Abstract
AIM: To investigate and evaluate the feasibility of the computer-aided screening diagnosis for enteric lesions in the capsule endoscopy (CE).
METHODS: After developing a series of algorithms for the screening diagnosis of the enteric lesions in CE based on their characteristic colors and contours, the normal and abnormal images obtained from 289 patients were respectively scanned and diagnosed by the CE readers and by the computer-aided screening for the enteric lesions with the image-processed software (IPS). The enteric lesions shown by the images included esoenteritis, mucosal ulcer and erosion, bleeding, space-occupying lesions, angioectasia, diverticula, parasites, etc. The images for the lesions or the suspected lesions confirmed by the CE readers and the computers were collected, and the effectiveness rate of the screening and the number of the scanned images were evaluated, respectively.
RESULTS: Compared with the diagnostic results obtained by the CE readers, the total effectiveness rate (sensitivity) in the screening of the commonly-encountered enteric lesions by IPS varied from 42.9% to 91.2%, with a median of 74.2%, though the specificity and the accuracy rates were still low, and the images for the rarely-encountered lesions were difficult to differentiate from the normal images. However, the number of the images screened by IPS was 5000 on average, and only 10%-15% of the original images were left behind. As a result, a large number of normal images were excluded, and the reading time decreased from 5 h to 1 h on average.
CONCLUSION: Though the total accuracy and specificity rates by the computer-aided screening for the enteric lesions with IPS are much lower than those by the CE readers, the computer-aided screening diagnosis can exclude a large number of the normal images and confine the enteric lesions to 5000 images on average, which can reduce the workload of the readers in the scanning of the images. This computer-aided screening technique can make a correct diagnosis as efficiently as possible in most of the patients.
Keywords: Enteric lesions, Image processing, Capsule endoscopy, Diagnosis
INTRODUCTION
Depiction of the small intestines has still been a challenge because of their length and tortuosity. In the past time when some lesions occurred in an intestinal segment, they were usually difficult to identify because of a shortage of the efficiently used tools[1,2]. When the capsule endoscopy (CE) was introduced to the clinical practice in 2000, it became a revolutionary diagnostic tool in diagnosing small intestinal diseases[3]. Besides, there is growing evidence that CE may be used in diagnosing some diseases occurring in the colon and the esophagus[4,5]. Because of the incapability of its use in the insufflation, affusion, biopsy and therapy, when compared with the balloon or push enteroscope[6-8], CE still has some limitations in diagnosing and treating the enteric lesions[9]; however, the patients are willing to accept it because it is painless and noninvasive. It is also much superior to the small intestinal radiology in management of some diseases[10].
CE is virtually a microcamera, which can create 2 images/s in the human gastrointestinal (GI) tract, obtain 40-60 thousand images of the GI lumen from the mouth to the anus before its batteries are exhausted[5].
The principal task of the CE readers is to scan all the images and find out what diseases occur in the GI tract, especially those occurring in the small intestines, because they cannot be detected by the routine gastroscopy or colonoscopy. So, the screening task is a heavy burden on the readers because there is a large number of images for them to screen. Though the number of the images for the lesions is smaller than 500 in most of the patients, the CE reader still has to scan ten thousands of the images one by one because the reader cannot make sure which images the lesions are in. As a rule, it would take 4-6 h at least for two readers (2-3 h for each reader) to finish this tiring work in our trial, and the 4-6 h was only the time for the two readers to finish scanning the images once. If there were some doubts and suspicions, much more time would be spent in repeating the scanning, which might do a great harm to the readers’ eyes. In order to solve this problem, the high-speed reading (HSR) technique is the commonly-used method at the present time[11], and the reading time can be decreased to 0.5-1 h for one reader[12], but the work intensity is much greater, and some lesions may be missed, too[12,13]. So, if the computer-aided screening diagnosis with image-processed software (IPS) can be used to identify the images for the enteric lesions from a large number of the images that contain the normal ones, it will greatly decrease the workload of the readers for doing this kind of work and make a correct diagnosis as soon as possible.
However, the previous studies showed that the computer-aided diagnosis could not be as accurate as the one made by the reader because of the complexity and multiformity of the lesions in the GI tract[14]. So, our research focused on the feasibility of “screening” the normal images. After the exclusion of a large number of the normal images with the help of IPS, the remaining images can efficiently be evaluated; whether the images indicate the confirmed lesions or indicate only the suspected lesions, they can efficiently be preserved for a further diagnosis.
MATERIALS AND METHODS
Image-sample collection and pretreatment
All the images, derived from the CE produced by OMOM Capsule Endoscopic Company (OCEC), Chongqing City, China, were obtained from the Endoscopic Center of West China Hospital of Sichuan University or provided by OCEC directly. Two hundred and eighty-nine patients, with a median age of 54.2 years (range, 17-83 years), were included. All the patients finished the CE examination without any capsule retention. No lesions were found in 86 of the patients, but 1 lesion was found in 182 patients, 2 in 12 patients, 3 in 6 patients, and 4 in 3 patients. The enteric lesions included esoenteritis, mucosal ulcer and erosion, bleeding, space-occupying lesions, angioectasia, diverticula, parasites, etc (Table 1). All the images would be converted to the bitmap (BMP) format directly before they were analyzed by IPS[15].
Table 1.
Proportions of enteric lesions and non-lesions in 289 patients
| Enteric lesion | No. of lesions and non-lesions | Proportion (%) |
| Non-lesion | 86 | 26.7 |
| Common lesion | ||
| Esoenteritis | 42 | 13.0 |
| Mucosal ulcer and erosion | 47 | 14.6 |
| Bleeding | 61 | 18.9 |
| Space-occupying lesion | 28 | 8.7 |
| Angioectasia | ||
| Telangiectasis | 5 | 1.6 |
| Angioectasia | 32 | 9.9 |
| Angioma | 7 | 2.2 |
| Rare lesion | ||
| Diverticulum | 3 | 0.9 |
| Parasite | 5 | 1.7 |
| Enteric stenosis | 2 | 0.6 |
| Intussusception | 2 | 0.6 |
| Enteric lymphangiectasis | 2 | 0.6 |
| Total | 322 | 100.0 |
Algorithm analysis
In every enteric image obtained by CE, all the pictorial contents could be grouped as one of the following three categories: enteric mucosa, enteric lesions, and enteric contents. Each of them had its own characteristic colors, coloring distribution or borderlines of the body to the surroundings, which might be distinguished from one another by the computer-aided IPS in most of the images. According to the Tricolor Theory, the color of every dot in the image consisted of three types of the primary pels: red (R), green (G) and blue (B), whose chromatic values varied from 0 to 255. The characteristic color (primary hue of the enteric mucosa was R, with a common value greater than 100 (average value, 150-200), which was different from that of the lesions or that of the contents in the small intestines in most of the images. Besides esoenteritis and bleeding, there was a series of algorithms developed to simulate the scanning of the human eyes to “screen” a large number of the images for the normal mucosa.
Algorithm principle
The first step was to decide the analyzed region in the image. The dots with an R value (Rv) less than 100 would be excluded because they formed the “dark region”, which was hard to be discerned even by the human eyes (Figure 1A and B), and the remaining dots would be contained for the analysis.
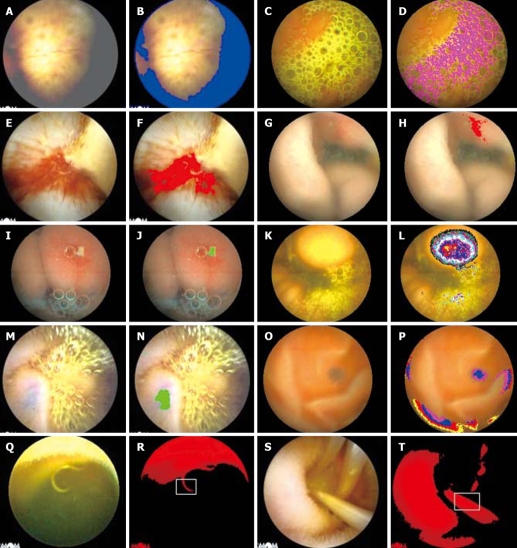
Figure 1.
The screened results of enteric lesions by the IPS. A: Space-occupying lesions; B: The blue area in the image for the “dark region”; C: The enteric bile; D: The purple area in the image for the enteric bile; E: The enteric blood (bleeding); F: The red area in the image for the enteric blood; G: Esoenteritis; H: The red area in the image for esoenteritis; I: Mucosal ulcer; J: The green area in the image for mucosal ulcer; K: Space-occupying lesions; L: Different coloring circle in the image for space-occupying lesions; M: Venous angioma in the bright background; N: The green area in the image for venous angioma; O: Venous angioma in the dark background; P: The loop-closed distribution of Gv of the venous angiomain image; Q: Parasites (hook worms); R: The image of red components (Rv > 150) and the curvature of the double limits in the pane calculated; S: Parasites (ascarides); T: The image of red components (Rv > 150) and the linearity of the double limits in the pane calculated.
The second step was to exclude the region of the enteric contents, such as bile, spume etc., by their own characteristic colors.
In the bile region, Rv was less than 230, and the ratio of Rv to Gv was less than 1.1, which led to the characteristic color of yellow or green (Figure 1C and D); in the blood region, the usual Rv of the dots was less than 180 but greater than 120, the ratio of Rv to Gv was greater than 2, and the B value (Bv) was greater than 30 at least (Figure 1E and F).
The third step was to extract the characteristic colors and the coloring distribution of the common lesions in the small intestines.
In the region of esoenteritis, the Rv (> 180) of the dots was greater than Gv, and the remaining value subtracted Gv from Rv was greater than 60-70, and Bv varied from 60 to 90 (Figure 1G and H); furthermore, the difference (60-70) between Rv and Gv in the inflammatory region was greater than that (30-40) between the background regions (mucosal regions) on average.
In the region of mucosal ulcer and erosion, Rv (> 170, < 200) was greater than Gv (> 150), but the remaining value subtracted Gv from Rv was less than 40, and Bv was greater than 100, so the mucosal ulcer and erosion in the image looked yellow and white appreciably, which was different from the color of the enteric bile. Rv in the region of mucosal ulcer and erosion was greater than that of the background region (mucosal region) by 30-40 on average (Figure 1I and J).
Generally speaking, in the region of space-occupying lesions, there was no characteristic color due to the lesions derived from the mucosal or submucosal tissues, but the space-occupying lesions had a characteristic protrusion into the enteric lumen, which led to a circular distribution of the degressive Gv, and Rv in the center of the circle was usually greater than 245 (Figure 1K and L).
Venous angioma was a special type of space-occupying lesions without any enteric protrusion, but it looked blue. In the bright background, the remaining value subtracted Gv from Rv (> 160, < 200) was less than 30, and Bv from Gv (> 150) less than 10 (Figure 1M and N). In the dark background, the remaining value subtracted Gv (> 70) from Rv (> 100, < 170) was less than 50. Bv was greater than 45, but the most important characteristic was that the lesion had the loop-closed distribution of Rv and Gv, which was different from that of the fringe of the image (Figure 1O and P).
Though the common parasites in the small intestines (Figure 1Q and S), mainly hook worms and ascarides, had no special body colors, but the double borderlines of the body to the surroundings (contour) usually existed clearly. Thus, a kind of the algorithm was developed to distinguish the parasites from the background mucosa or the enteric contents. Firstly, the chromatic pels of the parasites (Rv > 150, Gv > 120) in the bright visual field would be extracted. Secondly, as the double limits of the parasites in the extracted region had a similar curvature (hook worms) or linearity (ascarides), it could be calculated in some width and be judged by the software whether the parasites existed or not (Figure 1R and T). Finally, whether the screened results were accurate or not, the images about the esophagus, stomach and large intestine would be excluded if they were in the remaining images.
The rarely-encountered lesions, including diverticulum (Figure 2A), intussusception (Figure 2B), enteric stenosis (Figure 2C), enteric lymphangiectasis (Figure 2D), angioectasia (Figure 2E and F) etc., because of the absence of characteristic colors or contours compared with the background mucosa, could not be picked out by the algorithm principles mentioned above, which will be explained in Discussion.
Figure 2.
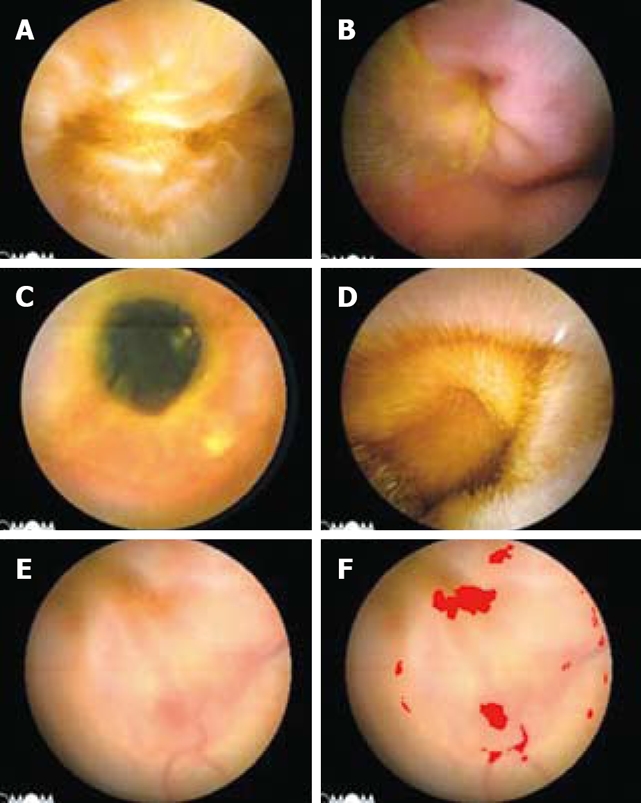
The enteric lesions not being recognised by the IPS. A: Enteric diverticulum; B: Intussusception; C: Enteric stenosis; D: Enteric lymphangiectasis; E: Angioectasia; F: The red area in the image for suspected bleeding, not angioectasia itself.
Supplementary algorithms
If there were several lesions in the image, the image processing would be terminated immediately for the saving of time if only one lesion or parts of the lesions were confirmed by any of the algorithms mentioned above.
Software development and operation
IPS was developed and operated in the common personal computer (memory 1G, Intel® Pentium® Core 2 CPU 1.73GHz, Hard Disk 60GB at least), with Windows Operation System Xp or Vista® home basic.
Screening standard and evaluation
The gold standard for enteric lesions was the diagnostic conclusion drawn by the CE reader (the physician who reviews the CE study). In order to decrease the rate of misdiagnosis to a lowest extent, the primary images and the screened images by IPS from the same patient would respectively be scanned by two readers[13] with a speed of 20 000 images/h, and the marked images by IPS would be extracted out and ranked by the time-index in the images from the jejunum to the ileum, which would be scanned again by two readers and assessed whether those images of the lesions were preserved in the remaining images (screened results) and whether the lesions accorded with those of the readers’ findings. If there was a series of continuous images of the confirmed lesions or the suspected lesions, only the first image and the last image would be marked out by IPS as shown by the index.
Inclusion standards for the screened results were: (1) A series of images for the lesions could be confirmed by IPS if one image was confirmed; (2) Only parts of the lesions in the images could be marked out by IPS.
Exclusion standards for the screened results were: None of the images for the lesions was picked out or none of the marked parts in the images was the lesion itself.
Statistical analysis
Statistical analysis was performed using the SPSS software (ver.11.5). The correlation between two variables was evaluated using Pearson’s χ2, Fisher’s exact test, and u test. Statistical significance was defined as P < 0.05.
RESULTS
Compared with the primary images in the left, the images in the right showed the suspected enteric lesions by different colors, such as red, blue, green, etc. (Figure 1F, H, J, F, N, P, R and T, and Figure 2F).
Screened results
In the 289 patients, the total number of the images varied from 41 358 to 62 874, with a median of 52 374 ± 4865 in the group with the confirmed lesions and 54 210 ± 3739 in the group without any lesions. Parts of the images in each patient were marked by at least one kind of color, and the number varied from 2353 to 18 732, with a median of 4156 ± 478 and 4219 ± 376, respectively, in the group with the lesions and the group without any lesions (Table 2). The mean time needed to read a CE study was 5.4 ± 1.2 h in the group with the lesions and 5.6 ± 1.3 h in the group without any lesions (Table 3). The mean time needed to read a CE study by a reader was 2.8 ± 0.7 h compared with the scanning time of 3.2 ± 0.6 h by IPS (most in 2.5-3.5 h, P > 0.05), and the number of the images for the lesions varied from 1 to 262 in the 236 patients.
Table 2.
Number of marked images in 289 patients
| Group (n) | Total No. of images | No. of marked images | P |
| With lesion (236) | 52 374 ± 4 865 | 4 156 ± 478b | < 0.001 |
| Without lesion (86) | 54 210 ± 3 739 | 4 219 ± 376d | < 0.001 |
| P | > 0.10 | > 0.10 |
P < 0.001, total No. of the images vs No. of the marked images in the group with lesions;
P < 0.001, total No. of the images vs No. of the marked images in the group without any lesions.
Table 3.
Scanning time (ST) spent in each patient on marked images in 289 patients
| Group (n) | ST for total images in each patient (h) | ST for marked images in each patient (h) | P |
| With lesion (236) | 5.4 ± 1.2 | 1.2 ± 0.2b | < 0.001 |
| Without lesion (86) | 5.6 ± 1.3 | 1.3 ± 0.1d | < 0.001 |
| P | > 0.10 | > 0.10 |
P < 0.001, ST for the total images vs ST for the marked images by ISP in the group with lesions;
P < 0.001, ST for the total images vs ST for the marked images by ISP in the group without any lesions.
For the common enteric lesions detected by CE, the effectiveness rate of the screened results (SRs) varied from 42.9% to 91.2%, with a median of 74.2%, but for the angioectasia and rare lesions, the effectiveness rate varied from 20% to 57.1%, with a median of only 22.9% (Table 4).
Table 4.
Screened results (SR) of IPS in 289 patients
| Enteric lesions | Effectiveness rate of SR (%) | False positive rate of SR (%) | P |
| Common lesion | |||
| Esoenteritis | 33/42 (78.6) | 188/280 (67.1) | > 0.05 |
| Mucosal ulcer/erosion | 31/47 (66.0) | 191/275 (69.5) | > 0.05 |
| Bleeding | 56/61 (91.8) | 121/261 (46.4) | > 0.05 |
| Space-occupying lesion | 12/28 (42.9) | 96/294 (32.7) | > 0.05 |
| Angioectasia | |||
| Telangiectasis | 1/5 (20.0) | 63/317 (19.9) | > 0.05 |
| Angioectasia | 3/32 (9.4) | 37/290 (12.8) | > 0.05 |
| Angioma | 4/7 (57.1) | 25/315 (7.9) | > 0.05 |
| Rare lesion1 | 3/14 (21.4) | 53/308 (17.2) | > 0.05 |
The samples of the rare lesions were not enough, so they were pooled together for the research.
The screened results showed that the sensitivity rate (effectiveness rate of SRs) was higher than the specificity rate and the accuracy rate in the commonly-encountered lesions but lower in angioectasia and rarely-encountered lesions; the positive predictive value was low and the negative predictive value was high in all the lesions (Tables 4 and 5).
Table 5.
Sensitivity, specificity, accuracy, positive/negative predictive value of SR for special lesions in 289 patients (%)
| Enteric lesions | Sensitivity | Specificity | Accuracy | Positive pv | Negative pv |
| Common lesion | |||||
| Esoenteritis | 78.6 | 32.9 | 38.8 | 14.9 | 91.1 |
| Mucosal 66.0ulcer/erosion | 30.5 | 35.7 | 14.0 | 84.0 | |
| Bleeding | 91.8 | 53.6 | 60.9 | 31.6 | 96.6 |
| Space- occupying lesion | 42.9 | 67.3 | 65.2 | 11.1 | 92.5 |
| Angioectasia | |||||
| Telangiectasis 20.0 | 80.1 | 79.2 | 1.6 | 98.4 | |
| Angioectasia | 9.4 | 87.2 | 79.5 | 7.5 | 89.7 |
| Angioma | 57.1 | 92.1 | 91.3 | 13.8 | 98.9 |
| Rare lesion1 | 21.4 | 82.8 | 80.1 | 5.4 | 5.9 |
pv: Predictive value.
Because the samples of the rare lesions were not enough, so they were pooled together for the research.
DISCUSSION
CE plays an important role in diagnosis of the small bowel diseases[16-18], such as bleeding[19-21], Crohn’s disease[21-23], tumors[20], angioectasia[20], etc. With the bowel peristalsis, CE passes through the small intestines and takes pictures of the intestinal cavity, though the total number of the images in each patient can vary from 40 000 to 60 000, with a median of 50 000, and the definite number of the images for the lesions is not greater than several hundreds in the patients. After the screening by the computer-aided diagnosis with the help of IPS, the number of the remaining images is only 10%-15% of the original ones (Table 2). Most of the lesions, especially the commonly-encountered enteric lesions, have been included and preserved, which can greatly reduce the workload on the readers and make an efficient diagnosis in most of the patients. However, one thing should be emphasized that the screened results provided by IPS are not the diseases themselves, but the lesions, because it was very difficult for IPS to make a diagnosis according to the lesions themselves just as the CE reader does; therefore, all the data in Table 4 only indicate the screening rates, not the diagnosis rates (Table 4).
The initial objective of this research was to make the diagnosis of the small bowel diseases directly, but the results were disappointing because of the complexity and the multiformity of the lesions. The complicated algorithm (Hidded Markov models, HMM[24,25] or artificial neural network, ANN[26-28]) was of a tryout. Though the diagnosis accuracy could be improved to some extent, the result was not satisfactory to the physicians, and the scanning time of IPS was much longer than that of the readers’ performance; therefore, it could not be accepted in the clinical practice. So, we had to turn our steps just to “screening” the images for the lesions and making the normal images left behind. The results showed that about 75% of the images for the common lesions could be screened out and the scanning-time of IPS was limited to 2.5-3.5 h (average, 3.2 h) in most of the patients after the simple algorithms were used based on the characteristic colors, coloring distribution or contours, which proved its feasibility and availability in the clinical application in our trial.
Though a large number of the normal images were excluded and the reading time was decreased from 5 h to 1 h on average (Table 3), the total time (scanning time of IPS plus reading time of CE reader) spent on average in each CE study did not decrease significantly when compared with that of HSR. But with the development of the CE technology, when the IPS is installed in the image-receiver and the images can be analyzed directly just as the Personal Digital Assistant (PDA) does[29-31], the total time can be decreased.
As the images were taken by CE at random, CE could not control the azimuth angles and the luminosity as efficiently as the gastroscope or the colonoscope. As a result, the contrast between the diseased mucosa and the normal mucosa in some images was incorrectly changed. In the screened images, the great majority of the normal images (> 90%) were misdiagnosed (false positive) and some of the images for the lesions were missed out by IPS; therefore, the specificity, accuracy, and predictive value for the positivity were very low for most of the lesions (Table 5). Another problem of IPS was the deficiency of self-adjustment of the borderline between the diseased mucosa and the normal mucosa, which resulted in the fact that only part of the lesions in the image could be marked out (Figure 1F) or part of the normal mucosa was mistakenly marked out (Figure 2F). These were the reasons why the readers would scan the excluded images if they thought that the results obtained from IPS did not accord with the clinical manifestations of the patients given by the CE examination.
For the rarely-encountered lesions by CE, there was still a lack of an ideal discriminating algorithm. These lesions were not the main indications for the use of CE if much more time was consumed, the screening procession was prolonged and the clinical significance was lost. So, we did not develop any special algorithm aimed at those lesions. Another kind of the lesions, i.e., angioectasia, was difficult to be directly recognized because of the deficiency of characteristic colors and contours unless the lesions were accompanied by bleeding (Figure 2F). The proper algorithms have still been under development.
In conclusion, the result of this pilot study has indicated that the computer-aided screening diagnosis can be used as an efficient auxiliary measurement for the commonly-encountered enteric lesions when the images are taken by CE. We suggest that a further research should focus on improvement of the specificity rate and the screening rate for the rarely-encountered enteric lesions. If the robotistic and controllable CE[32] is used and more images with a better contrast are provided, the screening rate can be significantly enhanced.
COMMENTS
Background
Capsule endoscopy (CE), which is virtually a microcamera, is a revolutionary diagnostic tool in diagnosing small bowel diseases, and CE can obtain 40-60 thousand images of the GI tract, though the number of the images for the lesions is smaller than 500 in most of the patients. The CE reader still has to scan ten thousands of the images one by one because the reader cannot make sure which images the lesions are in. So, it may be a big burden on the CE reader’s eyes and energy.
Research frontiers
In order to decrease the reading time, the high-speed reading (HSR) is the commonly-used method at present, the reading time can be decreased to 0.5-1 h for one reader, but the work intensity is much greater and some lesions may be missed.
Innovations and breakthroughs
In this pilot study, a kind of the image-processed software (IPS) aided by a computer was introduced to screen the large number of normal images, and only 10%-15% of the original images were left behind, of which most of the commonly-encountered lesions in the small intestines were preserved and diagnosed; therefore, the workload and the working time of the CE reader could be decreased significantly.
Applications
Though a large number of the normal images were excluded, and the reading time decreased significantly, the total time (scanning time of IPS plus reading time of a CE reader) spent on average in each CE study did not significantly decrease compared with that of HSR at present. But with the development of the CE technology and improvement of algorithms, when IPS is installed in the image-receiver and the images can be analyzed directly just as the Personal Digital Assistant (PDA) does, the total time can be decreased significantly.
Terminology
IPS is a kind of software installed in the computer workstation, which can identify the common lesions in the small intestines based on the characteristic colors and contours of the lesions. HSR system is a kind of software support system installed in the computer workstation, which can speed up the reading of the CE images.
Peer review
This article raises an interesting topic. Authors investigate and evaluate the feasibility of the computer-aided screening diagnosis for enteric lesions in the CE.
Acknowledgments
We thank Dr. Cheng-Wei Tang and Dr. Bing Hu for their technical assistance and Mrs. Ming-Hui Huang for her help in collecting image samples of the capsule endoscopy.
Footnotes
Supported by A Grant offered by West China Hospital, Sichuan University, No. 2007SZ018
Peer reviewer: Zvi Fireman, Professor, Department of Gastroenterology, Hillel-Yaffe Medical Center, PO Box 169, Gastroenterology Department, Hadera 38100, Israel
S- Editor Li DL L- Editor Ma JY E- Editor Ma WH
References
- 1.Meron GD. The development of the swallowable video capsule (M2A) Gastrointest Endosc. 2000;52:817–819. doi: 10.1067/mge.2000.110204. [DOI] [PubMed] [Google Scholar]
- 2.Swain P. Wireless capsule endoscopy. Gut. 2003;52 Suppl 4:iv48–iv50. doi: 10.1136/gut.52.suppl_4.iv48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzarolo S, Brady P. Small bowel capsule endoscopy: a systematic review. South Med J. 2007;100:274–280. doi: 10.1097/SMJ.0b013e31802fa05a. [DOI] [PubMed] [Google Scholar]
- 4.Seibel EJ, Carroll RE, Dominitz JA, Johnston RS, Melville CD, Lee CM, Seitz SM, Kimmey MB. Tethered capsule endoscopy, a low-cost and high-performance alternative technology for the screening of esophageal cancer and Barrett’s esophagus. IEEE Trans Biomed Eng. 2008;55:1032–1042. doi: 10.1109/TBME.2008.915680. [DOI] [PubMed] [Google Scholar]
- 5.Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 6.Jensen TM, Vilmann P, Hendel JW. [Double-balloon endoscopy for diagnosis and treatment of small bowel diseases. The first Danish experiences with 31 patients] Ugeskr Laeger. 2008;170:433–437. [PubMed] [Google Scholar]
- 7.Sidhu R, McAlindon ME, Kapur K, Hurlstone DP, Wheeldon MC, Sanders DS. Push enteroscopy in the era of capsule endoscopy. J Clin Gastroenterol. 2008;42:54–58. doi: 10.1097/01.mcg.0000225655.85060.74. [DOI] [PubMed] [Google Scholar]
- 8.Rondonotti E, Villa F, Mulder CJ, Jacobs MA, de Franchis R. Small bowel capsule endoscopy in 2007: indications, risks and limitations. World J Gastroenterol. 2007;13:6140–6149. doi: 10.3748/wjg.v13.i46.6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rondonotti E, Herrerias JM, Pennazio M, Caunedo A, Mascarenhas-Saraiva M, de Franchis R. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc. 2005;62:712–716; quiz 752, 754. doi: 10.1016/j.gie.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Marmo R, Rotondano G, Piscopo R, Bianco MA, Cipolletta L. Meta-analysis: capsule enteroscopy vs. conventional modalities in diagnosis of small bowel diseases. Aliment Pharmacol Ther. 2005;22:595–604. doi: 10.1111/j.1365-2036.2005.02625.x. [DOI] [PubMed] [Google Scholar]
- 11.Yagi Y, Vu H, Echigo T, Sagawa R, Yagi K, Shiba M, Higuchi K, Arakawa T. A diagnosis support system for capsule endoscopy. Inflammopharmacology. 2007;15:78–83. doi: 10.1007/s10787-006-0010-5. [DOI] [PubMed] [Google Scholar]
- 12.Levinthal GN, Burke CA, Santisi JM. The accuracy of an endoscopy nurse in interpreting capsule endoscopy. Am J Gastroenterol. 2003;98:2669–2671. doi: 10.1111/j.1572-0241.2003.08726.x. [DOI] [PubMed] [Google Scholar]
- 13.Lai LH, Wong GL, Chow DK, Lau JY, Sung JJ, Leung WK. Inter-observer variations on interpretation of capsule endoscopies. Eur J Gastroenterol Hepatol. 2006;18:283–286. doi: 10.1097/00042737-200603000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Gurudu SR, Vargas HE, Leighton JA. New frontiers in small-bowel imaging: the expanding technology of capsule endoscopy and its impact in clinical gastroenterology. Rev Gastroenterol Disord. 2008;8:1–14. [PubMed] [Google Scholar]
- 15.Gan T, Rao N. [Study on the computer-assisted real-time diagnosis for micro-focus of esophagus based on the change of region-gradation] Shengwu Yixue Gongchengxue Zazhi. 2007;24:756–759. [PubMed] [Google Scholar]
- 16.Pennazio M. Diagnosis of small-bowel diseases in the era of capsule endoscopy. Expert Rev Med Devices. 2005;2:587–598. doi: 10.1586/17434440.2.5.587. [DOI] [PubMed] [Google Scholar]
- 17.Ersoy O, Sivri B, Arslan S, Batman F, Bayraktar Y. How much helpful is the capsule endoscopy for the diagnosis of small bowel lesions? World J Gastroenterol. 2006;12:3906–3910. doi: 10.3748/wjg.v12.i24.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fireman Z, Kopelman Y. New frontiers in capsule endoscopy. J Gastroenterol Hepatol. 2007;22:1174–1177. doi: 10.1111/j.1440-1746.2007.04993.x. [DOI] [PubMed] [Google Scholar]
- 19.Leighton JA, Sharma VK, Hentz JG, Musil D, Malikowski MJ, McWane TL, Fleischer DE. Capsule endoscopy versus push enteroscopy for evaluation of obscure gastrointestinal bleeding with 1-year outcomes. Dig Dis Sci. 2006;51:891–899. doi: 10.1007/s10620-006-9350-7. [DOI] [PubMed] [Google Scholar]
- 20.Ohmiya N, Yano T, Yamamoto H, Arakawa D, Nakamura M, Honda W, Itoh A, Hirooka Y, Niwa Y, Maeda O, et al. Diagnosis and treatment of obscure GI bleeding at double balloon endoscopy. Gastrointest Endosc. 2007;66:S72–S77. doi: 10.1016/j.gie.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 21.Leighton JA, Triester SL, Sharma VK. Capsule endoscopy: a meta-analysis for use with obscure gastrointestinal bleeding and Crohn’s disease. Gastrointest Endosc Clin N Am. 2006;16:229–250. doi: 10.1016/j.giec.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Saurin JC. Capsule endoscopy. Endoscopy. 2007;39:986–991. doi: 10.1055/s-2007-966925. [DOI] [PubMed] [Google Scholar]
- 23.Pennazio M. Crohn’s disease: diagnostic and therapeutic potential of modern small-bowel endoscopy. Gastrointest Endosc. 2007;66:S91–S93. doi: 10.1016/j.gie.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 24.Pruteanu-Malinici I, Carin L. Infinite hidden Markov models for unusual-event detection in video. IEEE Trans Image Process. 2008;17:811–822. doi: 10.1109/TIP.2008.919359. [DOI] [PubMed] [Google Scholar]
- 25.Bali N, Mohammad-Djafari A. Bayesian approach with hidden Markov modeling and mean field approximation for hyperspectral data analysis. IEEE Trans Image Process. 2008;17:217–225. doi: 10.1109/TIP.2007.914227. [DOI] [PubMed] [Google Scholar]
- 26.La Cara GE, Ursino M. A model of contour extraction including multiple scales, flexible inhibition and attention. Neural Netw. 2008;21:759–773. doi: 10.1016/j.neunet.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Plagianakos VP, Magoulas GD, Vrahatis MN. Distributed computing methodology for training neural networks in an image-guided diagnostic application. Comput Methods Programs Biomed. 2006;81:228–235. doi: 10.1016/j.cmpb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Das A, Ben-Menachem T, Farooq FT, Cooper GS, Chak A, Sivak MV Jr, Wong RC. Artificial neural network as a predictive instrument in patients with acute nonvariceal upper gastrointestinal hemorrhage. Gastroenterology. 2008;134:65–74. doi: 10.1053/j.gastro.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 29.De Ville K. “The cure is in hand”? The brave new world of handheld computers in medicine. Camb Q Healthc Ethics. 2008;17:385–400. doi: 10.1017/S0963180108080523. [DOI] [PubMed] [Google Scholar]
- 30.Blaya JA, Gomez W, Rodriguez P, Fraser H. Cost and implementation analysis of a personal digital assistant system for laboratory data collection. Int J Tuberc Lung Dis. 2008;12:921–927. [PubMed] [Google Scholar]
- 31.Forjuoh SN, Reis MD, Couchman GR, Ory MG. Improving diabetes self-care with a PDA in ambulatory care. Telemed J E Health. 2008;14:273–279. doi: 10.1089/tmj.2007.0053. [DOI] [PubMed] [Google Scholar]
- 32.Park S, Park H, Park S, Jee C, Kim J, Kim B. Capsular locomotive microrobot for gastrointestinal tract. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2211–2214. doi: 10.1109/IEMBS.2006.259668. [DOI] [PubMed] [Google Scholar]