Abstract
Objective:
Delays in achieving blood pressure (BP) control may increase morbidity and mortality in patients with hypertension. Thus, deciding which antihypertensive agent to use and at what dosage, in addition to determining when to initiate combination therapy and which agents to combine, is important for achieving BP control.
Methods:
This randomized, double-blind, 14-week study was conducted to compare the efficacy and tolerability of various doses of valsartan ± hydrochlorothiazide (HCTZ) versus amlodipine ± HCTZ for maximizing BP control in 1,285 patients with uncontrolled hypertension. Patients with stage 1 hypertension and naïve to antihypertensive therapy (33.9%) started valsartan 160 mg or amlodipine 5 mg. Treatment-naïve patients with stage 2 hypertension (13.5%) or those uncontrolled on current antihypertensive monotherapy (52.6%) started valsartan 160 mg/HCTZ 12.5 mg or amlodipine 10 mg. At weeks 4, 8, and 11, patients not achieving BP control were up-titrated (maximum: valsartan 320 mg/HCTZ 25 mg, amlodipine 10 mg/HCTZ 25 mg).
Results:
At study end, 78.8% of patients on valsartan ± HCTZ were controlled (BP <140/90 mmHg) and still on study medication versus 67.8% on amlodipine ± HCTZ (P < 0.0001). Amlodipine-treated patients had a higher incidence of peripheral edema (22.4% vs 2.2%) and associated discontinuations (7.3% vs <1%). Initiating therapy earlier with valsartan/HCTZ, rather than titrating monotherapy to its maximum dose before adding a second agent, was superior to amlodipine monotherapy or amlodipine ± HCTZ for achieving BP control, and avoided excessive treatment adjustments and maintained tolerability.
Keywords: efficacy, hydrochlorothiazide, hypertension, combination therapy, titration, tolerability
Introduction
Numerous studies have demonstrated the importance of achieving blood pressure (BP) control in patients with hypertension.1 Delays in reaching BP goals may be associated with increased cardiovascular morbidity and mortality. Thus, determining which antihypertensive agent to use, and at what dosage, is important. Moreover, because most of the hypertensive population will require multiple drugs to attain BP control,2,3 physicians must determine when to initiate combination antihypertensive therapy (both in treatment-naïve patients and those on monotherapy) and must decide which antihypertensive agents are best to use in combination. Early use of combination antihypertensive therapy is associated with greater reductions in BP and earlier achievement of BP control, and is recommended as initial treatment for patients with BP ≥160/100 mmHg or high cardiovascular risk.2,3
The angiotensin-receptor blocker (ARB) valsartan and the calcium-channel blocker (CCB) amlodipine have proven to be safe and effective antihypertensive agents when used as monotherapy.4–7 The use of a thiazide diuretic in combination with an ARB or CCB is a commonly recommended option for the many patients who require more than a single antihypertensive agent.2,3 The addition of hydrochlorothiazide (HCTZ) to valsartan, for example, has been shown to provide greater antihypertensive efficacy than that of the individual components and to be well tolerated.8 Further, this combination has demonstrated safety and efficacy for the initial treatment of hypertension.9 The combination of valsartan ± HCTZ at a low dose (80–160/12.5 mg) also has demonstrated similar reductions in BP to a maximum dose of amlodipine.10–13 Titrating the combination of valsartan/HCTZ to its maximum dose may be more effective than adding HCTZ after the patient has reached the maximum dose of amlodipine.14
The study described herein compared the efficacy and tolerability of two strategies for maximizing BP control (>140/90 mmHg) in patients with uncontrolled hypertension: early initiation of the combination of a renin–angiotensin system (RAS) blocker with a thiazide diuretic (valsartan ± HCTZ) compared to a strategy of titrating monotherapy, using amlodipine, to maximum dose and then adding a second antihypertensive agent (HCTZ). To more closely mimic clinical practice, the study did not include a washout period (background medications were discontinued at baseline) or placebo run-in. Instead, eligible patients were immediately switched from their current antihypertensive therapy to study medication, including direct initiation of combination therapy (valsartan/HCTZ) or direct initiation with maximal dose (amlodipine) in appropriate patients.
Methods
The study protocol was approved by the Independent Ethics Committee or Institutional Review Board for each center, and the study was conducted in accordance with the ethical principles of the Declaration of Helsinki. All patients provided written informed consent prior to randomization.
Patients
Eligible patients were aged between 18 and 75 years. Treatment-naïve patients had stage 1/grade 1 hypertension (mean sitting systolic blood pressure [MSSBP] 140–159 mmHg and/or mean sitting diastolic blood pressure [MSDBP] 90–99 mmHg) or stage 2/grade 2 hypertension (MSSBP 160–179 mmHg and/or MSDBP 100–109 mmHg).2,3 Patients were considered treatment-naïve if they had received no antihypertensive medication in the previous 12 weeks. Patients on antihypertensive monotherapy were eligible provided their BP was uncontrolled (MSSBP 140–160 mmHg and/or MSDBP 90–100 mmHg) and they had been on monotherapy for ≥4 weeks and until ≤2 days before a pre-randomization visit. Both treatment-naïve and treated patients had to fulfill the BP criteria at both the pre-randomization visit and before randomization on day 1.
Key exclusion criteria included the following: MSSBP ≥ 180 mmHg or MSDBP ≥ 110 mmHg at any time between the pre-randomization visit and day 1; current treatment with a CCB; history of hypersensitivity to any of the study drugs; cerebrovascular accident or myocardial infarction within the previous 12 months or transient ischemic cerebral attack within the previous 6 months; presence of congestive heart failure, angina pectoris, significant valvular heart disease or arrhythmia, second or third degree heart block without a pacemaker, or diabetes; history of malignancy within the previous five years (except localized basal cell carcinoma of the skin); serum potassium level <3.5 or >5.5 mmol/L without medication; serum creatinine level >1.5 times above the upper limit of normal or a history of dialysis or nephrotic syndrome; and alanine or aspartate aminotransferase levels >2 times above the upper limit of normal or history of hepatic encephalopathy, esophageal varices, or portocaval shunt. Women were postmenopausal, surgically sterile, or using an adequate method of contraception.
Study design
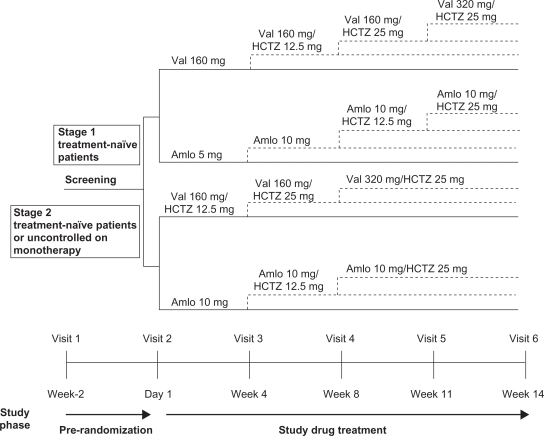
This was a randomized, double-blind, parallel-group, active-controlled study conducted in 11 countries (Argentina, Brazil, Columbia, Denmark, Ecuador, Spain, Finland, Germany, Ireland, Italy, and United Kingdom). Patients were assessed for eligibility within two weeks before randomization. Patients continued their antihypertensive medication during this period. Eligible patients were randomized in a 1:1 ratio and directly switched from their current antihypertensive therapy to either a valsartan strategy or an amlodipine strategy (Figure 1). Stage 1 treatment-naïve patients were started on either valsartan 160 mg once daily (o.d.) or amlodipine 5 mg o.d., whereas stage 2 treatment-naïve patients and those uncontrolled on current antihypertensive monotherapy were started on valsartan 160 mg/HCTZ 12.5 mg o.d. or amlodipine 10 mg o.d. Patients were instructed to take their study medication at approximately 8:00 am. To maintain blinding, all study medication was identical in packaging, labeling, appearance, and odor.
Figure 1.
Study design.
Abbreviations: Amlo, amlodipine; HCTZ, hydrochlorothiazide; Val, valsartan.
Patients visited the clinic at three- to four-week intervals during the 14-week treatment period for efficacy and tolerability assessments. As shown in Figure 1, up-titration or the addition of HCTZ was mandatory at visits in which the patient did not achieve a MSSBP <140 mmHg and a MSDBP <90 mmHg. Down-titration to the previous step was permitted for a MSSBP <100 mmHg or if the patient presented with symptomatic hypotension.
Use of monoamine oxidase inhibitors and tricyclic antidepressants was prohibited during the study, as was the chronic use of oral steroids, sympathomimetic drugs, and bronchodilators. Thyroid medication and hormone replacement therapy were allowed only if stable maintenance doses had been used in the previous six months.
Efficacy assessments
At each clinic visit, sitting BP measurements were obtained using a calibrated standard sphygmomanometer in accordance with the American Heart Association (AHA) Committee Report on blood pressure determination.15 Blood pressure readings were taken just before ingesting the morning dose of study medication (ie, at trough). After sitting for five minutes, three consecutive BP measurements were taken at one- to two-minute intervals. If the three MSSBP readings were not within ± 5 mmHg, the procedure was repeated until this criterion was met. The primary efficacy variable was the percentage of patients who achieved BP control (MSSBP/MSDBP < 140/90 mmHg) and were still on study medication at the end of the study (week 14).
Tolerability assessments
Adverse events (AEs) were monitored throughout the study and included spontaneous reports by the investigators and patients. In addition, standard laboratory tests (hematology and blood chemistry), vital signs, and physical examinations were performed.
Statistical methods
Efficacy analyses were performed using the intent-to-treat (ITT) population, which included all randomized patients who received ≥1 dose of study medication and had ≥1 postbaseline efficacy assessment. The safety population included all patients who received ≥1 dose of study medication and had ≥1 postbaseline tolerability assessment.
Demographic and baseline characteristics were compared between the two treatment strategies using the t-test (continuous variables) or chi-square test (categorical variables). For the primary efficacy variable, a logistic regression model was fitted including terms for treatment, country, and stage of hypertension or failed antihypertensive monotherapy. The number and percentage of patients whose BP was controlled and who were still on study medication within each treatment strategy, the point estimate for the odds ratio (valsartan/amlodipine), and the two-sided 95% confidence interval (CI) for the odds ratio were determined. The change from baseline to each visit and endpoint (week 14 or last observation carried forward value) in MSSBP and MSDBP was analyzed using analysis of covariance. Treatment, country, and stage of hypertension or failed antihypertensive monotherapy were included as fixed factors and baseline BP was included as a covariate in the model. The least-squares mean changes from baseline, treatment difference, 95% CI for the treatment difference, and P-value were determined.
Results
Patients
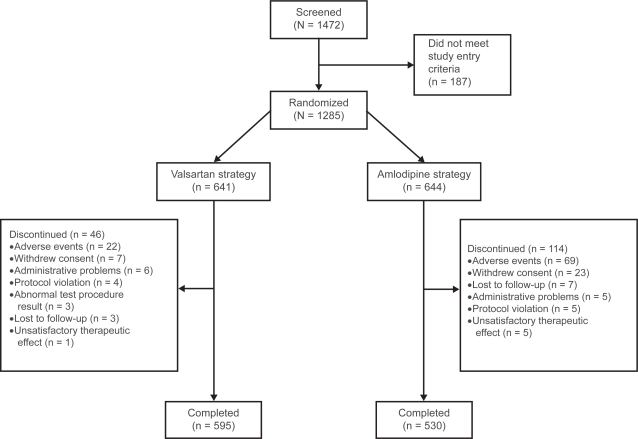
Patient disposition is presented in Figure 2. Of the 1,472 patients screened, 1,285 were randomized (641 valsartan, 644 amlodipine) and 1,125 completed the study (595 valsartan, 530 amlodipine). The ITT population was used for the primary analysis and comprised 1263 patients (632 valsartan, 631 amlodipine). There were more discontinuations with the amlodipine ± HCTZ strategy (114 patients, 17.7%) than with the valsartan ± HCTZ strategy (46 patients, 7.2%), primarily due to AEs (69 patients [10.7%] vs 22 patients [3.4%], respectively) and mostly attributed to peripheral edema (46 patients [7.3%] vs two patients [<1%]).
Figure 2.
Patient disposition.
Demographic and baseline characteristics were well balanced between the two treatment strategies, and no statistically significant differences were observed (Table 1). The study was conducted in 11 countries (seven in Europe and four in South America) at 122 research centers. Overall, mean age was 54.5 years and mean body mass index was 28.4 kg/m2. Most patients were male (55.2%), and the majority was Caucasian (86.2%). Patients were either stage 1 treatment naïve (33.9%), stage 2 treatment naïve (13.5%), or uncontrolled on current antihypertensive monotherapy (52.6%). At baseline, MSSBP/MSDBP was 150.2/93.9 mmHg.
Table 1.
Demographic and baseline characteristics
| Variable | Valsartan strategy (n = 641) | Amlodipine strategy (n = 644) | Total (n = 1285) |
|---|---|---|---|
| Mean (SD) age, y | 54.6 (10.8) | 54.3 (11.3) | 54.5 (11.1) |
| Age group, n (%) | |||
| < 65 y | 505 (78.8) | 505 (78.4) | 1,010 (78.6) |
| ≥ 65 y | 136 (21.2) | 139 (21.6) | 275 (21.4) |
| Gender, n (%) | |||
| Male | 362 (56.5) | 347 (53.9) | 709 (55.2) |
| Female | 279 (43.5) | 297 (46.1) | 576 (44.8) |
| Race, n (%) | |||
| Caucasian | 557 (86.9) | 551 (85.6) | 1,108 (86.2) |
| Native American | 16 (2.5) | 21 (3.3) | 37 (2.9) |
| Black | 10 (1.6) | 17 (2.6) | 27 (2.1) |
| Other | 58 (9.0) | 55 (8.5) | 113 (8.8) |
| Mean (SD) height, cm | 168.6 (10.0) | 168.6 (10.2)* | 168.6 (10.1)† |
| Mean (SD) weight, kg | 80.9 (15.8) | 81.0 (16.7)* | 80.9 (16.2)† |
| Mean BMI, kg/m2 | 28.4 (4.6) | 28.4 (5.0)* | 28.4 (4.8)† |
| Serum creatinine, umol/L | 79.4 (16) | 78.8 (15) | 79.1 (16) |
| Serum glucose, mmol/L | 5.56 (0.8) | 5.55 (0.8) | 5.55 (0.8) |
| Serum potassium, mmol/L | 4.38 (0.4) | 4.35 (0.8) | 4.36 (0.8) |
| MSSBP (SD), mmHg | 150.4 (9.0) | 150.0 (8.9) | 150.2 (9.0) |
| MSDBP (SD), mmHg | 93.9 (6.4) | 93.8 (6.2) | 93.9 (6.3) |
| Stage 1 treatment-naïve, n (%) | 220 (34.3) | 215 (33.4) | 435 (33.9) |
| Stage 2 treatment-naïve, n (%) | 90 (14.0) | 84 (13.0) | 174 (13.5) |
| Uncontrolled on current monotherapy, n (%) | 331 (51.6) | 345 (53.6) | 676 (52.6) |
Note:
n = 640;
N = 1281.
Abbreviations: BMI, body mass index; MSDBP, mean sitting diastolic blood pressure; MSSBP, mean sitting systolic blood pressure; SD, standard deviation.
Blood pressure measurements
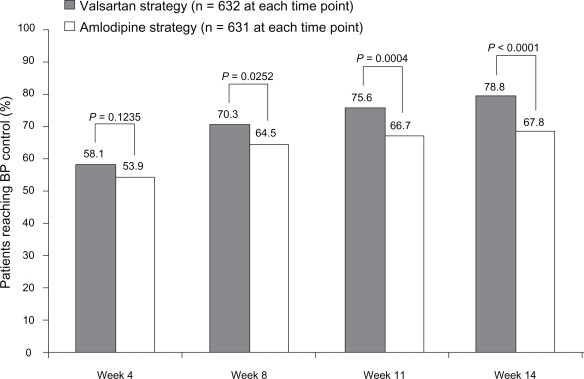
At week 14, 78.8% of patients in the valsartan ± HCTZ group and 67.8% in the amlodipine ± HCTZ group had their BP controlled and were still on study medication (P < 0.0001) (Figure 3). As shown in Figure 3, significant differences in favor of valsartan ± HCTZ also were apparent at weeks 8 (P = 0.0252) and 11 (P = 0.0004). More than 50% of patients had their BP controlled and were still on study medication by the time of their first on-therapy visit at week 4 (58.1% valsartan, 53.9% amlodipine; P = 0.1235).
Figure 3.
Percentage of patients who achieved blood pressure (BP) control (mean sitting systolic/diastolic BP <140/90 mmHg) and were still on study medication by visit.
Among patients who were uncontrolled on current antihypertensive monotherapy at the start of the study, a significantly greater percentage in the valsartan ± HCTZ group (79.4%) than in the amlodipine ± HCTZ group (62.9%) achieved BP control and were still on study medication at week 14 (P < 0.0001). The corresponding results for treatment-naïve patients, stage 1 and stage 2, (78.2% vs 73.5%) also tended to be better for valsartan ± HCTZ, but were not statistically superior to amlodipine ± HCTZ.
Least-squares mean reductions from baseline in MSSBP and MSDBP are shown in Table 2. Substantial reductions in MSSBP/MSDBP were apparent by week 4 with both treatment strategies (15.3/8.9 mmHg with valsartan, 13.5/8.0 mmHg with amlodipine). The magnitude of reduction was significantly greater with valsartan ± HCTZ at weeks 4, 8, 11, and endpoint for MSSBP and at weeks 4, 8, and endpoint for MSDBP (all P < 0.05).
Table 2.
Least-squares mean changes (SEM) from baseline in MSSBP and MSDBP by visit
|
MSSBP |
MSDBP |
|||||
|---|---|---|---|---|---|---|
| Week | Valsartan strategy* | Amlodipine strategy† | P | Valsartan strategy* | Amlodipine strategy† | P |
| 4 | −15.3 (0.5) | −13.5 (0.5) | 0.0029 | −8.9 (0.3) | −8.0 (0.3) | 0.0160 |
| 8 | −19.6 (0.5) | −18.0 (0.5) | 0.0078 | −10.6 (0.4) | −9.8 (0.4) | 0.0328 |
| 11 | −21.4 (0.5) | −19.4 (0.5) | 0.0006 | −12.1 (0.3) | −11.5 (0.3) | 0.1469 |
| 14 | −22.3 (0.5) | −21.3 (0.5) | 0.0630 | −12.8 (0.3) | −12.1 (0.3) | 0.0672 |
| Endpoint‡ | −21.7 (0.5) | −19.6 (0.5) | 0.0002 | −12.5 (0.3) | −11.1 (0.3) | <0.0001 |
Notes:
n = 632 at week 4, 614 at week 8, 603 at week 11, 600 at week 14, and 632 at endpoint.
n = 630 at week 4, 588 at week 8, 553 at week 11, 539 at week 14, and 631 at endpoint.
Week 14 or last observation carried forward value.
Abbreviations: MSDBP, mean sitting diastolic blood pressure; MSSBP, mean sitting systolic blood pressure; SEM, standard error of the least-squares mean.
Titration steps
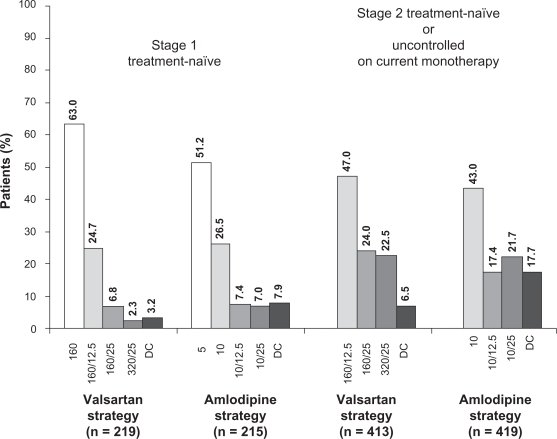
A numerically similar percentage of patients in each treatment strategy were up-titrated over the course of the study. At week 11, the last up-titration visit, the majority of stage 1 treatment-naïve patients were on their initial treatment or the first titration step: valsartan 160 mg alone or in combination with HCTZ 12.5 mg o.d. (192/219, 87.7%) or amlodipine 5 mg or 10 mg o.d. (167/215, 77.7%) (Figure 4). At the same time point, most stage 2 treatment-naïve patients and those uncontrolled on current antihypertensive monotherapy also were receiving their initial treatment or the first titration step: valsartan 160 mg in combination with either HCTZ 12.5 mg or 25 mg o.d. (293/413, 70.9%) or amlodipine 10 mg alone or in combination with HCTZ 12.5 mg o.d. (253/419, 60.4%). More patients were on combination treatment with valsartan/HCTZ than with amlodipine/HCTZ (Figure 4).
Figure 4.
Percentage of patients on each treatment regimen at week 11, the last up-titration visit. Percentages may not add up to 100 due to rounding. Numbers on x-axis represent doses (in mg) of valsartan, valsartan/hydrochlorothiazide (HCTZ), amlodipine, and amlodipine/HCTZ.
Abbreviation: DC, discontinuation.
Tolerability
Overall, AEs occurred in 41.5% and 53.3% of patients receiving valsartan ± HCTZ and amlodipine ± HCTZ, respectively. The most commonly reported AEs were peripheral edema (2.2% for valsartan vs 22.4% for amlodipine), headache (4.0%, 6.2%), and dizziness (3.8%, 1.7%). Peripheral edema resulted in the discontinuation of 46 (7.3%) patients treated with amlodipine ± HCTZ compared with two (<1.0%) patients on valsartan ± HCTZ. The incidence of all AE reports of edema is presented by clinic visit in Table 3. There were no deaths during the study. Mean changes in laboratory findings were minimal. Few patients experienced increases in serum creatinine levels ≥175 μmol/L (two valsartan, zero amlodipine) or serum potassium levels ≥5.8 mmol/L (four valsartan, two amlodipine). Twenty-four (3.8%) patients in the valsartan ± HCTZ group and 41 (6.5%) in the amlodipine ± HCTZ group experienced a >20% decrease in serum potassium levels at any postbaseline visit. No patient discontinued due to laboratory abnormalities. Vital signs did not reveal any clinically significant trends other than the expected improvements in BP.
Table 3.
Number (%) of patients with an adverse event of edema, by visit
| Day or Week | Valsartan strategy (n = 632) | Amlodipine strategy (n = 634) |
|---|---|---|
| Day 1 | 0 | 2 (<1.0) |
| Week 4 | 7 (1.1) | 88 (13.9) |
| Week 8 | 13 (2.1) | 132 (20.8) |
| Week 11 | 16 (2.5) | 147 (23.2) |
| Week 14 | 17 (2.7) | 153 (24.1) |
Notes: Patients were included in the visit occurring first after the onset of edema. Patients were counted in all subsequent visits, regardless of whether or not the edema had resolved.
Discussion
Current treatment guidelines acknowledge the need for combination therapy in the majority of patients with hypertension, and recommend combination therapy as initial treatment for most patients with baseline BP ≥160/100 mmHg or when total cardiovascular risk is high.2,3 Initial or early use of combination therapy using two drugs with complementary modes of action may allow patients to reach BP targets quicker, with fewer titration steps, and without an increase in the side effects associated with higher doses of monotherapy.16 Moreover, evidence from landmark trials suggests that more prompt BP control leads to better clinical outcomes.1
The present study employed algorithms consistent with current treatment guidelines, based on patients’ current BP level or previous history on antihypertensive drugs. We found that initiating therapy earlier with valsartan/HCTZ provided superior BP control rates (<140/90 mmHg) compared with titrating amlodipine monotherapy to its maximum dose before adding HCTZ. Significant differences in favor of valsartan ± HCTZ were observed at weeks 8, 11, and 14 (end of study). The differences were even greater for patients who at the start of the study were uncontrolled on previous monotherapy. In those patients who were naïve to antihypertensive therapy similar BP control rates were achieved using either treatment strategy approach. This finding may be explained by the greater number of patients using monotherapy in the treatment-naïve group compared to those in the previous monotherapy group since both regimens were associated with few titration steps. The higher incidence of peripheral edema with amlodipine ± HCTZ led to more frequent treatment discontinuations, resulting in an overall lower therapeutic success.
Our results support the use of the valsartan ± HCTZ strategy for the treatment of hypertension. This is consistent with the well-established role of the RAS in the pathogenesis of hypertension.17 Moreover, evidence from large outcomes trials (eg, HOPE, ALLHAT, LIFE, and VALUE) has consistently demonstrated that a RAS-inhibitor-based approach to treatment provides similar or greater cardiovascular and organ protection than regimens lacking this component.18 In contrast, our findings do not support the use of the amlodipine ± HCTZ strategy as this regimen was more poorly tolerated and yielded inferior BP control rates and BP reductions at most clinic visits. This is in line with current National Institute for Health and Clinical Excellence (NICE) guidance and the ABCD (A = angiotensin-converting enzyme inhibitor [ACE-I] or ARBs, B = beta-blockers, C = CCBs, and D = thiazide or thiazide-like diuretics) treatment algorithm, which suggests that patients starting on a CCB should add a RAS inhibitor.19,20 Although the most recent European guidelines advocate the use of a CCB/diuretic combination,2 our results and those of others14 suggest that this is a poor recommendation. The combination of an antihypertensive agent that blocks the RAS with one that does not is likely to be a more effective approach than using two agents that both block the RAS (eg, ACE-I+ARB)21,22 or two agents that do not affect the RAS (eg, CCB+diuretic). The effectiveness of amlodipine monotherapy has been demonstrated in some key hypertension outcome studies (eg, TOMHS, VALUE, and ALLHAT), but when patients with hypertension cannot be effectively controlled with amlodipine monotherapy, it would make most sense to use a complementary antihypertensive agent (eg, ACE-I or ARB). Recently, the ASCOT and ACCOMPLISH studies demonstrated the importance of combining amlodipine with an ACE-I as patients had significant reductions in cardiovascular events.23,24
Several previous randomized controlled studies have compared valsartan ± HCTZ and amlodipine ± HCTZ strategies in patients with essential hypertension, although none used the dose and titration schedules described herein. The combination of valsartan/HCTZ confers additional BP lowering over monotherapy with these agents,8 with low doses of this combination (80–160/12.5 mg) providing comparable BP reductions to high-dose amlodipine monotherapy (10 mg).10–13 Similar overall antihypertensive efficacy was demonstrated when a regimen of valsartan 80 mg o.d. titrated up to valsartan 160 mg/HCTZ 12.5 mg o.d. was compared with a regimen of amlodipine 5 mg o.d. titrated up to amlodipine 10 mg/HCTZ 12.5 mg o.d.25,26Lacourcière and colleagues conducted a 10-week, forced-titration, ambulatory BP monitoring study in which patients with stage 2 hypertension started therapy with valsartan 160 mg o.d. or amlodipine 5 mg o.d.14 The valsartan arm was titrated to valsartan 160 mg/HCTZ 12.5 mg o.d. at two weeks and valsartan 320 mg/HCTZ 25 mg o.d. at six weeks, while the amlodipine arm was titrated to double-dose at two weeks with the addition of HCTZ 25 mg o.d. at six weeks. At 10 weeks, the reduction from baseline BP was 3.8/2.7 mmHg greater with valsartan/HCTZ than amlodipine/HCTZ (both P < 0.01). The VALUE outcomes trial reported better BP control with an amlodipine-based strategy.27 However, several factors related to the study design may have influenced the results of VALUE. Patients were not randomized to different treatment strategies based on the severity of their hypertension or their prior treatment history or response. Moreover, the addition of HCTZ was not allowed before two months of monotherapy treatment, titration to the high-dose valsartan/HCTZ regimen (320 mg/25 mg o.d.) was not an option, and patients assigned to the valsartan strategy initiated treatment with a suboptimal starting dose (80 mg o.d.).4,28 The 160-mg dose of valsartan has been shown to be more effective than an 80-mg dose in reducing BP from baseline (14.3/11.1 mmHg vs 11.2/9.0 mmHg) and in providing BP control (39.3% vs 22.7%) after up to 8 weeks of therapy, with both doses having comparable AE and biochemical profiles.4Numerous ambulatory BP monitoring studies have demonstrated that valsartan 160 mg o.d. provides consistent reductions in BP throughout the 24-hour interval, with a preservation of the BP-lowering effect at the end of the dosing period.29,30 Further, compared with an 80-mg dose, 160-mg of valsartan resulted in more effective inhibition of the RAS over the 24-hour dosing period.31,32
Both treatments were well tolerated in the current study, with the exception of a relatively high incidence of peripheral edema in the amlodipine ± HCTZ group (22.4%) versus the valsartan ± HCTZ group (2.2%). Similarly, discontinuations due to this AE were higher in the former group (7.3% vs <1.0%). Other AEs were reported at a low and generally similar incidence with both treatment strategies. Peripheral edema is a known side effect of amlodipine. A pooled analysis of data from 40 placebo-controlled, double-blind studies in which 1,775 patients were treated with amlodipine (primarily 5 mg or 10 mg daily) and 1,213 with placebo found that the incidence of “edema” was fourfold greater with amlodipine than with placebo (P < 0.001).33 The rates of peripheral edema and associated discontinuations in our study (Table 3) were similar to those in Val-Syst study (4.8% and 0% for valsartan, respectively; 26.8% and 4.2% for amlodipine, respectively).26 Side effects can have a negative impact on patients’ persistence with antihypertensive treatment, which in turn may be associated with adverse clinical outcomes.34 Patients with hypertension who demonstrate poor persistence have increased morbidity and mortality and higher health care costs. Better tolerated treatment strategies should improve persistence and enable more patients to achieve protection against cardiovascular events.
Conclusion
Initiating therapy earlier with valsartan/HCTZ, rather than titrating monotherapy to its maximum dose before adding a second agent, was superior to amlodipine monotherapy or amlodipine ± HCTZ for achieving BP control (<140/90 mmHg) while avoiding excessive numbers of treatment adjustments and maintaining tolerability. The incidences of peripheral edema and associated discontinuations were greater with amlodipine ± HCTZ.
Footnotes
Disclosure
Results were presented in part at Hypertension 2008, the Joint Congress of the 18th Scientific Meeting of the European Society of Hypertension and the 22nd Scientific Meeting of the International Society of Hypertension, June 14–19, 2008, Berlin, Germany.
The following abstract has been published: Kolloch RE, Ferber P. A randomized, double-blind study to compare a valsartan-based vs an amlodipine-based treatment algorithm in achieving blood pressure control: the PROMPT study. J Clin Hypertens. 2008;10:509.
The authors wish to acknowledge Oxford PharmaGenesis, Inc. for their editorial support with the development of this manuscript.
Funding for this study was provided by Novartis Pharma AG, Basel, Switzerland.
Dion Zappe is an employee of Novartis Pharmaceuticals Corporation. Cheraz Cherif Papst is an employee of Novartis Pharma AG. Philippe Ferber was an employee of Novartis Pharma AG throughout the conduct of the study and during the preparation of this manuscript.
References
- 1.Basile JN, Chrysant S. The importance of early antihypertensive efficacy: the role of angiotensin II receptor blocker therapy. J Hum Hypertens. 2006;20(3):169–175. doi: 10.1038/sj.jhh.1001972. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25(6):1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 4.Weir MR, Crikelair N, Levy D, Rocha R, Kuturu V, Glazer R. Evaluation of the dose response with valsartan and valsartan/hydrochlorothiazide in patients with essential hypertension. J Clin Hypertens (Greenwich) 2007;9(2):103–112. doi: 10.1111/j.1524-6175.2007.06415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weir MR, Levy D, Crikelair N, Rocha R, Meng X, Glazer R. Time to achieve blood-pressure goal: influence of dose of valsartan monotherapy and valsartan and hydrochlorothiazide combination therapy. Am J Hypertens. 2007;20(7):807–815. doi: 10.1016/j.amjhyper.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Steffen HM. Amlodipine – a third generation dihydropyridine calcium antagonist. J Clin Basic Cardiol. 1999;2(1):45–52. [Google Scholar]
- 7.Tyler HM. Amlodipine: an effective once-daily antihypertensive agent. J Hum Hypertens. 1991;5(Suppl 1):61–66. [PubMed] [Google Scholar]
- 8.Wagstaff AJ. Valsartan/hydrochlorothiazide: a review of its use in the management of hypertension. Drugs. 2006;66(14):1881–1901. doi: 10.2165/00003495-200666140-00011. [DOI] [PubMed] [Google Scholar]
- 9.Pool JL, Glazer R, Weinberger M, Alvarado R, Huang J, Graff A. Comparison of valsartan/hydrochlorothiazide combination therapy at doses up to 320/25 mg versus monotherapy: a double-blind, placebo-controlled study followed by long-term combination therapy in hypertensive adults. Clin Ther. 2007;29(1):61–73. doi: 10.1016/j.clinthera.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Palatini P, Malacco E, Di SS, et al. Trough:peak ratio and smoothness index in the evaluation of 24-h blood pressure control in hypertension: a comparative study between valsartan/hydrochlorothiazide combination and amlodipine. Eur J Clin Pharmacol. 2002;57(11):765–770. doi: 10.1007/s00228-001-0393-6. [DOI] [PubMed] [Google Scholar]
- 11.Weir MR, Ferdinand KC, Flack JM, Jamerson KA, Daley W, Zelenkofske S. A noninferiority comparison of valsartan/hydrochlorothiazide combination versus amlodipine in black hypertensives. Hypertension. 2005;46(3):508–513. doi: 10.1161/01.HYP.0000180457.82483.6b. [DOI] [PubMed] [Google Scholar]
- 12.Ruilope LM, Malacco E, Khder Y, Kandra A, Bönner G, Heintz D. Efficacy and tolerability of combination therapy with valsartan plus hydrochlorothiazide compared with amlodipine monotherapy in hypertensive patients with other cardiovascular risk factors: the VAST study. Clin Ther. 2005;27(5):578–587. doi: 10.1016/j.clinthera.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Ruilope LM, Heintz D, Brandao AA, et al. 24-hour ambulatory blood-pressure effects of valsartan and hydrochlorothiazide combinations compared with amlodipine in hypertensive patients at increased cardiovascular risk: a VAST sub-study. Blood Press Monit. 2005;10(2):85–91. doi: 10.1097/00126097-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Lacourcière Y, Wright JT, Jr, Samuel R, Zappe D, Purkayastha D, Black HR. Effects of force-titrated valsartan/hydrochlorothiazide versus amlodipine/hydrochlorothiazide on ambulatory blood pressure in patients with stage 2 hypertension: the EVALUATE study. Blood Press Monit. 2009;14(3):112–120. doi: 10.1097/MBP.0b013e32832a9da7. [DOI] [PubMed] [Google Scholar]
- 15.Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry Circulation 199388(5 Pt 1):2460–2470. [DOI] [PubMed] [Google Scholar]
- 16.Neutel JM. The use of combination drug therapy in the treatment of hypertension. Prog Cardiovasc Nurs. 2002;17(2):81–88. doi: 10.1111/j.0889-7204.2002.01308.x. [DOI] [PubMed] [Google Scholar]
- 17.Nickenig G, Ostergren J, Struijker-Boudier H. Clinical evidence for the cardiovascular benefits of angiotensin receptor blockers. J Renin Angiotensin Aldosterone Syst. 2006;7(Suppl 1):S1–S7. doi: 10.3317/jraas.2006.017. [DOI] [PubMed] [Google Scholar]
- 18.Weir MR. Providing end-organ protection with renin-angiotensin system inhibition: the evidence so far. J Clin Hypertens (Greenwich) 2006;8(2):99–105. doi: 10.1111/j.1524-6175.2005.04648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Institute for Health and Clinical Excellence Hypertension Management of hypertension in adults in primary careNICE clinical guideline 34. 2006. Available at: http://www.nice.org.uk/nicemedia/pdf/cg034niceguideline.pdf Accessed June 2008.
- 20.Brown MJ, Cruickshank JK, Dominiczak AF, et al. Better blood pressure control: how to combine drugs. J Hum Hypertens. 2003;17(2):81–86. doi: 10.1038/sj.jhh.1001511. [DOI] [PubMed] [Google Scholar]
- 21.Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358(15):1547–1559. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 22.Sica DA. Combination ACE inhibitor and angiotensin receptor blocker therapy – future considerations. J Clin Hypertens (Greenwich) 2007;9(1):78–86. doi: 10.1111/j.1524-6175.2007.6359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dahlöf B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366(9489):895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 24.Jamerson K, Weber MA, Bakris GL, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359(23):2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 25.Palatini P, Mugellini A, Spagnuolo V, et al. Comparison of the effects on 24-h ambulatory blood pressure of valsartan and amlodipine, alone or in combination with a low-dose diuretic, in elderly patients with isolated systolic hypertension (Val-syst Study) Blood Press Monit. 2004;9(2):91–97. doi: 10.1097/00126097-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Malacco E, Vari N, Capuano V, Spagnuolo V, Borgnino C, Palatini P. A randomized, double-blind, active-controlled, parallel-group comparison of valsartan and amlodipine in the treatment of isolated systolic hypertension in elderly patients: the Val-Syst study. Clin Ther. 2003;25(11):2765–2780. doi: 10.1016/s0149-2918(03)80332-6. [DOI] [PubMed] [Google Scholar]
- 27.Julius S, Kjeldsen SE, Weber M, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363(9426):2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 28.Verdecchia P, Angeli F. Assessment of the optimal daily dose of valsartan in patients with hypertension, heart failure, or both. Clin Ther. 2004;26(4):460–472. doi: 10.1016/s0149-2918(04)90049-5. [DOI] [PubMed] [Google Scholar]
- 29.Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A. Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: a randomised, double-blind trial. Lancet. 2007;370(9583):221–229. doi: 10.1016/S0140-6736(07)61124-6. [DOI] [PubMed] [Google Scholar]
- 30.Hermida RC, Ayala DE, Khder Y, Calvo C. Ambulatory blood pressure-lowering effects of valsartan and enalapril after a missed dose in previously untreated patients with hypertension: A prospective, randomized, open-label, blinded end-point trial. Clin Ther. 2008;30(1):108–120. doi: 10.1016/j.clinthera.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Maillard MP, Würzner G, Nussberger J, Centeno C, Burnier M, Brunner HR. Comparative angiotensin II receptor blockade in healthy volunteers: the importance of dosing. Clin Pharmacol Ther. 2002;71(1):68–76. doi: 10.1067/mcp.2002.121425. [DOI] [PubMed] [Google Scholar]
- 32.Latif F, Tandon S, Obeleniene R, et al. Angiotensin II type 1 receptor blockade with 80 and 160 mg valsartan in healthy, normotensive subjects. J Card Fail. 2001;7(3):265–268. doi: 10.1054/jcaf.2001.26242. [DOI] [PubMed] [Google Scholar]
- 33.Osterloh I.The safety of amlodipine Am Heart J 1989118(5 Pt 2): 1114–1119. [DOI] [PubMed] [Google Scholar]
- 34.Elliott WJ. What factors contribute to the inadequate control of elevated blood pressure? J Clin Hypertens (Greenwich) 2008;10(1 Suppl 1):20–26. doi: 10.1111/j.1524-6175.2007.08028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]