Abstract
Sulfite oxidase catalyzes the terminal reaction in the degradation of sulfur amino acids. Genetic deficiency of sulfite oxidase results in neurological abnormalities and often leads to death at an early age. The mutation in the sulfite oxidase gene responsible for sulfite oxidase deficiency in a 5-year-old girl was identified by sequence analysis of cDNA obtained from fibroblast mRNA to be a guanine to adenine transition at nucleotide 479 resulting in the amino acid substitution of Arg-160 to Gln. Recombinant protein containing the R160Q mutation was expressed in Escherichia coli, purified, and characterized. The mutant protein contained its full complement of molybdenum and heme, but exhibited 2% of native activity under standard assay conditions. Absorption spectroscopy of the isolated molybdenum domains of native sulfite oxidase and of the R160Q mutant showed significant differences in the 480- and 350-nm absorption bands, suggestive of altered geometry at the molybdenum center. Kinetic analysis of the R160Q protein showed an increase in Km for sulfite combined with a decrease in kcat resulting in a decrease of nearly 1,000-fold in the apparent second-order rate constant kcat/Km. Kinetic parameters for the in vitro generated R160K mutant were found to be intermediate in value between those of the native protein and the R160Q mutant. Native sulfite oxidase was rapidly inactivated by phenylglyoxal, yielding a modified protein with kinetic parameters mimicking those of the R160Q mutant. It is proposed that Arg-160 attracts the anionic substrate sulfite to the binding site near the molybdenum.
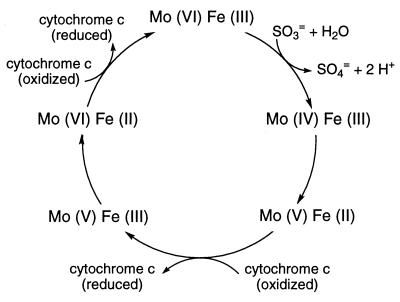
Sulfite oxidase catalyzes the oxidation of sulfite to sulfate, the terminal reaction in the oxidative degradation pathway of the sulfur-containing amino acids cysteine and methionine. The enzyme is a dimer of identical subunits and is located in the intermembrane space of mitochondria. Each 52-kDa subunit contains a small N-terminal heme domain and large C-terminal molybdopterin-binding domain (1). A single molybdenum atom at each active site of the enzyme is coordinated to the two dithiolene sulfurs of molybdopterin (2) and to the sulfur of Cys-207 (3). The reaction catalyzed by the enzyme is schematically represented in Fig. 1 and includes steps of sulfite binding, two-electron oxidative hydroxylation to form sulfate, two successive intramolecular one-electron transfers to the heme, and reoxidation of the heme by cytochrome c. Point mutations in the gene could generate enzyme molecules affected in any of these steps or in translocation to the mitochondrion. Thus the identification of such mutations in patients with sulfite oxidase deficiency could provide requisite information on the role of the affected amino acid residue in the catalytic activity of the enzyme.
Figure 1.
Schematic representation of the reaction catalyzed by sulfite oxidase. [Reproduced with permission from ref. 16 (Copyright 1997, Miami Children’s Hospital).]
Human sulfite oxidase deficiency is a debilitating disease characterized by severe neurological abnormalities, seizures, mental retardation, and dislocation of the ocular lenses that often leads to death in infancy (4). The phenotype of sulfite oxidase deficiency can arise from a mutation either in the sulfite oxidase gene (isolated sulfite oxidase deficiency) or in any of several genes involved in the synthesis of molybdopterin (molybdenum cofactor deficiency). In the latter case patients exhibit combined deficiencies of all molybdoenzyme activities. This article describes the design and application of procedures to isolate the mutant sulfite oxidase gene from patients diagnosed with isolated sulfite oxidase deficiency and to generate the recombinant form of the mutant protein for physicochemical characterization. In addition to the diagnostic utility of these studies, the identification of genetic defects causing sulfite oxidase deficiency has revealed point mutations leading to single amino acid changes that provide insight into the structure and function of the enzyme. One of those mutations, resulting in the replacement of Arg-160 with Gln, is described below.
MATERIALS AND METHODS
Chemicals and Reagents.
Reagents for RNA purification were from Promega, for PCR amplification from Perkin–Elmer/Cetus, and for DNA sequencing from Amersham. Reagents for cloning of PCR products were from Invitrogen. Cell culture media and supplements were from Life Technologies (Grand Island, NY). Phenylglyoxal was obtained from Sigma.
Quantitation of Urinary Metabolites.
Sulfite was measured using Macherey & Nagel sulfite test strips from Gallard Schlesinger and by monitoring at 550 nm the amount of cytochrome c reduced by aliquots of the urine sample in the presence of added rat liver sulfite oxidase. Thiosulfate (5) and S-sulfocysteine (6) were assayed as described. Oxypurines were quantitated using the procedure of Crawhall et al. (7) and urothione was measured as reported (8).
Fibroblast Culture Conditions.
Fibroblasts were cultured at the Cell Culture Facility, Duke University Comprehensive Cancer Center. Cells were grown at 37°C in an atmosphere of 5% CO2 to an approximate density of 2 × 107 cells per 150 cm2 flask in minimal essential media alpha with ribonucleosides supplemented with 10% bovine calf serum. Cells were assayed for sulfite oxidase activity as described (9).
Purification of mRNA from Cultured Fibroblasts.
RNA was routinely purified from ≈108 cells. The cell culture media was decanted from five flasks, and each flask was rinsed with 10 ml of ice-cold 140 mM NaCl, 8.1 mM Na2HPO4, 1.1 mM KH2PO4, 0.27 mM KCl, pH 7.4. The cells were released from the flasks by the addition of 8 ml of denaturing solution (25 g guanidine thiocyanate dissolved in 33 ml of 42 mM sodium citrate, 0.83% N-lauroylsarcosine, 0.2 mM 2-mercaptoethanol). The flask containing the denaturing solution was incubated on ice for ≈1 min; then the denaturing solution and cells were pipetted successively into the remaining four flasks allowing a 1-min incubation after each transfer. All flasks were then rinsed with an additional 4 ml of denaturing solution in reverse order relative to the initial cell disruption. The suspension was homogenized by five passes through a glass-Teflon homogenizer, and 1.2 ml of 2 M sodium acetate, pH 4.0, was added. The homogenate was extracted with 12 ml of phenol/chloroform/isoamyl alcohol (24:23:1) and centrifuged at 10,000 × g for 20 min at 4°C. The RNA in the aqueous phase was precipitated by the addition of an equal volume of isopropyl alcohol, and then pelleted by centrifugation at 10,000 × g for 15 min at 4°C. The RNA pellet was resuspended in 5 ml of denaturing solution, precipitated with an equal volume of isopropyl alcohol by centrifugation at 10,000 × g for 15 min at 4°C, rinsed with 75% ethanol, and resuspended in 0.5 ml 0.5× standard saline citrate (SSC; 1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7). Purification of mRNA was performed using the PolyATtract mRNA isolation system (Promega). The entire RNA sample from the previous step (≈1 mg) was heated to 65°C for 10 min, 13 μl of 20× SSC and 3 μl of biotinylated oligo(dT) probe were added and the solution was allowed to cool to room temperature. Streptavidin-paramagnetic particles preequilibrated with 0.5× SSC were added to the annealing mixture and incubated at room temperature for 10 min. The streptavidin-paramagnetic particles were washed four times with 300 μl 0.1× SSC by resuspension and magnetic capture. The mRNA was isolated by resuspension of the final streptavidin-paramagnetic particle pellet in 0.1 ml of water, magnetic recapture of the streptavidin-paramagnetic particles, and elution with an additional 0.15 ml of water. Total mRNA isolated was estimated to be 5 μg.
Synthesis of cDNA and PCR Amplification.
For first strand cDNA synthesis, a reaction mixture containing 3 μl mRNA, 4 μl 25 mM MgCl2, 2 μl 10× PCR buffer (500 mM KCl/100 mM Tris⋅HCl, pH 8.3), 2 μl each of dGTP, dATP, dTTP, dCTP (0.1 M), 20 units RNase inhibitor, 50 units reverse transcriptase, and 5 pmol of oligo(dT)16 in a total volume of 20 μl was incubated at room temperature for 10 min and then at 42°C for 15 min. The reverse transcriptase was denatured by heating to 99°C for 5 min, and the reaction was allowed to cool to room temperature. For PCR amplification of the cDNA, 4 μl of 25 mM MgCl2, 8 μl of 10× PCR buffer, 66.5 μl of water, and 0.5 unit of AmpliTaq DNA polymerase were added, and the mixture was heated to 95°C. Primers HN1B (TCGGTGTAGGGCTGCCATGGAGTCAACAC) and HN2B (GGTGGCTCCTTTCCATGGTCATGGGGAG) were added to a final concentration of 150 nM, and the reaction was cycled at 60°C for 30 s, 72°C for 90 s, 95°C for 30 s for a total of 35 cycles. The products of the PCR amplification were cloned into the pCR II vector and transformed into TA One Shot competent cells, and the inserts were sequenced.
Site-Directed Mutagenesis, Plasmids, and Strains.
Mutations were introduced into pRG118 as described (3). The human sulfite oxidase R160Q expression vector pRG118-R160Q was constructed by using primer 118R160Q (TCTTCTTCACCCAGAACCATCTGCCT) to replace the guanine at position 479 with adenine, and selection primer RG118S (GACCTGCAGTCTAGATTGGCTGTTTTGGCG), which changes the unique HindIII site in the multiple cloning region of pRG118 to XbaI. The human sulfite oxidase R160K expression plasmid pRG118-R160K was constructed by using selection primer RG118S and mutagenic primer 118R160K (TCTTCTTCACCAAGAACCATCTGCCT), which changes Cyt-478 to Ade and Gua-479 to Ade.
Analysis of the Molybdenum Center of Native Sulfite Oxidase and R160Q.
Molybdenum was quantitated by atomic absorption by using a Perkin–Elmer Z3030 instrument. Samples were prepared for analysis by wet ashing in nitric acid as described (10). The molybdenum domains of native sulfite oxidase and the R160Q mutant were prepared and spectrally analyzed as described (3).
Inhibition by Phenylglyoxal.
Native sulfite oxidase at a concentration of 2.4 μM in 0.1 M NaHCO3, 50 mM bicine, pH 8.3, was incubated with 5.2 mM phenylglyoxal at room temperature for 1 h. At intervals, aliquots were removed and assayed for sulfite oxidase activity. Activity measurements were corrected for the rate of nonenzymatic reduction of cytochrome c by sulfite.
RESULTS
Case Report.
The patient was born to first cousin consanguineous parents of Dutch descent and exhibited developmental delay and hypotonia during the first 2 years of life with regression beginning at ≈21 months. She has two healthy siblings. She had two seizures at ≈5 months of age but none since. At age 2 years bilateral dislocation of the lenses was detected, and calcification of the basal ganglia and hypoplasia of the cerebellar vermis were documented on computed tomography and magnetic resonance imaging scans. Ataxia, dystonia, and choreoathetoid movements became progressively worse. She had mild eczema, fine hair, and delayed teething but normal nails and joints. She had significant irritability and spasms needing sedation at night. At age 5 years, she had significant failure to thrive with feeding problems, aspiration, and generalized hypertonia.
Biochemical Laboratory Results.
Laboratory results obtained on urine and fibroblast cultures from the patient are summarized in Table 1. Sulfur metabolites, including sulfite, thiosulfate and S-sulfocysteine were all elevated, indicative of sulfite oxidase deficiency. Uric acid excretion was somewhat low; however, there was no elevation of urinary xanthine or hypoxanthine consistent with normal xanthine dehydrogenase activity in this individual. The presence of adequate amounts of molybdenum cofactor was confirmed by the finding of a normal level of urothione, the metabolic degradation product of the molybdenum cofactor (8). No sulfite oxidase activity could be detected in cultured fibroblasts. These results document the occurrence of isolated sulfite oxidase deficiency in this patient.
Table 1.
Biochemical laboratory results
| Result | Patient | Reference values |
|---|---|---|
| Urinary sulfur metabolites | ||
| Sulfite (test strip), μg/liter | 100–250 | None detected |
| Sulfite (quantitative assay), μg/liter | 108–211 | None detected |
| Thiosulfate, mM | 0.297–1.632 | <0.100 |
| S-Sulfocysteine, mM | 0.240 | 0.006–0.030 |
| Urinary oxypurines | ||
| Uric acid, mM | 0.14 | 0.44–4.50 |
| Xanthine, mM | 0.04 | 0–0.46 |
| Hypoxanthine, mM | 0.05 | 0–0.18 |
| Urothione, μM | 0.23 | 0.16–1.9 |
| Fibroblast sulfite oxidase activity, units/g soluble protein | None detected | 6–23 |
Identification of the Genetic Defect Causing Sulfite Oxidase Deficiency.
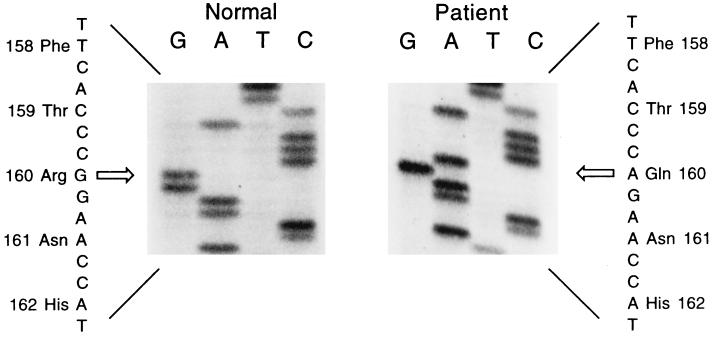
The mutant sulfite oxidase gene was cloned from fibroblasts isolated from the patient as described in Materials and Methods. Messenger RNA purified from fibroblasts was used to synthesize cDNA from which the mutant sulfite oxidase gene was amplified by PCR by using primers flanking the start and stop codons of the sulfite oxidase coding sequence. A single mutation in the sequence, a guanine to adenine transition, was discovered at nucleotide 479 (see Fig. 2). The result of the G479A mutation is the single amino acid substitution R160Q. The guanine to adenine transition at position 479 was identified in several clones obtained from the patient and was the only mutation observed. This finding suggests that both alleles of the sulfite oxidase gene in this patient harbor the same mutation and is consistent with the consanguinity of the parents.
Figure 2.
Nucleotide sequence analysis of sulfite oxidase. At nucleotide 479 a G → A transition of the patient’s gene is compared with a representative normal clone. The arrow indicates the position of the base substitution.
Expression and Characterization of the Sulfite Oxidase R160Q Mutant.
The R160Q genetic mutation identified in the patient was constructed and expressed in E. coli as described in Materials and Methods. The human recombinant sulfite oxidase R160Q protein was purified from E. coli as described (11). The mutant enzyme exhibited no unusual behavior throughout the purification procedure, although the specific activity of the enzyme (23.5 units/mg) was markedly lower than that of the native enzyme (1,100 units/mg) under the standard assay conditions described previously (11). Molybdenum analysis indicated that the R160Q mutant protein contained 0.91 mol Mo/mol heme, demonstrating that the mutation does not affect incorporation of the metal into the active site of the enzyme. However, the specific activity of the molybdenum center of the R160Q enzyme as measured by the sulfite-dependent reduction of ferricyanide, an activity not requiring the heme domain, was also significantly lower: 0.95 units/mg for the mutant vs. 66 units/mg for the native enzyme.
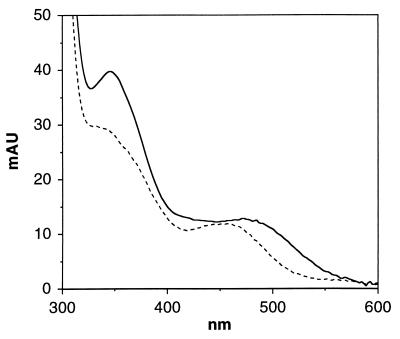
The molybdenum domains of native sulfite oxidase and of the R160Q mutant were prepared by using recombinant proteins, both with the K108R substitution introduced into the hinge region to enhance trypsin susceptibility at that specific site (3). The proteins were treated with trypsin at 4°C for 18 h, trypsin inhibitor was added, and the absorption spectra of the isolated domains were obtained by on-line diode array spectroscopy during HPLC chromatographic separation of the resultant molybdenum and heme domains. The absorption spectrum of the molybdenum domain of the R160Q mutant shows distinct differences from that of the native (see Fig. 3) including a blue shift in the 480-nm absorption band and a significant decrease in the intensity of the 350-nm band, suggestive of altered geometry of molybdenum coordination at the active site. The yield of intact molybdenum fragment from the R160Q mutant was ≈40% of that obtained from an equivalent amount of native protein using identical trypsin cleavage conditions, and several peaks of low molecular weight material were identified in the chromatogram of the R160Q cleavage mixture. This suggests that the R160Q mutation results also in a slightly more open structure that is somewhat susceptible to cleavage by trypsin at sites other than at residue 108. The molybdenum domain obtained from the R160Q mutant was eluted in the expected position from the HPLC gel filtration column and was unchanged in spectral or elution properties after incubation at 4°C for several days, indicating that the isolated domain was stable and that no further degradation occurred after the addition of trypsin inhibitor.
Figure 3.
Absorption spectra of the molybdenum domain of native sulfite oxidase (—) and the R160Q mutant (– – –). The samples were matched in absorbance at 280 nm.
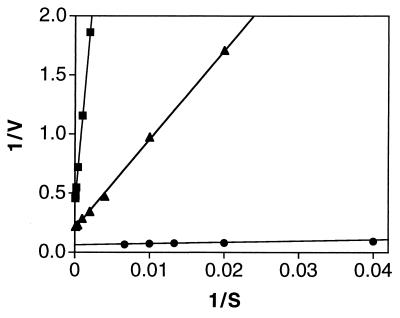
The kinetic constants Km (sulfite) and kcat (per subunit) indicated an extreme departure from the equivalent parameters associated with the native enzyme (see Fig. 4). The native enzyme was found to have a Km for sulfite of 17 μM, and a kcat of 16 s−1. The Km for sulfite and kcat of R160Q were determined to be 1.7 mM and 2.4 s−1, respectively. The increase in Km combined with the decrease in kcat results in a decrease of nearly 1,000-fold in the apparent second order rate constant kcat/Km.
Figure 4.
Kinetic analysis of native sulfite oxidase (•), sulfite oxidase R160Q (▪) and sulfite oxidase R160K (▴). The dependence of velocity on substrate concentration was plotted and fit to the equation V = kcat[S]/Km + [S] where V is the initial velocity of the reaction expressed as mol sulfite consumed per s per mol enzyme subunit. The following parameters were derived: Km = 17 μM for native, 1.7 mM for R160Q and 332 μM for R160K; kcat = 16 s−1 for native, 2.4 s−1 for R160Q and 4.8 s−1 for R160K; kcat/Km = 9.4 × 105 M−1·s−1 for native, 1.4 × 103 M−1·s−1 for R160Q and 1.4 × 104 M−1·s−1 for R160K. The reciprocal plots shown in the figure are based on a limited portion of the data set to illustrate the altered kinetic behavior of the two mutants.
The R160K mutant was constructed to determine whether a positively charged lysine could serve as a functional replacement for arginine in the active site. The kinetic parameters Km and kcat for the R160K mutant were found to be intermediate in value between those of the native protein and the R160Q mutant, with a Km for sulfite of 332 μM, and kcat of 4.8 s−1 (Fig. 4).
Arginine Modification Studies.
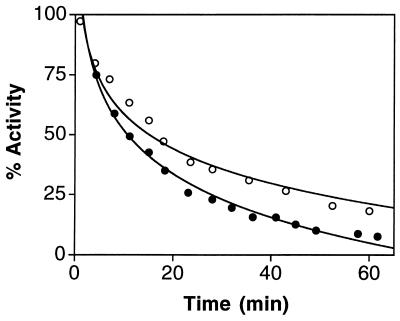
The observation that replacement of Arg-160 with Gln or Lys has a significant effect on the activity of sulfite oxidase suggested that this residue might be accessible to chemical modification by phenylglyoxal (12). Enzyme activity was assayed over a period of 60 min incubation with 5.2 mM reagent and showed a progressive decrease (see Fig. 5), approaching a state with nearly complete loss of activity when assayed under standard assay conditions (0.4 mM sulfite). Final residual activity was ≈20% that of wild type when enzyme assays were carried out by using 0.1 M sodium sulfite, as would be expected if the primary target of modification were the Arg-160 residue.
Figure 5.
Inactivation of sulfite oxidase by phenylglyoxal. Activity was measured using 0.4 mM sulfite (•) and 0.1 M sulfite (○). Other conditions are described in Materials and Methods.
DISCUSSION
The R160Q mutant of sulfite oxidase was derived from a patient exhibiting symptoms previously identified with deficiency of the enzyme. The high content of sulfite, thiosulfate, and S-sulfocysteine in the patient’s urine, the normal oxypurine profile and urothione level, and the absence of sulfite oxidase activity in fibroblast extracts confirmed the diagnosis of isolated sulfite oxidase deficiency. These findings indicated that a mutation in the sulfite oxidase gene has caused the absence of functional sulfite oxidase in this patient.
The use of recombinant techniques has allowed the identification of the single nucleotide replacement in the patient’s sulfite oxidase gene and the heterologous expression of the resulting R160Q mutant enzyme in E. coli. The mutant protein was expressed in E. coli at the same levels as native human sulfite oxidase and was amenable to purification by the procedure developed for the native enzyme. The purified enzyme is indistinguishable from the native in physical properties as well as in the content of heme, molybdopterin, and molybdenum. Differences in the molybdenum centers of the native and mutant proteins were revealed, however, by absorption spectroscopy of the isolated molybdenum domains. Studies of the molybdenum ligand field by extended x-ray absorption fine structure analysis carried out previously (13) have provided no evidence for Arg as a direct ligand to the metal; thus, it was somewhat of a surprise that the absorption properties of the R160Q mutant were altered to the extent observed. It would appear from these results that the modified residue is in fact quite near the metal or that it induces structural distortions at or near the catalytic site.
Detailed kinetic analysis has shown that the mutant protein has a greatly increased Km for sulfite and a marked decrease in kcat resulting in nearly a 1,000-fold decrease in the second order rate constant kcat/Km. The altered kinetic properties of the R160Q mutant protein are undoubtedly responsible for the in vivo state of sulfite oxidase deficiency in the patient. The residual catalytic activity of the R160Q enzyme, although readily monitored in samples of purified recombinant protein, was too low to be detected in cultured fibroblasts where sulfite oxidase expression is very limited. However, the residual catalytic competence of the R160Q sulfite oxidase may explain the relatively milder course of the disease in this particular patient. The more severe cases of sulfite oxidase deficiency, characterized by frequent seizures, often uncontrolled by medication, and death within a few days of birth, presumably result from a complete absence of the sulfite oxidase protein subsequent to gene mutations that lead to early termination of translation or regulatory mutations with drastic effects on transcription or translation.
The elevation in Km for sulfite in the R160Q mutant is in accord with a role for Arg-160 in attracting the negatively charged sulfite molecule to the catalytic site. Additional support for the presence of this residue at the active site was obtained from examination of the effect of phenylglyoxal on native enzyme. The enzyme was rapidly inactivated by this reagent yielding a modified protein with kinetic parameters mimicking those of the R160Q mutant. That is, the modified protein retained <5% residual activity when assayed with 0.4 mM sulfite (essentially saturating concentration for the native enzyme), but residual activity was close to 20% when the assays were carried out with 0.1 M sulfite, showing that the Km of the phenylglyoxal-modified protein for sulfite was increased just as was observed with the R160Q mutant. These data suggest that Arg-160 is accessible to and reactive with the phenylglyoxal.
Introduction of a positively charged lysine residue in place of arginine yielded a protein that exhibited kinetic parameters Km and kcat intermediate between those of the native and R160Q proteins. It is likely that the positive charge of the lysine residue does not occupy the same position in the active site of the enzyme as that of the arginine residue due to the altered length of the side chain; in addition, the arginine guanidino group may be better suited to attract the divalent sulfite anion.
Arg-160 is conserved in sulfite oxidases from numerous sources and also in the assimilatory nitrate reductases (14). It is reasonable to conclude that in the nitrate reductases, the arginine residue is involved in attracting the nitrate anion to the active site. The fact that the R160Q mutant displays a marked decrease in kcat in addition to the increase in Km, suggests, however, that in addition to its role of acting as an attractant for the substrate, the Arg residue may serve other functions in the overall catalytic mechanism of these enzymes.
Acknowledgments
We thank Mr. Ralph Wiley for assistance in purification of the recombinant sulfite oxidase. This work was supported by National Institutes of Health Grants GM00091 and GM44283.
Note
The crystal structure of chicken liver sulfite has recently been completed (15) and shows that the arginine residue corresponding to Arg-160 in the human sequence is localized in the molybdenum active site, in full accord with the findings reported here.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Rajagopalan K V. In: Molybdenum and Molybdenum-Containing Enzymes. Coughlan M P, editor. Oxford, U.K.: Pergamon; 1980. pp. 241–272. [Google Scholar]
- 2.Rajagopalan K V, Johnson J L. J Biol Chem. 1992;267:10199–10202. [PubMed] [Google Scholar]
- 3.Garrett R M, Rajagopalan K V. J Biol Chem. 1996;271:7387–7391. [PubMed] [Google Scholar]
- 4.Johnson J L, Wadman S K. In: The Metabolic and Molecular Bases of Inherited Disease, 7th edition. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw-Hill; 1995. pp. 2271–2283. [Google Scholar]
- 5.Shih V E, Carney M M, Mandell R. Clin Chim Acta. 1979;95:143–145. doi: 10.1016/0009-8981(79)90348-6. [DOI] [PubMed] [Google Scholar]
- 6.Johnson J L, Rajagopalan K V. J Inherited Metab Dis. 1995;18:40–47. doi: 10.1007/BF00711371. [DOI] [PubMed] [Google Scholar]
- 7.Crawhall J C, Itiaba K, Katz S. Biochem Med. 1983;30:261–270. doi: 10.1016/0006-2944(83)90092-3. [DOI] [PubMed] [Google Scholar]
- 8.Johnson J L, Rajagopalan K V. Proc Natl Acad Sci USA. 1982;79:6856–6860. doi: 10.1073/pnas.79.22.6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih V E, Abroms I F, Johnson J L, Carney M, Mandell R, Robb R M, Cloherty J P, Rajagopalan K V. N Engl J Med. 1977;297:1022–1028. doi: 10.1056/NEJM197711102971902. [DOI] [PubMed] [Google Scholar]
- 10.Johnson J L. Methods Enzymol. 1988;158:371–382. doi: 10.1016/0076-6879(88)58069-2. [DOI] [PubMed] [Google Scholar]
- 11.Garrett R M, Rajagopalan K V. J Biol Chem. 1994;269:272–276. [PubMed] [Google Scholar]
- 12.Takahashi K. J Biol Chem. 1968;243:6171–6179. [PubMed] [Google Scholar]
- 13.George G N, Garrett R M, Prince R C, Rajagopalan K V. J Am Chem Soc. 1996;118:8588–8592. [Google Scholar]
- 14.Meyer C, Gonneau M, Caboche M, Rouzé P. FEBS Lett. 1995;370:197–202. doi: 10.1016/0014-5793(95)00827-v. [DOI] [PubMed] [Google Scholar]
- 15.Kisker C, Schindelin H, Pacheco A, Wehbi W A, Garrett R M, Rajagopalan K V, Enemark J H, Rees D C. Cell. 1997;91:973–983. doi: 10.1016/s0092-8674(00)80488-2. [DOI] [PubMed] [Google Scholar]
- 16.Johnson J L, Garrett R M, Rajagopalan K V. Int Pediatr. 1997;12:22–26. [Google Scholar]