Abstract
AIM: To investigate the effects of fluoxetine on depression-induced changes of mast cell morphology and protease-1 (rMCP-1) expression in rats.
METHODS: A Sprague-Dawley rat model of chronic stress-induced depression was established. Fifty experimental rats were randomly divided into the following groups: normal control group, fluoxetine + normal control group, depressed model group, saline + depressed model group, and fluoxetine + depressed model group. Laser scanning confocal microscopy (LSCM) immunofluorescence and RT-PCR techniques were used to investigate rMCP-1 expression in gastric antrum. Mast cell morphology was observed under transmission electron microscopy. ANOVA was used for statistical analysis among groups.
RESULTS: Morphologic observation indicated that depression induced mast cell proliferation, activation, and granule hyperplasia. Compared with the normal control group, the average immunofluorescence intensity of gastric antrum rMCP-1 significantly increased in depressed model group (37.4 ± 7.7 vs 24.5 ± 5.6, P < 0.01) or saline + depressed model group (39.9 ± 5.0 vs 24.5 ± 5.6, P < 0.01), while there was no significant difference between fluoxetine + normal control group (23.1 ± 3.4) or fluoxetine + depressed model group (26.1 ± 3.6) and normal control group. The average level of rMCP-1mRNA of gastric antrum significantly increased in depressed model group (0.759 ± 0.357 vs 0.476 ± 0.029, P < 0.01) or saline + depressed model group (0.781 ± 0.451 vs 0.476 ± 0.029, P < 0.01 ), while no significant difference was found between fluoxetine + normal control group (0.460 ± 0.027) or fluoxetine + depressed model group (0.488 ± 0.030) and normal control group. Fluoxetine showed partial inhibitive effects on mast cell ultrastructural alterations and de-regulated rMCP-1 expression in gastric antrum of the depressed rat model.
CONCLUSION: Chronic stress can induce mast cell proliferation, activation, and granule hyperplasia in gastric antrum. Fluoxetine counteracts such changes in the depressed rat model.
Keywords: Depression model, Gastric antrum, Mast cell protease-1, Mast cells, Morphology, Fluoxetine hydrochloride
INTRODUCTION
Mast cells are now recognized as “granular cells of the connective tissue”, whose activation exacerbates allergic immune responses and as key players in the establishment of innate immunity as well as modulators of adaptive immune responses[1]. The role of mast cells in the gastrointestinal mucosa is not only to react to antigens, but also to actively regulate the barrier and transport properties of the intestinal epithelium. Mucosal mast cells respond to both IgE/antigen-dependent and non-IgE-dependent stimulation, releasing bioactive mediators into adjacent tissues where they induce physiological responses. Studies in models of hypersensitivity and stress have provided evidence that changes in mucosal function are due to either direct action of mast cell mediators on epithelial receptors and/or indirect action via nerves/neurotransmitters[2]. Intestinal anaphylaxis is associated with disturbances in gut function that are antigen-specific and dependent on mast cell degranulation. During mucosal immunoglobulin E-mediated reactions, rat mast cell protease II (rMCP-II) is released and is associated with ultrastructural changes in the intestinal mucosa. The systemic appearance of this specific protease provides a serum marker of intestinal anaphylaxis. Psychological stress may trigger this sensitive alarm system via the brain-gut axis[3]. In clinical studies, it has become clear that psychological factors, especially anxiety and depression, play an important role in gastrointestinal diseases by precipitating exacerbation of symptoms[4,5]. Several studies have shown that the prevalence of chronic stressed disorders in patients with gastrointestinal symptoms is 60%-85%[6,7]. Stress often worsens the symptoms of gastrointestinal diseases, which might be explained by altered neuroendocrine and visceral sensory responses to stress[8].
Fluoxetine hydrochloride (fluoxetine) is a kind of selective serotonin reuptake inhibitors (SSRIs), which belong to a class of antidepressants used in the treatment of depression and anxiety disorders. SSRIs increase the extracellular expression of the neurotransmitter serotonin by inhibiting its reuptake into the presynaptic cells. Studies have suggested that SSRIs may promote the growth of new neural pathways or neurogenesis[9]. SSRIs may also protect against neurotoxicity caused by other compounds as well as from depression itself. Recent studies showed that pro-inflammatory cytokine processes took place during depression in addition to somatic diseases and it was possible that symptoms manifested in these psychiatric illnesses were being attenuated by the pharmacological effects of antidepressants on the immune system[10] SSRIs have been found to be immunomodulatory and anti-inflammatory against pro-inflammatory cytokine processes[11,12].
The aim of this study is to investigate the effects of fluoxetine hydrochloride (fluoxetine) on mast cell morphology and rMCP-1 expression in gastric antrum in a rat model of depression.
MATERIALS AND METHODS
Animals
Fifty healthy male Sprague-Dawley rats, weighing 250 ± 300 g, from the Animal Center, Hubei Academy of Preventive Medical Sciences, were used in the present study. The animals were fed standard rat chow, allowed access to tap water and acclimatized to the surroundings for 1 wk prior to the experiments.
Reagents
Cy3-conjugated goat anti-rabbit IgG, rMCP-1 rabbit anti-mouse antibody were purchased from Sigma Co., USA. Fluoxitine hydrochloride capsule was purchased from Lilly Co. Ltd. Other reagents used in the study were all of analytical grade.
Experimental protocols
All procedures were approved by the Animal Care Committee at the Medical Department of Wuhan University. A rat model of chronic stress-induced depression was established[13,14]. The rats received a variety of stressors for 21 d, including tail nip for 1 min, cold water swimming at 4°C for 5 min, heat stress at 45°C for 5 min, water deprivation for 24 h, food deprivation for 24 h, 12-h inverted light/dark cycle (7:00 a.m. lights off and 7:00 p.m. lights on), paw electric shock (electric current 1.0 mA10 s, every 1 min, lasting 10 s, 30 times). The animals were randomly divided into five groups (10 rats per group): normal control, fluoxetine + normal control, depressed model control, saline + depressed model, and fluoxetine + depressed model. The depressed animals were treated with saline and fluoxetine (10 mg/kg), respectively. A normal control group of rats without receiving any stress was included and housed in a separate room; food and water were freely available in their home cage.
Immunofluorescence histochemistry
The rats were anesthetized with urethane (5 mg/kg ip.) and rapidly killed by decapitation. The gastric antrum samples (1 cm × 1 cm) were perfused with 4% paraformaldehyde for immunofluorescence histology from each group. Each sample was cut into 30 sections and each section was cut 50-μm thick using a vibratome. Serial sections were placed on slides, three to a slide. The sections were numbered from 1 to 30. Ten sections were incubated. The staining procedure was as follows: (1) the sections were washed in phosphate-buffered saline (PBS), then pretreated with 0.25% Triton X-100 for 30 min at 37°C and rinsed in PBS; (2) incubation for 12 h at 4°C in a 1:100 dilution of the primary antibody of rMCP-1 in PBS; and (3) incubation with 1:200 diluted secondary antibody (Cy3 -conjugated goat anti-rabbit IgG) in PBS for 1 h at 37°C.The sections were washed three times for 10 min after incubation steps 1 to 3, respectively, and were finally mounted in 50 g/L glycerin.
Detection was carried out according to the kit instructions (Leica SP2 TCS AOBS made in Germany). The specimens were excited with a laser beam at a wavelength of 492 nm (Cy3). The sections were observed under a laser scanning confocal microscope (LSCM) and analyzed with a Leica Q500IW image analysis system in terms of Cy3 fluorescent intensity.
Electron microscopic analysis
For electron microscopic analysis, gastric antrum tissue sections were fixed in modified Karnovsky's medium containing 2% paraformaldehyde, 3% glutaraldehyde and 0.1% tannic acid in 0.1 mmol/L phosphate buffer (pH 7.4) and processed as before[15]. Each electron microscopic sample was divided into 5 blocks. Each block was cut into 10 sections (200 μm thick). Five sections selected from 10 sections were observed. Ultrathin sections were placed onto copper gride, stained with uranyl acetate and lead citrate, and observed under a transmission electron microscope (Hitachi H-600, Japan). Mast cells were evaluated according to Letourneau[16]. Mast cells containing many intact electron-dense granules or containing empty granules were categorized as inactive and active cells, respectively. All mast cells were counted at magnification × 4000 in 30 visual fields.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
To quantify the expression of rMCP-1 RNA, we performed a RT-PCR assay as described previously[17]. Total mRNA was isolated with TRIZOL (Invitrogen) according to the instructions of manufacturer. The primers for RT-PCR were as follows: rMCP-1 (237 bp), forward primer 5′-GCCTGTAAAAACTATTTT-3′; reverse primer 5′-CAGGCTGGTCAGATCCTGC-3′. GAPDH (217bp), forward primer 5′-GAAACCTGCCAAGTATGATG-3′; reverse primer 5′-ACCAGGAAATGAGCTTGAGA-3′. The reaction mixture was added to the RNA solution and incubated at 42°C for 1 h, heated at 94°C for 5 min, and chilled at 48°C. For PCR, the cDNA reaction mixture was diluted with 40 ml of PCR buffer and mixed with 50 pmol of the primers. The reaction was carried out in a DNA thermal cycler under the following conditions: 94°C for 30 s, 58°C for 30 s and 72°C for 45 s. Following the reaction, the amplified products were analyzed by 1.5% agarose gel electrophoresis and visualized using ultravioletuorescence after staining with ethidium bromide. The relative content of rMCP-1 mRNA was calculated densitometrically based on the densitometric ratio between rMCP-1 and GAPDH.
Statistical analysis
Data were expressed as mean ± SE. Statistical analysis was performed using one-way ANOVA and the non-parametric Mann-Whitney U test between groups. P values less than 0.05 were considered statistically significant.
RESULTS
Immunofluorescence histochemical assay
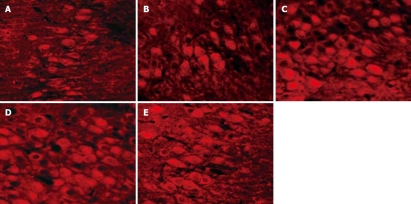
LSCM was used to prepare immunofluorescence picture, and LSCM imaging system was used to analyze the rMCP-1 immunofluorescence intensity among groups. Compared with the normal control group, the average immunofluorescence intensity of gastric antrum rMCP-1 significantly increased in depressed model group or saline + depressed model group (Figure 1, Table 1, P < 0.01), while there was no significant difference between fluoxetine + normal control group or fluoxetine + depressed model group and normal control group. Compared with depressed model group, the average immunofluorescence intensity of gastric antrum rMCP-1 significantly decreased in fluoxetine + depressed model group (Figure 1, Table 1, P < 0.01), while there was no significant difference between saline + depressed model group and depressed model group. This confirmed that chronic stress induced mast cells to secrete rMCP-1 and fluoxetine inhibited this effect.
Figure 1.
Gastric antrum tissue staining procedure include: (1) pretreatment with 0.25% Triton X-100; (2) incubation in the primary antibody of rMCP-1; (3) incubation with secondary antibody (Cy3 -conjugated goat anti-rabbit IgG). A: Expression of rMCP-1 in the normal control group; B: Expression of rMCP-1 in the fluoxetine + normal control group; C: Expression of rMCP-1 in the depressed model control group; D: Expression of rMCP-1 in the saline + depressed model group; E: Expression of rMCP-1 in the fluoxetine + depressed model group.
Table 1.
Average immunofluorescence intensity analysis of rMCP-1 alterations in gastric antrum of depressed rat model (n = 10, mean ± SE)
| Group | Fluorescence intensity of MCP-1 |
| Normal control | 24.8 ± 5.6 |
| Fluoxetine + control | 23.1 ± 3.4b |
| Depressed | 37.4 ± 7.7d |
| Saline + depressed | 39.9 ± 5.0d |
| Fluoxetine + depressed | 26.1 ± 3.6b |
rMCP-1: rat mast cell protease- 1.
P < 0.01 vs depressed model control group;
P < 0.01 vs normal control group.
Ultrastructural morphology analysis
Compared with the normal control rats, the total number of mast cells/30 visual fields and the percentage of activated mast cells increased significantly, while the percentage of normal mast cells decreased significantly in chronic stress-induced depressed rats or saline + depressed model rats (Table 2, P < 0.01). In depressed rats treated with fluoxetine, the changes in total number of mast cells/30 visual fields and the percentage of activated or normal mast cells were between normal control group and depressed model rats (Table 2, P < 0.05).
Table 2.
Ultrastructural morphologic changes of gastric tissue mast cells (n = 10, mean ± SE)
| Group | Mast cells/30 visual fields | Normal mast cells (%) | Activated mast cells (%) |
| Normal control | 24.0 ± 3.5 | 64.6 ± 9. 9 | 35.4 ± 3.7 |
| Fluoxetine + control | 22.2 ± 3.4c | 69.8 ± 6.1c | 30.2 ± 3.7c |
| Depressed | 40.6 ± 3.9b | 33.3 ± 4.7b | 66.7 ± 4.9b |
| Saline + depressed | 42.2 ± 3.7b | 30.4 ± 3.7b | 69.6 ± 4.7b |
| Fluoxetine + depressed | 28.1 ± 3.3ad | 44.4 ± 5.6ad | 55.6 ± 5.6ad |
P < 0.05,
P < 0.01 vs normal control group;
P < 0.05,
P < 0.01 vs depressed model control group.
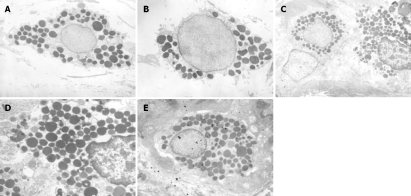
In morphology, ultrastructural observations indicated that gastric antrum mast cells were rich and strictly in perivasculitis. In normal control rats or fluoxetine + normal control rats, the mast cells were spherical in shape with round nucleus, and contained electron-dense granules. Some secretary granules were intact with homogeneous electron dense content (Figure 2A and B). In chronic stress-induced depressed rats or saline + depressed rats, the mast cells were elongated with a fusiform nucleus, granules maldistributed and contained altered electron-dense content. The mast cells were proliferative, while the granules were also hyperplastic. Mast cell secretary granules exposed to the surface of the target and mast cells contained fibrillar material, empty granules and lipid bodies (Figure 2C and D). In depressed rats treated with fluoxetine, the morphological alterations were between normal control rats and depressed rats (Figure 2E).
Figure 2.
Electron photomicrographs of mast cells from the rat gastric antrum among groups. A: A control group (× 4000); B: A fluoxetine + normal control group (× 4000); C: A depressed model group (× 4000); D: A saline + depressed model group (× 6000); E: A fluoxetine + control group (× 4000).
Effects of fluoxetine on gastric antrum rMCP-1mRNA by RT-PCR
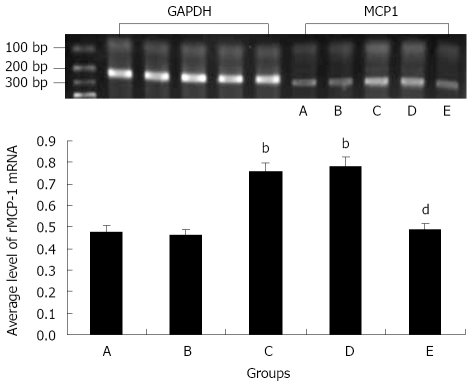
Compared with the normal control group, the average level of rMCP-1 mRNA of gastric antrum significantly increased in chronic stress-induced depressed model group or saline + depressed model group, while there was no significant difference between fluoxetine + normal control group or fluoxetine + depressed model group and normal control group. Compared with depressed model group, the average level of rMCP-1 mRNA of gastric antrum significantly decreased in fluoxetine + depressed model group, while there was no significant difference between saline + depressed model group and depressed model group (Figure 3, P < 0.01 ).
Figure 3.
Photograph and the average level of rMCP-1 mRNA of rat gastric antrum by RT-PCR among groups. A: Control group; B: Fluoxetine + control group; C: Depressed model group; D: Saline + depressed model group; E: Fluoxetine + depressed model group; bP < 0.01 vs normal control group; dP < 0.01 vs depressed model control group. rMCP-1: rat mast cell protease-1.
DISCUSSION
Mast cells are immunocytes, which are widely distributed throughout the gastrointestinal tract. Several stimuli (e.g. allergens, neuropeptides and stress) lead to mast cell activation with consequent mediator release (e.g. histamine, protease, tryptase, chymase and prostanoids)[18,19]. A number of reports indicate that mast cells can be activated by acute stress[20]. Mast cell activation has also been reported in the intestine after repetitive exposure to odors, and acute exposure to cold, which is largely used to study gastric erosion formation induced by stress, and activates both gastric and colonic motility and transit in rats[21]. In this study, chronic stress induced rMCP-1 to release in depressed rats. Chronic unpredictable heterotypic stressors appeared to induce mast cell proliferation and activation, while mast cell granules proliferated. Mast cell secretary granules in depressed rats occurred more often, exposing to the surface of the target and mast cells contained fibrillar material, empty granules and lipid bodies. Studies on mast cells illustrated the remarkable facility of mast cell population to respond to the changes in the environment by significant alterations in multiple aspects of their phenotype, including morphology, mediator content, degranulation pattern and proliferative potential[22].
On the other hand, fluoxetine showed partial inhibitive effects on mast cell ultrastructural alterations and de-regulated rMCP-1 mRNA expression in gastric antrum tissue in a rat model of depression. Fluoxetine is a serotonin reuptake inhibitor in the central nervous system as well as in mast cells. It depleted mast cell nuclear as well as cytoplasmic serotonin content[23]. Several researches found that antidepressants influenced histamine and serotonin secretion from rat peritoneal mast cells[24,25]. Maes[26] reported that various types of antidepressants, including SSRIs such as fluoxetine, had negative immunoregulatory effects. The negative immunoregulatory effects of antidepressants result from their effects on the cAMP-dependent protein kinase A (PKA) pathway. Chronic unpredicted mild stress can affect the PKA expression in rats and fluoxetine is antagonistic against it[27]. In addition, the effect of fluoxetine on rMCP-1 expression was related to neuropeptide [e.g. substance P (SP), corticotropin-releasing hormone (CRH), vasoactive intestinal polypeptide (VIP)]. In severe depression, SP serum levels are increased[28]. The data indicate that SP serum levels might be related to response to antidepressant therapy[29]. In other reports, fluoxetine can improve depressed behavior, increase VIP expression and decrease CRH expression in plasma and the duodenal tissue of depressed rats. Clinically effective therapy with antidepressants normalizes the disturbed activity of the hypothalamic-pituitary-adrenal (HPA) axis, in part by decreasing SP, CRH or increasing VIP synthesis[30].
These findings will conduce to understand that chronic heterotypic stress may induce the immune responses in gastric mucosa. Treatment with fluoxetine can ameliorate pathological changes in gastric antrum of depressed rat model, suggesting that SSRIs are an effective therapeutic agent for some gastroduodenal diseases caused by psychological factors.
COMMENTS
Background
In clinical studies, it has become clear that depression plays an important role in gastrointestinal diseases by precipitating exacerbation of symptoms. The stress may induce mast cell activation and degranulation.
Research frontiers
Some data strongly suggested that mast cells played an important role in pathophysiology of gastrointestinal diseases. Selective serotonin reuptake inhibitors (SSRIs) have been shown to be immunomodulatory and anti-inflammatory against proinflammatory cytokine processes.
Innovations and breakthroughs
The authors established a rat depression model, and observed the level of mast cell protease-1 (rMCP-1) expression in gastric antrum and the effects of fluoxetine on mast cell morphology and rMCP-1 expression in the depressed rats.
Applications
These findings will conduce to understand that chronic heterotypic stress may induce the immune responses in gastric mucosa. Treatment with fluoxetine can ameliorate pathological changes in gastric antrum of depressed rat model, suggesting that SSRIs are an effective therapeutic agent for some gastroduodenal diseases caused by psychological factors.
Terminology
Rat mast cell protease-1 (rMCP-1) is released and is associated with mast cell activation and degranulation.
Peer review
The authors of the present study showed that depression led to mast cell proliferation and activation in the gastric antrum. Fluoxetine counteracted such changes in gastric antrum in the depressed rat model.
Acknowledgments
We thank Dr. Shen-Lin Lei for his technical assistance.
Footnotes
Peer reviewer: Zong-Jie Cui, PhD, Professor, Institute of Cell Biology, Beijing Normal University, 19 Xin Jie Kou Wai Da Jie, Beijing 100875, China
S- Editor Tian L L- Editor Ma JY E- Editor Ma WH
References
- 1.Stelekati E, Orinska Z, Bulfone-Paus S. Mast cells in allergy: innate instructors of adaptive responses. Immunobiology. 2007;212:505–519. doi: 10.1016/j.imbio.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Yu LC, Perdue MH. Role of mast cells in intestinal mucosal function: studies in models of hypersensitivity and stress. Immunol Rev. 2001;179:61–73. doi: 10.1034/j.1600-065x.2001.790107.x. [DOI] [PubMed] [Google Scholar]
- 3.Gui XY. Mast cells: a possible link between psychological stress, enteric infection, food allergy and gut hypersensitivity in the irritable bowel syndrome. J Gastroenterol Hepatol. 1998;13:980–989. doi: 10.1111/j.1440-1746.1998.tb00558.x. [DOI] [PubMed] [Google Scholar]
- 4.Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28–36; discussion 37. [PubMed] [Google Scholar]
- 5.Kurina LM, Goldacre MJ, Yeates D, Gill LE. Depression and anxiety in people with inflammatory bowel disease. J Epidemiol Community Health. 2001;55:716–720. doi: 10.1136/jech.55.10.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haug TT, Mykletun A, Dahl AA. Are anxiety and depression related to gastrointestinal symptoms in the general population? Scand J Gastroenterol. 2002;37:294–298. doi: 10.1080/003655202317284192. [DOI] [PubMed] [Google Scholar]
- 7.Sykes MA, Blanchard EB, Lackner J, Keefer L, Krasner S. Psychopathology in irritable bowel syndrome: support for a psychophysiological model. J Behav Med. 2003;26:361–372. doi: 10.1023/a:1024209111909. [DOI] [PubMed] [Google Scholar]
- 8.Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–1108. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Brien SM, Scully P, Scott LV, Dinan TG. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients. J Affect Disord. 2006;90:263–267. doi: 10.1016/j.jad.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Obuchowicz E, Marcinowska A, Herman ZS. [Antide-pressants and cytokines--clinical and experimental studies] Psychiatr Pol. 2005;39:921–936. [PubMed] [Google Scholar]
- 11.Maes M. The immunoregulatory effects of antidepressants. Hum Psychopharmacol. 2001;16:95–103. doi: 10.1002/hup.191. [DOI] [PubMed] [Google Scholar]
- 12.Kubera M, Lin AH, Kenis G, Bosmans E, van Bockstaele D, Maes M. Anti-Inflammatory effects of antidepressants through suppression of the interferon-gamma/interleukin-10 production ratio. J Clin Psychopharmacol. 2001;21:199–206. doi: 10.1097/00004714-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Wang GH, Dong HY, Dong WG, Wang XP, Luo HS, Yu JP. Protective effect of Radix Acanthopanacis Senticosi capsule on colon of rat depression model. World J Gastroenterol. 2005;11:1373–1377. doi: 10.3748/wjg.v11.i9.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang PC, Jury J, Soderholm JD, Sherman PM, McKay DM, Perdue MH. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol. 2006;168:104–114; quiz 363. doi: 10.2353/ajpath.2006.050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimitriadou V, Lambracht-Hall M, Reichler J, Theoharides TC. Histochemical and ultrastructural characteristics of rat brain perivascular mast cells stimulated with compound 48/80 and carbachol. Neuroscience. 1990;39:209–224. doi: 10.1016/0306-4522(90)90234-u. [DOI] [PubMed] [Google Scholar]
- 16.Letourneau R, Rozniecki JJ, Dimitriadou V, Theoharides TC. Ultrastructural evidence of brain mast cell activation without degranulation in monkey experimental allergic encephalomyelitis. J Neuroimmunol. 2003;145:18–26. doi: 10.1016/j.jneuroim.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Ide H, Itoh H, Tomita M, Murakumo Y, Kobayashi T, Maruyama H, Osada Y, Nawa Y. Cloning of the cDNA encoding a novel rat mast-cell proteinase, rMCP-3, and its expression in comparison with other rat mast-cell proteinases. Biochem J. 1995;311(Pt 2):675–680. doi: 10.1042/bj3110675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbara G, Stanghellini V, De Giorgio R, Corinaldesi R. Functional gastrointestinal disorders and mast cells: implications for therapy. Neurogastroenterol Motil. 2006;18:6–17. doi: 10.1111/j.1365-2982.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 19.Brown JK, Knight PA, Wright SH, Thornton EM, Miller HR. Constitutive secretion of the granule chymase mouse mast cell protease-1 and the chemokine, CCL2, by mucosal mast cell homologues. Clin Exp Allergy. 2003;33:132–146. doi: 10.1046/j.1365-2222.2003.01571.x. [DOI] [PubMed] [Google Scholar]
- 20.Theoharides TC, Spanos C, Pang X, Alferes L, Ligris K, Letourneau R, Rozniecki JJ, Webster E, Chrousos GP. Stress-induced intracranial mast cell degranulation: a corticotropin-releasing hormone-mediated effect. Endocrinology. 1995;136:5745–5750. doi: 10.1210/endo.136.12.7588332. [DOI] [PubMed] [Google Scholar]
- 21.Enck P, Frieling T. Neurogastroenterology--information processing from the viscera to the brain in humans. Dtsch Tierarztl Wochenschr. 1998;105:468–471. [PubMed] [Google Scholar]
- 22.Galli SJ. New insights into “the riddle of the mast cells”: microenvironmental regulation of mast cell development and phenotypic heterogeneity. Lab Invest. 1990;62:5–33. [PubMed] [Google Scholar]
- 23.Csaba G, Kovacs P, Pallinger E. Hormones in the nucleus. Immunologically demonstrable biogenic amines (serotonin, histamine) in the nucleus of rat peritoneal mast cells. Life Sci. 2006;78:1871–1877. doi: 10.1016/j.lfs.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 24.Ferjan I, Erjavec F. Changes in histamine and serotonin secretion from rat peritoneal mast cells caused by antidepre-ssants. Inflamm Res. 1996;45:141–144. doi: 10.1007/BF02265168. [DOI] [PubMed] [Google Scholar]
- 25.Purcell WM, Hanahoe TH. The activity of amitriptyline as a differential inhibitor of amine secretion from rat peritoneal mast cells: the contribution of amine uptake. Agents Actions. 1990;30:41–43. doi: 10.1007/BF01968993. [DOI] [PubMed] [Google Scholar]
- 26.Maes M, Kenis G, Kubera M, De Baets M, Steinbusch H, Bosmans E. The negative immunoregulatory effects of fluoxetine in relation to the cAMP-dependent PKA pathway. Int Immunopharmacol. 2005;5:609–618. doi: 10.1016/j.intimp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Hu SY, Lei DL, Song WX. [Effect of chronic stress on PKA and P-CREB expression in hippocampus of rats and the antagonism of antidepressors] Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31:767–771. [PubMed] [Google Scholar]
- 28.Bondy B, Baghai TC, Minov C, Schule C, Schwarz MJ, Zwanzger P, Rupprecht R, Moller HJ. Substance P serum levels are increased in major depression: preliminary results. Biol Psychiatry. 2003;53:538–542. doi: 10.1016/s0006-3223(02)01544-5. [DOI] [PubMed] [Google Scholar]
- 29.Lieb K, Walden J, Grunze H, Fiebich BL, Berger M, Normann C. Serum levels of substance P and response to antidepressant pharmacotherapy. Pharmacopsychiatry. 2004;37:238–239. doi: 10.1055/s-2004-832599. [DOI] [PubMed] [Google Scholar]
- 30.Budziszewska B, Jaworska-Feil L, Tetich M, Basta-Kaim A, Kubera M, Leskiewicz M, Lason W. Regulation of the human corticotropin-releasing-hormone gene promoter activity by antidepressant drugs in Neuro-2A and AtT-20 cells. Neuropsychopharmacology. 2004;29:785–794. doi: 10.1038/sj.npp.1300379. [DOI] [PubMed] [Google Scholar]