Abstract
AIM: To determine if blockade of P-selectin in the isolated blood-perfused cold ex vivo rat liver model protects the liver from ischemia-reperfusion injury.
METHODS: The effect of P-selectin blockade was assessed by employing an isolated blood-perfused cold ex vivo rat liver with or without P-selectin antibody treatment before and after 6 h of cold storage in University of Wisconsin solution.
RESULTS: In our isolated blood-perfused rat liver model, pre-treatment with P-selectin antibody failed to protect the liver from ischemia-reperfusion injury, as judged by the elevated aspartate aminotransferase activity. In addition, P-selectin antibody treatment did not significantly reduced hepatic polymorphonuclear leukocyte accumulation after 120 min of perfusion. Histological evaluation of liver sections obtained at 120 min of perfusion showed significant oncotic necrosis in liver sections of both ischemic control and P-selectin antibody-treated groups. However, total bile production after 120 min of perfusion was significantly greater in P-selectin antibody-treated livers, compared to control livers. No significant difference in P-selectin and ICAM-1 mRNAs and proteins, GSH, GSSG, and nuclear NF-κB was found between control and P-selectin antibody-treated livers.
CONCLUSION: In conclusion, we have shown that blockade of P-selectin alone failed to reduced polymorphonuclear leukocyte accumulation in the liver and protect hepatocytes from ischemia-reperfusion injury in the isolated blood-perfused cold-ex vivo rat liver model.
Keywords: P-selectin, Ischemia-reperfusion, Antibody-blockade, Liver, Rat
INTRODUCTION
Ischemia-reperfusion (I/R)3 injury has been shown to play a major role in clinical and experimental hemorrhagic shock, organ resection, and transplantation[1-5]. The inflammatory component of I/R injury is mediated by pro-inflammatory cytokines such as TNF-α and IL-1β, and cellular adhesion molecules such as β2-integrins, ICAM-1, VCAM-1, and members of the selectin family, P-, E-, and L-selectin[6-8]. The sequence of events currently enjoying the most popularity as the mechanism responsible for I/R injury of the liver is: (1) KC are activated following I/R[9]; (2) During early reperfusion (0-2 h), KC are further activated by complement and produce significant vascular oxidative stress[10]; (3) KC also produce pro-inflammatory cytokines and chemokines, which is dependent on the activation of the redox-sensitive transcription factor NF-κB[11]. Activated hepatocytes and endothelial cells also produce reactive oxygen species (ROS) and contribute to the liver cytokine-chemokine milieu; (4) Cytokine mediated induction of adhesion molecules such as P- and E-selectins, ICAM-1, and VCAM-1 on the liver endothelium occur during reperfusion; (5) PMNs accumulate in the liver as a result of P- and E-selectin-mediated rolling and margination on the liver endothelium, followed by ICAM-1-dependent firm adhesion. Although PMNs accumulate in the liver during early reperfusion, they do not contribute to liver injury until the latter phase (6-24 h) of I/R injury[10,12,13]; and (6) PMNs transmigrate to the liver parenchyma via ICAM-1 and VCAM-1, bind to hepatocytes via ICAM-1/β2-integrins (CD11b/CD18), and engage in a sustained production of ROS to produce intracellular oxidative stress in hepatocytes and cell death[14-17].
Following I/R of several organs or tissues, a general mechanism of selectin-dependent rolling of PMNs followed by firmer adhesion to endothelial cells by integrins and ICAM-1 is applicable to their vasculature (heart, lung, intestine, and cremaster muscle). Accordingly, numerous studies reported that anti-P-selectin therapy afforded protection to the liver from I/R injury[18-21]. However, this general mechanism may not be applicable to the liver[13,14]. Numerous reports suggest that P-selectin attenuates I/R injury of the liver by mediating the recruitment of PMNs[18-20], while other reports minimize its role in liver I/R injury and its role in recruiting PMNs in the inflamed liver vasculature[21-26]. Furthermore, hepatic PMNs accumulation, mediated by P-selectin expressed on endothelial cells of postsinusoidal venules, might not contribute significantly to liver injury, because there is no experimental evidence supporting extravasation of these neutrophils to the liver parenchyma[23,26]. In addition, a recent report by Kubes et al. suggest that the protective effect observed in the liver with anti-P-selectin therapy may be mostly secondary to the anti-P-selectin therapy of accompanying intestinal I/R injury[27].
If the above scenario is to hold, then blockade of P-selectin should prevent or attenuate I/R injury, at least during the latter phase of I/R injury in the warm in vivo liver model. Therefore, to investigate if P-selectin blockade alone protects the liver from I/R injury, we employed an antibody to P-selectin and a cold-ex vivo I/R rat liver model. The present study demonstrates that while anti-P-selectin treatment may increase total bile flow in livers subjected to I/R, it failed to protect hepatocytes in the isolated blood-perfused rat liver model.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (250-350 g) were purchased Charles Rivers, Houston, TX). All an imals used in this study received a nutritionally balanced rodent diet, water ad libitum, and were cared for according to NIH guidelines.
Isolated-Perfused-Rat-Liver (IPRL) model
In brief, animals were anaesthetized with Nembutal (50-60 mg/kg bd. wt., ip, Sigma-Aldrich, St. Louis, MO), and under aseptic conditions, a laparotomy performed to access the liver for mobilization. Livers were carefully isolated from male Sprague-Dawley rats under Nembutal anesthesia after cannulation of the portal vein, common bile duct, and suprahepatic vena cava, while constantly perfused with oxygenated Krebs-Hensleit buffer (pH 7.4) via the portal vein[28]. Immediately after isolation, control and treated livers were flushed with 10 mL of pristine UW solution, and stored at 4°C for 6 h. Livers in the treated group received an additional flush of 1 mL of UW solution containing 420 μg of P-selectin Ab (CD62P, Cat.#553716, PharMingen, San Diego, CA) via the portal vein before cold-ex vivo ischemia (storage) and immediately before perfusion. This antibody has been show to inhibit the binding of neutrophils to rat P-selectin in both in vitro and in vivo studies. Control livers were also flushed with 1 mL of pristine UW solution immediately before perfusion. At the end of cold storage, livers were perfused with syngenic rat blood (diluted with Krebs-Hensleit buffer (pH 7.4) to a hematocrit of 12%, total volume 100 mL) in a re-circulating perfusion system using a fully-jacketed isolated-perfusion-rat-liver apparatus (RGT #130003, Radnoti Glass Technology, Inc., Monrovia, CA) for 120 min, as previously described[10]. Prior to perfusion, the perfusion apparatus was primed with blood perfusate at 37°C. Oxygenation was done with a membrane-oxygenating chamber (PO2 held > 250 mm Hg) monitored with inline-digital pressure transducer. Portal vein perfusate flow was continually adjusted to maintain portal pressures between 18 and 23 mm Hg, and monitored with inline-digital pressure transducer. Temperature, pH, and oxygen level were maintained throughout each experiment. Liver sections (snap-frozen in liquid nitrogen), blood perfusate, and bile (collected in pre-weighed eppendorf tubes) were collected every 30 min during perfusion. At the end of each experiment, sections of the liver were snap-frozen or placed in buffered-formalin, for blinded-histological evaluation of hematoxylin and eosin (HE) stained liver sections by a pathologist and determination of PMNs accumulation in the liver.
Plasma aspartate aminotransferase activity
Plasma AST activity was determined with a commercially available kit (#DG158K-U, Sigma Diagnostics, St. Louis, MO).
Histological analysis of liver injury
HE sections from formalin-fixed liver tissues obtained from sham-control and P-selectin Ab-treated livers were randomly selected and blindly analyzed for the degree of necrosis, hepatocellular vacuolization, glycogen depletion, zonal variations, and sinusoidal congestion, as measures of hepatic injury.
Polymorphonuclear leukocyte (PMNs) accumulation in the liver
PMNs accumulation in rat livers during perfusion was determined in formalin-fixed paraffin sections of the liver obtained at each perfusion-sampling time point as previously described[29]. A commercially available kit (91-C, Sigma-Aldrich, St. Louis, MO) was used to stain for sinusoidal-sequestrated PMNs using the well-established Naphthol AS-D Chloroacetate esterase procedure, according to the manufacturer’s directions. At least four random sections from each group were analyzed by viewing (blindly) fifty random high power fields (HPF, × 40) on each section. Results were expressed as number of PMNs/50HPF.
RT-PCR analysis of Liver P-selectin and ICAM-1 mRNAs
Total RNA was extracted from liver tissue using an UltraSpec Total RNA Isolation Kit (#BL-10050, Biotecx Laboratories Inc., Houston, TX). Complementary DNA (cDNA) was transcribed with 4 μg of total RNA, random hexamers, and a SuperScript II Preamplification System (#18089-011, GIBCO BRL, Life Technologies, Grand Island, New York) according to the manufacturer’s protocol. Using specific primers for p-selectin ICAM-1, and GAPDH, their cDNAs were amplified by polymerase chain reaction (PCR) under the following conditions: P-selectin and ICAM-1 (35 cycles, 94°C for 60 s, 56°C for 60 s, and 72°C for 120 s), and GAPDH, (28 cycles, 94°C for 60 s, 52°C for 60 s, and 72°C for 60 s). PCR reaction primers (Sigma-Genosys, Woodlands, TX) used were as follows: P-selectin forward primer (5'-TGTATCCAGCCTCTTGGGCATTCC-3') and P-selectin reverse primer (5'-TGGGACAGGAAGTGATGTTACACC-3') to give an 350-bp product; ICAM-1 forward primer (5'-AGGTGTGATATCCGGTAG-3') and ICAM-1 reverse primer (5'-TGGGACAGGAAGTGATGTTACACC-3') to give an 595-bp product; GAPDH forward primer (5'-GCCAAGTATGACATCAA-3') and GAPDH reverse primer (5'-CCATATTCATTGTCATACCA-3') to give a 203-bp product. All PCR products were electrophoresed on a 2% agarose gel (Fisher Scientific, Fair Lawn, NJ). Bands were visualized by post staining for 30 min with GelStar Nucleic Acid Gel Stain (FMC Bioproducts, Rockland, MA), and photographed. Photographs were digitized and evaluated as stated above. The relative expression of P-selectin and ICAM-1 messenger RNAs (mRNA) were assessed by taking the ratio of the intensity of the DNA bands of P-selectin and ICAM-1 to GAPDH band, and expressed as arbitrary units. To ensure an equal amount of RNA was used for all samples, RNA concentration was determined spectrophotometrically, and its integrity evaluate on agarose gel. DNA bands were digitized (Corel Photohouse 2.0, Ontario, Canada) and evaluated using an image analysis software (Scion Image Beta 3b, NIH Image modified for Windows by Scion Corporation, Frederick MD).
Western blot analysis of liver P-selectin and ICAM-1 proteins
Homogenates and supernatants of liver samples were prepared as described by Vural et al[30]. Proteins (P-selectin (14 μg) and ICAM-1 (27 μg)) in liver supernatants were separated on 6% NuPAGE gels (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membranes (Schleicher and Schuel, Dassel). Equal transfer to membranes was confirmed by staining the membranes with Ponceau S (Aldrich-Sigma, St. Louis, MO). P-selectin bands were detected on membranes by incubating with an anti-P-selectin-specific rabbit primary antibody diluted 1:100 (CD62P, PharMingen, San Diego, CA). A horseradish peroxidase-conjugated anti-rabbit secondary antibody (sc-2350, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) diluted 1:1000 and an enhanced chemiluminescence (ECL) kit (Amersham Life Sciences, Piscataway, NJ) were used to visualize bands. ICAM-1 was detected on membranes by incubating with an anti-ICAM-1-specific mouse primary antibody (MCA1333R, Serotec, Raleigh, NC) diluted 1:50. A horseradish peroxidase-conjugated anti-mouse secondary antibody (Amersham Life Sciences, Piscataway, NJ) diluted 1:1000 was used to reveal ICAM-1 as described above. To ensure equal loading and normalize Western-blot bands of P-selectin and ICAM-1, membranes were also probed for β-actin. Membranes were immunoblotted for β-actin with an affinity purified goat polyclonal antibody diluted 1:500 (sc-1616, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and a horseradish peroxidase-conjugated anti-goat secondary antibody (sc-2350, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) (diluted 1:1000). Actin bands were visualized with a commercially available ECL kit, as stated above.
Liver total (GSH + GSSG) and oxidized (GSSG) glutathione levels
Liver GSH + GSSG and GSSG levels were determined by the method of Tietze[31], as previously described by Jaeschke et al[14].
Liver nuclear factor-kappa B (NF-κB) activation
Activation of the redox-sensitive transcription factor NF-κB in control and P-selectin Ab-treated livers was measured using a commercially available ELISA kit (Trans-AMTM NF-κB p65 Transcription Factor Assay Kit, Active Motif, Carlsbad, CA). The assay was performed according to the manufacturer’s procedure and as described by Renard et al[32]. Nuclear proteins were extracted according to the procedure of Osarogiagbon et al[33]. Approximately 100 mg of snap-frozen liver tissue was homogenized in 0.4 mL of cold TM buffer (10 mmol/L Tris-HCl, 1 mmol/L MgCl2 (pH 7.0), containing completeTM protease inhibitors (Roche Diagnostics Corp., IN). Homogenates were centrifuged at 2000 r/min for 30 s, and the supernatant mixed with 200 μl of lysis buffer, incubated at 4°C for 5 min, and centrifuged at 5000 r/min for 10 min. The nuclear pellets were reconstituted with lysis buffer, and centrifuged at 14 000 r/min for 20 s at 4°C. Nuclear protein extract (15 μg) of each sample was used to assay for NF-κB activation. To ensure the specificity of the assay, the wild-type consensus oligonucleotide provided by the manufacturer served as a competitor to NF-κB binding.
Protein concentration
All protein concentrations were determined according to the method of Lowry et al[34].
Statistical analysis
Data were analyzed by unpaired Student’s t-test or ANOVA. After ANOVA, results were subsequently subjected to Tukey’s or Student-Newman Keuls non-parametric test to determined significant differences between groups.
RESULTS
Plasma AST activity, histologic analysis, and PMNs accumulation in the cold ex vivo -perfused liver
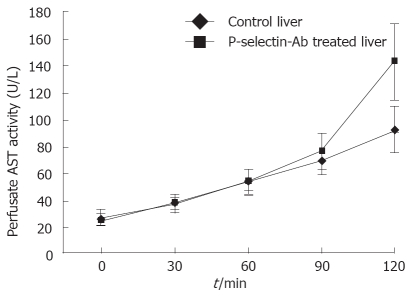
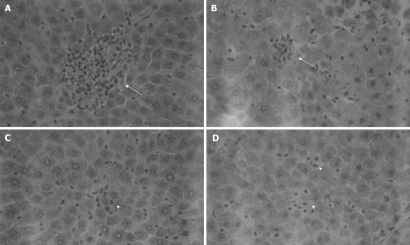
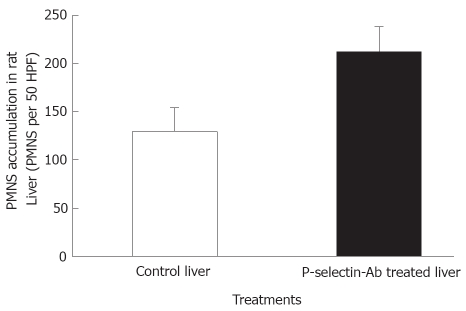
No significant difference in AST activity was found between control and P-selectin Ab-treated livers throughout the perfusion period (Figure 1). In addition, after 120 min of perfusion, similar degrees of point necrosis (solid arrows, Figure 2A, B) and inflammation (arrow heads, Figure 2C, D) were found in both cold ex vivo-perfused control and P-selectin Ab-treated livers. Similarly, no significant difference in hepatic PMNs accumulation was found between control and P-selectin Ab-treated livers after 120 min of perfusion (Figure 3).
Figure 1.
Perfusate aspartate aminotransferase (AST) activity in isolated-blood-perfused control and P-selectin-Ab treated rat livers after 6 h of cold ischemia.
Figure 2.
Histological analysis of isolated-blood-perfused control and P-selectin Ab-treated rat liver sections at 120 min perfusion after 6 h of cold ischemia (HE × 400). A, B: Point necroses in livers of control and P-selectin Ab-treated livers at 120 min perfusion (solid arrows); C, D: Inflammation in both control and P-selectin Ab-treated livers at 120 min of perfusion (arrow heads).
Figure 3.
Accumulation of polymorphonuclear leukocytes (PMNs) in isolated-blood-perfused control and P-selectin Ab-treated rat livers at 120 min perfusion after 6 h of cold ischemia.
Bile production in cold ex vivo-perfused liver
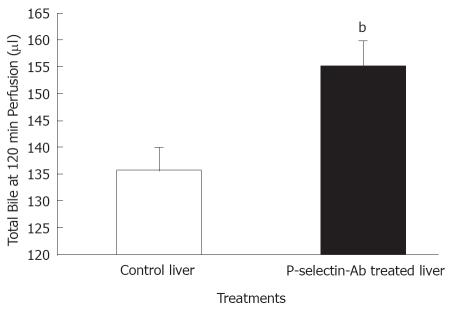
No significant difference in bile flow was found between control and P-selectin Ab-treated livers at individual sampling time-points throughout the perfusion period (data not shown). However, total bile production after 120 min of perfusion was significantly greater in P-selectin Ab-treated livers, compared to control (Figure 4).
Figure 4.
Bile production by isolated-blood-perfused control and P-selectin Ab-treated rat livers after 6 h of cold ischemia. bP = 0.009 vs control liver.
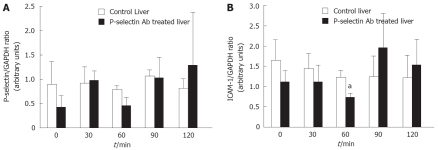
RT-PCR analysis of P-selectin and ICAM-1 mRNAs in the cold-ex vivo perfused liver
No significant difference in P-selectin mRNA and protein was found between cold-ex-vivo perfused control and P-selectin Ab-treated livers throughout the entire perfusion period (Figure 5A, B). In contrast, a significant reduction in ICAM-1 mRNA was found between cold-ex-vivo perfused control and P-selectin Ab-treated livers at 60 min of perfusion (Figure 5A). However, although a corresponding reduction in ICAM-1 protein was found, it did not reach statistical significance (Figure 5B). No significant difference in ICAM-1 mRNA and protein was found between cold ex-vivo-perfused control and P-selectin Ab-treated livers at all other time points of the perfusion period (Figure 5).
Figure 5.
Semi-quantitative RT-PCR analysis of P-selectin and ICAM-1 mRNA levels in isolated-blood-perfused control and P-selectin Ab-treated rat livers after 6 h of cold ischemia. aP < 0.05 vs control liver.
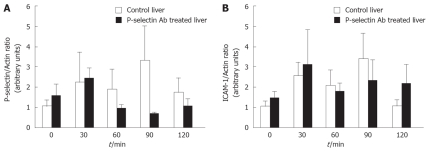
Western blot analysis of P-selectin and ICAM-1 proteins in the cold-ex-vivo perfused liver
No significant difference in P-selectin and ICAM-1 proteins was found between cold ex-vivo-perfused control and P-selectin Ab-treated livers throughout the entire perfusion period (Figure 6A, B). Although P-selectin expression at 90 min of perfusion is clearly greater in control livers compared to Ab-treated livers, the intra-group variance precluded statistical significance.
Figure 6.
Western blot analysis of P-selectin and ICAM-1 protein levels in isolated-blood-perfused control and P-selectin Ab-treated rat livers after 6 h of cold ischemia.
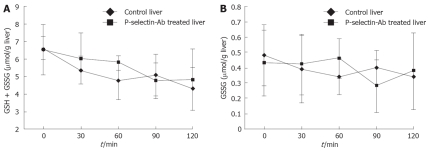
GSH + GSSG and GSSG levels in the cold-ex vivo-perfused liver
No significant difference in GSH + GSSG and GSSG levels was found between cold ex-vivo-perfused control and P-selectin Ab-treated livers throughout the entire perfusion period (Figure 7).
Figure 7.
Reduced (GSH + GSSG) and oxidized (GSSG) glutathione levels in isolated-blood-perfused control and P-selectin Ab-treated rat livers after 6 h of cold ischemia.
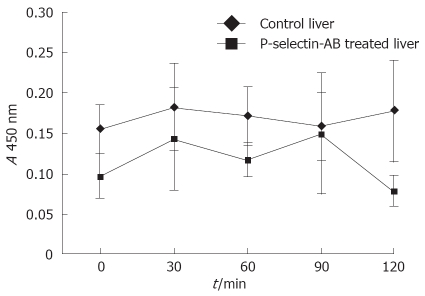
NF-κB activation in cold ex vivo-perfused liver
No significant difference in NF-κB activation was found between cold ex-vivo-perfused control and P-selectin Ab-treated livers throughout the entire perfusion period (Figure 8).
Figure 8.
ELISA analysis of nuclear p65 as a measure of liver NF-κB activation in isolated-blood-perfused control and P-selectin Ab-treated rat livers after 6 h of cold ischemia.
DISCUSSION
Our study demonstrates that antibody-blockade of P-selectin alone failed to protect the rat liver from I/R injury in the IPRL model. However, as stated earlier, considerable evidence exist that suggests that P-selectin plays a major role in I/R injury[18-21]. Existing reports also suggest that P-selectin mediates I/R injury of the liver by mediating initial rolling and margination of PMNs in the liver vasculature[18-20]. However, other reports minimize its role in liver I/R injury, and its role in recruiting PMNs in the inflamed liver vasculature[21-26].
At present, no convincing evidence exist for P-selectin expression on the mouse, human, and rat sinusoidal endothelia under normal and inflammatory conditions[22,35,36], although a more recent study reported immunohistochemical evidence of P-selectin protein expression on the rat liver sinusoid endothelium after cold storage and orthotopic liver transplantation[37]. In the present study, the accumulation of PMNs in livers after P-selectin blockade is probably due to physical trapping, resulting from the swelling of sinusoidal cells (e.g. endothelial and Kupffer cells), direct vasoconstriction, reduced deformability of PMNs exposed to activated complement factors, and the pre-existing intimacy (endothelium massaging) PMNs share with the sinusoidal endothelium, compared to the postsinusoidal endothelium. Once PMNs are slowed in the sinusoids, firm adhesion and transmigration may be mediated by ICAM-1/β2-integrins and VCAM-1/β1-integrins[23,38]. Alternatively, other, as yet unidentified, adhesion molecules may play a major role in the sequestration and transmigration of PMNs in liver sinusoids.
P-selectin blockade failed to protect the liver from I/R injury in our cold ex vivo model, as judged by the time dependent increase in AST activity, PMNs accumulation, and the similar histological-injury pattern found in control and P-selectin Ab-treated livers. Our findings are not in agreement with results of other studies that reported protection of the liver in cold ex vivo model with P-selectin blockade alone using the P-selectin ligands sPSGL-1and rPSGL-Ig[19,39] or an antibody to PSGL-1[40]. However, P-selectin blockade did enhance total bile production by 120 min of perfusion, which agrees with earlier reports[39,40]. Exactly how P-selectin blockade enhanced total bile production remains unclear.
P-selectin and ICAM-1 have been reported to be primary mediators of PMNs sequestration in the liver following I/R[19,20,38,41]. However, in this study, P-selectin blockade had little or no effect on P-selectin and ICAM-1 mRNA and protein levels except for the significant decrease in ICAM-1 mRNA found at 60 min perfusion, which had a concomitant decrease in ICAM-1 protein that did not reach statistical significance. These results support our finding that P-selectin blockade did not significantly alter PMNs accumulation in the liver at 120 min perfusion. In fact, P-selectin blockade caused an increase in PMNs accumulation in the liver, but not to a significant level. In addition, numerous studies have reported that ICAM-1 mediates firm adhesion and transmigration of PMNs in the liver following an inflammatory stimulus[42-47]. However, some studies have questioned the absolute role of ICAM-1 in PMNs sequestration in hepatic vasculature[21,48-52]. Furthermore, although the adhesion molecules ICAM-1 and VCAM-1 are expressed on cells lining the sinusoids, antibodies to selectins, integrins, and CAMs have all failed to prevent accumulation of PMNs in the liver sinusoids.
It is well documented that GSH plays a protective role in liver I/R injury[1,53-57]. GSH can react with ROS such as hydrogen peroxide, peroxynitrite, and hypochlorous acid generated by KC and PMNs. We measured liver GSH and GSSG levels in our cold ex vivo model and found no significant difference with P-selectin-blockade treatment. If significant oxidative stress occurred during reperfusion, an increase in liver GSSG should have occurred[1,53]. Although the major oxidative stress observed during perfusion is in the liver vasculature[1,53], we did not measure GSSG levels in the perfusate. Nevertheless, recent evidence from livers reperfused after cold storage has shown that hepatocytes may also be a source oxidative changes capable of impairing liver function during reperfusion[58]. Therefore, in this study we should have detected any significant change in GSH or GSSG as an intracellular oxidative stress that occurred during reperfusion. The lack of significant change in liver total GSH and GSSG in our IPRL model may be taken as the absence/attenuation of oxidative stress in the liver[53]. An alternate explanation for our failure to detect liver oxidative stress is that the above study used a more sensitive fluorescence detection compared to the colorimetric method employed in this study.
NF-κB activation in the liver following I/R has been reported[42,43,59]. Its activation has been reported as a requirement for I/R-dependent TNF-α induction in the liver[59]. The inflammatory response following I/R of the liver is primarily mediated by cytokines (e.g. TNF-α, IL-1β) and adhesion molecules P-selectin and ICAM-1, and VCAM-1. Induction of all the above inflammatory mediators requires activation of the transcription factor NF-κB. In addition, existing evidence suggest that redox-sensitive transcription factors NF-κB activation mediates the gene expression of pro-inflammatory cytokines such as TNF-α and IL-1β[11]. Therefore, this study addressed the activation of liver NF-κB in our cold ex vivo model, and failed to detect any significant reduction in liver NF-κB activation with P-selectin blockade throughout the perfusion period. This finding supports our results for P-selectin and ICAM-1 expression, with the exception of ICAM-1 mRNA levels at 60 min perfusion. A corresponding decrease in NF-κB activation was found at 60 min perfusion with P-selectin blockade, but did not achieve statistical significance.
Although most studies that characterized the benefit of P-selectin blockade in liver I/R injury used monoclonal antibodies or recombinant P-selectin ligands, in the present study we used a polyclonal antibody. Our use of a polyclonal antibody is not unusual, since other investigators have employed polyclonal antibodies to investigate blockade of mediators involved in I/R injury[60]. It is likely that our results are not in agreement with earlier studies because we employed a polyclonal antibody and at a different dose. Alternately, the antibody may have reacted with activated platelets and complement factors, as was later found for the monoclonal antibody PB1.3 used in the initial report that reported that P-selectin blockade alone protected the liver form I/R injury[20]. Nonetheless, while the general mechanism of selectin-dependent rolling of PMNs followed by firmer adhesion to endothelial cells by integrins and ICAM-1 is applicable to the vasculature of some organs and tissues (heart, lung, intestine, and cremaster muscle), this might not be the case for the entire vasculature of the liver[23,24].
In summary, this study demonstrates that while P-selectin blockade alone increased total bile flow in the IPRL model, it failed to protect the liver from I/R injury.
Footnotes
Supported by Grants from the American Liver Foundation, Naomi Judd Liver Scholar Award, The American Surgical Association Career Development Fellowship, and National I
Peer reviewer: Ravi S Chari, MD, Associate Professor, Division of Hepatobiliary Surgery and Liver Transplantation, Departments of Surgery and Cancer Biology, 1313 21st Avenue South Suite 801 Oxford House, Vanderbilt University Medical Center, Nashville, TN 37232-4753, United States
S- Editor Li JL L- Editor Stewart GJ E- Editor Zheng XM
References
- 1.Jaeschke H, Farhood A. Kupffer cell activation after no-flow ischemia versus hemorrhagic shock. Free Radic Biol Med. 2002;33:210–219. doi: 10.1016/s0891-5849(02)00867-5. [DOI] [PubMed] [Google Scholar]
- 2.Seekamp A, Jochum M, Ziegler M, van Griensven M, Martin M, Regel G. Cytokines and adhesion molecules in elective and accidental trauma-related ischemia/reperfusion. J Trauma. 1998;44:874–882. doi: 10.1097/00005373-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Kim YI, Song KE, Ryeon HK, Hwang YJ, Yun YK, Lee JW, Chun BY. Enhanced inflammatory cytokine production at ischemia/reperfusion in human liver resection. Hepatogastroenterology. 2002;49:1077–1082. [PubMed] [Google Scholar]
- 4.Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155–162. doi: 10.1097/00000658-200008000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilmakunnas M, Petäjä J, Höckerstedt K, Mäkisalo H, Fernandez JA, Griffin JH, Jansson SE, Repo H, Pesonen EJ. Activation of protein C during reperfusion in clinical liver transplantation. Transplantation. 2003;75:467–72. doi: 10.1097/01.TP.0000046529.83650.8A. [DOI] [PubMed] [Google Scholar]
- 6.Jaeschke H. Molecular mechanisms of hepatic ischemia-reperfusion injury and preconditioning. Am J Physiol Gastrointest Liver Physiol. 2003;284:G15–G26. doi: 10.1152/ajpgi.00342.2002. [DOI] [PubMed] [Google Scholar]
- 7.Jaeschke H, Smith CW, Clemens MG, Ganey PE, Roth RA. Mechanisms of inflammatory liver injury: adhesion molecules and cytotoxicity of neutrophils. Toxicol Appl Pharmacol. 1996;139:213–226. doi: 10.1006/taap.1996.0160. [DOI] [PubMed] [Google Scholar]
- 8.Lawson JA, Burns AR, Farhood A, Lynn Bajt M, Collins RG, Smith CW, Jaeschke H. Pathophysiologic importance of E- and L-selectin for neutrophil-induced liver injury during endotoxemia in mice. Hepatology. 2000;32:990–998. doi: 10.1053/jhep.2000.19068. [DOI] [PubMed] [Google Scholar]
- 9.Rymsa B, Wang JF, de Groot H. O2-. release by activated Kupffer cells upon hypoxia-reoxygenation. Am J Physiol. 1991;261:G602–G607. doi: 10.1152/ajpgi.1991.261.4.G602. [DOI] [PubMed] [Google Scholar]
- 10.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol. 1993;264:G801–G809. doi: 10.1152/ajpgi.1993.264.4.G801. [DOI] [PubMed] [Google Scholar]
- 11.Lin M, Rippe RA, Niemelä O, Brittenham G, Tsukamoto H. Role of iron in NF-kappa B activation and cytokine gene expression by rat hepatic macrophages. Am J Physiol. 1997;272:G1355–G1364. doi: 10.1152/ajpgi.1997.272.6.G1355. [DOI] [PubMed] [Google Scholar]
- 12.Jaeschke H, Farhood A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am J Physiol. 1991;260:G355–G362. doi: 10.1152/ajpgi.1991.260.3.G355. [DOI] [PubMed] [Google Scholar]
- 13.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic Res Commun. 1991;15:277–284. doi: 10.3109/10715769109105223. [DOI] [PubMed] [Google Scholar]
- 14.Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. [PubMed] [Google Scholar]
- 15.Shappell SB, Toman C, Anderson DC, Taylor AA, Entman ML, Smith CW. Mac-1 (CD11b/CD18) mediates adherence-dependent hydrogen peroxide production by human and canine neutrophils. J Immunol. 1990;144:2702–2711. [PubMed] [Google Scholar]
- 16.Jaeschke H, Bautista AP, Spolarics Z, Spitzer JJ. Superoxide generation by neutrophils and Kupffer cells during in vivo reperfusion after hepatic ischemia in rats. J Leukoc Biol. 1992;52:377–382. doi: 10.1002/jlb.52.4.377. [DOI] [PubMed] [Google Scholar]
- 17.Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- 18.Yadav SS, Howell DN, Steeber DA, Harland RC, Tedder TF, Clavien PA. P-Selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischemic mouse liver. Hepatology. 1999;29:1494–1502. doi: 10.1002/hep.510290505. [DOI] [PubMed] [Google Scholar]
- 19.Dulkanchainun TS, Goss JA, Imagawa DK, Shaw GD, Anselmo DM, Kaldas F, Wang T, Zhao D, Busuttil AA, Kato H, et al. Reduction of hepatic ischemia/reperfusion injury by a soluble P-selectin glycoprotein ligand-1. Ann Surg. 1998;227:832–840. doi: 10.1097/00000658-199806000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Criado FJ, Toledo-Pereyra LH, Lopez-Neblina F, Phillips ML, Paez-Rollys A, Misawa K. Role of P-selectin in total hepatic ischemia and reperfusion. J Am Coll Surg. 1995;181:327–334. [PubMed] [Google Scholar]
- 21.Young CS, Palma JM, Mosher BD, Harkema J, Naylor DF, Dean RE, Crockett E. Hepatic ischemia/reperfusion injury in P-selectin and intercellular adhesion molecule-1 double-mutant mice. Am Surg. 2001;67:737–744. [PubMed] [Google Scholar]
- 22.Essani NA, Fisher MA, Simmons CA, Hoover JL, Farhood A, Jaeschke H. Increased P-selectin gene expression in the liver vasculature and its role in the pathophysiology of neutrophil-induced liver injury in murine endotoxin shock. J Leukoc Biol. 1998;63:288–296. doi: 10.1002/jlb.63.3.288. [DOI] [PubMed] [Google Scholar]
- 23.Jaeschke H. Cellular adhesion molecules: regulation and functional significance in the pathogenesis of liver diseases. Am J Physiol. 1997;273:G602–G611. doi: 10.1152/ajpgi.1997.273.3.G602. [DOI] [PubMed] [Google Scholar]
- 24.Jaeschke H. Is anti-P-selectin therapy effective in hepatic ischemia-reperfusion injury because it inhibits neutrophil recruitment? Shock. 1999;12:233–234. doi: 10.1097/00024382-199909000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Wong J, Johnston B, Lee SS, Bullard DC, Smith CW, Beaudet AL, Kubes P. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J Clin Invest. 1997;99:2782–2790. doi: 10.1172/JCI119468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chosay JG, Essani NA, Dunn CJ, Jaeschke H. Neutrophil margination and extravasation in sinusoids and venules of liver during endotoxin-induced injury. Am J Physiol. 1997;272:G1195–G1200. doi: 10.1152/ajpgi.1997.272.5.G1195. [DOI] [PubMed] [Google Scholar]
- 27.Kubes P, Payne D, Woodman RC. Molecular mechanisms of leukocyte recruitment in postischemic liver microcirculation. Am J Physiol Gastrointest Liver Physiol. 2002;283:G139–G147. doi: 10.1152/ajpgi.00058.2002. [DOI] [PubMed] [Google Scholar]
- 28.Wyllie S, Seu P, Gao FQ, Goss JA. Deregulation of iron homeostasis and cold-preservation injury to rat liver stored in University of Wisconsin solution. Liver Transpl. 2003;9:401–410. doi: 10.1053/jlts.2003.50065. [DOI] [PubMed] [Google Scholar]
- 29.Wyllie S, Seu P, Gao FQ, Gros P, Goss JA. Disruption of the Nramp1 (also known as Slc11a1) gene in Kupffer cells attenuates early-phase, warm ischemia-reperfusion injury in the mouse liver. J Leukoc Biol. 2002;72:885–897. [PubMed] [Google Scholar]
- 30.Vural KM, Liao H, Oz MC, Pinsky DJ. Effects of mast cell membrane stabilizing agents in a rat lung ischemia-reperfusion model. Ann Thorac Surg. 2000;69:228–232. doi: 10.1016/s0003-4975(99)01052-8. [DOI] [PubMed] [Google Scholar]
- 31.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–22. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 32.Renard P, Ernest I, Houbion A, Art M, Le Calvez H, Raes M, Remacle J. Development of a sensitive multi-well colorimetric assay for active NFkappaB. Nucleic Acids Res. 2001;29:E21. doi: 10.1093/nar/29.4.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osarogiagbon UR, Choong S, Belcher JD, Vercellotti GM, Paller MS, Hebbel RP. Reperfusion injury pathophysiology in sickle transgenic mice. Blood. 2000;96:314–320. [PubMed] [Google Scholar]
- 34.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 35.Steinhoff G, Behrend M, Schrader B, Duijvestijn AM, Wonigeit K. Expression patterns of leukocyte adhesion ligand molecules on human liver endothelia. Lack of ELAM-1 and CD62 inducibility on sinusoidal endothelia and distinct distribution of VCAM-1, ICAM-1, ICAM-2, and LFA-3. Am J Pathol. 1993;142:481–488. [PMC free article] [PubMed] [Google Scholar]
- 36.Wisse E. An ultrastructural characterization of the endothelial cell in the rat liver sinusoid under normal and various experimental conditions, as a contribution to the distinction between endothelial and Kupffer cells. J Ultrastruct Res. 1972;38:528–562. doi: 10.1016/0022-5320(72)90089-5. [DOI] [PubMed] [Google Scholar]
- 37.Basile J, Wang L, Tarcsafalvi A, Han R, Boros P, Miller CM. Expression of GMP-140 (P-selectin) correlates with graft viability in cold-preserved rat livers. Transplantation. 2000;69:2440–2442. doi: 10.1097/00007890-200006150-00039. [DOI] [PubMed] [Google Scholar]
- 38.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ, Smith CW. Functional inactivation of neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology. 1993;17:915–923. [PubMed] [Google Scholar]
- 39.Amersi F, Farmer DG, Shaw GD, Kato H, Coito AJ, Kaldas F, Zhao D, Lassman CR, Melinek J, Ma J, et al. P-selectin glycoprotein ligand-1 (rPSGL-Ig)-mediated blockade of CD62 selectin molecules protects rat steatotic liver grafts from ischemia/reperfusion injury. Am J Transplant. 2002;2:600–608. doi: 10.1034/j.1600-6143.2002.20704.x. [DOI] [PubMed] [Google Scholar]
- 40.Tsuchihashi S, Fondevila C, Shaw GD, Lorenz M, Marquette K, Benard S, Shen XD, Ke B, Busuttil RW, Kupiec-Weglinski JW. Molecular characterization of rat leukocyte P-selectin glycoprotein ligand-1 and effect of its blockade: protection from ischemia-reperfusion injury in liver transplantation. J Immunol. 2006;176:616–624. doi: 10.4049/jimmunol.176.1.616. [DOI] [PubMed] [Google Scholar]
- 41.Farhood A, McGuire GM, Manning AM, Miyasaka M, Smith CW, Jaeschke H. Intercellular adhesion molecule 1 (ICAM-1) expression and its role in neutrophil-induced ischemia-reperfusion injury in rat liver. J Leukoc Biol. 1995;57:368–374. [PubMed] [Google Scholar]
- 42.Zwacka RM, Zhang Y, Zhou W, Halldorson J, Engelhardt JF. Ischemia/reperfusion injury in the liver of BALB/c mice activates AP-1 and nuclear factor kappaB independently of IkappaB degradation. Hepatology. 1998;28:1022–1030. doi: 10.1002/hep.510280417. [DOI] [PubMed] [Google Scholar]
- 43.Yoshidome H, Kato A, Edwards MJ, Lentsch AB. Interleukin-10 suppresses hepatic ischemia/reperfusion injury in mice: implications of a central role for nuclear factor kappaB. Hepatology. 1999;30:203–208. doi: 10.1002/hep.510300120. [DOI] [PubMed] [Google Scholar]
- 44.Ghobrial R, Amersi F, Stecker K, Kato H, Melinek J, Singer J, Mhoyan A, Busuttil RW, Kupiec-Weglinski JW, Stepkowski SM. Amelioration of hepatic ischemia/reperfusion injury with intercellular adhesion molecule-1 antisense oligodeoxynucleotides. Transplant Proc. 2001;33:538. doi: 10.1016/s0041-1345(00)02130-8. [DOI] [PubMed] [Google Scholar]
- 45.Viebahn R, Thoma M, Kinder O, Schenk M, Lauchart W, Becker HD. Analysis of intragraft adhesion molecules and their release in clinical liver transplantation: impact of reperfusion injury. Transplant Proc. 1998;30:4257–4259. doi: 10.1016/s0041-1345(98)01406-7. [DOI] [PubMed] [Google Scholar]
- 46.Yadav SS, Howell DN, Gao W, Steeber DA, Harland RC, Clavien PA. L-selectin and ICAM-1 mediate reperfusion injury and neutrophil adhesion in the warm ischemic mouse liver. Am J Physiol. 1998;275:G1341–G1352. doi: 10.1152/ajpgi.1998.275.6.G1341. [DOI] [PubMed] [Google Scholar]
- 47.Nakano H, Nagasaki H, Yoshida K, Kigawa G, Fujiwara Y, Kitamura N, Kuzume M, Takeuchi S, Sasaki J, Shimura H, et al. N-acetylcysteine and anti-ICAM-1 monoclonal antibody reduce ischemia-reperfusion injury of the steatotic rat liver. Transplant Proc. 1998;30:3763. doi: 10.1016/s0041-1345(98)01225-1. [DOI] [PubMed] [Google Scholar]
- 48.Colletti LM, Cortis A, Lukacs N, Kunkel SL, Green M, Strieter RM. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil-dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat. Shock. 1998;10:182–191. doi: 10.1097/00024382-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Marubayashi S, Oshiro Y, Maeda T, Fukuma K, Okada K, Hinoi T, Ikeda M, Yamada K, Itoh H, Dohi K. Protective effect of monoclonal antibodies to adhesion molecules on rat liver ischemia-reperfusion injury. Surgery. 1997;122:45–52. doi: 10.1016/s0039-6060(97)90263-4. [DOI] [PubMed] [Google Scholar]
- 50.Rentsch M, Post S, Palma P, Lang G, Menger MD, Messmer K. Anti-ICAM-1 blockade reduces postsinusoidal WBC adherence following cold ischemia and reperfusion, but does not improve early graft function in rat liver transplantation. J Hepatol. 2000;32:821–828. doi: 10.1016/s0168-8278(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 51.Kobayashi A, Imamura H, Isobe M, Matsuyama Y, Soeda J, Matsunaga K, Kawasaki S. Mac-1 (CD11b/CD18) and intercellular adhesion molecule-1 in ischemia-reperfusion injury of rat liver. Am J Physiol Gastrointest Liver Physiol. 2001;281:G577–G585. doi: 10.1152/ajpgi.2001.281.2.G577. [DOI] [PubMed] [Google Scholar]
- 52.Jaeschke H, Farhood A, Fisher MA, Smith CW. Sequestration of neutrophils in the hepatic vasculature during endotoxemia is independent of beta 2 integrins and intercellular adhesion molecule-1. Shock. 1996;6:351–356. doi: 10.1097/00024382-199611000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Jaeschke H. Vascular oxidant stress and hepatic ischemia/reperfusion injury. Free Radic Res Commun. 1991;12-13 Pt 2:737–743. doi: 10.3109/10715769109145853. [DOI] [PubMed] [Google Scholar]
- 54.Matsui N, Satsuki I, Morita Y, Inaizumi K, Kasajima K, Kanoh R, Fukuishi N, Akagi M. Xanthine oxidase-derived reactive oxygen species activate nuclear factor kappa B during hepatic ischemia in rats. Jpn J Pharmacol. 2000;84:363–366. doi: 10.1254/jjp.84.363. [DOI] [PubMed] [Google Scholar]
- 55.Amersi F, Nelson SK, Shen XD, Kato H, Melinek J, Kupiec-Weglinski JW, Horwitz LD, Busuttil RW, Horwitz MA. Bucillamine, a thiol antioxidant, prevents transplantation-associated reperfusion injury. Proc Natl Acad Sci USA. 2002;99:8915–8920. doi: 10.1073/pnas.132026099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumamoto Y, Suematsu M, Shimazu M, Kato Y, Sano T, Makino N, Hirano KI, Naito M, Wakabayashi G, Ishimura Y, et al. Kupffer cell-independent acute hepatocellular oxidative stress and decreased bile formation in post-cold-ischemic rat liver. Hepatology. 1999;30:1454–1463. doi: 10.1002/hep.510300601. [DOI] [PubMed] [Google Scholar]
- 57.Jaeschke H. Role of reactive oxygen species in hepatic ischemia-reperfusion injury and preconditioning. J Invest Surg. 2003;16:127–140. [PubMed] [Google Scholar]
- 58.Grattagliano I, Vendemiale G, Lauterburg BH. Reperfusion injury of the liver: role of mitochondria and protection by glutathione ester. J Surg Res. 1999;86:2–8. doi: 10.1006/jsre.1999.5620. [DOI] [PubMed] [Google Scholar]
- 59.Ballatori N, Truong AT, Ma AK, Boyer JL. Determinants of glutathione efflux and biliary GSH/GSSG ratio in perfused rat liver. Am J Physiol. 1989;256:G482–G490. doi: 10.1152/ajpgi.1989.256.3.G482. [DOI] [PubMed] [Google Scholar]
- 60.Ono K, Matsumori A, Furukawa Y, Igata H, Shioi T, Matsushima K, Sasayama S. Prevention of myocardial reperfusion injury in rats by an antibody against monocyte chemotactic and activating factor/monocyte chemoattractant protein-1. Lab Invest. 1999;79:195–203. [PubMed] [Google Scholar]