Abstract
Background
Postmenopausal conjugated equine estrogens (CEE) therapies increase the risk of cognitive impairment in women aged 65 years or older and are associated with smaller regional brain volumes; however, the link between these two phenomena has not been established.
Methods
Standardized magnetic resonance imaging was performed on 1,403 women, 1–4 years after they had participated in randomized placebo-controlled clinical trials of CEE-based therapies. Women included in this report were aged 65–80 years and free of dementia and mild cognitive impairment (MCI) when originally enrolled in the trials, which lasted an average of 4–6 years and were conducted at 14 academic U.S. medical centers. The associations that regional brain volumes and ischemic lesion volumes had with the development of cognitive impairment (i.e., dementia or MCI) were contrasted between treatment groups using analyses of covariance.
Results
Fifty-three women developed MCI or probable dementia during follow-up. Among women who had been prescribed CEE-based therapies, cognitive impairment was associated with relatively smaller hippocampal (p = .0002) and total brain volumes (p = .03). Qualitatively, these associations appeared to be independent of their level of pretreatment cognitive function. Among women who had been prescribed placebo, these relationships were not evident; instead, cognitive impairment was associated with greater ischemic lesion volume in the frontal lobe (p = .007) and overall (p = .02).
Conclusion
A mechanism by which CEE-based postmenopausal hormone therapy induces cognitive impairment appears to be through increased brain atrophy.
Keywords: Hormone therapy, Magnetic resonance imaging, Women's health
CONJUGATED equine estrogen (CEE) therapy, alone or combined with medroxyprogesterone acetate (MPA), increases the risks of dementia and mild cognitive impairment (MCI) in women aged 65 years or older (1,2); however, the neuroanatomical and neurochemical mechanisms underlying this effect are presently unknown. Recently, we reported that older women who had been prescribed CEE-based therapies, compared with placebo, had smaller mean frontal lobe volumes (p = .004), marginally smaller mean hippocampal (p = .05) and total brain volumes (p = .07), and comparable levels of ischemic lesion volumes (3,4). In this current article, we examine whether the phenomena of increased risk for cognitive impairment and decreased brain volumes are linked. We hypothesize that among women assigned to CEE-based hormone therapies, cognitive impairment would be more strongly associated with reduced brain volumes than among women assigned to placebo therapy.
We analyzed data from brain magnetic resonance imaging (MRI) scans that were performed on women, 1–4 years after they had participated in randomized placebo-controlled clinical trials of CEE-based therapies. After covariate adjustment to control for other risk factors for cognitive impairment, we examined whether the relationships that regional and total brain volumes had with cognitive impairment varied between treatment groups. We also conducted parallel analyses for ischemic lesion volumes. We then examined the consistency of our results among women grouped by baseline level of cognitive function, which may moderate the adverse effects of CEE-based therapies on global cognitive function (5).
PARTICIPANTS AND METHODS
The Women's Health Initiative Memory Study (WHIMS), an ancillary study to the Women's Health Initiative (WHI), consisted of parallel placebo-controlled randomized clinical trials of 0.625 mg/day CEEs with and without 2.5 mg/day MPA in women with a uterus or posthysterectomy (6). Participants were recruited from 39 clinical centers of the WHI CEE-alone and CEE + MPA clinical trials (7,8). They were 65–79 years of age and free of dementia, as ascertained per study protocol. Written informed consent was obtained; the National Institutes of Health and Institutional Review Boards of participating institutions approved the protocols and consent forms. Baseline demographic, lifestyle, and clinical factors were collected via self-report and standardized assessments. Physical activity was computed as metabolic equivalents per week. The CEE + MPA trial terminated earlier than planned (July 2002) due to an adverse risk-to-benefit profile (7). The CEE-alone trial also was terminated early (February 2004) due to an increased risk of stroke and lack of evidence for prevention of cardiovascular disease (8).
The WHIMS-MRI study was designed to contrast MRI outcomes among women who had been assigned to active versus placebo therapy during the WHIMS trials in 14 of its sites (9). Exclusion criteria included presence of pacemakers, other implants, foreign bodies, and other contraindications for MRI. None of those scanned had been classified as having cognitive impairment at WHIMS enrollment. Their mean (SD) age at scanning was 78.5 (3.7) years. On average (range), scans were collected 8.0 (6.5–10.2) and 3.0 (2.4–3.8) years after CEE + MPA trial enrollment and termination and 8.0 (6.5–10.1) and 1.4 (0.8–2.2) years after CEE-alone trial enrollment and termination.
Classification of Cognitive Impairment and Dementia
Modified Mini-Mental State (3MS) examinations (10) were administered annually to WHIMS participants by trained technicians who were masked to treatment and symptoms. Women scoring below cut-points based on education level proceeded to a second stage that included a neuropsychological battery and assessments of generalized anxiety, depression, and alcohol abuse (5). Experienced local physicians reviewed data, completed structured clinical evaluations, and classified women as having no dementia, MCI, or probable dementia based on standard Diagnostic and Statistical Manual of Mental Disorders criteria (11). If probable dementia was suspected, brain computerized tomography and laboratory blood tests were used to rule out possible reversible causes of cognitive decline and dementia. A central committee adjudicated all locally identified probable dementia cases, a 50% random sample of MCI cases and a 10% random sample of no dementia cases to make final classifications (1). Regardless of these findings, annual 3MS testing continued. Our analyses include all cases of cognitive impairment adjudicated as of January 1, 2008 that were triggered by pre-scan 3MS testing.
MRI Protocol
WHIMS-MRI contacted 2,345 WHIMS participants, of which 1,527 (65.1%) provided consent. Of these, 1,424 (93.3%) received brain MRI scans, of which 1,403 (98.5%) met central reading criteria for analysis. The women who were scanned differed from those who were not, but these differences were not related to treatment assignment (9).
Standardized protocols were used for obtaining and processing MRI scans and for measuring volumes (3,4). Series were acquired with field of view = 22 and matrix = 256 × 256. They included oblique axial spin density/T2-weighted spin echo (3200/0/30,120/3), FLAIR T2-weighted spin echo (8000/2000/100/3), and oblique axial 3D T1-weighted gradient echo (flip angle 30; 21/0/8/1.5) images from the vertex to skull base parallel to the anterior commissure–posterior commissure plane. T1-weighted volumetric MRI scans were first preprocessed according to a standardized protocol for alignment, removal of extracranial material, and segmentation of brain parenchyma into gray matter, white matter, and cerebrospinal fluid (CSF). Regional volumetric measurements were obtained using an automated computer-based template warping method that summed the number of respective voxels falling within each region. Volumes of brain lesions and periventricular abnormal white matter were measured separately, in parallel, using all three sets of images. Intracranial volume (ICV) was estimated as the total cerebral hemispheric volumes including ventricular CSF and the CSF within the sulcal spaces. The computer-assisted methodology we used has been validated against manual segmentation, that is, manual drawing of regions of interest, by an experienced expert neuroradiologist. Additional details on this methodology and its validation appear elsewhere (12).
Statistical Methods
The distributions of times until the first on-study classification of MCI or dementia, relative to the date of the MRI scan, were portrayed with Kaplan–Meier plots, and treatment groups were compared with a log-rank test. Because of their skewed distribution, log transformations (offset by 1) were applied to ischemic lesion volume measures; means and confidence intervals were reported in the original units using the delta method. Analyses of covariance were used to compare the mean brain volumes and ischemic lesion volumes between women who had and had not developed cognitive impairment, after adjustment for ICV. The analyses at the core of this article are comparisons of these differences for women grouped by treatment assignment, with additional covariate adjustment of pretreatment characteristics to limit the influence of factors other than hormone therapy.
We also wished to assess whether the influence that hormone therapy has on associations between brain volumes and the incidence of cognitive impairment only exists for women with pretreatment cognitive deficits, as measured with 3MS tests. We were limited in this because the development of cognitive impairment was quite rare among women who initially scored well (scores ≥95) on this test, and so our analyses have limited power and are presented as descriptive. Brain volumes were normalized by dividing with total ICV (13), expressed as a percent, and subtracted from 100%: this provided a measure of the CSF to ICV, for which total CSF was estimated by the difference between ICV and total brain volume, and thus serves as a measure of lost brain volume. We examined whether the relationship that this measure had with cognitive impairment and treatment assignment varied between women who had no evidence of pretreatment cognitive deficits (3MS scores ≥95) compared with those who had lower test scores and used plots of the cumulative distributions.
RESULTS
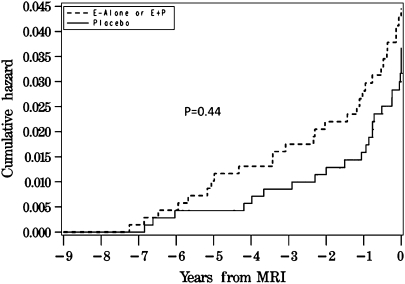
The 883 women from the CEE + MPA trial and 520 women from the CEE-alone trial who are described in this report included 43 who had been classified as having MCI at least once (20 placebo and 23 active) during follow-up and 23 who had been classified as having probable dementia (10 placebo and 13 active). All told, this involved 53 women who had at least one classification of cognitive impairment (24 placebo and 29 active). The time frame of the first classification of any impairment relative to the MRI scan for these 53 women appears in Figure 1, of which 21 (40%) occurred during the WHI trials and 32 occurred during post-trial follow-up.
Figure 1.
Incidence of cognitive impairment in relation to the timing of the magnetic resonance imaging (MRI) scan.
Table 1 describes characteristics at WHI enrollment, for women grouped by treatment assignment and cognitive impairment status. There was good balance between treatment groups. For example, the mean ages at enrollment for women assigned to active therapies was 70.6 years compared with 70.5 years for women assigned to placebo (p = .81). Cognitive impairment tended to occur among older women and for those with lower pretreatment 3MS scores (both p < .05). Fourteen women reported a prior stroke: five who had been assigned to CEE-based therapies and nine to placebo. One woman with prior stroke, who had been assigned to CEE-based therapies, developed cognitive impairment during follow-up. Because these women’s MRI images may have been be affected by these pretrial events, analyses were conducted with and without their inclusion.
Table 1.
Selected Dementia Risk Factors of the WHIMS-MRI Cohort at the Time of WHI Enrollment
| Variable | No Cognitive Impairment |
Cognitive Impairment |
p Values |
|||
| HT, N = 664 | Placebo, N = 686 | HT, N = 29 | Placebo, N = 24 | HT vs Placebo | Any CI vs No CI | |
| Age (y), n (%) | ||||||
| 65–69 | 342 (51.5) | 354 (51.6) | 11 (37.9) | 8 (33.3) | .81 | <.001 |
| 70–74 | 239 (36.0) | 236 (34.4) | 8 (27.6) | 9 (37.5) | ||
| 75+ | 83 (12.5) | 96 (14.0) | 10 (34.5) | 7 (29.2) | ||
| Education, n (%) | ||||||
| < High school | 31 (4.7) | 29 (4.2) | 1 (3.4) | 2 (8.3) | .97 | .78 |
| High school/GED | 149 (22.5) | 160 (23.4) | 8 (27.6) | 7 (29.2) | ||
| > High school < 4 y college | 263 (39.7) | 275 (40.2) | 11 (37.9) | 8 (33.3) | ||
| > 4 y college | 219 (33.1) | 220 (32.2) | 9 (31.0) | 7 (29.2) | ||
| Hypertension status, n (%) | ||||||
| No | 345 (52.0) | 366 (52.3) | 11 (37.9) | 13 (54.2) | .45 | .29 |
| Yes | 319 (48.0) | 320 (46.6) | 18 (62.1) | 11 (45.8) | ||
| Prior CVD | ||||||
| None | 623 (93.8) | 642 (93.6) | 26 (89.7) | 22 (91.7) | .56 | .33† |
| Stroke | 4 (0.6) | 9 (1.3) | 1 (3.4) | 0 (0.0) | ||
| Other CVD* | 37 (5.6) | 35 (5.1) | 2 (6.9) | 2 (8.3) | ||
| Diabetes, n (%) | ||||||
| No | 635 (95.6) | 645 (94.0) | 26 (89.7) | 22 (91.7) | .23 | .18 |
| Yes | 29 (4.4) | 41 (6.0) | 3 (10.3) | 2 (8.3) | ||
| Smoking status, n (%) | ||||||
| Never | 387 (59.0) | 391 (57.7) | 19 (59.4) | 9 (36.0) | .67 | .45 |
| Former | 243 (37.0) | 256 (37.8) | 12 (37.5) | 15 (60.0) | ||
| Current | 26 (4.0) | 31 (4.6) | 1 (3.1) | 1 (4.0) | ||
| METs/wk, n (%) | ||||||
| ≤3.5 | 217 (32.7) | 219 (31.9) | 4 (13.8) | 14 (58.3) | .90 | .12 |
| 3.6–12.5 | 223 (33.6) | 228 (33.2) | 9 (31.0) | 2 (8.3) | ||
| >12.6 | 224 (33.7) | 239 (34.8) | 16 (55.2) | 8 (33.3) | ||
| Prior hormone therapy, n (%) | ||||||
| No | 453 (68.2) | 461 (67.2) | 18 (62.1) | 12 (50.0) | .59 | .09 |
| Yes | 211 (31.8) | 225 (32.8) | 11 (37.9) | 12 (50.0) | ||
| 3MS score, n (%) | ||||||
| <90 | 31 (4.7) | 30 (4.4) | 11 (37.9) | 8 (33.3) | .36 | <.001 |
| 90–94 | 110 (16.6) | 130 (19.2) | 6 (20.7) | 8 (33.3) | ||
| 95–100 | 520 (78.7) | 518 (76.4) | 12 (41.4) | 8 (33.3) | ||
Notes: CABG = coronary artery bypass graft; CI = confidence interval; CVD = cardiovascular disease; GED = General Educational Development test; METs = metabolic equivalent; MI = myocardial infarction; PCTA = percutaneous transluminal coronary angioplasty; WHIMS-MRI = Women’s Health Initiative Memory Study-Magnetic Resonance Imaging; WHI = Women’s Health Initiative.
Other CVD defined as MI, angina, PCTA, or CABG.
Fisher’s exact test.
Table 2 examines the overall differences in MRI measures, comparing women who developed cognitive impairment during follow-up with those who did not. Those developing cognitive impairment had smaller total (p = .006) and hippocampal (p < .0001) volumes and greater total (p = .001) and frontal lobe (p = .0006) ischemic lesion volumes.
Table 2.
Fitted Mean Brain Volumes and Ischemic Lesion Volumes, With Adjustment for Total Intracranial Volume, for Women Grouped by Incidence of Cognitive Impairment
| No Cognitive Impairment, M (SE) | Cognitive Impairment, M (SE) | Difference, M (SE) | p Value | |
| Volume (cm3) | ||||
| Total brain | 800.75 (1.05) | 782.14 (5.20) | −18.61 (5.30) | .006 |
| Frontal lobe | 284.04 (0.44) | 280.78 (2.23) | −3.26 (2.27) | .15 |
| Hippocampus | 5.78 (0.03) | 4.74 (0.14) | −1.04 (0.14) | <.0001 |
| Ischemic lesion volume (cm3) | ||||
| Total brain | 4.977 (0.150) | 8.150 (1.159) | 3.173 (1.169) | .001 |
| Frontal lobe | 2.047 (0.062) | 3.366 (0.450) | 1.319 (0.454) | .0006 |
| Hippocampus | 0.054 (0.001) | 0.073 (0.004) | 0.019 (0.004) | .65 |
Table 3 examines whether the differences in mean brain volumes and ischemic lesion volumes associated with cognitive impairment varied depending on treatment assignment. Covariate adjustment for the cognitive impairment risk factors of Table 1 was used to limit their influence and to highlight differences related to CEE-based therapies. Among women who had been assigned to CEE-based therapy, the development of cognitive impairment was associated with significantly smaller total brain (p = .01) and hippocampal (p < .0001) volumes. Among women assigned to placebo, there were no significant relationships between the development of cognitive impairment and brain volumes: in this respect, they differed significantly from women assigned to CEE therapies for total (p = .03) and hippocampal (p = .0002) volumes. Among women who were assigned to placebo, development of cognitive impairment was associated with significantly greater ischemic lesion volumes in the frontal lobe (p = .007) and overall (p = .02). This association was not statistically significant among women who had been assigned to CEE-based therapies; however; the difference between the associations in the active and placebo groups also did not reach statistical significance (p = .23). Excluding women with a history of stroke from these analyses did not materially alter the above findings (Table 4). We also found little evidence that relationships varied between the CEE-alone and CEE + MPA trials (data not shown).
Table 3.
Differences in Adjusted Mean Brain Volumes and Ischemic Lesion Volumes Between Women With and Without Incident Cognitive Impairment by Treatment Assignment: All Women With Adjustment for Cognitive Impairment Risk Factors Listed in Table 1
| Region | No Cognitive Impairment, M (SE) | Cognitive Impairment, M (SE) | Difference, M (SE) | p Value |
| Brain volume (cm3) | ||||
| Total brain | ||||
| Hormone therapy | 799.11 (1.33) | 781.78 (6.58) | −17.33 (6.71) | .01 |
| Placebo | 801.58 (1.31) | 804.94 (7.29) | 3.36 (7.41) | .65 |
| p = .03 | ||||
| Frontal lobe | ||||
| Hormone therapy | 282.80 (0.59) | 280.87 (2.88) | −1.93 (2.94) | .51 |
| Placebo | 285.01 (0.58) | 287.41 (3.22) | 2.40 (3.27) | .46 |
| p = .32 | ||||
| Hippocampus | ||||
| Hormone therapy | 5.75 (0.04) | 4.40 (0.18) | −1.35 (0.18) | <.0001 |
| Placebo | 5.80 (0.04) | 5.43 (0.20) | −0.37 (0.20) | .06 |
| p = .0002 | ||||
| Ischemic lesion volume (cm3) | ||||
| Total brain | ||||
| Hormone therapy | 5.143 (0.210) | 6.648 (1.280) | 1.505 (1.297) | .20 |
| Placebo | 4.868 (0.198) | 8.029 (1.689) | 3.161 (1.701) | .02 |
| p = .39 | ||||
| Frontal lobe | ||||
| Hormone therapy | 2.102 (0.087) | 2.697 (0.506) | 0.595 (0.513) | .21 |
| Placebo | 2.011 (0.083) | 3.576 (0.700) | 1.565 (0.705) | .007 |
| p = .23 | ||||
| Hippocampus | ||||
| Hormone therapy | 0.004 (0.001) | 0.006 (0.005) | 0.002 (0.005) | .74 |
| Placebo | 0.007 (0.001) | 0.011 (0.006) | 0.004 (0.006) | .55 |
| p = .82 | ||||
Table 4.
Differences in Adjusted Mean Brain Volumes and Ischemic Lesion Volumes Between Women With and Without Incident Cognitive Impairment by Treatment Assignment: Women With no Prior History of Stroke With Adjustment for Cognitive Impairment Risk Factors Listed in Table 1
| Region | No Cognitive Impairment, M (SE) | Cognitive Impairment, M (SE) | Difference, M (SE) | p Value |
| Brain volume (cm3) | ||||
| Total brain | ||||
| Hormone therapy | 799.77 (1.34) | 783.04 (6.63) | −16.73 (6.76) | .01 |
| Placebo | 802.10 (1.32) | 805.48 (7.30) | 3.38 (7.41) | .65 |
| p = .04 | ||||
| Frontal lobe | ||||
| Hormone therapy | 283.08 (0.59) | 281.30 (2.93) | −1.78 (2.99) | .55 |
| Placebo | 285.21 (0.59) | 287.64 (3.23) | 2.43 (3.28) | .46 |
| p = .33 | ||||
| Hippocampus | ||||
| Hormone therapy | 5.75 (0.04) | 4.37 (0.18) | −1.38 (0.18) | <.0001 |
| Placebo | 5.81 (0.04) | 5.44 (0.20) | −0.37 (0.20) | .07 |
| p = .0001 | ||||
| Ischemic lesion volume (cm3) | ||||
| Total brain | ||||
| Hormone therapy | 5.173 (0.210) | 6.432 (1.259) | 1.259 (1.276) | .28 |
| Placebo | 4.854 (0.198) | 7.996 (1.680) | 3.142 (1.692) | .02 |
| p = .33 | ||||
| Frontal lobe | ||||
| Hormone therapy | 2.115 (0.087) | 2.643 (0.504) | 0.528 (0.511) | .27 |
| Placebo | 1.999 (0.083) | 3.558 (0.695) | 1.599 (0.700) | .007 |
| p = .20 | ||||
| Hippocampus | ||||
| Hormone therapy | 0.004 (0.001) | 0.006 (0.005) | 0.002 (0.005) | .73 |
| Placebo | 0.007 (0.001) | 0.011 (0.006) | 0.004 (0.006) | .53 |
| p = .81 | ||||
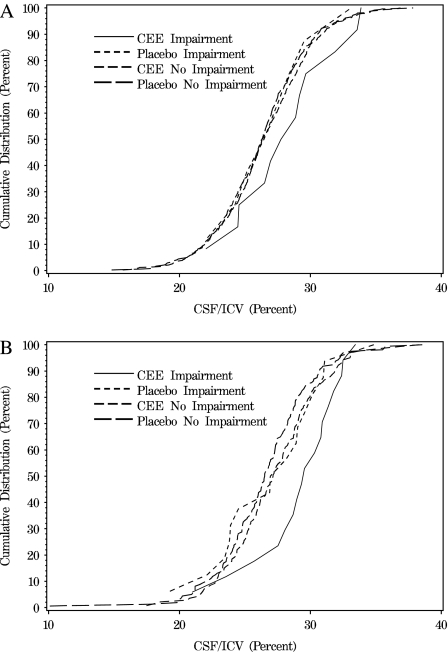
Figure 2 portrays the cumulative distributions of the percent CSF/ICV ratio (an index of lost brain volume) for women grouped by treatment assignment and cognitive impairment. The upper panel describes women whose 3MS score at WHI enrollment was 95 or more and who were thus measured to have high levels of pretreatment global cognitive function. The distribution of the index of lost brain volume for the eight (1.5%) placebo-assigned women who were classified with cognitive impairment was very similar to the distribution of the women in either treatment group who were not classified with cognitive impairment. However, those who were classified with cognitive impairment after they had been assigned to CEE-based therapy (N = 12; 2.3%) had a distribution of lost brain volumes that was markedly shifted toward higher values. The bottom panel describes women whose 3MS score at WHI enrollment was less than 95. These women tended to have smaller brain volumes compared with those who had higher baseline 3MS scores. Again, there was little difference in the distribution of lost brain volumes between cases who had been assigned to placebo (N = 16; 9.1%) and women who were not classified as having cognitive impairment; however, the distribution of lost brain volumes for women who had been assigned to CEE-based therapy (N = 17; 10.8%) was markedly shifted toward higher values. Mean CEE-related effects did not differ between women grouped by baseline 3MS (p = .66); however, power was quite low. Similar results were observed for hippocampal volumes (data not shown).
Figure 2.
Cumulative distribution of an index of lost brain volume—percent cerebrospinal fluid/intracranial volume (CSF/ICV) ratio—for women grouped by treatment assignment (conjugated equine estrogens [CEE] or placebo) and by whether they were classified with cognitive impairment (Imp or No Imp). The CSF/ICV ratios for women who developed cognitive impairment after assignment to CEE-based therapies tended to be shifted to reflect greater levels of lost brain volumes both for women whose baseline cognitive function was high (top panel) or lower (bottom panel). 3MS, Modified Mini-Mental State examinations.
DISCUSSION
When the WHI was designed, CEE-based therapies were the most commonly prescribed postmenopausal hormone therapy in the United States and were increasingly prescribed to older women for putative benefits related to chronic diseases (14,15). WHI findings that 0.625 mg/day CEE-based therapies conveyed no cardiovascular benefits for older women and, instead, conveyed health risks have subsequently reduced their prescription among older women (16).
The WHI also found that CEE-based therapies increased risk for stroke (in particular, ischemic stroke) by about 40% and that this risk appeared to be enhanced for women older than 60 years (15). Because of this, WHIMS-MRI was designed with the expectation that women who had been assigned to CEE-based therapies during the WHI would have increased levels of subclinical ischemic lesion burdens. Even though the scans were performed up to 4 years after the conclusion of the WHI trials, the enrolled women continued to exhibit treatment-attributable mean deficits in cognitive function that were of similar magnitudes to those observed at the conclusion of the trials (3) and differences in the numbers of cases of cognitive impairment between arms continued (Figure 1); these findings suggest that the cognitive deficits induced by CEE-based therapy remained after cessation of its use and could be studied by MRI. Only minor CEE-related differences were found in mean levels of ischemic lesion volumes; however, assignment to active therapy was associated with significant mean decrements in frontal lobe brain volumes and borderline significant decrements in total brain and hippocampal volumes (3,4).
We report that several of the MRI measures had strong overall associations with the development of cognitive impairment: these included smaller total and hippocampal volumes and greater total and frontal lobe ischemic lesion volumes. Of great interest is that some associations varied depending on treatment assignment: Women who had been assigned to CEE-based therapy and who developed cognitive impairment presented a different MRI profile than those assigned to placebo. These women had significantly smaller total and hippocampal volumes. Women who had been assigned to placebo did not express such associations: Among these women, cognitive impairment was associated with greater ischemic lesion volumes in the frontal lobe and overall. It thus appears that the increased development of cognitive impairment resulting from exposure to CEE-based therapies results from an increased rate of brain atrophy, overall and perhaps more critically in the hippocampus. Our results are drawn from women aged 65 years or older; it is unknown whether they extend to younger women using CEE-based therapies.
WHIMS investigators examined the consistency of the adverse effects of CEE-based therapies on many subgroups (including all listed in Table 1) but found only one factor that consistently appeared to have influence: women who scored relatively higher on the 3MS test at baseline had smaller mean treatment-related decrements in cognitive function (5). Only few of the women with baseline 3MS scores of 95 or more developed cognitive impairment, although the treatment-related hazard ratio (95% confidence interval) for incident dementia among these women appeared to be elevated: 4.04 (0.86–19.04) for CEE-alone and 2.82 (1.18–6.70) for CEE + MPA (2). The cases that emerged among women assigned to hormone therapy appeared to be associated with a treatment-related decrease in brain volume that qualitatively resembled the pattern seen for women with lower pretreatment levels of cognitive function. The limited number of these cases prevents us from developing strong conclusions, but our data open the possibility that CEE forms of hormone therapy may occasionally convey risks even among older women who score highly on tests of cognitive function.
The mechanisms whereby CEE-based therapies lead to decreased hippocampal volume are not well understood but may be related to an unfavorable balance of neurogenesis and apoptosis. Animal data demonstrate that short-term estrogen exposure stimulates neurogenesis (granule cell proliferation and survival) of the hippocampal dentate gyrus (17), whereas long-term exposure suppresses neurogenesis (18). WHIMS-MRI participants assigned to CEE-based therapies tended to be exposed long term, which theoretically may have suppressed neurogenesis. The neurotrophic, neuromodulatory, and neuroprotective effects of estrogen on hippocampal neurons appear to be both dependent on, as well as independent of, nuclear estrogen receptors (19) and require an optimal microenvironment of other important mediators including serotonin (20), adrenal steroids (21), brain-derived neurotrophic factor, and nerve growth factor (22). Assessing the content of the hippocampal microenvironment is beyond the scope of the WHIMS-MRI. Furthermore, the clinical relevance of neurogenesis to hippocampal volume is unknown, as this process slows considerably in aging animals and is likely to follow the same pattern in aging humans (23).
How is the finding of decreased hippocampal volume among women randomized to CEE-based therapy reconciled with earlier reports of increased hippocampal volume (24)? The latter may seem more intuitive given the myriad “neuroprotective” effects of estrogen in vitro and in animal studies (19). However, the large sample size of WHIMS-MRI and its randomized controlled design provide a particularly robust setting in which to address the role of estrogen in brain volume and cognitive impairment, in contrast to observational studies. There are several important aspects of the present study that may explain differing results. WHIMS-MRI participants were older than those in several other studies. Most (67%) had no prior exposure to hormone therapy (3). This raises the question of the so-called “window of opportunity effect” for estrogen therapy (24), a concept that has been used to explain beneficial cognitive effects of estrogen in certain subgroups, particularly younger women receiving estrogen therapy soon after surgical menopause (25). WHIMS-MRI was not designed to address this question, and the results of the current study may not generalize to such populations. There may also be a possible “threshold” effect in which women taking estrogen for fewer than ∼10 years have been shown to fare better cognitively than those exposed longer term (26,27). However, WHI participants were exposed to study-prescribed CEE-based therapies for fewer than 10 years, and no association between prior exposure and regional brain volumes was detected (3).
Another possible explanation for these results is that exposure to exogenous estrogens beyond the “window” following menopause may accelerate the negative effects of aging and hypertension on hippocampal atrophy and cognitive decline. For example, prolonged hypoestrogenicity leads to a loss of estrogen sensitivity and reintroduction of estrogens has detrimental rather than beneficial effects (22). Hypertension is also associated with cognitive decline and hippocampal and whole-brain atrophy (28,29). Whole-brain atrophy and white matter ischemic lesions may be early indications of cognitive decline and hippocampal atrophy may be a late sign (30). Therefore, it is possible that chronic estrogen exposure following a prolonged period of hypoestrogenicity in a woman with poor brain reserve and vascular risks such as hypertension contributes to hippocampal atrophy through a loss of neuroprotective mechanisms, leading to reduced hippocampal volumes and cognitive decline. It is also possible that the components of CEE, which include many active equine-specific estrogens, may affect brain function differently than endogenous estrogens (31,32).
One limitation of this study is that APOE genotype is unknown. The APOE-4 genotype not only increases the risk for Alzheimer’s disease but also modifies the anti-inflammatory effect of estrogens (33) and may explain the subset of women who were at increased risk of cognitive impairment or probable dementia. Another is that the volunteers for a clinical trial and an imaging study do not closely represent the population of elderly women at large. The women enrolled in WHIMS-MRI tended to be younger, more highly educated, healthier, and have better cognition than other WHIMS women (9). The incidence rates of cognitive impairment seen in WHIMS-MRI women is lower than those in more general populations (e.g., 34) so that our findings are based on a limited number of cases.
The WHI results have led to a marked decline in the use of postmenopausal hormone therapy in women of all ages, and its use is currently recommended only short term in women with menopausal symptoms that interfere with quality of life. The prevalence of moderate or severe vasomotor symptoms in women in the age group who were enrolled in WHIMS is relatively low (10%–15% of 60- to 69-year-old women and 5%–10% of 70- to 79-year-old women) (15), but these women may use hormone therapy over prolonged periods. The WHIMS and WHIMS-MRI study results suggest that women with 3MS scores less than 95 may be at particular risk for CEE-related atrophy and deterioration in cognitive function. However, the relatively rare development of cognitive impairment among women with initially good test scores may be facilitated by accelerated atrophy linked to CEE therapy.
FUNDING
The Women’s Health Initiative is funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health, U.S. Department of Health and Human Services. The Women’s Health Initiative Memory Study was funded in part by Wyeth Pharmaceuticals, Inc., St. Davids, PA. S.M.R. is supported by the Intramural Research Program, National Institute on Aging, National Institutes of Health.
APPENDIX
WHIMS-MRI Clinical Centers: Albert Einstein College of Medicine, Bronx, New York: Sylvia Wassertheil-Smoller, Mimi Goodwin, Richard DeNise, Michael Lipton, James Hannigan; Medical College of Wisconsin, Milwaukee, Wisconsin: Jane Morley Kotchen, Diana Kerwin, John Ulmer, Steve Censky; Stanford Center for Research in Disease Prevention, Stanford University, Stanford, California: Marcia L. Stefanick, Sue Swope, Anne Marie Sawyer-Glover; The Ohio State University, Columbus, Ohio: Rebecca Jackson, Rose Hallarn, Bonnie Kennedy; University of California at Davis, Sacramento: John Robbins, Sophia Zaragoza, Cameron Carter, John Ryan; University of California at Los Angeles: Lauren Nathan, Barbara Voigt, Pablo Villablanca, Glen Nyborg; University of Florida, Gainesville/Jacksonville: Marian Limacher, Sheila Anderson, Mary Ellen Toombs, Jeffrey Bennett, Kevin Jones, Sandy Brum, Shane Chatfield; University of Iowa, Davenport, Iowa: Jennifer Robinson, Candy Wilson, Kevin Koch, Suzette Hart; University of Massachusetts, Worcester, Massachusetts: Judith Ockene, Linda Churchill, Douglas Fellows, Anthony Serio; University of Minnesota, Minneapolis, Minnesota: K.L.M., Cindy Bjerk, Chip Truwitt, Margaret Peitso; University of Nevada, Reno, Nevada: Robert Brunner, Ross Golding, Leslie Pansky; University of North Carolina, Chapel Hill, North Carolina: Carol Murphy, Maggie Morgan, Mauricio Castillo, Thomas Beckman; University of Pittsburgh, Pennsylvania: L.H.K., Pat McHugh, Carolyn Meltzer, Denise Davis.
WHIMS-MRI Clinical Coordinating Center: Wake Forest University Health Sciences, Winston-Salem, North Carolina: Sally Shumaker, M.A.E., Laura Coker, Jeff Williamson, Debbie Felton, LeeAnn Andrews, Steve Rapp, Claudine Legault, Maggie Dailey, Julia Robertson, Patricia Hogan, S.A.J., Pam Nance, Cheryl Summerville, Josh Tan.
WHIMS-MRI Quality Control Center: University of Pennsylvania, Philadelphia: Nick Bryan, Christos Davatzikos, Lisa Desiderio.
WHIMS-MRI Working Group: Wake Forest University Health Sciences, Winston-Salem, North Carolina: LeeAnn Andrews; University of Pennsylvania, Philadelphia: Nick Bryan; Wake Forest University Health Sciences, Winston-Salem, North Carolina: Laura Coker; Wake Forest University Health Sciences, Winston-Salem, North Carolina: M.A.E.; Wake Forest University Health Sciences, Winston-Salem, North Carolina: Debbie Felton; University of Pittsburgh, Pennsylvania: L.H.K.; University of Minnesota, Minneapolis, Minnesota: K.L.M.; University of Minnesota, Minneapolis, Minnesota: Anne Murray; Gerontology Research Center, National Institute on Aging, Baltimore, Maryland: S.M.R.; Wake Forest University Health Sciences, Winston-Salem, North Carolina: Sally Shumaker; Wake Forest University Health Sciences, Winston-Salem, North Carolina: Jeff Williamson.
U.S. National Institutes of Health: National Institute on Aging, Bethesda, Maryland: Neil Buckholtz, Susan Molchan, S.M.R.; National Heart, Lung, and Blood Institute, Bethesda, Maryland, Jacques Rossouw, Linda Pottern.
References
- 1.Shumaker S, Legault C, Rapp S, et al. The effects of estrogen plus progestin on the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 2.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 3.Resnick SR, Espeland MA, Jaramillo SA, et al. Effects of postmenopausal hormone therapy on regional brain volumes in older women: the Women’s Health Initiative Magnetic Resonance Imaging Study (WHIMS-MRI) Neurology. 2009;72:135–142. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coker LH, Hogan PE, Bryan NR, et al. The effects of postmenopausal hormone therapy on volumetric sub-clinical cerebrovascular disease: the Women’s Health Initiative Memory Study-Magnetic Resonance Imaging Study (WHIMS-MRI) Neurology. 2009;72:125–134. doi: 10.1212/01.wnl.0000339036.88842.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 6.Shumaker SA, Reboussin BA, Espeland MA, et al. The Women’s Health Initiative Memory Study: a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 7.Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 9.Jaramillo SA, Felton D, Andrews LA, et al. Enrollment in a brain magnetic resonance study: results from the Women’s Health Initiative Memory Study Magnetic Resonance Imaging Study (WHIMS-MRI) Acad Radiol. 2007;14:603–612. doi: 10.1016/j.acra.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 12.Lao Z, Shen D, Liu D, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol. 2008;15:300–313. doi: 10.1016/j.acra.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitwell JL, Crum WR, Watt HC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR Am J Neuroradiol. 2001;22:1483–1489. [PMC free article] [PubMed] [Google Scholar]
- 14.The Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 15.Hersch AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2003;91:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 16.Rossouw JE, Prentice RL, Manson KE, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 17.Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1997;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ormerod BK, Lee TT, Galea LA. Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J Neurobiol. 2003;55:247–260. doi: 10.1002/neu.10181. [DOI] [PubMed] [Google Scholar]
- 19.Behl C. Oestrogen as a neuroprotective hormone. Nat Rev Neurosci. 2002;3:433–442. doi: 10.1038/nrn846. [DOI] [PubMed] [Google Scholar]
- 20.Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- 21.Galea LA, Spritzer MD, Barker JM, Pakluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- 22.Spencer JL, Waters EM, Romeo RD, et al. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kempermann G, Song H, Gage FH. Neurogenesis in the adult hippocampus, in adult neurogenesis. In: Gage FH, Kempermann G, Song H, editors. Adult Neurogenesis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2008. pp. 159–174. [Google Scholar]
- 24.Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: a possible window of opportunity effect. Neurobiol Aging. 2008;29:95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 26.Erickson KI, Colcombe SJ, Elavsky S, et al. Interactive effects of fitness and hormone treatment on brain health in postmenopausal women. Neurobiol Aging. 2007;28:179–185. doi: 10.1016/j.neurobiolaging.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Matthews K, Cauley J, Yaffe K, Zmuda JM. Estrogen replacement therapy and cognitive decline in older community women. J Am Geriatr Soc. 1999;47:518–523. doi: 10.1111/j.1532-5415.1999.tb02563.x. [DOI] [PubMed] [Google Scholar]
- 28.Wiseman RM, Saxby BK, Burton EJ, et al. Hippocampal atrophy, whole brain volume, and white matter lesions in older hypertensive subjects. Neurology. 2004;23:1892–1897. doi: 10.1212/01.wnl.0000144280.59178.78. [DOI] [PubMed] [Google Scholar]
- 29.Korf ES, White LR, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- 30.Walstein SE, Brown JR, Maier KJ, Katzel LI. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Ann Behav Med. 2005;29:174–180. doi: 10.1207/s15324796abm2903_3. [DOI] [PubMed] [Google Scholar]
- 31.Rozovsky I, Hoving S, Anderson CP, et al. Equine estrogens induce apolipoprotein E and glial fibrillary acidic protein in mixed glial cultures. Neurosci Lett. 2002;323:191–194. doi: 10.1016/s0304-3940(02)00146-5. [DOI] [PubMed] [Google Scholar]
- 32.Dhandapani KM, Wade FM, Mahesh VB, Brann DW. Astrocyte-derived transforming growth factor-β mediates the neuroprotective effects of 17β-estradiol involvement of nonclassical genomic signaling pathways. Endocrinology. 2005;146:2749–2757. doi: 10.1210/en.2005-0014. [DOI] [PubMed] [Google Scholar]
- 33.Colton CA, Brown CM, Vitek MP. Sex steroids, APOE genotype and the innate immune system. Neurobiol Aging. 2005;26:363–372. doi: 10.1016/j.neurobiolaging.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Hebert LE, Scherr PA, Beckett LA, et al. Age-specific incidence of Alzheimer’s disease in a community population. JAMA. 1995;273:1354–1359. [PubMed] [Google Scholar]