Abstract
Background
Few cohort studies have adequate numbers of carefully reviewed deaths to allow an analysis of unique and shared risk factors for cause-specific mortality. Shared risk factors could be targeted for prevention of premature death and the study of longevity.
Methods
A total of 5,888 community-dwelling persons aged 65 years or older living in four communities in the United States participated in the Cardiovascular Health Study cohort. Participants were initially recruited from 1989 to 1990; an additional 687 black participants were recruited in 1992–1993. The average length of follow-up was 16 years. Total and cause-specific mortality, including cardiovascular disease, stroke, cancer, dementia, pulmonary disease, infection, and other cause, were examined as outcomes. Variables previously associated with total mortality were examined for each cause of death using Cox proportional hazard models.
Results
Multiple risk factors were related to total mortality. When examining specific causes, many factors were related to cardiovascular death, whereas fewer were related to other causes. For most causes, risk factors were specific for that cause. For example, apolipoprotein E ϵ4 was strongly associated for dementia death and forced vital capacity with pulmonary death. Age, male sex, markers of inflammation, and cognitive function were related to multiple causes of death.
Conclusions
In these older adults, associations of risk factors with a given cause of death were related to specific deficits in that same organ system. Inflammation may represent a common pathway to all causes of death.
Keywords: Mortality risk, Cause of death, Elderly participants
OLDER adults are heterogeneous in the prevalence and patterns of chronic disease and related disability (1). We have previously shown that multiple aspects of chronic disease and lifestyle jointly and independently contributed to mortality risk in older adults (2). Together, a number of these factors explained a large part of the association of older age with mortality but did not explain the excess mortality in men relative to women. It is not known whether these associations would differ for specific causes of death and whether risk factors would be similar or different across causes. The extent to which risk factors might be shared could indicate common pathways to longevity and a potential for greater longevity through reduction of shared risk factors.
Most previous epidemiological studies have assessed risk factors for either total mortality or a specific cause of death, such as cardiovascular disease or a specific cancer. A literature review documents several risk factors that appear to be similar across diseases for some of the major causes of death. As examples, cigarette smoking is a risk factor for cardiovascular disease (3), stroke (4), chronic lung disease (5), and lung and other cancers (6), whereas obesity and glucose intolerance are risk factors for cardiovascular disease and stroke (7) and colon and other cancers (8,9). Education level and other markers of socioeconomic status are also risk factors that are common to several major causes of death (10). Inflammatory markers, such as C-reactive protein (CRP) and interleukin-6 (IL-6) (11), and the genetic marker, the apolipoprotein E ϵ4 (ApoE ϵ4) allele (12,13), appear to be nonspecific markers that may reflect an accelerated aging process and their absence may contribute to longevity. These associations for specific causes of death may or may not hold within a single cohort.
In this report, we examined mortality rates in the Cardiovascular Health Study (CHS) cohort after 16 years of follow-up and reevaluated risk factors for total and cause-specific mortality. We sought to determine the common and unique risk factors for several categories of specific causes of death.
METHODS
Study Population
The CHS is an ongoing, prospective observational study designed to determine the risk factors, consequences, and natural history of cardiovascular disease in men and women aged 65 years and older. A total of 5,888 men and women were enrolled in 1989–1990 (n = 5,201) and 1992–1993 (n = 687) from four U.S. communities: Sacramento County, California, Forsyth County, North Carolina, Washington County, Maryland, and Allegheny County, Pennsylvania. A random sample of age-eligible Medicare beneficiaries and age-eligible household members were recruited. Exclusion criteria were being wheelchair bound in the home, unable to participate in a clinic examination at the field center, undergoing active treatment for cancer, or planning to move in less than 3 years (2). The 5-year mortality report (14) included the original cohort of 5,201 men and women, whereas this report includes the original and added minority cohort. Protocols were approved by each participating institutional review board. All participants gave informed consent. Analysis including ApoE ϵ4 was restricted to those giving specific consent for analysis of genetic data.
Baseline Evaluation
Participants completed standardized interviews and an extensive examination at the field center in 1989–1990 for the original and in 1992–1993 for the minority cohort. The baseline data sets for the original and minority cohorts were combined. Although baseline data collection was comparable for many variables, echocardiography and nutritional assessment were not assessed in the minority cohort, thus these variables were not included in this analysis.
Examinations included demographic characteristics, medications used, health history, noninvasive testing, and blood assays along with self-assessed health status, health habits, physical activity, and physical function (2). Race was defined by self-report as white, black, or other. The few of other race were grouped with the whites for analysis purposes. Medication use in the past 2 weeks was assessed. Only diuretic use was included here based on a significant association in the previous 5-year follow-up. Health history included self-report of physician diagnosis of myocardial infarction, angina, congestive heart failure (CHF), intermittent claudication, stroke, transient ischemic attack, asthma, emphysema and chronic bronchitis (chronic lung disease), hypertension, diabetes, renal disease, arthritis, and cancer (2). Self-reported diagnoses of cardiovascular disease were validated according to standardized criteria including medications used and/or medical record review (15). Standardized examinations performed on all participants included electrocardiogram (15,16,17), spirometry (18), ankle–arm index (19), and carotid ultrasound (20) to measure the maximal stenosis and internal and common carotid artery wall thickness. Other assessments included blood pressure, height, and weight. Diabetes was defined by self-report and medication use or the presence of fasting glucose level greater than 126 mg/dl (21). Hypertension was defined as self-report of a diagnosis of hypertension confirmed by medication use or by a measured blood pressure of 140/90 mmHg or greater. Depressive symptoms were assessed using the Center for Epidemiological Studies-Depression scale questionnaire (22). Cognitive function was assessed with the Mini-Mental State Examination (23) and the Digit Symbol Substitution Test (DSST) (24). Performance-based measures of physical function included gait speed in meters per second at usual pace and grip strength in kilograms assessed with an isometric dynamometer (2). Phlebotomy was performed under fasting conditions, and the blood was analyzed by the Laboratory for Clinical Biochemistry Research at the University of Vermont for levels of glucose, total cholesterol, and high-density lipoprotein cholesterol, serum albumin, creatinine, and fibrinogen. Low-density lipoprotein cholesterol was calculated (25). ApoE ϵ4 genotype was assessed and grouped as the presence or absence of at least one ϵ4 allele (26,27). CRP (28) and IL-6 (29) were measured by use of enzyme-linked immunosorbent assays.
Ascertainment of Mortality and Cause of Death
Study participants were followed for an average of 13 years (<1 to 16 years), from 1989–1990 to 2006 (16 years) for the original cohort and from 1992–1993 to 2006 (13 years) for the minority cohort. Mortality, hospitalizations, and cardiovascular events were ascertained at annual examinations and 6-month telephone interviews. If the participant was unavailable, a proxy was interviewed. Deaths were also identified from obituaries. Data collection included information from medical records, proxy interviews, and death certificates. Follow-up for vital status was 100% complete. For all deaths, the cause of death was considered to be the underlying cause rather than the immediate cause of death. Cause of death was assigned by a committee of physicians who were without knowledge of prior examination findings. Cause of death was first determined to be cardiovascular or noncardiovascular. Cardiovascular deaths included atherosclerotic coronary disease, cerebrovascular disease (stroke), other atherosclerotic disease (such as aortic aneurysm), and other vascular disease (such as valvular heart disease or pulmonary embolism). The noncardiovascular deaths were first classified into 19 disease and organ system categories, then collapsed into five categories: dementia, cancer, pulmonary disease, infection, and “other” cause (30). Dementia death was classified when there was evidence of advanced disability due to dementia prior to death and no evidence of another cause. Cancer deaths included all cancer types. Pulmonary death included chronic obstructive pulmonary disease, pulmonary embolism, and respiratory failure. Infectious death included pneumonia, sepsis, and other infections. Death secondary to “other” causes included renal failure, liver failure, gastrointestinal (GI) disease (GI hemorrhage, perforated viscus, other GI disorders), trauma, amyotrophic lateral sclerosis, Parkinson’s disease, epilepsy, metabolic disease, amyloidosis, hip fracture, failure to thrive, myelodysplastic syndrome, and other musculoskeletal diseases.
Analysis
Mortality rates were calculated per 100 person-years. Survival curves for the CHS cohort were compared with an age-, race-, and sex-matched sample from the U.S. population. The CHS survival curves were computed using the Kaplan–Meier estimator with left truncation (entry) at baseline age. The population curves were computed using Hakulinen’s method (31), in which person-time was computed for each participant starting at recruitment and ending at the end of follow-up. In these curves, the probability of death was computed for each day from age–sex–race-specific mortality tables derived from the U.S. 1990 Census data by Therneau and Offord (32), and the expected numbers of deaths are aggregated across all study participants at risk. Both of the CHS and the U.S. estimated curves are based on assumptions that the risk at a certain age is the same regardless of age at entry into CHS. The census-based estimate was computed from the probability that a person of any given age would die during the following year.
The baseline characteristics described above that were previously examined as potential risk factors for total mortality were first reexamined using Cox proportional hazards models. Each risk factor was examined in either quintiles of events (weight, physical activity, forced vital capacity [FVC], systolic blood pressure [SBP], fasting glucose, albumin, creatinine, IL-6, and DSST) based on the 5-year models 2 or clinical meaningful groupings (age, smoking, carotid stenosis, ankle–brachial index, self-reported health, CRP, and instrumental activities of daily living [IADL] impairments). Newer models were compared with each other but could not be specifically compared with the previous 5-year mortality analysis for several reasons. First, we wanted to include race, which was not included in the previous report. Second, the risk factor assessment was slightly different in the minority cohort, and third, the coding of some variables had been changed. Thus, we could not directly replicate the previous analysis. However, in preliminary analysis, a model based on most variables from the 5-year mortality analysis was compared with (a) a full model with 70 variables, (b) a “best predictive” model, and (c) the original model with IL-6, CRP, and ApoE ϵ4 added. The best predictive model was selected from the 70 variables by all-subsets branch-and-bound search using the method of Lawless and Singhal (33). These analyses showed that the prediction from the original model was similar to those other models in that the areas under the receiver operating characteristic curves were not significantly different. Thus, for total mortality, we present a model based on the variables from the 5-year analysis plus the newer risk factors of race, IL-6, CRP, and ApoE ϵ4. Because informed consent was more limited for the genetics data, the sample size was decreased from 5,888 to 5,222 for models including ApoE ϵ4 but the models with the larger number were not substantively different.
To evaluate the commonality of risk factors across cause of death, separate models for each cause were tested using the same set of variables from the model for total mortality. Deaths from a cause other than the primary cause of interest were censored for each model, that is, we censored survival times at competing causes of death (34). We repeated the analyses modeling crude incidence of death, that is, when a participant died of one cause, we set time of death for competing causes to the indefinite future, representing the fact that death from any other cause is then impossible (35). These two analyses gave very similar hazard ratios, so we present the first approach. All analyses were conducted using R version 2.7.1 (R Foundation, Vienna, Austria).
RESULTS
Participants were aged 65 years and older at baseline with a mean age of 72.8 years; 15.7% were black, 84.3% were white or other ethnic group, and 57.6% were women. Though somewhat healthier than the general population, chronic conditions were common and more common in blacks than in whites. Table 1 provides a detailed description of men and women by race.
Table 1.
Characteristics of Cardiovascular Health Study Participants at Baseline
| White Women (n = 2,812) | Black Women (n = 581) | White Men (n = 2,152) | Black Men (n = 343) | All (n = 5,888) | |
| Demographics | |||||
| Age (y), M (SD) | 72.4 (5.4) | 73.1 (5.7) | 73.4 (5.7) | 72.7 (5.8) | 72.8 (5.6) |
| Currently married vs not (%) | 58 | 34 | 85 | 66 | 66 |
| High school graduate or higher level (%) | 74 | 54 | 73 | 56 | 71 |
| Annual household income >$50,000 (%) | 12 | 3 | 18 | 7 | 13 |
| Behavioral risk factors | |||||
| Weight (lbs), M (SD) | 146.3 (29.3) | 166.2 (35.6) | 174.4 (27.2) | 177.1 (31.5) | 160.3 (32.4) |
| BMI, M (SD) | 26.3 (5) | 29.6 (6) | 26.4 (3.7) | 26.7 (4.2) | 26.7 (4.7) |
| Smoking history (%) | |||||
| Never smoker | 57 | 59 | 32 | 32 | 47 |
| Current smoker | 12 | 13 | 10 | 20 | 12 |
| Former smoker | 31 | 28 | 58 | 48 | 42 |
| Pack-years (in smokers), M (SD) | 553.3 (7217.7) | 1393 (11647.5) | 326.2 (5341.2) | 3786.8 (19051.1) | 739.8 (8369.1) |
| Physical activity (kcal /wk), M (SD) | 1887.9 (4961.7) | 939.7 (1382.2) | 2352.9 (5997.7) | 2085.9 (9327.4) | 1975.7 (5504.9) |
| Health history | |||||
| Self-rated health (%) | |||||
| Excellent | 3 | 9 | 3 | 8 | 4 |
| Very good | 20 | 35 | 18 | 32 | 21 |
| Good | 37 | 33 | 38 | 35 | 37 |
| Fair | 26 | 17 | 25 | 17 | 24 |
| Poor | 14 | 6 | 15 | 9 | 13 |
| Coronary heart disease (prevalent), % | 15 | 20 | 26 | 17 | 20 |
| Congestive heart failure (prevalent), % | 4 | 7 | 5 | 6 | 5 |
| Stroke (%) | 3 | 6 | 5 | 9 | 4 |
| Diabetes (ADA criteria), % | 12 | 25 | 18 | 26 | 16 |
| Hypertension (%) | 57 | 78 | 54 | 65 | 59 |
| Noninvasive examinations | |||||
| Forced vital capacity (l), M (SD) | 2.5 (0.6) | 2.1 (0.6) | 3.6 (0.8) | 3.1 (0.8) | 2.9 (0.9) |
| Carotid stenosis >75% (%) | 1 | 1 | 1 | 2 | 1 |
| Ankle–arm index <0.9 (%) | 11 | 19 | 13 | 25 | 13 |
| Major ECG abnormality | 23 | 36 | 36 | 45 | 30 |
| Systolic BP (mmHg), M (SD) | 135.9 (21.7) | 143.8 (23.8) | 135.2 (21.1) | 139 (21.4) | 136.6 (21.8) |
| Diastolic BP (mmHg), M (SD) | 68.8 (11.1) | 74.4 (11) | 71.4 (11.3) | 76.2 (11.7) | 70.7 (11.4) |
| Blood tests | |||||
| Fasting glucose (mg/dl)*, M (SD) | 104.2 (1.2) | 112.5 (1.4) | 109.7 (1.3) | 111.3 (1.4) | 107.4 (1.3) |
| HDL cholesterol (mg/dl), M (SD) | 58.7 (16) | 60.8 (15.3) | 46.9 (12.5) | 52.2 (13.9) | 54.2 (15.7) |
| LDL cholesterol (mg/dl), M (SD) | 135 (36.6) | 132.8 (37.5) | 123.5 (33) | 122.3 (34) | 129.8 (35.7) |
| Serum creatinine (mg/dl), M (SD) | 0.9 (0.3) | 1.0 (0.5) | 1.2 (0.3) | 1.3 (0.8) | 1.1 (0.4) |
| Serum albumin (mg/dl), M (SD) | 4.0 (0.3) | 4.0 (0.3) | 4.0 (0.3) | 4.0 (0.3) | 4.0 (0.3) |
| CRP (mg/l)*, M (SD) | 2.5 (2.7) | 3.7 (3.0) | 2.4 (2.8) | 3.0 (2.8) | 2.6 (2.8) |
| IL-6 (pg/ml), M (SD) | 2.0 (1.8) | 2.5 (2.3) | 2.4 (1.9) | 2.3 (1.9) | 2.2 (1.9) |
| ApoE ϵ4 allele carrier (%) | 25 | 30 | 24 | 35 | 25 |
| Physical and cognitive function | |||||
| Gait speed (m/s), M (SD) | 0.8 (0.2) | 0.8 (0.3) | 0.9 (0.2) | 0.9 (0.3) | 0.9 (0.2) |
| ADL impairment (%) | 8 | 19 | 5 | 8 | 8 |
| IADL impairment (%) | 30 | 34 | 19 | 18 | 26 |
| Grip strength (lb), M (SD) | 21.7 (5.9) | 23.3 (6.6) | 37 (8.8) | 37.3 (9.7) | 28.4 (10.5) |
| MMSE score (max = 30), M (SD) | 28 (2.1) | 25.7 (3.6) | 27.7 (2.6) | 25 (3.9) | 27.5 (2.8) |
| DSST score, M (SD) | 39.7 (12.8) | 29.1 (13.6) | 35.5 (12.8) | 26.3 (12.6) | 36.4 (13.5) |
Notes: ADA = American Diabetes Association; ADL = activities of daily living; ApoE ϵ4 = apolipoprotein E e4; BP = blood pressure; BMI = body mass index; CRP = C-reactive protein; CVD = cardiovascular disease; DSST = Digit Symbol Substitution Test; ECG = electrocardiogram; HDL = high-density lipoprotein; IADL = instrumental activities of daily living; IL-6 = interleukin-6; LDL = low-density lipoprotein; MMSE = Mini-Mental State Examination.
*Geometric mean.
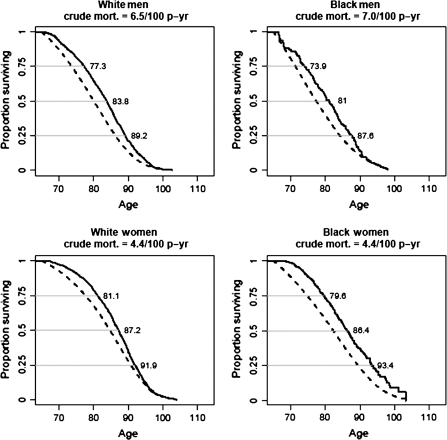
We first compared the survival curves from the CHS with the U.S. Census data. Figure 1 shows survival curves for black and white men and women in the CHS and for the U.S. population standardized to the CHS age–sex–race distribution. All the CHS survival curves were significantly different from the U.S. population curves (all p values <.001). Survival starting 5 years after recruitment was less different than the U.S. population but has remained statistically significantly lower. Median survival time was more than 87 years for the women and more than 80 years for the men in the CHS cohort. Men had lower median survival than women and blacks had significantly poorer median survival than whites, although older black women in CHS had better survival at the 75th percentile than older white women.
Figure 1.
Comparison of survival curves in Cardiovascular Health Study (solid lines) compared with U.S. census (dotted lines), 1990, by gender and race with age at death for the 25th, 50th, and 75th percentile. Gender difference in age at death: log-rank test X2 = 172, p < .0001. Race difference in age at death: log-rank test stratified by gender: X2 = 5.3, p = .0217. In women: X2 = 4.6, p = .03, in men: X2 = 4.1, p = .04.
Factors associated with total mortality at 16 years of follow-up are presented in Table 2. The risk factors for total mortality were consistent with the previously published findings at 5 years (2). Age was strongly associated with mortality. Smoking, pulmonary function, and body weight were each associated with risk of death. DSST, CHF history, coronary heart disease history, and self-reported health were also predictive of mortality. Male sex and most cardiovascular measures and blood measures were also associated with mortality. ApoE ϵ4 allele and IL-6 levels were both associated with higher total mortality risk. When compared with a model excluding these variables, the relationship of the other variables in the reported model with total mortality was not different (data not shown). Of note, black race was associated with a lower risk of death in these adjusted models.
Table 2.
Hazard Ratios for Total 16-Year Mortality in the Cardiovascular Health Study Cohort
| n | Total | p | |
| Demographics | |||
| Age (y) | |||
| >64 to ≤70 | 1,804 | 1.00 | <.001 |
| >70 to ≤75 | 1,659 | 1.4 (1.27–1.54) | |
| >75 to ≤80 | 1,069 | 2.01 (1.81–2.23) | |
| >80 to ≤85 | 496 | 2.65 (2.33–3.01) | |
| >85 | 194 | 3.62 (3.03–4.33) | |
| Sex | |||
| Men | 2,216 | 1.73 (1.54–1.95) | <.001 |
| Race | |||
| Black | 796 | 0.73 (0.66–0.82) | <.001 |
| Income ≥$50,000 | 663 | 0.9 (0.8–1.01) | .1 |
| Weight in pounds for men (women) | |||
| ≤142 (≤115) | 1,622 | 1.00 | <.001 |
| >142 to ≤156 (>115 to ≤131) | 868 | 0.9 (0.81–1) | |
| >156 to ≤172 (>131 to ≤145) | 991 | 0.79 (0.71–0.88) | |
| >172 to ≤190 (>145 to ≤168) | 880 | 0.69 (0.61–0.78) | |
| >190(>168) | 861 | 0.75 (0.66–0.85) | |
| Smoking | |||
| Never smoked | 2,426 | 1.00 | <.001 |
| >0 to ≤26 pack-years | 1,336 | 1.16 (1.06–1.27) | |
| >26 to ≤50 pack-years | 829 | 1.26 (1.14–1.4) | |
| >50 pack-years | 631 | 1.67 (1.5–1.86) | |
| Physical activity (kcal) | |||
| ≤67.5 | 602 | 1.00 | .01 |
| >67.5 to ≤472 | 916 | 0.9 (0.8–1.02) | |
| >472 to ≤980 | 976 | 0.92 (0.81–1.04) | |
| >980 to ≤1890 | 1,106 | 0.89 (0.79–1.01) | |
| >1890 | 1,622 | 0.82 (0.72–0.92) | |
| Health history | |||
| Self-rated health | |||
| Excellent | 706 | 1.00 | <.001 |
| Very good | 1,261 | 1.15 (1.01–1.31) | |
| Good | 1,955 | 1.25 (1.11–1.42) | |
| Fair | 1,100 | 1.41 (1.23–1.61) | |
| Poor | 200 | 1.76 (1.45–2.15) | |
| Congestive heart failure at baseline | 238 | 1.42 (1.22–1.65) | <.001 |
| Coronary heart disease at baseline | 1,008 | 1.1 (1.01–1.2) | .02 |
| Noninvasive examinations | |||
| Forced vital capacity (l) | |||
| <2.06 | 810 | 1.00 | <.001 |
| >2.06 to ≤2.54 | 1,143 | 0.84 (0.75–0.94) | |
| >2.54 to ≤3.00 | 1,067 | 0.75 (0.66–0.85) | |
| >3.00 to ≤3.60 | 1,133 | 0.65 (0.57–0.75) | |
| >3.60 | 1,069 | 0.56 (0.48–0.65) | |
| Major ECG abnormality | 1,484 | 1.31 (1.22–1.42) | <.001 |
| Carotid stenosis | |||
| 0 | 1,153 | 1.00 | <.001 |
| >0 to ≤25% | 1,566 | 1.24 (1.12–1.39) | |
| >25 to ≤50% | 2,287 | 1.41 (1.28–1.57) | |
| >50 to ≤75% | 163 | 1.38 (1.14–1.68) | |
| >75 to ≤100% | 53 | 1.62 (1.21–2.17) | |
| Ankle–arm index | |||
| <0.8 | 286 | 1.46 (1.27–1.67) | <.001 |
| >0.8 to ≤0.9 | 436 | 1.3 (1.16–1.46) | |
| >0.9 to ≤1.3 | 4,283 | 1.00 | |
| >1.3 | 217 | 1.11 (0.93–1.32) | |
| Systolic BP (mmHg) | |||
| <128 | 2,029 | 1.00 | .07 |
| >128 to ≤140 | 1,158 | 1.01 (0.92–1.11) | |
| >140 to ≤152 | 907 | 1.02 (0.92–1.13) | |
| >152 to ≤168 | 713 | 1.04 (0.93–1.16) | |
| >168 | 415 | 1.23 (1.08–1.39) | |
| Using diuretics | 1,401 | 1.06 (0.98–1.15) | .2 |
| Blood tests | |||
| Fasting glucose (mg/dl) | |||
| ≤94 | 1,395 | 1.00 | <.001 |
| >94 to ≤100 | 969 | 0.98 (0.88–1.09) | |
| >100 to ≤108 | 1,267 | 0.99 (0.9–1.1) | |
| >108 to ≤130 | 926 | 1.08 (0.97–1.21) | |
| >130 | 665 | 1.51 (1.35–1.7) | |
| Serum albumin (mg/dl) | |||
| ≤3.70 | 947 | 1.00 | <.001 |
| >3.70 to ≤3.90 | 1,265 | 0.89 (0.8–0.99) | |
| >3.90 to ≤4.00 | 748 | 0.99 (0.88–1.11) | |
| >4.00 to ≤4.20 | 1,242 | 0.85 (0.76–0.94) | |
| >4.20 | 1,020 | 0.83 (0.74–0.93) | |
| Serum creatinine (mg/dl) | |||
| ≤0.90 | 2,138 | 1.00 | <.001 |
| >0.90 to ≤1.10 | 776 | 1 (0.89–1.12) | |
| >1.10 to ≤1.20 | 1,186 | 1.12 (1.01–1.24) | |
| >1.20 to ≤1.50 | 664 | 1.31 (1.17–1.48) | |
| >1.50 | 458 | 1.6 (1.4–1.82) | |
| CRP (mg/l) | |||
| ≤1.0 | 1,379 | 1.00 | .43 |
| >1.00 to ≤3.00 | 2,264 | 0.95 (0.87–1.04) | |
| >3.00 | 1,579 | 0.98 (0.88–1.1) | |
| ApoE ϵ4 allele | 1,321 | 1.22 (1.12–1.32) | <.001 |
| IL-6 (pg/ml) | |||
| ≤1.06 | 977 | 1.00 | <.001 |
| >1.06 to ≤1.47 | 1,025 | 1.1 (0.96–1.25) | |
| >1.47 to ≤1.97 | 1,042 | 1.35 (1.19–1.53) | |
| >1.97 to ≤2.90 | 1,078 | 1.41 (1.24–1.6) | |
| >2.90 | 1,100 | 1.66 (1.45–1.91) | |
| Physical and cognitive function | |||
| IADL impairment | |||
| 0 or 1 | 4,890 | 1.00 | <.001 |
| 2 | 205 | 1.25 (1.07–1.47) | |
| 3+ | 127 | 1.12 (0.92–1.37) | |
| DSST score | |||
| ≤18 | 517 | 1.00 | <.001 |
| >18 to ≤25 | 720 | 0.97 (0.85–1.1) | |
| >25 to ≤33 | 889 | 0.83 (0.73–0.95) | |
| >33 to ≤40 | 1,147 | 0.72 (0.63–0.82) | |
| >40 | 1,949 | 0.59 (0.51–0.67) |
Note: ApoE ϵ4 = apolipoprotein E e4; CRP = C-reactive protein; DSST = Digit Symbol Substitution Test; ECG = electrocardiogram; IADL = instrumental activities of daily living; IL-6 = interleukin-6.
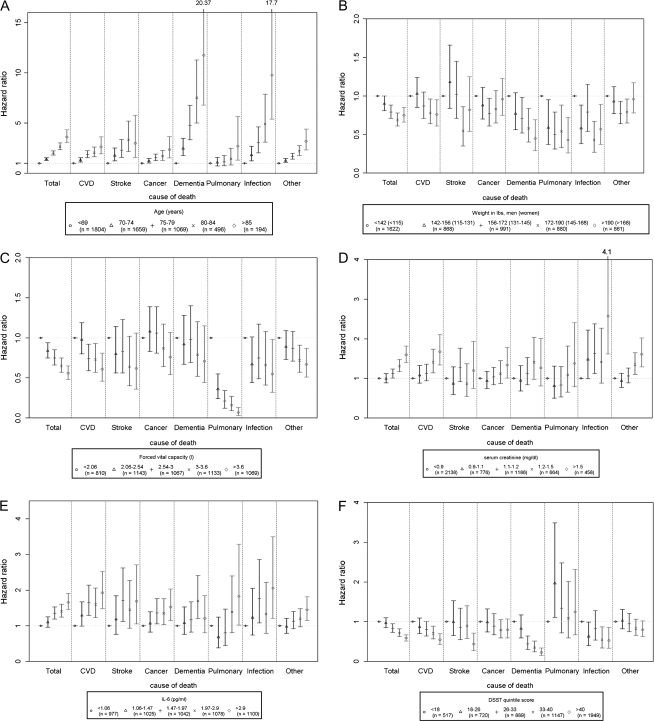
Cause-specific mortality was then examined using these same variables. Numbers and rates for each cause are shown in Table 3. Mean age at death shows that cancer deaths tended to occur at younger ages, whereas dementia death occurred at older ages. To illustrate the risk factors that tended to be related to multiple causes of death, we present a series of figures for each of these factors, adjusted for all other risk factors. Older age (Figure 2A) was associated with higher risk for all causes of death and was especially strong for dementia and infectious death, where the hazards ratios were approximately 10-fold higher compared with ages 65–69 years.
Table 3.
Mortality Rates and Age of Death for Each Cause of Death in the Cardiovascular Health Study Cohort
| CVD* | Stroke | Cancer | Dementia | Pulmonary | Infection | Other | |
| N deaths | 1,099 | 297 | 798 | 392 | 197 | 262 | 1,203 |
| Mortality rate per 100 person-years | 1.66 | 0.45 | 1.21 | 0.59 | 0.30 | 0.39 | 1.82 |
| Age at death (y) M (SD) | 83 (7) | 83 (6) | 81 (6) | 87 (5) | 83 (6) | 85 (6) | 82 (6) |
Note: CVD = cardiovascular disease. *CVD deaths are cardiovascular disease deaths other than stroke.
Figure 2.
Hazard ratios for each cause of death by risk factor: (A) age, (B) weight, (C) forced vital capacity (D) creatinine (E) interleukin-6 (IL-6), (F) Digit Symbol Substitution Test (DSST) score. All models included the risk factors shown in Table 2 for total mortality. CVD = cardiovascular disease.
Other than age, only a few other factors were consistently associated with multiple causes of death. Higher weight (Figure 2B) tended to be inversely associated with death from any cause and significantly so for cardiovascular, dementia, pulmonary, and infectious causes of death. FVC (Figure 2C) tended to be associated with all causes of death though only significant for cardiovascular disease, pulmonary, infectious, and other causes of death. Serum creatinine (Figure 2D) tended to be associated with all causes of death, but this was only significant for cardiovascular, cancer, infectious, and other cause. A higher DSST score (Figure 2E), a measure of cognitive speed, tended to be protective for all causes, except pulmonary death, and was significant for cardiovascular, stroke, dementia, and infectious death. IL-6 level (Figure 2F) was associated with all causes except dementia death.
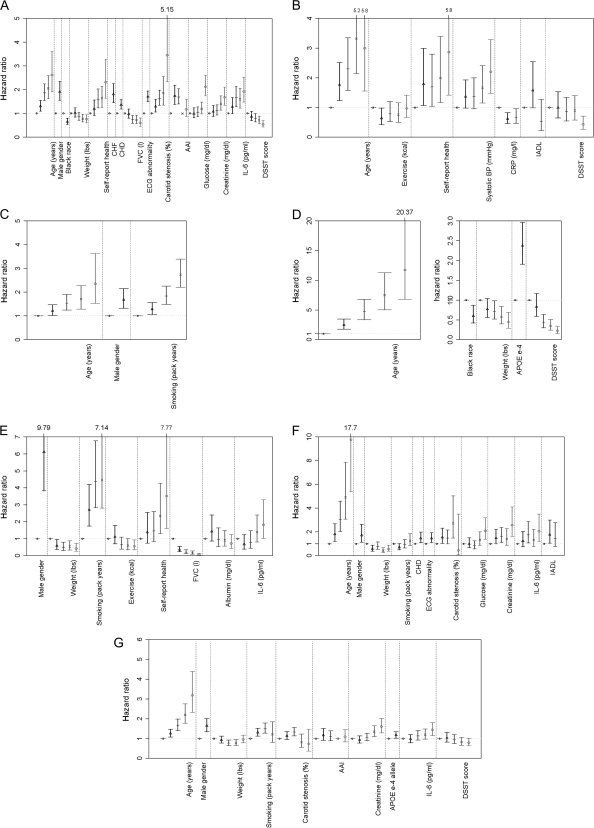
Within each cause of death, risk factors were examined and are presented in Figure 3A–G. Each model included all risk factors, although only significant associations are shown. Multiple risk factors were associated with cardiovascular death, whereas fewer factors were found to be associated with the other causes (Figure 3A). Although numerous noninvasive tests of cardiovascular function were considered, each contributed independently to prediction of cardiovascular disease mortality. Male sex, age, white race, lower physical activity, lower weight, and lower DSST score were also related to cardiovascular disease death, as were higher glucose, lower albumin, higher creatinine, and higher IL-6 level. Stroke death was associated with older age, poorer self-rated health, lower ankle–arm index, higher SBP, and higher serum creatinine and IL-6 (Figure 3B). Cancer death was significantly associated with older age, male sex, smoking, and higher creatinine (Figure 3C). Dementia death was associated with older age, white race, higher physical activity, ApoE ϵ4 allele, and lower DSST score (Figure 3D). Lower FVC as well as smoking and IL-6 were very strongly associated with pulmonary death, whereas higher weight, higher income, and higher physical activity were protective (Figure 3E). Infectious cause was associated with older age, male sex, lower weight, higher pulmonary function, higher glucose, poorer IADL performance, higher IL-6 level, and lower DSST score (Figure 3F). Other cause was related to older age, male sex, IL-6, glucose, and creatinine (Figure 3G).
Figure 3.
Hazard ratios for risk factors by each cause of death: (A) cardiovascular excluding stroke, (B) stroke, (C) cancer, (D) dementia, (E) pulmonary (F) infectious, (G) other causes. AAI = ankle arm index; CHD = coronary heart disease; CHF = congestive heart failure; FVC = forced vital capacity; interleukin-6 = IL-6, DSST = Digit Symbol Substitution Test.
CONCLUSIONS
Specific and shared risk factors for specific causes of death were identified in this long-term follow-up study of older adults. When examining each cause of death, it was clear that most of the risk factor associations with total mortality were due to the associations of multiple risk factors with cardiovascular death. Some other risk factors were quite specific to a given cause of death. For example, lung function was very strongly related to lung disease death, ApoE ϵ4 to dementia death, and noninvasive tests for cardiovascular disease were fairly specific in their prediction of cardiovascular disease death. Other than age, the factor that was most consistently associated with death across multiple causes was IL-6 level. Older age was especially strongly related to dementia and infectious death; the median age of death for these causes was 87 and 85 years, respectively.
In spite of the extensive baseline characterization of this cohort, there were few factors identified that were common risk factors for all causes of death and none trumped age itself in its consistency for predicting all cause categories. Dysfunction of two organ systems, the lung and kidney, tended to be related to multiple causes of death, though not all estimates were statistically significant. Others have noted that dysfunction of these two organ systems is strongly linked to mortality (36,37), although the reasons are not well understood. Lower weight tended to be associated with all causes of death, but especially for infectious and pulmonary death. This paradox of higher weight being protective in older adults has been previously noted in many studies of total mortality (38) and is now shown here to be consistent across many causes of death.
Higher IL-6, a marker of dysregulation of immune function and a chronic inflammatory state, was consistently related to all causes though it was not statistically significant for dementia death. This may be due to survival bias related to the very old age at death in that group. Higher IL-6 is related to frailty status (39), total mortality (40), and disability (41) and thus appears to capture the general decline of aging.
Association of mortality with the digit symbol score suggests that brain function is key to longevity (42,43). It was striking that ApoE ϵ4 allele was so strongly related to dementia death and not the other types. Our data show that its low prevalence in very long-lived people maybe specifically due to higher dementia mortality.
Interpreting cause-specific mortality is always complicated by the fact that the death of a participant from one cause makes it impossible to observe when they otherwise would have died from other causes. The effects of exposures on the observed time and cause of death may not have a simple relationship to their effects on physiological processes because of the steady removal of susceptible individuals from the cohort (44). There is no complete statistical solution to this problem, but we conducted a sensitivity analysis that suggested that the results are not very sensitive to the dependence between causes of death. Our primary analyses yielded similar hazard ratios to a model where we set time of death for competing causes to the indefinite future (35). This suggests that the risk estimates are robust to competing cause.
There are several limitations that should be kept in mind when examining these results. First, we did not attempt to identify all risk factors for death; rather, we reevaluated previously identified baseline risk factors with longer-term follow-up. Although other models might yield additional insights into causal relationships, the overall prediction of death was not improved with more complex models. Future models could also evaluate change in risk over time. Another limitation was the lack of detail on cancer death in these analyses. It is well known that risk factors differ by cancer type, so further analysis of cancer deaths is needed. Finally, there are certainly additional risk factors that would be important for the noncardiovascular causes of death that were not assessed as part of the CHS.
These findings have important implications for aging research. First, the specificity of some risk factors for the eventual cause of death suggests that death may be ultimately determined by the failure of the most limited organ system. In other words, we were able to identify the “weakest link” leading to death. Although comorbidity is common and other conditions certainly contribute to death, these findings provide support for the approach of assigning a specific cause of death in older adults (45). The findings of the common risk factor IL-6 support the concept that there is an underlying age-related state of decline that is not disease specific (11).
The importance of inflammation, cognitive function, kidney, and lung function across causes suggests that these systems have less reserve, decline more strongly with age over time or with risk factor exposure, or simply that these systems have fewer mechanisms for adaptation or repair of injury than do other systems. It is also possible that these systems were more accurately measured than others in this study. These findings also have important implications for clinical practice. Handheld portable spirometery and kidney function are easily measured and could be incorporated into risk assessment. Body weight is most easily monitored and of importance in predicting death across multiple causes.
The CHS survival has been somewhat better but similar to the U.S. population. The U.S. population would include many people who would be much sicker than people who enrolled in the CHS. In spite of this, the rates in the CHS were only slightly better and become closer over time, suggesting that the risk factor associations noted here are likely to be generalizable to the U.S. population. In terms of prevention, this study suggests that approaches that target cardiovascular disease and inflammation have the greatest potential to increase longevity.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at: http://biomed.gerontologyjournals.org/
FUNDING
The study was supported through R01-AG-023629 from the National Institute on Aging. The Cardiovascular Health Study was supported from contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through HL-075366 from the National Heart, Lung and Blood Institute, and the University of Pittsburgh Claude. D. Pepper Older Americans Independence Center P30-AG-024827.
Supplementary Material
Acknowledgments
Author Contributions: A.B.N., L.P.F., B.M.P., T.B.H., J.A.R., G.L.B., and L.H.K. participated in study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of the manuscript.
M.C.S. and A.M.A., R.K., and T.L. participated in study concept and design, data analysis and interpretation, and preparation of the manuscript.
M.C. participated in study concept and design, laboratory analysis of samples, and preparation of the manuscript.
Author Access to Data: A.B.N., A.M.A., T.L., and M.C.S. had full access to the data. Sponsor’s Role: the funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, and preparation of manuscript or in the decision to submit the manuscript for publication. Conflict of Interest: all authors report no conflict of interest.
References
- 1.Newman AB, Boudreau RM, Naydeck BL, Fried LF, Harris TB. A physiologic index of comorbidity—Relationship to mortality and disability. J Gerontol A Biol Sci Med Sci. 2008;63A:M603–M609. doi: 10.1093/gerona/63.6.603. PMCID: PMC2496995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 3.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years’ observations on male British doctors. BMJ. 1994;309:901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colditz GA, Bonita R, Stampfer MJ, et al. Cigarette smoking and risk of stroke in middle-aged women. N Engl J Med. 1988;318:937–941. doi: 10.1056/NEJM198804143181501. [DOI] [PubMed] [Google Scholar]
- 5.Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675–679. doi: 10.1164/rccm.2112096. [DOI] [PubMed] [Google Scholar]
- 6.Kuper H, Boffetta P, Adami HO. Tobacco use and cancer causation: association by tumour type. J Intern Med. 2002;252:206–224. doi: 10.1046/j.1365-2796.2002.01022.x. [DOI] [PubMed] [Google Scholar]
- 7.DeFronzo RA, Ferrannini E. Insulin resistance: a multifaced syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 8.Pischon T, Nothlings U, Boeing H. Obesity and cancer. Proc Nutr Soc. 2008;67:128–145. doi: 10.1017/S0029665108006976. [DOI] [PubMed] [Google Scholar]
- 9.Hsu IR, Kim SP, Kabir M, Bergman RN. Metabolic syndrome, hyperinsulinemia, and cancer. Am J Clin Nutr. 2007;86:s867–s871. doi: 10.1093/ajcn/86.3.867S. [DOI] [PubMed] [Google Scholar]
- 10.Sorlie P, Backlund E, Keller J. US mortality by economic, demographic, and social characteristics: the National Longitudinal Mortality Study. Am J Public Health. 1995;85:949–956. doi: 10.2105/ajph.85.7.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris TB, Kiel D, Roubenoff R, et al. Association of insulin-like growth factor-I with body composition, weight history, and past health behaviors in the very old: the Framingham Heart Study. J Am Geriatr Soc. 1997;45:133–139. doi: 10.1111/j.1532-5415.1997.tb04497.x. [DOI] [PubMed] [Google Scholar]
- 12.Louhija J, Miettinen HE, Kontula K, Tikkanen MJ, Miettinen TA, Tilvis RS. Aging and genetic variation of plasma apolipoproteins: relative loss of apolipoprotein E4 phenotype in centenarians. Arterioscler Thromb. 1994;14:1084–1089. doi: 10.1161/01.atv.14.7.1084. [DOI] [PubMed] [Google Scholar]
- 13.Kervinen K, Savolainen MJ, Salokannel J, et al. Apolipoprotein E and B polymorphisms—longevity factors assessed in nonagenarians. Atherosclerosis. 1994;105:89–95. doi: 10.1016/0021-9150(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Kronmal RA, Newman AB. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 15.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 16.Wolf H, MacInnis PJ, Stock S, Helppi RK, Rautaharju PM. The Dalhousie program: a comprehensive analysis program for rest and exercise electrocardiograms. In: Zywietz C, Schnieder B, editors. Computer Application on ECG and VCG Analysis. Amsterdam, The Netherlands: North Holland Publishing; 1973. pp. 231–232. [Google Scholar]
- 17.Gardin JM, Wong ND, Bommer W, et al. Echocardiographic design of a multicenter investigation of free living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 18.Enright PL, Kronmal RA, Higgins M, Schenker M, Haponik EF. Spirometry reference values for women and men 65 to 85 years of age. Am Rev Respir Dis. 1993;147:125–133. doi: 10.1164/ajrccm/147.1.125. [DOI] [PubMed] [Google Scholar]
- 19.Newman AB, Siscovick DS, Manolio TA, et al. The ankle-arm index as marker of atherosclerosis in the Cardiovascular Health Study. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 20.O’Leary DH, Polak JF, Wolfson SK, Jr, et al. Use of sonography to evaluate carotid atherosclerosis in the elderly: the Cardiovascular Health Study. Stroke. 1991;22:1155–1163. doi: 10.1161/01.str.22.9.1155. [DOI] [PubMed] [Google Scholar]
- 21.Barzilay JI, Spiekerman CF, Wahl PW. Cardiovascular disease in older adults with glucose disorders: comparison of American Diabetes Association criteria for diabetes mellitus with WHO criteria. Lancet. 1999;354:622–625. doi: 10.1016/s0140-6736(98)12030-5. [DOI] [PubMed] [Google Scholar]
- 22.Orme J, Reis J, Herz E. Factorial and discriminate validity of the Center for Epidemiological Studies Depression (CES-D) Scale. J Clin Psychol. 1986;42:28–33. doi: 10.1002/1097-4679(198601)42:1<28::aid-jclp2270420104>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 24.Weschler D. Weschler Adult Intelligence Scale-revised. New York, NY: Psychological Corporation; 1981. [Google Scholar]
- 25.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 26.Kuller LH, Shemanski L, Manolio T, et al. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke. 1998;29:388–398. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- 27.Hixon JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 28.Tracy RP, Lemaitre R, Psaty BM, Cushman M, Meilahn EN, Kuller LH. Relationship of C-reactive protein to risk of cardiovascular disease in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 1997;17:1121–1127. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 29.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated Interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 30.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 31.Hakulinen T. Cancer survival corrected for heterogeneity in patient withdrawal. Biometrics. 1982;38:933. [PubMed] [Google Scholar]
- 32.Therneau TM, Offord J. Mayo Clinic Section of Biostatistics: Rochester, MN; 1999. Expected survival based on hazard rates. Technical Report #63. http://www.mayo.edu/hsr/techrpt/63.pdf. Accessed August 18, 2009. [Google Scholar]
- 33.Lawless JF, Singhal K. Efficient screening of non-normal models. Biometrics. 1978;34:318–327. [Google Scholar]
- 34.Prentice RL, Kalbfleisch JD, Peterson AV, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 35.Pepe MS. Inference for events with dependent risks in multiple-endpoint studies. J Am Stat Assoc. 1991;86:770–778. [Google Scholar]
- 36.Young RP, Hopkins R, Eaton TE. Forced expiratory volume in one second: not just a lung function test but a marker of premature death from all causes. Eur Respir J. 2007;30:616–622. doi: 10.1183/09031936.00021707. [DOI] [PubMed] [Google Scholar]
- 37.Fried LF, Katz R, Sarnak MJ, et al. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 38.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 39.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 40.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 41.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 42.Kuller LH, Arnold AM, Longstreth WT, Jr, et al. White matter grade and ventricular volume on brain MRI as markers of longevity in the cardiovascular health study. Neurobiol Aging. 2007;28:1307–1315. doi: 10.1016/j.neurobiolaging.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 43.Rosano C, Newman AB, Katz R, Hirsch C, Kuller L. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56:1618–1625. doi: 10.1111/j.1532-5415.2008.01856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slud E, Byar D. How dependent causes of death can make risk factors appear protective. Biometrics. 1988;44:265–269. [PubMed] [Google Scholar]
- 45.Ives DG, Samuel P, Psaty BM, Kuller LH. Agreement between nosologist and cardiovascular health study review of deaths: implications of coding differences. 2009;57:133–139. doi: 10.1111/j.1532-5415.2008.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.