ Res. 1995;77:274–83. doi: 10.1161/01.res.77.2.274. [DOI] [PubMed] [Google Scholar]
- 74.Zou MH, Ullrich V. Peroxynitrite formed by simultaneous generation of nitric oxide and superoxide selectively inhibits bovine aortic prostacyclin synthase. FEBS Lett. 1996;382:101–4. doi: 10.1016/0014-5793(96)00160-3. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt P, Youhnovski N, Daiber A, Balan A, Arsic M, Bachschmid M, Przybylski M, Ullrich V. Specific nitration at tyrosine 430 revealed by high resolution mass spectrometry as basis for redox regulation of bovine prostacyclin synthase. J Biol Chem. 2003;278:12813–9. doi: 10.1074/jbc.M208080200. [DOI] [PubMed] [Google Scholar]
- 76.Cosentino F, Eto M, De Paolis P, van der Loo B, Bachschmid M, Ullrich V, Kouroedov A, Delli Gatti C, Joch H, Volpe M, et al. High Glucose Causes Upregulation of Cyclooxygenase-s and Alters Prostanoid Profile n Human Endothelial Cells: Role of Protein Kinase C and Reactive Oxygen Species. Circulation. 2003;107:1017–23. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- 77.Zou MH, Bachschmid M. Hypoxia-reoxygenation triggers coronary vasospasm in isolated bovine coronary arteries via tyrosine nitration of prostacyclin synthase. J Exp Med. 1999;190:135–9. doi: 10.1084/jem.190.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hink U, Oelze M, Kolb P, Bachschmid M, Zou MH, Daiber A, Mollnau H, August M, Baldus S, Tsilimingas N, et al. Role for peroxynitrite in the inhibition of prostacyclin synthase in nitrate tolerance. Journal of the American College of Cardiology. 2003;42:1826–34. doi: 10.1016/j.jacc.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Bachschmid M, Thurau S, Zou MH, Ullrich V. Endothelial cell activation by endotoxin involves superoxide/NO-mediated nitration of prostacyclin synthase and thromboxane receptor stimulation. FASEB J. 2003;17:914–6. doi: 10.1096/fj.02-0530fje. [DOI] [PubMed] [Google Scholar]
- 80.Zou MH, Leist M, Ullrich V. Selective nitration of prostacyclin synthase and defective vasorelaxation in atherosclerotic bovine coronary arteries. Am J Pathol. 1999;154:1359–65. doi: 10.1016/S0002-9440(10)65390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mais DE, Saussy DL, Chaikhouni A, Kochel PJ, Knapp DR, Hamanaka N, Halushka PV. Pharmacologic characterization of human and canine thromboxane A2/prostaglandin H2 receptors in platelets and blood vessels: evidence for different receptors. Journal of Pharmacology and Experimental Therapeutics. 1985;233:418–24. [PubMed] [Google Scholar]
- 82.Schildknecht S, Ullrich V. Peroxynitrite as regulator of vascular prostanoid synthesis. Arch Biochem Biophys. 2009;484:183–9. doi: 10.1016/j.abb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 83.Kühn H, O’Donnell VB. Inflammation and immune regulation by 12/15-lipoxygenases. Prog Lipid Res. 2006;45:334–56. doi: 10.1016/j.plipres.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Osher E, Weisinger G, Limor R, Tordjman K, Stern N. The 5 lipoxygenase system in the vasculature: emerging role in health and disease. Mol Cell Endocrinol. 2006;252:201–6. doi: 10.1016/j.mce.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 85.Noguchi N, Yamashita H, Hamahara J, Nakamura A, Kühn H, Niki E. The specificity of lipoxygenase-catalyzed lipid peroxidation and the effects of radical-scavenging antioxidants. Biol Chem. 2002;383:619–26. doi: 10.1515/BC.2002.064. [DOI] [PubMed] [Google Scholar]
- 86.Kühn H, Schewe T, Rapoport SM. The stereochemistry of the reactions of lipoxygenases and their metabolites. Proposed nomenclature of lipoxygenases and related enzymes. Adv Enzymol Relat Areas Mol Biol. 1986;58:273–311. doi: 10.1002/9780470123041.ch7. [DOI] [PubMed] [Google Scholar]
- 87.Kuhn H. Structural basis for the positional specificity of lipoxygenases. Prostaglandins Other Lipid Mediat. 2000;62:255–70. doi: 10.1016/s0090-6980(00)00084-8. [DOI] [PubMed] [Google Scholar]
- 88.Gardner HW. Soybean lipoxygenase-1 enzymically forms both (9S)- and (13S)-hydroperoxides from linoleic acid by a pH-dependent mechanism. Biochim Biophys Acta. 1989;1001:274–81. doi: 10.1016/0005-2760(89)90111-2. [DOI] [PubMed] [Google Scholar]
- 89.Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci U S A. 1997;94:6148–52. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mehrabian M, Allayee H, Wong J, Shih W, Wang XP, Shaposhnik Z, Funk CD, Lusis AJ. Circ Res. 2002;91:120–6. doi: 10.1161/01.res.0000028008.99774.7f. [DOI] [PubMed] [Google Scholar]
- 91.Spanbroek R, Hildner M, Köhler A, Müller A, Zintl F, Kühn H, Rådmark O, Samuelsson B, Habenicht AJ. IL-4 determines eicosanoid formation in dendritic cells by down-regulation of 5-lipoxygenase and up-regulation of 15-lipoxygenase 1 expression. Proc Natl Acad Sci U S A. 2001;98:5152–7. doi: 10.1073/pnas.091076998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Freeman A, Showell HJ. Leukotriene B4 receptor antagonism reduces monocytic foam cells in mice. Arterioscler Thromb Vasc Biol. 2002;22:443–9. doi: 10.1161/hq0302.105593. [DOI] [PubMed] [Google Scholar]
- 93.Berkowitz BA, Zabko-Potapovich B, Valocik R, Gleason JG. Effects of the leukotrienes on the vasculature and blood pressure of different species. J Pharmacol Exp Ther. 1984;229:105–12. [PubMed] [Google Scholar]
- 94.Allen SP, Chester AH, Dashwood MR, Tadjkarimi S, Piper PJ, Yacoub MH. Preferential vasoconstriction to cysteinyl leukotrienes in the human saphenous vein compared with the internal mammary artery. Implications for graft performance. Circulation. 1994;90:515–24. doi: 10.1161/01.cir.90.1.515. [DOI] [PubMed] [Google Scholar]
- 95.Munger KA, Montero A, Fukunaga M, Uda S, Yura T, Imai E, Kaneda Y, Valdivielso JM, Badr KF. Transfection of rat kidney with human 15-lipoxygenase suppresses inflammation and preserves function in experimental glomerulonephritis. Proc Natl Acad Sci U S A. 1999;96:13375–80. doi: 10.1073/pnas.96.23.13375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–78. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 97.Kühn H, Belkner J, Zaiss S, Fährenklemper T, Wohlfeil S. Involvement of 15-lipoxygenase in early stages of atherogenesis. J Exp Med. 1994;179:1903–11. doi: 10.1084/jem.179.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cyrus T, Witztum JL, Rader DJ, Tangirala R, Fazio S, Linton MF, Funk CD. Disruption of the 12/15-lipoxygenase gene diminishes atherosclerosis in apo E-deficient mice. J Clin Invest. 1999;103:1597–604. doi: 10.1172/JCI5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Harats D, Shaish A, George J, Mulkins M, Kurihara H, Levkovitz H, Sigal E. Overexpression of 15-lipoxygenase in vascular endothelium accelerates early atherosclerosis in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:2100–5. doi: 10.1161/01.atv.20.9.2100. [DOI] [PubMed] [Google Scholar]
- 100.Shen J, Kühn H, Petho-Schramm A, Chan L. Transgenic rabbits with the integrated human 15-lipoxygenase gene driven by a lysozyme promoter: macrophage-specific expression and variable positional specificity of the transgenic enzyme. FASEB J. 1995;9:1623–31. doi: 10.1096/fasebj.9.15.8529842. [DOI] [PubMed] [Google Scholar]
- 101.Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim HS, Kühn H, Guevara NV, Chan L. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–8. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rubbo H, Parthasarathy S, Barnes S, Kirk M, Kalyanaraman B, Freeman BA. Nitric oxide inhibition of lipoxygenase-dependent liposome and low-density lipoprotein oxidation: termination of radical chain propagation reactions and formation of nitrogen-containing oxidized lipid derivatives. Arch Biochem Biophys. 1995;324:15–25. doi: 10.1006/abbi.1995.9935. [DOI] [PubMed] [Google Scholar]
- 103.Nakatsuka M, Osawa Y. Selective inhibition of the 12-lipoxygenase pathway of arachidonic acid metabolism by L-arginine or sodium nitroprusside in intact human platelets. Biochem Biophys Res Commun. 1994;200:1630–4. doi: 10.1006/bbrc.1994.1638. [DOI] [PubMed] [Google Scholar]
- 104.Nelson MJ. The nitric oxide complex of ferrous soybean lipoxygenase-1. Substrate, pH, and ethanol effects on the active-site iron. J Biol Chem. 1987;262:12137–42. [PubMed] [Google Scholar]
- 105.Coffey M, Phare S, Peters-Golden M. Induction of inducible nitric oxide synthase by lipopolysaccharide/interferon gamma and sepsis down-regulates 5-lipoxygenase metabolism in murine alveolar macrophages. Exp Lung Res. 2004;30:615–33. doi: 10.1080/01902140490476391. [DOI] [PubMed] [Google Scholar]
- 106.Wiesner R, Rathmann J, Holzhütter HG, Stösser R, Mäder K, Nolting H, Kühn H. Nitric oxide oxidises a ferrous mammalian lipoxygenase to a pre-activated ferric species. FEBS Lett. 1996;389:229–32. doi: 10.1016/0014-5793(96)00591-1. [DOI] [PubMed] [Google Scholar]
- 107.Coffey MJ, Phare SM, Luo M, Peters-Golden M. Guanylyl cyclase and protein kinase G mediate nitric oxide suppression of 5-lipoxygenase metabolism in rat alveolar macrophages. Biochim Biophys Acta. 2008;1781:299–305. doi: 10.1016/j.bbalip.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 108.Coffey MJ, Phare SM, Peters-Golden M. Interaction between nitric oxide, reactive oxygen intermediates, and peroxynitrite in the regulation of 5-lipoxygenase metabolism. Biochim Biophys Acta. 2002;1584:81–90. doi: 10.1016/s1388-1981(02)00286-x. [DOI] [PubMed] [Google Scholar]
- 109.Scarborough PE, Ma J, Qu W, Zeldin DC. P450 subfamily CYP2J and their role in the bioactivation of arachnidonic acid in extrahepatic tissues. Drug Metab Rev. 1999;31:205–34. doi: 10.1081/dmr-100101915. [DOI] [PubMed] [Google Scholar]
- 110.Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–7. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- 111.Imig JD, Falck JR, Wei S, Capdevila JH. Epoxygenase metabolites contribute to nitric oxide-independent afferent arteriolar vasodilation in response to bradykinin. J Vasc Res. 2001;38:247–55. doi: 10.1159/000051053. [DOI] [PubMed] [Google Scholar]
- 112.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory Properties of Cytochrome P450 Epoxygenase-Derived Eicosanoids. Science. 1999;285:1276. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oyekan AO, McGiff JC. Functional response of the rat kidney to inhibition of nitric oxide synthesis: role of cytochrome p450-derived arachidonate metabolites. Br J Pharmacol. 1998;125:1065–73. doi: 10.1038/sj.bjp.0702171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Croft KD, McGiff JC, Sanchez-Mendoza A, Carroll MA. Angiotensin II releases 20-HETE from rat renal microvessels. Am J Physiol Renal Physiol. 2000;279:F544–51. doi: 10.1152/ajprenal.2000.279.3.F544. [DOI] [PubMed] [Google Scholar]
- 115.Eum HA, Yeom DH, Lee SM. Role of nitric oxide in the inhibition of liver cytochrome P450 during sepsis. Nitric Oxide. 2006;15:423–31. doi: 10.1016/j.niox.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 116.Kim YM, Bergonia HA, Müller C, Pitt BR, Watkins WD, Lancaster JR. Loss and degradation of enzyme-bound heme induced by cellular nitric oxide synthesis. J Biol Chem. 1995;270:5710–3. doi: 10.1074/jbc.270.11.5710. [DOI] [PubMed] [Google Scholar]
- 117.Wink DA, Osawa Y, Darbyshire JF, Jones CR, Eshenaur SC, Nims RW. Inhibition of cytochromes P450 by nitric oxide and a nitric oxide-releasing agent. Arch Biochem Biophys. 1993;300:115–23. doi: 10.1006/abbi.1993.1016. [DOI] [PubMed] [Google Scholar]
- 118.Takemura S, Minamiyama Y, Imaoka S, Funae Y, Hirohashi K, Inoue M, Kinoshita H. Hepatic cytochrome P450 is directly inactivated by nitric oxide, not by inflammatory cytokines, in the early phase of endotoxemia. Journal of Hepatoiogy. 1999;30:1035–44. doi: 10.1016/s0168-8278(99)80257-8. [DOI] [PubMed] [Google Scholar]
- 119.Zou MH, Daiber A, Peterson JA, Shoun H, Ullrich V. Rapid reactions of peroxynitrite with heme-thiolate proteins as the basis for protection of prostacyclin synthase from inactivation by nitration. Arch Biochem Biophys. 2000;376:149–55. doi: 10.1006/abbi.2000.1699. [DOI] [PubMed] [Google Scholar]
- 120.Roberts ES, Lin H, Crowley JR, Vuletich JL, Osawa Y, Hollenberg PF. Peroxynitrite-mediated nitration of tyrosine and inactivation of the catalytic activity of cytochrome P450 2B1. Chem Res Toxicol. 1998;11:1067–74. doi: 10.1021/tx980099b. [DOI] [PubMed] [Google Scholar]
- 121.Lee CM, Kim BY, Li L, Morgan ET. Nitric oxide-dependent proteasomal degradation of cytochrome P450 2B proteins. J Biol Chem. 2008;283:889–98. doi: 10.1074/jbc.M708821200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aitken AE, Lee CM, Morgan ET. Roles of nitric oxide in inflammatory downregulation of human cytochromes P450. Free Radic Biol Med. 2008;44:1161–8. doi: 10.1016/j.freeradbiomed.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alonso-Galicia M, Drummond HA, Reddy KK, Falck JR, Roman RJ. Inhibition of 20-HETE production contributes to the vascular responses to nitric oxide. Hypertension. 1997;29:320–5. doi: 10.1161/01.hyp.29.1.320. [DOI] [PubMed] [Google Scholar]
- 124.Fleming I, Michaelis UR, Bredenkötter D, Fisslthaler B, Dehghani F, Brandes RP, Busse R. Endothelium-derived hyperpolarizing factor synthase (Cytochrome P450 2C9) is a functionally significant source of reactive oxygen species in coronary arteries. Circ Res. 2001;88:44–51. doi: 10.1161/01.res.88.1.44. [DOI] [PubMed] [Google Scholar]
- 125.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–7. doi: 10.1161/01.cir.94.12.3341. [DOI] [PubMed] [Google Scholar]
- 126.López B, Moreno C, Salom MG, Roman RJ, Fenoy FJ. Role of guanylyl cyclase and cytochrome P-450 on renal response to nitric oxide. Am J Physiol Renal Physiol. 2001;281:F420–7. doi: 10.1152/ajprenal.2001.281.3.F420. [DOI] [PubMed] [Google Scholar]
- 127.Fischer D, Landmesser U, Spiekermann S, Hilfiker-Kleiner D, Hospely M, Müller M, Busse R, Fleming I, Drexler H. Cytochrome P450 2C9 is involved in flow-dependent vasodilation of peripheral conduit arteries in healthy subjects and in patients with chronic heart failure. Eur J Heart Fail. 2007;9:770–5. doi: 10.1016/j.ejheart.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 128.Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome P-450 2C9 signaling does not contribute to age-associated vascular endothelial dysfunction in humans. J Appl Physiol. 2008;105:1359–63. doi: 10.1152/japplphysiol.90629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Graier WF, Simecek S, Sturek M. Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. J Physiol. 1995;482 (Pt 2):259–74. doi: 10.1113/jphysiol.1995.sp020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA, Schwartzman ML. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2008;294:H1018–26. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- 131.Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–75. [PubMed] [Google Scholar]
- 132.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–50. [PubMed] [Google Scholar]
- 133.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87:9383–7. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marnett LJ, Wright TL, Crews BC, Tannenbaum SR, Morrow JD. Regulation of prostaglandin biosynthesis by nitric oxide is revealed by targeted deletion of inducible nitric-oxide synthase. J Biol Chem. 2000;275:13427–30. doi: 10.1074/jbc.275.18.13427. [DOI] [PubMed] [Google Scholar]
- 135.Ramsey KH, Sigar IM, Rana SV, Gupta J, Holland SM, Byrne GI, Morrow JD. Inducible Nitric Oxide Synthase Regulates Production of Isoprostanes In Vivo during Chlamydial Genital Infection in Mice. Infect Immun. 2003;71:7183–7. doi: 10.1128/IAI.71.12.7183-7187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moore KP, Darley-Usmar V, Morrow J, Roberts LJ. Formation of F2-isoprostanes during oxidation of human low-density lipoprotein and plasma by peroxynitrite. Circ Res. 1995;77:335–41. doi: 10.1161/01.res.77.2.335. [DOI] [PubMed] [Google Scholar]
- 137.Laskey RE, Mathews WR. Nitric oxide inhibits peroxynitrite-induced production of hydroxyeicosatetraenoic acids and F2-isoprostanes in phosphatidylcholine liposomes. Arch Biochem Biophys. 1996;330:193–8. doi: 10.1006/abbi.1996.0242. [DOI] [PubMed] [Google Scholar]
- 138.O’Byrne S, Forte P, II LJR, Morrow JD, Johnston A, Ängg\aard E, Leslie RDG, Benjamin N. Nitric Oxide Synthesis and Isoprostane Production in Subjects With Type 1 Diabetes and Normal Urinary Albumin Excretion. Diabetes. 2000;49:857. doi: 10.2337/diabetes.49.5.857. [DOI] [PubMed] [Google Scholar]
- 139.Montuschi P, Barnes P, Roberts LJ. Insights into oxidative stress: the isoprostanes. Curr Med Chem. 2007;14:703–17. doi: 10.2174/092986707780059607. [DOI] [PubMed] [Google Scholar]
- 140.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ. Increase in circulating products of lipid peroxidation (F 2-isoprostanes) in smokers. N Engl J Med. 1995;332:1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 141.Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brümmer J, Gutzki FM, Berger J, Frölich JC, Böger RH. Urinary 8-iso-prostaglandin F2alpha as a risk marker in patients with coronary heart disease: a matched case-control study. Circulation. 2004;109:843–8. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 142.Roberts LJ, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, Shyr Y, Morrow JD. The relationship between dose of vitamin E and suppression of oxidative stress in humans. Free Radic Biol Med. 2007;43:1388–93. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Praticò D, Lawson JA, Rokach J, FitzGerald GA. The isoprostanes in biology and medicine. Trends Endocrinol Metab. 2001;12:243–7. doi: 10.1016/s1043-2760(01)00411-8. [DOI] [PubMed] [Google Scholar]
- 144.Takahashi K, Nammour TM, Fukunaga M, Ebert J, Morrow JD, Roberts LJ, Hoover RL, Badr KF. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2 alpha, in the rat. Evidence for interaction with thromboxane A2 receptors. J Clin Invest. 1992;90:136–41. doi: 10.1172/JCI115826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kromer BM, Tippins JR. Coronary artery constriction by the isoprostane 8-epi prostaglandin F2 alpha. Br J Pharmacol. 1996;119:1276–80. doi: 10.1111/j.1476-5381.1996.tb16033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hou X, Gobeil F, Peri K, Speranza G, Marrache AM, Lachapelle P, Roberts J, Varma DR, Chemtob S, Ellis EF. Augmented vasoconstriction and thromboxane formation by 15-F(2t)-isoprostane (8-iso-prostaglandin F(2alpha)) in immature pig periventricular brain microvessels. Stroke. 2000;31:516–24. doi: 10.1161/01.str.31.2.516. discussion 525. [DOI] [PubMed] [Google Scholar]
- 147.Talati M, Meyrick B, Peebles RS, Davies SS, Dworski R, Mernaugh R, Mitchell D, Boothby M, Roberts LJ, Sheller JR. Oxidant stress modulates murine allergic airway responses. Free Radic Biol Med. 2006;40:1210–9. doi: 10.1016/j.freeradbiomed.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 148.Janssen LJ, Premji M, Netherton S, Catalli A, Cox G, Keshavjee S, Crankshaw DJ. Excitatory and inhibitory actions of isoprostanes in human and canine airway smooth muscle. J Pharmacol Exp Ther. 2000;295:506–11. [PubMed] [Google Scholar]
- 149.Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- 150.Patrono C, FitzGerald GA. Isoprostanes: potential markers of oxidant stress in atherothrombotic disease. Arterioscler Thromb Vasc Biol. 1997;17:2309–15. doi: 10.1161/01.atv.17.11.2309. [DOI] [PubMed] [Google Scholar]
- 151.Yura T, Fukunaga M, Khan R, Nassar GN, Badr KF, Montero A. Free-radical-generated F2-isoprostane stimulates cell proliferation and endothelin-1 expression on endothelial cells. Kidney Int. 1999;56:471–8. doi: 10.1046/j.1523-1755.1999.00596.x. [DOI] [PubMed] [Google Scholar]
- 152.Lynch SM, Morrow JD, Roberts LJ, Frei B. Formation of non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in plasma and low density lipoprotein exposed to oxidative stress in vitro. J Clin Invest. 1994;93:998–1004. doi: 10.1172/JCI117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tang M, Cyrus T, Yao Y, Vocun L, Praticò D. Involvement of thromboxane receptor in the proatherogenic effect of isoprostane F2alpha-III: evidence from apolipoprotein E-and LDL receptor-deficient mice. Circulation. 2005;112:2867–74. doi: 10.1161/CIRCULATIONAHA105.562223. [DOI] [PubMed] [Google Scholar]
- 154.Audoly LP, Rocca B, Fabre JE, Koller BH, Thomas D, Loeb AL, Coffman TM, FitzGerald GA. Cardiovascular responses to the isoprostanes iPF(2alpha)-III and iPE(2)-III are mediated via the thromboxane A(2) receptor in vivo. Circulation. 2000;101:2833–40. doi: 10.1161/01.cir.101.24.2833. [DOI] [PubMed] [Google Scholar]
- 155.Badr KF, Abi-Antoun TE. Isoprostanes and the Kidney. Antioxidants & Redox Signaling. 2005;7:236–43. doi: 10.1089/ars.2005.7.236. [DOI] [PubMed] [Google Scholar]
- 156.Morrow JD, Minton TA, Roberts LJ. The F2-isoprostane, 8-epi-prostaglandin F2 alpha, a potent agonist of the vascular thromboxane/endoperoxide receptor, is a platelet thromboxane/endoperoxide receptor antagonist. Prostaglandins. 1992;44:155–63. doi: 10.1016/0090-6980(92)90077-7. [DOI] [PubMed] [Google Scholar]
- 157.McNamara P, Lawson JA, Rokach J, FitzGerald GA. Isoprostane activation of the nuclear hormone receptor PPAR. Adv Exp Med Biol. 2002;507:351–5. doi: 10.1007/978-1-4615-0193-0_54. [DOI] [PubMed] [Google Scholar]
- 158.Fukunaga M, Makita N, Roberts LJ, Morrow JD, Takahashi K, Badr KF. Evidence for the existence of F2-isoprostane receptors on rat vascular smooth muscle cells. American Journal of Physiology- Cell Physiology. 1993;264:1619–24. doi: 10.1152/ajpcell.1993.264.6.C1619. [DOI] [PubMed] [Google Scholar]
- 159.Chen Y, Morrow JD, Roberts LJ. Formation of reactive cyclopentenone compounds in vivo as products of the isoprostane pathway. J Biol Chem. 1999;274:10863–8. doi: 10.1074/jbc.274.16.10863. [DOI] [PubMed] [Google Scholar]
- 160.Musiek ES, Gao L, Milne GL, Han W, Everhart MB, Wang D, Backlund MG, DuBois RN, Zanoni G, Vidari G, Blackwell TS, Morrow JD. Cyclopentenone isoprostanes inhibit the inflammatory response in macrophages. J Biol Chem. 2005;280:35562–70. doi: 10.1074/jbc.M504785200. [DOI] [PubMed] [Google Scholar]
- 161.Chiarpotto E, Domenicotti C, Paola D, Vitali A, Nitti M, Pronzato MA, Biasi F, Cottalasso D, Marinari UM, Dragonetti A, Cesaro P, Isidoro C, Poli G. Regulation of rat hepatocyte protein kinase C beta isoenzymes by the lipid peroxidation product 4-hydroxy-2,3-nonenal: A signaling pathway to modulate vesicular transport of glycoproteins. Hepatology. 1999;29:1565–72. doi: 10.1002/hep.510290510. [DOI] [PubMed] [Google Scholar]
- 162.Paola D, Domenicotti C, Nitti M, Vitali A, Borghi R, Cottalasso D, Zaccheo D, Odetti P, Strocchi P, Marinari UM, Tabaton M, Pronzato MA. Oxidative stress induces increase in intracellular amyloid beta-protein production and selective activation of betaI and betaII PKCs in NT2 cells. Biochem Biophys Res Commun. 2000;268:642–6. doi: 10.1006/bbrc.2000.2164. [DOI] [PubMed] [Google Scholar]
- 163.Parola M, Robino G, Marra F, Pinzani M, Bellomo G, Leonarduzzi G, Chiarugi P, Camandola S, Poli G, Waeg G, Gentilini P, Dianzani MU. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Invest. 1998;102:1942–50. doi: 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Uchida K, Shiraishi M, Naito Y, Torii Y, Nakamura Y, Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J Biol Chem. 1999;274:2234–42. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 165.Vacaresse N, Vieira O, Robbesyn F, Jürgens G, Salvayre R, Negre-Salvayre A. Phenolic antioxidants trolox and caffeic acid modulate the oxidized LDL-induced EGF-receptor activation. Br J Pharmacol. 2001;132:1777–88. doi: 10.1038/sj.bjp.0703981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Koren S, Fantus IG. Inhibition of the protein tyrosine phosphatase PTP1B: potential therapy for obesity, insulin resistance and type-2 diabetes mellitus. Best Pract Res Clin Endocrinol Metab. 2007;21:621–40. doi: 10.1016/j.beem.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 167.Wu RF, Terada LS. Oxidative modification of protein tyrosine phosphatases. Sci STKE 2006. 2006:pl2. doi: 10.1126/stke.3322006pl2. [DOI] [PubMed] [Google Scholar]
- 168.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–5. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Donath B, Fischer C, Page S, Prebeck S, Jilg N, Weber M, da Costa C, Neumeier D, Miethke T, Brand K. Chlamydia pneumoniae activates IKK/I kappa B-mediated signaling, which is inhibited by 4-HNE and following primary exposure. Atherosclerosis. 2002;165:79–88. doi: 10.1016/s0021-9150(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 170.Numazawa S, Ishikawa M, Yoshida A, Tanaka S, Yoshida T. Atypical protein kinase C mediates activation of NF-E2-related factor 2 in response to oxidative stress. Am J Physiol Cell Physiol. 2003;285:C334–42. doi: 10.1152/ajpcell.00043.2003. [DOI] [PubMed] [Google Scholar]
- 171.Cahuana GM, Tejedo JR, Jiménez J, Ramírez R, Sobrino F, Bedoya FJ. Involvement of advanced lipooxidation end products (ALEs) and protein oxidation in the apoptotic actions of nitric oxide in insulin secreting RINm5F cells. Biochem Pharmacol. 2003;66:1963–71. doi: 10.1016/j.bcp.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 172.Cristol JP, Thiemermann C, Guérin MC, Torreilles J, de Paulet AC. L-Arginine infusion after ischaemia-reperfusion of rat kidney enhances lipid peroxidation. J Lipid Mediat Cell Signal. 1996;13:9–17. doi: 10.1016/0929-7855(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 173.Sun Y, Carretero OA, Xu J, Rhaleb NE, Wang F, Lin C, Yang JJ, Pagano PJ, Yang XP. Lack of inducible NO synthase reduces oxidative stress and enhances cardiac response to isoproterenol in mice with deoxycorticosterone acetate-salt hypertension. Hypertension. 2005;46:1355–61. doi: 10.1161/01.HYP.0000192651.06674.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stadler K, Bonini MG, Dallas S, Jiang J, Radi R, Mason RP, Kadiiska MB. Involvement of inducible nitric oxide synthase in hydroxyl radical-mediated lipid peroxidation in streptozotocin-induced diabetes. Free Radic Biol Med. 2008;45:866–74. doi: 10.1016/j.freeradbiomed.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hattori Y, Hattori S, Kasai K. 4-hydroxynonenal prevents NO production in vascular smooth muscle cells by inhibiting nuclear factor-kappaB-dependent transcriptional activation of inducible NO synthase. Arterioscler Thromb Vasc Biol. 2001;21:1179–83. doi: 10.1161/hq0701.092135. [DOI] [PubMed] [Google Scholar]
- 176.Pope AJ, Druhan L, Guzman JE, Forbes SP, Murugesan V, Lu D, Xia Y, Chicoine LG, Parinandi NL, Cardounel AJ. Role of DDAH-1 in lipid peroxidation product-mediated inhibition of endothelial NO generation. Am J Physiol Cell Physiol. 2007;293:C1679–86. doi: 10.1152/ajpcell.00224.2007. [DOI] [PubMed] [Google Scholar]
- 177.Vaillancourt F, Morquette B, Shi Q, Fahmi H, Lavigne P, Di Battista JA, Fernandes JC, Benderdour M. Differential regulation of cyclooxygenase-2 and inducible nitric oxide synthase by 4-hydroxynonenal in human osteoarthritic chondrocytes through ATF-2/CREB-1 transactivation and concomitant inhibition of NF-kappaB signaling cascade. J Cell Biochem. 2007;100:1217–31. doi: 10.1002/jcb.21110. [DOI] [PubMed] [Google Scholar]
- 178.O’Donnell VB, Eiserich JP, Chumley PH, Jablonsky MJ, Krishna NR, Kirk M, Barnes S, Darley-Usmar VM, Freeman BA. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem Res Toxicol. 1999;12:83–92. doi: 10.1021/tx980207u. [DOI] [PubMed] [Google Scholar]
- 179.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d’Ischia M. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–9. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rudolph V, Schopfer FJ, Khoo NK, Rudolph TK, Cole MP, Woodcock SR, Bonacci G, Groeger AL, Golin-Bisello F, Chen CS, Baker PR, Freeman BA. Nitro-fatty acid metabolome: saturation, desaturation, beta-oxidation, and protein adduction. J Biol Chem. 2009;284:1461–73. doi: 10.1074/jbc.M802298200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ferreira AM, Ferrari M, Trostchansky A, Batthyány C, Souza JM, Alvarez MN, López GV, Baker PR, Schopfer FJ, O’Donnell V, Freeman BA, Rubbo H. Macrophage activation induces formation of the anti-inflammatory lipid cholesteryl-nitrolinoleate. Biochem J. 2008;417:223–34. doi: 10.1042/BJ20080701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Schopfer, Batthyany, Baker, Bonacci, Cole, Rudolph, Rudolph, Nadtochiy, Brookes, Freeman Detection and Quantification of Protein Adduction by Electrophilic Fatty Acid Nitration Products: Mitochondrial Generation of Fatty Acid Nitroalkene Derivatives. Free Radic Biol Med. 2009;46:1250–9. doi: 10.1016/j.freeradbiomed.2008.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nadtochiy SM, Baker PR, Freeman BA, Brookes PS. Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc Res. 2009;82:333–40. doi: 10.1093/cvr/cvn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Baker LM, Baker PR, Golin-Bisello F, Schopfer FJ, Fink M, Woodcock SR, Branchaud BP, Radi R, Freeman BA. Nitro-fatty acid reaction with glutathione and cysteine. Kinetic analysis of thiol alkylation by a Michael addition reaction. J Biol Chem. 2007;282:31085–93. doi: 10.1074/jbc.M704085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. J Biol Chem. 2006;281:35686–98. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Villacorta L, Zhang J, Garcia-Barrio MT, Chen XL, Freeman BA, Chen YE, Cui T. Nitro-linoleic acid inhibits vascular smooth muscle cell proliferation via the Keap1/Nrf2 signaling pathway. Am J Physiol Heart Circ Physiol. 2007;293:H770–6. doi: 10.1152/ajpheart.00261.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Schopfer FJ, Lin Y, Baker PR, Cui T, Garcia-Barrio M, Zhang J, Chen K, Chen YE, Freeman BA. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand. Proc Natl Acad Sci U S A. 2005;102:2340–5. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, Sweeney S, Long MH, Iles KE, Baker LM, Branchaud BP, Chen YE, Freeman BA. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–75. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Schopfer FJ, Baker PR, Giles G, Chumley P, Batthyany C, Crawford J, Patel RP, Hogg N, Branchaud BP, Lancaster JR, Freeman BA. Fatty acid transduction of nitric oxide signaling. Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. J Biol Chem. 2005;280:19289–97. doi: 10.1074/jbc.M414689200. [DOI] [PubMed] [Google Scholar]
- 190.Lima ES, Bonini MG, Augusto O, Barbeiro HV, Souza HP, Abdalla DS. Nitrated lipids decompose to nitric oxide and lipid radicals and cause vasorelaxation. Free Radic Biol Med. 2005;39:532–9. doi: 10.1016/j.freeradbiomed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 191.Lim DG, Sweeney S, Bloodsworth A, White CR, Chumley PH, Krishna NR, Schopfer F, O’Donnell VB, Eiserich JP, Freeman BA. Nitrolinoleate, a nitric oxide-derived mediator of cell function: synthesis, characterization, and vasomotor activity. Proc Natl Acad Sci U S A. 2002;99:15941–6. doi: 10.1073/pnas.232409599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Coles B, Bloodsworth A, Clark SR, Lewis MJ, Cross AR, Freeman BA, O’Donnell VB. Nitrolinoleate Inhibits Superoxide Generation, Degranulation, and Integrin Expression by Human Neutrophils: Novel Antiinflammatory Properties of Nitric Oxide-Derived Reactive Species in Vascular Cells. Circ Res. 2002;91:375–81. doi: 10.1161/01.res.0000032114.68919.ef. [DOI] [PubMed] [Google Scholar]
- 193.Coles B, Bloodsworth A, Eiserich JP, Coffey MJ, McLoughlin RM, Giddings JC, Lewis MJ, Haslam RJ, Freeman BA, O’Donnell VB. Nitrolinoleate inhibits platelet activation by attenuating calcium mobilization and inducing phosphorylation of vasodilator-stimulated phosphoprotein through elevation of cAMP. J Biol Chem. 2002;277:5832–40. doi: 10.1074/jbc.M105209200. [DOI] [PubMed] [Google Scholar]
- 194.Wright MM, Schopfer FJ, Baker PR, Vidyasagar V, Powell P, Chumley P, Iles KE, Freeman BA, Agarwal A. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid potently activates endothelial heme oxygenase 1 expression. Proc Natl Acad Sci U S A. 2006;103:4299–304. doi: 10.1073/pnas.0506541103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Iles KE, Wright MM, Cole MP, Welty NE, Ware LB, Matthay MA, Schopfer FJ, Baker PR, Agarwal A, Freeman BA. Fatty acid transduction of nitric oxide signaling: nitrolinoleic acid mediates protective effects through regulation of the ERK pathway. Free Radic Biol Med. 2009;46:866–75. doi: 10.1016/j.freeradbiomed.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]