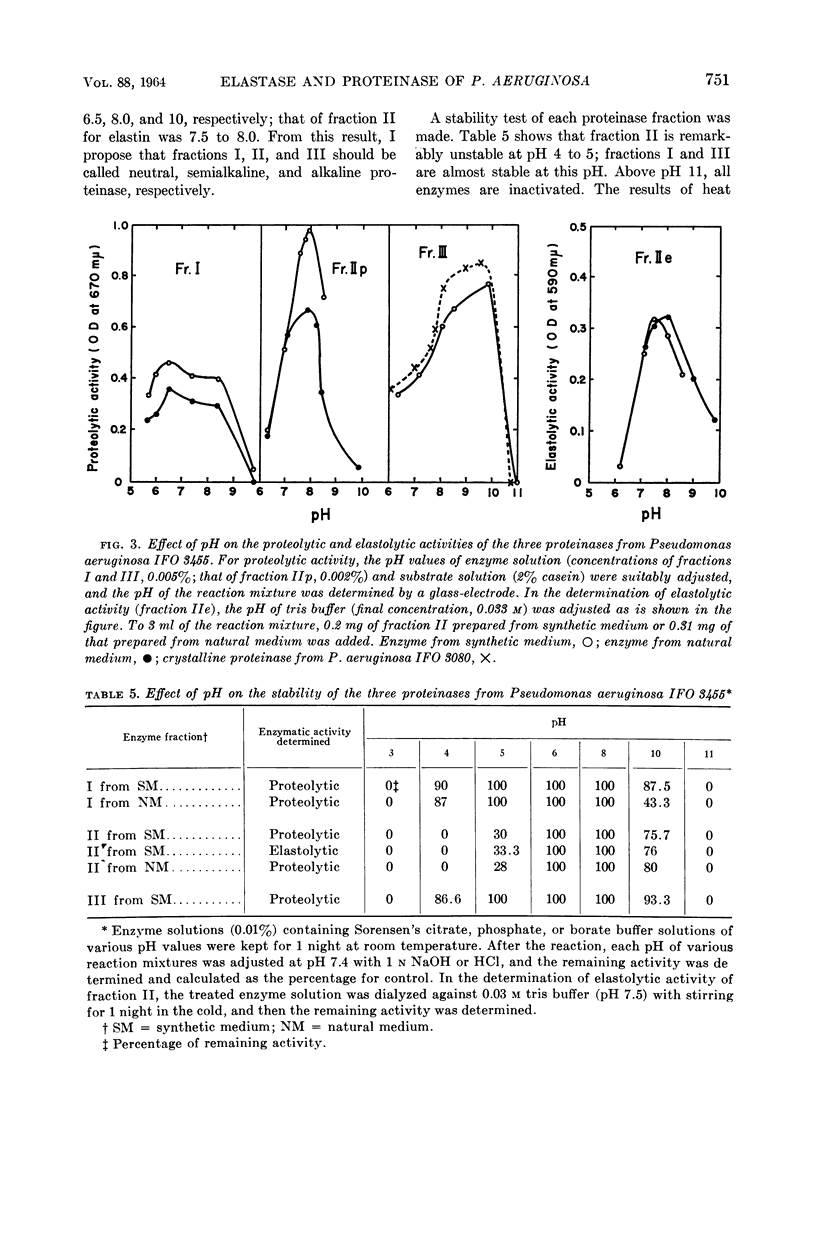
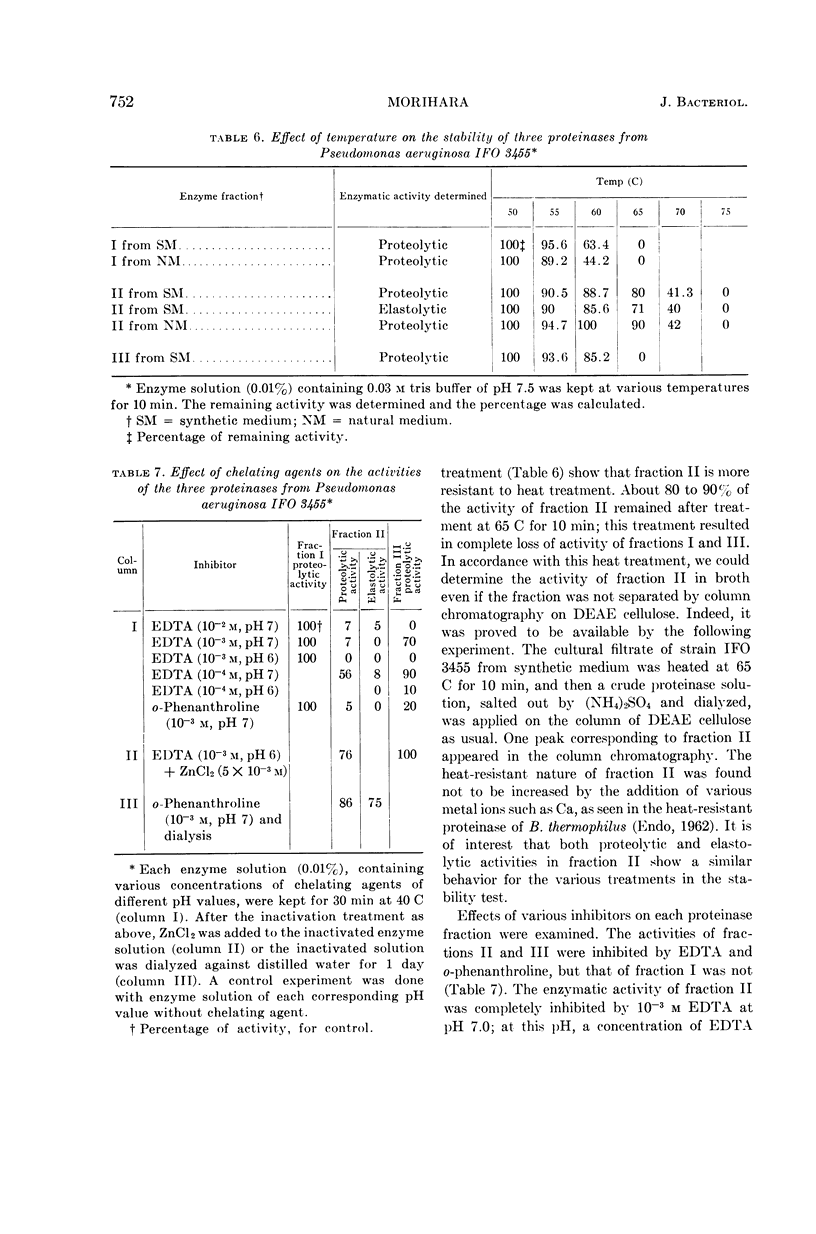
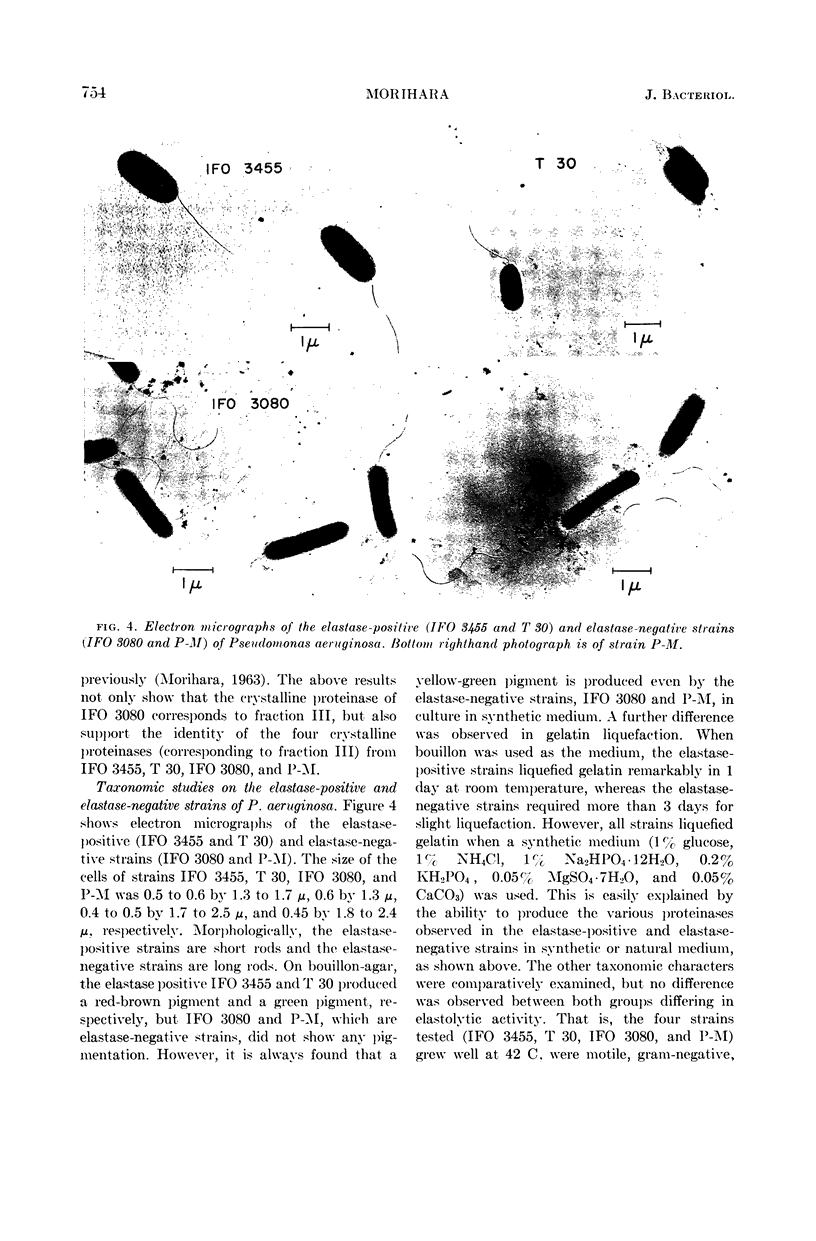
Abstract
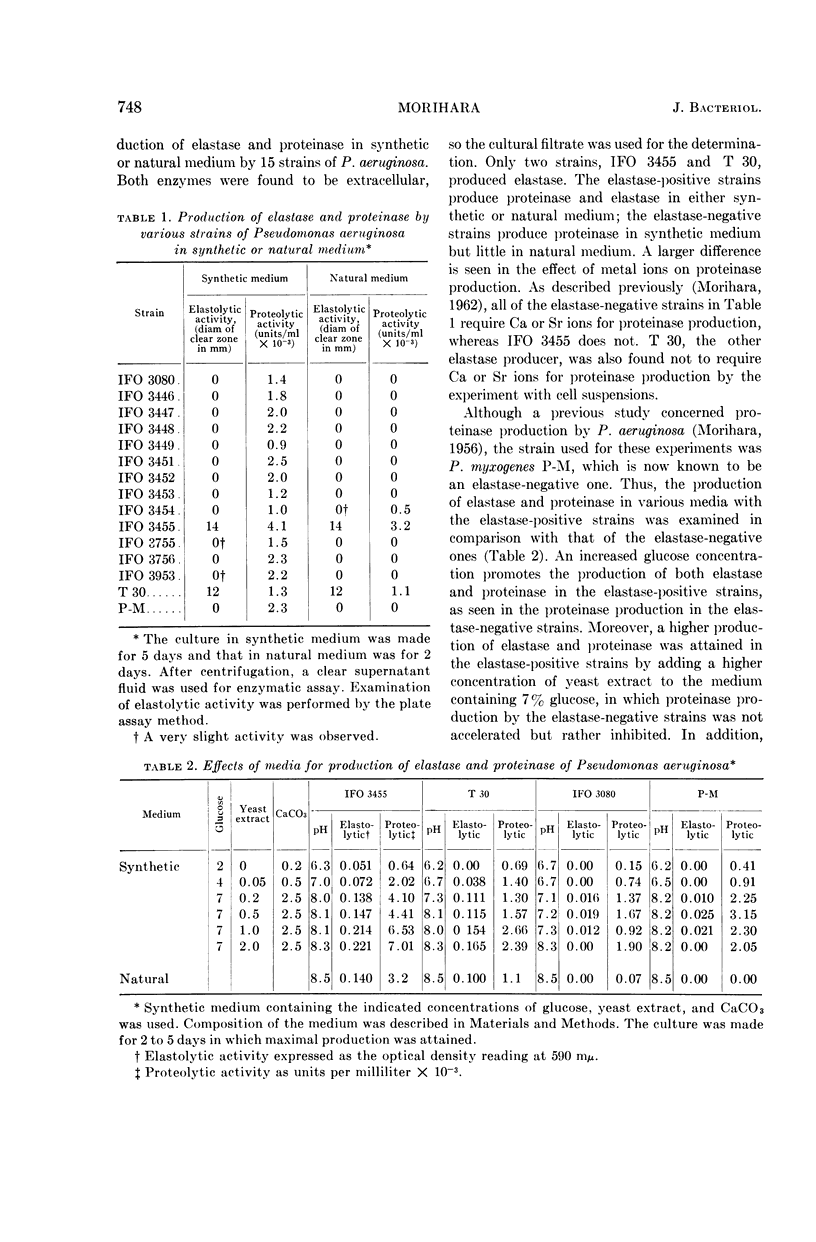
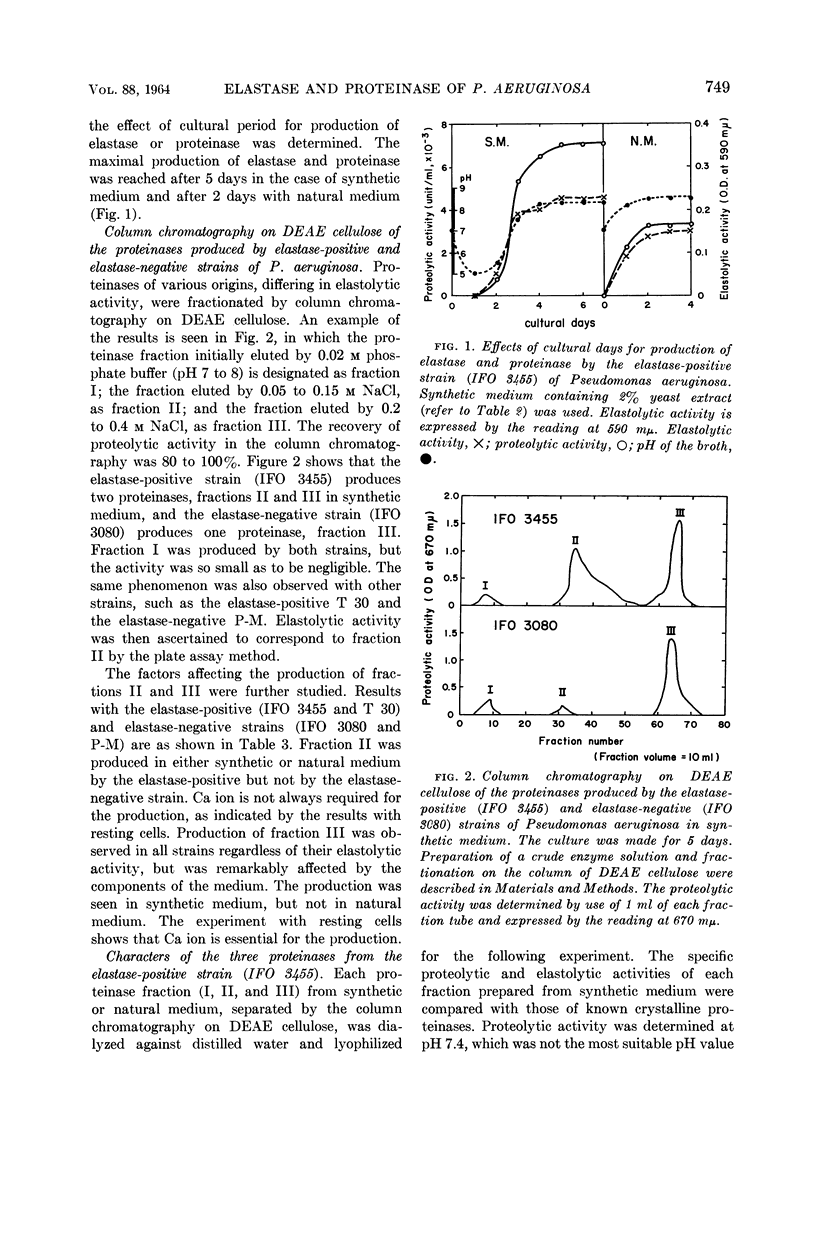
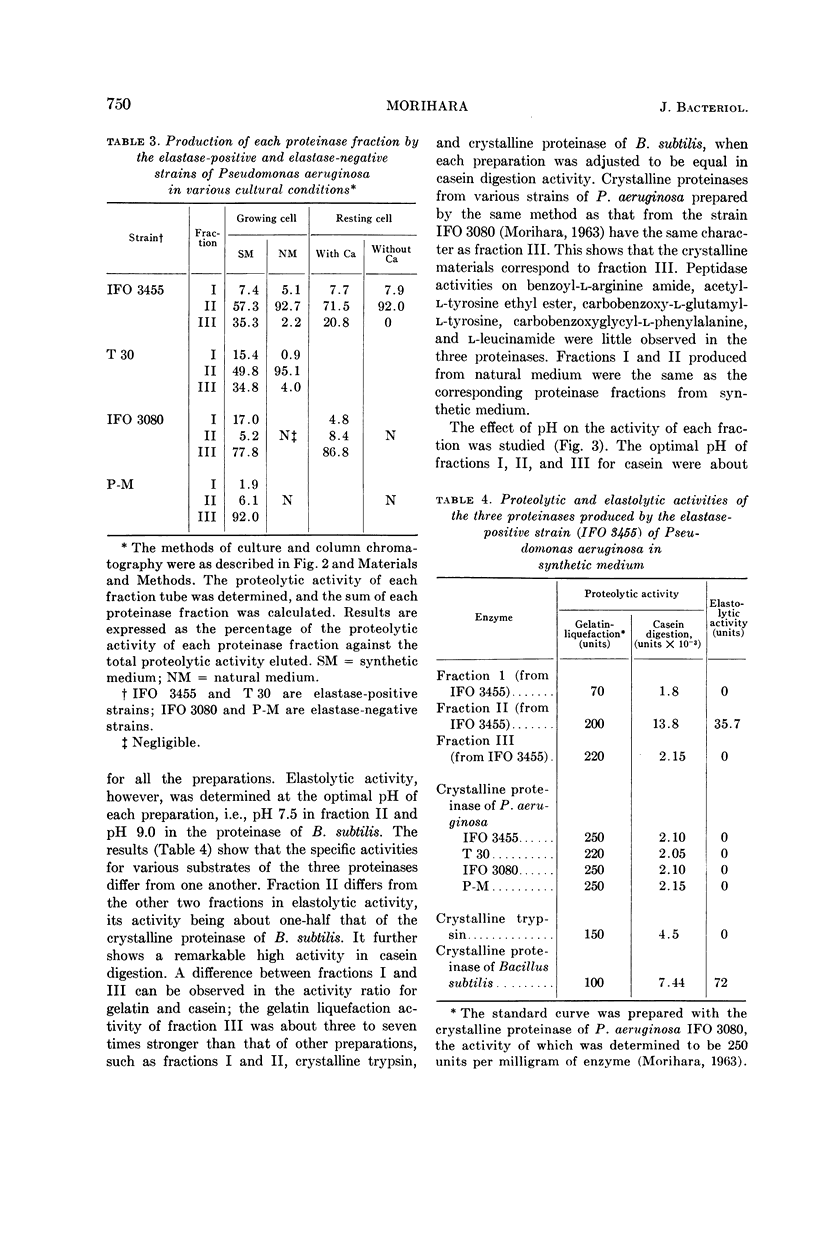
Morihara, Kazuyuki (Shionogi Research Laboratory, Osaka, Japan). Production of elastase and proteinase by Pseudomonas aeruginosa. J. Bacteriol. 88:745–757. 1964.—Some strains of Pseudomonas aeruginosa produced elastase, but the others did not. The elastase-positive strains produced two proteinases (fractions II and III), and the elastase-negative strains produced one proteinase (fraction III). Moreover, one proteinase (fraction I) was produced by both elastase-positive and elastase-negative strains, but the activity was small and negligible. The three proteinases were separated by column chromatography on diethylaminoethyl-cellulose, and elastolytic activity corresponded to fraction II. The effect of components of the medium was slight for production of fraction II; fraction III was produced only in synthetic medium containing Ca ion and not in natural medium. The optimal pH of fractions I, II, and III for casein was 6.5, 8.0, and 10.0, respectively; that of fraction II for elastin was 7.5 to 8.0. The other characters of the three proteinases also differed. Both the proteolytic and the elastolytic activities of fraction II showed a similar behavior for various treatments, except the inhibition test by NaCl and serum. Fraction III proteinases from various strains were identical in their enzymatic or other characters, showing that various strains were related regardless of the difference of their elastolytic activity. The reason that the elastolytic activity of P. aeruginosa differed according to the origin of the strain is discussed on the basis of a taxonomic study; results indicate that the ability to produce elastolytic activity (fraction II) may be a dissociative character of the species.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GABY W. L., HADLEY C. Practical laboratory test for the identification of Pseudomonas aeruginosa. J Bacteriol. 1957 Sep;74(3):356–358. doi: 10.1128/jb.74.3.356-358.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE H., NAKAGAWA T., MORIHARA K. Pseudomonas aeruginosa proteinase. II. Molecular weight and molecular dimension. Biochim Biophys Acta. 1963 May 7;73:125–131. doi: 10.1016/0006-3002(63)90427-x. [DOI] [PubMed] [Google Scholar]
- MANDL I., COHEN B. B. Bacterial elastase. I. Isolation, purification and properties. Arch Biochem Biophys. 1960 Nov;91:47–53. doi: 10.1016/0003-9861(60)90453-7. [DOI] [PubMed] [Google Scholar]
- MANDL I., KELLER S., COHEN B. Microbial elastases. A comparative study. Proc Soc Exp Biol Med. 1962 Apr;109:923–925. doi: 10.3181/00379727-109-27379. [DOI] [PubMed] [Google Scholar]
- MULL J. D., CALLAHAN W. S. ESTIMATION OF ELASTOLYTIC ACTIVITY OF STRAINS OF PSEUDOMONAS AERUGINOSA. J Bacteriol. 1963 May;85:1178–1179. doi: 10.1128/jb.85.5.1178-1179.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill A. T., Clark W. M. SOME CONDITIONS AFFECTING THE PRODUCTION OF GELATINASE BY PROTEUS BACTERIA. J Bacteriol. 1928 Apr;15(4):267–296. doi: 10.1128/jb.15.4.267-296.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OAKLEY C. L., BANERJEE N. G. Bacterial elastases. J Pathol Bacteriol. 1963 Apr;85:489–506. [PubMed] [Google Scholar]
- POMERANZ Y. Inactivation of alpha-amylases by synthetic detergents. Biochim Biophys Acta. 1963 May 7;73:105–112. doi: 10.1016/0006-3002(63)90425-6. [DOI] [PubMed] [Google Scholar]
- SACHAR L. A., WINTER K. K., SICHER N., FRANKEL S. Photometric method for estimation of elastase activity. Proc Soc Exp Biol Med. 1955 Nov;90(2):323–326. doi: 10.3181/00379727-90-22022. [DOI] [PubMed] [Google Scholar]
- SBARRA A. J., GILFILLAN R. F., BARDAWIL W. A. A plate assay for elastase. Nature. 1960 Oct 22;188:322–323. doi: 10.1038/188322b0. [DOI] [PubMed] [Google Scholar]
- THOMAS J., PARTRIDGE S. M. The chemistry of connective tissues. 5. The elastase activity of proteolytic enzymes. Biochem J. 1960 Mar;74:600–607. doi: 10.1042/bj0740600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALFORD R. L., KICKHOEFEN B. Selective inhibition of elastolytic and proteolytic properties of elastase. Arch Biochem Biophys. 1962 Aug;98:191–196. doi: 10.1016/0003-9861(62)90172-8. [DOI] [PubMed] [Google Scholar]
- Wilson E. D. STUDIES IN BACTERIAL PROTEASES I. THE RELATION OF PROTEASE PRODUCTION TO THE CULTURE MEDIUM. J Bacteriol. 1930 Jul;20(1):41–59. doi: 10.1128/jb.20.1.41-59.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]