Vesicles composed of lipids (liposomes) are widely used models of biomembranes and successful drug carriers.[1, 2] Retention of the encapsulated drug in the liposome is controlled by the lipid composition[3] and the two important lipid types are phospholipids and cholesterol. Cholesterol is important because its inclusion at 30 mole percent eliminates the phase transition of synthetic phospholipids,[4, 5] reduces membrane permeability,[6] and inhibits protein insertion into the bilayer.[7, 8] These are all factors that control liposome stability in blood.
However liposomes cannot be formed from pure cholesterol; in fact above 50 mole percent, cholesterol phase separates from the bilayer into crystals. Cholesterol can also rapidly transfer among biomembranes, lipoproteins and lipid vesicles.[9] Cholesterol transfer from the bilayer results in the decrease of liposome stability and subsequent loss of encapsulated contents from the liposomes.[3, 10] Therefore, to obtain desired membrane properties, it is important to regulate the sterol concentration in the liposome bilayer.
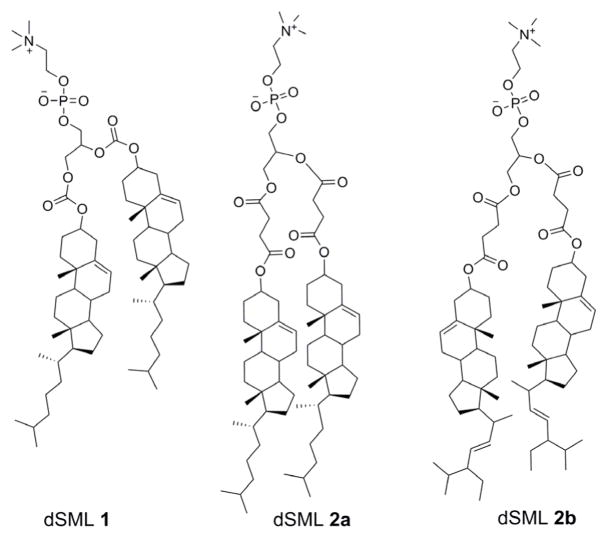
Chemistry has played a major role in the liposome research field, and synthetic efforts have focused on pegylated lipids,[11] cationic lipids[12], fluorinated lipids,[13] lipid prodrugs,[14] molecular umbrellas,[15] and to a lesser extent non-ionic amphiphiles.[16] Recently, we reported a new category of lipids: sterol-modified phospholipids (SMLs)[17] in which the sterol is covalently linked to the lyso-phospholipid at either sn-1 or sn-2 position. SMLs can form liposomes and do not transfer from the bilayer. We extend this concept to disterol-modified phospholipid (dSML) in which sterols are covalently attached to both the sn-1 and sn-2 positions of glycerophosphocholine (Figure 1).
Figure 1.
Structures of disterol-modified phospholipid
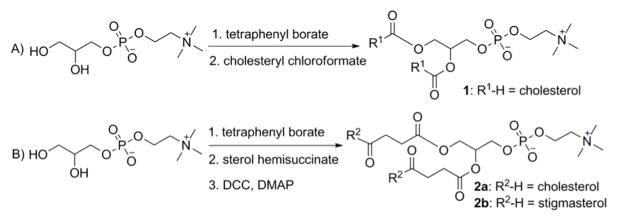
The synthesis of dSML is illustrated in Scheme 1 (See supporting information for detailed procedure). Glycerophosphocholine (GPC) was used as the starting material for the direct coupling of sterol derivatives such as: cholesteryl chloroformate, cholesterol hemisuccinate, and stigmasterol hemisuccinate. The use of GPC in the semi-synthesis of phospholipids generally requires a transition metal ion to increase the solubility of GPC in the organic solvents. We use tetraphenyl borate as the organic counter ion of GPC to generate a complex that is soluble in anhydrous pyridine. This procedure provides a general yield of about 50% for compounds 2 and 2a and a lower yield (17%) of compound 1, which may be due to steric hindrance between closely apposed sterols at the glycerol moiety.
Scheme 1.
Synthesis of disterol-modified phospholipids
To answer whether dSML can form liposomes with diacyl lipids, we formulated 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) and dSML at 2:1 mole ratio (an equivalent of 50% free cholesterol in conventional liposomes) either in pH 7.4 HEPES buffer saline or in 100 mM carboxyfluorescein (CF). Sonicated DPPC/2a suspension had a particle diameter around 80 nm measured by dynamic light scattering. Additionally, CF can be encapsulated and retained in the DPPC/dSML liposomes. The formation of DPPC/2a vesicles was further confirmed by freeze fracture electron microscope image with clear evidence of convex (shadow behind vesicles) and concave (shadow in front of vesicles) shapes of liposome spheres (Figure 2). Together the results suggest that dSML can form liposome with diacyl phospholipids. Liposomes can also be formed from the single component dSML 2a, but dispersions of single component dSML 1 or 2b aggregate upon hydration.
Figure 2.
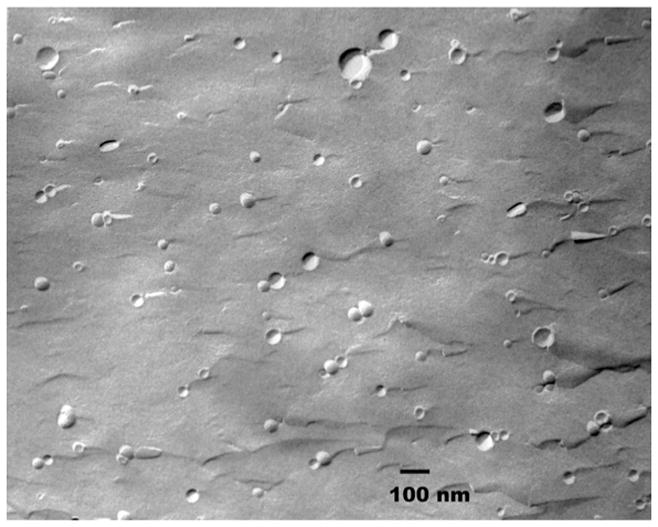
Freeze fracture electron microscope image of sonicated liposome DPPC/2a (2:1, mole ratio).
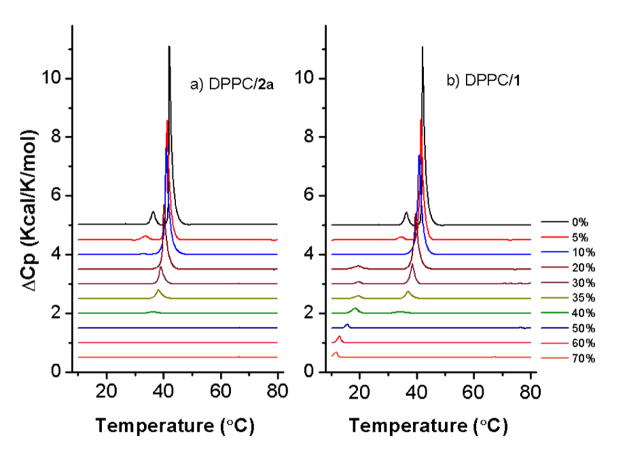
We investigated the thermotropic phase behavior of DPPC/dSML liposomes of various sterol mole ratios using differential scanning calorimetry (DSC). The inclusion of all three dSMLs into a DPPC dispersion influenced the transition of DPPC similar to unmodified cholesterol; a broadening of the transition peaks, a lowering of the mid-point of the transition temperature, and a complete elimination of transition at about 40% cholesterol (Figure 3). Incorporation of dSML 1 and 2b in the bilayer induced a small transition around 20 °C at the DSC traces of dSML 1 and 2b (supporting information) when the equivalent cholesterol was above 20 mol%. In the case of dSML 1 the transition appeared at a progressively lower temperature as the dSML mole percent increased.
Figure 3.
Differential scanning calorimetry thermograms of lipid dispersions. a) DPPC/dSML2a, b) DPPC/dSML1. Mole percentage of cholesterol in the liposome is indicated in the label.
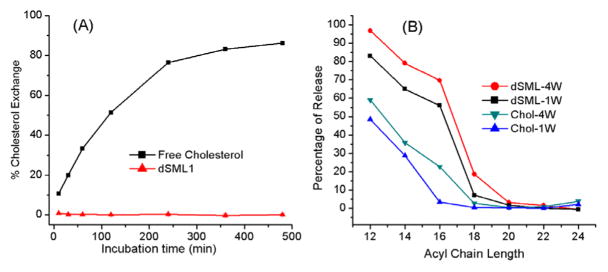
One of the major challenges for in vivo liposome drug delivery is the propensity of serum proteins and biological membranes to extract free cholesterol from the liposome bilayer. In nature, cholesterol exists as a free molecule, the 3-sulfate, or the 3-esters of fatty acids which cannot form bilayers by themselves. Synthetic cholesterol derivatives with a hydrophilic headgroup at 3-position such as phosphate,[18] phosphatidylcholine, hemisuccinate or ethylene oxide assemble into bilayers but can transfer between bilayers.[19] The transfer rate of dSML from the liposome bilayer was measured in a cholesterol exchange experiment. In this experiment, a 10 fold excess of neutral liposomes of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) was used as the acceptor, and the donor liposomes consisted of 50% DPPC, 40% cholesterol (or equivalent from dSML), and 10% negatively charged lipid 1,2-dipalmitoyl-sn-glycero-3-phosphatidylglycerol (DPPG). The donor and acceptor liposomes were mixed and incubated at 37 °C for various time, then separated on an anion exchange column. The amount of cholesterol transferred to the acceptor was determined by a cholesterol assay[20] (see supporting information). Free cholesterol exchanged with a half-life of 2 hours but there was no detectable transfer of dSML 1 from the donor liposome over the eight hour duration of the experiment (Figure 4A). The resistance of dSML to the extraction (similar properties for 2a and 2b) may be the result of the increased hydrophobicity of the disterol derivative as well as a strong interaction between dSML and other phospholipids in the bilayer. Such a mechanism has previously been suggested for the hydrophilic cholesterol derivatives.[19]
Figure 4.
(A) Cholesterol exchange rate from donor liposome to 10 fold excess acceptor liposome at 37 °C. (B) Leakage of carboxyfluorescein (CF) from liposomes in 30 volume % fetal bovine serum in pH 7.4 HEPES buffer saline at 37 °C. The release of CF from liposomes was monitored by measuring the change in the fluorescent intensity from the starting fluorescent intensity at different incubation times. The percentages of CF released from the liposome on day 7 (1W) and day 28 (4W) were calculated and plotted versus the alkyl chain length in the liposome formulations. Each data point is the average of triplicate measurements with a standard deviation less than 5%.
We studied the leakage of CF from liposomes consisting of dSML 2a and diacyl phosphocholines of different chain length in 30% fetal bovine serum at 37 °C (Figure 4B, see supporting information for the detailed procedure). Conventional liposomes composed of diacyl lipid and free cholesterol were used as controls. All liposome formulations have 45 mol % cholesterol or the equivalent from dSML 2a. Both dSML liposomes and conventional liposomes showed chain length dependent leakage profiles featuring higher stability with longer acyl chains. This trend is true for the leakage measured at either day 7 or day 28. The conventional liposome composition with free cholesterol was more stable up to an acyl chain length of 18 carbons, thereafter the dSML composition was as stable as free cholesterol containing vesicles.
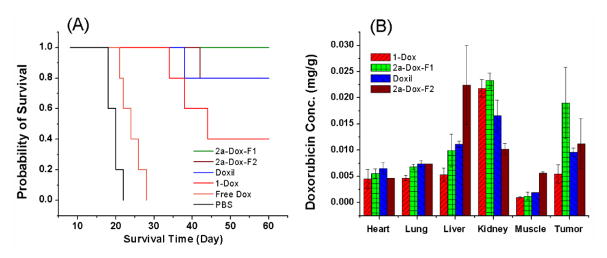
Based on the above properties of dSMLs, we chose to compare the drug carrier properties of liposome formulations containing dSML to those with free cholesterol for the delivery of the antitumor drug doxorubicin. Doxorubicin was encapsulated in the liposomes by the remote loading method[21] and the antitumor effect evaluated in a C26 murine adenocarcinoma model[22] (see supporting information). With a single i.v. dose of 15 mg/kg of doxorubicin on day 8 after the tumor inoculation, all the mice (n=5) treated with 2a-Dox-F1, a formulation similar to Doxil™ (FDA approved PEGylated liposomal doxorubicin), were cured without obvious toxicity and any palpable tumor (Figure 5, panel A, see supporting information for tumor growth curves). Noticeably, 2a-Dox-F2, a formulation having 94.8 mole % 2a and no 1,2-distearoyl-sn-glycero-3-phosphatidylcholine (DSPC), showed a comparable therapeutic effect as 2a-Dox-F1. The antitumor effects of both formulations were equivalent to Doxil™, and all were superior to the free doxorubicin. Liposomes composed of dSML 1-doxorubicin had a weaker antitumor effect and also a lower accumulation of the drug in the tumor (Fig 5, panel B). Formulation 2a-Dox-F2 has one less component (DSPC) than all other formulations, which could be an advantage in manufacture and quality control.
Figure 5.
Survival and biodistribution data of doxorubicin-loaded dSML liposomes on BALB/C mice bearing C-26 tumor. A single dose of 15 mg/kg liposomal doxorubicin or 10 mg/kg for free doxorubicin was administered i.v. on day 8 after the tumor inoculation. Panel (A): survival curves. 1-Dox: dSML1/DSPC/DSPE-PEG2000/α-tocopherol (33.0/61.8/5.0/0.2); 2a-Dox-F1: dSML2a/DSPC/DSPE-PEG2000/α-tocopherol (33.0/61.8/5.0/0.2); 2a-Dox-F2: dSML2a/DSPE-PEG2000/α-tocopherol (94.8/5.0/0.2) Log-rank analysis of the paired survival data: p = 0.0019 for 1-Dox, 2a-Dox-F1, 2a-Dox-F2, and Doxil vs PBS; P = 0.0018 for 1-Dox, 2a-Dox-F1, 2a-Dox-F2, and Doxil vs Free Dox. No significant difference between 1-Dox, 2a-Dox-F1, 2a-Dox-F2, and Doxil. Panel (B): liposomal doxorubicin biodistribution 48 hours after i.v. injection at 15 mg/kg.
In summary, we have created a new category of sterol-modified lipids (dSML) by linking sterols to both hydroxyl groups of glycerophosphocholine using a one pot synthesis. The library of dSML can be expanded by varying the combination of sterols, linkage types, and headgroups. dSML lipids preserve the condensing effect on lipid chain packing but do not transfer. They extend the lipid compositions that can be employed to control contents release from the liposome and dSML 2a can be used by itself to form a lipid vesicle. Doxorubicin encapsulated in dSML liposomes accumulated in murine C26 tumor and showed therapeutic effect equivalent to Doxil™. The promising results open the way for further therapeutic studies with water-soluble drugs such as, gemcitabine, erlotinib, vinorelbine, topotecan, floxuridine, vincristine and platinum compounds which are not yet therapeutically successful formulations in traditional liposome compositions. [1–3]
Supplementary Material
Footnotes
This work was supported by NIH EB003008, GM61851, and CA119343. We thank Nichole Macaraeg for the animal study, and Katherine Jerger for the leakage study.
Supporting information for this article is available on the WWW under http://www.angewandte.org.
References
- 1.Lammers T, Hennink WE, Storm G. Br J Cancer. 2008;99:392. doi: 10.1038/sj.bjc.6604483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torchilin VP. Nat Rev Drug Discov. 2005;4:145. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 3.Drummond DC, Noble CO, Hayes ME, Park JW, Kirpotin DB. J Pharm Sci. 2008;97:4696. doi: 10.1002/jps.21358. [DOI] [PubMed] [Google Scholar]
- 4.McMullen TP, Lewis RN, McElhaney RN. Biochemistry. 1993;32:516. doi: 10.1021/bi00053a016. [DOI] [PubMed] [Google Scholar]
- 5.Lai MZ, Duzgunes N, Szoka FC. Biochemistry. 1985;24:1646. doi: 10.1021/bi00328a012. [DOI] [PubMed] [Google Scholar]
- 6.Papahadjopoulos D, Nir S, Oki S. Biochim Biophys Acta. 1972;266:561. doi: 10.1016/0006-3002(72)90001-7. [DOI] [PubMed] [Google Scholar]
- 7.Senior J, Gregoriadis G. Life Sci. 1982;30:2123. doi: 10.1016/0024-3205(82)90455-6. [DOI] [PubMed] [Google Scholar]
- 8.Semple SC, Chonn A, Cullis PR. Biochemistry. 1996;35:2521. doi: 10.1021/bi950414i. [DOI] [PubMed] [Google Scholar]
- 9.Phillips MC, Johnson WJ, Rothblat GH. Biochim Biophys Acta. 1987;906:223. doi: 10.1016/0304-4157(87)90013-x. [DOI] [PubMed] [Google Scholar]
- 10.Torchilin VP, Weissig V. Liposomes: a practical approach. 2. Oxford University Press; Oxford; New York: 2003. [Google Scholar]
- 11.Lasic DD. Angew Chem Int Ed Engl. 1994;33:1685. [Google Scholar]
- 12.Martin B, Sainlos M, Aissaoui A, Oudrhiri N, Hauchecorne M, Vigneron JP, Lehn JM, Lehn P. Curr Pharm Des. 2005;11:375. doi: 10.2174/1381612053382133. [DOI] [PubMed] [Google Scholar]
- 13.Krafft MP, Riess JG. Biochimie. 1998;80:489. doi: 10.1016/s0300-9084(00)80016-4. [DOI] [PubMed] [Google Scholar]
- 14.Linderoth L, Peters GH, Madsen R, Andresen TL. Angew Chem Int Ed Engl. 2009;48:1823. doi: 10.1002/anie.200805241. [DOI] [PubMed] [Google Scholar]
- 15.Janout V, Regen SL. Bioconj Chem. 2009;20:183. doi: 10.1021/bc800296g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taubert A, Napoli A, Meier W. Curr Opin Chem Biol. 2004;8:598. doi: 10.1016/j.cbpa.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Huang Z, Szoka FC., Jr J Am Chem Soc. 2008;130:15702. doi: 10.1021/ja8065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis SC, Szoka FC., Jr Bioconjug Chem. 1998;9:783. doi: 10.1021/bc980047y. [DOI] [PubMed] [Google Scholar]
- 19.Kan CC, Yan J, Bittman R. Biochemistry. 1992;31:1866. doi: 10.1021/bi00121a040. [DOI] [PubMed] [Google Scholar]
- 20.Wybenga DR, Pileggi VJ, Dirstine PH, Di Giorgio J. Clin Chem. 1970;16:980. [PubMed] [Google Scholar]
- 21.Bolotin EM, Cohen R, Bar LK, Emanuel N, Ninio S, Lasic DD, Barenholz Y. J Liposome Res. 1994;4:455. [Google Scholar]
- 22.Huang SK, Mayhew E, Gilani S, Lasic DD, Martin FJ, Papahadjopoulos D. Cancer Res. 1992;52:6774. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.