Abstract
The structure and dynamics of actin cytoskeleton are factors important for regulation of cell adhesion, spreading, and migration. TRIP6 is a LIM domain-containing protein interacting with many actin cytoskeleton- associated proteins and modulating the activity of certain transcription factors. To study the functions of TRIP6, we inhibited its expression in A549 and A431 cells with short interfering RNAs (siRNAs). TRIP6 knockdown led to an increase in the number and length of stress fibers and acquisition of the locomotor phenotype. Staining for paxillin demonstrated a decrease in the number of focal adhesion zones and their reorganization, while staining for E-cadherin revealed a loss of cell-to-cell contacts. These morphological changes were accompanied by a twofold increase in cell motility rate, as determined by the wound-healing assay. Thus, downregulation of TRIP6 in the above cell lines led to development of more malignant phenotype of epithelial cells. Possible mechanisms underlying the effects observed are discussed.
Keywords: TRIP6, actin cytoskeleton, focal adhesions, cell-to-cell contacts, cell migration, RNA interference
Changes in the structure and dynamics of actin cytoskeleton influence many signal pathways, some of which are involved in the regulation of cell adhesion, spreading, and migration. Regulation of the cytoskeleton also affects cell division and malignant transformation [1].
Interplay between the cytoskeleton and the transcriptional machinery may be mediated by adaptor proteins containing LIM domains [2], which are highly conserved cysteine-rich structures with two tandem zinc fingers [3]. These domains are not involved in DNA binding but are responsible for protein–protein interactions. Sequence and spacing between conservative residues are highly variable and confer specificity of interaction and a wide spectrum of interacting proteins. Thus, LIM domains function as scaffolds to support the assembly of large macromolecular complexes [2–4].
TRIP6 (thyroid hormone receptor interacting protein 6) is a 476-aa zyxin family protein [5, 6] expressed predominantly in epithelial cells [6–9]. Analysis of the TRIP6 sequence shows two distinct regions: the aproline- rich N-terminal half and a C-terminal region containing three LIM domains. Similarly to other zyxin family members, TRIP6 is a cytoplasmic protein primarily localized to focal adhesion plaques. However, under certain conditions, proteins of this family may shuttle to the nucleus and then back to the cytoplasm due to the nuclear export signal (NES) [4, 8, 9]. To date, zyxin family members are recognized as actin-associated adaptor proteins relaying signals between the cytoplasm and the nucleus, where they may play a role of transcriptional regulators [2].
To date, a number of TRIP6 interaction partners have been identified. In the cytoplasm, TRIP6 binds to tyrosine phosphatase PTP-BL [6, 10]; to the adaptor protein RIL involved in the actin stress fiber assembly [9, 11]; and to p130Cas, which is required for outside–in integrin signaling [12]. The wide range of TRIP6 interaction partners suggests its role in the regulation of the actin cytoskeleton and cell motility. On the other hand, it is known that TRIP6 can interact with certain transcriptional factors, such as thyroid hormone receptor THR-β, retinoid X receptor [7], glucocorticoid receptor, c-Fos, the NFκB subunit p65 (RelA) [13, 14], and v-Rel [8]. Note that TRIP6 may function both as a positive and a negative transcription regulator. However, despite the ample data on the properties of TRIP6, its biological role remains obscure.
To study the role of TRIP6 in the regulation of actin cytoskeleton and cell adhesion and migration, we downregulated the expression of the TRIP6 gene using small interfering RNAs (siRNAs). RNA interference is a process of sequence-specific posttranscriptional inhibition of gene expression by homologous double-stranded RNAs [15]. The siRNAs were expressed using the lentiviral pLSLP constructs, from which a short (64 nucleotides in length) hairpin RNA was synthesized under the control of the H1 RNA promoter. Inhibition of endogenous expression of TRIP6 (GenBank accession no. BC004249) was carried out using the sequence 5′-gaagctggttcacgacatgaa (siTRIP6). The 5′-ctaacactgggttatacaa siRNAs (siE6) specific for the E6 gene of human papilloma virus type 18 (GenBank accession no. X04354) was used as a negative control. The procedure of virus packaging and cell transduction was described elsewhere [16]. The effectiveness of suppression of target genes was assessed by RT-PCR (data not shown), Northern blot hybridization, and Western blot analysis (Fig. 1A). A549 lung carcinoma cells and A431 epidermoid carcinoma cells were used to obtain stable cell lines with introduced siTRIP6 and siE6.
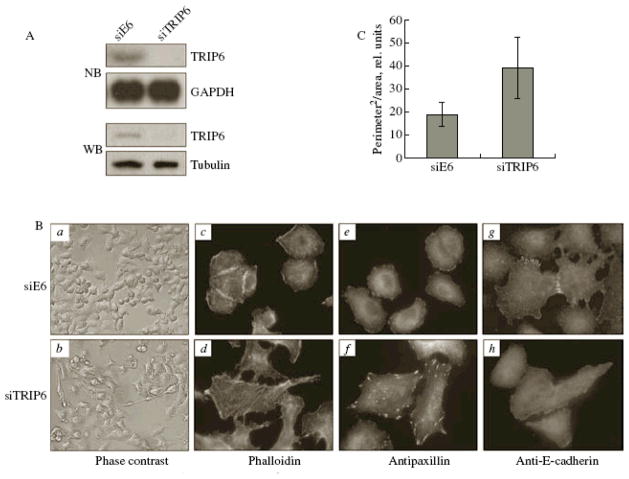
Figure 1.
Morphological changes in A549 cells upon TRIP6 downregulation by siRNA. (A) Cells were transduced with the lentiviral vector pLSLP containing either the control siRNA (targeting the E6 papilloma virus gene) or TRIP6-specific siRNA. The inhibition of TRIP6 expression was assessed by Northern blot hybridization (NB) and Western blot analysis (WB). (B) Cells were transduced with the lentiviral vector pLSLP containing either the control siRNA (a, c, e, g ) or TRIP6-specific siRNA (b, d, f, h ): (a, b ) phase contrast microscopy; (c, d ) staining of polymerized actin with FITC-conjugated phalloidin; (e, f ) detection of focal contacts with antibodies to paxillin; (g, h ) staining of cell-to-cell contacts with antibodies to E-cadherin; (e–h) TRITC-labeled antibodies to mouse immunoglobulins were used as secondary antibodies. (C) The ratio between the squared cell perimeter to cell area was determined after photographing the cells. Thirty randomly selected cells from the control and experimental groups were analyzed using the ImagePro program. The procedure of virus packaging and cell transduction was described earlier [16].
Light microscopy of A549 and A431 cells, in which TRIP6 expression was inhibited by siRNA, revealed visible morphological changes in these cells, compared to the control cells (Fig. 1B, panels a, b ). The control siE6-transduced A549 cells were either discoid or polygonal; they retained cell-to-cell contacts and high degree of spreading. The siTRIP6-containing A549 cells were spindle- or star-shaped; the degree of their spreading decreased, and extended stable- edge regions appeared, which led to an increased ratio of the squared perimeter of cells to their area (Fig. 1C). As a result of inhibition of TRIP6 expression, many cells acquired fibroblast-like polarized phenotype characteristic of migrating cells, with a lamellopodia formed on the front edge and clearly distinguishable body and tail (Fig. 1B, panels a, b ). Such changes, along with the loss of epithelial markers, are features associated with epithelial–mesenchymal transition [17]. In addition, the presence of crisscrossed cells may indicate the loss of contact inhibition. Similar morphological changes were also observed in the siTRIP6-transduced A431 cells (data not shown).
The actin cytoskeleton is implicated in the maintenance of cell shape and motility. We analyzed the changes in the actin cytoskeleton caused by the inhibition of TRIP6 expression (Fig. 1B, panels c, d ). Actin phalloidin staining revealed enhanced stress- fiber formation in cells with downregulated TRIP6, while actin bundles were almost absent in the control (siE6-transduced) A549 cells. Tractions in the cytoskeleton developed by actin filaments trigger the assembly of focal adhesions. Focal contacts were detected by immunofluorescent staining for paxillin (Fig. 1B, panels e, f ). In the control cells, focal contacts were uniformly distributed over the entire perimeter of the cell, forming a thin rim. In cells with suppressed TRIP6 expression, conversely, large zones of focal adhesion were observed, which were localized sparsely and along the perimeter. A decrease in the number of focal contacts and their reorganization may take place in transformed cells. Another sign of an increased transformation of epithelial cells, the loss of cell-to-cell contacts, was observed upon immunofluorescent staining of siTRIP6-transduced cells using antibodies to E-cadherin (Fig. 1B, panels g, h ). Similar changes were observed in A431 cells upon TRIP6 knockdown (data not shown).
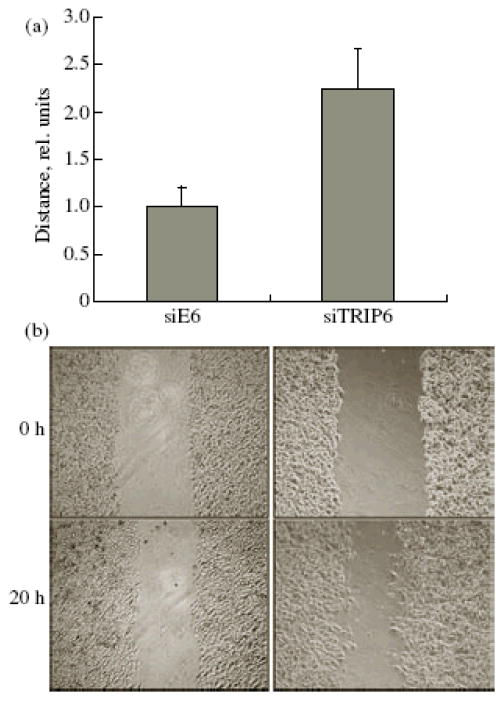
Taking into account the acquisition of the locomotor phenotype and the reorganization of actin cytoskeleton and focal adhesion zones in cells with suppressed TRIP6 expression, we analyzed the ability of these cells to migrate. A linear wound in a cell monolayer was inflicted with a plastic pipette tip; the cells were allowed to migrate for 20 h and were photographed (Fig. 2). The migration rate of A549 cells transduced with siTRIP6 was twice as high as that of the control siE6-transduced cells (Fig. 2a). Similar results were obtained with A431 cells (data not shown).
Figure 2.
Migration of A549 cells in the wound-healing test. A linear wound in a cell monolayer was inflicted with a plastic pipette tip; the cells were allowed to migrate for 20 h and were photographed. (a) Cell migration rate was normalized to the control (siE6) (M ± SD). The results of three independent experiments performed in triplicate are shown. (b) A typical “wound” at the beginning of experiment (upper row) and 20 h later (lower row), phase contrast microscopy.
Thus, suppression of TRIP6 expression by siRNA in the studied carcinoma cell lines led to the formation of actin stress fibers, reorganization of focal adhesions and a decrease in their number, and the loss of cell-tocell contacts and contact inhibition. These changes were accompanied by the acquisition of the locomotor phenotype and increased motility of cells, which are indicative of an increased degree of epithelial cell transformation [18]. These effects may be due to either cytoplasmic or nuclear TRIP6. In the nucleus, TRIP6 modulates the activation of MEK/ERK signaling pathway and activity of AP-1 transcriptional factor [14], which are involved in morphological transformation and may lead to upregulation of the epidermal growth factor receptor (EGFR) [19] and induction of small Rho-GTPases and actin cytoskeleton reorganization. In the cytoplasm, TRIP6 may be involved in the regulation of actin polymerization by competing with α-actinin for the binding with the adaptor protein RIL [9]. TRIP6 suppression leads to enhanced association of α-actinin with F-actin by RIL [9] and an increase in the number of stress fibrils. In addition, TRIP6 may be involved in the regulation of interaction of Crk with the p130Cas family proteins [12]. It was shown that the Cas–Crk complex plays a crucial role in membrane ruffling and cell migration due to activation of the Rac–JNK signaling [21]. TRIP6 also interacts with the tyrosine phosphatase PTP-BL, competing with the tumor suppressor APC [6, 10, 22], which is responsible for the binding and degradation of free β-catenin. Free β-catenin forms a complex with the transcriptional factor Tcf/Lef and activates expression of target genes [18, 23], as well as induces ERK and Wnt signaling [24, 25]. It is possible that TRIP6 downregulation changes the stoichiometry of interaction between APC and β-catenin and leads to the accumulation of the latter in the cytoplasm. An increase in β-catenin dephosphorylation by the tyrosine phosphatase PTP-BL, which enhances its stability, cannot be ruled out either. Thus, TRIP6 may affect the dynamics of the actin cytoskeleton, cell adhesion, and migration by regulating different signaling pathways. However, additional studies are required to clarify particular mechanisms of this regulation.
Acknowledgments
The study was supported by grants from the Russian Foundation for Basic Research (P.M.C.), grants from National Institutes of Health (R01 CA104903 and R01 AG025278 (P.M.C.) and grant from the Howard Hughes Medical Institute (P.M.C.).
References
- 1.Alberts B, Johnson A, Levis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. N.Y: Garland Publishing; 2002. [Google Scholar]
- 2.Kadrmas JL, Beckerle MC. The LIM domain: From the cytoskeleton to the nucleus. Nature Rev Mol Cell Biol. 2004;5:920–931. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]
- 3.Dawid IB, Breen JJ, Toyama R. LIM domains: Multiple roles as adapters and functional modifiers in protein interactions. Trends Genet. 1998;14:156–162. doi: 10.1016/s0168-9525(98)01424-3. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Gilmore TD. LIM domain protein TRIP6 has a conserved nuclear export signal, nuclear targeting sequences, and multiple transactivation domains. Biochim Biophys Acta. 2001;1538:260–272. doi: 10.1016/s0167-4889(01)00077-5. [DOI] [PubMed] [Google Scholar]
- 5.Yi J, Beckerle MC. The human TRIP6 gene encodes a LIM domain protein and maps to chromosome 7q22, a region associated with tumorigenesis. Genomics. 1998;49:314–316. doi: 10.1006/geno.1998.5248. [DOI] [PubMed] [Google Scholar]
- 6.Murthy KK, Clark K, Fortin Y, Shen SH, Banville D. ZRP-1, a zyxin-related protein, interacts with the second PDZ domain of the cytosolic protein tyrosine phosphatase hPTP1E. J Biol Chem. 1999;274:20679–20687. doi: 10.1074/jbc.274.29.20679. [DOI] [PubMed] [Google Scholar]
- 7.Lee JW, Choi HS, Gyuris J, Brent R, Moore DD. Two classes of proteins dependent on either the presence or absence of thyroid hormone for interaction with the thyroid hormone receptor. Endocrinology. 1995;9:243–254. doi: 10.1210/mend.9.2.7776974. [DOI] [PubMed] [Google Scholar]
- 8.Koedood Zhao M, Wang Y, Murthy K, Yi J, Beckerle MC, Gilmore TD. LIM domain-containing protein TRIP6 can act as a coactivator for the v-Rel transcription factor. Gene Expr. 1999;8:207–217. [PMC free article] [PubMed] [Google Scholar]
- 9.Cuppen E, van Ham M, Wansink DG, de Leeuw A, Wieringa B, Hendriks W. The zyxin-related protein TRIP6 interacts with PDZ motif in the adaptor protein RIL and the protein tyrosine phosphatase PTP-BL. Eur J Cell Biol. 2000;79:283–293. doi: 10.1078/S0171-9335(04)70031-X. [DOI] [PubMed] [Google Scholar]
- 10.Erdmann KS. The protein tyrosine phosphatase PTP-Basophil/Basophil-like: Interacting proteins and molecular functions. Eur J Biochem. 2003;270:4789–4798. doi: 10.1046/j.1432-1033.2003.03895.x. [DOI] [PubMed] [Google Scholar]
- 11.Bashirova AA, Markelov ML, Shlykova TV, Levshenkova EV, Alibaeva RA, Frolova EI. The human RIL gene: Mapping to human chromosome 5q31.1, genomic organization and alternative transcripts. Gene. 1998;210:239–245. doi: 10.1016/s0378-1119(98)00080-8. [DOI] [PubMed] [Google Scholar]
- 12.Yi J, Kloeker S, Jensen CC, Bockholt S, Honda H, Hirai H, Beckerle MC. Members of the zyxin family of LIM proteins interact with members of the p130 Cas family of signal transducers. J Biol Chem. 2002;277:9580–9589. doi: 10.1074/jbc.M106922200. [DOI] [PubMed] [Google Scholar]
- 13.Kassel O, Schneider S, Heilbock C, Litfin M, Gottlicher M, Herrlich P. A nuclear isoform of the focal adhesion LIM-domain protein TRIP6 integrates activating and repressing signals at AP-1- and NFκB-regulated promoters. Genes Dev. 2004;18:2518–2528. doi: 10.1101/gad.322404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Bin LH, Liu Y, Chen D, Zhai Z, Shu HB. TRIP6 is RIP2-associated common signaling component of multiple NF-| B activation pathways. J Cell Sci. 2005;118:555–563. doi: 10.1242/jcs.01641. [DOI] [PubMed] [Google Scholar]
- 15.Dykxhoorn DM, Novina CD, Sharp PA. Killing the messenger: Short RNAs that silence gene expression. Nature Rev Mol Cell Biol. 2003;4:457–467. doi: 10.1038/nrm1129. [DOI] [PubMed] [Google Scholar]
- 16.Razorenova OV, Agapova LS, Budanov AV, Ivanov AV, Strunina SM, Chumakov PM. Retroviral reporter systems for assessing the activity of stressinducible signal transduction pathways controlled by the p53, HIF-1, and HSF-1 transcription factors. Mol Biol. 2005;39:286–293. [PMC free article] [PubMed] [Google Scholar]
- 17.Thiery JP. Epithelial–mesenchymal transitions in tumor progression. Nature Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 18.Kopnin BP. Targets for oncogenes and tumor suppressors: A key to understanding basic mechanisms of carcinogenesis. Biokhimiya. 2000;65:5–33. [PubMed] [Google Scholar]
- 19.Malliri A, Symons M, Hennigan RF, Hurlstone AFL, Lamb RF, Wheeler T, Ozanne BW. The transcription factor AP-1 is required for EGF-induced activation of Rho-like GTPases, cytoskeletal rearrangements, motility, and in vitro invasion of A431 cells. J Cell Biol. 1998;143:1087–1099. doi: 10.1083/jcb.143.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallenius T, Scharm B, Vesikansa A, Luukko K, Schafer R, Makela T. The PDZ-LIM protein RIL modulates actin stress fiber turnover and enhances the association of α-actinin with F-actin. Exp Cell Res. 2004;293:117–128. doi: 10.1016/j.yexcr.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Bouton AH, Riggins RB, Bruce-Staskal PJ. Functions of the adapter protein Cas: Signal convergence and determination of cellular responses. Oncogene. 2001;20:6448–6458. doi: 10.1038/sj.onc.1204785. [DOI] [PubMed] [Google Scholar]
- 22.Erdmann KS, Kuhlmann J, Lessmann V, Herrmann L, Eulenburg V, Muller O, Heumann R. The Adenomatous Polyposis Coli-protein (APC) interacts with the protein tyrosine phosphatase PTP-BL via an alternatively spliced PDZ domain. Oncogene. 2000;19:3894–3901. doi: 10.1038/sj.onc.1203725. [DOI] [PubMed] [Google Scholar]
- 23.Nelson WJ, Nusse R. Convergence of Wnt, β-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yun MS, Kim SE, Jeon SH, Lee JS, Choi KY. Both ERK and Wnt/β-catenin pathways are involved in Wnt3a-induced proliferation. J Cell Sci. 2005;118:313–322. doi: 10.1242/jcs.01601. [DOI] [PubMed] [Google Scholar]
- 25.Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS, Clarke AR, Winton DJ. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]