Abstract
The cyclic adenosine monophosphate–dependent protein kinase A (PKA) pathway helps regulate both cell growth and division, and triglyceride storage and metabolism in response to nutrient status. Studies in yeast show that disruption of this pathway promotes longevity in a manner similar to caloric restriction. Because PKA is highly conserved, it can be studied in mammalian systems. This report describes the metabolic phenotype of mice lacking the PKA catalytic subunit Cβ. We confirmed that Cβ has high levels of expression in the brain but also showed moderate levels in liver. Cβ-null animals had reduced basal PKA activity while appearing overtly normal when fed standard rodent chow. However, the absence of Cβ protected mice from diet-induced obesity, steatosis, dyslipoproteinemia, and insulin resistance, without any differences in caloric intake or locomotor activity. These findings have relevant pharmacological implications because aging in mammals is characterized by metabolic decline associated with obesity, altered body fat distribution, and insulin resistance.
Keywords: Aging, PKA Cβ, Metabolic syndrome, Obesity resistance, Insulin sensitivity
THERE is increasing interest in the reduction and/or redistribution of adiposity in conjunction with delayed aging. Obesity is associated with impaired function of most organ systems and is a strong risk factor for shortened life span (1). Therefore, genes that promote adiposity are potential inhibitory targets for aging intervention. Studies in invertebrate organisms have indicated an important role for nutrient responsive kinase signaling pathways in response to nutrient deprivation. One such pathway in yeast is defined by cyclic adenosine monophosphate (cAMP)–dependent protein kinase A (PKA) (2,3). Several mutations that reduce PKA activity lead to increased life span in yeast and interestingly map to the same longevity pathway as caloric restriction (CR). PKA regulates cell growth and division in response to environmental cues (4,5) and is controlled by intracellular cAMP levels that are in turn modulated by adenylyl cylase (AC) activity (6). Loss of function of CYR1, an AC ortholog, extends life span in yeast, as do mutations in adenylate cyclase CDC35, the GTP–GDP exchange factor CDC25, or the PKA catalytic subunits TPK1, TPK2, and TPK3 (2,3). These invertebrate studies suggested that PKA may play a role in mammalian aging because in mammals PKA is thought to be important in the genetic regulation of obesity and energy balance, with a central role in the regulation of triglyceride storage and metabolism in response to nutrient status (7).
Mammalian PKA is a tetrameric holoenzyme composed of four subunits: two catalytic and two regulatory (6). In mice, separate genes encode each of the four regulatory isoforms (RIα, RIβ, RIIα, and RIIβ) and two catalytic isoforms (Cα and Cβ) (8). Each R subunit binds one C subunit, and binding of cAMP to R subunits causes the dissociation of active C subunits. In general, α-subunits are expressed ubiquitously in all tissues, whereas β-subunits show a more restricted pattern of expression (9). Cβ has been reported to be expressed at low levels in many tissues but found in high levels only in the nervous system (9). The Cβ gene encodes three isoforms: Cβ1, Cβ2, and Cβ3 (10,11). The last two are transcribed from neural-specific promoters, and knocking out all three isoforms (Cβall−/−) in mice reduces basal PKA activity in the brain (12). While Cβall−/− mice appear overtly normal on a regular diet, we speculated that they may show a more substantial phenotype in conditions of nutrient overload. Here, we report that Cβall−/− animals are significantly protected against diet-induced obesity, steatosis, dyslipoproteinemia, and insulin resistance when fed a high-caloric diet. These effects cannot be explained solely by reduced food consumption or enhanced activity and suggest that reduced brain and liver PKA Cβ activity confers a potential anti-aging phenotype in mammals.
METHODS
PKA Cβ-Deficient (Cβall−/−) Mice
Cβall−/− mice lack expression of all PKA Cβ isoforms. Mice were originally generated on a 50:50 (129XC57BL/6) genetic background and backcrossed to C57BL/6J (Jackson Laboratory, Bar Harbor, ME) for five generations (98% C57BL/6J) (12). We continued to backcross these mice to C57BL/6J for our studies. For this study, heterozygotes from congenic C57BL/6J mice were interbred to produce Cβall−/− and wild-type littermates. Cβall−/− mice have a neomycin expression cassette in lieu of exon 2. To identify the mutant allele, a reverse primer was designed inside the neomycin gene (5′-TGC TCT AGT AGC TTT ACG GAG C-3′) to be used with a forward primer (5′-TTG TGA CTT GCT TCC AAC TAA TG-3′) located approximately 435 bp upstream in intron 1–2. To identify the wild-type allele, a reverse primer was designed inside intron 1–2, in a region removed by the neomycin cassette (5′-GGA TTT GCC ATG GTC GTC TA-3′); a 345-bp product was obtained when used with the forward primer, 5′-TCT AAG GAT AGT CGT CAG TTA-3′. Mice were maintained in a 25°C-specific pathogen-free barrier facility with 12-hour alternating light and dark cycles and were given free access to food and water. All procedures used in this study were approved by the Animal Care and Use Committee of the University of Washington.
Specialized Diet Cohorts
The two diets used in our studies were standard rodent chow (5053; Picolab, Richmond, IN) containing 20% (wt/wt) protein, 4.5% fat (ether extract), and 55% carbohydrate (primarily starch), and a high-fat, high-sucrose (high-caloric) diet (S3282; Bio-Serv, Frenchtown, NJ) containing 20% protein, 36% fat (primarily lard), and 36% carbohydrate (primarily sucrose). Mice were maintained on standard rodent chow until 12 weeks of age, at which time they were either switched to the high-caloric diet or continued on the standard chow for an additional 13–16 weeks. Both the regular diet and high-caloric diet cohorts had a minimum of seven mice per gender per genotype. Food intake was measured every 3 weeks. Two to three mice of the same genotype were housed per cage, and food intake was measured as the weight of food a cage consumed over a 24-hour period, averaged between the number of mice in each cage. Food was weighed three times in a row over the course of 3 days and averaged.
Immunoblotting
Whole brains and right lobes of livers were harvested from 3-month-old female and 4-month-old male mice, respectively, both maintained on a regular diet. The whole brains and half of the lobe of each of the livers each were ground in liquid nitrogen followed by homogenization in 1 mL of ice-cold RIPA containing 25 μL each of the following protease and phosphatase inhibitor cocktails: P8340, P2850, P5726 (Sigma-Aldrich, St Louis, MO). Thirty micrograms of protein from the cytosolic fraction was loaded to each lane of a NuPAGE Novex 4–12% Bis–Tris Gel (NP0322BOX; Invitrogen, Grand Island, NY). Blots were probed with primary antibodies to PKA Cα (NB100-92207; Novus Biological [Littleton, CO]; diluted 1:500 in tris-buffer saline tween-20 [TBST]) and Cβ (obtained from G.S.M., University of Washington, Seattle; diluted 1:1000 in TBST). A goat anti-rabbit, horseradish peroxidase-conjugated secondary antibody (ab6721; Abcam, Cambridge, MA) was used at a dilution of 1:3000, and detection was accomplished using ECL (95038-560; VWR Scientific, Brisbane, CA).
Body and Liver Composition
Body weights were measured weekly. Body and liver composition analyses were performed on all cohorts at the end of the experiment, following 13 weeks on either the regular or the diabetogenic diet (25 weeks of age). Composition measurements were measured using quantitative nuclear magnetic resonance imaging (QNMR), with an EchoMRI 3-in-1 Animal Tissue Composition Analyzer, at the University of Washington Mouse Metabolic Phenotyping Center (MMPC). Body composition measurements were performed on live mice. Mice were then euthanized by CO2, and body length (from tip of nose to anus) and weights were measured. The liver and the following fat pads were dissected out and weighed: inguinal, reproductive, retroperitoneal, mesenteric, and brown adipose. The large lobe of the liver was embedded in paraffin and sectioned; a small (approximately 30 mg) piece was set aside for QNMR composition measurements.
Serum Triglycerides and Cholesterol
Before dissection and following euthanasia, blood was collected by cardiac puncture. Upon collection, serum was immediately separated using serum separator tubes (365956; Becton Dickinson, Franklin Lakes, NJ) and stored at −80°C until analysis. Triglyceride concentration (measured from glycerol derived by hydrolytic action of lipase on glycerides) was measured using a serum triglyceride determination kit (TR0100; Sigma Aldrich, St Louis, MO). Serum high-density lipoprotein (HDL) and low and very low–density lipoprotein (LDL/VLDL) concentrations were also quantified using a kit (K613-100; BioVision, Mountain View, CA). Both kits were used as per the manufacturer’s instructions.
Glucose Homeostasis
Blood glucose levels were measured weekly. For blood glucose measurements, food was removed from mice 6 hours before blood was drawn by tail pricking. Analyses were performed using a glucometer and Comfort Curve Test Strips (Advantage; Accu-Chek, Roche, Basel, Switzerland). For intraperitoneal glucose tolerance testing (IPGTT) and insulin resistance assays, mice were first fasted overnight for 16 hours. Mice were then injected intraperitoneally with either 20% D-(+)-glucose (G7021; Sigma Aldrich) in phosphate buffered saline (14190; Invitrogen) at a dose of 2 g glucose/kg body weight for the IPGTT or human insulin (Humulin R; Eli Lilly, Indianapolis, IN) diluted with sterile diluent (Eli Lilly) at a dose of 1.0 U insulin/kg body weight for the insulin resistance assay. Blood glucose measurements were performed as previously described at 0, 30, 60, 90, 120, and 240 and 0, 30, 60, and 90 minutes after injection for the IPGTT and insulin resistance assay, respectively. For serum insulin measurements, food was removed from mice 12 hours before the collection of blood from the retro-orbital sinus into serum separator tubes (365956; Becton Dickinson); after separation, plasma was either used immediately or stored at −80°C until analysis. Plasma insulin was measured using an ELISA kit (EZRMI-13K; LINCO, St Charles, MO) as per the manufacturer’s instructions.
Stastistics
Data are presented as means. Error bars, where present, indicate ± standard deviation. Differences between genotypes were determined using the Student’s t test. Probabilities of individual data points being different are indicated on graphs as p ≤ .0001 (***), p ≤ .001 (**), p ≤ .05 (*), p ≤ .1 or borderline significant (b) and p > .1 or insignificant (no indicator).
RESULTS
Cβall−/− Mice Have No Detectable Levels of PKA Cβ and Unaltered Levels of Cα
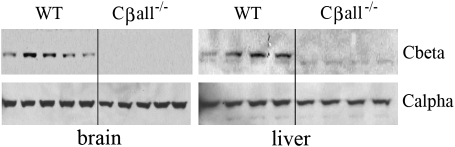
Loss of the Cβ subunit in tissues of Cβall−/− mice was confirmed. Immunoblotting detected no Cβ protein in either brains or livers of Cβall−/− mice (Figure 1). Because compensatory increases in Cα in the brain in response to loss of Cβ have been reported (12), we also looked at Cα levels in these tissues. We did not find any noticeable alterations in expression levels of the α-catalytic subunit (Figure 1).
Figure 1.
Cα and Cβ protein levels in brain and liver. Immunoblots showed no detectable levels of Cβ in either total brain or in liver of Cβall−/− mice. Cα levels were found to be unaltered by disruption of Cβ in these tissues. Each lane represents an individual mouse.
Fat Mass Accumulation Is Altered in PKA Cβ-Null Mice
Male Cβall−/− mice at 12 weeks of age on standard rodent chow showed body weights approximately 10% less than wild-type littermates, and this size difference persisted over the 14-week course of the regular diet treatment (Figure 2A). When maintained on a diabetogenic diet for 15 weeks, body weights between genotypes continued to diverge (Figure 2B). There were no significant differences in body weight found between genotypes in females on standard chow (Figure 2C). Fat acquisition was reflected by a pronounced difference in the rate of weight gain between female Cβall−/− mice and their wild-type littermates over the course of their maintenance on the diabetogenic diet. After 6 weeks, significant differences in body weight were seen between genotypes (Figure 2D). By 12 weeks on the high-caloric diet, wild-type female mice had almost doubled their weight, whereas body weights of female mutants had only increased by about 30% (Figure 2D).
Figure 2.
Weight gain and adiposity of male and female wild-type and Cβall−/− mice on regular and diabetogenic diets. (A and B) Body weights of males maintained on a regular (A) and diabetogenic (B) diet for 14–15 weeks. Mutants weighed about 10% less than wild-type littermates when maintained on a regular diet; this weight difference became more pronounced if mice were raised on a diabetogenic diet. (C and D) Body weights of female wild-type and Cβall−/− mice fed either a regular (A) or a diabetogenic (B) diet for 14–15 weeks. There was no difference in body weight between genotypes if maintained on a regular diet. Mutants had a slower rate of weight gain than wild-type littermates if raised on a diabetogenic diet. (E) Quantitative nuclear magnetic resonance imaging body composition analysis. There was no difference in percent fat or percent lean mass between genotypes for male mice, regardless of diet. Female mutants showed increased slightly higher adiposity compared with wild types when on a regular diet but reduced adiposity when maintained on the diabetogenic diet. (F–I) Final fat pad percent body weights of male and female wild-type and Cβall−/− mice on regular and diabetogenic diets. (F) No differences were seen in fat pad percent body weights between genotypes of males on a regular diet. (G) Fat distribution was different in mutant males raised on a diabetogenic diet compared with wild-type mice. Reproductive fat pads comprised a larger percentage of the body weight in mutants than wild-type mice, whereas retroperitoneal, mesenteric and brown adipose fat pads had lower percent body weights. (H) Borderline significant to no significant differences was seen in fat pad weights between genotypes of females on a regular diet. Inguinal fat pads had slightly higher percent body weights in the mutants. (I) All fat pads of female mutants on the diabetogenic diet were much smaller than those of their wild-type littermates. *p < .05, **p < .001, ***p < .0001, and b represents borderline significance. Error bars represent standard deviations. n = 7–10 mice per set.
QNMR analysis showed no differences in percent body weight of fat and lean tissue between genotypes for 25-week-old males maintained on a regular diet, whereas Cβall−/− females of the same age and on the regular diet had a slightly higher percentage of body fat than their wild-type littermates (Figure 2E). For mice maintained on the diabetogenic diet until 25 weeks of age, only females showed a significant difference in percent body fat and lean weight between genotypes. At the end of the diabetogenic diet treatment, Cβall−/− females had significantly lower body fat percentages, and higher lean weight percentages, than their wild-type littermates. Wild-type littermates were found to have body fat percentages that were approximately 25% higher than mutants (Figure 2E).
Fat pad percent body weights between genotypes of male mice kept on the regular diet were similar (Figure 2F). However, and even though QNMR did not show differences in total percent body fat between male mutant and wild-type mice after 13 weeks on the high-caloric diet, fat distribution differed between genotypes. Mutants had larger reproductive pads, but smaller retroperitoneal, mesenteric, and brown adipose fat pads (Figure 2G). Fat pad percent body weights between genotypes of female mice kept on the regular diet showed slightly larger inguinal and brown adipose fat pads in Cβall−/− females (Figure 2H), but after being maintained on the diabetogenic diet, all fat pads in the mutants were smaller when compared with those of the wild-type animals (Figure 2I). There were no significant differences in food intake between males of either genotype on the regular diet (Table 1). Male Cβall−/− mice on the diabetogenic diet were sometimes seen to be slightly hypophagic compared with wild-type mice. Female mutants were not found to eat less than their wild-type littermates. If anything, females on a regular diet were found to be borderline hyperphagic. With the exception of 1 week during the dietary treatment, no significant differences were observed between genotypes for females on the diabetogenic diet. No differences in locomotor activity were seen between genotypes for either gender at any time of day (data not shown).
Table 1.
Food Intake in Male and Female Wild-type and Cβall−/− Mice on Regular and Diabetogenic Diets
| Males |
Females |
||||||||||
| Regular Diet (g) |
Diabetogenic Diet (g) |
Regular Diet (g) |
Diabetogenic Diet (g) |
||||||||
| Week | WT | Cβall−/− | Week | WT | Cβall−/− | Week | WT | Cβall−/− | Week | WT | Cβall−/− |
| 3 | 4.9 ± 0.5 | 5.2 ± 0.9 | 3 | 3.4 ± 0.3 | 3.4 ± 0.5 | 0 | 4.6 ± 0.3 | 4.5 ± 0.3 | 0 | 2.9 ± 0.3 | 2.6 ± 0.5 |
| 5 | 6.0 ± 0.9 | 6.0 ± 1.2 | 7 | 2.8 ± 0.1 | 2.7 ± 0.4 | 3 | 4.7 ± 0.1 | 4.9 ± 0.7 | 3 | 2.7 ± 0.1 | 3.0 ± 0.4 |
| 7 | 5.3 ± 0.2 | 4.9 ± 0.7 | 10 | 3.6 ± 0.2 | 3.0 ± 0.5* | 5 | 4.1 ± 0.2 | 4.7 ± 0.5† | 7 | 2.8 ± 0.1 | 3.4 ± 0.04* |
| 14 | 6.3 ± 0.7 | 6.1 ± 1.3 | 12 | 4.0 ± 0.2 | 3.1 ± 0.8* | 7 | 4.9 ± 0.3 | 5.5 ± 0.3* | 10 | 2.2 ± 0.3 | 2.3 ± 0.5 |
| 15 | 6.3 ± 0.8 | 5.6 ± 1.3 | 14 | 3.2 ± 0.2 | 3.3 ± 0.4 | 12 | 4.4 ± 0.4 | 5.0 ± 0.1† | 15 | 3.1 ± 0.4 | 3.0 ± 0.4 |
Notes: Males, regular diet: no differences in food intake were seen over the course of the study. Males, diabetogenic diet: only on two occasions (at 10 and 12 weeks) was some hypophagia seen in the mutants when compared with wild types. Females, regular diet: mutant females showed slight hyperphagy in weeks 5, 7, and 12 of the study. Females, diabetogenic diet: food intake was the same between mutants and wild types, except in week 7, where slight hyperphagia was observed in the mutants. *p < .05, and dagger represents borderline significance. Errors are presented as standard deviations. n = 7–10 mice per set.
PKA Cβ-Null Mice Are Protected Against Hepatic Adiposity
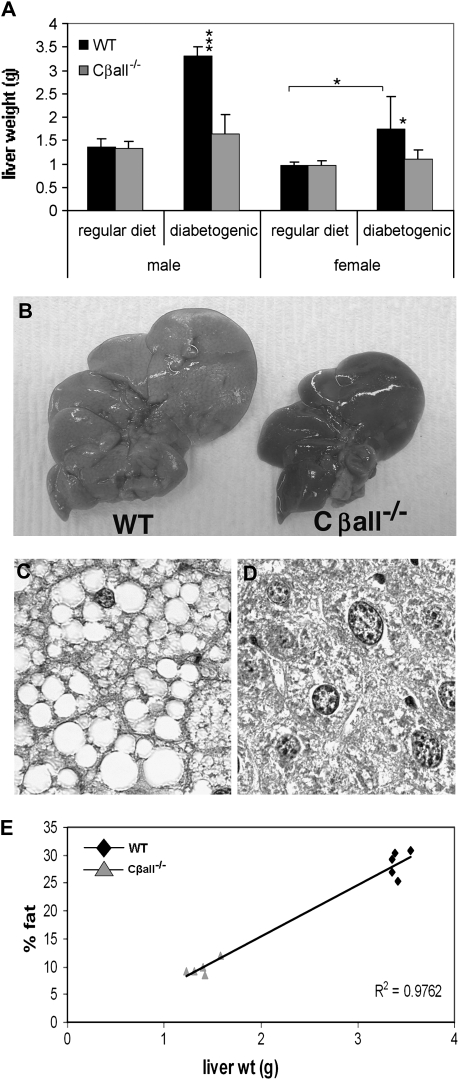
No differences in liver weights or liver fat content were found between genotypes of either gender when raised on a regular diet. On the diabetogenic diet, however, wild-type mice of both genders developed larger livers, whereas Cβall−/− mice did not (Figure 3A and B). The cells of livers from wild-type mice fed the high-caloric diet contained large fat-filled vacuoles (Figure 3C) that were absent from the livers of mutant mice (Figure 3D). QNMR analysis revealed a strong and direct correlation between total liver weight and percent fat content (Figure 3E).
Figure 3.
Cβall−/− mutants are protected against fatty liver disease. (A) Wild-type (WT) mice fed the diabetogenic diet developed large livers, whereas livers from mutants remained the same size as those from mice on a regular diet. (B) Livers from WT mice fed a diabetogenic diet were large and pale in color compared with those of mutants. (C and D) Periodic acid/Schiff-stained sections of paraffin-embedded livers from male mice fed a diabetogenic diet. Livers from WT mice were full of fat-filled vacuoles, absent from livers of Cβall−/− mice. (E) Correlation of percent fat content of livers from male mice maintained on a diabetogenic diet (measured with quantitative nuclear magnetic resonance imaging), with total liver weight. R2 = .9762. *p < .05, **p < .001, ***p < .0001. Error bars represent standard deviations. n = 7–10 mice per set.
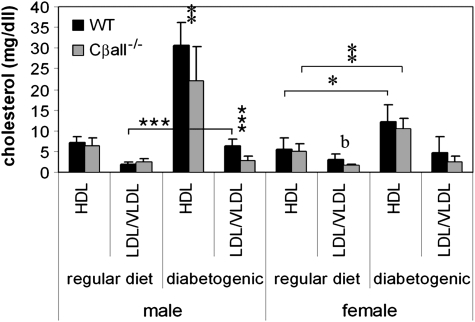
Deletion of PKA Cβ Alters Serum Cholesterol but Not Triglyceride Levels
There were no differences in serum triglyceride levels between genotypes of either gender, regardless of diet (data not shown). No differences in HDL or LDL/VLDL were seen between genotypes of males maintained on the regular diet (Figure 4). LDL/VLDL serum levels were slightly lower in Cβall−/− females compared with wild-type littermates when maintained on a regular diet, but with borderline significance. The diabetogenic diet caused significant increases in HDL in both genotypes of both genders. In males, however, this increase in HDL was not as high for Cβall−/− mice compared with wild-type littermates. LDL/VLDL serum levels increased in male wild types fed the diabetogenic diet but not in mutants. Neither female mutants nor their wild-type littermates experienced increases in serum LDL/VLDL when switched to the diabetogenic diet. By the end of the diabetogenic diet treatment, only males showed a difference in HDL and LDL/VLDL serum levels between genotypes. Both HDL and LDL/VLDL serum levels were significantly lower in Cβall−/− males when compared with wild types.
Figure 4.
Lipoproteins in male (left side) and female (right side) wild-type and Cβall−/− mice on regular and diabetogenic diets. All mice on the diabetogenic diet experienced rises in serum HDL levels, but male mutants had significantly lower levels than wild types. Male wild-type mice, but not mutant mice, also showed increases in low-density lipoprotein (LDL) and very low–density lipoprotein (VLDL) when fed the diabetogenic diet. *p < .05, **p < .001, ***p < .0001. Error bars indicate standard deviations. n = 7–10 mice per set.
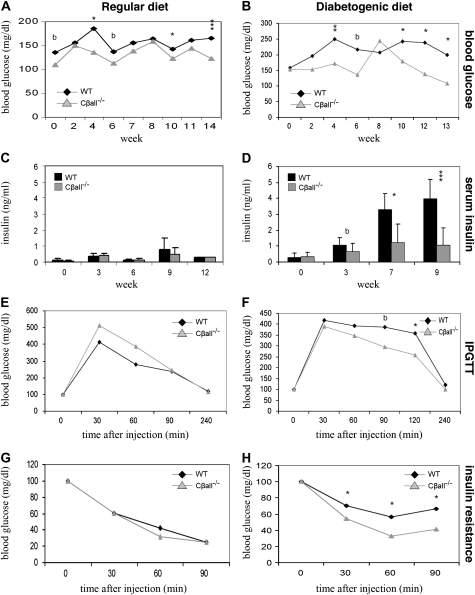
Absence of PKA Cβ Enhances Glucose Clearance and Improves Insulin Sensitivity
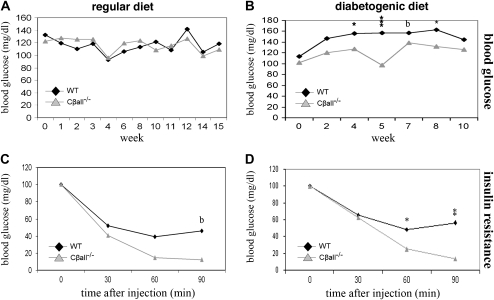
The diabetogenic diet tends to cause insulin resistance in BL/6 mice. Blood glucose levels of male Cβall−/− mice were frequently found to be lower than wild types (Figure 5A), and these differences became more pronounced when mice were switched to the diabetogenic diet (Figure 5B). Serum insulin levels in males were barely detectable and not different between genotypes on the regular diet (Figure 5C), but on the diabetogenic diet, serum levels increased in wild-type males while remaining low in Cβall−/− mice (Figure 5D). Cβall−/− male mice on the diabetogenic diet showed superior glucose clearance and insulin sensitivity compared with wild types (Figure 5F and H), with no differences seen between genotypes fed the regular diet (Figure 5E and G). Females showed no difference between genotypes in blood glucose levels when fed the regular diet (Figure 6A), but wild-type mice showed elevated blood glucose compared with the mutants when switched to the diabetogenic diet (Figure 6B). Serum insulin differences were not seen between genotypes in females, regardless of diet (data not shown). Differences in glucose clearance between genotypes in females were not seen, regardless of diet (data not shown), but Cβall−/− female mice on either diet were found to be more insulin sensitive than their wild-type littermates when insulin sensitivity tests were performed (Figure 6C and D).
Figure 5.
Glucose homeostasis in male wild-type and Cβall−/− mice on regular and diabetogenic diets. (A) Blood glucose levels of male mutants on the regular diet were frequently found to be lower than those of wild-type mice over the course of the study. Differences between genotypes were even greater when mice were fed the diabetogenic diet (B). (C) Serum insulin in both genotypes remained very low (barely detectable) over the course of the study when mice were fed the regular diet. (D) Serum insulin levels in wild-type mice fed the diabetogenic diet increased 10-fold by the end of the study but remained low in mutants. (E and F) Intraperitoneal glucose tolerance testings (IPGTTs) on mice fed regular (E) and diabetogenic (F) diets. There were no significant differences between genotypes of mice fed the regular diet in the ability to clear intraperitoneally injected glucose, but mutants were found to have better glucose disposal than wild types if fed the diabetogenic diet for 12 weeks. (G and H) Insulin resistance of mice fed regular (G) and diabetogenic (H) diets. Differences were only seen between genotypes if mice were fed the diabetogenic diet; mutants were found to be more sensitive to insulin than wild-type mice. *p < .05, **p < .001, ***p < .0001, and b represents borderline significance. Error bars represent standard deviations. n = 7–10 mice per set. For IPGTT and insulin resistance analyses, results were standardized by setting initial blood glucose levels at 100%.
Figure 6.
Glucose homeostasis in female wild-type and Cβall−/− mice on regular and diabetogenic diets. (A) Blood glucose levels of female mutants on the regular diet were found to be similar to those of wild-type mice over the course of the study, but mutants were frequently found to have lower blood glucose levels when mice were fed the diabetogenic diet. (C and D) Insulin resistance of mice fed regular (C) and diabetogenic (D) diets. Mutant mice on both diets were found to be more insulin sensitive than wild-type mice when injected intraperitoneally with insulin. *p < .05, **p < .001, ***p < .0001, and b represents borderline significance. Error bars represent standard deviations. n = 7–10 mice per set. For IPGTT and insulin resistance analyses, results were standardized by setting initial blood glucose levels at 100%.
DISCUSSION
We show that disruption of the PKA catalytic subunit Cβ protects mice from diet-induced obesity, steatosis, dyslipoproteinemia, and insulin resistance. We also show gender-specific effects. Female Cβall−/− mice are more insulin sensitive than wild-type mice even when fed a regular diet. On a high-caloric diet, female Cβall−/− mice display reduced weight gain, adiposity, resistance to fatty liver disease, and improved insulin sensitivity when compared with their wild-type littermates. Although male Cβall−/− mice on a high-caloric diet show reduced weight gain compared with wild-type littermates, this is probably due to a growth defect rather than obesity resistance because they do not show a lower overall body fat percentage. They do, however, deposit fat differently and maintain their insulin sensitivity. They display reduced plasma cholesterol, and, like females, they are also resistant to the development of a fatty liver. While PKA is known to be involved in the regulation of food intake, dramatic differences in caloric intake were not found between genotypes and are not believed to be responsible for the differences in growth and fat accumulation seen between Cβall−/− and wild-type mice. Locomotor activity was also not found to be different between genotypes.
Sexual dimorphism is common in mouse models of obesity and insulin resistance, but interestingly, it is usually the males that show susceptibility to genetic mutations in this regard (13). For example, mice lacking peroxisome proliferator-activated receptor-alpha, a transcription factor involved in fatty acid oxidation gene expression, show an obesity susceptible phenotype in males only, which can be rescued by a 2-week pretreatment with β-estradiol (14). A female-specific phenotype points to a hormonal effect and may be due to a role for estrogen in the regulation of lipid metabolism. It has been found that in general, male mice are more susceptible to diet-induced obesity than female mice; in keeping with the idea that estrogen is involved in obesity resistance, ovariectomies removed protection of the females against this weight gain (15). The gender-specific nature of the obesity resistance observed in Cβall−/− mice points to the potential involvement of an estrogen signaling pathway.
We have confirmed that the Cβall mutation results in a complete loss of the PKA Cβ subunit. While the genetic mechanism behind the obesity resistance in Cβ−/− mice remains unknown, some insight may be provided by studies of mutants lacking the RIIβ regulatory subunit of PKA. As with Cβall−/− mice, RIIβ−/− mice are protected against diet-induced obesity (16,17). Cβ, like RIIβ, has high levels of expression in the brain, but unlike RIIβ, it is not present at high levels in adipose tissue (17). It is thus likely that PKA activity in the brain plays a very important role in the obesity resistance and some of the other phenotypes shared by both of these mutants. The arcuate nucleus region of the hypothalamus contains leptin-responsive neurons that control metabolic rate through the activation of Gs-coupled melanocortin receptors. These receptors are thought to increase energy expenditure through stimulation of the cAMP pathway and activation of PKA (18). It is known that disruption of the RIIβ subunit rescues the obesity phenotype seen in agouti lethal yellow mice, believed to suffer from antagonism of these receptors (18), and loss of RIIβ also rescues the mutant phenotype of the obese leptin-deficient ob/ob mouse (19). It is most likely that RIIβ acts downstream of leptin receptors in the leptin melanocortin pathway, and it is possible that PKA Cβ acts in a similar manner. Gender specificity has not been reported for phenotypes demonstrated by mice lacking the RIIβ subunit, however, and both genders of RIIβ−/− mice are resistant to age-induced obesity (20). However, estrogen acts in the hypothalamus in a very similar manner to leptin, regulating food intake and energy expenditure in both animals and humans (21,22,23). Loss of Cβ may thus mimic some of the RIIβ−/− leptin–mediated phenotypes by altering a hypothalamic estrogen signaling pathway, in which case disruption of both of these genes should have a synergistic effect on obesity resistance. Future studies will examine the effects of a dietary challenge on double knockouts of these genes.
In keeping with the idea that these two proteins may have somewhat different roles in obesity resistance, loss of either RIIβ or Cβ has a very different effect on overall PKA activity. Disruption of RIIβ causes an increase in basal PKA activity (19). Disruption of Cβ causes a decrease in basal PKA activity in the brain in spite of a reported compensatory increase in the amount of Cα protein in the cortex, amygdala, and hippocampus (12). However, the catalytic subunits Cα and Cβ have highly conserved amino acid differences across species, and they are thus believed to have unique functions (24). Total PKA activity may very likely not be as important as the individual levels of activity by each of these subunits. While we were not able to show increases in Cα protein in total brain extracts of Cβ−/− mice, loss of the Cβ subunit could still create a stoichiometric shift in Cβ/Cα activity, representing an increase in a particular type of PKA catalytic function. Cα- and Cβ-specific PKA activity assays need to be developed to test this hypothesis.
There were significant differences in how fat was distributed in males on a high-caloric diet, even though the overall percentage of body fat was not different between genotypes. Mutants accumulated more fat in their reproductive fat pads and less in other fat pads than wild-type mice. Importantly, the mesenteric fat pad was found to be smaller in mutants. The accrual of mesenteric fat is a common feature of aging, and its removal extends life span in rats (25). Mesenteric fat is the source of several hormones and cytokines that induce inflammation and oxidative damage in vascular tissue (26) and is known to play a critical role in the pathogenesis of metabolic syndrome, its thickness correlating well with cardiovascular risk factors (27,28). Mesenteric fat thickness also correlates with cholesterol levels in humans (27), and Cβall−/− male mice were found to be resistant to increases in HDL and LDL/VLDL when maintained on a high-caloric diet. Thus, while Cβall−/− male mice raised on a high-caloric diet are not less obese in terms of percent body fat, they still enjoy health benefits. Obesity resistance in female mutants was more overt. QNMR revealed a much higher fat-to-lean tissue ratio, as well as dramatically larger fat pads, in wild-type mice raised on the high-caloric diet compared with Cβall−/− mice. Lack of weight gain in female mutants on the high-caloric diet is thus largely due to a lack of fat accumulation. Cβ may have evolved to be important in the regulation of fat accumulation of females during reproductive life.
Livers of Cβall−/− mice of both genders were found to be protected from steatosis. This was not entirely due to a secondary effect of protection against general obesity as percent body fat in males on the high-caloric diet was similar between genotypes, but percent liver fat was dramatically different. Although male Cβall−/− mice did not show reduced body fat percentages after 12 weeks on the high-caloric diet compared with wild types, all mutants showed lack of steatosis. Protection against fatty livers in Cβall−/− mice is thus due to additional genetic factors not related to the peripheral anti-obesity phenotype. Potential roles for PKA Cβ in fatty liver resistance are diverse and need to be investigated. Reduced lipolysis and fatty acid transfer to the liver is one mechanism by which steatosis can be prevented, but we did not find differences in serum triglyceride levels between the Cβall−/− and wild-type mice. Another explanation for the lack of fatty liver seen in the Cβall−/− mutants is altered gluconeogenesis. It is known that the transcription factor, cAMP response element–binding protein (CREB) does play a major and direct role in hepatic lipid and glucose metabolism, acting as a regulatory checkpoint and promoter for gluconeogenesis and fatty acid oxidation (29,30,31). However, we did not find changes in CREB phosphorylation levels in the livers of Cβ−/− mice (unpublished observations). PKA is also involved in the regulation of carbohydrate responsive element–binding protein (ChREBP), a transcription factor that is activated in response to high glucose and that upregulates the expression of lipogenic genes (32,33,34,35). Phosphorylation of ChREBP by cAMP-dependent PKA results in its inactivation (32). Inhibition of ChREBP improves hepatic steatosis and insulin resistance in the leptin-deficient obese ob/ob mouse (36). A potential role for PKA Cβ in the ChREBP pathway will be the focus of future studies. Alternatively, rather than playing a direct role in fatty liver resistance, changes in PKA signaling in the brain could lead indirectly to changes in liver fat content, for example, by affecting the release of pituitary hormones such as adrenocorticotropic hormone (ACTH). ACTH release is stimulated by a cAMP-dependent PKA-mediated pathway (37,38), and its secretion leads to the release of hormones from the adrenal cortex that ultimately stimulate liver gluconeogenesis by inducing key gluconeogenic enzymes PEPCK and G6Pase (39,40,41). Fatty liver resistance could also be linked to protection against high-caloric diet-induced insulin resistance. Insulin resistance is the most common and reproducible factor associated with nonalcoholic fatty liver disease (42) and is believed to increase the intrahepatic production of free fatty acids (43).
Cβall−/− mice were protected against diet-induced insulin resistance. Both genders displayed insulin sensitivity, another unusual finding among diabetic rodent models (17). This effect was not merely due to obesity resistance because even under a regular diet regimen and with slightly higher adiposity, female mutants showed improved glucose disposal in response to peritoneally injected insulin compared with wild types. After being on the high-caloric diet, Cβall−/− male mice shared the same percent body fat as their wild-type littermates but were also more responsive to insulin. Cβ thus directly influences insulin sensitivity. It has been proposed that because PKA is known to antagonize insulin activation of the mitogen-activated protein kinase cascade, mutants with decreased PKA activity may have enhanced insulin sensitivity (17). The insulin sensitive phenotype of Cβall−/− mice may thus indicate a specific role for the Cβ subunit in the regulation of this pathway. Because insulin inhibits the assembly and release of VLDLs from the liver (44), it is possible that the reduced serum VLDL levels seen in Cβall−/− mice are an indirect effect of the insulin sensitivity. Alternatively, as with fatty liver resistance, disruption of pituitary PKA Cβ may lead to these phenotypes because it is known that ACTH stimulates insulin secretion and inhibits VLDL secretion (45,46).
Studies in divergent organisms have implicated PKA as being important in the regulation of aging in response to nutrient status (47). Theories for how PKA disruption promotes longevity in yeast are diverse, including enhanced resistance to heat and oxidative stress (48,49). In yeast, it was initially proposed that reduced PKA increased life span by activating the Sir2 histone deacetylase and reducing the accumulation of extrachromosomal ribosomal DNA circles (3), but subsequent studies have shown that PKA and CR both act via Sir2-independent mechanisms (50,51). More recently, reduced signaling through TOR, PKA, and the yeast homolog of ribosomal S6 kinase, Sch9, has been shown to increase life span in part by reducing messenger RNA (mRNA) translation (52). Because regulation of mRNA translation is also a conserved mechanism of longevity control in multicellular eukaryotes (53), it is tempting to speculate that PKA may influence mammalian aging in a similar manner.
The role of PKA in regulating metabolism may be particularly relevant for mammalian longevity. CR extends life span in many organisms. It is unknown exactly which of the physiological changes and what genetic mechanisms are responsible for life span extension in CR mice, but like Cβall−/− and RIIβ−/− animals, they do not develop altered adiposity and increased insulin efficiency, hallmarks associated with metabolic syndrome (54,55). RIIβ−/− animals have been found to be both resistant to age-related obesity as well as long lived (20). Aging in people is often characterized by metabolic decline associated with obesity, altered body fat distribution, and insulin resistance (56,57,58). The increased obesity and insulin resistance found in the majority of middle-aged people in the Western world are major risk factors for diminished life span and enhanced age-related disease conditions such as diabetes, cardiovascular disease, neurodegeneration, and cancer (59,60). These observations suggest that PKA may be a viable pharmacological target for treating age-associated diseases, and it will be of particular interest to determine whether Cβall−/− animals have an extended life span.
FUNDING
Supported in part by the Ellison Medical Foundation at the National Insititute on Aging (Nathan Shock Center) and at the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health.
Acknowledgments
QNMR was performed by the Seattle MMPC at the University of Washington. We acknowledge the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases/National Heart Blood and Lung Institute), U24 DK076126, for their support of this facility. Presented in part at the 2008 Gerontological Society of America Annual Meeting.
References
- 1.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289:187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 2.Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292:288–290. doi: 10.1126/science.1059497. [DOI] [PubMed] [Google Scholar]
- 3.Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen P, Rupes I, Sharom JR, Schneper L, Broach JR, Tyers M. A dynamic transcriptional network communicates growth potential to ribosome synthesis and critical cell size. Genes Dev. 2004;18:2491–2505. doi: 10.1101/gad.1228804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin DE, Soulard A, Hall MN. TOR regulates ribosomal protein gene expression via PKA and the Forkhead transcription factor FHL1. Cell. 2004;119:969–979. doi: 10.1016/j.cell.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 6.McKnight GS, Cummings DE, Amieux PS, et al. Cyclic AMP, PKA, and the physiological regulation of adiposity. Recent Prog Hormone Res. 1998;53:139–161. [PubMed] [Google Scholar]
- 7.Robinson SW, Dinulescu DM, Cone RD. Genetic models of obesity and energy balance in the mouse. Annu Rev Genet. 2000;34:687–745. doi: 10.1146/annurev.genet.34.1.687. [DOI] [PubMed] [Google Scholar]
- 8.McKnight GS. Differential expression of mRNAs for protein kinase inhibitor isoforms in mouse brain. Curr Opin Cell Biol. 1991;3:213–217. [Google Scholar]
- 9.Brandon EP, Idzerda RL, McKnight GS. PKA isoforms, neural pathways, and behaviour: making the connection. Curr Opin Neurobiol. 1997;7:397–403. doi: 10.1016/s0959-4388(97)80069-4. [DOI] [PubMed] [Google Scholar]
- 10.Desseyn JL, Burton KA, McKnight GS. Expression of a nonmyristylated variant of the catalytic subunit of protein kinase A during male germ-cell development. Proc Natl Acad Sci U S A. 2000;97:6433–6438. doi: 10.1073/pnas.97.12.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guthrie CR, Skalhegg BS, McKnight GS. Two novel brain-specific splice variants of the murine Cbeta gene of cAMP-dependent protein kinase. J Biol Chem. 1997;272:29560–29565. doi: 10.1074/jbc.272.47.29560. [DOI] [PubMed] [Google Scholar]
- 12.Howe DG, Wiley JC, McKnight GS. Molecular and behavioural effects of a null mutation in all PKA Cβ isoforms. Mol Cell Neurosci. 2002;20:515–524. doi: 10.1006/mcne.2002.1119. [DOI] [PubMed] [Google Scholar]
- 13.Leiter EH, Le PH, Coleman DL. Susceptibility to db gene and streptozotocin-induced diabetes in C57BL mice: control by gender-associated, MHC-unlinked traits. Immunogenetics. 1987;26:6–13. doi: 10.1007/BF00345448. [DOI] [PubMed] [Google Scholar]
- 14.Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, Kelly DP. A gender-related defect in lipid metabolism and glucose homeostastis in peroxisome proliferator-activated receptor α-deficient mice. J Clin Invest. 1998;102:1083–1091. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong J, Stubbins RE, Smith RR, Harvey AE, Núñez NP. Differential susceptibility to obesity between male, female and ovariectomized female mice. Nut J. 2009;8:11–15. doi: 10.1186/1475-2891-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings DE, Brandon EP, Planas JV, Motamed K, Idzerda RL, McKnight GS. Genetically lean mice result from targeted disruption of the RIIβ subunit of protein kinase A. Nature. 1996;382:622–626. doi: 10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- 17.Schreyer SA, Cummings DE, McKnight GS, LeBoeuf RC. Mutation of the RIIβ subunit of protein kinase A prevents diet-induced insulin resistance and dyslipidemia in mice. Diabetes. 2001;50:2555–2562. doi: 10.2337/diabetes.50.11.2555. [DOI] [PubMed] [Google Scholar]
- 18.Czyzyk TA, Sikorski MA, Yang L, McKnight GS. Disruption of the RIIβ subunit of PKA reverses the obesity syndrome of agouti lethal yellow mice. Proc Natl Acad Sci U S A. 2008;105:276–281. doi: 10.1073/pnas.0710607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newhall KJ, Cummings DE, Nolan MA, Mcknight GS. Deletion of the RIIβ-subunit of protein kinase A decreases body weight and increases energy expenditure in the obese, leptin-deficient ob/ob mouse. Mol Endocrinol. 2005;19:982–991. doi: 10.1210/me.2004-0343. [DOI] [PubMed] [Google Scholar]
- 20.Enns LC, Morton JF, Treuting PR, et al. Disruption of protein kinase A in mice enhances healthy aging. PLoS ONE. 2009;4:e5963. doi: 10.1371/journal.pone.0005963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain Res. 1984;322:41–48. doi: 10.1016/0006-8993(84)91178-8. [DOI] [PubMed] [Google Scholar]
- 22.Dubuc PU. Effects of estrogen on food intake, body weight, and temperature of male and female obese mice. Proc Soc Exp Biol Med. 1985;180:468–473. doi: 10.3181/00379727-180-42204. [DOI] [PubMed] [Google Scholar]
- 23.Palmer K, Gray JM. Central vs peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiol Behav. 1986;37:187–189. doi: 10.1016/0031-9384(86)90404-x. [DOI] [PubMed] [Google Scholar]
- 24.Gamm DM, Baude EJ, Uhler MD. The major catalytic subunit isoforms of cAMP-dependent protein kinase have distinct biochemical properties in vitro and in vivo. J Biol Chem. 1996;271:15736–15742. doi: 10.1074/jbc.271.26.15736. [DOI] [PubMed] [Google Scholar]
- 25.Muzumdar R, Allison DB, Huffman DM, et al. Visceral adipose tissue modulates mammalian longevity. Aging Cell. 2008;7:438–440. doi: 10.1111/j.1474-9726.2008.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panagiotakos DB, Pitsavos C, Yannakoulia M, Chrysohoou C, Stefanadis C. The implication of obesity and central fat on markers of chronic inflammation: the ATTICA study. Atherosclerosis. 2005;183:308–315. doi: 10.1016/j.atherosclerosis.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Liu KH, Chan YL, Chan WB, Kong WL, Kong MO, Chan JCN. Sonographic measurements of mesenteric fat thickness is a good correlate with cardiovascular risk factors: comparison with subcutaneous and preperitoneal fat thickness, magnetic resonance imaging and anthropometric indexes. Int J Obes. 2003;27:1267–1273. doi: 10.1038/sj.ijo.0802398. [DOI] [PubMed] [Google Scholar]
- 28.Liu KH, Chan YL, Chan WB, Chan JCN, Chu CWW. Mesenteric fat thickness is an independent determinant of metabolic syndrome and identifies subjects with increased carotid intima-media thickness. Diabetes Care. 2006;29:379–384. doi: 10.2337/diacare.29.02.06.dc05-1578. [DOI] [PubMed] [Google Scholar]
- 29.Exton JH. Mechanisms of hormonal regulation of hepatic glucose metabolism. Diabetes Metab Rev. 1987;3:163–183. doi: 10.1002/dmr.5610030108. [DOI] [PubMed] [Google Scholar]
- 30.Pilkis SJ, Claus TH. el-Maghrabi MR. The role of cyclic AMP in rapid and long-term regulation of gluconeogenesis and glycolysis. Adv Sec Mess Phosphoprot Res. 1988;22:175–191. [PubMed] [Google Scholar]
- 31.Herzig S, Long F, Jhala US, et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita H, Takenoshita M, Sakurai M, et al. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc Natl Acad Sci U S A. 2001;98:9116–9121. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uyeda K, Yamashita H, Kawaguchi T. Carbohydrate responsive element-binding protein (ChREBP): a key regulator of glucose metabolism and fat storage. Biochem Pharmacol. 2002;63:2075–2080. doi: 10.1016/s0006-2952(02)01012-2. [DOI] [PubMed] [Google Scholar]
- 34.Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishii S, Iizuka K, Miller BC, Uyeda K. Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc Natl Acad Sci U S A. 2004;101:15597–15602. doi: 10.1073/pnas.0405238101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dentin R, Benhamed F, Hainault I, et al. Liver-specific inhibition of ChREBP improves hepatic steatosis and insulin resistance in ob/ob mice. Diabetes. 2006;55:2159–2170. doi: 10.2337/db06-0200. [DOI] [PubMed] [Google Scholar]
- 37.Hadley AJ, Flack JD, Buckingham JC. Effects of selective phosphodiesterase inhibitors on the release of ACTH and LH from the rat pituitary gland in vitro. Pharmacol Comm. 1993;3:283–295. [Google Scholar]
- 38.Hadley AJ, Kumari M, Cover PO, Osborne J, Flack JD, Buckingham JC. Stimulation of the hypothalamo-pituitary-adrenal axis in the rat by the type 4-phosphodiesterase (PDE-4) inhibitor denbufylline. Br J Pharmacol. 1996;119:463–470. doi: 10.1111/j.1476-5381.1996.tb15695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamers WH, Hanson RW, Meisner HM. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc Natl Acad Sci U S A. 1982;79:5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lange AJ, Argaud D, el Maghrabi MR, Pan W, Maitra SR, Pilkis SJ. Isolation of a cDNA for the catalytic subunit of rat liver glucose-6-phosphatase: regulation of gene expression in FAO hepatoma cells by insulin, dexamethasone and cAMP. Biochem Biophys Res Commun. 1994;201:302–309. doi: 10.1006/bbrc.1994.1702. [DOI] [PubMed] [Google Scholar]
- 41.Schmoll D, Wasner C, Hinds CJ, Allan BB, Walther R, Burchell A. Identification of a cAMP response element within the glucose-6-phosphatase hydrolytic subunit gene promoter which is involved in the transcriptional regulation by cAMP and glucocorticoids in H4IIE hepatoma cells. Biochem J. 1999;338:457–463. [PMC free article] [PubMed] [Google Scholar]
- 42.Marchesini G, Brizi M, Morselli-Labate AM, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 43.Patrick L. Nonalcoholic fatty liver disease: relationship to insulin sensitivity and oxidative stress. Treatment approaches using vitamin E, magnesium, and betaine. Altern Med Rev. 2002;7:276–291. [PubMed] [Google Scholar]
- 44.Koo S-H, Montminy M. Fatty acids and insulin resistance: A perfect storm. Mol Cell. 2006;21:449–450. doi: 10.1016/j.molcel.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Al-Majed HT, Jones PM, Persaud SJ, Sugden D, Huang GC, Amiel S, Whitehorse BJ. ACTH stimulates insulin secretion from MIN6 cells and primary mouse and human islets of Langerhans. J Endocrinol. 2004;180:155–166. doi: 10.1677/joe.0.1800155. [DOI] [PubMed] [Google Scholar]
- 46.Xu N, Ekstrom U, Nilsson-Ehle P. ACTH decreases the expression and secretion of apolipoprotein B in HepG2 cell cultures. J Biol Chem. 2001;276:38680–38684. doi: 10.1074/jbc.M104659200. [DOI] [PubMed] [Google Scholar]
- 47.Kenyon C. A conserved regulatory system for aging. Cell. 2001;105:165–168. doi: 10.1016/s0092-8674(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 48.Longo VD, Fabrizio P. Regulation of longevity and stress resistance: a molecular strategy conserved from yeast to humans? Cell Mol Life Sci. 2002;59:903–908. doi: 10.1007/s00018-002-8477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan L, Vatner DE, O’Connor JP, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 50.Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo V. Sir2 blocks extreme life-span extension. Cell. 2005;123:655–667. doi: 10.1016/j.cell.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 51.Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2004;2:E296. doi: 10.1371/journal.pbio.0020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steffen KK, MacKay VL, Kerr EO, et al. Yeast life span extension by depletion of 60s ribosomal subunits is mediated by Gcn4. Cell. 2008;133:292–302. doi: 10.1016/j.cell.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaeberlein M, Kennedy BK. Protein translation. Aging Cell. 2007;6:731–734. doi: 10.1111/j.1474-9726.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- 54.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu BP. Aging and oxidative stress: modulation by dietary restriction. Free Radical Biol Med. 1996;21:651–668. doi: 10.1016/0891-5849(96)00162-1. [DOI] [PubMed] [Google Scholar]
- 56.Kuczmarski RJ, Flegal KM, Campbell SM, Johson CL. Increasing prevalence of overweight among U.S. adults. The National Health and Nutrition Examination Surveys, 1960 to 1991 (NHANES III) JAMA. 1994;272:238–239. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 57.Enzi G, Gasparo M, Binodetti PR, Fiore D, Semisa M, Zurlo F. Subcutaneous and visceral fat distribution according to sex, age and overweight, evaluated by computed tomography. Am J Clin Nutr. 1986;44:739–746. doi: 10.1093/ajcn/44.6.739. [DOI] [PubMed] [Google Scholar]
- 58.Fraze E, Chlou M, Chen Y, Reaven GM. Age related changes in postprandial plasma glucose, insulin, and FFA concentrations in non-diabetic individuals. J Am Geriatr Soc. 1987;35:212–218. doi: 10.1111/j.1532-5415.1987.tb02313.x. [DOI] [PubMed] [Google Scholar]
- 59.Calle EE, Thun JM, Petrelli JM, Rodriguez C, Heath JCW. Body-mass index and mortality in a prospective cohort. N Engl J Med. 1999;341:1097–1102. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 60.Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in never-smoking overweight U.S. white women aged 40-64 years. Am J Epidemiol. 1995;141:1128–1141. doi: 10.1093/oxfordjournals.aje.a117386. [DOI] [PubMed] [Google Scholar]