Abstract
Antidepressant-, cocaine- and 3,4-methylenedioxymethamphetamine-sensitive serotonin (5-hydroxytryptamine, 5-HT) transporters (SERTs) are expressed on presynaptic membranes of 5-HT-secreting neurons to provide efficient uptake of the biogenic amine after release. SERTs also support 5-HT transport across platelet, placental, gastrointestinal and pulmonary membranes and thus play a critical role in central nervous system and peripheral nervous system 5-HT signaling. SERTs are subject to multiple levels of posttranslational regulation that can rapidly alter 5-HT uptake and clearance rates. Specific cell surface receptors are now known to regulate SERT trafficking and/or catalytic function, with pathways activating protein kinase C, protein kinase G and p38 mitogen-activated protein kinase receiving the greatest attention. Remarkably, disease-associated mutations in SERT not only impact basal SERT activity but also selectively impact one or more SERT regulatory pathway(s). In this review, we describe both trafficking-dependent and trafficking-independent modes of SERT regulation and also the suspected roles played in regulation by SERT-associated proteins. Elucidation of the SERT ‘regulome’ provides important depth to our understanding of the likely origins of 5-HT-associated disorders and may help orient research to develop novel therapeutics.
Keywords: A3 adenosine receptor, interleukin-1β, p38 mitogen-activated protein kinase, protein kinase C, protein kinase G, protein phosphatase 2A, serotonin, trafficking, transporter
The tryptophan derivative 5-hydroxytryptamine (5-HT) is an important signaling molecule in the brain and periphery (1,2). Like many other neurotransmitters, 5-HT is predominantly inactivated by transporter-mediated clearance. 5-HT transport activity associated with neuronal terminals has been extensively investigated using synaptosomal preparations, beginning with the pioneering studies of Snyder (3). This preparation permitted the development of the first serotonin transporter (SERT)-selective reuptake inhibitor (SSRI) antidepressants (e.g. fluoxetine or Prozac™) well before SERT genes could be cloned (4). SERT proteins (also termed 5-HT transporters) are encoded by a single gene in man (Htt, SLC6A4) as well as in commonly used rodent models (5). SERTs bear closest identity with norepinephrine transporters (NET, SLC6A2) with which they also share sensitivity to tricyclic antidepressants (6). Topologically, SERT is a polytopic, integral membrane protein comprised of twelve transmembrane (TM) domains (5,7,8). The crystal structure of a bacterial SLC6A4 transporter, LeuTAa (9-11), is consistent with the predicted topology of SERT (12,13) and has permitted the use of homology-based modeling approaches to provide a more realistic inspection of key helices that participate in 5-HT recognition as well as how 5-HT transport across membranes is achieved (Figure 1). SERT mediates secondary active, ion-coupled 5-HT transport, deriving energy for inward 5-HT transport largely from the TM Na+ gradient. SERT exhibits a coupling stoichiometry of one 5-HT: one Na+ and one Cl−, with one K+ molecule believed to be effluxed on a separate step of the transport cycle (14).
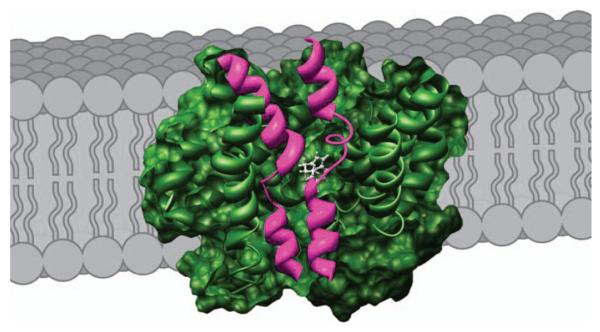
Figure 1. Model of SERT in the plasma membrane based on the LeuTAa structure.
SERT (green) is depicted in a cutaway view exposing the proposed 5-HT-binding site. TMs 1 and 6 (magenta) comprise central aspects of the substrate permeation pathway in LeuTAa, and homology-based modeling studies support similar relationships for the SERT 5-HT transport pathway (12). Figure courtesy of Julie R. Field and John Steiner.
Localization of SERT
Before discussing the mechanisms by which SERT appears to be regulated, we briefly review aspects of the cellular and subcellular localization of the transporter as revealed through immunocytochemical studies and cell fractionation techniques. SERT is distributed in the rat central nervous system (CNS) within or on cell bodies and dendrites of the raphe nuclei as well as along fibers coursing through many regions of the forebrain (15). Glial expression was not evident in initial studies, although higher resolution immunoelectronmicroscopy studies have provided evidence for a small degree of SERT expression in astrocytes that may contribute to 5-HT clearance (16). Additionally, these high-resolution studies provided evidence that SERT is not enriched on the plasma membranes of active zones; rather, SERT immunoreactivity is localized perisynaptically, along axonal membranes or on intracellular vesicles, presumably compartments related to SERT membrane trafficking (17,18). A further study noted that axonal SERT immunoreactivity is preferentially located at the plasma membrane, whereas SERT in raphe neuron soma and dendrites is predominantly cytoplasmic in nature (19). As was recently shown for NET, this localization is likely but one snapshot of a dynamic localization pattern that is influenced by regulatory stimuli (20). Although our focus in this study is on more rapid regulatory control of SERT surface expression and transport activity, we also recognize that we have an insufficient understanding of mechanisms that dictate how SERT traffics to, and becomes enriched in, presynaptic membrane compartments. Important leads in this direction are evident in the work of Sitte and colleagues who have elucidated important roles for transporter oligomerization in endoplasmic reticulum export (21) as well as important interactions with SERT by members of the Sec24D family (22). The export of SERT homologues [e.g. gamma aminobutyric acid (GABA) transporter (GAT)-1] from biosynthetic compartments relies on targeting to exocyst-associated trafficking vesicles, and similar mechanisms may apply to dictate SERT axonal localization (23).
Qian et al. first evaluated SERT subcellular distribution by biotinylation studies and subcellular fractionation, noting a predominantly cell surface expression (~75%) of SERT in transfected human embryonic kidney (HEK) 293 cells (15). Using the same model, Ramamoorthy and colleagues established SERT to be a phosphoprotein where phosphorylation paralleled SERT internalization. Transporter phosphorylation occurs with protein kinase C (PKC) activation or protein phosphatase 2A (PP2A) inhibition (and thus is likely to involve Ser/Thr phosphorylation) (24,25). No kinetic biotinylation studies have yet been performed nor have characterizations of SERT recycling pathways been achieved similar to studies found focused on the dopamine transporter (DAT) and the GAT-1 GABA transporter (26-28). Whereas several groups have shown clathrin-mediated DAT endocytosis in transfected cells and distinct internalization signals in the carboxy terminal of DAT, Deken et al. demonstrated in rat brain that GAT-1 transporters underwent endocytosis through a clathrin-dependent mechanism, trafficked on distinct vesicles and were reinserted into the plasma membrane within minutes. Thus, by analogy with other transporters in the SLC6 family, SERTs are likely to undergo regulated downregulation, although the specific pathways remain to be defined.
Magnani et al. have noted that the steady-state surface expression of SERT is consistent with localization to cholesterol-rich lipid raft domains (29). The authors found reduced SERT activity following cholesterol depletion because of a decrease in the maximal transport velocity (Vmax) and an increase in the 5-HT Km. SERT (and NET) largely sediments in detergent-resistant fractions enriched for lipid raft markers, and NET activity has been reported to be insensitive to clathrin-dependent treatments (30,31). Carneiro and Blakely performed analogous studies of the subcellular localization of SERT in human and mouse platelets, adopting fractionation approaches common in the analysis of platelet membrane proteins (32). In this study, the authors found SERT to be localized predominantly to intracellular membranes devoid of extensive cytoskeletal associations that are soluble to Triton-X-100 (TS). These depots are observable by immunocytochemistry as puncta localized to the cytoplasm of resting platelets (Figure 2) that engage in relocation to plasma membrane sites, identified as focal adhesions using marker proteins that localize to this domain. The latter compartments are also evident upon subcellular fractionation as a Triton-X-100-insoluble, actin-rich membrane skeleton (MS) domain, distinct from higher density membranes (both surface and intracellular membranes) associated with cytoskeletal (termed CS) proteins. Analogous to lipid raft studies, SERT in the platelet MS domain appears to be more active as SERT protein associations that predominate in the CS fraction (e.g. Hic-5, see subsequently) reduce SERT catalytic activity (32). Thus, the platelet studies of SERT localization indicate that steady-state transporter expression is intracellular and associated with CS, whereas the majority of SERT that is found on the plasma membrane is associated with the MS. These studies demonstrate a more complex localization of SERT to multiple membrane subdomains that likely support distinct phases of a regulatory cycle. In summary, SERT is found predominantly in the plasma membrane of transfected cells and in axons, whereas the transporter is expressed intracelluarly in raphe neuron cell bodies and platelets, suggesting that basal control of SERT localization depends on cell type and cellular subcompartments.
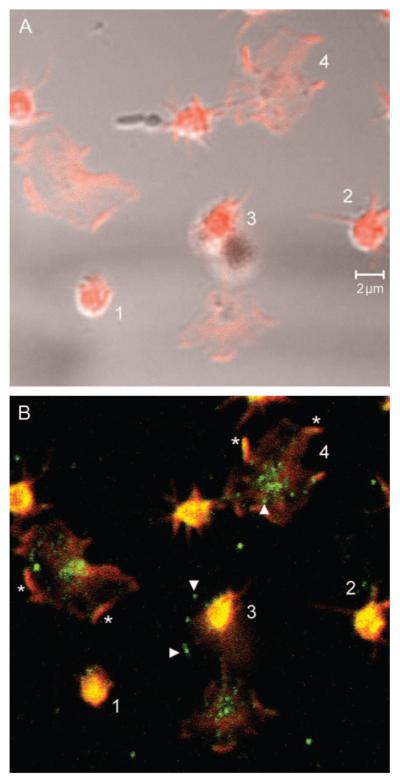
Figure 2. Adhesion-dependent changes in SERT localization in human platelets.
Fresh human platelets were isolated from whole blood by centrifugation and seeded on collagen coverslips. After fixation, platelets were stained with rabbit anti-SERT affinity-purified immunoglobulin G (IgG) and washed, followed by incubation with Cy2 (green) anti-rabbit IgG (Jackson Immunoresearch Laboratories) and phalloidin Alexa 688 (red) (Molecular Probes). As they adhere, platelets begin to spread, which coincides with relocalization of SERT from cytoplasmic pools to focal adhesions. Panel (A) demonstrates phalloidin staining. Panel (B) demonstrates SERT and phalloidin double staining. ‘1’ denotes a newly adherent platelet with largely cytoplasmic SERT staining. ‘2’ denotes a platelet in the early steps of extension with SERT found primarily in the cell soma. ‘3’ reveals movement of SERT peripherally along fine membrane extensions. ‘4’ reveals SERT concentrated in domains consistent with focal adhesions. Arrowheads identify punctate labeling, presumably denoting SERT trafficking vesicles. Asterisks identify putative focal adhesions where SERT colocalizes with phalloidin.
Rapid Reductions in SERT Activity
As noted above, initial observations of rapid (2-30 min) downregulation of SERT Vmax following phorbol ester treatments provided an initial mechanistic framework that supported loss of SERT surface expression in parallel with SERT phosphorylation (25,33). Because extracellular 5-HT acting through the transporter (as SSRIs block 5-HT effects) could prevent phorbol ester-triggered SERT internalization as well as phosphorylation, our group advanced a ‘use it or lose it’ model for SERT regulation. In this study, the presence of extracellular 5-HT is proposed to help establish the appropriate level of surface expression of SERT proteins needed for amine clearance, presumably through reduced endocytosis (34,35).
Trafficking-dependent modulation
The membrane compartments that harbor SERT are distinguished by one or more proteins that contribute structural and/or regulatory properties. The first SERT-associated protein defined was the catalytic subunit of the serine/threonine phosphatase PP2A (PP2Ac). Bauman et al. (36) established that okadaic acid-dependent phosphatase activity is enriched in SERT immunoprecipitations, consistent with the formation of SERT complexes with either protein phosphatase 1 or PP2A. Subsequent immunoblotting of SERT immunoprecipitations from transfected cells and brain membranes revealed the presence of PP2Ac. Interestingly, the authors found that PP2Ac associations could be eliminated by pretreatment of cells with okadaic acid but were stabilized by extracellular 5-HT incubation, thus providing one explanation for reduced b-phorbol 12-myristate 13-acetate (PMA)-induced SERT phosphorylation that occurs with coincident 5-HT treatments (34,36). The simplest scenario (Figure 3) consistent with the existing data thus involves SERT/PP2Ac associations that are enriched in plasma membrane domains containing active SERT, whereas less active SERT pools and inactivated (and possibly recycling) SERTs being relatively deficient in SERT/PP2Ac complexes.
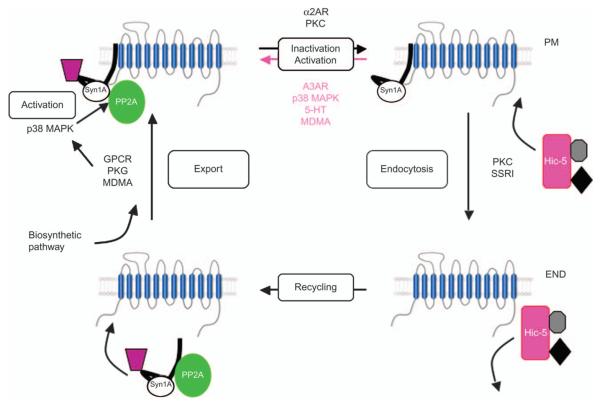
Figure 3. Schematic illustration of SERT regulation.
SERT traffics in and out of the plasma membrane (PM) where it resides in distinct membrane compartments and recycles through endosomal (END) compartments. Various SERT-associated proteins, including Syn1A, PP2A and Hic-5 (among others), differentially associate with SERT in distinct compartments where they can influence transporter trafficking and/or catalytic function. MDMA, 3,4-methylenedioxymethamphetamine.
The plasma membrane SNARE protein syntaxin 1A (Syn1A) has been a well-documented associate of a number of neurotransmitter transporters in the SLC6 family including GAT-1, the GLYT2 glycine transporter and NET (37-40). Haase et al. provided evidence that Syn1A also associates with SERT (41). Sung et al. described analogous associations of NET with Syn1A that can be disrupted by PKC activators in parallel with loss of surface NET expression (39). The latter studies also demonstrated that Syn1A restricts the catalytic function of NET. Comparable evidence for catalytic modulation of SERT by Syn1A has not been advanced. Quick (42) showed that Syn1A associations with SERT dictate whether the transporter moves 5-HT in an electroneutral mode, tightly coupled with co-transported ions (Na+/Cl−). When SERT lacks Syn1A associations, 5-HT transport occurs in an electrogenic mode, characterized by nonstoichiometric ion flux. Possibly, as Sung et al. have proposed for NET, Syn1A associations with SERT dictate whether the transporter contributes to membrane excitability and/or whether the transporter enters a higher capacity 5-HT transport mode (Figure 3).
Through yeast two-hybrid screening with the carboxy terminus of DAT, Carneiro et al. identified the focal adhesion protein Hic-5 as a transporter-binding partner that also binds SERT (43). Hic-5 is a Lin 11, Isl 1, Mec 3 (LIM) domain scaffolding protein that binds the carboxy terminus of SERT, similarly to DAT (32). Hic-5 is found in association with SERT in platelets where the majority of Hic-5/SERT associations are localized to the CS fraction. Because, like SERT, the bulk of Hic-5 is found in the TS fraction, unassociated with the transporter, rather than in the CS domain, Carneiro and Blakely advanced the hypothesis that PKC activation recruits Hic-5 to SERT (Figure 2). In this study, Hic-5 could support or stabilize catalytic inactivation of the transporter as well as translocation of SERT to the CS compartment, followed by SERT internalization and dissociation of Hic-5. The degree to which this model supports SERT function at synapses is currently being investigated.
Using the yeast two-hybrid assay, Müller et al. identified secretory carrier membrane protein 2 (SCAMP2) as a protein that interacts with the amino terminus of SERT (44). After confirmation of a native SCAMP2/SERT complex in glutathione S-transferase pull-down assays, these investigators documented a decreased Vmax of 5-HT transport when SCAMP2 and SERT were coexpressed in transfected cells. This change was paralleled by reduced transporter surface expression. SCAMP2 localizes to lipid rafts where, as noted above, SERT is also localized (28,32). Possibly, SCAMP2 binding could facilitate exit of SERT from lipid rafts to achieve endocytosis of SERT and a reduction in SERT activity. How SCAMP2 associations are established or are modulated and how this interaction relates to other SERT amino-terminal associations, such as that of Syn1A, are unknown.
Chanrion et al. (45) recently identified a carboxy-terminal association of SERT with neuronal nitric oxide synthase (nNOS or NOS1). Cotransfection of yellow fluorescent protein-tagged SERT and nNOS leads to reduced 5-HT transport ascribed to lower surface expression. Evidence of a functional nNOS/SERT association is evident in brain, and synaptosomal 5-HT transport assays using nNOS−/−>mice display increased SERT activity and plasma membrane expression. These studies are particularly intriguing given evidence, noted below, that a NOS-dependent pathway supports rapid, A3 adenosine receptor (A3AR)-dependent elevations of SERT activity, both in mast cells and in serotonergic terminals. Possibly, nNOS associations may contribute to entry of SERT into an intracellular, regulated pathway. In the absence of nNOS, SERT may remain at the cell surface or target the plasma membrane through a constitutive pathway and thus exhibit the observed elevations in basal 5-HT transport activity. One expectation arising from this hypothesis may be achieved at the expense of regulation of SERT through cyclic GMP (cGMP)- and protein kinase G (PKG)-linked pathways.
Another cell surface receptor whose activation has been linked to acute SERT downregulation is the α2 adrenergic receptor (α2AR) (46). Treatment of forebrain synaptosomes with 5-bromo-N-[4,5-dihydro-1H-imidazol-2-yl]-6-quinoxalinamine (UK14304), an α2AR agonist, reduces 5-HT transport, an effect derived from a reduced sensitivity to 5-HT (increased Km). UK14304 reductions in 5-HT transport were found to be dependent on the activity of voltage-sensitive Ca++ channels, suggesting that Ca++-mediated signaling events downstream of α2AR activation downregulate SERT. Chronoamperometry studies documented an ability of acute UK14304 injection to diminish 5-HT clearance in vivo. These findings support the idea that the well-established adrenergic regulation of 5-HT release (47,48) is accompanied by modulation of 5-HT clearance and suggests further consideration of α2AR-directed medications in the pharmacotherapy of mood disorders.
Trafficking-independent modulation
Ramamoorthy and colleagues made a striking discovery that SERT downregulation by PKC activators in platelets is actually biphasic, with both trafficking-dependent and trafficking-independent phases of downregulation evident (49). Short (≤5 min) treatment of rat platelets with PMA leads to rapid inhibition of SERT activity through decreased Vmax and increased Km, yet no change in surface expression of the transporter is observed. In contrast, longer incubations of platelets with PMA (≥30 min) produce a further reduction in 5-HT transport Vmax that coincides with a reduction in SERT cell surface abundance. With short PMA treatments, phosphoamino acid analyses indicate phosphorylation of SERT only at Ser residues, whereas longer treatments lead to both Ser and Thr phosphorylation. Taken together, these studies indicate that PKC activation can influence two sequential modes of SERT regulation, one linked to catalytic downregulation and a second to transporter endocytosis (Figure 3). Although specific sites of PKC-dependent SERT phosphorylation are as yet undefined, Ser and Thr residues in human NET intracellular loop 2 – residues conserved in SERT – were identified as necessary for phorbol ester-triggered phosphorylation (50).
Rapid Elevations in SERT Activity
The examples of acute SERT regulation discussed up to this point involve a decrease in SERT catalytic activity or diminished cell surface expression. Below, we describe examples of rapid upregulation of 5-HT transport and consider how supporting mechanisms lend credence to a bidirectional SERT regulatory cycle that is poised to respond to needs for both elevated and diminished 5-HT clearance.
Trafficking-dependent modulation
Miller and Hoffman were the first to identify a rapid, receptor-dependent mechanism of increasing SERT function in cultured cells (51), research corroborated and extended to the CNS by Zhu et al. (52). When rat basophilic leukemia (RBL-2H3) cells, derived from serotonergic mast cells, are treated with a broad-spectrum adenosine receptor agonist, 5′-N-ethylcarboxamido-adenosine, 5-HT transport capacity (Vmax) increases (51,53). Zhu et al. utilized specific A3AR agonists and antagonists and transporter–receptor-cotransfected cells to validate the role of A3ARs in this effect (53). SERT stimulation by A3ARs requires activation of PKG by a phospholipase C, Ca++, NOS and cGMP-dependent mechanism (53). Miller and Hoffman initially ascribed A3AR regulation of SERT to a change in catalytic function because of no detectible changes in SERT antagonist binding (51). Zhu et al. recognized that the latter measure might have failed to discriminate SERT trafficking from changes in total SERT protein. These investigators opted for membrane impermeant binding conditions using 5-HT displacement at 4°C and the cocaine analog methyl 3β-(4-iodophenyl) tropane-2β-carboxic acid methyl ester (RTI-55) (as well as biotinylation measures in transfected cells) to define subcellular pools of SERT. These approaches revealed an increase in SERT surface expression following treatment with A3AR agonists, 8-BrcGMP or the endogenous cGMP potentiator sildenafil (54) comparable to the elevations in 5-HT uptake. Use of membrane impermeant [2-(trimethylammonium) ethyl]-methanethiosulfonate bromide to inactivate surface SERTs prior to A3AR agonist treatments offers evidence that activation of the A3AR-PKG pathway results in the recruitment of intracellular SERTs rather than in retention of surface transporters (53).
Using mouse brain synaptosomes derived from A3AR−/− mice and chronoamperometry in rat brain, Zhu and colleagues further established that A3ARs regulate SERT in the CNS (52). PKG antagonists, including the PKG1 isoform-specific peptide inhibitor DT-2 (YGRKKRRQRRRPPLRK5H), block stimulation of SERT by the A3AR agonist N6-(3-iodobenzyl)-N-methyl-5′ carbamoyladenosine (IB-MECA) in these native models, as in RBL-2H3 cells (52,53,55). Although these studies with native preparations document the presence of a SERT regulatory cycle in serotonergic terminals, other preparations are needed to advance our understanding of the specific mechanisms that support synaptic transporter localization and function. McDonald et al. (56) recently demonstrated how direct visualization of Caenorhabditis elegans synaptic DAT proteins can be achieved using enhanced green fluorescent proteinlabeled transporters expressed in transgenic nematodes, taking advantage of the transparency and identified synapses of this model system (Figure 4). Studies are currently underway to adapt this framework to investigation of the regulation of the C. elegans SERT, MOD-5 (57).
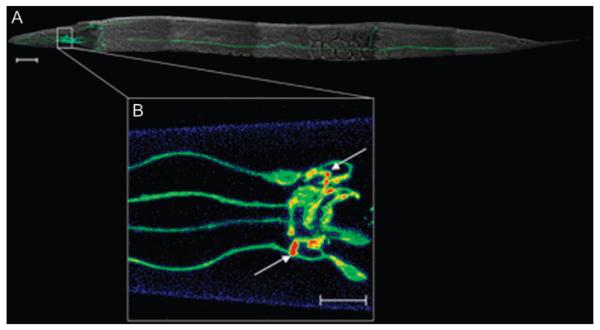
Figure 4. Synaptic localization of biogenic amine transporters can be directly visualized in C. elegans synapses.
The SERT-related DAT-1 in C. elegans as an enhanced green fluorescent protein (EGFP) translational fusion (Pdat-1:EGFP::DAT-1) after production of transgenic nematodes is shown (53). Three-dimensional (3D) reconstructions of confocal images were obtained using Zeiss lsm 510 software to visualize the dopamine neurons in vivo. A) Image stacks were taken at 1 μm using two-channel imaging for EGFP and combined with a whole animal image obtained by differential interference contrast microscopy. Scale bar = 50 μm. B) Pseudocolored 3D reconstruction of synapses of the dopaminergic CEP (cephalic) neurons. Image stacks were taken at 0.5 μm using the EGFP channel only. Arrows indicate dopamine synapses. Scale bar = 10 μm. Figure courtesy of Shannon L. Hardie.
Stimulation of SERT through a PKG-linked pathway raises the question as to whether SERTs are phosphorylated as a consequence of PKG activation. Using metabolic labeling, immunoprecipitations and phosphoamino acid analysis of rat midbrain synaptosomes, Ramamoorthy et al. documented SERT incorporation of [32P] after 8-Br-cGMP treatments specifically on Thr residues (58). After comprehensive mutagenesis of cytosolic Thr residues, Thr276 of human SERT was found to be the only Thr residue essential for 8-Br-cGMP stimulation of phosphorylation. Importantly, mutation of this site to Ala blocked 8-Br-cGMP stimulation of 5-HT uptake. Future studies are needed to define whether PKG directly phosphorylates SERT at Thr276.
In contrast to the studies of Zhu et al. who had defined a trafficking-dependent mode of regulation sustained by PKG activation, Ramamoorthy et al. suggested that PKG activation leads to catalytic activation of SERT (58). Thus, although treatment of synaptosomes with 8-Br-cGMP led to increased 5-HT transport by an elevation in 5-HT transport capacity (Vmax), no increase in surface SERT was detected using cell surface biotinylation approaches. Three differences between the works of these two groups may help clarify this issue. First, Zhu et al. predominantly used stimulation of SERT by A3ARs, stimulation likely to activate only a limited pool or specific isoforms of cellular PKG. Use of 8-Br-cGMP as in the Ramamoorthy's studies to regulate SERT may activate additional pools of PKG whose actions could lead to other effects. Second, Zhu et al. employed much lower concentrations of 8-Br-cGMP (10 μM) versus that of Ramamoorthy et al. (250 μM). Third, although both groups utilize transfected cell models, levels of expression of SERT in the Zhu studies are kept low to match those originally encountered in RBL-2H3 cells that express rat SERT from the native SERT promoter. With a biochemical end-point in mind, (the isolation of SERT phosphorylation sites) Ramamoorthy et al. were compelled to work with SERT in culture models at much higher SERT protein levels. Our group has found (Zhu and Blakely, unpublished data) that 8-Br-cGMP-induced trafficking and enhancement of SERT activity are difficult to monitor at high expression levels, possibly because of saturation of a limited number of transport vesicles that helps shuttle SERT in and out of the membrane. It is possible, therefore, that Ramamoorthy's studies define only part of the regulatory system through which SERT is controlled. Indeed, as we note below, Zhu et al. have found that PKG stimulation leads to p38 mitogen-activated protein kinase (MAPK) activation that triggers SERT catalytic activation.
Trafficking-independent modulation
Based on the ability of p38 MAPK to support trafficking-independent regulation of NET (59) and of PKG to phosphorylate and activate p38 MAPK (53), Zhu et al. asked whether p38 MAPK was involved in SERT upregulation that follows A3AR activation. Remarkably, the p38 MAPK inhibitor SB203580 blocked fully the increase in SERT activity seen with IB-MECA, although it did not prevent elevations in SERT surface expression (60).
These findings suggest that PKG enhances both surface trafficking of SERT and SERT catalytic activity through two distinct pathways. Indeed, it is possible to bypass PKG altogether by using direct activators of p38 MAPK, such as anisomycin, which predictably increase 5-HT transport. Whereas PKG activation alters SERT transport capacity, p38 MAPK activation increases 5-HT affinity (61). One clue to the mechanism of this effect may be found in the recent study of Zhang et al. who show that phosphorylation at Thr276 after PKG activation produces an altered conformation of TM5 (62). This finding leads naturally to the idea that PKC-induced SERT catalytic downregulation may derive from phosphorylation of a nearby residue whose modification stabilizes a conformation of TM5 that is associated with a low 5-HT transport rate and/or prevents PKG-induced phosphorylation at Thr276. Consistent with this idea, PKC activation triggers dissociation of PP2Ac from SERT (36). Because p38 MAPK activation triggers catalytic activation of SERT and this effect is dependent on PP2A activity (61), PKG may phosphorylate Thr276 of SERT as well as other proteins that control SERT trafficking. PKG could then generate a conformation of SERT of higher 5-HT affinity as well as activate p38 MAPK, leading to PP2A activation that sustains the inhibitory Ser in a dephosphorylated state so that SERT activation is unopposed. Conversely, with p38 MAPK inhibited, PP2Ac activation may be insufficient to oppose basal inhibitory phosphorylation at this Ser.
Such a model could explain the actions of inflammatory cytokines such as interleukin-1β and tumor necrosis factor-α that activate p38 MAPK in a PKG-independent manner leading to enhanced SERT activity, without inducing SERT trafficking (63). By this model, the removal of tonic PKC inhibition is all that is needed to elevate SERT catalytic activity. Further research is needed to understand how transit of SERT between distinct protein complexes or membrane subdomains places SERT in a context for catalytic modulation.
Samuvel et al. have also suggested that basal p38 MAPK activity supports SERT trafficking. Rat brain synaptosomes treated with the p38 MAPK inhibitors SB203580 or PD169316 display decreased basal 5-HT transport that coincides with reduced SERT cell surface levels (31). Additionally, p38 MAPK antagonists reduce basal SERT phosphorylation. Again, it is likely that p38 MAPK-supported basal trafficking and p38 MAPK-supported SERT catalytic activity can be reconciled if one considers the differences in pharmacology (activator versus inhibitor), the alternate serotonergic preparations and the timing of drug application utilized in various p38 MAPK studies. As Zhu et al. have shown, acute p38 MAPK activation leading to enhanced catalytic regulation is monitored when p38 MAPK is acutely activated, whereas the studies of Samuvel et al. derive from studies where basal p38 MAPK is inhibited. Possibly, changes in basal SERT phosphorylation observed with p38 MAPK inhibitors reflect an indirect consequence of reductions in basal surface expression; for example, alterations in SERT phosphorylation may lead to PP2Ac association in compartments that prepare SERT to recycle back to the plasma membrane.
Recently, Carneiro et al. described a novel platelet SERT interactor that supports a bidirectional regulation of the transporter (64). Using platelets derived from mice lacking integrin β3 (Itgb3−/−), the authors determined that 5-HT transport is severely reduced, although total and plasma membrane SERT expression is unchanged. Conversely, αIIβ3 activation by immobilized fibrinogen leads to an increase in 5-HT uptake without affecting SERT surface expression. These phenomena appear to be supported, in part, through direct physical interactions of the SERT carboxy terminus with integrin β3. Remarkably, cotransfection of human SERT and a hyperserotonemia- and thrombosisassociated integrin β3 variant, Leu33Pro, leads to increased 5-HT uptake as well as to enhanced SERT surface expression. These effects may well relate to some of the same mechanisms of SERT regulation described above as the impact of the Leu33Pro variant on SERT requires active PP2A and p38 MAPK. Because integrin proteins help establish focal adhesions (Figure 2), these studies draw additional attention not only to the trafficking pathways between cytosolic and plasma membrane for SERTs but also to the movements between plasma membrane subdomains as a critical component of SERT regulation.
Impact of Disease-Associated SERT Mutations on SERT Regulation
The emerging understanding of how SERT is regulated has afforded important opportunities to ask whether dysregulated SERT contributes to 5-HT-linked disorders. Glatt et al. laid a foundation for such an inspection in an initial analysis of genetic variation in SERT coding sequences, documenting multiple rare variants, although no functional studies were presented (65). One of these variants, Ile425Val, was reisolated by Ozaki et al. in two unrelated pedigrees populated by subjects with obsessive–compulsive disorder, a condition often treated with SSRIs (66). Kilic et al. (67) studied the Ile425Val variant in transfected cells and found it to be a gain-of-function variant, findings replicated by Prasad et al. (68) in a comprehensive study of all the Glatt study's rare variants. Surprisingly, many of the rare SERT variants (although not Ile425Val) were found to be insensitive to PKG or p38 MAPK activation. One of these variants, Gly56Ala, although relatively uncommon, is estimated to be carried by 1–2 million Americans. Prasad et al. showed that Gly56Ala is also a gain-of-function SERT variant and exhibits elevated basal phosphorylation as well as being as refractory to phosphorylation by PKG activation. These findings took on additional significance when these investigators found enrichment of this variant in a cohort of subjects with autism, along with multiple other rare SERT coding variants including Ile425Leu (same position as Ileu425Val above) (68,69). Autism has long been linked to altered 5-HT signaling, in part because of the well-replicated observation of whole blood hyperserotonemia in a sizeable fraction of autistic children as well as the finding that 5-HT precursor depletion worsens autistic symptoms (70,71). These results suggest that dysregulated SERT expression during development may be a shared mechanism for multiple 5-HT-linked disorders.
The findings reviewed above point to specific regulatory steps in the modulation of SERT that should be examined for further assessment of disease risk alleles and/or therapeutic intervention. The complexity of SERT regulation will likely require attention to conditions encountered by SERT in native environments. Several lines of evidence, including that presented for PKG, SCAMP2, Hic-5, and integrin β3, suggest complex and dynamic compartmentation of SERT. Kinases such as PKG and p38 MAPK as well as the multiple interacting proteins noted have many targets besides neurotransmitter transporters, including CS proteins and transcription factors. It will therefore be critical to evaluate specifically the pools of SERT-interacting proteins that act to regulate SERT under conditions that limit overexpression or other nonphysiological manipulations often required in biochemical analyses. Use of cell surface receptors such as the A3AR to trace the pathways that modulate SERT and development of knock-in mouse lines expressing dysregulated human SERT alleles should offer important opportunities to navigate through this complexity. Regardless, the recent discovery of new players in SERT modulation and the elucidation of human SERT mutations that disrupt transporter regulation offer promise for important progress in a host of devastating mental disorders long associated with disrupted 5-HT signaling.
Acknowledgments
We thank Shannon L. Hardie, Julie R. Field and John Steiner for assistance with figure preparation. J. A. S. was supported by MH064913. A. M. D. C. was supported by a NARSAD Young Investigator Award and National Institutes of Health (NIH) award NS049261. R. D. B. was supported by NIH awards DA07390, MH078028 and MH058921.
References
- 1.Sodhi MS, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. doi: 10.1016/S0074-7742(04)59006-2. [DOI] [PubMed] [Google Scholar]
- 2.McLean PG, Borman RA, Lee K. 5-HT in the enteric nervous system: gut function and neuropharmacology. Trends Neurosci. 2007;30:9–13. doi: 10.1016/j.tins.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Snyder SH. Putative neurotransmitters in the brain: selective neuronal uptake, subcellular localization, and interactions with centrally acting drugs. Biol Psychiatry. 1970;2:367–389. [PubMed] [Google Scholar]
- 4.Fuller RW, Perry KW, Molloy BB. Effect of an uptake inhibitor on serotonin metabolism in rat brain: studies with 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine (Lilly 110140) Life Sci. 1974;15:1161–1171. doi: 10.1016/s0024-3205(74)80012-3. [DOI] [PubMed] [Google Scholar]
- 5.Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker EL, Blakely RD. Norepinephrine and serotonin transporters: molecular targets of antidepressant drugs. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 321–333. [Google Scholar]
- 7.Blakely RD, Berson HE, Fremeau RT, Jr, Caron MG, Peek MM, Prince HK, et al. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman BJ, Mezey E, Brownstein MJ. Cloning of a serotonin transporter affected by antidepressants. Science. 1991;254:579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na(+)/Cl(−)-dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- 10.Chen JG, Liu-Chen S, Rudnick G. Determination of external loop topology in the serotonin transporter by site-directed chemical labeling. J Biol Chem. 1998;273:12675–12681. doi: 10.1074/jbc.273.20.12675. [DOI] [PubMed] [Google Scholar]
- 11.Torres GE, Amara SG. Glutamate and monoamine transporters: new visions of form and function. Curr Opin Neurobiol. 2007;17:304–312. doi: 10.1016/j.conb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Henry LK, Defelice LJ, Blakely RD. Getting the message across: a recent transporter structure shows the way. Neuron. 2006;49:791–796. doi: 10.1016/j.neuron.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Henry LK, Meiler J, Blakely RD. Bound to be different: neurotransmitter transporters meet their bacterial cousins. Mol Interv. 2007;7:306–309. doi: 10.1124/mi.7.6.4. [DOI] [PubMed] [Google Scholar]
- 14.Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- 15.Qian Y, Melikian HE, Rye DB, Levey AI, Blakely RD. Identification and characterization of antidepressant-sensitive serotonin transporter proteins using site-specific antibodies. J Neurosci. 1995;15:1261–1274. doi: 10.1523/JNEUROSCI.15-02-01261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickel VM, Chan J. Ultrastructural localization of the serotonin transporter in limbic and motor compartments of the nucleus accumbens. J Neurosci. 1999;19:7356–7366. doi: 10.1523/JNEUROSCI.19-17-07356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou FC, Tao-Cheng JH, Segu L, Patel T, Wang Y. Serotonin transporters are located on the axons beyond the synaptic junctions: anatomical and functional evidence. Brain Res. 1998;805:241–254. doi: 10.1016/s0006-8993(98)00691-x. [DOI] [PubMed] [Google Scholar]
- 18.Miner LH, Schroeter S, Blakely RD, Sesack SR. Ultrastructural localization of the serotonin transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to dopamine terminals. J Comp Neurol. 2000;427:220–234. doi: 10.1002/1096-9861(20001113)427:2<220::aid-cne5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 19.Tao-Cheng JH, Zhou FC. Differential polarization of serotonin transporters in axons versus soma-dendrites: an immunogold electron microscopy study. Neuroscience. 1999;94:821–830. doi: 10.1016/s0306-4522(99)00373-5. [DOI] [PubMed] [Google Scholar]
- 20.Miner LH, Jedema HP, Moore FW, Blakely RD, Grace AA, Sesack SR. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J Neurosci. 2006;26:1571–1578. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farhan H, Freissmuth M, Sitte HH. Oligomerization of neurotransmitter transporters: a ticket from the endoplasmic reticulum to the plasma membrane. Handb Exp Pharmacol. 2006;175:233–249. doi: 10.1007/3-540-29784-7_12. [DOI] [PubMed] [Google Scholar]
- 22.Farhan H, Reiterer V, Korkhov VM, Schmid JA, Freissmuth M, Sitte HH. Concentrative export from the endoplasmic reticulum of the gamma-aminobutyric acid transporter 1 requires binding to SEC24D. J Biol Chem. 2007;282:7679–7689. doi: 10.1074/jbc.M609720200. [DOI] [PubMed] [Google Scholar]
- 23.Farhan H, Korkhov VM, Paulitschke V, Dorostkar MM, Scholze P, Kudlacek O, Freissmuth M, Sitte HH. Two discontinuous segments in the carboxyl terminus are required for membrane targeting of the rat gamma-aminobutyric acid transporter-1 (GAT1) J Biol Chem. 2004;79:28553–28563. doi: 10.1074/jbc.M307325200. [DOI] [PubMed] [Google Scholar]
- 24.Qian Y, Galli A, Ramamoorthy S, Risso S, DeFelice LJ, Blakely RD. Protein kinase C activation regulates human serotonin transporters in HEK-293 cells via altered cell surface expression. J Neurosci. 1997;17:45–47. doi: 10.1523/JNEUROSCI.17-01-00045.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramamoorthy S, Giovanetti E, Qian Y, Blakely RD. Phosphorylation and regulation of antidepressant-sensitive serotonin transporters. J Biol Chem. 1998;273:2458–2466. doi: 10.1074/jbc.273.4.2458. [DOI] [PubMed] [Google Scholar]
- 26.Holton KL, Loder MK, Melikian HE. Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat Neurosci. 2005;8:881–888. doi: 10.1038/nn1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sorkina T, Hoover BR, Zahniser NR, Sorkin A. Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic. 2005;6:157–170. doi: 10.1111/j.1600-0854.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 28.Deken SL, Wang D, Quick MW. Plasma membrane GABA transporters reside on distinct vesicles and undergo rapid regulated recycling. J Neurosci. 2003;23:1563–1568. doi: 10.1523/JNEUROSCI.23-05-01563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magnani F, Tate CG, Wynne S, Williams C, Haase J. Partitioning of the serotonin transporter into lipid microdomains modulates transport of serotonin. J Biol Chem. 2004;279:38770–38778. doi: 10.1074/jbc.M400831200. [DOI] [PubMed] [Google Scholar]
- 30.Jayanthi LD, Samuvel DJ, Ramamoorthy S. Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters: evidence for localization in lipid rafts and lipid raft mediated internalization. J Biol Chem. 2004;279:19315–19324. doi: 10.1074/jbc.M311172200. [DOI] [PubMed] [Google Scholar]
- 31.Samuvel DJ, Jayanthi LD, Bhat NR, Ramamoorthy S. A role for p38 mitogen-activated protein kinase in the regulation of the serotonin transporter: evidence for distinct cellular mechanisms involved in transporter surface expression. J Neurosci. 2005;25:29–41. doi: 10.1523/JNEUROSCI.3754-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carneiro AM, Blakely RD. Serotonin-, protein kinase C-, and Hic-5-associated redistribution of the platelet serotonin transporter. J Biol Chem. 2006;281:24769–24780. doi: 10.1074/jbc.M603877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blakely RD, Ramamoorthy S, Schroeter S, Qian Y, Apparsundaram S, Galli A, DeFelice LJ. Regulated phosphorylation and trafficking of antidepressant-sensitive serotonin transporter proteins. Biol Psychiatry. 1998;44:169–178. doi: 10.1016/s0006-3223(98)00124-3. [DOI] [PubMed] [Google Scholar]
- 34.Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999;285:763–766. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- 35.Blakely RD, Bauman AL. Biogenic amine transporters: regulation in flux. Curr Opin Neurobiol. 2000;10:328–336. doi: 10.1016/s0959-4388(00)00088-x. [DOI] [PubMed] [Google Scholar]
- 36.Bauman AL, Apparsundaram S, Ramamoorthy S, Wadzinski BE, Vaughan RA, Blakely RD. Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J Neurosci. 2000;20:7571–7578. doi: 10.1523/JNEUROSCI.20-20-07571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beckman ML, Bernstein EM, Quick MW. Protein kinase C regulates the interaction between a GABA transporter and syntaxin 1A. J Neurosci. 1998;18:6103–6112. doi: 10.1523/JNEUROSCI.18-16-06103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geerlings A, Lopez-Corcuera B, Aragon C. Characterization of the interactions between the glycine transporters GLYT1 and GLYT2 and the SNARE protein syntaxin 1A. FEBS Lett. 2000;470:51–54. doi: 10.1016/s0014-5793(00)01297-7. [DOI] [PubMed] [Google Scholar]
- 39.Sung U, Apparsundaram S, Galli A, Kahlig KM, Savchenko V, Schroeter S, Quick MW, Blakely RD. A regulated interaction of syntaxin 1A with the antidepressant-sensitive norepinephrine transporter establishes catecholamine clearance capacity. J Neurosci. 2003;23:1697–1709. doi: 10.1523/JNEUROSCI.23-05-01697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blakely RD, Sung U. SNARE-ing neurotransmitter transporters. Nat Neurosci. 2000;3:969–971. doi: 10.1038/79898. [DOI] [PubMed] [Google Scholar]
- 41.Haase J, Killian AM, Magnani F, Williams C. Regulation of the serotonin transporter by interacting proteins. Biochem Soc Trans. 2001;29:722–728. doi: 10.1042/0300-5127:0290722. [DOI] [PubMed] [Google Scholar]
- 42.Quick MW. Regulating the conducting states of a mammalian serotonin transporter. Neuron. 2003;40:537–549. doi: 10.1016/s0896-6273(03)00605-6. [DOI] [PubMed] [Google Scholar]
- 43.Carneiro AM, Ingram SL, Beaulieu JM, Sweeney A, Amara SG, Thomas SM, Caron MG, Torres GE. The multiple LIM domain-containing adaptor protein Hic-5 synaptically colocalizes and interacts with the dopamine transporter. J Neurosci. 2002;22:7045–7054. doi: 10.1523/JNEUROSCI.22-16-07045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Müller HK, Wiborg O, Haase J. Subcellular redistribution of the serotonin transporter by secretory carrier membrane protein 2. J Biol Chem. 2006;281:28901–28909. doi: 10.1074/jbc.M602848200. [DOI] [PubMed] [Google Scholar]
- 45.Chanrion B, Mannoury la Cour C, Bertaso F, Lerner-Natoli M, Freissmuth M, Millan MJ, Bockaert J, Marin P. Physical interaction between the serotonin transporter and neuronal nitric oxide synthase underlies reciprocal modulation of their activity. Proc Natl Acad Sci U S A. 2007;104:8119–8124. doi: 10.1073/pnas.0610964104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ansah TA, Ramamoorthy S, Montanez S, Daws LC, Blakely RD. Calcium-dependent inhibition of synaptosomal serotonin transport by the alpha 2-adrenoceptor agonist 5-bromo-N-[4,5-dihydro-1H-imidazol-2-yl]-6-quinoxalinamine (UK14304) J Pharmacol Exp Ther. 2003;305:956–965. doi: 10.1124/jpet.102.047134. [DOI] [PubMed] [Google Scholar]
- 47.Galzin AM, Moret C, Langer SZ. Evidence that exogenous but not endogenous norepinephrine activates the presynaptic alpha-2 adrenoceptors on serotonergic nerve endings in the rat hypothalamus. J Pharmacol Exp Ther. 1984;228:725–732. [PubMed] [Google Scholar]
- 48.Adell A, Celada P, Abellan MT, Artigas F. Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res Brain Res Rev. 2002;39:154–180. doi: 10.1016/s0165-0173(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 49.Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol Pharmacol. 2005;67:2077–2087. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- 50.Jayanthi LD, Annamalai B, Samuvel DJ, Gether U, Ramamoorthy S. Phosphorylation of the norepinephrine transporter at threonine 258 and serine 259 is linked to protein kinase C-mediated transporter internalization. J Biol Chem. 2006;281:23326–23340. doi: 10.1074/jbc.M601156200. [DOI] [PubMed] [Google Scholar]
- 51.Miller KJ, Hoffman BJ. Adenosine A3 receptors regulate serotonin transport via nitric oxide and cGMP. J Biol Chem. 1994;269:27351–27356. [PubMed] [Google Scholar]
- 52.Zhu CB, Steiner JA, Munn JL, Daws LC, Hewlett WA, Blakely RD. Rapid stimulation of presynaptic serotonin transport by A(3) adenosine receptors. J Pharmacol Exp Ther. 2007;322:332–340. doi: 10.1124/jpet.107.121665. [DOI] [PubMed] [Google Scholar]
- 53.Zhu CB, Hewlett WA, Feoktistov I, Biaggioni I, Blakely RD. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol Pharmacol. 2004;65:1462–1474. doi: 10.1124/mol.65.6.1462. [DOI] [PubMed] [Google Scholar]
- 54.Zhu CB, Hewlett WA, Francis SH, Corbin JD, Blakely RD. Stimulation of serotonin transport by the cyclic GMP phosphodiesterase-5 inhibitor sildenafil. Eur J Pharmacol. 2004;504:1–6. doi: 10.1016/j.ejphar.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 55.Taylor MS, Okwuchukwuasanya C, Nickl CK, Tegge W, Brayden JE, Dostmann WR. Inhibition of cGMP-dependent protein kinase by the cell-permeable peptide DT-2 reveals a novel mechanism of vaso-regulation. Mol Pharmacol. 2004;65:1111–1119. doi: 10.1124/mol.65.5.1111. [DOI] [PubMed] [Google Scholar]
- 56.McDonald PW, Hardie SL, Jessen TN, Carvelli L, Matthies DS, Blakely RD. Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci. 2007;27:14216–14227. doi: 10.1523/JNEUROSCI.2992-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ranganathan R, Sawin ER, Trent C, Horvitz HR. Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J Neurosci. 2001;21:5871–5884. doi: 10.1523/JNEUROSCI.21-16-05871.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramamoorthy S, Samuvel DJ, Buck ER, Rudnick G, Jayanthi LD. Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J Biol Chem. 2007;282:11639–11647. doi: 10.1074/jbc.M611353200. [DOI] [PubMed] [Google Scholar]
- 59.Apparsundaram S, Sung U, Price RD, Blakely RD. Trafficking-dependent and -independent pathways of neurotransmitter transporter regulation differentially involving p38 mitogen-activated protein kinase revealed in studies of insulin modulation of norepinephrine transport in SK-N-SH cells. J Pharmacol Exp Ther. 2001;299:666–677. [PubMed] [Google Scholar]
- 60.Blakely RD, Defelice LJ, Galli A. Biogenic amine neurotransmitter transporters: just when you thought you knew them. Physiology (Bethesda) 2005;20:225–231. doi: 10.1152/physiol.00013.2005. [DOI] [PubMed] [Google Scholar]
- 61.Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, PP2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- 62.Zhang YW, Gesmonde J, Ramamoorthy S, Rudnick G. Serotonin transporter phosphorylation by cGMP-dependent protein kinase is altered by a mutation associated with obsessive compulsive disorder. J Neurosci. 2007;27:10878–10886. doi: 10.1523/JNEUROSCI.0034-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 64.Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest. 2008;118:1544–1552. doi: 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glatt CE, DeYoung JA, Delgado S, Service SK, Giacomini KM, Edwards RH, Risch N, Freimer NB. Screening a large reference sample to identify very low frequency sequence variants: comparisons between two genes. Nat Genet. 2001;27:435–438. doi: 10.1038/86948. [DOI] [PubMed] [Google Scholar]
- 66.Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, Murphy DL. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- 67.Kilic F, Murphy DL, Rudnick G. A human serotonin transporter mutation causes constitutive activation of transport activity. Mol Pharmacol. 2003;64:440–446. doi: 10.1124/mol.64.2.440. [DOI] [PubMed] [Google Scholar]
- 68.Prasad HC, Zhu CB, McCauley JL, Samuvel DJ, Ramamoorthy S, Shelton RC, Hewlett WA, Sutcliffe JS, Blakely RD. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc Natl Acad Sci U S A. 2005;102:11545–11550. doi: 10.1073/pnas.0501432102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson GM, Freedman DX, Cohen DJ, Volkmar FR, Hoder EL, McPhedran P, Minderaa RB, Hansen CR, Young JG. Whole blood serotonin in autistic and normal subjects. J Child Psychol Psychiatry. 1987;28:885–900. doi: 10.1111/j.1469-7610.1987.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 71.McDougle CJ, Naylor ST, Goodman WK, Volkmar FR, Cohen DJ, Price LH. Acute tryptophan depletion in autistic disorder: a controlled case study. Biol Psychiatry. 1993;33:547–550. doi: 10.1016/0006-3223(93)90011-2. [DOI] [PubMed] [Google Scholar]