Abstract
AIM: To develop a simplified and quick protocol to induce cirrhosis and standardize models of partial liver resection in rats.
METHODS: In Fischer F344 rats two modified protocols of phenobarbital-carbon tetrachloride (CCl4) (dilution 50%) gavage to induce cirrhosis (frequency adjusted according to weight, but each subsequent dose was systematically administered) were tested, i.e. the rapid and slow protocols. Prothrombin time (PT) and total bilirubin (TB) were also evaluated. Animals from the rapid group underwent 15% hepatectomy and animals from the slow group underwent 70% hepatectomy.
RESULTS: Rapid protocol: This corresponded to 1 gavage/4 d over 6 wk (mortality 30%). Mean PT was 35.2 ± 2.8 s (normal: 14.5 s), and mean TB was 1.8 ± 0.2 mg/dL (normal: 0.1 mg/dL). Slow protocol: This corresponded to 1 gavage/6 d over 9 wk (mortality 10%). Mean PT was 11.8 ± 0.2 s (normal: 14.5 s), and mean TB was 0.4 ± 0.04 mg/dL (normal: 0.1 mg/dL). Pathological analyses were performed in both protocols which showed persistent cirrhosis at 3 mo. Rat mortality in the rapid gavage group who underwent 15% hepatectomy and in the slow gavage group who underwent 70% hepatectomy was 50% and 70%, respectively.
CONCLUSION: Our modified model is a simplified method to induce cirrhosis which is rapid (6 to 9 wk), efficient and stable up to 3 mo. Using this method, “Child Pugh A” or “Child Pugh BC” cirrhotic rats were obtained. Our models of cirrhosis and hepatectomy can be used in various situations focusing on postoperative survival.
Keywords: Carbon tetrachloride, Cell therapy, Hepatectomy, Liver cirrhosis, Liver failure acute, Mortality, Surgery
INTRODUCTION
There is currently a worldwide epidemic of chronic liver disease (chronic viral hepatitis B and C, and hepatopathy associated with obesity via the non alcoholic steatohepatitis (NASH) syndrome. At the same time, the number of patients presenting with hepatocellular carcinoma and/or persistent end-stage hepatic function alteration have increased. These two complications have led to more frequent discussions on hepatic resection and transplantation. However, the scope for both these solutions is limited by postoperative mortality and the constantly growing shortage of donors.
This situation led physicians and surgeons to propose new therapeutic solutions[1]. These solutions include: (1) Reduced-sized orthotopic liver graft[2,3], hepatic transplantation with living donor[4] and xenogenic transplantation[5,6]; (2) Cellular therapy by transplantation of hepatocytes into the liver, spleen and peritoneum (isolated or encapsulated)[7,8] (autologous, allogeneic, xenogenic, free or encapsulated, genetically modified ex vivo)[9].
It is thus necessary to have animal models of cirrhosis, which make it possible to obtain compensated (equivalent to Child Pugh A) or decompensated cirrhosis (equivalent to Child Pugh BC). At best, liver cirrhosis must be rapidly obtainable, and durable. It is also necessary to have standardized models of partial hepatic resection in these cirrhotic animals with reproducible mortality.
The most validated cirrhosis model in the rat is the carbon tetrachloride (CCl4) (induced by phenobarbital) cirrhosis model[10]. Many protocols exist, which differ in the route of administration (gavage, intraperitoneal or subcutaneous injection), the dilution of CCl4 (1/20 to 1/10), the frequency (1 to 4 wk) and the duration (8 to 28 wk) of CCl4 administration. The efficiency of these protocols (70% to 100%), as well as the inherent toxic-mortality (20% to 90%) is variable in the literature[11]. The study in which the CCl4 protocol for cirrhosis is best described is that of Kobayashi et al[12,13]. Here CCl4 was given by gavage twice weekly and diluted to 10%. The amount of CCl4 was decreased if body weight remained constant, and not administered if the weight decreased. The study was carried out over a 28-wk period. The animals were studied only when evidence of liver failure did not improve after CCl4 was withheld for 4 wk. However, this reference protocol is too restrictive particularly for pilot studies[14].
The aim of this study was to establish a restrictive CCl4 cirrhosis model which resulted in “Child Pugh A” or “Child Pugh BC” cirrhotic rats, which could be carried out easily, quickly and in a reproducible and durable way by modulating the dilution of CCl4 and the duration and frequency of gavage. In addition, we tested different types of hepatectomy in terms of the resected volume (“minor or major”) to obtain reproducible postoperative mortality.
MATERIALS AND METHODS
Animals
Male Fischer F344 rats (Iffa Credo, France) weighing 150-180 g were used. The rats were given free access to standard laboratory food and water throughout the experiments. Rats were acclimatized to our laboratory conditions for 7 d. All the procedures performed on the animals were in accordance with the European Committee on the Use and Care of Animals.
Induction of phenobarbital-carbon tetrachloride-induced liver cirrhosis
Rats were given phenobarbital (lyophilized 200 mg, Rhône-Poulenc Rorer, France) in their drinking water at a concentration of 0.5 g/L throughout the establishment of liver cirrhosis. Two weeks later, CCl4 (diluted 50% in olive oil) was given intragastrically using a gavage needle at different intervals determined during preliminary experiments.
Rat weight was approximately 200 g at the time of first gavage. The initial dose was 0.08 mL (0.20 mL/kg of CCl4). Each subsequent dose was systematically administered (even if weight remained stable or decreased) but adjusted based on changes in body weight: from 150 to 220 g, 0.06 to 0.09 mL (0.20 mL/kg of CCl4); from 230 to 270 g, 0.12 to 0.14 mL (0.25 mL/kg); from 280 to 300 g, 0.17 to 0.18 mL (0.30 mL/kg); from 310 to 330 g, 0.22 to 0.23 mL (0.35 mL/kg); from 340 to 360 g, 0.27 to 0.29 mL (0.40 mL/kg); from 370 to 390 g, 0.33 to 0.35 mL (0.45 mL/kg). Various gavage protocols (frequency, duration of gavage, dose adjustment, mortality during gavage) were tested leading to the selection of two protocols (rapid protocol, slow protocol).
Once these two protocols (rapid and slow) were selected, 3 groups of 10 animals for each protocol were used as test groups to affirm that these protocols were reproducible in terms of mortality related to gavage, gravity of cirrhosis (ascites, splenomegaly, portal hypertension, biology, pathology) and persistence of the histological lesions with time.
Biology
Prothrombin time (PT) and total bilirubin (TB) were assessed in 5 rats in the rapid and slow protocol groups 3 wk after gavage interruption.
Histological examination
Representative liver blocks, obtained when the rats were sacrificed, were prepared to establish the gavage protocols or after partial hepatectomy. After routine paraffin wax processing, hematoxylin eosin and Masson’s trichromie stained sections were obtained and the presence of cirrhosis was assessed under light microscopy. Pathological analyses were performed in rats 3 wk after gavage interruption (T0) and at regular intervals (2 wk, 5, 9, 13, 18 s).
Anesthesia
Partial hepatectomy was performed under ether inhalation anesthesia. The day before intervention, the animals were fasted for 12 h prior to surgery. Postoperatively, animals received glucose supplementation (immediate postoperative subcutaneous glucose 30%, and glucose 10% added to drinking water during the first 24 h) and animals were heated during the first 24 h.
Hepatectomy
Hepatectomy was performed in cirrhotic rats 3 wk after gavage interruption. The extent of hepatectomy was adjusted by excising various combinations of liver lobes that are known to have a predictable volume[15] according to the nature of the protocol and the observed postoperative mortality: 15% hepatectomy (epiploïc lobes, i.e. minor hepatectomy) and 70% hepatectomy (left lateral and median lobes, i.e. major hepatectomy) were chosen after preliminary experiments.
Two groups of 10 animals from the rapid group underwent 15% hepatectomy and two groups of 10 animals from the slow group underwent 70% hepatectomy. The postoperative day 1 mortality was noted. Pathologic examination of the resected specimen was carried out systematically.
RESULTS
Rapid and slow protocol gavage mortality
Rapid protocol: This corresponded to 1 gavage/4 d over 42 d (6 wk). Mortality in the rapid protocol was 30%, 30% and 40% (mean: 30%), from the first gavage to 3 wk after gavage interruption, respectively. In the surviving rats, 3 wk after gavage interruption, at the time of sacrifice, there were macroscopic micro nodular cirrhosis aspects in all animals, and ascites, splenomegaly and portal hypertension signs (at the level of the stomach) in 85%, 65% and 60% of animals, respectively.
Slow protocol: This corresponded to 1 gavage/6 d over 63 d (9 wk). Mortality in the rapid protocol was 10%, 20% and 10% (mean: 10%), from the first gavage to 3 wk after gavage interruption, respectively. In the surviving rats, 3 wk after gavage interruption, at the time of sacrifice, there were macroscopic micro nodular cirrhosis aspects in all animals, and ascites, splenomegaly and portal hypertension signs (at the level of the stomach) in 5%, 5% and 0% of animals, respectively.
Biology
Rapid protocol: In the surviving rats, 3 wk after gavage interruption, mean PT was 35.2 ± 2.8 s (normal: 14.5 s), and mean bilirubin was 1.8 ± 0.2 mg/dL (normal: 0.1 mg/dL) (n = 5).
Slow protocol: In the surviving rats, 3 wk after gavage interruption, mean PT was 11.8 ± 0.2 s (normal: 14.5 s), and mean bilirubin was 0.4 ± 0.04 mg/dL (normal: 0.1 mg/dL) (n = 5).
Pathological assessment
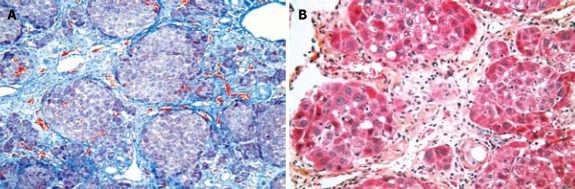
After both the rapid and slow protocols, all surviving rats presented with cirrhosis (T0 = 3 wk after gavage interruption) with extensive mutilating fibrosis and nodules (Figure 1). The cirrhosis aspects persisted on analyses performed at regular intervals (2 wk, 5, 9, 13, 18 s).
Figure 1.
Rat liver after CCl4 gavage. A: Rat liver [9 wk after T0 (12 wk after CCl4 gavage interruption)] extensive mutilating fibrosis, with nodules (cirrhosis). In the nodules separated by fibrous septa, hepatocytes with a basophil cytoplasm and a round nucleus are organized in fine spans (Masson's trichromie, × 10); B: Rat liver [6 wk after T0 (9 wk after CCl4 gavage interruption)] extensive mutilating fibrosis, with nodules (cirrhosis) (HE, × 10).
Mortality after partial hepatectomy
Mortality in the two groups of 10 animals from the rapid gavage group, who underwent 15% hepatectomy, was 50% at postoperative day 1. Mortality in the two groups of 10 animals from the slow gavage group, who underwent 70% hepatectomy, was 70% at postoperative day 1.
DISCUSSION
CCl4 is widely used to induce experimental liver damage. The data from this study showed that our modified model is a simplified and improved method for inducing cirrhosis which was rapid (6 to 9 wk), efficient, stable up to 3 mo and which resulted in “Child Pugh A” or “Child Pugh BC” cirrhotic rats. Fifteen percent hepatectomy (“minor hepatectomy”) in decompensated cirrhotic animals and 70% hepatectomy (“major hepatectomy”) in compensated cirrhotic animals resulted in a postoperative mortality of 50% and 70%, respectively. These reproducible and high postoperative mortality rates can be used in protocols devoted to cellular therapy in chronic liver diseases[1,8]. Moreover, different series have studied the biological[16,17] and hemodynamic[18-20] variations induced by liver cirrhosis. Other authors have investigated various treatments in rats with liver cirrhosis[21-24].
In the Kobayashi et al[12,13], CCl4 reference induced cirrhosis model, animals were studied only when evidence of liver failure did not improve after CCl4 was withheld for 4 wk, following 28 wk of gavage. Overall, the length of the protocol was 8 mo. However, this protocol ensured the induction of cirrhosis which was slow, stable and very close to cirrhosis found in clinical practice. The main pitfall of this protocol was its length which is not compatible with small “pilot” or “preliminary” studies. In our study, serum albumin level, serum ammonia level and degree of encephalopathy (coma scale), are lacking, however, persistent histological alteration at 3 mo is, in our view, a major argument for the use of this method. To reinforce the pertinence of modified protocols for inducing cirrhosis in rats, at a recent congress, Quadrelli et al[25] presented a new approach to improve the model of cirrhotic liver induced by CCl4, which associated gavage and subcutaneous injection of CCl4. This new approach, associated with 45% mortality allowed cirrhosis induction in 60% of rats in a period of 9 wk.
We have found only 5 papers in the literature which reported mortality after hepatectomy (rational for the study of cirrhotic rats focusing on postoperative mortality) in various models of compensated “Child Pugh A” CCl4 induced cirrhosis in rats. One of these studies reported a postoperative mortality of 70% after 30% hepatectomy[26]. In the remaining four studies after 70% hepatectomy, a postoperative mortality of 15%, 20%, 25% and 40%, respectively was reported[27-30]. In this series, rat mortality after 70% hepatectomy in compensated “Child Pugh A” rats was higher, about 70% and could be explained by the more intensive gavage protocol used. However, the extent of the resection, i.e. 15% in decompensated cirrhotic animals, and 70% in compensated cirrhotic animals chosen in this series are compatible with minor and major hepatic resections performed in clinical practice in cirrhotic patients.
In conclusion, our modified model is a simplified method for inducing cirrhosis that is rapid (6 to 9 wk), efficient and stable up to 3 mo. It resulted in “Child Pugh A” or “Child Pugh BC” cirrhotic rats. Our models of cirrhosis and hepatectomy can be used in various situations focusing on postoperative survival.
COMMENTS
Background
It is necessary to have animal models of compensated (equivalent to Child Pugh A) and decompensated cirrhosis (equivalent to Child Pugh BC) for various studies. At best, cirrhosis must be rapidly obtainable and durable. The most validated cirrhosis model in the rat is the carbon tetrachloride (CCl4) (induced by phenobarbital) cirrhosis model. Many protocols exist, which differ in the route of administration (gavage, intraperitoneal, subcutaneous), the dilution of CCl4, and in the frequency and the duration of CCl4 administration. The efficiency of these protocols (70% to 100%), as well as the inherent toxic-mortality (20% to 90%) is variable in the literature.
Research frontiers
The establishment of an efficient and quick model of cirrhosis in rat with an acceptable rate of mortality has been the topic of several investigations in recent years.
Innovations and breakthroughs
This paper has reported a reproducible, rapid (6 to 9 wk), efficient and stable up to 3 mo model of liver cirrhosis, which resulted in “Child Pugh A” or “Child Pugh BC” cirrhotic rats.
Applications
Based on the results of this work, it is possible to standardize models of partial hepatic resection in cirrhotic rats with reproducible mortality. This rapid model is compatible with small “pilot” or “preliminary” studies. It can be used in protocols devoted to cellular therapy in chronic liver diseases, and in biological and hemodynamic variations induced by liver cirrhosis.
Terminology
The extent of hepatectomy was adjusted by excising various combinations of liver lobes that are known to have a predictable volume according to the nature of the protocol and the observed postoperative mortality: 15% hepatectomy (epiploïc lobes, i.e. minor hepatectomy) and 70% hepatectomy (left lateral and median lobes, i.e. major hepatectomy) were chosen after preliminary experiments.
Peer review
The data presented by the authors supports the possibility of a simplified method for inducing cirrhosis that is rapid, efficient and stable. The topic is very interesting because the model of "Child Pugh A" or "Child Pugh BC" cirrhosis and hepatectomy can be used in various situations focusing on postoperative survival.
Acknowledgments
We thank Ms Fon and Ms Boudet for their precious help during the gavage protocols.
Footnotes
Peer reviewer: Hiroshi Yoshida, MD, First Department of Surgery, Nippon Medical School, 1-1-5 Sendagi, Bunkyo-ku, Tokyo 113-8603, Japan
S- Editor Li DL L- Editor Webster JR E- Editor Ma WH
References
- 1.Regimbeau JM, Mallet VO, Bralet MP, Gilgenkrantz H, Houssin D, Soubrane O. [Transplantation of isolated hepatocytes. Principles, mechanisms, animal models, clinical results] Gastroenterol Clin Biol. 2002;26:591–601. [PubMed] [Google Scholar]
- 2.Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery. 1984;95:367–370. [PubMed] [Google Scholar]
- 3.Soubrane O, Houssin D. [All out search of graft for liver transplantation] Gastroenterol Clin Biol. 1993;17:845–850. [PubMed] [Google Scholar]
- 4.Raia S, Nery JR, Mies S. Liver transplantation from live donors. Lancet. 1989;2:497. doi: 10.1016/s0140-6736(89)92101-6. [DOI] [PubMed] [Google Scholar]
- 5.White SA, Nicholson ML. Xenotransplantation. Br J Surg. 1999;86:1499–1514. doi: 10.1046/j.1365-2168.1999.01340.x. [DOI] [PubMed] [Google Scholar]
- 6.Malassagne B, Regimbeau JM, Taboit F, Troalen F, Chereau C, Moire N, Attal J, Batteux F, Conti F, Calmus Y, et al. Hypodermin A, a new inhibitor of human complement for the prevention of xenogeneic hyperacute rejection. Xenotransplantation. 2003;10:267–277. doi: 10.1034/j.1399-3089.2003.02030.x. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Hodgson HJ. Transplantation of isolated hepatocytes. Indian J Gastroenterol. 1985;4:97–100. [PubMed] [Google Scholar]
- 8.Ochenashko OV, Volkova NA, Mazur SP, Somov AY, Fuller BJ, Petrenko AY. Cryopreserved fetal liver cell transplants support the chronic failing liver in rats with CCl4-induced cirrhosis. Cell Transplant. 2006;15:23–33. doi: 10.3727/000000006783982232. [DOI] [PubMed] [Google Scholar]
- 9.Panis Y, Cardoso J, Houssin D. [Gene therapy in Hepatology. Experimental results and clinical perspectives] Gastroenterol Clin Biol. 1994;18:262–276. [PubMed] [Google Scholar]
- 10.Proctor E, Chatamra K. Standardized micronodular cirrhosis in the rat. Eur Surg Res. 1984;16:182–186. doi: 10.1159/000128407. [DOI] [PubMed] [Google Scholar]
- 11.Mullen KD, McCullough AJ. Problems with animal models of chronic liver disease: suggestions for improvement in standardization. Hepatology. 1989;9:500–503. doi: 10.1002/hep.1840090326. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi N, Ito M, Nakamura J, Cai J, Hammel JM, Fox IJ. Treatment of carbon tetrachloride and phenobarbital-induced chronic liver failure with intrasplenic hepatocyte transplantation. Cell Transplant. 2000;9:671–673. doi: 10.1177/096368970000900512. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi N, Ito M, Nakamura J, Cai J, Gao C, Hammel JM, Fox IJ. Hepatocyte transplantation in rats with decompensated cirrhosis. Hepatology. 2000;31:851–857. doi: 10.1053/he.2000.5636. [DOI] [PubMed] [Google Scholar]
- 14.Rivera-Huizar S, Rincon-Sanchez AR, Covarrubias-Pinedo A, Islas-Carbajal MC, Gabriel-Ortiz G, Pedraza-Chaverri J, Alvarez-Rodriguez A, Meza-Garcia E, Armendariz-Borunda J. Renal dysfunction as a consequence of acute liver damage by bile duct ligation in cirrhotic rats. Exp Toxicol Pathol. 2006;58:185–195. doi: 10.1016/j.etp.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Madrahimov N, Dirsch O, Broelsch C, Dahmen U. Marginal hepatectomy in the rat: from anatomy to surgery. Ann Surg. 2006;244:89–98. doi: 10.1097/01.sla.0000218093.12408.0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borkham-Kamphorst E, Kovalenko E, van Roeyen CR, Gassler N, Bomble M, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. Platelet-derived growth factor isoform expression in carbon tetrachloride-induced chronic liver injury. Lab Invest. 2008;88:1090–1100. doi: 10.1038/labinvest.2008.71. [DOI] [PubMed] [Google Scholar]
- 17.Lavina B, Gracia-Sancho J, Rodriguez-Vilarrupla A, Chu Y, Heistad DD, Bosch J, Garcia-Pagan JC. Superoxide dismutase gene transfer reduces portal pressure in ccl4 cirrhotic rats with portal hypertension. Gut. 200;58:118–125. doi: 10.1136/gut.2008.149880. [DOI] [PubMed] [Google Scholar]
- 18.Cardenas A, Lowe R, Oh S, Bodkin S, Kenney T, Lamorte WW, Afdhal NH. Hemodynamic effects of substance P and its receptor antagonist RP67580 in anesthetized rats with carbon tetrachloride-induced cirrhosis. Scand J Gastroenterol. 2008;43:328–333. doi: 10.1080/00365520701685691. [DOI] [PubMed] [Google Scholar]
- 19.Maksan SM, Ryschich E, Ulger Z, Gebhard MM, Schmidt J. Disturbance of hepatic and intestinal microcirculation in experimental liver cirrhosis. World J Gastroenterol. 2005;11:846–849. doi: 10.3748/wjg.v11.i6.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsugawa K, Hashizume M, Migou S, Kishihara F, Kawanaka H, Tomikawa M, Tanoue K, Sugimachi K. Role of nitric oxide and endothelin-1 in a portal hypertensive rat model. Scand J Gastroenterol. 2000;35:1097–1105. doi: 10.1080/003655200451243. [DOI] [PubMed] [Google Scholar]
- 21.Jang JH, Kang KJ, Kim YH, Kang YN, Lee IS. Reevaluation of experimental model of hepatic fibrosis induced by hepatotoxic drugs: an easy, applicable, and reproducible model. Transplant Proc. 2008;40:2700–2703. doi: 10.1016/j.transproceed.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 22.Fang HL, Lai JT, Lin WC. Inhibitory effect of olive oil on fibrosis induced by carbon tetrachloride in rat liver. Clin Nutr. 2008;27:900–907. doi: 10.1016/j.clnu.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Perez R, Garcia-Fernandez M, Diaz-Sanchez M, Puche JE, Delgado G, Conchillo M, Muntane J, Castilla-Cortazar I. Mitochondrial protection by low doses of insulin-like growth factor- I in experimental cirrhosis. World J Gastroenterol. 2008;14:2731–2739. doi: 10.3748/wjg.14.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan LP, Chen FH, Ling L, Bo H, Chen ZW, Li F, Zhong MM, Xia LJ. Protective effects of total flavonoids of Bidens bipinnata L. against carbon tetrachloride-induced liver fibrosis in rats. J Pharm Pharmacol. 2008;60:1393–1402. doi: 10.1211/jpp/60.10.0016. [DOI] [PubMed] [Google Scholar]
- 25.Quadrelli L, Secchi MA, Consagra MF, Rossi L, Peralta E, Muniagurria C, Gabriele M, Figallo G. New approach to improved model of cirrhotic liver induced by carbon tetrachloride. HPB. 2005;7:35. [Google Scholar]
- 26.Kaido T, Seto S, Yamaoka S, Yoshikawa A, Imamura M. Perioperative continuous hepatocyte growth factor supply prevents postoperative liver failure in rats with liver cirrhosis. J Surg Res. 1998;74:173–178. doi: 10.1006/jsre.1997.5243. [DOI] [PubMed] [Google Scholar]
- 27.MacIntosh E, Gauthier T, Pettigrew N, Minuk G. Liver regeneration and the effect of exogenous putrescine on regenerative activity after partial hepatectomy in cirrhotic rats. Hepatology. 1992;16:1428–1433. doi: 10.1002/hep.1840160620. [DOI] [PubMed] [Google Scholar]
- 28.Hwang TL, Yu HC, Chen PC, Chen MF. Liver regeneration following partial hepatectomy and stimulation by hepatic stimulatory substance in cirrhotic and non-cirrhotic rats. Res Exp Med (Berl) 1995;195:201–208. doi: 10.1007/BF02576789. [DOI] [PubMed] [Google Scholar]
- 29.Moser M, Zhang M, Gong Y, Johnson J, Kneteman N, Minuk GY. Effect of preoperative interventions on outcome following liver resection in a rat model of cirrhosis. J Hepatol. 2000;32:287–292. doi: 10.1016/s0168-8278(00)80074-4. [DOI] [PubMed] [Google Scholar]
- 30.Andiran F, Ayhan A, Tanyel FC, Abbasoglu O, Sayek I. Regenerative capacities of normal and cirrhotic livers following 70% hepatectomy in rats and the effect of alpha-tocopherol on cirrhotic regeneration. J Surg Res. 2000;89:184–188. doi: 10.1006/jsre.2000.5825. [DOI] [PubMed] [Google Scholar]