Abstract
Focal Adhesion Kinase (FAK) is overexpressed in a number of tumors, including breast cancer. Another marker of breast cancer tumorigenesis is the tumor suppressor gene p53 that is frequently mutated in breast cancer. In the present study, our aim was to find a correlation between FAK overexpression, p53 expression and mutation status in a population-based series of invasive breast cancer tumors from the Carolina Breast Cancer Study. Immunohistochemical analyses of 622 breast cancer tumors revealed that expression of FAK and p53 were highly correlated (P = 0.0002) and FAK positive tumors were 1.8 times more likely to be p53 positive compared to FAK negative tumors [odds ratio (OR) = 1.8; 95% Confidence Interval (CI) 1.2 – 2.8, adjusted for age, race and stage at diagnosis]. Tumors positive for p53 expression showed higher intensity of FAK staining (P<0.0001) and higher percent of FAK positive staining (P<0.0005). From the same study, we evaluated 596 breast tumors for mutations in the p53 gene, using SSCP (single strand conformational polymorphism) and sequencing. Statistical analyses were performed to determine the correlation between p53 mutation status and FAK expression in these tumors. We found that FAK expression and p53 mutation were positively correlated (P<0.0001) and FAK positive tumors were 2.5 times more likely to be p53 mutation positive compared to FAK negative tumors [adjusted OR = 2.5, 95% CI 1.6–3.9]. This is the first analysis demonstrating a high correlation between FAK expression and p53 mutations in a population-based series of breast tumors.
Keywords: p53, Focal Adhesion Kinase, breast cancer, tumor
Introduction
Tumor suppressor p53 is one of the major markers of human tumorigenesis and is mutated in almost 50% of all tumors 1. Following induction by a variety of cell stresses such as DNA damage, hypoxia, and oncogene activation, p53 up-regulates a number of genes that can promote cell death and growth arrest, such as p21, Bax, cyclin G, GADD45 (reviewed in 2). Recently, it was shown that p53 can also repress promoter activities of a number of anti-apoptotic genes and cell-cycle genes (survivin, cyclin B1, cdc2, cdc25 c, stathmin, Map4, bcl-2 and FAK) 3.
Focal adhesion kinase (FAK) is a non-receptor cytoplasmic protein tyrosine kinase that controls a number of cellular signaling pathways, including proliferation, cell spreading, motility, angiogenesis, invasion and survival 4. FAK is overexpressed in many types of tumors, including breast cancer tumors 5. We have shown that FAK up-regulation occurred in early stages of breast tumorigenesis 5, 6. In addition, increased FAK mRNA and protein expression has been demonstrated in adenomatous tissues and in matched samples of colorectal carcinoma and liver metastases 7, 8.
The first report on indirect functional link between FAK and tumor suppressor gene p53 was reported by Ilic et al. 9. The authors demonstrated that p53 controls survival signals from the extracellular matrix transduced by FAK in anchorage-dependent cells 9. Recently, cloning and characterization of the FAK promoter demonstrated p53 transcription factor binding sites that had potential importance for regulation of FAK transcription 3. In addition, we and other group demonstrated direct physical interaction of the FAK and p53 proteins 10, 11 providing a novel mechanism for FAK-p53-mediated signaling in tumorigenesis 12, 13.
Recently, global characterization of 65,572 p53 ChIP DNA fragments was done on 5-fluorouracil-treated HCT116 colorectal cancer cells to determine potential targets of activated p53 14. The authors identified a number of novel targets, including PTK2 (FAK) that were involved in cell adhesion, migration and metastasis 14. These 5-fluouracil-treated HCT116 cells inhibited FAK 14, leading the authors to suggest that p53 can suppress metastasis through repression of FAK. We performed ChIP assays on HCT116 colon cancer cells and demonstrated that p53 can bind the FAK promoter both in vitro 3 and in vivo 15 and repress its promoter activity 3. Furthermore, recently we demonstrated down-regulation of FAK mRNA and protein levels by adenoviral overexpression of p53 and by endogenous induction of p53 with chemotherapy15. The aim of this report was to analyze the p53 status and FAK expression in a population-based series of breast cancers. We clearly show for the first time that high FAK expression and the presence of p53 mutations positively correlated in a population-based series of invasive breast cancer.
Materials and Methods
Study Population
The Carolina Breast Cancer Study (CBCS) is a population-based, case-control study of breast cancer in African American and white women16, 17. The CBCS included women 20–74 years of age from 24 contiguous counties of central and eastern North Carolina. This research was approved by the University of North Carolina School of Medicine IRB and University of Florida IRB. Women with a first diagnosis of invasive breast cancer (Phase 1 of the CBCS) were identified by the North Carolina Central Cancer Registry through a rapid case ascertainment system. Increased cases of African American women and women younger than 50 years were included so that these subpopulations would represent approximately 50% of the study population 17.
Tumor specimens for FAK staining
Formalin-fixed, paraffin-embedded tumor blocks were obtained from participating hospital’s Pathology Departments and tumors were sectioned by the Specialized Programs of Research Excellence (SPORE) Immunohistochemiostry Core facility. Total 629 breast cancer formalin-fixed, paraffin-embeded tissue cases were used for FAK immunohistochemical analysis.
Immunohistochemistry
FAK immuniohistochemistry was performed using the FAK monoclonal antibody 4.47 (Upstate Biotechnology, USA), as described in 16. In brief, slides were heated at 60°C for one hour prior to deparafinization, rehydration and quenching of endogenous peroxidase activity (3% hydrogen peroxide in methanol). The hydration process was completed by rinsing in DAKO TBS buffer (DAKO Corporation, Carpinteria, CA), containing 0.05% Tween 20 (DAKO Corporation, Carpinteria, CA). During heat-induced epitope recovery, sections were steam-heated while submerged in antigen retrieval Citra buffer (BioGenex, San Ramon, CA) for 30 minutes. Sections were blocked in normal horse serum (Vectastatin Elite Kit, Vector Laboratories, Burlingame, CA) for 15 minutes and then incubated with anti-FAK 4.47 monoclonal antibody (1:250 dilution) overnight at 4°C in a humidity chamber. Sections were washed in DAKO TBS buffer containing 0.05% Tween 20 and then loaded into the DAKO Autostainer for application and incubation of the biotinylated horse antimouse IgG (Vectastatin Elite Kit, Vector Laboratories, Burlingame, CA) for 30 minutes at room temperature. The Avidin-biotin complex (Vectastatin Elite Kit, Vector Laboratories, Burlingame, CA) was applied to the slides for 30 minutes at room temperature. The chromagenic reaction was performed with SG (Vector Laboratories, Burlingame, CA) for 10 minutes at room temperature. Slides were removed from the autostainer and counterstained with Nuclear Fast Red (Vector Laboratories, Burlingame, CA) for 10 minutes and coverslipped with PerMount (Fisher Scientific). Positive and negative controls were included with each run. P53 monoclonal antibody (clone DO-7; Dako, CA, USA) was used for p53 immunohistochemical staining, as described in 18. In brief, the slides were dried and p53 antibody was applied 1:100 dilution to the slides for 30 minutes at 37C. Then slides were processed the same as above. Control sections were incubated with a comparable concentration of isotype-matched IgG1 monoclonal antibody, MOPC-21 (Sigma, St Louis, Mo).
Immunohistochemistry Scoring
A single experienced board certified pathologist scored blindly each tissue section for FAK expression based on a scoring system that measured intensity (0, none; 1, borderline; 2, weak; 3, moderate; 4, strong) and percentage of positive cells (1–100), as described in 16. FAK was considered high with intensity equal 3 or 4 and with percent (%) of positive cells greater than or equal to 90% and FAK was considered low (0,1 or 2 intensity and/or < 90% positive cells16,18. Positive p53 was considered as at least 2+ nuclear intensity in > 10% of cancer cells.
P53 Mutation Screening
Mutations in exons 4–8 of the p53 gene were evaluated by SSCP analysis and DNA sequencing, as described before 17. P53 positive tumor contained any p53 mutation, excluding silent non-clinical-relevant mutations and p53-negative included tumors without mutation and tumors with silent mutations, as defined in 17.
Statistical analysis
Statistical analyses were performed with SAS statistical software, Version 9.2, SAS Institute Inc., Cary, NC, USA. Fisher’s exact test was used to determine the correlation between FAK expression and p53 mutation. Chi-square test was used to determine correlation between FAK and p53 expression. Unconditional logistic regression was implemented to estimate odds ratios for the association of high FAK expression and p53 mutation. For OR, odds ratio and CI confidence interval calculations unadjusted or adjusted for age (11-ordinal variable value), race (African American, non African American) and stage at diagnosis (1, 2, 3+4) models were used.
Results and Discussion
In the present report we analyzed p53 status and FAK expression in a population-based series of breast cancer tumors. We screened 596 breast tumors from Phase I of Carolina Breast Cancer Study 17 for mutation status and found p53 mutations in 169 patients (28%) of the 596 tumors. This group we called p53 mutation positive (Table 1). The group did not include negative silent mutation of p53, as described in 17. Another group 427 (72%) out of 596 included no p53 mutations and silent p53 mutation, called p53 mutation-negative group (Table 1). Immunohistochemical analysis of FAK expression was performed as described in 16. High FAK expression was considered high with intensity 3+ or 4+ and ≥90% positive cells and not high (0,1 or 2 intensity and/or <90% positive cells 16. Statistical analysis by Fisher’s exact test shows that among p53 mutation-positive tumors FAK expression was high in 38%, while in p53-mutation negative cases FAK expression was high in 18% of cases and low in 82%. Thus, high FAK expression positively correlated with p53 mutation (P<0.0001). An example of a breast tumor with hot-spot mutation and FAK expression is shown on Fig 1. We used logistic regression analysis to calculate odds ratio (OR) and found that tumors with p53 mutation were significantly more likely to have FAK overexpression than tumors without p53 mutation (unadjusted OR=2.8; 95% confidence interval CI, 1.8–4.2); adjusted OR = 2.5, 95% CI 1.6–3.9; (adjusted for age (11- level ordinal variable), race (African American, non African American) and stage at diagnosis (1, 2, 3+4). Thus, analysis of 596 breast cancer patients showed that p53 mutation was correlated with high FAK expression in the breast cancers.
Table 1.
Correlation between FAK and p53 mutations in breast cancer tumors.
| FAK expression | p53 mutation status | |
|---|---|---|
| p53 positive (p53+) | p53 negative (p53−) | |
| N = 169 | N = 427 | |
| N (%) | N (%) | |
| High FAK | 65 (38%) | 78 (18%) |
| Low FAK | 104 (62%) | 349 (82%) |
| P<0.0001 | ||
| OR=2.8 (95% CI, 1.8–4.2) unadjusted | ||
| OR=2.5 (95% CI, 1.6–3.9) adjusted for age, race and stage | ||
| P a<0.0001 | ||
Fisher’s exact test
Figure 1. Immunostaining of FAK and p53 expression in breast cancer tumor with p53 mutation.
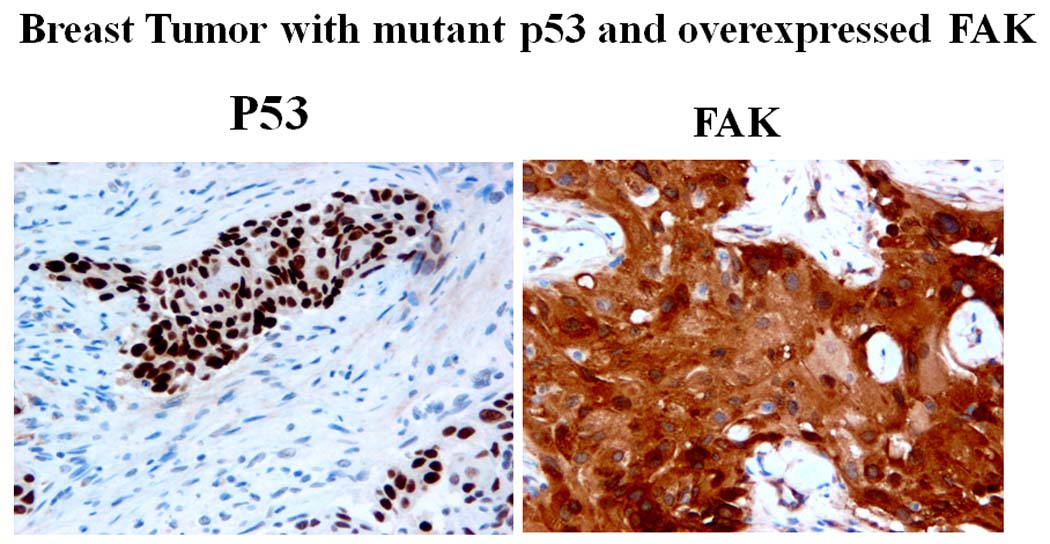
Example of high FAK and p53 staining in breast cancer tumor in patients positive for p53 mutation. Immunostaining of FAK and p53 was performed on different sections of tumors as described in Materials and Methods. FAK 4.47 antibody was used at dilution 1:250; p53 antibody was used (1:500). High FAK expression and p53 are shown. P53 was overexpressed in tumor with p53 mutation.
We also performed immunohistochemical analysis of FAK and p53 expression in 622 tumors from the Carolina Breast Cancer Study (Table 2). We found that tumors with high FAK expression were more likely to be p53-positive (unadjusted OR=2.0, 95% CI 1.4–3.0; adjusted OR = 1.8, 95% CI 1.2 – 2.8). The correlation was statistically significant (Chi square test, P=0.0002) (Table 2). P53-positive tumors showed higher intensity of FAK staining and higher percent of positive cells (Table 2). These data are consistent with data on endometrial cancers that demonstrated correlation with FAK and p53 overexpression 18. There were also supporting our data reports that showed a positive correlation between p53 mutation and p53 overexpression in breast cancers 19 20. In addition, p53 mutations and overexpression has been demonstrated to be a marker of poor patent prognosis and breast cancer aggressiveness. One of the mechanisms of positive correlation between p53 mutation and FAK overexpression is based on the repressive function of wild type of p53 on the FAK promoter 3. Once p53 has either a DNA-binding domain mutation or a frameshift mutation, it is not able to bind the FAK promoter, so the repression of p53 is abrogated, and FAK transcription increases due to the non-function of p53.
Table 2.
Correlation between FAK and p53 expression in breast cancer tumors.
| FAK expression | p53 expression | |
|---|---|---|
| p53 positive (p53+) | p53 negative (p53−) | |
| N = 292 | N = 330 | |
| N (%) | N (%) | |
| High FAK | 91 (31%) | 60 (18%) |
| Low FAK | 201 (69%) | 270 (82%) |
| OR = 2.0 (95% CI, 1.4–3.0) unadjusted | ||
| OR = 1.8 (95% CI, 1.2–2.8) adjusted for age, race and stage | ||
| P b =0.0002 | ||
| FAK intensity b | Mean | Median | |
|---|---|---|---|
| p53 expression positive (p53+) | 2.5 | 2.0 | 0–4 |
| p53 expression negative (p53−) | 1.9 | 2.0 | 0–4 |
| Pc<0.0001 | |||
| FAK staining | |||
|---|---|---|---|
| % positive c | Mean | Median | |
| p53-positive | 71.8% | 80 | 0–99 |
| p53-negative | 62.9% | 80 | 0–99 |
| Pc=0.0005 | |||
Chi-square test
Student’s t-test
In summary, this is the first analysis on population-based series of 596 breast cancer tumors demonstrating the high positive correlation between p53 expression and mutations with FAK overexpression and provides an additional basis for FAK-p53 signaling during tumorigenesis. Since FAK overexpression and p53 mutation are associated with worse prognosis, the combined abrogation of these pathways will be important for breast cancer therapeutics.
Acknowledgments
We would like to thank Nicole Massoll for providing an image of immunohistochemical staining of breast cancer tumor. Research is supported by NIH grant CA65910 (W. G. Cance) and Susan G. Komen for the Cure grant BCTR0707148 (V.M.Golubovskaya).
Abbreviations
- FAK
Focal Adhesion Kinase
- ChIP
chromatin immunoprecipitation
Footnotes
Novelty and Impact: This is the first analysis demonstrating a high correlation between FAK expression and p53 mutations in a population-based series of breast tumors. Since FAK overexpression highly correlate with p53 mutation, the combined abrogation of these pathways may be important for breast cancer therapeutics.
References
- 1.Vogelstein B, Kinzler KW. p53 function and dysfunction. Cell. 1992;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- 2.Giaccia AJ, Kastan MB. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 3.Golubovskaya V, Kaur A, Cance W. Cloning and characterization of the promoter region of human focal adhesion kinase gene: nuclear factor kappa B and p53 binding sites. Biochim et Biophys Acta (BBA) - Gene Structure and Expression. 2004;1678:111–125. doi: 10.1016/j.bbaexp.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Hanks SK, Ryzhova L, Shin NY, Brabek J. Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front Biosci. 2003;8:d982–d996. doi: 10.2741/1114. [DOI] [PubMed] [Google Scholar]
- 5.Lightfoot HM, Lark A, Livasy CA, Moore DT, Cowan D, Dressler L, Craven RJ, Cance WG. Upregulation of focal adhesion kinase (FAK) expression in ductal carcinoma in situ (DCIS) is an early event in breast tumorigenesis. Breast Cancer Res Treat. 2004;88:109–116. doi: 10.1007/s10549-004-1022-8. [DOI] [PubMed] [Google Scholar]
- 6.Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, Simkins S, Xu L. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res. 2000;6:2417–2423. [PubMed] [Google Scholar]
- 7.Weiner TM, Liu ET, Craven RJ, Cance WG. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993;342:1024–1025. doi: 10.1016/0140-6736(93)92881-s. [DOI] [PubMed] [Google Scholar]
- 8.Lark AL, Livasy CA, Calvo B, Caskey L, Moore DT, Yang X, Cance W. Overexpression of focal adhesion kinase in primary colorectal carcinomas and colorectal liver metastases: immunohistochemistry and real-time PCR analyses. Clin Cancer Res. 2003;9:215–222. [PubMed] [Google Scholar]
- 9.Ilic D, Almeida EA, Schlaepfer DD, Dazin P, Aizawa S, Damsky CH. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J Cell Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golubovskaya VM, Finch R, Cance WG. Direct interaction of the N-terminal domain of focal adhesion kinase with the N-terminal transactivation domain of p53. J Biol Chem. 2005;280:25008–25021. doi: 10.1074/jbc.M414172200. [DOI] [PubMed] [Google Scholar]
- 11.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, Larocque N, Fisher SJ, Schlaepfer DD, Ilic D. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29:9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golubovskaya VM, Cance WG. Focal adhesion kinase and p53 signaling in cancer cells. Int Rev Cytol. 2007;263:103–153. doi: 10.1016/S0074-7696(07)63003-4. [DOI] [PubMed] [Google Scholar]
- 13.Cance WG, Golubovskaya VM. Focal Adhesion Kinase Versus p53: Apoptosis or Survival? Sci Signal. 2008;1:pe22. doi: 10.1126/stke.120pe22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei CL, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, Shahab A, Yong HC, Fu Y, Weng Z, Liu J, Zhao XD, Chew JL, Lee YL, Kuznetsov VA, Sung WK, Miller LD, Lim B, Liu ET, Yu Q, Ng HH, Ruan Y. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124:207–219. doi: 10.1016/j.cell.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 15.Golubovskaya VM, Finch R, Kweh F, Massoll NA, Campbell-Thompson M, Wallace MR, Cance WG. p53 regulates FAK expression in human tumor cells. Mol Carcinog. 2008;47:373–382. doi: 10.1002/mc.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lark AL, Livasy CA, Dressler L, Moore DT, Millikan RC, Geradts J, Iacocca M, Cowan D, Little D, Craven RJ, Cance W. High focal adhesion kinase expression in invasive breast carcinomas is associated with an aggressive phenotype. Mod Pathol. 2005;18:1289–1294. doi: 10.1038/modpathol.3800424. [DOI] [PubMed] [Google Scholar]
- 17.Conway K, Edmiston SN, Cui L, Drouin SS, Pang J, He M, Tse CK, Geradts J, Dressler L, Liu ET, Millikan R, Newman B. Prevalence and spectrum of p53 mutations associated with smoking in breast cancer. Cancer Res. 2002;62:1987–1995. [PubMed] [Google Scholar]
- 18.Livasy CA, Moore D, Cance WG, Lininger RA. Focal adhesion kinase overexpression in endometrial neoplasia. Appl Immunohistochem Mol Morphol. 2004;12:342–345. doi: 10.1097/00129039-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Fukushima T, Onda M, Abe R, Otake T, Kimijima I, Tsuchiya A. p53 mutations and overexpressions in Japanese breast cancer. Eur J Surg Oncol. 1995;21:595–600. doi: 10.1016/s0748-7983(95)95047-8. [DOI] [PubMed] [Google Scholar]
- 20.Lien HC, Lin CW, Mao TL, Kuo SH, Hsiao CH, Huang CS. p53 overexpression and mutation in metaplastic carcinoma of the breast: genetic evidence for a monoclonal origin of both the carcinomatous and the heterogeneous sarcomatous components. J Pathol. 2004;204:131–139. doi: 10.1002/path.1624. [DOI] [PubMed] [Google Scholar]


